Abstract
Excessive liver production of ketone bodies is one of many metabolic complications that can arise from diabetes, and in severe untreated cases, it can result in ketoacidosis, coma, and death. Mitochondrial HMG-CoA synthase (HMGCS2), the rate-limiting enzyme in ketogenesis, has been shown to interact with PPARα and act as a coactivator to up-regulate transcription from the PPRE of its own gene. Although protein palmitoylation is typically a cytosolic process that promotes membrane association, we recently identified 21 palmitoylated proteins in rat liver mitochondria, including HMGCS2. Herein, our data support a mechanism whereby palmitate is first added onto HMGCS2 active site Cys166 and then transacylated to Cys305. Palmitoylation promotes the HMGCS2/PPARα interaction, resulting in transcriptional activation from the Hmgcs2 PPRE. These results, together with the fact that 8 of the 21 palmitoylated mitochondrial proteins that we previously identified have nuclear receptor interacting motifs, demonstrate a novel—and perhaps ubiquitous—role for palmitoylation as a modulator of transcription.—Kostiuk, M. A., Keller, B. O., Berthiaume, L. G. Palmitoylation of ketogenic enzyme HMGCS2 enhances its interaction with PPARα and transcription at the Hmgcs2 PPRE.
Keywords: HMG-CoA synthase 2, ketone bodies, mitochondria
Fasting, prolonged exercise, or low-carbohydrate high-fat ketogenic diets lead to increased production (ketogenesis) and use of the ketone bodies acetoacetate and β-hydroxybutyrate generated from the breakdown of fatty acids and certain amino acids (1,2,3). In diabetes, excess ketone production can occur as a result of absolute or relative insulin deficiency and the subsequent increased levels of counterregulatory molecules, such as catecholamines and glucagon (4, 5). This disregulation can lead to ketoacidosis, a life-threatening condition characterized by hyperketonemia, hyperglycemia, and metabolic acidosis (5, 6).
Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase (HMGCS2) (EC 2.3.3.10) is the rate-limiting enzyme in ketogenesis, catalyzing the condensation reaction between acetoacetyl-CoA and acetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), which then is converted by 3-hydroxy-3-methylglutaryl-CoA lysase into the ketone bodies (2, 3). The Hmgcs2 gene contains a peroxisome proliferator response element (PPRE) in its promoter region and is under the transcriptional regulation of the peroxisome proliferator activated receptor α (PPARα) (7). Its expression is up-regulated in the diabetic state (8), and overexpression of HMGCS2 in transgenic mice leads to hyperketogenesis (9).
PPARα is one of three PPAR subtypes (α, γ, and β) that are ligand-activated transcription factors belonging to the nuclear receptor superfamily (10, 11). These proteins use fatty acids and their derivatives as natural ligands and play a major role in the expression of genes involved in energy homeostasis. PPARα is the major PPAR expressed in the liver and controls the transcription of many genes involved in fatty acid metabolism, as well as ketogenesis (7, 10, 12, 13). In comparison to wild-type mice, mice with PPARα-null alleles reveal a failure to up-regulate several mitochondrial genes, including Hmgcs2, during food deprivation and under hypoglycemic conditions (reviewed in ref. 14). Typically, transcriptional coactivators that directly bind PPARα and other nuclear receptors contain one or more conserved LXXLL nuclear receptor interaction motifs (nuclear receptor box; reviewed in ref. 15), although recent evidence suggests LXXLL-containing proteins can also act as corepressors of agonist-bound nuclear receptors (reviewed in ref. 16). Of special interest, HMGCS2 contains an LXXLL nuclear receptor box and was shown to bind PPARα, translocate to the nucleus, and up-regulate transcription from the PPRE of its own gene (17).
Along with their major roles as energy sources and building blocks for various lipids, fatty acids can also be covalently attached to proteins in a process known as protein fatty acylation. N-myristoylation and S-acylation (commonly referred to as palmitoylation) are the two main types of fatty acylation. Myristoylation occurs at N-terminal glycine residues through an irreversible amide bond, while palmitoylation occurs at internal cysteine residues through a reversible thioester bond. The specific reactions are typically catalyzed by two families of transferases: N-myristoyl transferases (NMTs) and protein fatty acyltransferases (PATs) (reviewed in refs. 18,19,20,21). Palmitoylation can also occur spontaneously (22,23,24,25,26,27,28).
Using direct labeling methods, we and others have found several proteins to be palmitoylated in mitochondria (22, 24, 29), including ∼40 in rat liver mitochondria (24). The spontaneous palmitoylation of 2 mitochondrial enzymes involved in amino acid metabolism, methylmalonyl semialdehyde dehydrogenase (MMSDH), and carbamoyl phosphate synthetase 1 (CPS 1), has been characterized. In both cases, fatty acylation was shown to occur on an active site cysteine residue, thereby inhibiting these catabolic enzymes and suggesting that a metabolic crosstalk involving protein fatty acylation exists between fatty acid and amino acid degradation pathways (22, 24). We recently identified 19 palmitoylated proteins from rat liver mitochondria (26), one of which being HMGCS2.
Palmitoylation can also be detected indirectly using the acyl-biotin exchange reaction, a method that allows the replacement of a palmitoyl moiety bound to a protein by a biotinyl moiety (30). Using variations of this method, three other groups (31,32,33) have performed large-scale screens for palmitoylated proteins. Altogether, 44 putative palmitoylated mitochondrial proteins were identified by these three groups. Nine of these 44 mitochondrial proteins (including HMGCS2) were previously identified in Kostiuk et al. (26) and are listed in Supplemental Table 1, thereby validating our previous work and warranting the need to further characterize the role of their palmitoylation as it pertains to mitochondrial metabolism.
We have previously shown that palmitoylation of HMGCS2 occurred in vitro in a solution containing only buffer, palmitoyl-CoA, and purified enzyme, suggesting that the acylation occurs in a spontaneous, palmitoyl-CoA concentration-dependent fashion (26). Palmitoylation of HMGCS2 was also detected in situ, indicating it also occurs in cells and tissue (33). Since HMGCS2 was shown to act as a coactivator for PPARα (17), we sought to investigate whether palmitoylation of HMGCS2 could be involved in the interaction with PPARα and subsequent transcriptional activation from the Hmgcs2 PPRE.
Herein, we report that palmitoylation of HMGCS2 is required for the HMGCS2/PPARα interaction and the resulting transcriptional activation at the Hmgcs2 PPRE. The level of interaction between HMGCS2 and PPARα increased in a long-chain fatty acyl-CoA concentration-dependent fashion. The ability of a palmitoylation-deficient HMGCS2 mutant to bind PPARα was drastically reduced, and consequently, it lost the ability to up-regulate transcription at the Hmgcs2 PPRE. These results demonstrate a novel role for palmitoylation as a requirement for the interaction between transcription factor and coactivator and for the first time implicate palmitoylation as a regulator of transcription.
MATERIALS AND METHODS
Materials
[125I]NaI (2.14 Ci/mmol) was from Amersham Biosciences (Little Chalfont, UK). PVDF membrane and Centricon filters were from Millipore Corp. (Temecula, CA, USA). ECL Plus was obtained from Amersham Biosciences. Leupeptin and Complete protease inhibitor cocktail were obtained from Roche Molecular Biochemicals (Indianapolis, IN, USA). Azido-tetradecanoic acid (an isosteric analog of palmitate; ref. 26) and triarylphosphine-biotin were kind gifts of Dr. Camille Falck (University of Texas Southwestern, Dallas, TX, USA) and Dr. Carolyn Bertozzi (University of California, Berkeley, CA, USA), respectively, and were described previously (26). N-ethylmaleimide (NEM), bovine trypsin, dithiothreitol (DTT), iodoacetamide (IAA), α-cyano-4-hydroxy-cinnamic acid (4-HCCA), and trifluoroacetic acid (TFA) were all of highest available quality and purchased from Sigma-Aldrich (Oakville, ON, Canada). Solvents were all HPLC grade and purchased from Fisher Scientific (Ottawa, ON, Canada). All chemicals were used without further purification.
Plasmids and mutagenesis
C-terminal hexa-His-tagged versions of the mature cDNA for human mHMG-CoA synthase (HMGCS2-His6) were constructed in the bacterial expression vector pET19b (Novagen, Madison, WI, USA) and have been described previously (26). pSG5PPARα and pGEX-GST/PPARα were a kind gift from Dr. John Capone (McMaster University, Hamilton, ON, Canada). To produce the cysteine to serine mutants of HMGCS2-His6, site-directed mutagenesis was performed using appropriate oligonucleotides and the Stratagene Quickchange II site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). To engineer a C-terminal HA tag on HMGCS2, the vectors pCDNA-HMGCS2-HA (wild type and mutants) were assembled by PCR using the HMGCS2 coding region from the pET19b-HMGCS2-His6 (wild type and mutant) plasmids as templates with the appropriate primers. The sequences of the oligonucleotides used in the mutagenesis follow; all mutations and encoding sequences were fully confirmed by DNA sequencing.
Engineering of HMGCS2 mutants: Cysl66Ser mutation, 5′-GATACCACCAATGCCCCTACGGTGGTACTGCC and 5′-GGCAGTACCACCGTAGGAGGCATTGGTGGTATC; Cys305Ser mutation, 5′-CTTTCATACACCCTTTTCCAAGATGGTCCAGAAGTCTCTGG and 5′-CAGAGACTTCTGGACCATCTTGGAAAAGGGTGTATGAAAG. The bases that were exchanged resulting in the substitution of the original codon encoding for a cysteine residue to a codon encoding for a serine residue are underscored.
Engineering a C-terminal HA tag on HMGCS2: forward 5′-AAGCTTCGAATTCATGCAGCGTCTGTTGACTCCAGTGAAGCGC and reverse 5′-ACTTCGGATCCTTAAGCGTAATCTGGAACATCGTATGGGTAGACGGGACGCCGGGCATACTTTCG. Amplified PCR products were cut with BamH1 and EcoR1 and subcloned in appropriately digested pCDNA 3.1 plasmid.
Preparation of radiolabled fatty acids
Radioiodination of the iodopalmitate with [125I]NaI (2.14 Ci/mmol; Amersham Pharmacia Biotech) was performed as described previously (34), without the high-pressure liquid chromatography purification step.
Synthesis of the [125I]-iodopalmitoyl-CoA and azidotetradecanoyl-CoA
[125I]-iodopalmitoyl-CoA and azidotetradecanoyl-CoA (azido-palmitoyl-CoA) were generated from [125I]-iodopalmitate and azidotetradecanoic acid (azido-palmitate) following the protocol reported previously (34).
Labeling of proteins with [125I]-iodopalmitoyl-CoA or azido-palmitoyl-CoA
Incubations were carried out in a final volume of 50–100 μl using 1 μg HMGCS2-His6 with typically a 50-μM final concentration of [125I]-iodopalmitoyl-CoA or 100 μM azido-palmitoyl-CoA (in some assays concentration acyl-CoA may vary) for 30 min at 25°C in 50 mM Tris (pH 7.4) or 50 mM MOPS (pH 7.4). The radioactive reactions were stopped by the addition of 0.25 vol 5× SDS-PAGE loading buffer containing 200 mM DTT, incubated for 2 min at 100°C, and loaded onto a 10% SDS-polyacrylamide gel. Following staining and drying, we exposed the gels to film, typically 12 to 24 h at −80°C. Alternatively, the gels were exposed to a phosphorimager screen (Molecular Dynamics, Sunnyvale, CA, USA) for typically 4 to 12 h and scanned on a Storm 840 phosphorimager (Molecular Dynamics).
The reactions containing the azido-palmitate analog were carried out as described in Kostiuk et al. (26). Briefly, following acylation, the reactions were adjusted to 1% SDS, and the triarylphosphine-biotin was added in a 2:1 mol:mol ratio to that of azido-palmitoyl-CoA. The reaction was allowed to proceed for another 2 h at room temperature, stopped by the addition of 0.25 vol 5× SDS-PAGE-loading buffer containing 200 mM DTT, and separated by electrophoresis. For the palmitoyl-CoA competition experiments, purified HMGCS2-His6 was preincubated for 45 min in various concentrations of palmitoyl-CoA as detailed in the figure legends prior to labeling as above. The resulting biotinylated-azido-palmitoylated proteins were detected with neutravidin-conjugated HRP (1:20,000; Pierce Biotechnology, Rockford, IL, USA) using enhanced chemiluminescence (ECL).
NEM treatment
All NEM treatments were carried out with freshly prepared NEM at the concentrations indicated in the figure legends in 50 mM HEPES or MOPS (pH 7.4) for 30 min to 1 h at room temperature in the dark.
Expression, purification, and enzymatic assay of HMGCS2-His6
Expression and purification of HMGCS2-His6 was carried out as described previously (26). Purified HMGCS2-His6 was assayed for activity using the method described by Lowe and Tubbs (35), with the exception that the incubation temperature was lowered from 30°C to 24°C.
Purification of GST-PPARα
pGEX-GST-PPARα was expressed in BL21(DE3) bacteria. Midlogarithmic 500-ml cultures were induced with 0.1 mM IPTG and allowed to express GST-PPARα for 4 h at 22°C. Pelleted bacteria were resuspended in 25 ml PBS containing 1 tablet of Complete protease inhibitor (Roche Molecular Biochemicals) per 50 ml PBS and lysed with 2 passes through a French press. Following centrifugation, the supernatant was rocked for 30 min at room temperature with 1 ml glutathione-Sepharose beads; following extensive washes, the amount of GST-PPARα bound to the beads was assessed by SDS-PAGE analysis. The beads containing known amounts of GST-PPARα were portioned into aliquots and either used immediately for binding assays or stored at −80°C.
In vitro GST-PPARα binding assays
One microgram of GST-PPARα or GST alone bound to the glutathione-Sepharose beads (typically 10 to 20 μl of beads) was incubated for 1 h in GST-binding buffer at room temperature with rocking, with amounts of HMGCS2 wild-type or mutant proteins as detailed in the figure legends. Following extensive washes in PBS, the beads were boiled in SDS loading buffer containing 40 mM DTT for 2 min, followed by SDS-PAGE. For the binding reactions following palmitoylation, 1 μg of HMGCS2-His6 wild-type or mutants was first incubated in reactions containing palmitoyl-CoA, various acyl-chain-length acyl-CoAs, or the palmitoyl-CoA analogues in concentrations shown in the figure legends, and 10% of the reaction (100 ng of protein) was used for the binding assays.
Detection of HMCS2-His6
Following SDS-PAGE and Western blot analysis, the membranes containing the proteins were probed with mouse monoclonal anti-penta-His (Qiagen, Valencia, CA, USA) in a 1:1000 ratio, followed by sheep anti-mouse IgG-HRP (GE Healthcare, New York, NY, USA) at a ratio of 1:10,000 and developed with ECL. Typical exposure to film was 5 to 10 s.
In vitro transcription and translation
In vitro transcription and translation reactions were carried out using a TNT T7 Coupled Reticulocyte Lysate System (Promega, Madison, WI, USA) according to manufacturer’s specifications, using 1 μg of plasmid DNA pSG5 HMGCS2, and 50 μl of the reaction was used in the GST-PPARα binding assays as described above.
Luciferase assays
Twenty-five percent confluent COS-7 cells in DMEM, 10% FBS, 100 U/ml penicillin G, and 100 mg/ml streptomycin were transfected with Fugene 6 transfection reagent (Roche Applied Science, Mannheim, Germany) in a ratio of 1 μg DNA/3 μl as per manufacturer’s specifications with the amounts of DNA as detailed in the figure legends 4 h after plating. All transfections were equalized as to plasmid DNA concentrations using empty pCDNA 3.1, where required. Forty hours post-transfection, luciferase assays were performed using the Promega Luciferase assay system (Promega; E1501), as per manufacturer’s specifications, and relative light units were read on a Lumat LB 9507 (Berthold Technologies, Bad Wildbad, Germany). The relative light units were normalized to the transfection efficiency (HMGCS2 protein content), as assessed by quantification of immunoprecipitates using the Imagequant program (Molecular Dynamics) following scanning of the membranes on a Storm 840 phosphorimager in the fluorescence mode. Normalizations were carried out by multiplying the relative light units of the HMGCS2 C166,305S by the differential wild-type/mutant HMGCS2 ratio. Immunoprecipitations were carried out using rat monoclonal anti-HA affinity matrix clone 3F10 (Roche), and PVDF membranes were probed with mouse anti-HA clone 12CA5 (Roche) at a concentration of 1:1000 followed by sheep anti-mouse IgG-HRP (GE Healthcare) at a concentration of 1:5000.
Immunoprecipitation and coimmunoprecipitation experiments
Postnuclear supernatants from COS-7 cells cotransfected with pcDNA-HMGCS2-HA wild-type or pcDNA-HMGCS2-HA-Cys166Ser mutant and pSG5-PPARα lysed with Promega Passive Lysis Buffer (Promega; E194A) were incubated with 30 μl Anti-HA affinity matrix (Roche; clone 3F10) for 2 h at room temperature with rocking. Alternatively, cells were lysed with RIPA 0.1% SDS buffer and incubated with 5 μg affinity-purified rabbit anti-HMGCS2 from our laboratory for 2 h at room temperature with rocking, followed by incubation with 20 μl protein G sepharose. After extensive washes with PBS, the beads were then boiled 2 min in 1× SDS loading buffer containing 40 mM DTT and subjected to Western blotting and ECL using a mouse anti-HA monoclonal (12CA5) antibody, chicken anti-HMGCS2 (Sigma-Aldrich) or rabbit polyclonal anti-mouse PPARα antibodies (PA1-822A; Affinity BioReagents, Golden, CO, USA).
Mass spectrometry methodology
All chemical modifications (reduction, alkylation, and digestion of proteins) and mass spectrometric analysis of tryptic and chymotryptic peptides were performed as previously published (36) and are described in greater detail in Supplemental Material.
RESULTS
Palmitoylation is required for the interaction between HMGCS2/PPARα
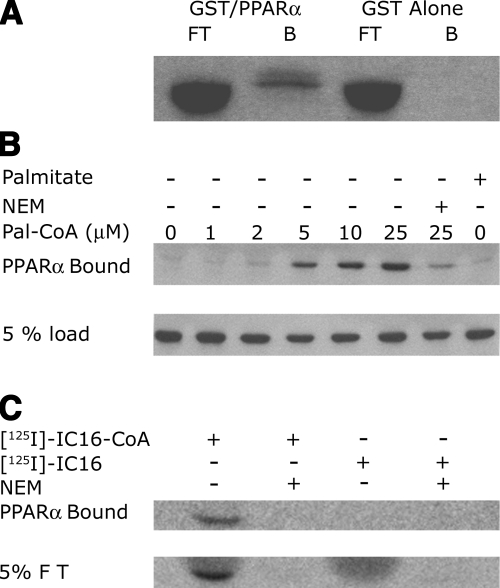
Using a yeast 2-hybrid screen, HMGCS2 was identified as a PPARα binding protein, and the interaction was confirmed using in vitro transcription and translation in reticulocyte lysates followed by GST/PPARα binding experiments (17). Because the concentration of long-chain fatty acyl-CoAs is unknown in reticulocyte lysates and could vary between preparations, we not only performed PPARα binding experiments using reticulocyte lysates (Fig. 1A), but also using well-defined conditions in vitro with highly purified recombinant proteins and increasing concentrations of long-chain fatty acyl-CoAs of varying acyl-chain length. We found that the level of interaction between HMGCS2 and PPARα increased in a palmitoyl-CoA concentration-dependent manner (Fig. 1B). Replacing the palmitoyl-CoA with palmitate in our binding assay resulted in no significant HMGCS2 binding to PPARα, showing that the presence of an esterified long-chain fatty acyl-CoA moiety is a prerequisite for the interaction with GST-PPARα.
Figure 1.
Level of interaction between HMGCS2 and PPARα increases in a palmitoyl-CoA concentration-dependent manner. A) In vitro transcribed and translated [35S]HMGCS2 was incubated with 1 μg of GS/PPARα prebound to glutathione Sepharose beads or GST Sepharose alone as shown, followed by SDS-PAGE. Fluorogram of the dried gel representing 10% of the flow through (FT) or the bound protein (B) is shown. B) One milligram of purified HMGCS2-His6 was incubated with various concentrations of palmitoyl-CoA or palmitate or the combination of 30 min of pretreatment in 10 mM NEM followed by palmitoyl-CoA as shown for 30 min. One hundred nanograms of HMGCS2 (10% of the reaction) was incubated with immobilized GSTPPARα followed by SDS PAGE and Western blot analysis with anti-His5 antibody. C) One microgram of purified HMGCS2-His6 was incubated with 25 μM [125I]-iodopalmitoyl-CoA (lanes 1 and 3) or 25 μM [125I]-iodopalmitate (lanes 2 and 4) following a 30-min preincubation with buffer alone (lanes 1 and 2) or 10 mM NEM (lanes 3 and 4). Ten percent of these reactions (100 ng protein) were incubated with immobilized GST-PPARα, followed by SDS PAGE gel. Autoradiography of the dried gels from the bound fractions and 5% of eluted fractions is shown.
When covalent acylation of HMGCS2 was prevented by NEM cysteine alkylation, the binding to GST-PPARα was abrogated in the presence of long-chain fatty acyl-CoA, suggesting that the presence of acylatable free cysteine residues available for covalent modification of HMGCS2-His6 are involved in the interaction with PPARα. To determine whether the palmitoylated HMGCS2-His6 was indeed binding to PPARα, we preincubated HMGCS2-His6 with radioactive [125I]-iodopalmitoyl-CoA or [125I]-iodopalmitate (34) prior to performing the PPARα binding assays. As shown in Fig. 1C, the HMGCS2-His6 eluted from the GST-PPARα beads had incorporated the radioactive fatty acid label when [125I]-iodopalmitoyl-CoA was used in the preincubation mixture but failed to when [125I]-iodopalmitate was used. Alkylation of HMGCS2-His6 with NEM prior to [125I]-iodopalmitoyl-CoA labeling not only prevented incorporation of the label onto HMGCS2-His6 but also its binding to PPARα. Taken together, these results show that palmitoylation of HMGCS2-His6 on a cysteine residue is a requirement for the interaction between HMGCS2-His6 and PPARα and rule out a simple role for long-chain fatty acyl-CoA as PPARα ligands responsible for this interaction.
Palmitoylation of HMGCS2 and subsequent PPARα interaction is specific for long-chain fatty acids
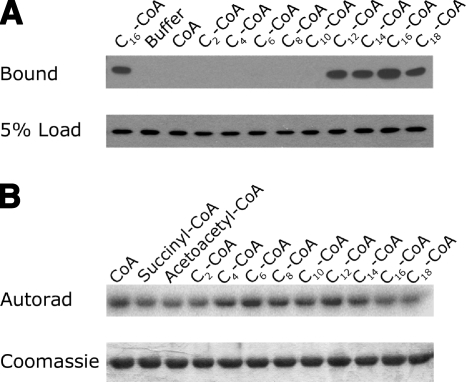
The acylation of a previously characterized palmitoylated mitochondrial enzyme, MMSDH, was shown to be specific for long-chain fatty acids (>10 carbon chain length) (22). The reaction catalyzed by HMGCS2 is thought to be carried out in 3 steps, the first of which is a nucleophilic attack of Cys166 on the thioester bond of acetyl-CoA forming a thioester acetyl-enzyme intermediate (37). Because of the nucleophilic nature of Cys166, we sought to find out whether fatty acyl-CoA of any acyl-chain length could acylate HMGCS2-His6 and mediate the interaction between HMGCS2-His6 and PPARα. To do so, we incubated HMGCS2-His6 with 25-μM acyl-CoAs of varying acyl-chain lengths prior to performing the GST-PPARα binding assays. As revealed in Fig. 2A, a chain length of ≥12 carbons is required to promote binding of HMGCS2-His6 to PPARα, therefore ruling out the possibility that the acetyl-enzyme intermediate or any acyl-enzyme intermediate is sufficient to mediate the interaction with PPARα. To confirm this observation, we performed a competition assay by preincubating HMGCS2-His6 in the presence of acyl-CoAs (50 μM) of varying acyl-chain lengths prior to labeling with [125I]-iodopalmitoyl-CoA. The data in Fig. 2B show that palmitoylation of HMGCS2-His6 by [125I]-iodopalmitoyl-CoA can be reduced by preincubation with succinyl-CoA, acetoacetyl-CoA, acetyl-CoA (C2-CoA), and acyl-CoAs of 14 to 18 carbons, but not with acyl-CoAs of between 4 and 12 carbons, suggesting that the acylation of HMGCS2 is specific to long-chain fatty acids ≥ 12 carbons. Interestingly, preincubation with the known substrates, acetyl-CoA and acetoacetyl-CoA, as well as the known covalent inhibitor of HMGCS2, succinyl-CoA (38), can also reduce access to the HMGCS2 palmitoylation site, suggesting that palmitoylation may occur at, or involve, the active site cysteine (Cys166).
Figure 2.
Palmitoylation of HMGCS2 and its interaction with PPARα are specific to acylation of HMGCS2 by long-chain fatty acids. A) One microgram of HMGCS2-His6 was incubated with 50 μM acyl-CoAs of varying acyl-chain lengths as shown, and 10% of the reaction (100 ng HMGCS2) was incubated with 1 μg GST-PPARα immobilized on glutathione-Sepharose beads followed by SDS PAGE and Western blot analysis with anti-His5 antibody. Results from Western blots of the bound protein and 5% of the protein loaded in the binding reaction are shown. B) One microgram of HMGCS2-His6 was incubated in reactions containing 50 μM succinyl-CoA, acetoacetyl-CoA, or 50 μM of acyl-CoAs of varying acyl-chain lengths as shown for 30 min, followed by reaction with 100 μM [125I]-iodopalmitoyl-CoA for 30 min. Following SDS-PAGE, the dried Coomassie-stained gels were subjected to autoradiography.
Palmitoylation of HMGCS2 at cysteine residue 305 requires active site cysteine residue 166
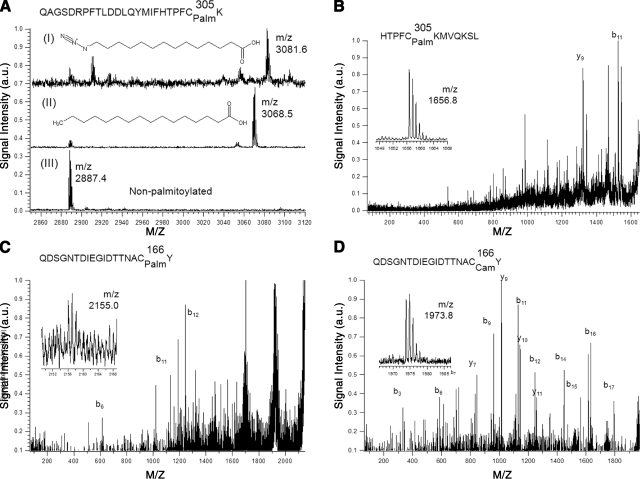
In an effort to determine which of the 9 cysteine residues found in HMGCS2 were palmitoylated, we performed mass spectrometry analyses on fatty acylated HMGCS2-His6 and nontreated HMGCS2-His6. Figure 3A shows that both a palmitoyl moiety and an azido-palmitate analog (26) were attached to a tryptic peptide containing Cys305 (m/z=3068.5, Fig. 3A, I; and m/z=3081.6, Fig. 3A, II, respectively) when HMGCS2-His6 was preincubated with 50 μM palmitoyl-CoA or azido-palmitoyl-CoA, but not in its absence (Fig. 3A, III). Tryptic peptides containing Cys166 could not be observed. This could be explained by a lack of tryptic cleavage sites in the vicinity of the sequence stretch containing Cys166, leading to relevant peptides too large (>5 kDa) for efficient detection with our applied method. We therefore followed up with a chymotryptic protein digestion of HMGCS2-His6 with or without acylation with palmitoyl-CoA to yield smaller peptides for investigation. As shown in Fig. 3B, when HMGCS2-His6 was acylated with palmitoyl-CoA but not in its absence, we could again readily detect a strong signal for the palmitoylated chymotryptic peptide containing Cys305 (m/z=1656.8) and confirmed its identity by MS/MS analysis (fragment ion labeling for all MS/MS spectra was done according to the scheme developed by Roepstorff and Fohlman, ref. 39). In the latter experiment, the alkylated, nonpalmitoylated counterpart peptide containing Cys305 could not be detected. Interestingly, MS/MS analysis of a very low abundant signal for the expected palmitoylated chymotryptic peptide containing Cys166 (m/z=2155.0) confirmed its presence (Fig. 3C). However, in contrast to Cys305, the alkylated, nonpalmitoylated counterpart peptide (m/z=1973.8) was observed in high abundance, and its identity was also confirmed by MS/MS (Fig. 3D). Although not necessarily quantitative, these results suggest that Cys305 might be the main acylated cysteine residue in HMGCS2.
Figure 3.
HMGCS2 is palmitoylated on cysteines 166 and 305. A) MALDI mass spectra excerpt showing tryptic peptides containing Cys305 (sequence: QAGSDRPFTLDDLQYMIFHTPFCK) from wild-type HMGCS2-His6 after S-acylation with azidopalmitoyl-CoA (I), palmitoyl-CoA (II), or control (no palmitoylation; III). Signals at m/z 2887 ([M+H]+) and m/z 2909 ([M+Na]+) stem from a tryptic peptide containing no cysteines (sequence: GTHMENVYDFYKPNLASEYPIVDGK). B–D) MALDI MS/MS spectra (with MS spectra excerpts of precursor ions) of chymotryptic peptides of wild-type HMGCS2-His6 after S-acylation with palmitoyl-CoA. B) Palmitoylated peptide containing Cys305. C). Signal from palmitoylated peptide containing Cys166. D) Signal from carbamidomethylated peptide containing Cys166.
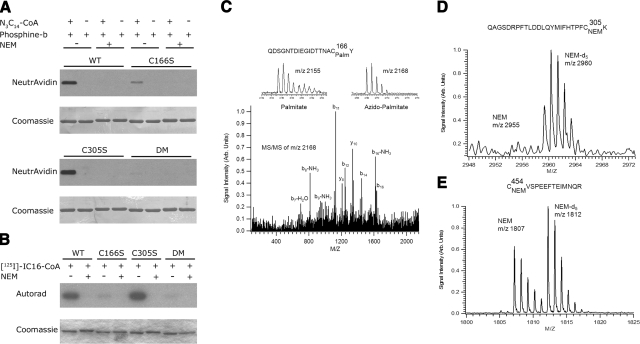
To confirm whether HMGCS2-His6 is palmitoylated on these distinct sites, we engineered, expressed in Escherichia coli, and purified the wild-type HMGCS2-His6 or three mutants in which the putative palmitoylated cysteine residues were changed to serine residues (HMGCS2-His6-Cys166Ser, -Cys305Ser, and -Cys166,305Ser). The three purified mutant proteins were equally soluble to the wild-type HMGCS2 and showed near-identical gel filtration elution patterns (data not shown), indicating that the conservative Cys-Ser mutations apparently did not significantly affect the structure of HMGCS2. We then labeled the four proteins with azido-palmitoyl-CoA (26) or the radioactive [125I]-iodopalmitoyl-CoA (34) with or without prior treatment in NEM (Figs. 4A, B, respectively). After chemical ligation of the enzymes labeled with azido-palmitoyl-CoA with a biotinylated triarylphosphine followed by protein blotting and ECL detection, we showed that azido-palmitoyl-CoA analog is readily incorporated in the wild-type HMGCS2-His6 in the absence of NEM, but not in its presence. Similar results were obtained when [125I]-iodopalmitoyl-CoA was used as a label (Fig. 4B). Unexpectedly, we showed that HMGCS2-His6-Cys166Ser had the most significant reduction in acylation of the single mutants in comparison to wild-type HMGCS2-His6, while HMGCS2-His6-Cys305Ser was acylated to levels similar to that of or even slightly higher than the wild-type enzyme. One possible interpretation of these results is that the nucleophilic active site Cys166 is required for a transacylation reaction involving Cys305 as the final palmitoyl acceptor. In the absence of Cys305 in the HMGCS2-His6-Cys305Ser mutant, the palmitoyl moiety could accumulate on the active site Cys166 as a trapped transient acyl-intermediate.
Figure 4.
Palmitoylation of Cys 305 requires the active site Cys 166. A) One milligram of HMGCS2-His6 wild-type or mutant as shown was incubated with or without azidopalmitoyl-CoA or NEM, followed by reaction with phosphine-biotin. Acylation was detected by protein blotting with neutravidin-HRP/ECL, and corresponding protein was visualized by Coomassie blue staining of the membrane. B) One milligram of HMGCS2-His6 wild-type or mutant as shown was labeled using [125I]-iodopalmitoyl-CoA in the presence or absence of NEM, followed by SDS-PAGE. Acylation was detected by autoradiography and corresponding protein by Coomassie blue staining. C) MALDI MS/MS spectrum (with MS spectra excerpt of precursor ions) of azido-palmitoylated chymotryptic peptide containing Cys166 from the HMGCS2-His6 Cys305Ser mutant. For this mutant, a strong signal is observed compared to the wild type shown in figure 3C. D, E) Palmitoylation, reduction, alkylation with NEM for wild-type, and NEM-d5 for Cys166Ser were done before mixing of the two proteins and tryptic digestion after mixing. D) Tryptic peptides containing Cys305. Only the peptide originating from the Cys166Ser mutant is clearly observable (m/z=2960), further supporting our findings that S-palmitoylation of Cys305 is more enhanced in the wild-type protein compared to the Cys166Ser mutant. E) Signals for the tryptic peptides containing Cys454 (which is not involved in palmitoylation), confirming that both wild-type and Cys166Ser are present in approximately similar amounts.
To test whether this was the case, we labeled HMGCS2-His6-Cys305Ser with 50 μM palmitoyl-CoA or azido-palmitoyl-CoA and subjected the labeled protein to mass spectrometric analysis after digestion with chymotrypsin. We showed an increased detection of the palmitoylated (m/z=2155) or azido-palmitoylated (m/z= 2168) peptide at Cys166 and confirmed its identity by MS/MS (Fig. 4C). Currently, there are no mass spectrometric methods available to directly quantify the extent of palmitoylation; thus, we devised an indirect experiment employing stable-isotope-labeled NEM for relative quantitative measurement of nonpalmitoylated thiol residues. To support our finding that Cys166 is necessary for enhanced or more complete palmitoylation of Cys305, we acylated both wild-type HMGCS2-His6 and HMGCS2-His6-Cys166Ser with 50 μM palmitoyl-CoA, then subjected both samples to reduction with DTT and alkylation with NEM for wild-type HMGCS2-His6 and NEM-d5 for HMGCS2-His6-Cys166Ser, respectively. After mixing equal aliquots of both samples, we subjected the mixture to tryptic digestion overnight. MALDI MS of the resulting peptide mixture revealed the presence of the NEM-d5-labeled tryptic peptide containing Cys305, originating from HMGCS2-His6-Cys166Ser but no NEM-labeled tryptic peptide containing Cys305 originating from wild-type HMGCS2-His6 (Fig. 4D). Figure 4E shows the signals for the tryptic peptides containing Cys454 (which is not involved in palmitoylation), confirming that both wild-type and Cys166Ser are present in approximately similar amounts. This indirect, quantitative experiment supports our above-described findings that acylation at Cys305 is significantly enhanced in the presence of Cys166 but is much less prominent in its absence. This observation further supports a transacylation mechanism involving the active site Cys166 as a palmitoyl donor and Cys305 as a palmitoyl acceptor.
Mutation of palmitoylation sites abrogates HMGCS2/PPARα interaction
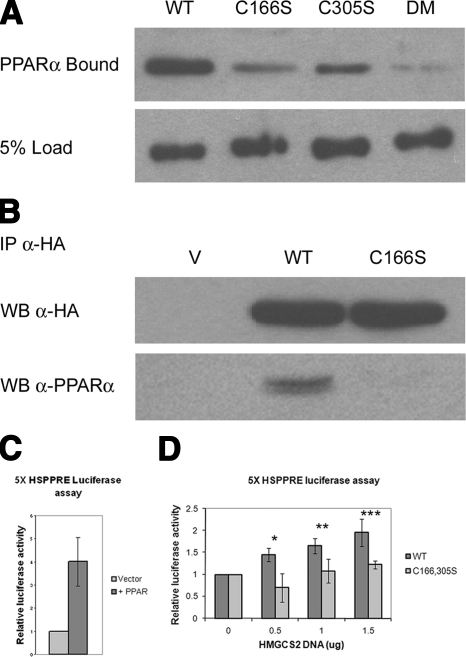
Next, we wanted to assess the importance each of these palmitoylation events incurred on HMGCS2’s ability to bind PPARα in vitro and in cells. To do so, we preincubated wild-type HMGCS2-His6 and HMGCS2-His6-Cys166Ser, -Cys305Ser, and -Cys166,305Ser mutants with 25 μM palmitoyl-CoA prior to performing the GST-PPARα binding assay. We found a major reduction in the ability of HMGCS2 to bind to immobilized GST-PPARα when cysteine residue 166 was mutated to a serine residue and a significant but less dramatic loss of binding when cysteine residue 305 was mutated to a serine residue (Fig. 5A). The HMGCS2-His6-Cys166,305Ser double mutant showed very little binding to GST-PPARα, which we suspect might correspond to the basal level of binding incurred by the LXXLL nuclear receptor box found in HMGCS2, or to the very low level of apparent palmitoylation seen in the double mutant in Fig. 4A, B.
Figure 5.
Palmitoylation of HMGCS2 stimulates the HMGCS2/PPARα interaction and transcriptional up-regulation at the Hmgcs2 PPRE. A) One milligram of purified HMGCS2-His6 and the Cys to Ser mutants as shown was reacted with 25 mM palmitoyl-CoA for 30 min, and 10% of the reaction (100 ng HMGCS2) was incubated with 1 mg GST-PPARα immobilized on glutathione-Sepharose beads, followed by SDS PAGE and Western blot analysis with anti-His5 antibody. B) COS 7 cells transfected with PPARα and HA-tagged wild-type or C166S HMGCS2 were immunoprecipitated with an anti-HA antibody, followed by SDS-PAGE and transfer to a PVDF membrane, and probed with anti-HA and anti-PPARα. C, D) COS 7 cells were cotransfected with pGL3HSPPRE and pSG5PPARα with or without increasing amounts of pcDNAHMGCS2-WT or pcDNA-HMGCS2-C166,305S, and resulting luciferase activity was measured. C) Stimulation of the transcriptional up-regulation of the reporter vector by PPARα normalized to the transfection with the reporter vector alone set to 1. D) Typical increases of luciferase activity in cells transfected with increasing amounts of pcDNA-HMGCS2-WT or pcDNA-HMGCS2-C166,305S normalized as fold over cells transfected with PPARα alone set to 1 (n=4). Error bars = se.
We then confirmed that the interaction between HMGCS2 and PPARα is physiological and occurs in cells by performing a coimmunoprecipitation using COS-7 cells cotransfected with plasmids expressing PPARα- and HA-tagged wild-type HMGCS2 or the HMGCS2-Cys166Ser mutant lacking the catalytic residue critical for palmitoylation. In Fig. 5B, we show that PPARα coimmunoprecipitated with the wild-type HMGCS2, but not the Cys166Ser mutant, thereby not only further confirming their interaction in cells, but that this interaction is dependent on HMGCS2 palmitoylation.
Palmitoylation enhances the up-regulation of a HMGCS2 PPRE luciferase reporter vector
Hmgcs2 contains a PPRE in its promoter region and has previously been shown to promote its own transcription through its interaction with PPARα (17). Having clearly shown that palmitoylation of HMGCS2 is a requirement for the interaction with PPARα, we next assessed the importance of HMGCS2 palmitoylation on transcription from the Hmgcs2 PPRE. We cotransfected COS-7 cells with a pGL3 luciferase reporter vector containing 5 copies of the Hmgcs2 PPRE (pGL3HSPPRE) and a vector expressing PPARα (pSG5-PPARα) with or without increasing amounts of plasmid expressing either wild-type HMGCS2 or the palmitoylation-deficient HMGCS2-Cys166, 305Ser mutant. Our results, normalized for HMGCS2 protein expression levels, show an increase in the transcription at the Hmgcs2 PPRE in cells transfected with the wild-type HMGCS2, but not the palmitoylation-deficient HMGCS2-Cys166,305Ser (Fig. 5). In response to cotransfection with pGL3HSPPRE and pSG5-PPARα, the luciferase expression was increased 4-fold over the pGL3HSPPRE reporter vector alone (Fig. 5C). When increasing concentrations of HMGCS2 wild-type DNA were added to cells cotransfected with pGL3HSPPRE and pSG5-PPARα, we observed a progressive increase in luciferase expression reaching up to an average 2-fold increase over the expression seen in cells transfected with pGL3HSPPRE and pSG5-PPARα (8-fold overall in comparison to cells transfected with pGL3HSPPRE) (Fig. 5D). Cotransfection of pGL3HSPPRE and PPARα with the HMGCS2 Cys166,305Ser palmitoylation-deficient mutant resulted in a much lower level of up-regulation of luciferase expression when compared to the wild type, reaching an average maximum of 1.2-fold over that seen in cells transfected with pGL3HSPPRE and pSG5-PPARα (4.8-fold overall in comparison to cells transfected with pGL3HSPPRE) (Fig. 5D). Along with the requirement for palmitoylation in the HMGCS2/PPARα interaction in cultured cells, as assessed by coimmunoprecipitation, these additional results demonstrate that the HMGCS2/PPARα interaction and the subsequent transcriptional up-regulation at the Hmgcs2 PPRE rely heavily on the palmitoylation of HMGCS2. As shown in Supplemental Fig. 1, expression levels of HMGCS2 in transfected COS-7 cells were optimized to match the levels of endogenous HMGCS2 found in rat primary hepatocytes, thus confirming that the interaction we are seeing is not simply the result of overexpression of HMGCS2.
DISCUSSION
Herein, we demonstrate for the first time a role for palmitoylation as a regulator of transcription. Since protein palmitoylation typically leads to membrane attachment and nuclear exclusion (40), these results came as a surprise and represent a breakthrough in our understanding of the functions of palmitoylation. More specifically, we show that palmitoylation promotes the interaction between the mitochondrial HMGCS2 protein and the nuclear receptor PPARα resulting in transcriptional activation from the Hmgcs2 PPRE.
Interestingly, HMGCS2 is not acylated via an enzymatic transfer by a palmitoyl acyltransferase; rather, its acylation occurs spontaneously. Palmitoylation indeed required only the presence of highly purified recombinant HMGCS2 and palmitoyl-CoA. In vitro, the HMGCS2/PPARα interaction proceeded and increased in a palmitoyl-CoA concentration-dependent manner. Therefore, cellular concentrations of palmitoyl-CoA could regulate the acylation level of HMGCS2, as well as its transcriptional involvement in cells and tissues.
Using in vitro labeled recombinant HMGCS2 and novel mass spectrometry methodology, we demonstrate that the palmitoyl moiety is first incorporated onto the active site cysteine (Cys166) of HMGCS2 and then transferred onto the final acceptor cysteine (Cys305). We named this new mechanism catalytic cysteine palmitoyl relay (CCPR). This elegant palmitoyl relay mechanism allows active site Cys166 to perform a dual function: one as a catalyst in the production of ketone body precursors and one in the transfer of palmitate to Cys305 to signal the requirement for more HMGCS2 via PPARα-mediated transcriptional activation. Palmitoylation of HMGCS2, thus, results in a feed-forward mechanism of HMGCS2 transcription.
Unlike the inner leaflet of the plasma membrane and the surface of endosomes, the mitochondrial compartment is not known for its content in palmitoylated proteins, and only a few papers have been published on this topic (22, 24, 26, 29). Recent large-scale “palmitoylome” screens have also identified numerous putative palmitoylated mitochondrial proteins (31,32,33), suggesting that palmitoylation of mitochondrial proteins occurs in cells and tissues. The palmitoylation of two mitochondrial enzymes involved in amino acid metabolism, MMSDH and CPS1, has previously been shown to occur on an active-site cysteine residue, inhibiting these catabolic enzymes. This suggested a possible metabolic crosstalk between fatty acid and amino acid degradation pathways (22, 24). In addition to MMSDH and CPS1, we recently identified 19 other putative palmitoylated proteins from rat liver mitochondria (26) and are striving to identify the possible roles for palmitoylation of these mitochondrial proteins. From our data herein, it appears that the interaction between PPARα and HMGCS2 is more dependent on palmitoylation than the well-established role of the LXXLL nuclear receptor box. Perhaps these two modes of protein interactions act synergistically. The fact that 8 of the 21 palmitoylated mitochondrial proteins identified so far contain the LXXLL nuclear receptor box (Supplemental Table 2) suggests that the involvement of palmitoylation as a regulator of transcription may perhaps be ubiquitous. Because palmitoylation of HMGCS2 altered its transcription, we believe that the transcription of other palmitoylated mitochondrial proteins, or other yet to be discovered functions for palmitoylation of mitochondrial proteins, might also be altered in cases where cellular levels of palmitoyl-CoA are increased, such as in obesity or diabetes. The fact that obesity is reaching epidemic proportions in Western society (41) definitely warrants further investigation on the roles played by palmitoylation of mitochondrial proteins in the dysregulation of metabolism in obese animals and humans.
MMSDH is also a palmitoylated mitochondrial protein that contains an LXXLL nuclear receptor box (Supplemental Table 2). To test the possibility that MMSDH could bind PPARα, and to determine whether palmitoylation is was a requirement for any interaction, we subjected the wild-type MMSDH and a palmitoylation-deficient (Cys319Ser) mutant (22) to our PPARα binding assay and found that the palmitoylation-deficient form of MMSDH exhibited a dramatic reduction in PPARα binding (Supplemental Figure 2). Because MMSDH is inhibited by active-site fatty acylation (22), we feel it is unlikely that the palmitoylation-dependent interaction between MMSDH and PPARα would increase the production of MMSDH, as seen with HMGCS2. Rather, in this case, the interaction may lead to the formation of a transcriptional corepressor complex. Indeed, recent studies have challenged the generally accepted role of nuclear receptor box-containing proteins as strictly one of coactivator by showing that certain proteins containing the LXXLL motif can also act as corepressors through their interaction with agonist-bound nuclear receptors, including PPARα (reviewed in ref. 16). Alternatively, palmitoylation of MMSDH could possibly result in the formation of a transcriptional coactivator complex for other gene products required under conditions of high palmitoyl-CoA concentrations.
The LXXLL nuclear receptor-interacting motif is located at aa 390–394 in HMGCS2. Interestingly, when the interaction between PPARα and HMGCS2 was first discovered using a yeast 2-hybrid assay, a partial HMGCS2 clone encoding aa 1–345 and therefore lacking the LXXLL motif was originally found as capable of interacting with PPARα (17). This prompted the researchers to suggest that another structural determinant was important in the interaction between PPARα and HMGCS2. We now strongly believe this second determinant to be palmitoylation, whether occurring at cysteine residue 166 or 305 or both.
The glycolytic enzyme GAPDH has been shown to play an essential role in a transcriptional complex (42) required for the S-phase-specific transcription of histone H2B. GAPDH’s function was also regulated by the NAD+/NADH ratio, possibly linking the S-phase specific transcription of histones to the metabolic/redox state of the cell. In the same study, Zheng et al. (42) also reported that HA-tagged monomeric GAPDH purified from a nuclear preparation could restore transcriptional activity in GAPDH-depleted nuclear extracts, while the “cytosolically” purified monomeric GAPDH or the commercially available octomeric GAPDH could not. In an attempt to explain this phenomenon, the authors suggested that conformational changes and/or post-translational modifications may be required for GAPDH’s nuclear function (42). In 2005, GAPDH was shown to become palmitoylated at physiological concentrations of palmitoyl-CoA (28), leaving us to wonder whether palmitoylation could be the modification required for the nuclear localization and transcriptional function of GAPDH.
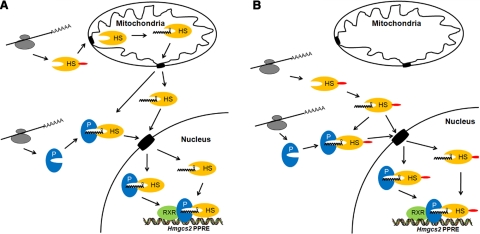
Two major questions remain and will require further investigation: where in the cell palmitoylation of HMGCS2 occurs, and how HMGCS2 enters the nucleus. In Fig. 6A, we propose that palmitoylation of HMGCS2 could occur in the mitochondria, followed by retrotranslocation to the cytosol and then transport to the nucleus, with or without prior binding to PPARα. In previous reports, the mitochondrial proteins aspartate aminotransferase (43) and the 60-kDa heat-shock chaperone protein (44) were shown to be localized to the cytoplasm in their mature processed form, lacking the mitochondrial leader sequence, therefore establishing some possible precedent to mitochondrial retrotranslocation. In a second scenario, shown in our model in Fig. 6B, HMGCS2 could become palmitoylated in the cytosol and actively or passively transported to the nucleus with or without prior binding to PPARα.
Figure 6.
Models of the HMGCS2-PPARα palmitoylation-dependent interaction and nuclear localization, showing the 4 possible PPARα-binding scenarios leading to nuclear localization of HMGCS2. A) We postulate that processing of the leader sequence and palmitoylation both occur in the mitochondria prior to the interaction with PPARα. Once palmitoylated, HMGCS2 exits the mitochondria, albeit via an unknown mechanism, the nuclear entry could occur either directly or following the binding to PPARα. B) We postulate that palmitoylation of HMGCS2 occurs in the cytosol and results in direct nuclear translocation or results in the binding of HMGCS2 to PPARα prior to nuclear import. HMGCS2 (HS) shown in yellow, PPARα (P) shown in blue, RXRα (RXR) shown in green; grey structures represent ribosomes.
On the basis of our new data, under ketogenic conditions, increasing concentrations of cellular palmitoyl-CoA would lead to increasing levels of HMGCS2 palmitoylation and therefore increased transcription of Hmgcs2. Thus, palmitoylation of HMGCS2 would be regulating a feed-forward mechanism resulting in increased ketogenesis. Our results could also explain why under conditions of chronic increased levels of free fatty acids, as seen in cases of obesity or diabetes, the levels of HMGCS2 are elevated (8). Of note, in extreme cases, relative or absolute insulin deficiency can be coupled with an excess of counterregulatory hormones, such as catecholamines and glucagon, leading to further disregulation of carbohydrate, amino acid, and fatty acid metabolism (4) and to possible life-threatening ketoacidosis (5, 6). The data presented herein provide a possible explanation of how increased concentrations of palmitoyl-CoA under the above-mentioned conditions could lead to an increased output of ketone bodies and therefore further exacerbate the acidification of the blood.
From the evolutionary point of view, considering the endosymbiotic relationship between mitochondria and eukaryotic cells, and the subsequent transfer of mitochondrial genes to the nucleus over time, a continuous communication between the mitochondria and the nucleus for the coordination of gene expression would be beneficial. We propose that palmitoylation of transcriptional coregulators could represent such a means of communication that would enable the nucleus to fine-tune the transcription levels of certain nuclear encoded mitochondrial genes to fulfill the metabolic requirements of the mitochondria. In this context, the palmitoylation of transcriptional coregulators could represent an elegant means developed by mammalian cells to transmit environmental nutritional cues from the mitochondria to the nucleus ultimately resulting in changes in cellular metabolism.
Supplementary Material
Acknowledgments
This work was funded by Canadian Institutes of Health Research grant MOP 81248 and a Heart and Stroke Foundation grant to L.G.B. M.A.K. held a Province of Alberta graduate scholarship. The authors thank Megan C. Yap for technical assistance. B.O.K. thanks the University of British Columbia’s Child and Family Research Institute for an Establishment Award. The authors thank Sheila Innis, Roger Dyer, Jürgen Kast, Jason Rogalski, and Suzanne Perry (University of British Columbia) for access to and help with the mass spectrometric equipment, which is funded through grants from the Canada Foundation for Innovation and the Michael Smith Foundation for Health Research.
References
- Cullingford T E. The ketogenic diet; fatty acids, fatty acid-activated receptors and neurological disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;70:253–264. doi: 10.1016/j.plefa.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Fukao T, Lopaschuk G D, Mitchell G A. Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids. 2004;70:243–251. doi: 10.1016/j.plefa.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hegardt F G. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J. 1999;338:569–582. [PMC free article] [PubMed] [Google Scholar]
- Eledrisi M S, Alshanti M S, Shah M F, Brolosy B, Jaha N. Overview of the diagnosis and management of diabetic ketoacidosis. Am J Med Sci. 2006;331:243–251. doi: 10.1097/00000441-200605000-00002. [DOI] [PubMed] [Google Scholar]
- Wolfsdorf J, Craig M E, Daneman D, Dunger D, Edge J, Lee W R, Rosenbloom A, Sperling M A, Hanas R. Diabetic ketoacidosis. Pediatr Diabetes. 2007;8:28–43. doi: 10.1111/j.1399-5448.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- Casteels K, Mathieu C. Diabetic ketoacidosis. Rev Endocr Metab Disord. 2003;4:159–166. doi: 10.1023/a:1022942120000. [DOI] [PubMed] [Google Scholar]
- Rodriguez J C, Gil-Gomez G, Hegardt F G, Haro D. Peroxisome proliferator-activated receptor mediates induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by fatty acids. J Biol Chem. 1994;269:18767–18772. [PubMed] [Google Scholar]
- Serra D, Casals N, Asins G, Royo T, Ciudad C J, Hegardt F G. Regulation of mitochondrial 3-hydroxy-3-methylglutaryl-coenzyme A synthase protein by starvation, fat feeding, and diabetes. Arch Biochem Biophys. 1993;307:40–45. doi: 10.1006/abbi.1993.1557. [DOI] [PubMed] [Google Scholar]
- Valera A, Pelegrin M, Asins G, Fillat C, Sabater J, Pujol A, Hegardt F G, Bosch F. Overexpression of mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in transgenic mice causes hepatic hyperketogenesis. J Biol Chem. 1994;269:6267–6270. [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Reddy J K. Transcription coactivators for peroxisome proliferator-activated receptors. Biochim Biophys Acta. 2007;1771:936–951. doi: 10.1016/j.bbalip.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Flores A M, Aneskievich B J. Corepressors of agonist-bound nuclear receptors. Toxicol Appl Pharmacol. 2007;223:288–298. doi: 10.1016/j.taap.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meertens L M, Miyata K S, Cechetto J D, Rachubinski R A, Capone J P. A mitochondrial ketogenic enzyme regulates its gene expression by association with the nuclear hormone receptor PPARα. EMBO J. 1998;17:6972–6978. doi: 10.1093/emboj/17.23.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlmakers M J, Marsh M. The on-off story of protein palmitoylation. Trends Cell Biol. 2003;13:32–42. doi: 10.1016/s0962-8924(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Linder M E, Deschenes R J. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- Resh M D. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Smotrys J E, Linder M E. Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- Berthiaume L, Deichaite I, Peseckis S, Resh M D. Regulation of enzymatic activity by active site fatty acylation. A new role for long chain fatty acid acylation of proteins. J Biol Chem. 1994;269:6498–6505. [PubMed] [Google Scholar]
- Bizzozero O A, Bixler H A, Pastuszyn A. Structural determinants influencing the reaction of cysteine-containing peptides with palmitoyl-coenzyme A and other thioesters. Biochim Biophys Acta. 2001;1545:278–288. doi: 10.1016/s0167-4838(00)00291-0. [DOI] [PubMed] [Google Scholar]
- Corvi M M, Soltys C L, Berthiaume L G. Regulation of mitochondrial carbamoyl-phosphate synthetase 1 activity by active site fatty acylation. J Biol Chem. 2001;276:45704–45712. doi: 10.1074/jbc.M102766200. [DOI] [PubMed] [Google Scholar]
- Duncan J A, Gilman A G. Autoacylation of G protein alpha subunits. J Biol Chem. 1996;271:23594–23600. doi: 10.1074/jbc.271.38.23594. [DOI] [PubMed] [Google Scholar]
- Kostiuk M A, Corvi M M, Keller B O, Plummer G, Prescher J A, Hangauer M J, Bertozzi C R, Rajaiah G, Falck J R, Berthiaume L G. Identification of palmitoylated mitochondrial proteins using a bio-orthogonal azido-palmitate analogue. FASEB J. 2008;22:721–732. doi: 10.1096/fj.07-9199com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien P J, St. Jules R S, Reddy T S, Bazan N G, Zatz M. Acylation of disc membrane rhodopsin may be nonenzymatic. J Biol Chem. 1987;262:5210–5215. [PubMed] [Google Scholar]
- Yang J, Gibson B, Snider J, Jenkins C M, Han X, Gross R W. Submicromolar concentrations of palmitoyl-CoA specifically thioesterify cysteine 244 in glyceraldehyde-3-phosphate dehydrogenase inhibiting enzyme activity: a novel mechanism potentially underlying fatty acid induced insulin resistance. Biochemistry. 2005;44:11903–11912. doi: 10.1021/bi0508082. [DOI] [PubMed] [Google Scholar]
- Stucki J W, Lehmann L H, Siegel E. Acylation of proteins by myristic acid in isolated mitochondria. J Biol Chem. 1989;264:6376–6380. [PubMed] [Google Scholar]
- Drisdel R C, Green W N. Labeling and quantifying sites of protein palmitoylation. BioTechniques. 2004;36:276–285. doi: 10.2144/04362RR02. [DOI] [PubMed] [Google Scholar]
- Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey A O, Thompson J X, Roth A F, Drisdel R C, Mastro R, Green W N, Yates J R, 3rd, Davis N G, El-Husseini A. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature. 2008;456:904–909. doi: 10.1038/nature07605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B R, Cravatt B F. Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods. 2009;6:135–138. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Di Vizio D, Kirchner M, Steen H, Freeman M R. Proteome-scale characterization of human s-acylated proteins in lipid raft-enriched and non-raft membranes. Mol Cell Proteomics. 2009;9:54–70. doi: 10.1074/mcp.M800448-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume L, Peseckis S M, Resh M D. Synthesis and use of iodo-fatty acid analogs. Methods Enzymol. 1995;250:454–466. doi: 10.1016/0076-6879(95)50090-1. [DOI] [PubMed] [Google Scholar]
- Lowe D M, Tubbs P K. 3-Hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Purification, molecular and catalytic properties. Biochem J. 1985;227:591–599. doi: 10.1042/bj2270591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Whittal R M, Li L. Two-layer sample preparation: a method for MALDI-MS analysis of complex peptide and protein mixtures. Anal Chem. 1999;71:1087–1091. doi: 10.1021/ac980684h. [DOI] [PubMed] [Google Scholar]
- Miziorko H M, Lane M D. 3-Hydroxy-3-methylgutaryl-CoA synthase. Participation of acetyl-S-enzyme and enzyme-S-hydroxymethylgutaryl-S-CoA intermediates in the reaction. J Biol Chem. 1977;252:1414–1420. [PubMed] [Google Scholar]
- Lowe D M, Tubbs P K. Succinylation and inactivation of 3-hydroxy-3-methylglutaryl-CoA synthase by succinyl-CoA and its possible relevance to the control of ketogenesis. Biochem J. 1985;232:37–42. doi: 10.1042/bj2320037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff P, Fohlman J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed Mass Spectrom. 1984;11:601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- McCabe J B, Berthiaume L G. Functional roles for fatty acylated amino-terminal domains in subcellular localization. Mol Biol Cell. 1999;10:3771–3786. doi: 10.1091/mbc.10.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou I, Chrousos G P, Tsigos C. Stress, visceral obesity, and metabolic complications. Ann N Y Acad Sci. 2006;1083:77–110. doi: 10.1196/annals.1367.008. [DOI] [PubMed] [Google Scholar]
- Zheng L, Roeder R G, Luo Y. S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell. 2003;114:255–266. doi: 10.1016/s0092-8674(03)00552-x. [DOI] [PubMed] [Google Scholar]
- Isola L M, Zhou S L, Kiang C L, Stump D D, Bradbury M W, Berk P D. 3T3 fibroblasts transfected with a cDNA for mitochondrial aspartate aminotransferase express plasma membrane fatty acid-binding protein and saturable fatty acid uptake. Proc Natl Acad Sci U S A. 1995;92:9866–9870. doi: 10.1073/pnas.92.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys B J, Gupta R S. Immunoelectron microscopic localization of the 60-kDa heat shock chaperonin protein (Hsp60) in mammalian cells. Exp Cell Res. 1996;222:16–27. doi: 10.1006/excr.1996.0003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.