Abstract
The uptake of dietary lipids from the small intestine is a complex process that depends on the activities of specific membrane receptors with yet unknown regulatory mechanisms. Using both mouse models and human cell lines, we show here that intestinal lipid absorption by the scavenger receptor class B type 1 (SR-BI) is subject to control by retinoid signaling. Retinoic acid via retinoic acid receptors induced expression of the intestinal transcription factor ISX. ISX then repressed the expression of SR-B1 and the carotenoid-15,15′-oxygenase Bcmo1. BCMO1 acts downstream of SR-BI and converts absorbed β,β-carotene to the retinoic acid precursor, retinaldehyde. Using BCMO1-knockout mice, we demonstrated increased intestinal SR-BI expression and systemic β,β-carotene accumulation. SR-BI-dependent accumulation of β,β-carotene was prevented by dietary retinoids that induced ISX expression. Thus, our study revealed a diet-responsive regulatory network that controls β,β-carotene absorption and vitamin A production by negative feedback regulation. The role of SR-BI in the intestinal absorption of other dietary lipids, including cholesterol, fatty acids, and tocopherols, implicates retinoid signaling in the regulation of lipid absorption more generally and has clinical implications for diseases associated with dyslipidemia.—Lobo, G. P., Hessel, S., Eichinger, A., Noy, N., Moise, A. R., Wyss, A., Palczewski, K., von Lintig, J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal β,β-carotene absorption and vitamin A production.
Keywords: carotenoids, retinoids, nuclear receptors, scavenger receptors
The small intestine is responsible for absorbing dietary lipids and delivering them to the organism as triglyceride-rich lipoproteins. Inadequate dietary supplies of essential lipids as well as sustained consumption of diets rich in bulk lipids such as fatty acids and cholesterol are associated with a broad spectrum of diseases, including neurodegenerative disease, cardiovascular disease, and type 2 diabetes. Emerging evidence indicates that intestinal absorption of dietary lipids involves specific membrane transporters, such as the Niemann-Pick C1-like 1 protein, scavenger receptors, and ABC cassette transporters (1). These proteins have become targets for the development of clinical antihyperlipdemic drugs, e.g., the cholesterol-lowering drug Ezetimibe (1, 2). Large variability in the absorption of dietary lipids such as cholesterol and β,β-carotene also exists among individuals (3,4,5), but endogenous regulatory mechanisms that control lipid absorption are largely unknown.
Animal studies indicate an important role of the scavenger receptor B type 1 (SR-B1; also denoted as SCARB1) in the intestinal absorption of dietary lipids (6). In SR-BI-deficient mice, intestinal absorption of tocopherols (vitamin E) is decreased (7), and biliary cholesterol secretion is impaired (8). However, overexpression of SR-BI in the intestine results in enhanced absorption of cholesterol and fatty acids (9). SR-BI also is important for vitamin A production; thus, a gene encoding a protein that is homologous to SR-BI is essential for the absorption of the vitamin A carotenoid precursors in Drosophila (10, 11). Studies in SR-B1-deficient mice and cell lines provide evidence that the role of SR-B1 in carotenoid absorption is well conserved in mammals (12,13,14).
Recently, the gut specific homeodomain transcription factor ISX has been identified as a putative repressor of intestinal SR-BI expression (15). SR-B1 is normally found on the apical surfaces of absorptive epithelial cells, and its levels decrease from the duodenum to ileum (6, 16, 17), in contrast to the increasing duodenum-ileum gradient for ISX (15). In ISX-deficient mice, SR-BI expression is significantly enhanced and its expression extends to more distal parts of the intestine (15). ISX also has been shown to repress the intestinal expression of the carotenoid-15,15′-monooxygenase, BCMO1 (18). In intestinal enterocytes, BCMO1 acts downstream of SR-B1 and converts absorbed β,β-carotene to vitamin A-aldehyde (for recent review, see ref. 19). This compound can be metabolized into the unique series of endogenous vitamin A metabolites, including retinoic acid (RA). RA is a hormone-like compound that regulates gene expression by activating nuclear receptors termed retinoic acid receptors (RARs), which are ligand-controlled transcription factors that function as heterodimers with the retinoid X receptor (RXR). RAR-RXR heterodimers bind to regulatory regions of target genes harboring response elements (REs) composed of two direct repeats of the motif 5′-PuG(G/T)TCA spaced by 2 or 5 bp (DR-2, DR-5), and they activate gene expression on ligand binding (20).
Animal model data also suggest that dietary β,β-carotene and its retinoid metabolites repress intestinal BCMO1 enzymatic activity and that this regulation, involving RA and RARs, appears to be exerted at the transcriptional level (21, 22). ISX expression also is influenced by dietary retinoids, being low in vitamin A deficiency and high in vitamin A sufficiency (18). These findings indicate that the transcription factor ISX lies at the intersection between the retinoid signaling pathway and the regulation of intestinal lipid absorption, thus making it a promising therapeutic target for treating patients with dyslipidemia.
However, the molecular mechanisms involved in the crosstalk between retinoid signaling and ISX activity have yet to be elucidated in functional detail. In addition, the putative role of ISX in controlling lipid absorption via SR-BI and vitamin A homeostasis lacks experimental testing in animal models. To address these questions, we analyzed the role of ISX and retinoid signaling for the regulation of intestinal lipid absorption using both human colonic cell lines and mouse models with impaired β,β-carotene and retinoid metabolism.
MATERIALS AND METHODS
All reagents unless indicated were purchased from Sigma Chemical Co. (Portland, OR, USA). Platinum Pfx polymerase, Prolong Gold antifade mounting medium, mammalian expression vector pCDNA 3.1 V5/His-TOPO, and TOP10 competent cells were obtained from Invitrogen/Molecular Probes (Carlsbad, CA, USA). All reagents for quantitative real-time PCR (qRT-PCR) were purchased from Applied BioSystems (ABI; Foster City, CA, USA). DMEM and fetal bovine serum (FBS) was obtained from Gibco Life Technologies, Inc. (Hercules, CA, USA). The chromatin immunoprecipitation (ChIP) assay kit was purchased from Millipore (Billerica, MA, USA). The M-PER mammalian protein extraction reagent, BCA, and Bradford protein assay kits were from Pierce Biotechnology Inc. (Rockford, IL, USA). ECL or enhanced ECL chemiluminescence reagents were obtained from either Pierce Biotechnology or Pharmacia (Erlangen, Germany). Antibodies anti-SR-BI (H-180), anti-ISX (C-16), and anti-RAR (M-454) were from Santa Cruz Biotechnologies (Santa Cruz, CA, USA), and anti-RAN was from Abcam (Cambridge, MA, USA). The anti-mouse-HRP and anti-rabbit-HRP conjugated secondary antibodies were purchased from Promega (Madison, WI, USA). Oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA, USA).
Animals, diets, and experimental procedures
Mice were maintained in accordance with the Swiss and American animal protection laws throughout these experimental studies. B6;129S6-Bcmo1tm1dnp-knockout (Cmo1−/−) and control (B6;129S6) mice were housed individually under environmentally controlled conditions (24°C, 12-h light-dark cycle) with ad libitum access to feed and water. Powdered “vitamin A-free” diet Ssniff®EF 1/51, containing a residual amount of 0.15 IU vitamin A/g (Ssniff GmbH, Soest, Germany) was used as the basal diet. This was supplemented with control beadlets (DSM Ltd, Sisseln, Switzerland) in group 1 or with β,β-carotene-containing beadlets (10% CWS; DSM Ltd) in groups 2 and 3 to provide 150 μg/g β,β-carotene. Mice of group 3 also received 300 IU vitamin A in the form of retinyl palmitate (Dr. Ehrenstorfer GmbH, Augsburg, Germany) by weekly oral gavage in Miglyol 812 (Sasol, Witten, Germany). Lrat−/− mice used in this study have been described previously (23). For determination of the effects of vitamin A deficiency (VAD) and vitamin A sufficiency (VAS) on ISX expression in the duodenum and jejunum of these mice, they were fed AIN-93 formulation diets supplemented with 10 IU vitamin A (VAS diet) and without vitamin A supplementation (VAD diet). Diets were purchased from ResearchDiets (New Brunswick, NJ, USA). For determination of the effect of RA on intestinal ISX mRNA expression, 8 wk old Lrat−/− mice were subjected to a vitamin A-deficient diet for 10 d (AIN-93G Growing Rodent Diet Without Added Vitamin A; Research Diets, New Brunswick, NJ, USA). Animals (n=3 each) were gavaged twice, 24 h apart, with 0.5 mg RA (Sigma) dissolved in 100 μl of corn oil or with vehicle alone. After 24 h, animals were sacrificed, and the small intestine and liver were removed and snap-frozen in liquid nitrogen until further analyses.
Cell lines, cell cultures, and transient transfections
Human intestinal CaCo-2 and hepatocyte HepG2 cells were maintained in high-glucose DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin sulfate and cultured at 37°C in 5% CO2. For the cycloheximide experiment, cells were pretreated with 20 μg/ml of cycloheximide for 20 min, and RA (1 μM) was added and cells were incubated for another 4–6 h at 37°C in 5% CO2. For the RAR pan-antagonist LE540 experiment, CaCo-2 cells were pretreated with 1 μM of LE540 for 1 h; after which 1 μM RA was added, and cells were incubated for an additional 4 h. Control cells were not treated or were treated with 1 μM RA alone for 4 h. After harvesting, cells were then processed for total RNA. mRNA expression of relevant genes was determined by qRT-PCR with gene-specific probe sets (ABI). HepG2 cells were cultured in 100-cm2 dishes and transfected with 4–6 μg of purified plasmid DNA (pISX-WT) by using LipofectAMINE 2000 (L2000) and Opti-MEM according to manufacturer’s instructions (Invitrogen). Cells were harvested 48–72 h post-transfection, and total protein was extracted and processed as detailed below.
Protein isolation and Western blot analyses
Total protein from animal tissue or cultured cells was isolated at indicated time points by using the M-PER mammalian protein extraction reagent with protease inhibitors (Roche, Basel, Switzerland) according to manufacturer’s instructions (Pierce). Mice were sacrificed by cervical dislocation. Small intestines were collected, rinsed in ice-cold phosphate buffered saline (PBS; pH 7.4), and snap-frozen in liquid nitrogen. Mouse intestine (∼100 mg) was then homogenized in liquid nitrogen using a mortar and pestle and transferred directly into M-PER buffer (500 μl) containing protease inhibitors. Proteins (30–50 μg) were fractionated on 4–10% SDS-PAGE gels using the Bio-Rad Minigel system and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore). Equal protein loading was confirmed by routine immunoblotting of the membranes with Ponceau S staining and by Western blot analysis using anti-RAN as the loading control. PVDF membranes were blocked with 5% milk prepared in Tris-buffered saline (pH 7.4) containing 0.05% Tween (TBS-T) for 1 h and then probed with either anti-ISX, anti-SR-BI, or anti-BCMO1 (1:1000 dilution) antibody overnight at 4°C, followed by incubation with the appropriate HRP-conjugated secondary antibody, before being visualized with the enhanced ECL chemiluminescence detection system (Pierce or Pharmacia). For determination of liver retinol binding protein (RBP4) levels, a commercially available polyclonal antiserum raised against human RBP4 (DakoCytomation, Hamburg, Germany) was used in a 1:1000 dilution.
WT ISX plasmid construction
Total RNA (1 μg) from the human colonic CaCo-2 cell line was reverse transcribed by using the SuperScript One-Step RT-PCR for LongTemplates system (Invitrogen). The full-length ISX open reading frame (ORF) was amplified by PCR using the ISX-forward primer (5′-ATGTGTGCTGAGGTGGGCCCTG-3′) and the ISX-reverse-primer (5′-TGTTGAAGTAGCACAGATGCTG-3′) with the Expand High Fidelity PCR system (Roche) according to the manufacturer’s instructions. The amplified ISX cDNA product then was cloned into the pCDNA 3.1 V5/His TOPO vector by following the manufacturer’s instructions (Invitrogen) and transformed into TOP10 cells according to the manufacturer’s instructions (Invitrogen). Positive clones were selected and subjected to plasmid DNA minipreparation according to manufacturer’s instructions (Qiagen, Valencia, CA, USA). Finally, appropriate construction of wild-type (WT) ISX ORF (pISX-WT) in the pCDNA 3.1 V5/His TOPO vector was verified by sequence analysis on both strands (Genomics Core Sequencing Facility, Case Western Reserve University, Cleveland, OH, USA).
Indirect immunofluorosence and confocal microscopy
For immunostaining experiments, CaCo-2 cells were seeded at a density of 2 × 105 cells on coverslips in 6-well plates and allowed to adhere for 24 h. Cells were treated with RA (1 μM) or control vehicle (ethanol) and incubated for 12 h. Then cells were fixed in a freshly prepared mixture of 4% formalin in phosphate buffered saline (PBS; pH 7.4) for 20 min at room temperature. After multiple washes with PBS, cells were incubated with blocking buffer (2% BSA and 0.2% Triton-X 100 in PBS) for 15 min at room temperature. The primary polyclonal rabbit antiserum anti-ISX prepared at a 1:500 dilution in blocking buffer was then added to these cells and incubated for 1 h at room temperature. The primary antibody was removed, and cells were washed gently 3 times with PBS for a total of 30 min. The secondary antibody anti-rabbit conjugated Alexa 488 was diluted 1:500 in blocking buffer, added to the cells, and incubated for 1 h at room temperature in darkness. After further washing in PBS, coverslips with cells were mounted facedown onto glass slides (Labtek, Scotts Valley, CA, USA) with a small drop of ProLong Gold antifade mounting medium containing DAPI (Molecular Probes). The next day, cells were examined at room temperature under a Zeiss LSM 510 UVMETA confocal microscope with an HCX Plan ×40 numerical aperture 1.4 oil-immersion objective lens (Zeiss, Jena, Germany). Images were acquired with Zeiss confocal software version 2.0. All experiments were performed in triplicate, and ∼100 cells/experiment were counted.
ChIP assay and PCR
CaCo-2 cells were cultured in 150-cm2 dishes and, on 90% confluence, were treated with 1% formaldehyde at 37°C with gentle swirling for 10 min to enable crosslinking of nuclear proteins with genomic DNA. Then the ChIP assay, to evaluate binding of RARs to the ISX promoter, was performed essentially as described by the manufacturer (Millipore). About 4 μg of RAR antibody (M-454; Santa Cruz Biotechnologies) was used for immunoprecipitation, and IgG was used as the negative control. ISX promoter primers used for PCR were ISX-hum-prom-Fwd-1 (5′-AGCCGTGGGCACAGGATACC-3′) with ISX-hum-prom-Rev-1 (5′-GATGATCCAAACAGGATTTC-3′) and ISX-hum-prom-Fwd-2 (5′-TGGTAAGGGCTGAGCCGTGG-3′) with ISX-hum-prom-Rev-2 (5-CAAACAGGATTTCGTGTCCA-3′). PCR products were electrophoresed on 2% agarose gels.
HPLC separation of retinoids and carotenoids from tissues and plasma
Retinoids and carotenoids were extracted from tissues and plasma under a dim red safety light (600 nm). Briefly, tissues (20–40 mg) were homogenized in 200 μl 2 M hydroxylamine (pH 6.8) and 200 μl methanol with a glass homogenizer. For determination of β,β-carotene blood levels, 200 μl plasma was added to 200 μl methanol. Then 400 μl acetone was added to either the plasma or tissue extracts. Extraction of carotenoids and retinoids was performed with petroleum ether. The extraction was repeated 3 times, and the collected organic phases were dried under a stream of nitrogen and dissolved in HPLC solvent. HPLC separation of carotenoids and retinoids and quantification of peak integrals was performed as described previously (24). Solvents for HPLC and extraction were of HPLC-grade and purchased from Merck (Darmstadt, Germany).
RNA preparation and qRT-PCR
RNA preparation and qRT-PCR analyses were performed as described previously (25). The following primers were used for qRT-PCR analysis of target genes: ISX, 5′-TTCCACTTCACCCATTACCC-3′ and 5′-CTCTTCTCCTGCTTCCTCCA-3′; SR-BI, 5′-CTCTCCCACCCCCACTTT-3′ and 5′-TTCCCTGTTTGCCCGATG-3′, BCMO1, 5′-ACACCATCCCCGACTTCAC-3′ and 5′-GTTTACCGCCACATACTTCC-3′; and BCMO2, 5′-ATCGCCCAGTTTTGAAGGAG-3′ and 5′-ACCCGAGCAGAGACAGCA-3′. As a housekeeping gene, we used β-actin: 5′-ACGGGCATTGTGATGGACTC-3′ and 5′-GTGGTGGTGAAGCTGTAGCC-3′. RNA was extracted from mouse intestine or cultured cells with the Trizol reagent (Invitrogen) and purified by using the RNeasy system (Qiagen). Approximately 2 μg of total RNA was reverse transcribed with the High Capacity RNA-to-cDNA kit (ABI) following the manufacturer’s instructions. Quantitative PCR (Q-PCR) was carried out using TaqMan chemistry, TaqMan Gene Expression Master Mix and Assays on Demand probes (ABI) for mouse ISX (Mm01243745_m1), mouse Bcmo1 (Mm00502437_m1), mouse Scarb1/SR-BI (Mm00450236_m1), and human ISX (Hs01368145_m1), respectively. The 18s rRNA (4319413E) or β-actin probe set (ABI) were used as endogenous controls. All real-time experiments were performed with the ABI Step-One Plus qRT-PCR machine. Gene expression analysis was accomplished by the relative standard curve method (ABI Technical Bulletin No. 2).
In silico promoter analysis
We used NUBIScan version 2.0 software (http://www. nubiscan.unibas.ch/software) and AliBaba 2.1 (http://www. gene-regulation.com) to identify putative nuclear receptor binding sites in the human ISX gene.
Statistical analysis
Student’s t test was used to analyze the data, presented as means ± sd. Values of P ≤ 0.05 were considered significant.
RESULTS
ISX expression is under the control of RA and RARs
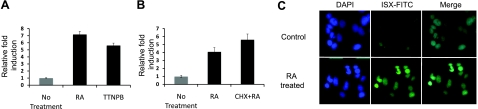
Animal studies indicate that ISX represses intestinal SR-BI and BCMO1 expression (15, 18). BCMO1 converts absorbed β,β-carotene to retinaldehyde, which can serve as a precursor for RA that, in turn, activates RARs (25). Because RA and RARs are implicated in regulating intestinal expression of BCMO1, it was suggested that RARs and ISX independently control BCMO1 gene activity (18). However, BCMO1 expression is not altered in ISX-deficient mice subjected to vitamin A restriction (18), suggesting instead that the effect of RA on BCMO1 expression is dependent on ISX. Therefore, we reasoned that ISX rather than BCMO1 expression is directly controlled by RA and RARs. To test this hypothesis, we treated human colonic CaCo-2 cells with RA or the synthetic RAR agonist, TTNPB. After 4 h, we isolated total RNA and determined ISX mRNA expression by qRT-PCR analysis. In both RA- and RAR agonist-treated CaCo-2 cells, ISX mRNA increased 4–6 fold over levels to those in untreated or vehicle-treated control cells (Fig. 1A). To provide further evidence for RAR regulation of ISX, we treated CaCo-2 cells with LE540, a well-known RAR pan-antagonist. This treatment prevented RA-dependent induction of ISX mRNA expression (Supplemental Fig. S1). To examine whether induction of ISX was a direct effect or whether it involved the synthesis of other protein factors, we treated CaCo-2 cells with RA in the presence of the protein synthesis inhibitor cycloheximide (20 μg/ml). RA still induced ISX expression ∼4-fold in the presence of cycloheximide, indicating direct transcriptional regulation (Fig. 1B). We also performed immunohistochemical staining to determine whether RA also increased ISX expression at the protein level in CaCo-2 cells. In this case, CaCo-2 cells were treated with RA for 12 h and then cells were fixed and stained with an ISX-specific antiserum. By confocal imaging, we detected strong staining for ISX in the nucleus of RA-treated CaCo-2 cells. In contrast, vehicle-treated control CaCo-2 cells showed only weak ISX staining (Fig. 1C). This analysis provides evidence that ISX is a RA-inducible target gene, an activity that likely reflects direct positive control of RARs.
Figure 1.
RA induces ISX expression through RARs in CaCo-2 cells. CaCo-2 cells were seeded in DMEM and 10% FBS. After allowing the cells to adhere for 24 h, cells were treated with 1 μM RA or 1 μM 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid (TTNPB) (a specific RAR agonist) or pretreated with 1 μM cycloheximide (CHX) and then 1 μM RA as indicated. After 4 h, total RNA was extracted and reverse transcribed, and qRT-PCR was performed with a specific probe set for ISX. mRNA levels were normalized for 18S rRNA expression. A) ISX mRNA expression in CaCo-2 cells treated with either RA or TTNPB. B) ISX mRNA levels in CaCo-2 cells pretreated with CHX and then with RA. Results are presented as fold induction vs. untreated control cells (n=3/condition). C) CaCo-2 cells treated with 1 μM RA or without (vehicle control) for 12 h were subjected to indirect immunostaining using the ISX primary antibody and Alexa Fluor-488 secondary antibody as indicated. ISX expression is detected as green fluorescence. Nuclei were also concurrently stained with DAPI, which was included in the mounting medium. Approximately 100 cells/experiment were counted; representative images from 3 independent experiments are shown. Images were acquired at ×40.
RARs directly bind to the ISX promoter
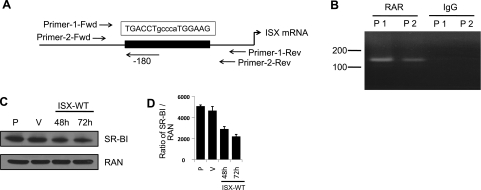
RARs function by binding to specific retinoic acid response elements (RAREs) within regulatory regions of target genes. We used NubiScanV 2.0 and AliBaba 2.1 software to identify putative RAREs in the human ISX gene. This program predicted a DR-5 RARE located 180 bp upstream of the ATG start codon (Fig. 2A). To investigate whether RARs associate with this element in CaCo-2 cells, we performed ChIP assays using antiserum specific for human RARs and two primer pairs designed for detection of the putative RARE in precipitated DNA fractions (Fig. 2A). Using these primer pairs, we amplified by PCR the respective regions in immunoprecipitated chromatin from CaCo-2 cells. PCR products were clearly detectable in chromatin fractions immunoprecipitated with RAR antiserum (Fig. 2B). In contrast, no PCR products were detected when we used IgG as a control in this assay (Fig. 2B). Thus, our analysis provides evidence that RARs specifically bind to the predicted RARE within the human ISX promoter.
Figure 2.
RARs directly bind to the ISX promoter in the ChIP assay. A) Schematic representation of the retinoic acid response element (RARE) in the human ISX promoter region, along with PCR primer pair locations. B) ChIP assays in CaCo-2 cells were performed with the anti-RAR (M-454) antibody specific for human RARs, while IgG was used as a control in the IP reaction. To show binding of RARs to the putative RARE in the ISX promoter region, we designed two primer pairs for its detection in precipitated DNA fractions as indicated. C) Overexpression of ISX in human hepatocyte cells HepG2 decreased SR-BI protein levels. Human hepatocyte HepG2 cells were transiently transfected with either WT ISX or empty vector (V) using Lipofectamine 2000 as indicated. Untransfected parental (P) HepG2 cells were also included as a control. Cells were harvested at 48 h and 72 h post-transfection; total protein was isolated and then subjected to immunoblot analysis as indicated. RAN (Ras-related nuclear protein) was used as the protein loading control. D) Densiometric quantification of SR-BI protein levels as expressed by SR-BI to RAN levels (means ± sd, n=3 independent experiments).
ISX represses SR-BI expression in HepG2 cells
Previous studies in mice provide evidence that ISX directly controls BCMO1 expression (18). It has been reported that the expression of SR-BI is highly increased in ISX deficiency (15), but it is unknown whether SR-BI is a direct downstream target of ISX. To address this question, we cloned the full-length human ISX from CaCo-2 cells and tranfected it into the human hepatocyte cell line HepG2, a cell line that expresses SR-BI (26). The transfection efficiency was determined to be 67 to 72% using a GFP-reporter construct (data not shown). We performed immunoblot analysis for SR-BI using total protein extracts of these transfected HepG2 cells. As shown in Fig. 2C, SR-BI protein levels decreased in HepG2 cells ectopically overexpressing ISX as compared to cells transfected with the vector alone. Quantification revealed a decrease of SR-BI protein levels to 57% after 48 h and 43% after 72 h of the levels in nontransfected cells (Fig. 2D). Considering the transfection efficiency, the remaining SR-BI expression can be attributed largely to nontransfected cells (33%). Thus, we conclude that ISX repressed the expression of SR-BI in HeG2 cells.
RA induces ISX expression in vitamin A-deficient mice
Our studies in human cell lines showed that ISX is an RA target gene. Therefore, we next analyzed retinoid dependency of ISX expression in a mouse model. The lecithin:retinol acyl transferase (LRAT)-deficient mouse model cannot convert retinol to retinyl esters; hence, it lacks liver vitamin A stores and possesses only trace amounts of retinyl esters in most other tissues (23, 27, 28). Due to impaired vitamin A storage, LRAT-knockout mice are highly susceptible to dietary vitamin A-deficiency (27, 28). To induce vitamin A deficiency in these mice, we fed 8-wk-old LRAT-deficient animals a diet lacking any source of vitamin A (n=5) or a vitamin A sufficient diet (n=5). After 2 wk, we sacrificed animals and performed qRT-PCR analysis with duodenal and jejunal RNA preparation. In animals subjected to vitamin A depletion, ISX mRNA levels were decreased (5.2- and 3.6-fold in duodenum and jejunum, respectively), whereas SR-BI mRNA levels (4.2- and 3.8-fold in duodenum and jejunum, respectively) and BCMO1 mRNA levels were increased (25- and 8.5-fold in duodenum and jejunum, respectively) as compared to vitamin A sufficient animals (Supplemental Fig. S2). Thus, this study confirmed vitamin A dependency of ISX mRNA expression (18).
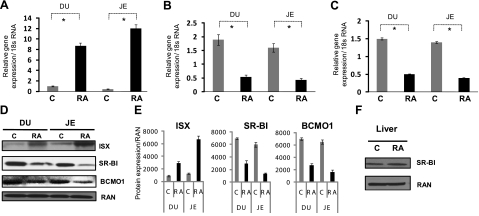
Our studies in CaCo-2 cells revealed that the effect of vitamin A on ISX expression is mediated by its derivative RA. Therefore, we asked whether RA treatment can induce intestinal ISX expression in vitamin A-deficient animals. For this experiment, we maintained 8-week-old LRAT-deficient animals (n=6) on a diet lacking any source of vitamin A. After 10 d, these mice were gavaged orally with RA (n=3) or the vehicle control (n=3). We then determined ISX mRNA levels in the small intestine of these mice. qRT-PCR quantification revealed that RA treatment resulted in a 9- and 11-fold increase of duodenal and jejunal mRNA levels of this transcription factor, respectively (Fig. 3A). Immunoblot analysis additionally showed that the increase of ISX mRNA was paralleled by a 3.2- and 4.3-fold increase of ISX protein levels in the duodenum and jejunum, respectively (Fig. 3D, E).
Figure 3.
RA induces ISX expression in vitamin A-deficient Lrat−/− mice. Eight-week-old Lrat−/− mice were maintained on a diet lacking vitamin A. After 10 d on this diet, mice were orally gavaged with either RA (0.5 mg/animal) or vehicle control as indicated (n=3/condition). After 24 h, the gavage was repeated. After an additional 24 h, animals were sacrificed, and their small intestines were removed. Total RNA was then extracted; expression of relevant genes was quantified as indicated. A–C) mRNA expression of ISX (A) and its downstream targets SR-BI (B) and BCMO1 (C) was determined using gene specific probe sets (ABI) and qRT-PCR. Values are means ± sd from 2 independent experiments carried out in triplicate. *P ≤ 0.001. Gray bars indicate vehicle-gavaged control mice; solid black bars indicate RA-gavaged mice. D) Total protein from RA or vehicle-treated Lrat−/− mice (n=3/condition) was obtained from intestinal tissue (duodenum and jejunum), subjected to immunoblot analysis, and probed with the respective antibodies as indicated. E) Densiometric quantification of ISX, SR-BI, and BCMO1 protein levels (means ± sd, n=3). F) Expression of SR-BI in the liver of RA gavaged or control (vehicle-treated) Lrat−/− mice (n=3/condition) was also estimated by immunoblot analysis as indicated. RAN (Ras-related nuclear protein) was used as the protein loading control.
We next analyzed the effects of RA-dependent induction of ISX expression on its downstream target genes. qRT-PCR analysis showed that the mRNA levels of SR-BI and BCMO1 were significantly reduced in the intestine of RA-treated as compared to vehicle-treated Lrat-knockout mice (Fig. 3B, C). The decrease in SR-BI and BCMO1 expression was also detectable at the protein level as shown by immunoblot analysis of total duodenal and jejunal protein extracts (Fig. 3D). SR-BI protein levels were 2.3- and 5.4-fold and BCMO1 protein levels were 2.3- and 3.2-fold decreased in the duodenum and jejunum, respectively (Fig. 3E). No such effect of RA treatment on SR-B1 expression was found in the livers of RA treated animals, where ISX is not expressed (Fig. 3F). Hence, RA-induced down-regulation of SR-BI is mediated by ISX and displays a corresponding tissue-specificity.
ISX regulates β,β-carotene uptake levels in a BCMO1-dependent manner
SR-B1 facilitates the absorption of dietary lipids, including β,β-carotene (12, 14), whereas BCMO1 converts absorbed β,β-carotene to retinaldehyde (19) that can be oxidized to RA. From the above study, we found that, by activating RAR, RA induces the expression of ISX and thus represses intestinal SR-BI and BCMO1 expression. Taken together, these findings suggest that intestinal vitamin A uptake and production are under negative feedback control via induction of ISX expression by the β,β-carotene metabolite RA. We used BCMO1-knockout mice to test this hypothesis. Because this mouse strain cannot convert β,β-carotene to retinoids (25), ISX-dependent regulation of β,β-carotene absorption via SR-BI should be impaired. For this experiment, we maintained 8-wk-old BCMO1-knockout and WT mice on different diets (n=6/group and genotype). Animals in group 1 were continuously fed a VAD diet and served as controls to determine baseline levels of β,β-carotene and retinoids. Animals in groups 2 and 3 received diets supplemented with β,β-carotene (0.15 mg/g). To analyze the effect of preformed retinoids on β,β-carotene absorption, mice in group 3 were additionally gavaged weekly with dietary vitamin A (300 UI), i.e., VAS diet.
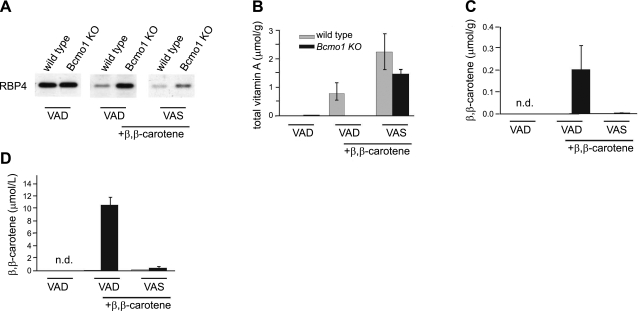
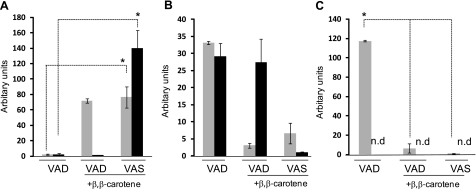
After 15 wk, we sacrificed these mice and determined β,β-carotene and retinoid levels in liver and blood (Fig. 4). In animals of group 1, retinoid stores in the liver were largely depleted (Fig. 4B), demonstrating that the nonsupplemented diet effectively induced systemic vitamin A deficiency in these mice. This deficiency was also evidenced by an accumulation of serum RBP4 in the liver (Fig. 4A). Retinol bound to RBP4 is the major transported form of retinoid in the blood, and RBP4 is secreted from the liver in a vitamin A-dependent manner (29). In β,β-carotene supplemented WT mice (group 2), vitamin A levels in the liver were significantly increased over baseline levels of animals subjected to dietary vitamin A deprivation (Fig. 4B). Accordingly, liver RBP4 levels were decreased significantly compared to animals of group 1 (Fig. 4A). As expected, β,β-carotene supplementation of BCMO1-knockout mice did not result in vitamin A production. Livers of these animals lacked retinoids, and they showed elevated hepatic RBP4 levels similarly to the vitamin A-deficient animals of group 1 (Fig. 4A, B). Instead, this mouse mutant accumulated large amounts of β,β-carotene in the liver and blood (Fig. 4C, D). Supplementation with a combination of vitamin A and β,β-carotene (group 3) significantly increased liver retinoid levels in BCMO1-knockout mice (Fig. 4B). Vitamin A-replenishment of BCMO1-knockout mice in group 3 was also evidenced by a decrease in liver RBP4 levels (Fig. 4A). Most important, dietary vitamin A prevented high levels of β,β-carotene accumulation in BCMO1-knockout mice. Even though these animals were supplemented with the same amount of β,β-carotene as their littermates of group 2, β,β-carotene levels in the blood and liver were 22- and 20-fold lower, respectively.
Figure 4.
Retinoids control intestinal β,β-carotene absorption levels. A) Immunoblot analysis for RBP4 in liver protein extracts from control and BCMO1−/− mice. B, C) Levels of total vitamin A (all-trans-retinol and retinyl esters) (B) and β,β-carotene in livers of Bcmo1-knockout and WT mice (C). D) β,β-carotene levels in plasma of Bcmo1-knockout and WT mice. Gray bars indicate WT; solid black bars indicate Bcmo1-knockout mice. Values are means ± sd; n = 6 animals/genotype and supplementation group. Group 1: VAD diet with no supplementation; group 2: VAD diet with 150 μg/g β,β-carotene; group 3: VAS diet with 150 μg/g β,β-carotene and a weekly oral dose of 300 IU vitamin A.
β,β-Carotene accumulation is correlated with decreased ISX and increased SR-BI expression in BCMO1-knockout mice
We observed that dietary vitamin A can prevent β,β-carotene accumulation in BCMO1-deficient mice. This finding is likely explained by the RA dependent induction of ISX that repressed intestinal SR-BI expression (see above) required for β,β-carotene absorption (12). To test this hypothesis, we determined the effects of different supplementations on intestinal ISX, SR-BI, and BCMO1 mRNA expression in mice of different genotypes (Fig. 5). ISX mRNA expression was highly reduced in vitamin A-deficient control animals (group 1) (Fig. 5A). In contrast, ISX mRNA expression was >30-fold increased in animals that received dietary vitamin A supplementation (group 3) (Fig. 5A). Analysis of the effect of β,β-carotene on the ISX expression levels (group 2 vs. 1) revealed a strict dependency of ISX expression on the BCMO1 genotype, i.e., the ability to covert β,β-carotene to retinoids for RA production. In WT animals, ISX mRNA expression was increased 26-fold by supplementation with β,β-carotene. In contrast, mRNA levels of this transcription factor, in BCMO1-knockout mice, remained as low as in vitamin A-deficient animals regardless of supplementation with β,β-carotene (Fig. 5A).
Figure 5.
β,β-Carotene induces ISX expression in a BCMO1-dependent manner. Relative intestinal mRNA levels of ISX (A), SR-BI (B), and BCMO1 as determined by qRT-PCR (C). Gray bars indicate WT; solid black bars indicate Bcmo1-knockout mice. Values are means ± sd; n = 3 animals/genotype and supplementation group. Group 1: VAD diet with no supplementation; group 2: VAD diet with 150 μg/g β,β-carotene; group 3: VAS diet with 150 μg/g β,β-carotene and a weekly oral dose of 300 IU vitamin A. n.d., not detectable. *P ≤ 0.001.
As expected for a downstream target repressed by ISX activity, SR-BI showed an inverse pattern of expression as compared to ISX (Fig. 5B). Moreover, the same result held true for the second ISX target gene, BCMO1, in WT mice. The mRNA levels dictated by these genes were significantly increased in vitamin A-deficient animals (group 1), but inversely, were decreased in animals that received vitamin A supplementation (group 3) (Fig. 5C). Again, the effect of β,β-carotene supplementation alone was dependent on the presence of BCMO1. β,β-Carotene supplementation decreased intestinal SR-BI expression in WT but not in BCMO1-knockout mice (Fig. 5B, C). Thus, BCMO1-dependent retinoid production from β,β-carotene induced ISX expression paralleled by down-regulation of SR-BI and BCMO1 expression. In BCMO1 deficiency, such a regulation did not occur leading to increased SR-BI activity and β,β-carotene accumulation.
DISCUSSION
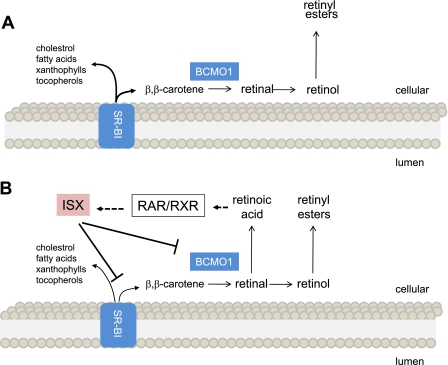
Here we describe a diet-responsive regulatory network that controls the intestinal activity of SR-BI. This 82-kDa membrane protein facilitates the absorption of various lipids, including β,β-carotene, a major source of retinoids in the human diet. On absorption, β,β-carotene is cleaved oxidatively to retinoids by intestinal BCMO1 (see Fig. 6 for the current model). We demonstrate that the β,β-carotene metabolite RA, via RARs, induces the expression of the transcription factor ISX in the small intestine. In turn, ISX represses the intestinal gene expression of both SR-BI and BCMO1. Mouse models showed that this crosstalk between retinoid and ISX signaling elegantly controls vitamin A production from β,β-carotene by negative feedback regulation. The role of SR-BI in the absorption of additional lipids suggests that this regulation may extend to lipids other than β,β-carotene.
Figure 6.
Crosstalk between RAR and ISX signaling controls lipid absorption. A) VAD: SR-BI and BCMO1 expression are increased significantly throughout the small intestine. Enhanced SR-BI activity facilitates the absorption of various lipids, including cholesterol, fatty acids, xanthophylls, tocopherols, and β,β-carotene (7, 9, 12, 14). B) VAS: retinoids derived either from β,β-carotene conversion or preformed dietary retinoids promote the production of RA. RA binds to RARs, inducing ISX expression. Induction of ISX then leads to the repression of intestinal expression of SR-BI and BCMO1.
ISX is a RA inducible target gene that represses intestinal SR-BI and BCMO1 expression
ISX, first characterized in a screen for gut-restricted transcription factors (15), is expressed in an increasing intestinal gradient, from lower levels in the duodenum to high levels in the ileum (15). ISX-deficient mice develop no gross abnormalities but show significantly elevated intestinal mRNA expression of SR-B1 and BCMO1 (15, 18) that normally exhibits the opposite intestinal gradient of expression to ISX (6, 8, 21). These genes encode key components required for absorption of dietary lipids and conversion of absorbed β,β-carotene to vitamin A, respectively (12, 25). Animal studies indicated that ISX expression depends on dietary intake of vitamin A (18), thereby implicating crosstalk between ISX and dietary retinoids. Here we show that the molecular basis of this crosstalk is a RA/RAR-dependent regulation of ISX expression. On RA treatment, mRNA expression of this transcription factor was induced in human colonic CaCo-2 cells. This induction was mediated by RARs because treatment of cells with the RAR agonist TTNPB had the same effect, whereas a specific RAR pan-antagonist inhibited this effect. Using cycloheximide, we showed that this effect of RA on ISX expression is direct and does not require the synthesis of additional protein factors. ChIP analysis demonstrated that RARs bind directly to an RARE within the human ISX promoter. This RAR-dependent induction of gene expression was paralleled by an increase in ISX protein level and, as expected for a transcription factor, ISX was localized to the nuclei of CaCo-2 cells.
In vivo evidence is also provided in this study for RA-dependent regulation of ISX expression. RA treatment of vitamin A-deficient mice induced ISX expression in both the duodenum and jejunum, whereas treatment with the vehicle control had no such effect. Induction of ISX expression was paralleled by a decrease in intestinal SR-B1 and BCMO1 expression both at the mRNA and protein level. No such alteration of SR-B1 protein levels was observed in the liver of these animals, where ISX is not expressed, which indicated that the effects of RA was dependent on ISX. This finding suggested that ISX is an RA-inducible repressor of SR-BI and BCMO1 expression. Genetic studies in mice had previously demonstrated that BCMO1 is an ISX target gene (18). As shown here, overexpression of ISX in Hep2G cells decreased SR-BI protein levels, indicating that SR-BI is also a direct target of ISX. Thus, we conclude that the effects of RA on intestinal SR-BI and BCMO1 expression are mediated by the induction of ISX expression. This regulation of key proteins involved in vitamin A production via the intestine-specific transcription factor ISX not only confers tissue-specificity but also allows for SR-BI and BCMO1 to be controlled by additional regulatory mechanisms. Both the human and murine BCMO1 are induced by PPARs (30, 31). In a vitamin A-deficient state, increased uptake of fatty acids may lead to an increase in the expression of BCMO1 via the activation of PPAR signaling and absence of repression via ISX. In tissues where ISX is absent, BCMO1 expression is most likely regulated independently of RA via other mechanisms, which might include PPAR.
ISX controls β,β-carotene absorption and vitamin A production
Our findings in cell lines and mice suggest that β,β-carotene absorption and conversion to retinoids is under negative feedback control by the β,β-carotene derivative RA. Studies in BCMO1-deficient mice provided in vivo experimental evidence for this notion. This mouse mutant cannot convert β,β-carotene to retinoids, including RA, and thus ISX-dependent regulation of SR-BI expression should be abrogated. When β,β-carotene was provided as the major dietary source of retinoids, this mouse mutant accumulated large amounts of β,β-carotene systemically as evidenced by highly elevated levels of intestinal SR-BI expression. In contrast, WT mice efficiently converted β,β-carotene to retinoids accompanied by induction of ISX expression and decreased expression of intestinal SR-B1 and BCMO1. Gavage with preformed retinoids prevented β,β-carotene accumulation in BCMO1-deficient mice that also exhibited an induction of ISX expression and decreased expression of intestinal SR-BI.
Our studies in human colonic cell lines indicate that the ISX-dependent regulation of intestinal vitamin A production is well conserved in humans. This regulatory mechanism should be considered in future recommendations regarding the efficiency of β,β-carotene for vitamin A production. Based on our findings, it can be predicted that differences would be significant in β,β-carotene utilization in populations with a high vs. a low vitamin A status. In vitamin A deficiency, enhanced and distally extended expression of SR-BI and BCMO1 in the small intestine ensures that even small amounts of dietary β,β-carotene can be absorbed efficiently and used for vitamin A production. When sufficient dietary vitamin A is available, absorption of β,β-carotene is repressed at the level of SR-B1 (Fig. 6). This ISX-dependent regulation of intestinal SR-BI and BCMO1 expression prevents excess β,β-carotene uptake and the production of toxic amounts of RA that can prove detrimental (32).
In addition, our findings imply that dietary vitamin A affects lipid absorption more generally. SR-BI facilitates the intestinal absorption not only of β,β-carotene (12), but also of fatty acids, cholesterol, vitamin E, and nonprovitamin A carotenoids (7, 9, 14). Studies in mice showed that elevated intestinal SR-BI levels accompany enhanced absorption of cholesterol and fatty acids (9). Animal studies indicate that diets low in vitamin A promotes adiposity (33, 34) and epidemiological studies link low vitamin A intake with a high incidence of obesity in certain human populations (35, 36). Notably, BCMO1-knockout mice develop hepatic steatosis and are highly susceptible to diet-induced obesity, pathology enhanced by vitamin A deficiency (25). Genetic variability is considerable in β,β-carotene metabolism in the general population. Two common polymorphisms, R267S and A379V in the BCMO1 gene, occur with allele frequencies of 42 and 24%, respectively. Moreover, 267S/379V double-mutant individuals can have a reduced ability to convert β,β-carotene to vitamin A derivatives (37). In addition, a polymorphism in the putative BCMO1 promoter region has been identified that is associated with elevated plasma carotenoid levels (38). Recently, an association of elevated plasma carotenoid levels with risk factors and biomarkers related to cardiovascular disease has been reported (39). Our studies indicate that increased intestinal SR-BI expression and enhanced lipid uptake likely contribute to these impairments. Thus, an inadequate supply of dietary vitamin A and/or genetic polymorphisms in BCMO1 may constitute risk factors for human dyslipidemia.
In summary, a diet-responsive regulatory network that controls intestinal lipid absorption and vitamin A production is described. In this process, we provide experimental evidence that the intestine-specific homeodomain transcription factor ISX acts as a RA-sensitive “gatekeeper” that controls vitamin A production by repressing gene expression of SR-BI and BCMO1. These findings indicate that intestinal lipid absorption is regulated, and they identify vitamin A as important dietary signal that influences this process. The prevalence of diseases associated with dyslipidemia has increased, including cardiovascular and neurodegenerative disorders. The role of dietary vitamin A in preventing lipid-induced disorders needs further research and is of unquestionable relevance for human health.
Supplementary Material
Acknowledgments
The authors thank Dr. Elwin Morgan (Case Western Reserve University) and Dr. Kristi Bennett (Cleveland Clinic Foundation, Cleveland, OH, USA) for their technical advice in the ChIP assay. This work was supported by U.S. National Institutes of Health grants EY019641 to J.V.L. and EY009339 to K.P.
References
- Wang D Q. Regulation of intestinal cholesterol absorption. Annu Rev Physiol. 2007;69:221–248. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- Rosenblum S B, Huynh T, Afonso A, Davis H R, Jr, Yumibe N, Clader J W, Burnett D A. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4 -hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J Med Chem. 1998;41:973–980. doi: 10.1021/jm970701f. [DOI] [PubMed] [Google Scholar]
- Sehayek E, Nath C, Heinemann T, McGee M, Seidman C E, Samuel P, Breslow J L. U-shape relationship between change in dietary cholesterol absorption and plasma lipoprotein responsiveness and evidence for extreme interindividual variation in dietary cholesterol absorption in humans. J Lipid Res. 1998;39:2415–2422. [PubMed] [Google Scholar]
- McNamara D J, Kolb R, Parker T S, Batwin H, Samuel P, Brown C D, Ahrens E H., Jr Heterogeneity of cholesterol homeostasis in man. Response to changes in dietary fat quality and cholesterol quantity. J Clin Invest. 1987;79:1729–1739. doi: 10.1172/JCI113013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel P, Grolier P, Mekki N, Boirie Y, Rochette Y, Le Roy B, Alexandre-Gouabau M C, Lairon D, Azais-Braesco V. Low and high responders to pharmacological doses of beta-carotene: proportion in the population, mechanisms involved and consequences on beta-carotene metabolism. J Lipid Res. 1998;39:2250–2260. [PubMed] [Google Scholar]
- Hauser H, Dyer J H, Nandy A, Vega M A, Werder M, Bieliauskaite E, Weber F E, Compassi S, Gemperli A, Boffelli D, Wehrli E, Schulthess G, Phillips M C. Identification of a receptor mediating absorption of dietary cholesterol in the intestine. Biochemistry. 1998;37:17843–17850. doi: 10.1021/bi982404y. [DOI] [PubMed] [Google Scholar]
- Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, Margotat A, Lagrost L, Collet X, Borel P. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J Biol Chem. 2006;281:4739–4745. doi: 10.1074/jbc.M509042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardones P, Quinones V, Amigo L, Moreno M, Miquel J F, Schwarz M, Miettinen H E, Trigatti B, Krieger M, VanPatten S, Cohen D E, Rigotti A. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J Lipid Res. 2001;42:170–180. [PubMed] [Google Scholar]
- Bietrix F, Yan D, Nauze M, Rolland C, Bertrand-Michel J, Comera C, Schaak S, Barbaras R, Groen A K, Perret B, Terce F, Collet X. Accelerated lipid absorption in mice overexpressing intestinal SR-BI. J Biol Chem. 2006;281:7214–7219. doi: 10.1074/jbc.M508868200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer C, Sumser E, Wernet M F, Von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci U S A. 2002;99:10581–10586. doi: 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, von Lintig J. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry. 2006;45:13429–13437. doi: 10.1021/bi060701u. [DOI] [PubMed] [Google Scholar]
- Van Bennekum A, Werder M, Thuahnai S T, Han C H, Duong P, Williams D L, Wettstein P, Schulthess G, Phillips M C, Hauser H. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry. 2005;44:4517–4525. doi: 10.1021/bi0484320. [DOI] [PubMed] [Google Scholar]
- During A, Dawson H D, Harrison E H. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005;135:2305–2312. doi: 10.1093/jn/135.10.2305. [DOI] [PubMed] [Google Scholar]
- During A, Harrison E H. Mechanisms of provitamin A (carotenoid) and vitamin A (retinol) transport into and out of intestinal Caco-2 cells. J Lipid Res. 2007;48:2283–2294. doi: 10.1194/jlr.M700263-JLR200. [DOI] [PubMed] [Google Scholar]
- Choi M Y, Romer A I, Hu M, Lepourcelet M, Mechoor A, Yesilaltay A, Krieger M, Gray P A, Shivdasani R A. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development. 2006;133:4119–4129. doi: 10.1242/dev.02537. [DOI] [PubMed] [Google Scholar]
- Cai S F, Kirby R J, Howles P N, Hui D Y. Differentiation-dependent expression and localization of the class B type I scavenger receptor in intestine. J Lipid Res. 2001;42:902–909. [PubMed] [Google Scholar]
- Voshol P J, Schwarz M, Rigotti A, Krieger M, Groen A K, Kuipers F. Down-regulation of intestinal scavenger receptor class B, type I (SR-BI) expression in rodents under conditions of deficient bile delivery to the intestine. Biochem J. 2001;356:317–325. doi: 10.1042/0264-6021:3560317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, Seino S. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15′-monooxygenase (Bcmo1) expression. J Biol Chem. 2008;283:4905–4911. doi: 10.1074/jbc.M707928200. [DOI] [PubMed] [Google Scholar]
- Von Lintig J, Hessel S, Isken A, Kiefer C, Lampert J M, Voolstra O, Vogt K. Towards a better understanding of carotenoid metabolism in animals. Biochim Biophys Acta. 2005;1740:122–131. doi: 10.1016/j.bbadis.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- Bachmann H, Desbarats A, Pattison P, Sedgewick M, Riss G, Wyss A, Cardinault N, Duszka C, Goralczyk R, Grolier P. Feedback regulation of beta, beta-carotene 15,15′-monooxygenase by retinoic acid in rats and chickens. J Nutr. 2002;132:3616–3622. doi: 10.1093/jn/132.12.3616. [DOI] [PubMed] [Google Scholar]
- Takitani K, Zhu C L, Inoue A, Tamai H. Molecular cloning of the rat beta-carotene 15,15′-monooxygenase gene and its regulation by retinoic acid. Eur J Nutr. 2006;45:320–326. doi: 10.1007/s00394-006-0601-3. [DOI] [PubMed] [Google Scholar]
- Batten M L, Imanishi Y, Maeda T, Tu D C, Moise A R, Bronson D, Possin D, Van Gelder R N, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem. 2000;275:11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, von Lintig J, Wyss A. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem. 2007;282:33553–33561. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- Webb N R, de Villiers W J, Connell P M, de Beer F C, van der Westhuyzen D R. Alternative forms of the scavenger receptor BI (SR-BI) J Lipid Res. 1997;38:1490–1495. [PubMed] [Google Scholar]
- O'Byrne S M, Wongsiriroj N, Libien J, Vogel S, Goldberg I J, Baehr W, Palczewski K, Blaner W S. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Gudas L J. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- Paik J, Vogel S, Quadro L, Piantedosi R, Gottesman M, Lai K, Hamberger L, Vieira Mde M, Blaner W S. Vitamin A: overlapping delivery pathways to tissues from the circulation. J Nutr. 2004;134:276S–280S. doi: 10.1093/jn/134.1.276S. [DOI] [PubMed] [Google Scholar]
- Boulanger A, McLemore P, Copeland N G, Gilbert D J, Jenkins N A, Yu S S, Gentleman S, Redmond T M. Identification of beta-carotene 15, 15′-monooxygenase as a peroxisome proliferator-activated receptor target gene. FASEB J. 2003;17:1304–1306. doi: 10.1096/fj.02-0690fje. [DOI] [PubMed] [Google Scholar]
- Gong X, Tsai S W, Yan B, Rubin L P. Cooperation between MEF2 and PPARgamma in human intestinal beta, beta-carotene 15,15′-monooxygenase gene expression. BMC Mol Biol. 2006;7:7. doi: 10.1186/1471-2199-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Ghyselinck N B, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- Ribot J, Felipe F, Bonet M L, Palou A. Changes of adiposity in response to vitamin A status correlate with changes of PPAR gamma 2 expression. Obes Res. 2001;9:500–509. doi: 10.1038/oby.2001.65. [DOI] [PubMed] [Google Scholar]
- Kawada T, Kamei Y, Sugimoto E. The possibility of active form of vitamins A and D as suppressors on adipocyte development via ligand-dependent transcriptional regulators. Int J Obes Relat Metab Disord. 1996;20:S52–S57. [PubMed] [Google Scholar]
- Wolfe W S, Sanjur D. Contemporary diet and body weight of Navajo women receiving food assistance: an ethnographic and nutritional investigation. J Am Diet Assoc. 1988;88:822–827. [PubMed] [Google Scholar]
- Vaughan L A, Benyshek D C, Martin J F. Food acquisition habits, nutrient intakes, and anthropometric data of Havasupai adults. J Am Diet Assoc. 1997;97:1275–1282. doi: 10.1016/S0002-8223(97)00305-2. [DOI] [PubMed] [Google Scholar]
- Leung W C, Hessel S, Meplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh J E, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding β-carotene 15,15′-monoxygenase alter β-carotene metabolism in female volunteers. FASEB J. 2008 doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Perry J R, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried L P, Albanes D, Corsi A M, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba R D, Frayling T M. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84:123–133. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gaziano J M, Norkus E P, Buring J E, Sesso H D. Associations of plasma carotenoids with risk factors and biomarkers related to cardiovascular disease in middle-aged and older women. Amer J Clin Nutr. 2008;88:747–754. doi: 10.1093/ajcn/88.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.