Abstract
Resistance to endocrine therapies remains a major problem in the management of estrogen receptor-α (ER)-positive breast cancer. We show that inhibition of NF-κB (p65/RELA), either by overexpression of a mutant IκB (IκBSR) or a small-molecule inhibitor of NF-κB (parthenolide; IC50=500 nM in tamoxifen-resistant cells), synergistically restores sensitivity to 4-hydroxytamoxifen (4HT) in resistant MCF7/RR and MCF7/LCC9 cells and further sensitizes MCF-7 and MCF7/LCC1 control cells to 4HT. These effects are independent of changes in either cell cycle distribution or in the level of autophagy measured by inhibition of p62/SQSTM1 expression and cleavage of LC3. NF-κB inhibition restores the ability of 4HT to decrease BCL2 expression, increase mitochondrial membrane permeability, and induce a caspase-dependent apoptotic cell death in resistant cells. Each of these effects is reversed by a caspase 8 (CASP8)-specific inhibitor that blocks enzyme-substrate binding. Thus, increased activation of NF-κB can alter sensitivity to tamoxifen by modulating CASP8 activity, with consequent effects on BCL2 expression, mitochondrial function, and apoptosis. These data provide significant new insights into how molecular signaling affects antiestrogen responsiveness and strongly suggest that a combination of parthenolide and tamoxifen may offer a novel therapeutic approach to the management of some ER-positive breast cancers.—Nehra, R., Riggins, R. B., Shajahan, A. N., Zwart, A., Crawford, A. C., Clarke, R. BCL2 and CASP8 regulation by NF-κB differentially affect mitochondrial function and cell fate in antiestrogen-sensitive and -resistant breast cancer cells.
Keywords: apoptosis, autophagy, breast neoplasms, RELA, selective estrogen receptor modulators, tamoxifen
Antiestrogen therapy has been used to treat women with breast cancer for >30 yr, and it remains among the most effective and least toxic of the systemic therapies currently available for the treatment of estrogen receptor-α (ER)-positive breast cancers (1, 2). The triphenylethylene tamoxifen (TAM), a selective estrogen receptor modulator (SERM), is still the most widely used antiestrogen in clinical settings. However, acquired drug resistance is a major limitation to the effectiveness of current antiestrogen/hormonal treatments. Approximately 70% of newly diagnosed breast cancers are ER positive (3) of which 50% do not respond to TAM and exhibit de novo or intrinsic resistance (1, 2). Most patients that initially respond are at risk for relapse and the development of antiestrogen-resistant breast cancer.
Despite >10 million patient yr of experience with TAM, the precise mechanisms that contribute to progression to acquired antiestrogen resistance remain uncertain. Resistance mechanisms may include heterogeneity of ER expression within tumors, ER mutation, mitogenic growth factor production, and loss of ER expression, culminating in the deregulation of cell survival and cell cycle progression functions (1, 2, 4). ER-regulated functions appear to be important; most tumors that become antiestrogen resistant still express ER (5,6,7) and inhibition of ER in antiestrogen-resistant cells is growth inhibitory (8). However, it is also likely that breast cancer cells that acquire resistance to antiestrogens have altered the expression and/or function of some key components of the gene network that controls cell proliferation and cell fate (9).
We previously generated a novel series of genetically related variants from the MCF-7 human breast cancer cell line to identify new antiestrogen-resistance mechanisms. Differences in the transcriptomes of estrogen-independent (aromatase-inhibitor-resistant-like phenotype) but antiestrogen-sensitive (MCF7/LCC1) (10) and estrogen-independent TAM (SERM) and fulvestrant [selective estrogen receptor degrader (SERD)] cross-resistant (MCF7/LCC9; ref. 11) cells have been explored by serial analysis of gene expression (SAGE) and gene expression microarrays. These studies showed NF-κB p65 mRNA expression and transcriptional activation to be significantly increased in the cross-resistant MCF7/LCC9 cells (12). NF-κB is a transcription factor associated with several aspects of oncogenesis, including control of apoptosis, cell cycle progression, differentiation, and cell migration (13). Elevated NF-κB activity is detected during early stages of neoplastic transformation in the rat mammary gland (14). Widely expressed in human and rat mammary tumors (15, 16), NF-κB expression is increased in breast cancer cells that exhibit an estrogen-independent phenotype (17, 18).
NF-κB antiapoptotic activity appears to be crucial for tumor development and resistance to several antineoplastic drugs (13, 19, 20). Parthenolide (Par), a sesquiterpene lactone isolated from the European herb feverfew (Tanaceteum parthenium), is a potent small-molecule inhibitor of NF-κB (21). Recently, Par has attracted considerable attention for its antitumor activity in vitro and in vivo. Par is well tolerated with no significant toxicity in patients with cancer (22), and several studies (23, 24) have shown that Par, either alone or in combination with cytotoxic drugs, can induce apoptosis.
We have reported that the expression of the NF-κB regulator NEMO/IKKγ is up-regulated in antiestrogen-resistant MCF7/LCC9 cells, likely explaining their increased expression of NF-κB mRNA. Pharmacological inhibition of NF-κB by Par restores sensitivity to the SERD fulvestrant (Faslodex; ICI 182,780) by synergistically enhancing apoptosis (25), perhaps as a consequence of its actions as a transcription factor acting alone or in cooperation with other transcription factors including IRF1 (26, 27) and AP-1 (28). However, resistance to TAM and fulvestrant often occurs independently (29, 30) and a role for NF-κB in affecting TAM responsiveness has not been previously studied. Thus, the primary goals of the current study were to explore a potential role for NF-κB in TAM resistance, to establish its mechanism of action, and then to explore whether interfering with NF-κB activity might provide a means to improve responses to TAM therapy.
Our results show that both molecular (mutant IκB; IκBSR) and pharmacological (Par) approaches are highly effective in down-regulating NF-κB activity, further sensitize TAM-sensitive MCF7/LCC1 cells to TAM, and synergistically restore sensitivity to TAM in resistant cells. Combined treatment with Par and TAM restores TAM-induced cell death in resistant MCF7/LCC9 by decreasing the expression of the key antiapoptotic protein BCL2. Inhibition of BCL2 expression alters the ratio of BCL2:BAX expression in favor of an increased destabilization of the mitochondrial membrane and leads to an increase in mitochondrial membrane permeability. Finally, we show that these events are caspase dependent and involve the regulation of caspase-8 (CASP8) activity upstream of mitochondria. A role for executioner caspases downstream of mitochondria also is likely. Together, our data strongly suggest that NF-κB plays a critical role in the development of the antiestrogen-resistant phenotype and that NF-κB inhibition provides a means to overcome resistance to both SERMs and SERDs. These findings provide strong support for designing clinical trials to combine Par and antiestrogens in ER-positive breast cancer patients.
MATERIALS AND METHODS
Cell culture and reagents
MCF-7 cells (ER positive, estrogen dependent for growth, and antiestrogen sensitive) were routinely grown in improved minimal essential medium (IMEM; Biofluids, Rockville, MD, USA) with phenol red and supplemented with 5% FBS (FBS-IMEM). MCF-7 cells were originally obtained from Dr. Marvin Rich (Barbara Ann Karmanos Cancer Institute, Detroit, MI, USA). MCF-7 derived MCF7/LCC1 (ER-positive, estrogen independent for growth, and antiestrogen sensitive), MCF7/LCC9 cells (ER positive, estrogen independent for growth, and antiestrogen cross-resistant; refs. 10, 11), and MCF7/RR cells (ER positive, estrogen independent for growth, TAM resistant, and fulvestrant sensitive; MCF-7 variant generated directly from MCF-7 cells by selection against TAM; ref. 31) were routinely grown in phenol red-free IMEM (Biofluids) supplemented with 5% charcoal-stripped calf serum (CCS; CCS-IMEM). We confirmed the genetic lineage of the 3 variant cell lines as being derived from the original MCF-7 cell line by DNA fingerprinting using genetic markers at 9 different loci. MDA-MB-231 cells (ER negative and antiestrogen cross resistant) were obtained from the Lombardi Comprehensive Cancer Center Tissue Culture Shared Resource and were routinely grown in IMEM with phenol red and supplemented with 5% FBS. These cells represent a standard model of de novo resistance. All cells were shown to be free of Mycoplasma spp. contamination and were maintained in a humidified incubator at 37°C in an atmosphere containing 95% air-5% CO2.
4-Hydroxytamoxifen (4HT) and Par were purchased from Sigma-Aldrich (St. Louis, MO, USA), and fulvestrant was obtained from Tocris Bioscience (Ellisville, MO, USA). The concentrations of 4HT and Par used were 1 μM and 500 nM respectively, unless otherwise indicated. The Insolution caspase inhibitor I [cell-permeable, irreversible, pancaspase inhibitor (PI), catalog no. 627609] and the CASP8/caspase-8 Inhibitor II (C8I; catalog no. 218759, potent, cell-permeable, irreversible inhibitor of CASP8; the Z-IETD-FMK sequence binds to CASP8 and blocks its binding to the substrate) were purchased from Calbiochem (San Diego, CA, USA); a 20 μM concentration of each was used. All experiments in this manuscript were repeated ≥3 times unless explicitly stated otherwise.
Stable transfection with IκBSR
MCF7/LCC9 cells were seeded at a density of 8 × 105 cells/dish in 10 cm2 dishes and grown for 24 h before transfection. Cells were stably transfected with 4 μg of either an empty pCMV4 plasmid or S32/36A mutant pCMV4-FLAG IκBα, a mutant IκB that acts as a dominant-negative NF-κB inhibitor also referred to as IκBSR (kindly provided by Dr. Marty Mayo, University of Virginia, Charlottesville, VA, USA) and 1 μg of the puromycin-resistance cassette (pBABE plasmid), using Fugene 6 (Roche Diagnostics, Indianapolis, IN, USA) as recommended by the manufacturer. Stably transfected cells were selected for growth in the presence of 1 μg/ml puromycin. Puromycin-resistant colonies were selected and expanded from 10 cm2 dishes to 6-well dishes and then to T-75 75-cm2 plastic tissue culture flasks.
For each colony selected, cells were lysed as described below and screened for FLAG and IκBα protein expression by Western blot analysis. Cells transfected with the control empty vector (EV) were designated LCC9/EV, and those transfected with the S32/36A mutant pCMV4-FLAG IκBα were designated LCC9/IκBSR.
Cell lysis, immunoblotting, and coimmunoprecipitation
To determine the effects of 4HT, Par, PI, and C8I on protein expression, MCF7/LCC1 and MCF7/LCC9 cells were seeded into 6-well dishes at 3 × 105 cells/well and cultured in normal growth medium for 24 h. Cells were then treated with vehicle, 1 μM 4HT (IC50 for the parental MCF7/LCC1 cells), or 500 nM Par (IC50 for the cross-resistant MCF7/LCC9 cells) singly or in combination with or without the 20 μM caspase inhibitor (PI or C8I as indicated) in CCS-IMEM for 72 h. For the determination of basal p65 NF-κB and BCL2 protein expression, cells were grown in T-25 cm2 tissue culture flasks. Cells were washed once with saline solution (1× PBS) and then lysed on ice in modified RIPA buffer (150 mM NaCl; 50 mM Tris, pH 7.5; 1% Igepal CA-630; and 0.5% deoxycholate) supplemented with Complete Mini protease inhibitor cocktail tablets (Roche) and 1 mM sodium orthovanadate phosphatase inhibitor (Sigma). Lysates were clarified by centrifugation at 4°C, and total protein was quantified using the bicinchoninic acid assay purchased from Pierce Biotechnology (Rockford, IL, USA).
Whole-cell lysate (20–40 μg) was resolved by PAGE using NuPAGE 10% precast acrylamide gels (Invitrogen, Carlsbad, CA, USA) and then transferred onto nitrocellulose membranes. Nitrocellulose membranes were washed briefly in Tris-buffered saline and Tween-20 (TBST) and blocked in a solution of TBST containing 5% nonfat dry milk for 15 min with constant agitation. After blocking, the nitrocellulose membrane was incubated with the following primary antibodies overnight at 4°C: mouse monoclonal BCL2 primary antibody AAM-072 (1:500; Stressgen Biotechnologies, Vancouver, BC, Canada), rabbit polyclonal BAX primary antibody 06-499 (1:500; Upstate Biotechnology, Charlottesville, VA, USA), rabbit polyclonal p65 NF-κB primary antibody 06-418 (1:1000, Upstate Biotechnology), rabbit p50 NF-κB primary antibody 06-886 (1:500; Upstate Biotechnology), rabbit polyclonal LC3B primary antibody 2775 (1:1000, Cell Signaling, Beverly, MA, USA), rabbit polyclonal p62/SQSTM1 primary antibody ab64134 (1:1000; Abcam, Cambridge, MA, USA), mouse monoclonal ERα primary antibody VP-E613 (1:1000; Vector Laboratories, Burlingame, CA, USA), mouse monoclonal p300 primary antibody 554215 (1:1000; BD Biosciences, San Jose, CA, USA), rabbit polyclonal c-Jun NH2-terminal kinase (JNK) primary antibody 06-748 (1:1000; Upstate Biotechnology), and rabbit active-JNK pAb primary antibody v-7931(1:500; Promega, Madison, WI, USA). Membranes were washed in TBST (3× for 15 min) and were incubated for 1 h with horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences, Piscataway, NJ, USA) at a 1:5000 dilution at room temperature with constant agitation before enhanced chemiluminescence (Amersham Biosciences) and exposure to film. For rabbit polyclonal BAX primary antibody 06-499 (1:500; Upstate Biotechnology), the nitrocellulose membrane was blocked in 10% horse serum in TBST for 1 h and incubated with the primary antibody overnight at 4°C in this solution. The secondary antibody was also diluted in 10% horse serum in TBST, incubated for 1 h at room temperature. Finally, membranes were reprobed as above with β-actin monoclonal antibody (1:5000; Sigma) to confirm equal loading of the gels.
Quantification was done by densitometry. Data are means ± se, presented as the protein:β-actin ratio. To screen LCC9/EV and S32/36A mutant pCMV4-FLAG IκBα (LCC9/IκBSR) clones for FLAG and IκBα protein expression, mouse monoclonal FLAG-M5 primary antibody F4042 (1:500; Sigma) and rabbit polyclonal IκBα primary antibody sc-371 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used, respectively.
For coimmunoprecipitations, 400 μg of treated (as indicated) cell lysate was incubated with 2.5 μl of p65 NF-κB antibodies overnight at 4°C with rotation. The following day, 50 μl of protein A-Sepharose beads (Amersham Biosciences) was added, and the tubes were returned for additional rotation for 1 h at 4°C to recover the immune complexes. The samples were centrifuged, and the supernatant was aspirated. The beads were then washed once with lysis buffer and twice with Tris-saline (TN; 50 mM Tris, pH 7.5, and 150 mM NaCl), resuspended in 2× Laemmli sample buffer, and boiled for 5 min. The immune complexes and 20 μg of corresponding cell lysate were then resolved by PAGE as described above.
p65/RELA small inhibitory RNA (siRNA)
siRNA oligonucleotides directed against p65/RELA (NF-κB.2, HARDC-000003) were purchased from Dharmacon (Lafayette, CO, USA), nonsilencing control oligonucleotides (si-ctrl) were purchased from Qiagen (Valencia, CA, USA), and the Lipofectamine 2000 reagent was purchased from Invitrogen. MCF7/LCC9 cells were seeded in 6-well plastic tissue culture dishes in CCS-IMEM at 1 × 105 cells/well. The following day, cells were transfected with 100 nM si-p65/RELA or nonsilencing control siRNA oligonucleotides (si-ctrl) for either 24 or 96 h before trypsinization and lysis using RIPA buffer supplemented with phosphatase and protease inhibitors. Lysates were then separated by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted for p65/RELA and BCL2. GAPDH goat polyclonal antibody sc-20357 (1:5000; Santa Cruz) was used as the loading control.
Transient transfections and luciferase reporter assays
Cells were seeded at a density of ∼8 × 104 cells/well into 12-well dishes and allowed to grow for 24 h before transfection. Cells were cotransfected with 0.4 μg/well of pNF-κB-Luc reporter plasmid (Stratagene, La Jolla, CA, USA) and 0.1 μg/well of pRL-SV40 plasmid (Promega) containing the Renilla luciferase gene under the control of a constitutive SV40 promoter using the Fugene 6 transfection reagent (Roche). To determine whether IκBSR inhibits NF-κB-dependent transcription transiently in MCF7/LCC1 and MCF7/LCC9 cells, these cells were also cotransfected with a third plasmid that was either a standard control comprising an empty pCMV4 plasmid, or S32/36A mutant pCMV4-FLAG IκBα (IκBSR). Three hours post-transfection, cells were treated with either 500 nM Par and/or 1 μM 4HT in CCS-IMEM, or medium was changed to IMEM containing no drug as indicated for 24 h. Subsequently, cells were lysed, and activation of the pNF-κB-Luciferase construct was measured using the Dual Luciferase Assay Kit (Promega) according to the manufacturer’s instructions. Luminescence was quantified using a Lumat LB 9501 luminometer (EG&G Berthold, Bundoora, VIC, Australia). Three independent experiments were done each at least in quadruplicate, and the luciferase values were normalized to Renilla luminescence. Data are presented as means ± se.
Cell proliferation
To study effects of NF-κB inhibition on response to 4HT or to caspase inhibition (using 20 μM of either PI or C8I), the TAM-resistant (MCF7/LCC9, MCF7/RR, and MDA-MB-231) and the LCC9/IκBSR cells were seeded at a density of 1 to 1.8 × 104 cells/well in 24-well plates; 24 h later, they were treated with the indicated concentrations of drug in appropriate medium for 7 d, with redosing on d 3 and 5. On the day of counting, cells were washed twice with warm 1× PBS, trypsinized, resuspended in PBS, and counted using a Beckman Coulter Counter (Beckman Coulter Corp., Fullerton, CA, USA). To study the effects of NF-κB inhibition in response to 4HT in TAM-sensitive (MCF-7 and MCF7/LCC1) cells and in response to fulvestrant in TAM-resistant but fulvestrant-sensitive (MCF7/RR) cells, these cells were seeded at a density of 2 × 104 cells/well in 24-well plates. Twenty-four hours postplating, cells were treated with increasing concentrations of 4HT (0–1 μM) or fulvestrant (0–1 μM) in the presence or absence of 500 nM Par as shown in appropriate medium for 5 d, with redosing once on d 3. On d 5, cells were trypsinized, resuspended in PBS, and counted as described above. Data were normalized to vehicle-treated (control) cells, and 3 independent experiments were done each at least in quadruplicate. Data are presented as means ± se.
Mitochondrial membrane permeability
Cells were seeded at a density of 5 × 105 cells/well in 6-well dishes and cultured in normal growth medium. Twenty-four hours later, cells were treated with ethanol vehicle, 4HT (1 μM), Par (500 nM), singly or in combination with or without the caspase inhibitor (20 μM PI and 20 μM C8I) for 18–20 h. Cells were gently washed once with warm 1× PBS and trypsinized to remove adherent cells from the culture dish. Cells were then centrifuged at 350 g for 5 min. One microliter of MitoSensor reagent (Clontech Laboratories, Mountain View, CA, USA) was added to 1 ml incubation buffer/1 ml 1× warm PBS (final concentration: 5 μg/ml). The reagent was vortexed and then centrifuged for 5 min at 14,000 rpm. The cell pellet was gently resuspended in 1 ml diluted Mitosensor reagent and incubated at 37°C in a 5% CO2 incubator for 15–20 min. Cells were again centrifuged at 350 g for 5 min to obtain a cell pellet and resuspended in 500 μl of 1× PBS, and fluorescence was measured by the Lombardi Comprehensive Cancer Center Flow Cytometry Shared Resource. Apoptotic cells show primarily green fluorescence (due to collapse of the electrochemical gradient across the mitochondrial membrane) and are easily differentiated from healthy cells that exhibit red and green fluorescence. Data are presented as means ± se for ≥3 independent experiments.
Cell cycle and apoptosis analyses
Cells were seeded at a density of 5 × 105 cells/dish in 10 cm2 dishes and cultured in growth medium (CCS-IMEM) for 24 h. The following day, cells were treated with ethanol vehicle, 4HT (1 μM), and/or Par (500 nM) in CCS-IMEM for an additional 72 h. For cell cycle analysis, cells were harvested, fixed in ethanol, and analyzed for alterations in cell cycle via FACS at the Lombardi Comprehensive Cancer Center Flow Cytometry Shared Resource according to the method of Vindelov et al. (32). Data are presented as means ± se for ≥3 independent experiments. For detecting apoptosis, staining for annexin V was performed according to the manufacturer’s instructions as described in the TACS Annexin V Kit (4830-250-K; In Situ Cell Detection Kit; Trevigen, Gaithersburg, MD, USA). After being stained with FITC annexin V and PI in the binding buffer provided, apoptotic cells showed green fluorescence that was measured by the Flow Cytometry Shared Resource (Lombardi Comprehensive Cancer Center). Data are presented as means ± se for ≥3 independent experiments.
Statistical analyses
One-way ANOVA was used to compare the effects of treatment on cell proliferation, apoptosis, mitochondrial membrane permeability assay, and immunoblot assays with >2 treatment groups. Student’s t test was used to compare 2 group-design experiments. The nature of the interactions between 4HT and Par and between fulvestrant and Par was defined by determining the R index (RI) (33). RI values were obtained by calculating the expected cell survival (Sexp; the product of survival obtained with drug A alone and the survival obtained with drug B alone) and dividing this Sexp by the observed cell survival in the presence of both drugs (Sobs). Sexp/Sobs >1.0 indicates a potentially synergistic interaction, <1.0 indicates a potentially antagonistic interaction, and =1 is indicative of an additive interaction between the 2 drugs used.
RESULTS
p65/RELA is up-regulated and basal NF-κB activity is increased in antiestrogen-resistant MCF7/RR cells
We first measured the expression of p65/RELA protein in MCF7/RR cells, an antiestrogen-resistant variant of MCF-7 generated following in vitro selection against TAM (TAM resistant but retains sensitivity to fulvestrant; ref. 31). As seen in MCF7/LCC9 cells (12), MCF7/RR cells also exhibit an increase in p65/RELA protein expression by 8-fold compared with their MCF-7 cell controls (Fig. 1A; P<0.05). Expression of the p50 and p52 NF-κB subunits is not different between the 2 cell lines (data not shown). To measure transcriptional activation of NF-κB, we used a dual-luciferase promoter-reporter assay where MCF-7 and MCF7/RR cells were cotransfected with a NF-κB-luciferase reporter vector and a phRL-SV40-Renilla control vector. Figure 1B shows that the basal activity of the NF-κB promoter is increased by ∼3-fold (P<0.001) in antiestrogen-resistant MCF7/RR cells compared with the antiestrogen-sensitive MCF-7 control cells. These data strongly implicate increased NF-κB signaling in reduced TAM responsiveness.
Figure 1.
Basal p65/RELA protein expression and the basal transcriptional activity of NFκB are both increased in antiestrogen-resistant MCF7/RR cells. A) Quantification and representative immunoblot of p65/RELA expression in MCF-7 and MCF7/RR cells. Whole-cell lysates (20 μg) were separated by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted. β-Actin was loading control. Data are mean ± se relative p65:actin ratio (normalized to MCF-7 control cells) for 3 independent experiments. *P<0.05; Student’s t test. B) Basal transcriptional activity of NF-κB in MCF-7 and MCF7/RR cells. Data are mean ± se fold induction relative to MCF-7 cells for 4 determinations. *P < 0.001; Student’s t test.
Inhibition of NF-κB by Par restores sensitivity to 4HT in resistant MCF7/LCC9 and MCF7/RR cells
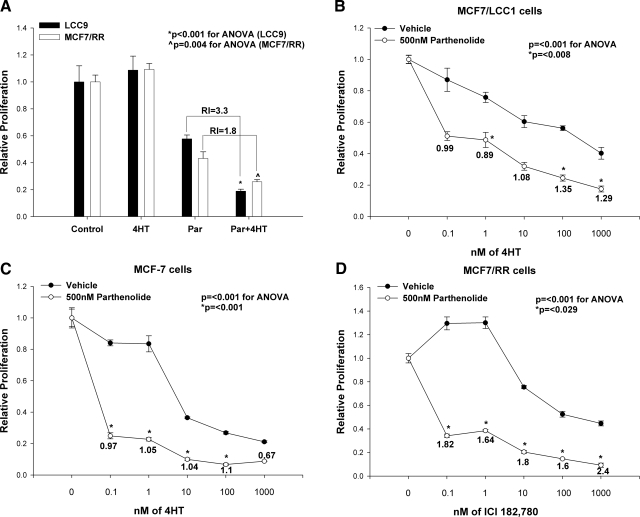
We determined whether NF-κB inhibition reverses resistance to 4HT in resistant cells (MCF7/LCC9 and MCF7/RR) and increases sensitivity to 4HT in highly responsive cells (MCF7/LCC1 and MCF-7). These 4 models were each treated with increasing concentrations of antiestrogen 4HT (0–1 μM; 0=vehicle control) in the presence or absence of 3 different concentrations of Par (50, 100, and 500 nM). While 1 μM 4HT alone is ineffective in both the resistant MCF7/LCC9 and MCF7/RR cells (Fig. 2A; 1-way ANOVA: LCC9, P<0.001; MCF7/RR, P=0.004), a combination of 4HT and exposure to the IC50 of Par (500 nM) significantly inhibits growth and restores sensitivity to 4HT in both resistant variants (MCF7/LCC9 and MCF7/RR). Concentrations of Par that are inactive as a single agent for cell proliferation (50 and 100 nM) do not restore sensitivity to 4HT in these cells (data not shown). This result is to be expected, as some antiestrogen-resistant cells are dependent on NF-κB (25). Thus, concentrations of Par that inhibit NF-κB activity should also inhibit basal proliferation in other antiestrogen-resistant cell models. The interaction between Par and 4HT is synergistic in both resistant cells (MCF7/LCC9 and MCF7/RR), generating an RI of 3.3 for MCF7/LCC9 and an RI of 1.8 for MCF7/RR cells (values>1.0 indicate synergy; ref. 33).
Figure 2.
A) Par inhibits proliferation and synergistically restores 4HT sensitivity in antiestrogen-resistant MCF7/LCC9 and MCR7/RR cells. Cells were seeded in quadruplicate and treated with ethanol vehicle, 4HT (1 μM), or Par (500 nM) in the presence or absence of 4HT (1 μM) in CCS-IMEM for 7 d before counting. Data are mean ± se proliferation relative to ethanol-treated control of 4 determinations. P = 0.029 for MCF7/LCC9 vs. control; P = 0.015 for MCF7/RR vs. control. *P < 0.001, ∧P = 0.004; ANOVA; RI = 3.3, 1.8, respectively. B–D) Par increases sensitivity to 4HT in antiestrogen-sensitive MCF7/LCC1 and MCF-7 cells (B, C) and synergistically enhances sensitivity to ICI 182,780 (fulvestrant) in TAM-resistant MCF7/RR cells (D). Cells were seeded in quadruplicate and treated with either 0–1000 nM (1 μM), 4HT (B, C), or 0–1000 nM ICI 182,780 (D) in the presence or absence of 500 nM Par in CCS-IMEM for 5 d before counting. Data are mean ± se proliferation relative to ethanol-treated control of 4 determinations. P ≤ 0.001 for all treatment groups; 1-way ANOVA. RI values for each treatment doseage are indicated. B) MCF7/LCC1 cells. *P ≤ 0.008; Student’s t test. C) MCF-7 cells. *P ≤ 0.001; Student’s t test. D) MCF7/RR cells. *P ≤ 0.029; Student’s t test.
MCF7/RR cells were also subjected to increasing concentrations of fulvestrant in the presence or absence of 500 nM Par. While MCF7/RR cells are sensitive to fulvestrant (Fig. 2D), the combined treatment with Par and fulvestrant further robustly and synergistically increases their sensitivity to fulvestrant, as is evident from their RI values (RI=2.4 for 1 μM fulvestrant; Fig. 2D).
While inhibition of endogenous NF-κB by Par increases sensitivity to 4HT in the antiestrogen-sensitive cells (MCF7/LCC1 and MCF-7), this interaction is additive rather than synergistic (Fig. 2B, C). Par also does not restore sensitivity to 4HT in MDA-MB-231 cells (an ER-negative model of de novo endocrine cross-resistance; Supplemental Fig. S1). Thus, increased NF-κB transcriptional activity contributes to an ER-positive, TAM-resistant phenotype. Furthermore, reducing NF-κB activation by Par both reverses the antiestrogen-resistant phenotype and can also increase (but to a lesser degree) TAM sensitivity in cells that are already sensitive to 4HT. Since some ER-positive breast cancers are heterogeneous and may contain both TAM-sensitive and TAM-resistant cells, the ability to concurrently reverse resistance in resistant cells and increase sensitivity in sensitive cells implies a potentially significant advantage to including Par in TAM regimens.
Par inhibits NF-κB-dependent transcription in resistant MCF7/LCC9 and MCF7/RR cells
Par restores 4HT sensitivity in resistant MCF7/LCC9 and MCF7/RR cells. Thus, we determined whether this exposure also inhibits NF-κB transcriptional activity. As shown in Fig. 3, Par inhibits NF-κB-dependent transcription in both resistant (MCF7/LCC9 and MCF7/RR) and sensitive (MCF7/LCC1 and MCF-7) cells. However, inhibition of NF-κB activity is significantly greater in resistant cells (MCF7/LCC9 and MCF7/RR; ∼50% inhibition) compared with sensitive cells (MCF7/LCC1 and MCF-7; ∼30% inhibition). These observations are consistent with the effects of Par on responsiveness to TAM as measured by changes in cell proliferation (Fig. 2) and with the effects of Par on fulvestrant sensitivity (25).
Figure 3.
Par inhibits NF-κB-dependent transcriptional activity in all breast cancer cell lines. Cells were cotransfected with pNF-κB-Luc and pRL-SV40 Renilla constructs before treatment with 500 nM Par for 24 h before lysis and luminescence detection. Data are mean ± se relative luciferase:Renilla activity (relative light units) for 4 determinations. *P = 0.011, ∧P = 0.016; Student’s t test.
Dominant-negative NF-κB inhibitor (mutant IκB; IκB super-repressor IκBSR) mimics the effects seen with Par
Since Par may have “off-target” effects, we took a molecular approach to obtain independent confirmation of the role of Par in NF-κB inhibition. A constitutively active mutant IκB (pCMV4-FLAG-tagged IκBSR) was stably introduced into MCF7/LCC9 cells; we used these cells because they exhibit the strongest interaction between Par and 4HT. IκBSR cannot be phosphorylated and targeted for degradation and so acts as a dominant-negative NF-κB inhibitor (34). Figure 4A shows the characterization of these cells and their EV controls. Expression of the appropriate constructs was measured using antibodies to FLAG and IκBα, respectively. MCF7/LCC9/IκBSR clone 5 cells express FLAG and overexpress IκBα/IκBSR (∼2 fold), whereas MCF7/LCC9/EV clone 11 control cells do not express FLAG (Fig. 4A). The ability of IκBSR to restore 4HT-induced inhibition of cell proliferation was then measured (Fig. 4B). Control cells (MCF7/LCC9/EV clone 11) remain resistant to 4HT. In marked contrast, IκBSR expression restores TAM sensitivity in the resistant MCF7/LCC9 cells (Fig. 4B; P<0.001), confirming the effects seen with Par. These data show that the effects of Par are primarily driven by its inhibition of NF-κB.
Figure 4.
IκBSR (mutant IκBα; dominant-negative NF-κB inhibitor) expression sensitizes the resistant MCF7/LCC9 cells to antiestrogen 4HT and inhibits NF-κB dependent transcription. A) Characterization of MCF7/LCC9 cells stably expressing IκBSR and their EV controls. Whole-cell lysates (40 μg) from IκBSR stable transfectants and EV controls were separated by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with mouse monoclonal FLAG-M5 and rabbit polyclonal IκBα antibodies, respectively. β-Actin was loading control. LCC9/IκBSR clone 5 cells overexpress IκBα/IκBSR and express FLAG, as shown in a representative immunoblot. B) IκBSR expression restores 4HT-induced inhibition of cell proliferation in MCF7/LCC9 cells. Cells were seeded in quadruplicate and treated with either ethanol vehicle or with 4HT (1 μM) for 7 d before counting. Data are mean ± se relative proliferation of 4 determinations. *P < 0.001; Student’s t test. C) IκBSR significantly inhibits NF-κB-dependent transcription in both MCF7/LCC9 and MCF7/LCC1 cells. Cells were transiently transfected in quadruplicate with pNF-κB-Luc and pRL-SV40 Renilla constructs with or without IκBSR for 24 h before lysis and luminescent detection. Data are mean ± se relative luciferase:Renilla activity (relative light units) for 4 determinations. *P = 0.001, ∧P = 0.001; Student’s t test.
We also measured NF-κB-driven luciferase activity in cells transiently transfected with IκBSR. The dominant-negative NF-κB inhibitor (IκBSR) almost completely inhibits NF-κB-dependent transcription in both the resistant MCF7/LCC9 and the sensitive MCF7/LCC1 cells (Fig. 4C; P=0.001 relative to LCC1/EV; P=0.001 relative to LCC9/EV). These data further imply that inhibition of endogenous NF-κB by both molecular (IκBSR) and pharmacological (Par) approaches effectively reverse the TAM-resistant phenotype.
Combined treatment with Par and 4HT increases mitochondrial membrane permeability and induces apoptosis in resistant cells
Par inhibits NF-κB-dependent transcription in TAM-resistant cells (Fig. 3). Thus, we asked whether the combination of Par and 4HT is more effective than Par alone in inhibiting NF-κB-dependent transcription. We measured NF-κB activity in the resistant cells (MCF7/LCC9 and MCF7/RR) as in Fig. 1B. While Par alone significantly inhibits NF-κB-dependent transcription in both the resistant cells, Par in combination with 4HT has no additional effect on NF-κB-luciferase activity in the resistant MCF7/LCC9 and MCF7/RR cells (Supplemental Fig. S2). We measured the effects of Par ± 4HT or ethanol vehicle on ER expression (Supplemental Fig. S3). We found no consistent or statistically significant effect on protein levels of ER, irrespective of the treatment in both resistant variants (MCF7/LCC9 and MCF7/RR). The physical association of p65/RELA with either NF-κB p50 or with the coactivator p300 was also measured using coimmunoprecipitation. We found no change in the association of p65/RELA with either p50 or with the coactivator p300 following any of the treatments (Supplemental Fig. S4).
Since Par can reverse the resistance of breast cancer cells to TNF-related apoptosis by inducing JNK independent of NF-κB inhibition (24), we determined whether Par-induced JNK activity may play a role in its restoration of 4HT sensitivity. Antiestrogen-sensitive-MCF7/LCC1 and -resistant-MCF7/LCC9 cells were treated with ethanol vehicle, Par, or 4HT in the presence or absence of Par for 72 h. Whole cell lysates were then collected and immunoblotted for active-JNK. While the protein levels of JNK and p-JNK were both higher in resistant MCF7/LCC9 cells compared with sensitive MCF7/LCC1 cells, the protein levels of JNK and p-JNK did not change in MCF7/LCC9 cells following treatment (Supplemental Fig. S5). These data suggest that the synergistic interaction between Par and antiestrogen 4HT may not involve activation of the JNK pathway.
Because 4HT can affect proliferation by affecting both cell cycle changes and apoptosis, we measured these end points in vitro. MCF7/LCC9 cells were treated with ethanol vehicle or 4HT in the presence or absence of Par for 72 h, and the relative proportions of cells in different phases (G0/G1; S; G2/M) were analyzed by flow cytometry. Par alone or in combination with the antiestrogen 4HT does not alter the cell cycle profile of MCF7/LCC9 cells (Supplemental Fig. S6), suggesting that the synergistic interaction between Par and 4HT is not due to changes in cell cycling. Since 4HT can also induce apoptosis (4), we studied the effects of ethanol vehicle or 4HT in the presence or absence of Par on apoptosis as measured by FITC-conjugated annexin V and propidium iodide staining. As expected, 4HT alone does not induce apoptosis in resistant MCF7/LCC9 cells (Fig. 5A), whereas 4HT in the presence of Par statistically significantly induces apoptosis by 1.7-fold (Fig. 5A; 1-way ANOVA: P=0.042; P<0.05 relative to 4HT; P<0.05 relative to control). These effects are independent of any changes in autophagy as measured by LC3 cleavage or inhibition of p62/SQSTM1 expression (35) (Supplemental Fig. S9).
Figure 5.
Combined treatment with Par and 4HT induces apoptosis and enhances MMP in MCF7/LCC9 cells. Cells were treated with Par (500 nM), 4HT (1 μM), Par + 4HT, or ethanol vehicle in CCS-IMEM for 72 h before determination of apoptosis by annexin V assay (A) or for 18–20 h before measuring MMP (B). Data are means ± se normalized to ethanol-treated cells for ≥3 independent experiments. P = 0.042 (A), P=0.008 (B) for all treatment groups; 1-way ANOVA. *P < 0.05.
The induction of apoptosis is often accompanied by the mitochondrial permeability transition, where the electrochemical gradient across the mitochondrial membrane collapses due to the formation of pores driven by the activation of proapoptotic members of the BCL2 family. Once the mitochondrial membrane is permeable, cytochrome c is released into the cytoplasm. To determine whether the combination of Par and 4HT in resistant MCF7/LCC9 cells induces changes in the mitochondrial membrane integrity, we measured the effects of Par on mitochondrial membrane permeability (MMP). Consistent with the effects on apoptosis shown in Fig. 5A, Fig. 5B shows a statistically significant increase in MMP in the presence of both drugs (Fig. 5B; 1-way ANOVA: P=0.008; P<0.05 relative to 4HT; P<0.05 relative to control). No significant increase in MMP was seen in response to either Par or 4HT alone. Thus, combined treatment with Par and 4HT synergistically reduces cell growth and restores 4HT-induced cell death in resistant cells, at least in part, by activating the intrinsic apoptotic pathway, leading to permeabilization of the outer mitochondrial membrane.
Par in combination with 4HT inhibits expression of BCL2 in resistant cells
The changes in MMP seen in Fig. 5B implicate the BCL2 family of proteins as key players in signaling downstream from NF-κB in the context of antiestrogen responsiveness. The BCL2 family of proteins can be divided into the antiapoptotic (such as BCL2, BCL-XL, and BCL-W) and proapoptotic proteins (including BAX and BID). The balance between proapoptotic and the antiapoptotic members of the BCL2 family can determine cell fate. To confirm how BCL2 protein expression is affected by 4HT and Par, MCF7/LCC1 and MCF7/LCC9 cells were treated with ethanol vehicle, Par, and 4HT in the presence or absence of Par for 72 h. Whole-cell lysates were collected and subjected to Western blot analysis. While 4HT strongly inhibits BCL2 protein expression in the sensitive MCF7/LCC1 cells (Fig. 6C, D; P<0.05 relative to control), it does not affect BCL2 expression in MCF7/LCC9 cells (Fig. 6A, B). In contrast, BCL2 protein expression is significantly inhibited in resistant MCF7/LCC9 cells by Par (Fig. 6A, B; 50% inhibition; P=0.001 relative to control), and Par in combination with antiestrogen 4HT further inhibits BCL2 protein expression in resistant MCF7/LCC9 cells (Fig. 6A, B; 75% inhibition; P=0.003 relative to Par). Similar results were seen in the sensitive MCF7/LCC1 cells (Fig. 6D; P<0.05 relative to 4HT), which are consistent with the effects of Par on responsiveness to 4HT (Fig. 2B) measured by changes in cell proliferation, but RI values were additive. To evaluate whether this decrease in BCL2 protein expression is a transcriptional or posttranscriptional event, BCL2 mRNA levels were measured by RT-PCR in the resistant MCF7/LCC9 cells following treatment. Similar to the protein levels, BCL2 mRNA was inhibited by ≥70% when cells were treated with the combination of Par and 4HT (data not shown). Thus, the changes in BCL2 protein expression closely follow changes in BCL2 mRNA transcription.
Figure 6.
BCL2 protein expression is down-regulated by the combined treatment with Par and 4HT in MCF7/LCC1 and MCF7/LCC9 cells. MCF7/LCC1 and MCF7/LCC9 cells were each treated with 500 nM Par, 1 μM 4HT, Par + 4HT, or ethanol vehicle in CCS-IMEM for 72 h before cell lysis. Whole-cell lysates (20–40 μg) were separated by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted for BCL2. β-Actin was loading control. Data are means ± se from 3 independent experiments. A) Representative immunoblot for MCF7/LCC9 cells. B) Relative BCL2:β-actin ratio in MCF7/LCC9 cells. P ≤ 0.001 for all treatment groups, P = 0.032 for 4HT vs. control; 1-way ANOVA. *P = 0.003; ∧P = 0.001. C) Representative immunoblot for MCF7/LCC1 cells. D) Relative BCL2:β-actin ratio in MCF7/LCC1 cells. P = 0.005 for all treatment groups; 1-way ANOVA. *P < 0.05; ∧P < 0.05. −I, without caspase inhibitor (pancaspase or CASP8).
While Par alone or in combination with 4HT has no statistically significant effect on expression of the proapoptotic protein BAX (data not shown), there is a marked 5-fold increase in the BAX:BCL2 ratio when MCF7/LCC9 cells are treated with a combination of Par and 4HT (Supplemental Fig. S7), primarily because of the decrease in BCL2 expression. These data strongly suggest that Par may synergize with 4HT by acting to decrease the expression of BCL2 such that a marked increase in the overall BAX:BCL2 ratio occurs (36, 37), shifting the balance toward cell death and restoring the 4HT-induced apoptotic response in resistant cells.
Stable expression of IκBSR and siRNA to p65/RELA; both inhibit BCL2 protein expression in MCF7/LCC9 cells
To establish whether the dominant-negative NF-κB inhibitor (IκBSR) mimics the effect of Par on BCL2, whole-cell lysates were collected from LCC9/EV and LCC9/IκBSR cells, subjected to SDS-PAGE, and immunoblotted to measure the protein expression of BCL2. Similar to the effects of Par seen in MCF7/LCC9 cells in Fig. 6A, BCL2 protein expression is significantly inhibited in LCC9/IκBSR cells (Fig. 7A; ∼65% inhibition; P=0.029). While there is again no significant effect on BAX expression (data not shown), the overall BAX:BCL2 ratio was increased 2-fold (Supplemental Fig. S8). These data confirm the effects seen with Par and in turn suggest that the effects of Par as seen in Fig. 6A are primarily driven by its inhibition of NF-κB.
Figure 7.
Stable expression of IκBSR and siRNA to p65/RELA: both inhibit BCL2 protein expression in MCF7/LCC9 cells. A) Whole-cell lysates (40 μg) from LCC9/IκBSR cells (cells stably expressing IκBSR) and EV controls were separated by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted for BCL2. β-Actin was loading control. Representative immunoblot is shown. Data are mean ± se relative BCL2:β-actin ratio normalized to EV controls from 3 independent experiments. *P = 0.029; Student’s t test. B) BCL2 protein expression is inhibited in resistant MCF7/LCC9 cells by siRNA p65/RELA NF-κB knockdown at 96 h. MCF7/LCC9 cells were seeded in 12-well dishes and were transfected with control (si-ctrl) or p65-specific (si-p65/RELA) oligonucleotides for either 24 or 96 h before lysis. Lysates were separated by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted for p65/RELA and BCL2 as shown. GAPDH is loading control. Representative immunoblots for p65/RELA and BCL2 are shown.
In addition to the dominant-negative inhibitor IκBSR, an antisense approach to inhibiting NF-κB expression was also used. We inhibited NF-κB expression by transiently transfecting MCF7/LCC9 cells with siRNA oligonucleotides directed against p65/RELA NF-κB (si-p65) or a nonsilencing control (si-Ctrl) and measured the expression after 24–96 h. We observed a ∼2-fold decrease in p65/RELA expression at 24 h and an almost complete p65/RELA knockdown at 96 h (Fig. 7B). Notably, endogenous BCL2 protein expression is significantly inhibited in MCF7/LCC9 cells when p65/RELA NF-κB expression is knocked down by siRNA at 96 h (Fig. 7B). These data are consistent with the effects of NF-κB inhibition on BCL2 (Figs. 6A and 7A), which has appropriate responsive element sequences (2 κB sites located at −2306 and −1896) in its promoter (38).
Caspase inhibitors reverse the effects of NF-κB and 4HT on cell proliferation and mitochondrial membrane permeability
Caspases are central components of the intrinsic apoptotic pathway that ultimately leads to apoptotic cell death. Since we have shown that NF-κB inhibition affects apoptosis by disrupting BCL2-mediated signaling and changing the MMP in TAM-resistant cells, we sought to determine whether restoration of 4HT-induced cell death by Par is caspase dependent. We first used a PI that inhibits all caspases upstream and downstream of the mitochondria. We then measured the effects of caspase inhibition on 2 major end points: cell proliferation and MMP.
To measure the effects on cell proliferation, MCF7/LCC9 cells were treated as shown in Fig. 2A for 7 d in the presence or absence of a PI. In the absence of the PI, combined treatment with 4HT and Par synergistically inhibits growth and restores sensitivity to 4HT in MCF7/LCC9 cells. However, this effect of NF-κB inhibition is fully reversed by the PI (Fig. 8A; 1-way ANOVA: P≤0.001; P=0.029 relative to Par; P=0.029 relative to Par+4HT). We then measured the effects of the PI on MMP. MCF7/LCC9 cells were treated as shown in Fig. 5B before measuring the changes in MMP in the presence or absence of the PI. While combined treatment of resistant MCF7/LCC9 cells with 4HT and Par significantly increases MMP in the absence of the PI (1.6-fold; P<0.05 relative to 4HT), the effects on MMP are completely reversed by the PI (Fig. 8C; P=0.012 relative to LCC9/Par +4HT). These data show that a caspase-dependent cell death mechanism is active when cells are treated with 4HT and the NF-κB inhibitor Par. Notably, these data strongly implicate caspases upstream of the mitochondria because the effects of treatment on MMP were fully reversed by the PI.
Figure 8.
PI and the specific inhibitor to CASP8: both reverse the effects of NF-κB inhibition on cell proliferation and MMP in MCF7/LCC9 cells. Cells were seeded in quadruplicate and treated with 1 μM 4HT, 500 nM Par ± 4HT (1 μM), or ethanol vehicle in CCS-IMEM in the presence or absence of either a PI (A) or a CASP8 inhibitor (B) for 7 d before counting. Data are mean ± se relative proliferation of 4 determinations. A) PI fully reverses the effects of NF-κB inhibition on cell proliferation. P ≤ 0.001 for all treatment groups; 1-way ANOVA. *P = 0.029; ∧P = 0.029. B) CASP8 inhibitor partially reverses the effects of NF-κB inhibition on cell proliferation. P ≤ 0.001 for all treatment groups; 1-way ANOVA. *P = 0.01; ∧P ≤ 0.001. C) Cells were treated as above in the presence or absence of either a PI or a CASP8 inhibitor for 18–20 h before MMP was measured. Data are mean ± se relative MMP normalized to ethanol-treated cells for ≥3 independent experiments. *P = 0.012; ∧P = 0.006.
To further clarify which upstream caspases are involved in restoring 4HT-induced cell death in MCF7/LCC9 cells, we first measured the effects of a specific CASP8 inhibitor, C8I, on cell proliferation. C8I is an irreversible inhibitor of CASP8; the Z-IETD-FMK sequence binds only to CASP8 and blocks substrate binding. Antiestrogen-resistant MCF7/LCC9 cells were treated as shown in Fig. 2A for 7 d in the presence or absence of C8I. In the absence of C8I, combined treatment with 4HT and Par synergistically inhibited growth and restored sensitivity to 4HT in MCF7/LCC9 cells. This effect of NF-κB inhibition is at least partially reversed by the CASP8 inhibitor (Fig. 8B; 1-way ANOVA: P≤0.001; P=0.01 relative to LCC9/Par; P≤0.001 relative to LCC9/Par+4HT). We then measured the effects of C8I on mitochondrial membrane permeability. MCF7/LCC9 cells were treated as shown in Fig. 5B for 20 h before we measured the changes in MMP in the presence or absence of C8I. The CASP8 inhibitor data (Fig. 8C; P=0.006 relative to LCC9/Par+4HT) mimic the effects seen with the PI, implying that CASP8, acting upstream of the mitochondria, is involved in restoring 4HT-induced caspase-dependent cell death in TAM-resistant breast cancer cells. Since we did not fully reverse resistance, we cannot exclude a minor role for CASP2 and/or CASP10 in 4HT-induced apoptosis as modified by NF-κB. Studies to address the role of other caspases are currently ongoing.
Caspase inhibitors reduce sensitivity to the antiproliferative effects of 4HT and prevent mitochondrial membrane permeabilization in response to 4HT in MCF7/LCC9 cells stably expressing IκBSR
To establish whether the dominant-negative NF-κB inhibitor (IκBSR) mimics the effects seen in Fig. 8, LCC9/IκBSR cells were treated with ethanol vehicle or 4HT for 7 d in the absence or presence of either PI or C8I. In the absence of both the pancaspase and C8I caspase inhibitors, 4HT effectively inhibits LCC9/IκBSR cell growth by ∼40%. However, addition of the PI reverses the effects of NF-κB inhibition (Fig. 9A; P=0.014). The CASP8 inhibitor data shown in Fig. 9A (P=0.036) mimic the effects seen with the PI. To determine whether the 2 caspase inhibitors pancaspase and C8I affect the permeability of the mitochondrial membrane in LCC9/IκBSR cells, MMP was measured following treatment with ethanol vehicle or 4HT for ∼20 h in the absence or presence of either PI or C8I. Figure 9B shows a significant increase in MMP (≥1.5-fold) in response to antiestrogen 4HT in LCC9/IκBSR cells in the absence of caspase inhibitors. In marked contrast, addition of either PI or C8I fully reverses the increase in MMP (Fig. 9B; P=0.024 for PI; P=0.037 for C8I).
Figure 9.
PI and the specific inhibitor of CASP8 both reduce sensitivity to the antiproliferative effects of 4HT and prevent mitochondrial membrane permeabilization in response to 4HT in LCC9/IκBSR cells (cells stably expressing IκBSR). A) LCC9/IκBSR cells were seeded in quadruplicate and treated with either ethanol vehicle, or with 4HT (1 μM) for 7 d in the absence or presence of either a PI or a CASP8 inhibitor before counting. Data are mean ± se relative proliferation of 4 determinations. *P = 0.014, 0.036; Student’s t test. B) LCC9/IκBSR cells were treated with either ethanol vehicle or 4HT (1 μM) for 18–20 h in the absence or presence of either a PI or a CASP8 inhibitor before MMP was measured. Data are mean ± se relative MMP normalized to ethanol-treated cells for ≥3 independent experiments. *P = 0.024; ∧P = 0.037; Student’s t test.
These data confirm that NF-κB inhibition by both pharmacological (Par) and molecular (IκBSR) approaches effectively restore 4HT-induced cell death and sensitivity to 4HT in resistant cells, likely acting through a CASP8-dependent intrinsic apoptotic pathway.
Caspase inhibitors block the ability of Par and 4HT to inhibit BCL2 expression
Finally, to determine whether the down-regulation of BCL2 seen when MCF7/LCC9 cells were treated with 4HT and Par (Fig. 6A, B) was also caspase dependent, we treated MCF7/LCC9 cells with ethanol vehicle or 4HT in the presence or absence of Par, but in the presence of PI for 72 h. Whole-cell lysates were collected and subjected to SDS-PAGE and immunoblotted for BCL2. While in the absence of the PI Par alone and in combination with 4HT strongly inhibits BCL2 protein expression in resistant MCF7/LCC9 cells (Fig. 6A, B; 50% inhibition with Par alone; 75% inhibition achieved with combined treatment of Par and 4HT), addition of the PI to Par alone or in combination with 4HT no longer inhibits the expression of antiapoptotic protein BCL2 (Fig. 10A, B; 1-way ANOVA: P=0.818). Furthermore, the significant down-regulation of BCL2 protein expression observed with 4HT in the sensitive MCF7/LCC1 cells (Fig. 6C) is also no longer seen in the sensitive MCF7/LCC1 cells in the presence of the PI (Fig. 10C, D; 1-way ANOVA: P=0.748). Notably, C8I mimics the effects seen with the PI (Fig. 11; 1-way ANOVA: P=0.377 for LCC9; P=0.143 for LCC1).
Figure 10.
Combined treatment with Par and 4HT no longer inhibits BCL2 protein expression in the presence of PI in MCF7/LCC1 and MCF7/LCC9 cells. MCF7/LCC1 and MCF7/LCC9 cells were each treated with 500 nM Par or 1 μM 4HT, Par + 4HT, or ethanol vehicle in CCS-IMEM for 72 h in the presence of PI before cell lysis. Whole-cell lysates (20–40 μg) were separated by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted for BCL2. β-Actin was loading control. Data are means ± se from 3 independent experiments. A) Representative immunoblot for MCF7/LCC9 cells. B) Relative BCL2:β-actin ratio in MCF7/LCC9 cells. P = 0.818 for all treatment groups; 1-way ANOVA. C) Representative immunoblot for MCF7/LCC1 cells. D) Relative BCL2:β-actin ratio in MCF7/LCC1 cells. P = 0.748 for all treatment groups; 1-way ANOVA.
Figure 11.
Combined treatment with Par and 4HT no longer inhibits BCL2 protein expression in the presence of a specific inhibitor to CASP8 (C8I) in MCF7/LCC1 and MCF7/LCC9 cells. MCF7/LCC1 and MCF7/LCC9 cells were each treated with 500 nM Par or 1 μM 4HT, Par + 4HT, or ethanol vehicle in CCS-IMEM for 72 h in the presence of C8I before cell lysis. Whole-cell lysates (20–40 μg) were separated by SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted for BCL2. β-Actin was loading control. Data are means ± se from 3 independent experiments. A) Representative immunoblot for MCF7/LCC9 cells. B) Relative BCL2:β-actin ratio in MCF7/LCC9 cells. P = 0.377 for all treatment groups; 1-way ANOVA. C) Representative immunoblot for MCF7/LCC1 cells. D) Relative BCL2:β-actin ratio in MCF7/LCC1 cells. P = 0.143 for all treatment groups; 1-way ANOVA.
Thus, NF-κB inhibition restores 4HT-induced cell death and sensitivity to 4HT in resistant cells by increasing MMP, decreasing the expression of specific antiapoptotic proteins (BCL2), and altering the BAX:BCL2 ratio, contributing directly to a caspase-dependent intrinsic apoptotic cell death pathway.
DISCUSSION
NF-κB may play a role in determining endocrine responsiveness in breast cancer (12), most likely as a consequence of its actions as a transcription factor that acts alone or in heterodimers with other transcription factors such as IRF1 or AP1 (12, 26,27,28). TAM-resistant cells do not exhibit any change in the expression of either the p50 or p52 subunits of NF-κB (data not shown), but NF-κB p65/RELA protein expression (8-fold) and the basal activity of the NF-κB promoter (3-fold) are both significantly increased. Since increased NF-κB activation is also associated with acquired estrogen independence (17), NF-κB may be a central component in cellular signaling in a multiple endocrine-resistance phenotype.
To determine the functional relevance of these observations, we now show that inhibiting NF-κB resensitizes TAM-resistant cells to TAM-induced growth inhibition. Both molecular (mutant IκB; IκBSR) and pharmacological (Par) approaches to inhibit NF-κB are highly effective in down-regulating NF-κB activity and synergistically restoring sensitivity to 4HT. This reversal of resistance is seen in both MCF7/RR and MCF7/LCC9 cells. While Par alone strongly inhibits the proliferation of resistant MCF7/LCC9 and MCF7/RR cells, the interaction between Par and 4HT is strongly synergistic (RI=3.3 for MCF7/LCC9 cells; RI=1.8 for MCF7/RR cells). NF-κB inhibition by Par further sensitizes antiestrogen-sensitive MCF-7 and MCF7/LCC1 cells to 4HT; the interaction between these 2 drugs is additive in sensitive cells. Par with or without 4HT does not affect ER expression (Supplemental Fig. S3) and does not induce sensitivity to 4HT in ER-negative cells (MDA-MB-231 cells; see Supplemental Fig. S1), implying that the reversal of resistance in ER-positive cells requires expression of functional ER protein.
The ability of reduced NF-κB activity to affect proliferation endpoints in antiestrogen-resistant cells could reflect the effects of 4HT on either cell cycle distribution and/or apoptosis (4, 39). Combined treatment with Par and 4HT does not affect the cell cycle profile of the resistant MCF7/LCC9 (Supplemental Fig. S6) and MCF7/RR cells (data not shown). Thus, an effect of Par and 4HT on cell survival seems likely. Figure 5A shows that NF-κB inhibition by Par significantly induces apoptosis in MCF7/LCC9 cells (Fig. 5A). Samaddar et al. (40) have recently implicated autophagy in responsiveness to TAM. We measured light chain 3 (LC3) cleavage (35) and p62/A170/SQSTM1 (p62; scaffold protein/adaptor protein) expression (41) as markers of autophagy (Supplemental Fig. S9). During autophagy, LC3-I converts to LC3-II that subsequently associates with autophagic vesicles. We found no evidence of either LC3-II conversion or depleted levels of p62 in MCF7/LCC9 cells when treated with Par in the presence or absence of 4HT (Supplemental Fig. S9), suggesting that the ability of NF-κB inhibition to restore 4HT-induced cell death most likely involves an apoptotic, rather than autophagic, cell death pathway.
The mitochondrial membrane permeability transition is a critical step in the induction of intrinsic apoptosis. Small pores form in the outer membrane of the mitochondria, a process driven in part either by dimerized BAX or activated BID, BAK, or BAD proteins (the proapoptotic members of the BCL2 family). These proteins cause the mitochondrial membrane potential across the membrane to collapse, leading in turn to the release of cytochrome c into the cytoplasm and an increase in effector caspase activities (42,43,44). Consistent with the observed effects on apoptosis, NF-κB inhibition by both IκBSR and Par markedly increases 4HT-induced permeability of the mitochondrial membrane in MCF7/LCC9 cells (Figs. 5B and 9B). Expression of the antiapoptotic protein BCL2 is also up-regulated in MCF7/LCC9 cells compared with their sensitive MCF7/LCC1 controls (45). 4HT treatment alone strongly inhibits BCL2 protein expression in MCF7/LCC1 cells by 60%, (Fig. 6C, D) but not in the resistant MCF7/LCC9 cells. Par alone inhibits BCL2 protein expression in MCF7/LCC9 cells by 50%, and a greater inhibition (75%) is achieved with the combined treatment of Par and 4HT (Fig. 6A, B). These observations imply at least a partial resensitization to 4HT. Similar responses are seen in the antiestrogen-sensitive MCF7/LCC1 cells (Figs. 6C, D), also consistent with the effects of Par on responsiveness to 4HT as measured by changes in cell proliferation (Fig. 2A–C) and with the effects of Par on fulvestrant sensitivity (25).
An increase in BCL2 expression and a decrease in BAX expression can predict the response to chemotherapy in breast cancer cells (46), where the proapoptotic effect of cytotoxic drugs is linked to either a low BCL2:BAX or a high BAX:BCL2 ratio (36, 37). While Par alone or in combination with 4HT does not affect the expression of the proapoptotic BAX protein, its inhibition of BCL2 leads to a 5-fold increase in the overall BAX:BCL2 ratio (Supplemental Fig. S7) driving the cell toward apoptosis.
The ability of antiestrogens to induce apoptosis through changing NF-κB and BCL2 activity is likely mediated through changes in caspase activation. However, the precise role of caspases in this regard is unknown. Activation of effector caspases following an increase in MMP and cytochrome c release is expected, but a role for the activity of caspases upstream of mitochondria cannot be excluded. To establish the role of caspases, we first used a broad spectrum PI that inhibits caspases both upstream and downstream of the mitochondria. The effects of NF-κB inhibition (by both Par and IκBSR) on cell proliferation and mitochondrial membrane permeability are statistically significantly reversed by the PI (Figs. 8A, C and 9). Since the effects on mitochondrial membrane permeability are also reversed, caspases upstream of the mitochondria, such as CASP2, CASP8, or CASP10, are strongly implicated in regulating the effects of NF-κB signaling through BCL2 on mitochondrial function and cellular responsiveness to antiestrogens.
We now show that CASP8 is functionally involved in NF-κB signaling in the context of affecting endocrine responsiveness. Using a specific inhibitor to CASP8 that works by blocking the binding of CASP8 to its substrate, we obtained similar results (Figs. 8B, C and 9) as seen with the use of a PI. In addition, the effects of Par alone or in combination with 4HT on BCL2 expression in both MCF7/LCC9 and MCF7/LCC1 cells are reversed with either the PI or the specific CASP8 inhibitor (Figs. 10 and 11). Notably, the decrease in BCL2 expression with 4HT treatment observed in MCF7/LCC1 cells is no longer seen in the presence of either the PI or the specific CASP8 inhibitor (Figs. 10C, D and 11C, D). While we cannot exclude a minor role for CASP2 and/or CASP10, these are unlikely to be dominant given the significant effects evident with inhibition of CASP8 alone. Thus, NF-κB signaling to events upstream of its effects on mitochondrial permeability appears to be transduced primarily by CASP8. Precisely how this signaling flows is unclear. One possible explanation is that CASP8 also cleaves BID (47) leading to a conformational change in BAX (48). Insertion of activated BAX into the outer mitochondrial membrane would increase mitochondrial membrane permeability, facilitate the release of cytochrome c, and ultimately induce apoptosis (49, 50). While beyond the scope of the work presented here, studies to test this hypothesis are currently ongoing.
Our data strongly suggest that Par may be a useful therapeutic strategy, acting through its effects on NF-κB. However, Par is also known to activate JNK and reverse resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL; ref. 24). Protein levels of JNK and phospho-JNK are higher in MCF7/LCC9 cells, but these do not change when these resistant cells are growth inhibited by treatment with either Par alone or the synergistic combination of Par and 4HT (Supplemental Fig. S5). Thus, JNK-dependent pathways are unlikely to account for the synergistic interaction between Par and 4HT in MCF7/LCC9 cells. Loss of p53 or its reduced expression can activate NF-κB (51, 52), but the levels of p53 mRNA and protein expression are comparable in MCF7/LCC1 and MCF7/LCC9 cells; loss of p53 is unlikely to account for the increased NF-κB activity.
In summary, our data show that antiestrogen-sensitive and -resistant cells differentially use NF-κB to affect cell fate. In resistant cells, NF-κB expression and function are increased and act by signaling through CASP8 and BCL2 to affect prevent mitochondrial membrane destabilization and the consequent induction of an apoptotic and/or autophagic cell death. These data further provide a compelling rationale to initiate clinical trials of Par in combination with a SERM or SERD in ER-positive breast cancers. Such combination therapy is predicted to be better than an antiestrogen alone in sensitive cells; Par should also inhibit some ER-negative cells that may also be present in these often heterogeneous breast tumors. Furthermore, in heavily pretreated patients where residual tumor cells are ER positive but have become estrogen independent and cross-resistant to TAM and ICI, this combination could prove useful in producing further clinical responses.
Supplementary Material
Acknowledgments
This work was supported by Department of Defense Awards W81XWH-04-1-0378 and W81XWH-04-1-0570 from the U.S. Army Medical Research and Materiel Command and R01-CA131465 and R21-CA139246 from the U.S. Department of Health and Human Services, National Institutes of Health. Technical services were provided by the Lombardi Comprehensive Cancer Center Flow Cytometry and Cell Sorting and Macromolecular Shared Resources. The authors thank Dr. Marty Mayo (University of Virginia, Charlottesville, VA, USA) for providing the empty pCMV4 and the S32/36A mutant pCMV4-FLAG IκBα (IκBSR) plasmids. The authors also thank Drs. Stephen Byers, Dean Rosenthal, Thomas G. Sherman, and Bassem R. Haddad for thoughtful comments in carrying out this research.
References
- Clarke R, Leonessa F, Welch J N, Skaar T C. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev. 2001;53:25–71. [PubMed] [Google Scholar]
- Clarke R, Liu M C, Bouker K B, Gu Z, Lee R Y, Zhu Y, Skaar T C, Gomez B, O'Brien K, Wang Y, Hilakivi-Clarke L A. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22:7316–7339. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun M J. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Riggins R, Bouton A H, Liu M C, Clarke R. Antiestrogens, aromatase inhibitors, and apoptosis in breast cancer. Vitam Horm. 2005;71:201–237. doi: 10.1016/S0083-6729(05)71007-4. [DOI] [PubMed] [Google Scholar]
- Encarnacion C A, Ciocca D R, McGuire W L, Clark G M, Fuqua S A, Osborne C K. Measurement of steroid hormone receptors in breast cancer patients on tamoxifen. Breast Cancer Res Treat. 1993;26:237–246. doi: 10.1007/BF00665801. [DOI] [PubMed] [Google Scholar]
- Kuukasjarvi T, Kononen J, Helin H, Holli K, Isola J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol. 1996;14:2584–2589. doi: 10.1200/JCO.1996.14.9.2584. [DOI] [PubMed] [Google Scholar]
- Bachleitner-Hofmann T, Pichler-Gebhard B, Rudas M, Gnant M, Taucher S, Kandioler D, Janschek E, Dubsky P, Roka S, Sporn E, Jakesz R. Pattern of hormone receptor status of secondary contralateral breast cancers in patients receiving adjuvant tamoxifen. Clin Cancer Res. 2002;8:3427–3432. [PubMed] [Google Scholar]
- Kuske B, Naughton C, Moore K, Macleod K G, Miller W R, Clarke R, Langdon S P, Cameron D A. Endocrine therapy resistance can be associated with high estrogen receptor alpha (ERalpha) expression and reduced ERalpha phosphorylation in breast cancer models. Endocr Relat Cancer. 2006;13:1121–1133. doi: 10.1677/erc.1.01257. [DOI] [PubMed] [Google Scholar]
- Clarke R, Shajahan A N, Riggins R, Cho Y, Crawford A C, Xuan J, Wang Y, Zwart A, Nehra N, Liu M C. Gene network signaling in hormone responsiveness modifies apoptosis and autophagy in breast cancer cells. J Steroid Biochem Mol Biol. 2009;114:8–20. doi: 10.1016/j.jsbmb.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner N, Boulay V, Fojo A, Freter C E, Lippman M E, Clarke R. Acquisition of hormone-independent growth in MCF-7 cells is accompanied by increased expression of estrogen-regulated genes but without detectable DNA amplifications. Cancer Res. 1993;53:283–290. [PubMed] [Google Scholar]
- Brunner N, Boysen B, Jirus S, Skaar T, Holst-Hansen C, Lippman J, Frandsen T, Spang-Thomsen M, Fuqua S A W, Clarke R. MCF7/LCC9: An antiestrogen-resistant MCF-7 variant in which acquired resistance to the steroidal antiestrogen ICI 182, 780 confers an early cross-resistance to the nonsteroidal antiestrogen tamoxifen. Cancer Res. 1997;57:3486–3493. [PubMed] [Google Scholar]
- Gu Z, Lee R Y, Skaar T C, Bouker K B, Welch J N, Lu J, Liu A, Zhu Y, Davis N, Leonessa F, Brunner N, Wang Y, Clarke R. Association of interferon regulatory factor-1, nucleophosmin, nuclear factor-kappaB, and cyclic AMP response element binding with acquired resistance to faslodex (ICI 182,780) Cancer Res. 2002;62:3428–3437. [PubMed] [Google Scholar]
- Baldwin A S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D W, Sovak M A, Zanieski G, Nonet G, Romieu-Mourez R, Lau A W, Hafer L J, Yaswen P, Stampfer M, Rogers A E, Russo J, Sonenshein G E. Activation of NF-kappaB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis. 2000;21:871–879. doi: 10.1093/carcin/21.5.871. [DOI] [PubMed] [Google Scholar]
- Sovak M A, Bellas R E, Kim D W, Zanieski G J, Rogers A E, Traish A M, Sonenshein G E. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell P C, Guttridge D C, Funkhouser W K, Baldwin A S., Jr Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene. 2000;19:1123–1131. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- Pratt M A C, Bishop T E, White D, Yasvinski G, Menard M, Niu M Y, Clarke R. Estrogen withdrawal-induced NF-kappaB activity and bcl-3 expression in breast cancer cells: roles in growth and hormone independence. Mol Cell Biol. 2003;23:6887–6900. doi: 10.1128/MCB.23.19.6887-6900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakshatri H, Bhat-Nakshatri P, Martin D A, Goulet R J, Sledge G W. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishodia S, Aggarwal B B. Nuclear factor-kappaB: a friend or a foe in cancer? Biochem Pharmacol. 2004;68:1071–1080. doi: 10.1016/j.bcp.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Verma I M. Nuclear factor (NF)-kappaB proteins: therapeutic targets. Ann Rheum Dis. 2004;63:ii57–ii61. doi: 10.1136/ard.2004.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehner S P, Heinrich M, Bork P M, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz M L. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- Curry E A, III, Murry D J, Yoder C, Fife K, Armstrong V, Nakshatri H, O'Connell M, Sweeney C J. Phase I dose escalation trial of feverfew with standardized doses of parthenolide in patients with cancer. Invest New Drugs. 2004;22:299–305. doi: 10.1023/B:DRUG.0000026256.38560.be. [DOI] [PubMed] [Google Scholar]
- Wen J, You K R, Lee S Y, Song C H, Kim D G. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem. 2002;277:38954–38964. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]
- Nakshatri H, Rice S E, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene. 2004;23:7330–7344. doi: 10.1038/sj.onc.1207995. [DOI] [PubMed] [Google Scholar]
- Riggins R, Zwart A, Nehra R, Agarwal P, Clarke R. The NFκB inhibitor parthenolide restores ICI 182,780 (Faslodex; fulvestrant)-induced apoptosis in antiestrogen resistant breast cancer cells. Mol Cancer Ther. 2005;4:33–41. [PubMed] [Google Scholar]
- Bouker K B, Skaar T C, Fernandez D R, O'Brien K A, Clarke R. Interferon regulatory factor-1 mediates the proapoptotic but not cell cycle arrest effects of the steroidal antiestrogen ICI 182,780 (Faslodex, fulvestrant) Cancer Res. 2004;64:4030–4039. doi: 10.1158/0008-5472.CAN-03-3602. [DOI] [PubMed] [Google Scholar]
- Drew P D, Franzoso G, Becker K G, Bours V, Carlson L M, Siebenlist U, Ozato K. NF kappa B and interferon regulatory factor 1 physically interact and synergistically induce major histocompatibility class I gene expression. J Interferon Cytokine Res. 1995;15:1037–1045. doi: 10.1089/jir.1995.15.1037. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yau C, Gray J W, Chew K, Dairkee S H, Moore D H, Eppenberger U, Eppenberger-Castori S, Benz C C. Enhanced NF kappa B and AP-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007;7:59. doi: 10.1186/1471-2407-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünner N, Frandsen T L, Holst-Hansen C, Bei M, Thompson E W, Wakeling A E, Lippman M E, Clarke R. MCF7/LCC2: A 4-hydroxytamoxifen resistant human breast cancer variant which retains sensitivity to the steroidal antiestrogen ICI 182,780. Cancer Res. 1993;53:3229–3232. [PubMed] [Google Scholar]
- Howell A, DeFriend D, Robertson J F R, Blamey R W, Walton P. Response to a specific antioestrogen (ICI 182,780) in tamoxifen-resistant breast cancer. Lancet. 1995;345:29–30. doi: 10.1016/s0140-6736(95)91156-1. [DOI] [PubMed] [Google Scholar]
- Butler W B, Fontana J A. Responses to retinoic acid of tamoxifen-sensitive and -resistant sublines of human breast cancer cell line MCF-7. Cancer Res. 1992;52:6164–6167. [PubMed] [Google Scholar]
- Vindelov L L, Christensen I J, Nissen N I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983;3:323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Romanelli S, Perego P, Pratesi G, Carenini N, Tortoreto M, Zunino F. In vitro and in vivo interaction between cisplatin and topotecan in ovarian carcinoma systems. Cancer Chemother Pharmacol. 1998;41:385–390. doi: 10.1007/s002800050755. [DOI] [PubMed] [Google Scholar]
- Patel N M, Nozaki S, Shortle N H, Bhat-Nakshatri P, Newton T R, Rice S, Gelfanov V, Boswell S H, Goulet R J, Jr, Sledge G W, Jr, Nakshatri H. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene. 2000;19:4159–4169. doi: 10.1038/sj.onc.1203768. [DOI] [PubMed] [Google Scholar]
- Klionsky D J, others Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilling G, Hacohen H, Nordenberg J, Livnat T, Rotter V, Sidi Y. Differential sensitivity of MCF-7 and LCC2 cells, to multiple growth inhibitory agents: possible relation to high bcl-2/bax ratio? Cancer Lett. 2000;161:27–34. doi: 10.1016/s0304-3835(00)00579-6. [DOI] [PubMed] [Google Scholar]
- Teixeira C, Reed J C, Pratt M A. Estrogen promotes chemotherapeutic drug resistance by a mechanism involving Bcl-2 proto-oncogene expression in human breast cancer cells. Cancer Res. 1995;55:3902–3907. [PubMed] [Google Scholar]
- Viatour P, Bentires-Alj M, Chariot A, Deregowski V, de Leval L, Merville M P, Bours V. NF- kappa B2/p100 induces Bcl-2 expression. Leukemia. 2003;17:1349–1356. doi: 10.1038/sj.leu.2402982. [DOI] [PubMed] [Google Scholar]
- Taylor I W, Hodson P J, Green M D, Sutherland R L. Effects of tamoxifen on cell cycle progression of synchronous MCF-7 human mammary carcinoma cells. Cancer Res. 1983;43:4007–4010. [PubMed] [Google Scholar]
- Samaddar J S, Gaddy V T, Duplantier J, Thandavan S P, Shah M, Smith M J, Browning D, Rawson J, Smith S B, Barrett J T, Schoenlein P V. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol Cancer Ther. 2008;7:2977–2987. doi: 10.1158/1535-7163.MCT-08-0447. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, Sou Y S, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Narita M, Shimizu S, Ito T, Chittenden T, Lutz R J, Matsuda H, Tsujimoto Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood K A, Hsu Y, Zimmerberg J, Youle R J. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc Natl Acad Sci U S A. 1999;96:5492–5497. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A C, Riggins R B, Shajahan A N, Zwart A, Clarke R. Co-inhibition of BCL-W and BCL2 restores antiestrogen sensitivity through BECN1 and promotes an autophagy-associated necrosis. PLoS ONE. 2010;5:e8604. doi: 10.1371/journal.pone.0008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J C. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7:541–546. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu C J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou J C. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Martinou J C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- Goonesinghe A, Mundy E S, Smith M, Khosravi-Far R, Martinou J C, Esposti M D. Pro-apoptotic Bid induces membrane perturbation by inserting selected lysolipids into the bilayer. Biochem J. 2005;387:109–118. doi: 10.1042/BJ20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Chung H, Kim H J, Lee J Y, Oh M Y, Kim Y, Kong G. Id-1 regulates Bcl-2 and Bax expression through p53 and NF-kappaB in MCF-7 breast cancer cells. Breast Cancer Res Treat. 2008;112:287–296. doi: 10.1007/s10549-007-9871-6. [DOI] [PubMed] [Google Scholar]
- Kawauchi K, Araki K, Tobiume K, Tanaka N. p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nat Cell Biol. 2008;10:611–618. doi: 10.1038/ncb1724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.