Abstract
Digoxin and other cardiac glycosides inhibit hypoxia-inducible factor-1 (HIF-1) transcriptional activity in cultured cells and suppress tumor xenograft growth. We tested the hypothesis that digoxin reduces HIF-1 levels in ischemic tissue in vivo and suppresses neovascularization. Well-established murine models of ocular neovascularization were used to test our hypothesis. In mice with ischemic retinopathy, intraocular or intraperitoneal injection of digoxin markedly reduced retinal levels of HIF-1α protein and mRNAs encoding multiple hypoxia-regulated proangiogenic proteins and their receptors. Daily intraperitoneal injection of 2 mg/kg starting at postnatal day (P) 12 or a single intravitreous injection of 100 ng of digoxin at P12 reduced retinal neovascularization by >70% at P17. Digoxin also reduced the number of CXCR4+ cells and F4/80+ macrophages in ischemic retina and significantly reduced choroidal neovascularization at Bruch’s membrane rupture sites. Digoxin suppresses retinal and choroidal neovascularization by reducing HIF-1α levels, which blocks several proangiogenic pathways. Since digoxin suppresses multiple pathways in addition to VEGF signaling, it may provide advantages over specific VEGF antagonists for treatment of patients with retinal and choroidal diseases complicated by neovascularization and/or excessive vascular permeability. It may also be useful for treatment of neovascular diseases in other tissues.—Yoshida, T., Zhang, H., Iwase, T., Shen, J., Semenza, G. L., Campochiaro, P. A. Digoxin inhibits retinal ischemia-induced HIF-1α expression and ocular neovascularization.
Keywords: age-related macular degeneration, angiogenesis, cardiac glycosides, diabetic retinopathy
Ischemic retinopathies are the most prevalent cause of moderate and severe vision loss in working-age Americans (1). They are a group of diseases in which different primary insults cause damage to retinal vessels leading to areas of poorly perfused retina. In diabetic retinopathy, the primary insult is hyperglycemia; in retinopathy of prematurity, it is exposure to levels of oxygen that are higher than normal for a particular stage of retinal vascular development; in central and branch retinal vein occlusion, it is obstruction of venous outflow which increases hydrostatic pressure and reduces perfusion; in sickle cell retinopathy, it is obstruction of retinal vessels with nondeformable sickled red blood cells; and in retinal vasculitis, it is inflammation. Regardless of the underlying cause, retinal ischemia is a point of convergence after which all of these diseases share critical pathogenic events. One such event is stabilization of hypoxia-inducible factor 1 (HIF-1), which then activates transcription of genes that contain a hypoxia response element (2,3,4,5). The products of these HIF-1-responsive genes act together to promote retinal neovascularization (NV), choroidal NV, and/or excessive retinal vascular leakage (6).
A particularly important HIF-1-responsive gene encodes vascular endothelial growth factor (VEGF), which plays a critical role in retinal and choroidal NV, both of which are strongly suppressed by VEGF antagonists (7,8,9,10,11,12). However, the products of other HIF-1-inducible genes have been implicated in the development of retinal and/or choroidal NV, including angiopoietin 2 (ANGPT2) (13,14,15,16), platelet-derived growth factor-B (PDGF-B) (17,18,19), placental growth factor-1 (PGF) (20, 21), and erythropoietin (EPO) (22, 23). Recruitment of bone marrow-derived cells to ischemic retina also contributes to ocular NV and may be based in part on the hypoxia-induced production of stem cell factor (SCF) and stromal-derived growth factor-1 (SDF-1), which bind to cognate receptors, CD117 (cKit), and CXCR4, on the surface of recruited cells (24, 25). HIF-1 is necessary and sufficient for the expression of VEGF, PGF, PDGF-B, ANGPT2, SCF, and SDF-1 in mouse models of limb, cutaneous, and ocular neovascularization (6, 26). Thus, multiple HIF-1-responsive genes appear to contribute to ocular NV. Most important, whereas increased levels of VEGF alone induce NV only in the deep capillary bed of the retina (8), increased levels of HIF-1α are sufficient to induce NV in the choroid and all vascular beds of the retina (6).
We recently utilized a cell-based reporter assay to screen for drugs that inhibit HIF-1 transcriptional activity. This screen identified digoxin and 10 other cardiac glycosides as potent inhibitors of HIF-1α protein synthesis, with IC50 values < 100 nM, in a wide range of transformed human hepatic, lymphoid, and prostate cell lines (27). Treatment of mice with digoxin resulted in an inhibition of HIF-1 transcriptional activity and VEGF mRNA expression in tumor xenografts, resulting in significant inhibition of tumor growth. When tumor cells were engineered to express HIF-1α from an expression vector that was not inhibited by digoxin, tumor growth in vivo was not inhibited by digoxin treatment, demonstrating that the anticancer effect of digoxin was based on its ability to inhibit HIF-1α synthesis in the tumor (27). On the basis of those data and the critical role of HIF-1 in mediating ischemia-induced neovascularization by activating the expression of a number of proangiogenic proteins, we hypothesized that digoxin may also function as an inhibitor of pathological angiogenesis. In the present study, we tested that hypothesis using well-established mouse models of ocular NV.
MATERIALS AND METHODS
Mice
Pathogen-free C57BL/6 mice (Charles River, Wilmington, MA, USA) were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines of the Johns Hopkins University Animal Care and Use Committee.
Mouse model of choroidal NV
Choroidal NV was induced by laser photocoagulation-induced rupture of Bruch’s membrane, as described previously (28). Briefly, 5- to 6-wk-old female C57BL/6 mice were anesthetized with ketamine hydrochloride (100 mg/kg body weight) and pupils were dilated. Laser photocoagulation (75-μm spot size, 0.1-s duration, 120 mW) was performed in the 9, 12, and 3 o’clock positions of the posterior pole of each eye with the slit lamp delivery system of an OcuLight GL diode laser (Iridex, Mountain View, CA, USA) and a handheld coverslip as a contact lens to view the retina. Production of a tissue bubble by the laser, which indicates rupture of Bruch’s membrane, is an important factor in obtaining choroidal NV; therefore, only burns in which a bubble was produced were included in the study. Immediately after laser-induced rupture of Bruch’s membrane, mice were randomized to various treatment groups, including intravitreous injections of 1 μl of PBS or PBS containing various concentrations of digoxin, or daily intraperitoneal (i.p.) injection of vehicle or 2 mg/kg of digoxin. Intravitreous injections were done under a dissecting microscope with a Harvard Pump Microinjection System (Harvard Apparatus, Holliston, MA, USA) and pulled glass micropipettes, as described previously (29).
After 14 d, the mice were perfused with 1 ml of PBS containing 50 mg/ml of fluorescein-labeled dextran (2×106 Da average molecular mass; Sigma-Aldrich, St. Louis, MO, USA) and choroidal flat mounts were examined by fluorescence microscopy. Images were captured with a Nikon Digital Still Camera DXM1200 (Nikon Instruments Inc., New York, NY, USA). Image analysis software (Image-Pro Plus; Media Cybernetics, Silver Spring, MD, USA) was used to measure the total area of choroidal NV at each rupture site with the investigator masked with respect to treatment group.
Oxygen-induced ischemic retinopathy
C57BL/6 mice placed in 75% O2 at postnatal day (P) 7 and at P12 were returned to room air and given an intraocular injection of 1 μl of PBS or PBS containing 0.0001, 0.001, 0.005, 0.01, 0.05, 0.1, or 0.25 μg of digoxin, or daily i.p. injection of vehicle or 2 mg/kg of digoxin. At P17, the area of retinal NV on the surface of the retina was measured as described previously (30,31,32). Briefly, P17 mice were given an intraocular injection of 1 μl of rat anti-mouse platelet endothelial cell adhesion molecule-1 (PECAM-1) antibody (Pharmingen, San Jose, CA, USA); after 12 h, they were euthanized, and eyes were fixed in PBS-buffered formalin for 5 h at room temperature. Retinas were dissected, washed, and incubated with goat-anti rat polyclonal antibody conjugated with Alexa 488 (Invitrogen, Carlsbad, CA, USA) at 1:500 dilution at room temperature for 45 min and flat mounted. An observer masked with respect to treatment group measured the area of NV per retina by image analysis.
Assessment of bone marrow-derived cells in the retina
C57BL/6 mice were placed in 75% O2 at P7, and at P12, they were returned to room air and received an intravitreous injection of 1 μl of PBS or PBS containing 0.05 μg of digoxin. At P17, the mice were given an intraocular injection of 1 μl of phycoerythrin-conjugated anti-CXCR4 (Pharmingen, San Jose, CA, USA) or F4/80 (EBioscience, San Diego, CA, USA) antibody; after 8 h, mice were euthanized, and their eyes were removed and fixed in phosphate-buffered formalin at room temperature for 5 h. Retinas were dissected, washed with PBS containing 0.25% Triton X-100, and flat mounted. Slides were viewed with a Nikon fluorescence microscope, and the number of CXCR4+ cells per square millimeter or the number of F4/80+ macrophages per retina was counted by image analysis, with the investigator masked with respect to treatment group.
Immunoblot assays
Three retinas from three different mice were pooled and lysed in RIPA buffer. Equal amounts of protein were fractionated by 10% SDS-PAGE. Monoclonal anti-HIF-1α antibody H1α67 (Novus Biologicals, Littleton, CO, USA) was used for immunoblot assays (27). Blots were stripped and reprobed with a polyclonal antibody against β-actin to confirm equal protein loading.
Quantitative real-time RT-PCR (qrtPCR)
RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) followed by DNase (Ambion, Austin, TX, USA) treatment according to the manufacturer’s instructions. Oligonucleotide primers (Table 1) were designed using Beacon Designer software (Premier Biosoft, Palo Alto, CA, USA), and cDNA was prepared using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). cDNA samples were diluted 1:10, and real-time PCR was performed using iQ SYBR Green Supermix and the iCycler real-time PCR detection system (Bio-Rad). For each primer pair, annealing temperature was optimized by gradient PCR and linearity of all assays was established by strict criteria (27). The expression of each target mRNA relative to 18S rRNA was calculated based on the threshold cycle (Ct) as 2−Δ(ΔCt), where ΔCt = Ct target − Ct 18S and Δ(Δ Ct) = Δ Ct digoxin − Δ Ct PBS.
TABLE 1.
Primers for qrtPCR
| Name | FWD | REV |
|---|---|---|
| PLGF | GGATGTGCTCTGTGAATGC | CCTCTGAGTGGCTGGTTAC |
| VEGF | GGCTGCTGTAACGATGAAG | CTCTCTATGTGCTGGCTTTG |
| SDF1 | GAGAGCCACATCGCCAGAG | TTTCGGGTCAATGCACACTTG |
| SCF | CCTTAGGAATGACAGCAGTAGC | AGCCAATTACAAGCGAAATGAG |
| EPO | CGAGTTCTGGAGAGGTACATC | TGACTTTGGTATCTGGGACTG |
| ANGPT2 | CTGTGCGGAAATCTTCAAGTC | TGCCATCTTCTCGGTGTTG |
| CD117 | GCCACGTCTCAGCCATCTG | GTCGCCAGCTTCAACTATTAACT |
| EPOR | GGGCTCCGAAGAACTTCTGTG | ATGACTTTCGTGACTCACCCT |
| Tie-2 | CGGCCAGGTACATAGGAGGAA | TCACATCTCCGAACAATCAGC |
| CXCR4 | GAAGTGGGGTCTGGAGACTAT | TTGCCGACTATGCCAGTCAAG |
| VEGFR1 | TGGCTCTACGACCTTAGACTG | CAGGTTTGACTTGTCTGAGGTT |
| VEGFR2 | TTTGGCAAATACAACCCTTCAGA | GCAGAAGATACTGTCACCACC |
| RPL13A | GAGGCCCCTACCATTTCCGA | GGCTTCAGCCGAACAACCTT |
ELISA
SDF-1 protein levels in mouse retinal tissue lysates were measured using a commercial kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions. Retinas were lysed by sonication in PBS, and a 50-μl aliquot of lysate from each retina was used for the assay. SDF-1 levels were determined based on a standard curve and expressed as nanograms per microgram of total protein.
Statistical analysis
Data are expressed as means ± se. Statistical analysis was performed using Student’s t test or ANOVA with Dunnett’s correction for multiple comparisons, and P < 0.05 was considered significant.
RESULTS
Digoxin inhibits HIF-1α protein expression in ischemic retina
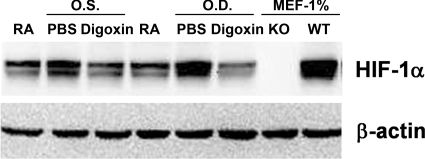
When mice are exposed to 75% O2 at P7 through P12, expression of VEGF is reduced, thereby halting retinal vascular development and causing regression of newly developed retinal vessels, which results in areas of nonperfused retina (33). After return to room air at P12, the retina becomes hypoxic, leading within 2 h to increased HIF-1α levels, which remain increased for ≥5 d (5). Compared to the HIF-1α levels in retinas of P15 mice raised in room air, HIF-1α levels were substantially increased in the retinas of P15 mice with ischemic retinopathy that received an intraocular injection of PBS at P12 (Fig. 1). The retinal level of HIF-1α in P15 mice with ischemic retinopathy that had received an intraocular injection of 0.25 μg of digoxin was less than that seen in the eyes of mice injected with PBS and was comparable to that seen in the retinas of room-air controls.
Figure 1.
Digoxin reduces HIF-1α protein levels in ischemic retina. At P12, mice with ischemic retinopathy were given an intraocular injection of 0.25 μg of digoxin or PBS in the left (O.S.) and right (O.D.) eyes. At P15, pooled whole tissue lysates were prepared from hypoxic retinas of 3 PBS- or Digoxin-treated mice and from control mice maintained in room air (RA). Immunoblot assays were performed using a monoclonal antibody that recognizes HIF-1α. Lysates of mouse embryo fibroblasts (MEF) that were derived from HIF-1α knockout (KO) or wild-type (WT) mice cultured at 1% O2 served as negative and positive controls, respectively. Blots were stripped, and immunoblot assays were performed using an antibody against β-actin as a loading control.
Digoxin selectively inhibits expression of HIF-1 target genes
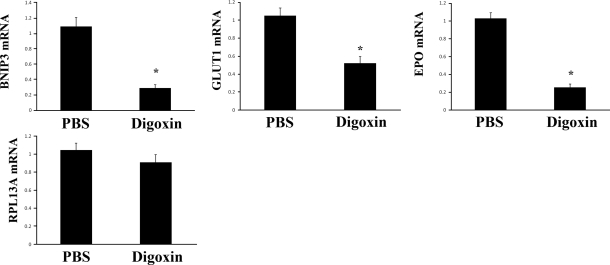
To determine whether HIF-1 transcriptional activity in the ischemic retina was inhibited by digoxin, we utilized qrtPCR to determine the levels of BNIP3, GLUT1, and EPO mRNA, which are encoded by known HIF-1 target genes. The levels of BNIP3, GLUT1, and EPO mRNA in ischemic retina were significantly reduced by digoxin treatment (Fig. 2). In contrast, the expression of RPL13A mRNA, which encodes a ribosomal protein and is not regulated by HIF-1, was not inhibited by digoxin.
Figure 2.
Digoxin specifically inhibits HIF-1 target gene expression in ischemic retina. Mice with ischemic retinopathy were given daily i.p. injections of PBS or 2 mg/kg of digoxin between P12 and P15 and then euthanized. Total retinal RNA was isolated, and qrtPCR assays were performed using primers specific for BNIP3, GLUT1, erythropoietin (EPO), or RPL13A mRNA. Fold change in retinal mRNA levels from mice injected with digoxin compared to retinas from eyes injected with PBS is shown (means±se; n=16 retinas each). *P < 0.05 vs. PBS; unpaired t test.
Digoxin reduces mRNA levels for proangiogenic factors and/or their receptors in ischemic retina
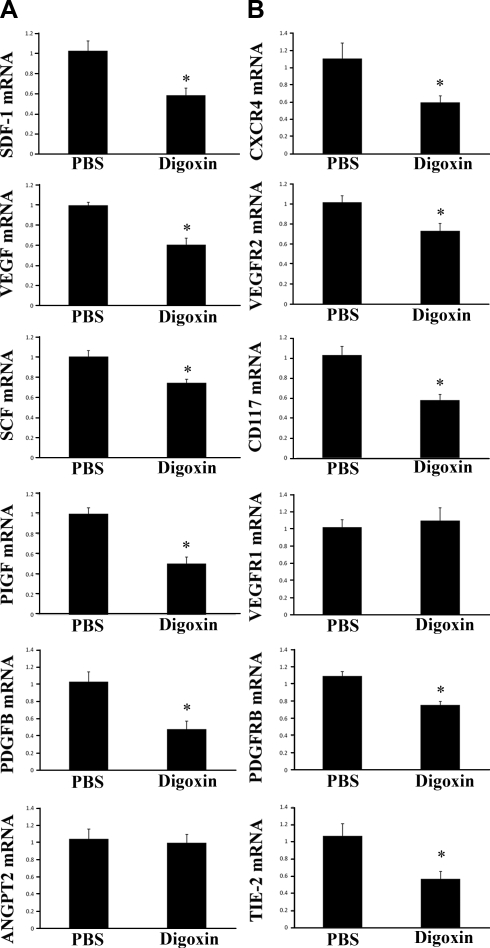
Multiple genes encoding proteins that stimulate NV are transcriptionally activated by HIF-1 (6). In P15 mice with ischemic retinopathy that had received an intraocular injection of 0.25 μg of digoxin at P12, the mRNA for several proangiogenic ligands, including VEGF, SDF-1, SCF, PDGF-B, and PGF, was significantly reduced compared to that seen in eyes of mice with ischemic retinopathy that received an intraocular injection of PBS at P12 (Fig. 3A). The mRNA for ANGPT2 was not significantly reduced in digoxin-treated ischemic retinas, but the mRNA level for its receptor Tie2 was reduced (Fig. 3B). The mRNA for the receptors for VEGF (VEGFR2), SDF-1 (CXCR4), SCF (CD117), and PDGF-B (PDGFRβ) were significantly decreased by digoxin (Fig. 3B). The mRNA level for VEGFR1, the receptor for PGF, was not reduced.
Figure 3.
Intraocular digoxin reduces levels of mRNA encoding angiogenic growth factors and their cognate receptors in ischemic retina. At P12, mice with ischemic retinopathy were given an intraocular injection of 0.25 μg of digoxin or PBS, and at P15, the mice were euthanized. Total retinal RNA was isolated (n=8/group), and qrtPCR assays were performed using primers specific for mRNAs encoding angiogenic growth factors (A), including SDF-1, VEGF, SCF, PGF, PDGF-B, and ANGPT2 or the cognate receptors for these factors, CXCR4, VEGF receptor 2 (VEGFR2), CD117 (CKIT), VEGF receptor 1 (VEGFR1), PDGF receptor-β (PDGFRB), or TIE2 (B). Fold change in retinal mRNA levels from mice injected with digoxin compared to retinas from eyes injected with PBS is shown (means±se; n=8 retinas each). *P<0.05 by unpaired t test vs. PBS).
Digoxin reduces SDF-1 protein in ischemic retina
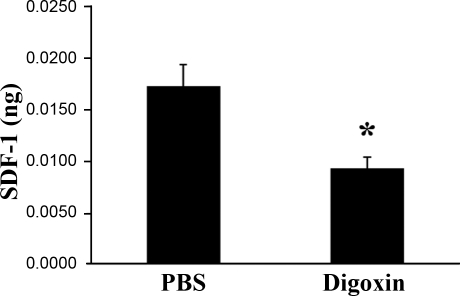
Because digoxin reduces the mRNA for multiple proangiogenic factors and their receptors in ischemic retina, it is reasonable to assume that the proteins codes by these mRNAs are reduced. To check this assumption, ELISAs were performed to measure SDF-1 protein levels. The level of SDF-1 protein in the retinas of mice with ischemic retinopathy at P15 given an intraocular injection of 0.25 μg of digoxin at P12 was significantly less than that seen in mice with ischemic retinopathy given an intraocular injection of PBS (Supplemental Fig. 1A). Likewise, mice with ischemic retinopathy given i.p. injections of 2 mg/kg of digoxin at P12, P13, and P14 had a significant reduction in SDF-1 compared to those treated with PBS (Fig. 4).
Figure 4.
Digoxin reduces SDF-1 protein levels in ischemic retina. At P12, mice with ischemic retinopathy were given an intraocular injection of 0.25 μg of digoxin or PBS. At P15, the mice were euthanized, retinal lysates were prepared, and mean ± se (n=4) SDF-1 levels (ng/μg of total protein) were determined by ELISA. *P < 0.05 vs. PBS; unpaired t test.
Digoxin suppresses ischemia-induced retinal NV
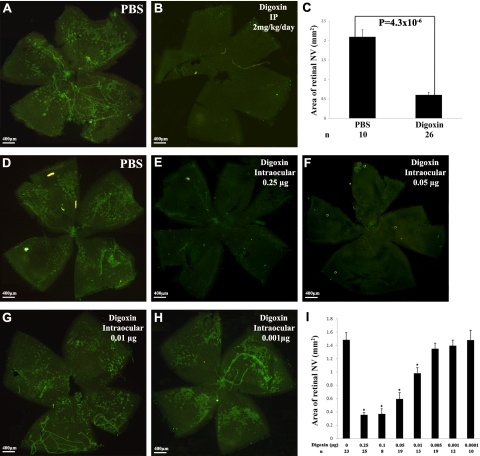
When mice with ischemic retinopathy were given daily i.p. injections of PBS between P12 and P17, retinal flat mounts stained for PECAM-1 by a technique that selectively stains NV on the surface of the retina (30) showed numerous patches of NV (Fig. 5A). Mice given daily i.p. injection of 2 mg/kg of digoxin between P12 and P17 showed almost no NV on the surface of the retina (Fig. 5B). Measurement of NV by image analysis showed a 75% reduction in the area of retinal NV in digoxin-treated mice (Fig. 5C; P<0.00001 by unpaired t test). Compared to mice given an intraocular injection of PBS at P12, those given an intraocular injection of digoxin ≥ 0.01 μg had significant reduction in NV, with strong inhibition after injection of 0.05 μg or more of digoxin (Fig. 5D–I).
Figure 5.
Digoxin suppresses ischemia-induced retinal neovascularization. At P7, litters of C57BL/6 mice were placed in 75% oxygen; at P12, they were returned to room air and given daily i.p. injections of PBS or 2 mg/kg/d of digoxin, or a single intraocular injection of 0 (PBS), 0.0001, 0.001, 0.005, 0.01, 0.05, 0.1, or 0.25 μg of digoxin. At P17, retinal flat mounts immunofluorescently stained for PECAM-1 by a technique that selectively stains new vessels showed extensive neovascularization on the surface of retinas from mice given i.p. injections of PBS (A), but almost no neovascularization on the surface of retinas from mice given i.p. injections of digoxin (B). Image analysis confirmed that compared to PBS-treated mice, mice treated with digoxin had a statistically significant reduction (unpaired t test) in the area of retinal neovascularization (C). Compared to mice given an intraocular injection of PBS at P12 (D), those given an injection of 0.25 (E), 0.1, 0.05 (F), or 0.01 μg (G) of digoxin, but not those given an injection of 0.005, 0.001 (H), or 0.0001 μg, showed significant reduction (ANOVA with Dunnett’s correction for multiple comparisons) in the area of retinal neovascularization (I).
Digoxin reduces bone marrow-derived cells in ischemic retina
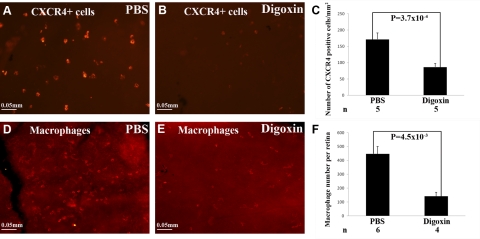
Increased production of SDF-1 in ischemic retina promotes the recruitment and retention of CXCR4+ bone marrow-derived cells, which contribute to the pathogenesis of retinal NV (24, 25). Compared to mice given an intraocular injection of PBS at P12, which showed many CXCR4+ cells on the surface of the retina at P17 (Fig. 6A), mice given an intraocular injection of 0.05 μg of digoxin showed a significant reduction in CXCR4+ cells in the retina (Fig. 6B, C). Retinas from digoxin-treated mice also showed a significant reduction in F4/80+ macrophages (Fig. 6D–F).
Figure 6.
Digoxin blocks recruitment of bone marrow-derived cells into ischemic retina. At P7, litters of C57BL/6 mice were placed in 75% oxygen; at P12, they were returned to room air and given an intraocular injection of PBS or 0.05 μg of digoxin. At P17, retinal flat mounts immunofluorescently stained with anti-CXCR4 (A, B) showed a reduction in CXCR4+ cells in retinas from digoxin-treated (B) vs. PBS-treated mice (A, C). Macrophages are CXCR4+ cells that are selectively stained with the F4/80 antibody; F4/80-stained retinas (D, E) showed a reduction in macrophages in ischemic retinas from digoxin-treated mice (E) compared to those treated with PBS (D, F). Statistical comparisons were made by unpaired t test.
Digoxin suppresses choroidal NV at Bruch’s membrane rupture sites
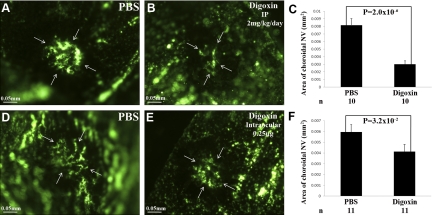
Choroidal NV occurs in diseases of Bruch’s membrane and the retinal pigmented epithelium, such as age-related macular degeneration (AMD), and although it is not clear whether hypoxia plays a role, HIF-1 has been implicated (34). Rupture of Bruch’s membrane with laser photocoagulation causes choroidal NV, and inhibition of VEGF strongly suppresses NV in this model and in patients with choroidal NV due to AMD (10, 12, 35, 36). Compared to mice that received daily i.p. injections of PBS for 14 d after laser photocoagulation, in which there were large areas of choroidal NV at Bruch’s membrane rupture sites (Fig. 7A), mice that were treated with daily i.p. injections of 2 mg/kg of digoxin for 14 d had a significant reduction in the area of choroidal NV lesions (Fig. 7B, C). A single intraocular injection of 0.25 μg of digoxin immediately after laser photocoagulation also resulted in significant reduction in the area of choroidal NV 14 d later when compared to eyes injected with PBS (Fig. 7D–F).
Figure 7.
Digoxin suppresses choroidal neovascularization at Bruch’s membrane rupture sites. Adult C57BL/6 mice had rupture of Bruch’s membrane with laser photocoagulation at 3 locations in each eye and then were treated with daily i.p. injections of PBS (A) or 2 mg/kg of digoxin (B), or with a single intraocular injection of PBS (D) or 0.25 μg of digoxin (E). After 14 d, choroidal flat mounts from fluorescein-labeled dextran-perfused mice showed large areas of choroidal neovascularization in mice that received i.p. injections of PBS (A) that were significantly reduced in mice that received i.p. injections of digoxin (B, C). Compared to eyes treated with an intraocular injection of PBS (D), the area of choroidal neovascularization at Bruch’s membrane rupture sites was significantly less in eyes given an intraocular injection of digoxin (E, F). Statistical comparisons were made by unpaired t test.
DISCUSSION
Digoxin is an inhibitor of Na+/K+ ATPase that has been used for decades to treat patients with congestive heart failure or arrhythmias. In a cell-based screen to identify drugs that inhibit HIF-1 transcriptional activity, digoxin and 10 other cardiac glycosides were found to reduce HIF-1 activity by >88% (27). Digoxin also inhibited tumor xenograft growth, and this effect was dependent on its ability to inhibit HIF-1α expression (27). In this study, we have demonstrated that digoxin blocks hypoxia-induced up-regulation of HIF-1α in the retina and suppresses hypoxia-induced expression of multiple HIF-1 target genes. Systemic administration or intraocular injection of digoxin strongly suppressed ischemia-induced retinal NV and choroidal NV at Bruch’s membrane rupture sites. Dose dependency with high potency and efficacy were observed in the ischemic retinopathy model in which maximal inhibition of about >70% was achieved with a single intraocular injection of 100 ng of digoxin. Retinal NV was also reduced by >70% by systemic administration of 2 mg/kg/d of digoxin and choroidal NV was reduced by >60%.
VEGF plays a critical role in the pathogenesis of retinal and choroidal NV, and the development of potent VEGF antagonists has revolutionized the treatment of choroidal NV due to AMD (7,8,9,10,11, 35, 36). However, treatment with monthly injections of ranibizumab results in a major improvement in vision in less than half of all patients with neovascular AMD; proangiogenic factors other than VEGF may be mediating NV in nonresponding patients (35, 36). Other factors that have been implicated in retinal and choroidal NV include PDGF-B (17,18,19, 37), SDF-1 (24, 25), and ANGPT2 (13, 14, 16). PGF and EPO have been shown to stimulate retinal NV, but there is no information regarding their effect on choroidal NV (20,21,22,23).
In view of the many proangiogenic factors that may contribute to retinal and choroidal NV, it is reasonable to consider combination therapy. In animal models, combined use of VEGF and PDGF antagonists is more effective than either alone (19). Antagonists of VEGF do not cause regression of choroidal neovascularization (35, 36), and a study in which ranibizumab was combined with an aptamer that binds PDGF-B provided preliminary evidence of regression of choroidal neovascularization and strong efficacy in patients with neovascular AMD (38, 39). An efficient way to simultaneously block VEGF and PDGF signaling is with drugs that block VEGF and PDGF receptor tyrosine kinase (RTK) activity, which constitute the most effective class of agents that we have identified for suppression of retinal and choroidal NV (10, 40,41,42). The RTK inhibitors that have been tested have different kinase inhibition profiles, but they all block VEGFR1, VEGFR2, and PDGFRβ. The levels of suppression of retinal and choroidal NV achieved with digoxin are similar to those achieved with RTK inhibitors, suggesting that the simultaneous suppression of multiple proangiogenic agents, which is achieved by blocking HIF-1 may provide advantages over monotherapy.
In addition to its direct effects on retinal cells to reduce production of proangiogenic factors, digoxin also substantially reduced the number of macrophages in ischemic retina, which have been shown to play a major contribution to retinal and choroidal NV (25, 30, 43). Digoxin inhibited the expression of SCF in ischemic retina, which may reduce recruitment of other bone marrow-derived cells, such as endothelial progenitor cells, which have been suggested to play a role in ocular NV (44, 45). Thus, digoxin blocks stimulation of retinal and choroidal NV via multiple mechanisms, which reflects the role of HIF-1 as a master regulator of ischemia-induced angiogenesis (46) and provides a means to achieve the effects of combination therapy while administering a single agent.
Digoxin is a cardioactive drug with a relatively narrow therapeutic window for treatment of congestive heart failure. However, the strong effect of a single intraocular injection of 100 ng of digoxin suggests potency, and dose-escalation studies should be considered to determine whether oral doses below those used for congestive heart failure are effective in blocking ocular NV. Ultimately, intraocular administration of a slow-release formulation of digoxin could provide an effective and sustained therapy for a large group of blinding diseases that are complicated by ocular NV or excessive vascular permeability.
Acknowledgments
This work was supported by U.S. National Institutes of Health/National Eye Institute grant EY012609 (P.A.C.) and the Johns Hopkins Institute for Cell Engineering (G.L.S.) G.L.S. is the C. Michael Armstrong Professor of Johns Hopkins University. P.A.C. is the George and Dolores Eccles Professor of Ophthalmology and Neuroscience.
References
- Klein R, Klein B E K, Moss S E, Davis M D, DeMets D L. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- Semenza G L, Wang G L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G L, Semenza G L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G L, Jiang B-H, Rue E A, Semenza G L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Yu A, Della N, Ozaki K, Luna J D, Yamada H, Hackett S F, Okamoto N, Zack D J, Semenza G L, Campochiaro P A. Hypoxia inducible factor-1a is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40:182–189. [PubMed] [Google Scholar]
- Kelly B D, Hackett S F, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro P A, Semenza G L. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- Aiello L P, Pierce E A, Foley E D, Takagi H, Chen H, Riddle L, Ferrara N, King G L, Smith L E H. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N, Tobe T, Hackett S F, Ozaki H, Vinores M A, LaRochelle W, Zack D J, Campochiaro P A. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997;151:281–291. [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Seo M-S, Ozaki K, Yamada H, Yamada E, Hofmann F, Wood J, Campochiaro P A. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000;156:679–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak N, Okamoto N, Wood J M, Campochiaro P A. VEGF is an important stimulator in a model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–3164. [PubMed] [Google Scholar]
- Kryzstolik M G, Afshari M A, Adamis A P, Gaudreault J, Gragoudas E S, Michaud N M, Li W, Connolly E, O'Neill C A, Miller J W. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- Saishin Y, Saishin Y, Takahashi K, Lima Silva R, Hylton D, Rudge J, Wiegand S J, Campochiaro P A. VEGF-TRAPR1R2 suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- Hackett S F, Ozaki H, Strauss R W, Wahlin K, Suri C, Maisonpierre P, Yancopoulos G, Campochiaro P A. Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184:275–284. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hackett S F, Wiegand S J, Yancopoulos G, Campochiaro P. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192:182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Deering T, Oshima S, Nambu H, Reddy P S, Kaleko M, Connelly S, Hackett S F, Campochiaro P A. Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J Cell Physiol. 2004;199:412–417. doi: 10.1002/jcp.10442. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Oshima S, Nambu H, Kachi S, Takahashi K, Umeda N, Shen J, Dong A, Apte R S, Duh E, Hackett S F, Okoye G, Ishibashi K, Handa J, Melia M, Wiegand S, Yancopoulos G, Zack D J, Campochiaro P A. Different effects of angiopoietin 2 in different vascular beds in the eye; new vessels are most sensitive. FASEB J. 2005;19:963–965. doi: 10.1096/fj.04-2209fje. [DOI] [PubMed] [Google Scholar]
- Seo M-S, Okamoto N, Vinores M A, Vinores S A, Hackett S F, Yamada H, Yamada E, Derevjanik N L, LaRochelle W, Zack D J, Campochiaro P A. Photoreceptor-specific expression of PDGF-B results in traction retinal detachment. Am J Pathol. 2000;157:995–1005. doi: 10.1016/S0002-9440(10)64612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Gehlbach P, Ando A, Dyer G, Lipinsky E, Chaudhry A G, Hackett S F, Campochiaro P A. Retina-specific expression of PDGF-B versus PDGF-A: vascular versus nonvascular proliferative retinopathy. Invest Ophthalmol Vis Sci. 2002;43:2001–2006. [PubMed] [Google Scholar]
- Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson G S, Adamis A P, Shima D T. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006;168:2036–2053. doi: 10.2353/ajpath.2006.050588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewechin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate K H, Foidart J M, Schaper W, Charnock-Jones D S, Hicklin D J, Herbert J M, Collen D, Persico M G. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- Rakic J-M, Lamber V, Devy L, Luttun A, Carmeliet P, Claes C, Nguyen L, Foidart J M, Noel A, Munaut C. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3186–3193. doi: 10.1167/iovs.02-1092. [DOI] [PubMed] [Google Scholar]
- Morita H, Ohneda O, Yamashita T, Takahashi S, Suzuki S, Nakajima O, Kawauchi S, Ema M, Shibahara S, Udono T, Tomita K, Tamai M, Sogawa K, Yamamoto M, Fujii-Kuriyama Y. HLF/HIF-2α is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 2003;22:1134–1146. doi: 10.1093/emboj/cdg117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, Suzuma I, Ohashi H, Ojima T, Murakami T, Kobayashi T, Masuda S, Nagoa M, Yoshimura N, Takagi H. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Eng J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- Butler J M, Guthrie S M, Koc M, Afzal A, Caballero S, Brooks H L, Mames R N, Seqal M S, Grant M B, Scott E W. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima e Silva R, Shen J, Hackett S F, Kachi S, Akiyama H, Kiuchi K, Yokoi K, Hatara C, McLauer T, Aslam S, Gong Y Y, Xiao W H, Khu N H, Thut C, Campochiaro P. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21:3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- Patel T H, Kimura H, Weiss C R, Semenza G L, Hofmann L V. Constitutively active HIF-1α improves perfusion and arterial remodeling in an endovascular model of limb ischemia. Cardiovasc Res. 2005;68:144–154. doi: 10.1016/j.cardiores.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Zhang H, Qian D Z, Tan Y S, Lee K, Gao P, Ren Y R, Rey S, Hammers H, Chang D, Pili R, Dang C V, Liu J O, Semenza G L. Digoxin and other cardiac glycosides inhibit HIF-1alpha sythesis and block tumor growth. Proc Natl Acad Sci U S A. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T, Ortega S, Luna J D, Ozaki H, Okamoto N, Derevjanik N L, Vinores S A, Basilico C, Campochiaro P A. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, Mori K, Yang H S, Zack D J, Ettyreddy D, Brough D E, Wei L L, Campochiaro P A. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- Shen J, Xie B, Dong A, Swaim M, Hackett S F, Campochiaro P A. In vivo immunostaining demonstrates macrophages associate with growing and regressing vessels. Invest Ophthalmol Vis Sci. 2007;48:4335–4341. doi: 10.1167/iovs.07-0113. [DOI] [PubMed] [Google Scholar]
- Xie B, Shen J, Dong A, Rashid A, Stoller G, Campochiaro P A. Blockade of sphingosine-1-phosphate reduces macrophage influx and retinal and choroidal neovascularization. J Cell Physiol. 2009;218:192–198. doi: 10.1002/jcp.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong A, Xie B, Shen J, Yoshida T, Yokoi K, Hackett S F, Campochiaro P A. Oxidative stress promotes ocular neovascularization. J Cell Physiol. 2009;219:544–552. doi: 10.1002/jcp.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L E H, Wesolowski E, McLellan A, Kostyk S K, D'Amato R, Sullivan R, D'Amore P A. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Vinores S A, Xiao W H, Aslam S, Shen J, Oshima Y, Nambu H, Liu H, Carmeliet P, Campochiaro P A. Implication of the hypoxia response element of the VEGF promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development. J Cell Physiol. 2006;206:749–758. doi: 10.1002/jcp.20525. [DOI] [PubMed] [Google Scholar]
- Brown D M, Kaiser P K, Michels M, Soubrane G, Heier J S, Kim R Y, Sy J P, Schneider S, Group A S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P J, Brown D M, Heier J S, Boyer D S, Kaiser P K, Chung C Y, Kim R Y, Group M S. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Battegay E J, Rupp J, Iruela-Arispe L, Sage E H, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125:917–928. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer D S, Ophthotech Anti-PGDF in AMD Study Group Combined inhibition of platelet-derived (PDGF) and vascular endothelial (VEGF) growth factors for the treatment of neovascular age-related macular degeneration (NV-AMD). Results of a phase 1 study. Invest Ophthalmol Vis Sci. 2009 Online ARVO Abstract 1260. [Google Scholar]
- Cousins S W, Csaky K G, Ophthotech Study Group Patterns of CNV fluorescein aind indocyanine green angiographic regression responses after anti-VEGF monotherapy or anti-VEGF plus anti-PDGF combotherapy. Invest Ophthalmol Vis Sci. 2009 Online ARVO Abstract 1261. [Google Scholar]
- Seo M-S, Kwak N, Ozaki H, Yamada H, Okamoto N, Fabbro D, Hofmann F, Wood J M, Campochiaro P A. Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am J Pathol. 1999;154:1743–1753. doi: 10.1016/S0002-9440(10)65430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas J, Mahesh S, Umeda N, Kachi S, Akiyama H, Yokoi K, Cao J, Chen Z, Dellamary L, Tam B, Racanelli-Layton A, Hood J, Martin M, Noronha G, Soll R, Campochiaro P A. Topical administration of a multi-targeted kinase inhibitor suppresses choroidal neovasculaization and retinal edema. J Cell Physiol. 2008;216:29–37. doi: 10.1002/jcp.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Saishin Y, Saishin Y, King A, Levin R, Campochiaro P A. The multi-targeted kinase inhibitor pazopanib causes suppression and regression of choroidal neovascularization. Arch Ophthalmol. 2009;127:494–499. doi: 10.1001/archophthalmol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi C, Sonoda K H, Egashira K, Qiao H, Hisatomi T, Nakao S, Ishibashi M, Charo I F, Sakamoto T, Murata T, Ishibashi T. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74:25–32. doi: 10.1189/jlb.0902436. [DOI] [PubMed] [Google Scholar]
- Grant M B, May W S, Caballero S, Brown G A, Guthrie S M, Mamee R N, Byrne B J, Vaught T, Spoerri P E, Peck A B, Scott E W. Adult hematopoietic stem cells provide functional hemangioblastic activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- Otani A, Kinder K, Ewait K, Otero F J, Schimmel P, Friedlander M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med. 2002;8:1004–1010. doi: 10.1038/nm744. [DOI] [PubMed] [Google Scholar]
- Semenza G L. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]