Abstract
The cellular sources that contribute to the renewal of human endometrium are largely unknown. It has been suggested that endometrial stem cells originate from bone marrow-derived mesenchymal stem cells (MSC), with subsequent development into endometrial stromal fibroblasts (hESF). We hypothesized that if bone marrow-derived MSC contribute to endometrial regeneration and are progenitors of hESF, their treatment with agents known to regulate hESF differentiation could promote their differentiation down the stromal fibroblast lineage. To this end, we treated bone marrow-derived MSC with estradiol and progesterone, bone morphogenetic protein 2 (BMP2), and activators of the protein kinase A (PKA) pathway and investigated specific markers of hESF differentiation (decidualization). Furthermore, we investigated the transcriptome of these cells in response to cAMP and compared this to the transcriptome of hESF decidualized in response to activation of the PKA pathway. The data support the idea that MSC can be differentiated down the hESF pathway, as evidenced by changes in cell shape and common expression of decidual markers and other genes important in hESF differentiation and function, and that bone marrow-derived MSC may be a source of endometrial stem/progenitor cells. In addition, we identified MSC-specific markers that distinguish them from other fibroblasts and, in particular, from hESF, which is of biologic relevance and practical value to the field of endometrial stem cell research.
Keywords: cyclic adenosine monophosphate, differentiation, endometrium, fibroblasts, mesenchymal stem cells
Mesenchymal stem cells (MSC) can be differentiated down the human endometrial stromal fibroblast (hESF) pathway as evidenced by changes in cell shape and expression of decidual markers and other genes important in hESF differentiation and function.
INTRODUCTION
Human endometrium is a unique tissue that undergoes regular dynamic processes of growth, differentiation, sloughing, and renewal during a woman's reproductive years, generating 4–14 mm of mucosal tissue each menstrual cycle [1]. To date, the cellular sources that contribute to the complete renewal of endometrium are not known with certainty. The recently identified small population of clonogenic, self-renewing, multipotent mesenchymal stem cells (MSC) with high proliferative potential in human endometrium is a candidate stem/progenitor cell population [2, 3]. Key properties of MSC found in other tissues, such as bone marrow, adipose, dental pulp, amniotic fluid, umbilical cord blood, placenta, skeletal muscle, and pancreas [4, 5], include differentiation in vitro into multiple mesodermal cell types, such as osteoblasts, adipocytes, myoblasts, and chondroblasts [6, 7]. Wolff et al. [8] demonstrated that freshly isolated human endometrial stromal fibroblasts (hESF) can differentiate down the chondrogenic lineage, thus indicating the presence of multipotent stem/progenitor cells in human endometrium [8]. Also, isolated hESF plated at clonal density can differentiate toward adipogenic, osteogenic, myogenic, and chondrogenic lineages [9]. Thus, the stromal fibroblast may derive from a stem/progenitor cell in human endometrium.
Whereas endometrial MSC have properties of progenitors with the capacity to differentiate down multiple lineages, their origin remains unclear. One potential source is bone marrow-derived MSC, which are multipotent cells that, together with hematopoietic stem cells, construct the bone marrow. Osteoblasts, adipocytes, muscle cells, chondrocytes, and neurons originate from MSC in vivo and in vitro [10–12]. Clonogenic endometrial MSC express surface markers typical of bone marrow MSC and fat MSC (CD29+, CD44+, CD73+, CD90+, CD105+, CD146+, PDGFRβ+, CD31−, CD34–, CD45−, and Stro-1−) and were found in a perivascular location, suggesting they may be a subset of pericytes involved in regulating both endometrial stromal repair and angiogenesis [9]. Furthermore, Taylor [13] provided evidence that endometrial regeneration may occur as a result of bone marrow-derived stem cells in bone marrow transplant recipients [13]. Donor-derived cells were detected in endometrial samples of bone marrow recipients, and these cells were histologically similar to endometrial epithelial and stromal fibroblasts and expressed appropriate markers of endometrial cell differentiation. It has been proposed that cyclic mobilization of bone marrow-derived stem cells may be a normal physiologic process [13, 14]. Interestingly, bone marrow-derived MSC have been suggested to differentiate not only to stromal fibroblasts but also to endometrial epithelial cells [15, 16].
In the current study, we hypothesized that if bone-marrow derived MSC contribute to endometrial regeneration and are progenitors to hESF, their treatment with agents known to regulate hESF differentiation could promote their differentiation down the stromal fibroblast lineage. To this end, we treated bone marrow-derived MSC with estradiol (E2) and progesterone (P4), bone morphogenetic protein 2 (BMP2), and activators of the protein kinase A (PKA) pathway and investigated specific markers of hESF differentiation (decidualization). Furthermore, we investigated the transcriptome of these cells in response to cAMP and compared this to the transcriptome of hESF decidualized in response to activation of the PKA pathway. The data support the idea that MSC can be differentiated down the hESF pathway, as evidenced by changes in cell shape and common expression of decidual markers and other genes important in hESF differentiation and function.
MATERIALS AND METHODS
MSC Culture
Bone marrow-derived MSC (Cambrex Biosciences) were expanded in high-glucose Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS; HyClone, Thermo Scientific, Inc.), 1% penicillin and 1% streptomycin. At 80% confluency, MSC were trypsinized and plated at 5 × 103 cells/cm2 in 6-cm plates. Nearly confluent cells were serum-starved overnight and thereafter treated for 0, 3, 7, 14, and 21 days in low-serum medium (2% FBS) with the following treatments: 1) 10 nM E2 and 1 mM P4 (E2+P4), 2) 0.5 or 1 mM 8-Br-cAMP (Sigma-Aldrich), 3) 50 or 100 nM BMP2 (R&D Systems), 4) 0.5 and 1 mM 8-Br-cAMP plus BMP2, 5) 0.5 and 1 mM 8-BrcAMP plus BMP2 plus E2+P4, and 6) 50 or 100 nM human follicle-stimulating hormone (FSH; Sigma-Aldrich). Vehicle controls were present at each time point for all treatments listed. Each experiment was conducted in duplicate and repeated at least three times. Media were changed every third day. Endpoints were prolactin (PRL) and insulin-like growth factor-binding protein-1 (IGFBP1) mRNA, assessed by quantitative real-time RT-PCR, and protein in conditioned media, assessed by ELISA (see below), after 3, 7, 14, and 21 days of incubation. Immunostaining for vimentin, cytokeratin 7, and Oct-4 was performed before and after MSC treatments to assess the stromal lineage of MSC and their differentiation status [17–19]. MSC expressed vimentin and did not express cytokeratin or Oct-4 independent of culture conditions or duration of culture or treatment. The morphological appearance of MSC under different treatment conditions was photographed using a Leica CTR 6500 microscope (Leica Microsystems, Ltd.). Scanned images were processed using the Leica TCS SP5 software package.
Isolation and Culture of hESF
Isolation and culture of hESF from endometrial samples (biopsy and hysterectomy specimens) from subjects without endometrial disease, which were used herein for comparison with MSC, were performed as previously described in detail [20–22]. The University of California, San Francisco (UCSF) Committee on Human Research and the Stanford University Committee on the Use of Human Subjects in Research approved the study. Written informed consent was obtained from subjects. Samples were also obtained through the UCSF National Institutes of Health Human Endometrial Tissue and DNA Bank with appropriate institutional review, approvals, and informed consent from all subjects.
Culture of Human Dermal Neonatal Fibroblasts
To determine specificity of the differentiative response, human dermal neonatal fibroblasts (HDFn; Cascade Biologics, Inc., Invitrogen) were used as a comparator. They were expanded in high-glucose DMEM containing 10% FBS (HyClone), 1% penicillin and 1% streptomycin. At 90% confluency, HDFn were trypsinized and plated at 5 × 103 cells/cm2 in 6-cm plates. At near confluency, cells were serum-starved overnight and thereafter treated with 1 mM 8-Br-cAMP for 0, 3, 7, 14, and 21 days in triplicate. Endpoint analyses were measurements of PRL, IGFBP1, and stanniocalcin 1 (STC1) mRNA, assessment using quantitative RT-PCR, and PRL and IGFBP1 protein in conditioned media, assessed using specific ELISA assays (see below).
Enzyme-Linked Immunosorbent Assay
Conditioned media from MCS treated under different conditions and for different time periods were subjected to ELISA to determine PRL and IGFBP1 protein concentrations according to the manufacturers' instructions (Alpha Diagnostic International, Inc., and Diagnostic Systems Laboratories, respectively) and as described previously [20, 21]. Inter- and intra-assay coefficients of variation were 5–7.4% and 2.4–3.4%, respectively, for the IGFBP1 ELISA and 6.7–10.4% and 7.8–8.2%, respectively, for PRL. Minimum detection limits for PRL and IGFBP1 assays were 0.14 and 0.4 ng/ml, respectively. Levels of PRL and IGFBP1 were normalized to total RNA.
Total RNA Isolation, Quantitative Real-Time RT-PCR, and Microarray Hybridization
Total RNA from all cell cultures was purified using the Qiagen RNeasy Plus Mini kit according to the manufacturer's instructions. Samples stored in RNase-free H2O were quantified by spectroscopy, and the purity was analyzed by the 260:280 absorbance ratio. RNA quality was assessed by an A2100 Bioanalyzer (Agilent Technologies). For real-time RT-PCR analysis, 1 μg of RNA was converted to cDNA using the iScript cDNA Synthesis kit (Bio-Rad Laboratories). The mRNA preparations from technical duplicates for each set of treatments were pooled together. The real-time RT-PCR reaction was carried out in duplicates for 40 cycles with the primers listed in Supplemental Table S1 (all supplemental data are available online at www.biolreprod.org).
Microarray analysis was performed with MSC (n = 3) at time zero (t = 0; untreated, uncultured control), 14-day vehicle control, and 14-day MSC treated with 8-Br-cAMP. Only high-quality samples (RNA integrity number = 9.1–10) were used for microarray analysis. Hybridization was performed with Affymetrix Human Gene 1.0 ST arrays, as described earlier [23]. For each sample, 100 ng of total RNA were reverse transcribed to cDNA using 500 ng of T7-(N6) primers and SuperScript II. A second strand of DNA was generated using DNA polymerase, followed by overnight in vitro transcription to generate cRNA. After processing through cRNA clean-up spin columns, 10 μg of cRNA were reverse transcribed using random primers and SuperScript II. Mixtures were digested with RNase H, and the cDNA was purified by the cDNA clean-up spin columns. Lastly, 5.5 μg of sense cDNA were fragmented and labeled by using the GeneChip WT terminal labeling kit. The quality of cDNA and fragmented cDNA was assessed in the Agilent bioanalyzer. Microarrays were hybridized, washed, stained, and scanned according to the protocol described in WT Sense Target Labeling Assay Manual from Affymetrix (Version 4; FS450_0007) at the UCSF Gladstone Genomics Core Facility.
Statistical Evaluation
Statistical analysis of data generated in the ELISA assays was performed using a two-tailed, type 3 Student t-test. For quantitative RT-PCR, the nonparametric Mann-Whitney test was used. Significance was determined at P ≤ 0.05.
Microarray Gene Expression Data Analysis and Statistical Analysis
As described previously [23], to remove variation of a nonbiological origin, densitometry values among arrays were normalized using the RMA function and further transformed to the logarithmic 2 (log2) scale. Probes with known correspondence to a GenBank accession ID were selected for functional analysis. Statistically significant differences between groups were determined using the Bioconductor (http://www.bioconductor.org/) packages multtest (for the parametric MSC intragroup comparison, with a multiple test correction cutoff false discovery rate < 0.01) and RankProd (for the nonparametric inter-MSC-hESF group comparison, with a multiple test correction cutoff pfp [P-value corrected] < 0.05), both run under R software (http://www.r-project.org/). Functional annotations were carried out using Ingenuity Pathway Analysis (Ingenuity Systems; http://www.ingenuity.com/), where gene symbols and fold-changes of the up- and down-regulated genes were imported. The raw data files of current experiments are uploaded to the National Center for Biotechnology Information Gene Expression Omnibus database (number GSE20631).
Hierarchical Clustering
Herein, we conducted hierarchical cluster analysis of differentially expressed genes from all samples (the combined gene list) using the smooth correlation for distance measure algorithm (GeneSpring 7.3) to identify samples with similar patterns of gene expression (Eisen et al.; see Note Added in Proof). The output data are displayed graphically as a Heatmap, based on the measured intensity values of the genes in the gene list, and are represented as a hierarchical tree with branches to indicate the relationships among different groups. Principal component analysis (PCA) was performed to further evaluate similarities in gene expression patterns in our experimental groups. An unsupervised pattern recognition and visualization tool used to analyze large amounts of data derived from array gene expression analysis, PCA displays a multidimensional data set in a reduced dimensionality (three dimensions) to capture as much of the variation in the data as possible [22]. Each dimension represents a component to which a certain percentage of variance in the data is attributed. We applied the unbiased PCA algorithm in GeneSpring to all the samples before and after the treatment, using all 19 492 gene probes on the Affymetrix Human Gene 1.0 ST arrays to look for similar expression patterns and underlying cluster structures [24].
The most highly up- or down-regulated genes, as well as some potentially interesting genes, were chosen for validation by quantitative real-time RT-PCR as described above.
RESULTS
Morphological Transformation of MSC
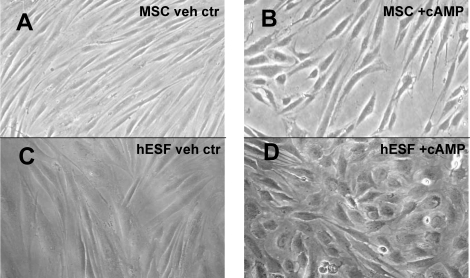
Human endometrial stromal fibroblasts undergo distinct morphologic changes, from a spindle-like to more epithelial appearance with more rounded shape and large nuclei, as they differentiate in response to treatment with cAMP analogues and/or E2+P4 [19]. Because MSC have been postulated to be progenitors of hESF, we investigated whether 8-Br-cAMP treatment of MSC elicited similar changes in cell shape and morphology compared to those of similarly treated hESF (Fig. 1). Indeed, the morphological changes of 8-Br-cAMP-treated MSC were very similar to those observed with hESF treated in vitro with 8-Br-cAMP (Fig. 1). No other treatment of the MSC resulted in changes in shape or morphology throughout the culture period (data not shown).
FIG. 1.
Morphological transformation of MSC. A) MSC cultured for 14 days, vehicle control. B) MSC treated with 1 mM 8-Br-cAMP for 14 days. C) Human endometrial stromal fibroblasts (hESF) cultured for 4 days, vehicle control. D) hESF treated with 8-Br-cAMP for 4 days. Magnification ×40.
Up-regulation of Decidualization Markers
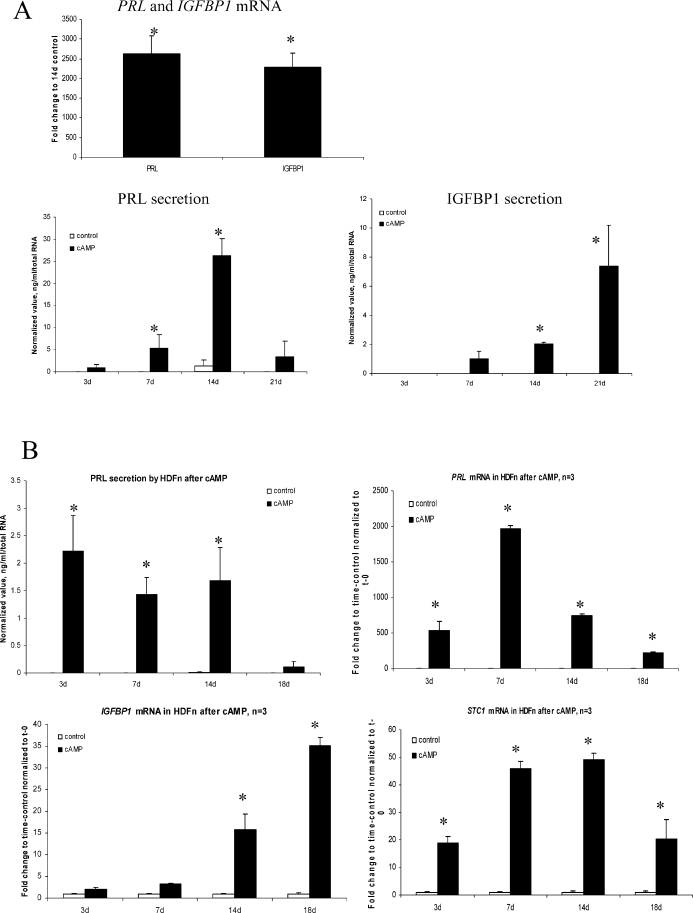
The classical markers of hESF differentiation (decidualization) in response to 8-Br-cAMP, PRL and IGFBP1, were assessed in MSC treated with 1 mM 8-Br-cAMP. A robust expression of these markers was noted, with maximum mRNA and protein expression observed at 14 days of treatment. PRL and IGFBP1 mRNAs were significantly up-regulated (2600- and 2200-fold, respectively) compared to vehicle controls cultured for the same time period, and corresponding proteins in MSC conditioned media were also increased significantly (Fig. 2A). Treatment with E2+P4, BMP2, or FSH did not elicit up-regulation of PRL or IGFBP1 (data not shown). Also, no significant difference in IGFBP1 and PRL mRNA or protein expression was found in MSC treated with cAMP plus E2+P4 versus 8-Br-cAMP alone (data not shown), suggesting that the MSC were not responsive to the steroid hormone therapy and that expression of IGFBP1 and PRL was specifically induced by activation of the PKA pathway by the cAMP analogue.
FIG. 2.
A) PRL and IGFBP1 mRNA levels in MSC treated with 1 mM 8-Br-cAMP for 14 days expressed as fold-change compared to 14 days, vehicle control. Time course of PRL and IGFBP1 protein expression in conditioned media from MSC treated with 1 mM 8-Br-cAMP were normalized to total RNA levels (n = 3). B) Expression of PRL, IGFBP1, and STC1 mRNA and/or protein in HDFn (human dermal fibroblasts, neonatal) treated with 1 mM cAMP for 14 days. PRL, IGFBP1, and STC1 mRNA levels are expressed as fold-change to time control (vehicle control at 3, 7, 14, and 18 days) normalized to t = 0. Note the difference in scale between A and B. PRL protein secretion at different culture time points was normalized to total RNA. No IGFBP1 secretion by HDFn occurred with or without cAMP treatment. Asterisk indicates statistically significant difference compared to vehicle control at each time point (P ≤ 0.05). Error bars represent the SEM.
Alternative Fibroblast Cell Line Response to cAMP Treatment
To determine whether the 8-Br-cAMP-induced up-regulation of IGFBP1 and PRL in bone marrow-derived MSC was specific to this cell type (and hESF) or was a general feature of fibroblasts, we treated HDFn with 8-Br-cAMP and assessed expression of PRL and IGFBP1 mRNAs and IGFBP1 and PRL protein secretion. Interestingly, HDFn secreted PRL protein at low levels, confirming a previous report [25], whereas no secretion of IGFBP1 by these cells was observed in response to cAMP treatment (Fig. 2B). However, HDFn expressed high levels of PRL mRNA with very low IGFBP1 mRNA expression upon cAMP treatment (Fig. 2B) compared to levels in cAMP-treated hESF [20, 23] or MSC (Fig. 2A). We also investigated another cAMP-regulated gene, STC1, and found that its mRNA was expressed at very low levels in HDFn (Fig. 2B).
Expression of Hormone Receptors and Selected Steroidogenic Enzymes
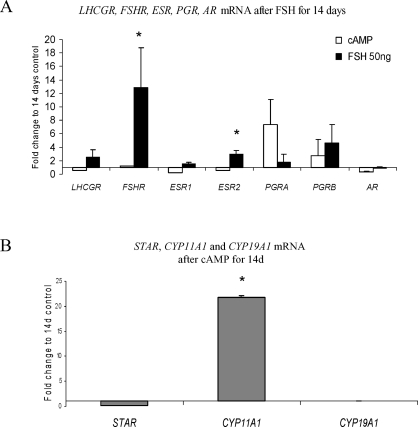
We and others have previously found that hESF express estrogen receptors (ESR), P4 receptors (PGR), androgen receptors (AR), and gonadotropin receptors, as well as key steroidogenic enzymes [20, 26–29]. We found that MSC expressed ESR, PGR, and AR mRNAs, as well as luteinizing hormone (LH) receptor and FSH receptor (LHCGR and FSHR) transcripts in t = 0, vehicle control, and treatment groups at different time points, without significant influence of cAMP on their expression (Fig. 3A). Treatment with recombinant human FSH at low doses (50 nM) significantly up-regulated mRNA for FSHR and ESR2 (12.5- and 2.5-fold, respectively) compared to vehicle control, whereas changes in LHR, ESR1, PGR, and AR were not significant (Fig. 3A). Expression of key steroidogenic enzyme STAT, CYP11A1 (P450scc), and CYP19A1 (aromatase) transcripts were evaluated. CYP11A1 mRNA was significantly up-regulated by 1 mM 8-Br-cAMP after 14 days of treatment (Fig. 3B), consistent with our earlier report on CYP11A1 mRNA in cAMP-treated hESF [20].
FIG. 3.
A) LH receptor (LHCGR), FSH receptor (FSHR), estrogen receptors α and β (ESR1 and ESR2), progesterone receptors A and B (PGRA and PGRB, respectively), and androgen receptor (AR) mRNA expression in MSC treated with 1 mM 8-Br-cAMP for 14 days or 50 nM FSH for 14 days expressed as fold-change compared to 14 days, vehicle control. B) Messenger RNA expression for steroidogenic enzymes STAR, CYP11A1, and CYP19A1 in MSC treated with 1 mM 8-Br-cAMP for 14 days expressed as fold-change compared to 14 days, vehicle control. Error bars represent the SEM. *P ≤ 0.05.
Microarray Analysis
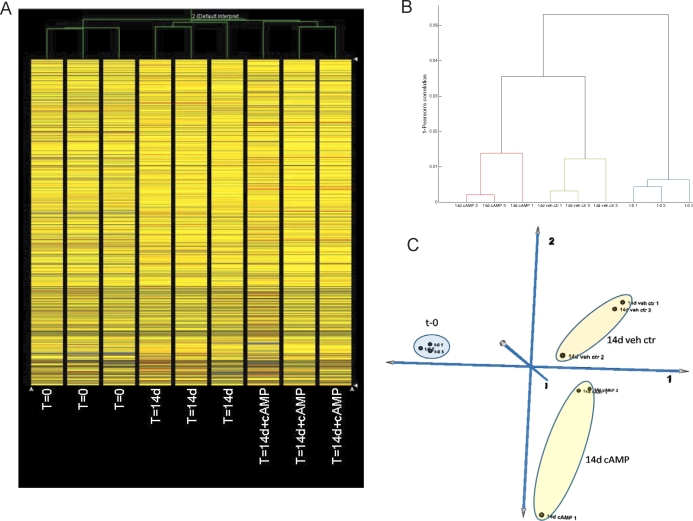
To comprehensively assess changes in gene expression in MSC, we performed comparative microarray analysis of MSC untreated and uncultured (t = 0; group A), MSC untreated and cultured for 14 days with vehicle control (group B), and MSC treated with 1 mM 8-Br-cAMP for 14 days (group C). Hierarchical clustering analysis of all genes demonstrated that samples clustered according to their assigned group (Fig. 4, A and B). PCA also demonstrated that group A clustered separately from groups B and C, with the latter two groups closer to each other but still distinct, demonstrating a distinctive effect of the culture process in general and 8-Br-cAMP in particular (Fig. 4C).
FIG. 4.
A) Hierarchical clustering analysis of MSC at t = 0 (T=0), MSC treated with vehicle for 14 days (T=14d), and MSC treated with 8-Br-cAMP for 14 days (T=14d+cAMP) are also shown. B) Dendogram of hierarchical clustering analysis of MSC at t = 0 (t-0) and 14 days after vehicle (14d veh ctr) or 8-Br-cAMP (14d cAMP) treatment. C) PCA of MSC at t = 0 and 14 days after vehicle or 8-Br-cAMP treatment.
Network and Pathway Analysis and Gene Regulation
To assess the effect of 8-Br-cAMP on MSC, group C was compared to group B (Table 1). Results of the other comparisons can be found in Supplemental Tables S2 and S3. Ingenuity Pathway Analysis of group C versus group B identified 91 regulated genes, of which 78 were eligible for generating networks. Top networks activated by cAMP in MSC included 1) tissue development, cancer, and cellular growth and proliferation; 2) cell cycle, molecular transport, and gene expression; and 3) organismal functions, skeletal and muscular development and function, and connective tissue development and function, indicating the presence of differentiation signs.
TABLE 1.
List of genes regulated in MSC after 14 days of treatment with 1mM 8-br-cAMP vs. MSC treated for 14 days with vehicle (control).a
Of note, the microarray data (Table 1) support our findings of cAMP induction in MSC of the decidualization biomarkers PRL and IGFBP1 (Fig. 2A). Furthermore, they demonstrate up-regulation of other cAMP-regulated genes with known functions in endometrial physiology—specifically, solute carrier family 16, member 6 (monocarboxylic acid transporter 7) (SLC16A6); IGF1; inhibin beta A (INHBA); VEGFA; pappalysin (PAPPA); parathyroid hormone-like hormone (PTHLH); dual-specificity phosphatase 4 (DUSP4); and others. Moreover, 40 genes, presented in Table 1, were uniquely regulated in group C (cAMP-treated MSC) only and were not found in groups A and B.
Multiple canonical pathways were regulated in all three comparison groups of the present study, with involved genes being both up- and down-regulated. One should note that several molecules can participate in different pathways simultaneously. Treatment with cAMP compared to the vehicle control (group C vs. group B comparison) revealed the following pathways: activated hepatic fibrosis/hepatic stellate cell activation, caveolar-mediated endocytosis, arginine and proline metabolism, glycine, serine and threonine metabolism, and LXR/RXR activation pathways.
Microarray Result Validation with Quantitative Real-Time RT-PCR
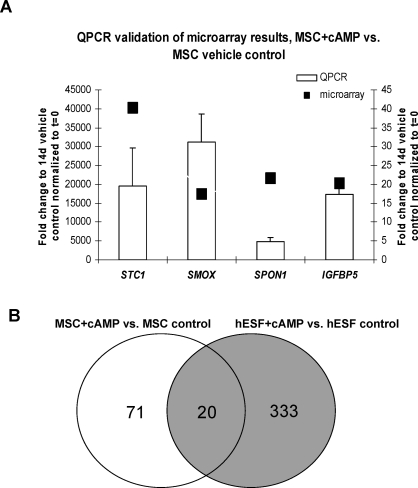
Expression of selected genes (STC1, SMOX, SPON1, and IGFBP5) was validated with quantitative real-time RT-PCR (Fig. 5A) using the same samples that were used for microarray analysis. These data are consistent with and validate the microarray results, even though the scale of the fold-change is different.
FIG. 5.
A) Real-time RT-PCR validation of selected genes in MSC treated for 14 days with cAMP versus vehicle control. Correspondent microarray data for stanniocalcin 1 (STC1), spermine oxidase (SMOX), spondin 1 (SPON1), and IGF-binding protein 5 (IGFBP5) are presented. Data are presented as fold-change. Error bars represent the SEM. The left y-axis addresses quantitative PCR results; the right y-axis addresses microarray data. B) Venn diagram of common and unique genes expressed in cAMP-treated MSC and hESF compared to their respective controls.
Comparison of Response to 8-Br-cAMP in MSC Versus hESF
We compared two independent gene lists, MSC plus 8-Br-cAMP versus control with hESF plus 8-Br-cAMP versus control. Both used the same platform for analysis (Affymetrix Human Gene 1.0 ST arrays) [23] (unpublished data). We found 484 genes were up- and down-regulated in MSC treated with cAMP versus hESF plus cAMP (Supplemental Table S4). Of 484 genes, 333 were unique to the hESF response, 71 were unique to the MSC response, and 20 were in common (Fig. 5B and Table 1). Interestingly, in MSC, cAMP did not induce expression of somatostatin (SST), a known cAMP-regulated gene that is highly up-regulated in hESF upon cAMP treatment [19, 30]. The 20 genes in common are known to be involved in endometrial physiology, underscoring that activation of the PKA pathway in MSC directs this cell type down the hESF lineage, with genes in common that have physiologic relevance to the differentiation and function of the hESF (see below).
Novel Genes Highly Up-regulated in Both MSC Plus cAMP and hESF Plus cAMP
Stanniocalcin 1 (STC1), a calcium-regulating glycoprotein strongly expressed in decidualized stromal cells in rat uterus [31], was uniquely regulated in MSC only after cAMP treatment, with 40.2-fold up-regulation (Table 1). Comparably, it was 19-fold up-regulated in hESF from women without endometrial pathology upon decidualization with cAMP [23].
Spermine oxidase (SMOX), an enzyme that preferentially oxidizes spermine, a polyamine involved in cellular metabolism and suggested to play a role in immune privilege in the decidua [32], increased 17.4-fold in cAMP-treated MSC compared to cells treated with vehicle for 14 days. It was also 5.8-fold up-regulated in hESF in response to cAMP (Table 1) [23].
SLC16A6 (solute carrier family 16, member 6 [monocarboxylic acid transporter 7], MCT5) belongs to the monocarboxylate cotransporter (MCT) family, several members of which are important for lactate, pyruvate, and ketone body metabolism [33]. It was up-regulated 23.0-fold by cAMP in MSC of the present study. We found expression of SLC16A6 in normal human endometrium using microarray, real-time RT-PCR, and immunohistochemistry [23, 34] (unpublished data). It was also up-regulated by 8-Br-cAMP in hESF (Table 1) [23].
Comparison of MSC and hESF Gene Signatures
Another important question when studying bone marrow-derived MSC and their differentiation toward the endometrial stromal lineage is how to distinguish the two cells types. Both share the same known stromal cell markers, and identification of genes specific for undifferentiated derived MSC are of great importance for both endometrial and stem cell biology.
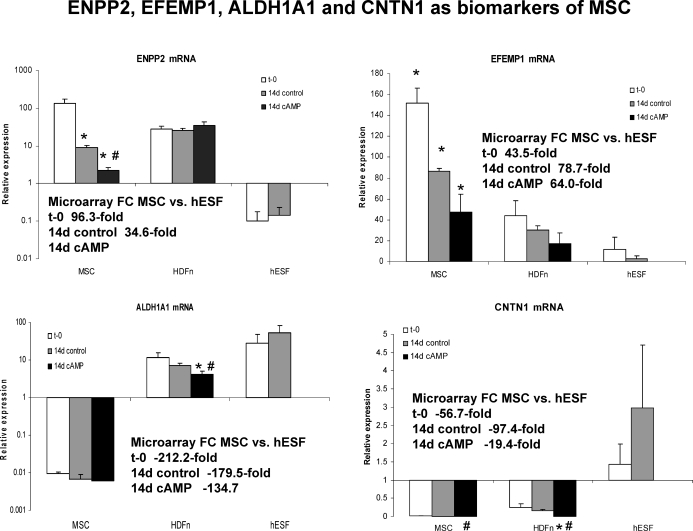
Of 19 492 genes on the Affymetrix Human Gene 1.0 ST arrays, 281 genes were different between untreated-uncultured MSC (t = 0) and untreated-uncultured hESF (t = 0). Based on this gene list (Supplemental Table S5), several genes have been identified as molecular markers that distinguish bone marrow-derived MSC from endometrial stromal fibroblasts (before any differentiation agents). The most highly up-regulated gene (96-fold) was ENPP2 (autotoxin, ectonucleotide pyrophosphatase/phosphodiesterase 2), which encodes a protein that functions as both a phosphodiesterase and phospholipase. FOXD4L1 (forkhead box D4-like 1) was up-regulated 78.7-fold in MSC versus hESF; it belongs to FOX gene family. EFEMP1 (epidermal growth factor [EGF]-containing fibulin-like extracellular matrix protein 1, fibulin 3), an antagonist of angiogenesis [35], was 43.5-fold up-regulated in MSC versus hESF at t = 0. It is a partner with TIMP3 (tissue inhibitor of metalloproteinases-3) [36], which was 4.2-fold up-regulated under the same conditions. Interestingly, several MMPs were significantly down-regulated in MSC versus hESF at t = 0, including MMP1, MMP3, MMP10, and MMP12, which were more than 30-fold down-regulated in MSC.
We found that 481 genes were different between untreated MSC cultured for 14 days (vehicle control) and untreated hESF cultured for 14 days (vehicle control) (unpublished gene list) (Supplemental Table S6). ENPP2, FOXD4L1, and EFEMP1 were 34.6-, 90.3-, and 78.8-fold, respectively, higher in cultured MSC versus hESF, indicating the sustained differences between MSC and hESF between t = 0 and 14 days. LOC391706 (ribosomal protein S3a pseudogene 18), ALDH1A1 (aldehyde dehydrogenase 1 family, member A1), CNTN1 (contactin 1, glycoprotein gP135), and WNT2 were the most (>390-, >179-, >56-, and >71-fold, respectively) down-regulated genes in MSC versus hESF in both comparisons.
Based on the above, we analyzed the mRNA expression of several potential markers distinguishing MSC from hESF and HDFn. As shown in Figure 6, ENPP2 and EFEMP1 mRNAs are highly expressed in undifferentiated, uncultured MSC, and their expression significantly decreases when MSC are differentiated with cAMP. Also, ENPP2 and EFEMP1 mRNA expression is very low in highly differentiated fibroblasts, such as hESF, and their expression in HDFn is modest and does not depend on culture conditions or cAMP. Similarly, ALDH1A1 and CNTN1 mRNA expression in MSC confirmed the microarray data and demonstrated that expression of these genes in undifferentiated, uncultured MSC is very low compared to that in more differentiated fibroblast cell types (Fig. 6). These data suggest that ENPP2, EFEMP1, ALDH1A1, and CNTN1 may be used as markers of multipotent bone marrow-derived MSC.
FIG. 6.
Relative expression of ENPP2, EFEMP1, ALDH1A1, and CNTN1 mRNA in MSC (t = 0 [t-0], 14-day control [14d control], and 14 days treated with cAMP [14d cAMP]), HDFn (t-0, 14d control, and 14d cAMP), and hESF (t-0 and 14d control). Inserts indicate the fold-change (FC) expression of genes in the microarray data set in the present study. The y-axis is presented as a log scale in the two graphs on the left. An asterisk indicates a statistically significant difference (P ≤ 0.05) compared to t-0 control for each gene and each cell type; a number symbol indicates a statistically significant difference (P ≤ 0.05) between vehicle control and cAMP-treated cells. Error bars represent the SEM.
DISCUSSION
Activation of the PKA Pathway, Cell Shape, and Decidualization Markers
The present study has demonstrated that PKA pathway activation in bone marrow-derived MSC results in 1) differentiation of this cell type; 2) expression of classical markers of decidualization, PRL and IGFBP1; 3) cell shape changes consistent with the decidualized endometrial stromal fibroblast (hESF) phenotype; and 4) expression of markers of differentiation down the hESF lineage in MSC in response to 8-Br-cAMP, as determined by a transcriptomic approach. Also, untreated MSC expressed mRNA for steroid hormone and gonadotropin receptors. Importantly, of all the different differentiation stimuli investigated, 8-Br-cAMP was the only agent able to stimulate the expression of decidualization markers in MSC and trigger the morphological transformation of these cells. In addition, we have discovered new markers of multipotent MSC that can distinguish them from hESF and, likely, other fibroblast cell types and enable tracking of the differentiation process down various lineages in vitro and in vivo.
As noted, treatment with 8-Br-cAMP resulted in the up-regulation of PRL and IGFBP1 decidualization marker mRNAs in MSC and secretion of the respective proteins. Pathway and network analyses demonstrated progression from activation of metabolism to differentiation in MSC upon culture and treatment with cAMP, accompanied by decidual morphologic transformation of MSC in vitro. Remarkably, comparison of the cAMP-regulated gene lists in MSC versus hESF demonstrated 20 genes in common, all of which are known to be involved in endometrial physiology [23, 34]. Thus, the data strongly support the idea that MSC can differentiate in response to cAMP down the endometrial stromal fibroblast lineage.
The question naturally arises about what stimulus of the PKA pathway initiates the responses observed herein with MSC. (In fact, the stimulus of activation of the PKA pathway is unclear in human endometrial tissue.) We have considered that differentiation of progenitor cells may begin in the menstrual/postmenstrual repair phase, which is the time of the rise of FSH in the circulation. For that reason, we treated MSC with different doses of recombinant human FSH. However, no effect on decidualization gene expression, similar to the cAMP effect, was observed. Nevertheless, FSH was able to stimulate FSH receptor mRNA and tended to stimulate LH receptor mRNA, although this was not statistically significant. One may speculate that differentiation starts in the previous cycle with the rise of FSH so that cells are fully differentiated by menstruation and the early proliferative phase, when they are needed for regeneration.
Steroid Hormone and Gonadotropin Receptor mRNA Expression
It was unanticipated that multipotent MSC express ESR, PGR, AR, FSHR, and LHCGR mRNAs, and to the best of our knowledge, this is the first report on the expression of reproductive hormone receptors in human bone marrow-derived MSC. It was reported previously that bone marrow-derived MSC express specific neural proteins before any differentiation [37, 38], indicating that undifferentiated MSC residing in bone marrow express a variety of markers and receptors, probably in anticipation of a stimulus to trigger their differentiation.
Do All Fibroblasts Differentiate Similarly in Response to Identical Stimuli?
Controversial data exist regarding the ability of mature fibroblasts to differentiate in vitro toward different lineages. Some authors report that skin fibroblasts possess stem/progenitor cell properties and that differentiation toward adipocytes or osteoblasts does not distinguish MSC from more mature fibroblasts [39]. Others, however, have demonstrated that human skin or gingival fibroblasts are unable to differentiate in osteogenic medium [40], in contrast to MSC, which do differentiate in this medium [11, 41, 42]. A study by Richards and Hartman [25] demonstrated that dermal fibroblasts express PRL, but not IGFBP1, after prolonged treatment with dibutyryl-cAMP. Cervical fibroblasts decidualize after cAMP plus P4 treatment for 6 and 10 days [43], and fibroblasts from term human decidua closely resemble endometrial stromal cells by induction of PRL and IGFBP1 expression [44]. However, decidual fibroblasts may be considered to have properties similar to those of hESF because of their “endometrial niche.”
Herein, we demonstrated that human neonatal dermal fibroblasts are not capable of comprehensive response to 8-Br-cAMP treatment compared to hESF, demonstrating that fully matured fibroblasts are probably restricted in their differentiation ability. This also makes us confident that the potential to differentiate down the endometrial-stromal lineage observed in cAMP-treated MSC is specific to the multipotent bone-marrow derived MSC.
MSC and the Endometrial Niche
The hypothesis that circulating bone marrow-derived MSC are a source of endometrial regeneration is to be considered. Hypothetically, the arrival of circulating MSC to different tissues may be driven by a combination of different chemoattractants and, thereafter, reside in a specific tissue, where they differentiate as needed. Final differentiation toward different lineages may be a result of the niche to which the MSC have migrated and settled. Thus, the niche may ultimately determine the lineage down which the MSC differentiate. Siddappa et al. [45] reported that activation of the PKA pathway by dibutyryl-cAMP in osteogenic medium resulted in increased expression of osteogenic markers and enhanced in vivo bone formation by bone marrow-derived MSC from orthopedic patients. With regard to endometrial stromal fibroblasts, we postulate that they may be derived/repopulated from bone marrow-derived circulating MSC, with the latter potentially differentiating upon arrival to endometrium in response to local stimuli and in the appropriate contextual niche. The longer time required to observe the decidualization response in MSC may result from the fact that MSC in their undifferentiated, naïve state require more time to acquire the endometrial phenotype compared to endometrial-stromal fibroblasts, which are already “assigned” a specific pheno- and genotype in endometrial niche.
The question arises whether sufficient numbers of circulating MSC and cells exiting from the bone marrow are available to regenerate and maintain the endometrium on a cyclic basis. It is of interest that in the study by Bian et al. [46], the percentage of circulating MSC (CD105+, CD34−, and CD45−) in control subjects was 0.0028 ± 0.0016 (mean ± SEM). Similarly, Mansilla et al. [47] demonstrated that in healthy donors, 0.0078% ± 0.0044% (mean ± SEM) of circulating cells are MSC. Furthermore, those studies demonstrated that the number of circulating MSC increases significantly during acute large skin damage (e.g., burns), with bone sarcomas, and with ischemic heart disease [48]. However, no information is available regarding the sufficient numbers of MSC needed to regenerate endometrium each month. Pelliccia et al. [49] showed increased numbers of subpopulations of circulating endothelial progenitor cells in normal postmenopausal women compared to men, whereas Herrmann et al. [50] showed that levels of circulating endothelial progenitor cells are greater in premenopausal women compared to postmenopausal women. Almost all of these studies were performed to determine the basis of cardioprotection in women compared to men and in premenopausal versus postmenopausal women. Whether these results may be applied to the numbers of MSC circulating with the purpose of endometrial regeneration is not known. One may assume that the numbers of circulating MSC would be higher in premenopausal women, particularly at the end of the menstrual cycle. Nevertheless, a quantitative study on the numbers of MSC in the circulation, doubling times in situ in the endometrium, and rates of steroid hormone-dependent differentiation would be needed to attempt to assess whether sufficient numbers of circulating MSC are available to regenerate and maintain endometrial stroma on a cyclic basis.
Unique MSC Biomarkers
The use of markers that will help to distinguish human bone marrow-derived MSC from fibroblasts of any origin, and from hESF in particular, is of value to understand the development and differentiation/regeneration processes of a variety of tissues. Several genes identified herein may serve as potential markers to distinguish MSC from hESF. Ishii et al. [40] showed that several markers differ between MSC and fibroblasts, such as higher expression of tissue factor pathway inhibitor-2, major histocompatibility complex-DR-alpha, major histocompatibility complex-DR-beta, and neuroserpin as well as lower expression of adrenomedullin, apolipoprotein D, C-type lectin superfamily member-2, collagen type XV alpha1, CUG triplet repeat RNA-binding protein, matrix metalloproteinase-1, protein tyrosine kinase-7, and Sam68-like phosphotyrosine protein/T-STAR. None of these genes, except for neuroserpin, MMP1, and protein tyrosine kinase-7, were regulated in the present study when comparing untreated-uncultured (t = 0) MSC and hESF or vehicle-treated cultured cells, suggesting that these genes are not as valuable in comparing MSC with hESF, as they are in other systems (e.g., periodontal ligament cells or human skin and gingival fibroblasts [40, 51]). Wagner et al. [52] demonstrated that ALDH1A1 transcript was down-regulated in human bone marrow-derived MSC versus human newborn foreskin fibroblasts, in agreement with our results. Shahdadfar et al. [53] identified ENPP2 as a human MSC-specific marker. However, we were not able to find data regarding the expression of EFEMP1 and CNTN1 in MSC versus other fibroblast sources. Herein, we suggest that high expression of ENPP2 (prevents apoptosis [54]) and EFEMP1, as well as low or no expression of ALDH1A1 (data are similar to human limbal stem cells [55]) and CNTN1 (neural adhesion molecule and neuromuscular junction protein [56]), mRNA are characteristic for multipotent MSC and will aid in distinguishing MSC from endometrial stromal fibroblasts, in particular in studies with endometrial stem/progenitor cells. The level of expression of these markers in stem/progenitor cells isolated from endometrium is currently under investigation in our laboratory.
In summary, marked up-regulation of decidualization markers and changes in cell shape of MSC upon cAMP treatment support the hypothesis that bone marrow-derived MSC may be a potential source of endometrial stem/progenitor cells. Identification of MSC-specific markers distinguishing them from other fibroblasts and, in particular, from endometrial stromal cells is of biologic relevance and practical value to the field of endometrial stem cell research.
NOTE ADDED IN PROOF
Additional information regarding hierarchical cluster analysis of gene expression can be found in an article by Eisen et al., 1988 [57].
Footnotes
Supported by the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreement U54 HD 055764-03 (to L.C.G.) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and the California Institute for Regenerative Medicine SEED grant RS1-00207-1 (to L.C.G.).
REFERENCES
- Hess A, Nayak N, Giudice LC.Oviduct and endometrium: cyclic changes in primate oviduct and endometrium. Knobil E, Neill JD.The Physiology of Reproduction, 3rd ed San Diego:Academic Press;2005: 337–381. [Google Scholar]
- Chan RW, Schwab KE, Gargett CE.Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod 2004; 70: 1738–1750. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D.Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod 2009; 80: 1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI.Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007; 213: 341–347. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008; 3: 301–313. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E.Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A.Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy 2005; 7: 393–395. [DOI] [PubMed] [Google Scholar]
- Wolff EF, Wolff AB, Hongling D, Taylor HS.Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod Sci 2007; 14: 524–533. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Gargett CE.Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod 2007; 22: 2903–2911. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV.Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology 1994; 134: 277–286. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Martin BJ.Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res 2004; 95: 9–20. [DOI] [PubMed] [Google Scholar]
- Cho KJ, Trzaska KA, Greco SJ, McArdle J, Wang FS, Ye JH, Rameshwar P.Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin-1 alpha. Stem Cells 2005; 23: 383–391. [DOI] [PubMed] [Google Scholar]
- Taylor HS.Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 2004; 292: 81–85. [DOI] [PubMed] [Google Scholar]
- Du H, Taylor HS.Stem cells and female reproduction. Reprod Sci 2009; 16: 126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Taylor HS.Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 2007; 25: 2082–2086. [DOI] [PubMed] [Google Scholar]
- Bratincsak A, Brownstein MJ, Cassiani-Ingoni R, Pastorino S, Szalayova I, Toth ZE, Key S, Nemeth K, Pickel J, Mezey E.CD45-positive blood cells give rise to uterine epithelial cells in mice. Stem Cells 2007; 25: 2820–2826. [DOI] [PubMed] [Google Scholar]
- Ruiz ME, Freeman J, Bouhasin JD, Knutsen AP, Hendrix MJ.Arrest of in vitro T cell differentiation of normal bone marrow-derived CD34+ stem cells with thymic epithelial fragments from children with AIDS. Stem Cells 1996; 14: 533–547. [DOI] [PubMed] [Google Scholar]
- Lysy PA, Smets F, Najimi M, Sokal EM.Leukemia inhibitory factor contributes to hepatocyte-like differentiation of human bone marrow mesenchymal stem cells. Differentiation 2008; 76: 1057–1067. [DOI] [PubMed] [Google Scholar]
- Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I, Muceniece R, Ancans J.Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev Rep 2009; (in press). Published online ahead of print September 22, 2009. [DOI] [PubMed]
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC.Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod 2009; 80: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Velarde MC, Giudice LC.The progesterone receptor co-activator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology 2009; 150: 3863–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC.Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab 2006; 91: 1453–1461. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Horcajadas JA, Weeks JL, Esteban FJ, Nezhat C, Conti M, Giudice LC.The PKA pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis Endocrinology 2010; 151: 1341–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliffe IT, Morgan BJ.Principal component analysis and exploratory factor analysis. Stat Methods Med Res 1992; 1: 69–95. [DOI] [PubMed] [Google Scholar]
- Richards RG, Hartman SM.Human dermal fibroblast cells express prolactin in vitro. J Invest Dermatol 1996; 106: 1250–1255. [DOI] [PubMed] [Google Scholar]
- La Marca A, Carducci Artenisio A, Stabile G, Rivasi F, Volpe A.Evidence for cycle-dependent expression of follicle-stimulating hormone receptor in human endometrium. Gynecol Endocrinol 2005; 21: 303–306. [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura K, Imai K, Liao S, Mori T.Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Hum Reprod 1992; 7: 1461–1466. [DOI] [PubMed] [Google Scholar]
- Apparao KB, Lovely LP, Gui Y, Lininger RA, Lessey BA.Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod 2002; 66: 297–304. [DOI] [PubMed] [Google Scholar]
- Bukovsky A, Cekanova M, Caudle MR, Wimalasena J, Foster JS, Henley DC, Elder RF.Expression and localization of estrogen receptor-alpha protein in normal and abnormal term placentae and stimulation of trophoblast differentiation by estradiol. Reprod Biol Endocrinol 2003; 1: 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney EP, Tulac S, Huang ST, Giudice LC.Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics 2003; 16: 47–66. [DOI] [PubMed] [Google Scholar]
- Xiao LJ, Yuan JX, Song XX, Li YC, Hu ZY, Liu YX.Expression and regulation of stanniocalcin 1 and 2 in rat uterus during embryo implantation and decidualization. Reproduction 2006; 131: 1137–1149. [DOI] [PubMed] [Google Scholar]
- Illei G, Morgan DM.The distribution of polyamine oxidase activity in the fetomaternal compartments. Br J Obstet Gynaecol 1979; 86: 873–877. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Meredith D.The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 2004; 447: 619–628. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC.Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006; 147: 1097–1121. [DOI] [PubMed] [Google Scholar]
- Albig AR, Schiemann WP.Fibulin-5 function during tumorigenesis. Future Oncol 2005; 1: 23–35. [DOI] [PubMed] [Google Scholar]
- Klenotic PA, Munier FL, Marmorstein LY, Anand-Apte B.Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1). Implications for macular degenerations. J Biol Chem 2004; 279: 30469–30473. [DOI] [PubMed] [Google Scholar]
- Tondreau T, Lagneaux L, Dejeneffe M, Massy M, Mortier C, Delforge A, Bron D.Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation 2004; 72: 319–326. [DOI] [PubMed] [Google Scholar]
- Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED.Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells 2006; 24: 1054–1064. [DOI] [PubMed] [Google Scholar]
- Haniffa MA, Collin MP, Buckley CD, Dazzi F.Mesenchymal stem cells: the fibroblasts' new clothes? Haematologica 2009; 94: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Koike C, Igarashi A, Yamanaka K, Pan H, Higashi Y, Kawaguchi H, Sugiyama M, Kamata N, Iwata T, Matsubara T, Nakamura K, et al. Molecular markers distinguish bone marrow mesenchymal stem cells from fibroblasts. Biochem Biophys Res Commun 2005; 332: 297–303. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM.Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol 2002; 30: 896–904. [DOI] [PubMed] [Google Scholar]
- Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ.Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A 1995; 92: 4857–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S, Calder AA, Kelly RW.Decidualization of cervical stromal cells. Eur J Obstet Gynecol Reprod Biol 2004; 114: 189–196. [DOI] [PubMed] [Google Scholar]
- Richards RG, Brar AK, Frank GR, Hartman SM, Jikihara H.Fibroblast cells from term human decidua closely resemble endometrial stromal cells: induction of prolactin and insulin-like growth factor binding protein-1 expression. Biol Reprod 1995; 52: 609–615. [DOI] [PubMed] [Google Scholar]
- Siddappa R, Martens A, Doorn J, Leusink A, Olivo C, Licht R, van Rijn L, Gaspar C, Fodde R, Janssen F, van Blitterswijk C, de Boer J.cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci U S A 2008; 105: 7281–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian ZY, Li G, Gan YK, Hao YQ, Xu WT, Tang TT.Increased number of mesenchymal stem cell-like cells in peripheral blood of patients with bone sarcomas. Arch Med Res 2009; 40: 163–168. [DOI] [PubMed] [Google Scholar]
- Mansilla E, Marin GH, Drago H, Sturla F, Salas E, Gardiner C, Bossi S, Lamonega R, Guzman A, Nunez A, Gil MA, Piccinelli G, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc 2006; 38: 967–969. [DOI] [PubMed] [Google Scholar]
- Ripa RS, Wang Y, Goetze JP, Jorgensen E, Johnsen HE, Tagil K, Hesse B, Kastrup J.Circulating angiogenic cytokines and stem cells in patients with severe chronic ischemic heart disease—indicators of myocardial ischemic burden? Int J Cardiol 2007; 120: 181–187. [DOI] [PubMed] [Google Scholar]
- Pelliccia F, Pasceri V, Cianfrocca C, Vitale C, Meoni G, Pristipino C, Speciale G, Mercuro G, Rosano G.Circulating endothelial progenitor cells in postmenopausal women with and without coronary artery disease. Climacteric 2009; 12: 259–265. [DOI] [PubMed] [Google Scholar]
- Herrmann JL, Abarbanell AM, Weil BR, Manukyan MC, Poynter JA, Wang Y, Coffey AC, Meldrum DR.Gender dimorphisms in progenitor and stem cell function in cardiovascular disease. J Cardiovasc Trans Res 2010; 3: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Iwata T, Shiba H, Igarashi A, Hirata R, Takeda K, Mizuno N, Tsuji K, Kawaguchi H, Kato Y, Kurihara H.Identification of marker genes distinguishing human periodontal ligament cells from human mesenchymal stem cells and human gingival fibroblasts. J Periodontal Res 2007; 42: 283–286. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD.Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005; 33: 1402–1416. [DOI] [PubMed] [Google Scholar]
- Shahdadfar A, Fronsdal K, Haug T, Reinholt FP, Brinchmann JE.In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 2005; 23: 1357–1366. [DOI] [PubMed] [Google Scholar]
- Song J, Clair T, Noh JH, Eun JW, Ryu SY, Lee SN, Ahn YM, Kim SY, Lee SH, Park WS, Yoo NJ, Lee JY, Nam SW.Autotaxin (lysoPLD/NPP2) protects fibroblasts from apoptosis through its enzymatic product, lysophosphatidic acid, utilizing albumin-bound substrate. Biochem Biophys Res Commun 2005; 337: 967–975. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Kolli S, Li DQ, de Paiva CS, Pryzborski S, Dimmick I, Armstrong L, Figueiredo FC, Lako M.A putative role for RHAMM/HMMR as a negative marker of stem cell-containing population of human limbal epithelial cells. Stem Cells 2008; 26: 1609–1619. [DOI] [PubMed] [Google Scholar]
- Compton AG, Albrecht DE, Seto JT, Cooper ST, Ilkovski B, Jones KJ, Challis D, Mowat D, Ranscht B, Bahlo M, Froehner SC, North KN.Mutations in contactin-1, a neural adhesion and neuromuscular junction protein, cause a familial form of lethal congenital myopathy. Am J Hum Genet 2008; 83: 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D.Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 1998; 95: 14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]