Abstract
Continual spermatogenesis relies on a pool of spermatogonial stem cells (SSCs) that possess the capacity for self-renewal and differentiation. Maintenance of this pool depends on survival of SSCs throughout the lifetime of a male. Response to extrinsic stimulation from glial cell line-derived neurotrophic factor (GDNF), mediated by the PIK3/AKT signaling cascade, is a key pathway of SSC survival. In this study, we found that expression of the POU domain transcription factor POU3F1 in cultured SSCs is up-regulated via this mechanism. Reduction of Pou3f1 gene expression by short interfering RNA (siRNA) treatment induced apoptosis in cultured germ cell populations, and transplantation analyses revealed impaired SSC maintenance in vitro. POU3F1 expression was localized to spermatogonia in cross-sections of prepubertal and adult testes, implying a similar role in vivo. Through comparative analyses, we found that expression of POU5F1, another POU transcription factor implicated as essential for SSC self-renewal, is not regulated by GDNF in cultured SSCs. Transplantation analyses following siRNA treatment showed that POU5F1 expression is not essential for SSC maintenance in vitro. Additionally, expression of NODAL, a putative autocrine regulator of POU5F1 expression in mouse germ cells, could not be detected in SSCs isolated from testes or cultured SSCs. Collectively, these results indicate that POU3F1, but not POU5F1, is an intrinsic regulator of GDNF-induced survival and self-renewal of mouse SSCs.
Keywords: GDNF, POU3F1, POU5F1, self-renewal, spermatogonial stem cell
Reduction of POU3F1 expression in mouse SSCs impairs their self-renewal and survival in vitro, whereas similar reduction of POU5F1 expression has no effect.
INTRODUCTION
Continual spermatogenesis from puberty until old age in males relies on maintenance of a pool of spermatogonial stem cells (SSCs). Similar to other tissue-specific stem cell populations, SSCs maintain tissue homeostasis by retaining the capacity for self-renewal and differentiation. In general, SSC fate decisions are controlled by intrinsic molecular pathways activated by extrinsic signals such as growth factor stimuli. Underlying stem cell fate decisions is cell survival; thus, growth factor stimulation must also activate antiapoptotic pathways. The self-renewal and survival of rodent SSCs, including mouse [1], rat [2, 3], and hamster [4], depend on response to the growth factor glial cell line-derived neurotrophic factor (GDNF). Maintenance of SSCs in vitro for extended periods requires GDNF stimulation of self-renewal and induction of cell survival. Several studies [5–7] have examined the self-renewal mechanism stimulated by GNDF, but understanding of the intrinsic survival pathway is limited.
Rarity of SSCs in the testis and lack of specific markers to unequivocally identify these cells present major challenges for studying survival mechanisms in vivo. Fortunately, long-term in vitro culture methods that support self-renewal and differentiation of rodent SSCs and a robust transplantation assay to unequivocally quantify SSCs based on their functional ability to reestablish spermatogenesis are available [8]. In mice, culture of THY1+ germ cells in serum-free medium conditions with GDNF and fibroblast growth factor 2 (FGF2) supplementation supports survival and expansion of SSC numbers for extended periods [1]. Importantly, these cultures consist of SSCs and non-stem cells that are likely spermatogonia produced by differentiation given that expression of spermatogonial markers is homogeneous within the cell population [1]. Thus, these culture conditions support self-renewal, differentiation, and survival of mouse SSCs, providing an important in vitro model system to study mechanisms regulating SSC biology. However, because of the heterogeneous nature of the cultured cell populations, experimental treatments must be coupled with transplantation assays to unequivocally draw conclusions about effects on SSCs. Using these two approaches, major advances in knowledge of SSC biology can be made, and specific factors can be intensively examined for importance in promoting SSC functions.
The octamer (OCT)-binding family of transcription factors, also referred to as POU domain transcription factors, has diverse roles in cellular processes and stem cell functions, and individual members display a spatiotemporal expression pattern. In embryonic stem (ES) cells, maintenance of pluripotency and self-renewal relies on expression of one such member, POU5F1 [9–11]. Global knockout of this molecule causes embryonic lethality in mice [9], and selective disruption in primordial germ cells causes germ cell death during embryonic development [12]. Thus, POU5F1 has been regarded as an essential regulator of germ cell survival. Additionally, recent findings suggest that this molecule has an important role in regulating self-renewal of mouse SSCs [13]. Our previous investigations identified GDNF-regulated genes within cultured mouse THY1+ germ cells enriched for SSCs [5]. Mining of that database for OCT transcription factors revealed that expression of POU3F1, also referred to as TST-1 or OCT6, is influenced by GDNF stimulation. In comparison, the expression of POU5F1 is not regulated by exposure to GDNF in cultured mouse THY1+ germ cells [5, 6]. Pou3f1 gene expression was first identified in the testis [14] and has been examined as a regulator of neural cell development [15–17]. Targeted disruption of POU3F1 expression in mice causes neonatal lethality in which pups display abnormal myelination of the axon sheath [17]. While POU5F1 expression and function have been investigated in the male germline, similar analysis of POU3F1 has not been conducted to date. The objective of this study was to determine whether POU3F1 has an important role in SSC self-renewal and survival.
MATERIALS AND METHODS
Animals and Reagents
Donor mice used for establishment of THY1+ germ cell cultures were B6.129S7-Gtrosa26 (designated Rosa; The Jackson Laboratory, Bar Harbor, ME). Most cells, including all germ cells, in this strain of mouse express the LacZ marker transgene, making reestablished spermatogenesis after transplantation clearly identifiable. Recipient mice used for transplantation assays were F1 progeny from mating of 129SvCP × C57BL/6 that were treated with busulfan (60 mg/kg of body weight) at least 6 wk before transplantation to deplete endogenous germ cells. Donor mice used for immunohistochemistry analysis of POU3F1 and POU5F1 expression were inbred C57BL/6. All animal procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Inhibition of PIK3 and AKT function was achieved by treatment with 10 μM LY294002 (Cell Signaling Technologies, Danvers, MA) and 20 nM AKT Inhibitor IV (Calbiochem, San Diego, CA), respectively.
Apoptosis Assays
Determination of the percentage of apoptotic cells in cultured THY1+ germ cells treated with Pou3f1 short interfering RNA (siRNA) was conducted using a Nexin Apoptosis labeling kit (Guava Technologies, Hayward, CA) and flow cytometric analysis. Briefly, 2 × 105 THY1+ germ cells were seeded per well in a 12-well plate format and transfected with Pou3f1 or control siRNA oligonucleotides as described herein. Cells were then harvested by trypsin-edetic acid (EDTA) digestion at 30 h after transfection and incubated with Annexin V-PE (Guava Technologies) to label apoptotic cells according to the manufacturer's instructions. The percentage of apoptotic cells was then quantified using a Guava Technologies PCA personal cytometer.
ES Cell Cultures
The ES cells were acquired from Dr. Alan Bradley (AB1 mouse ES cell line). Cells were cultured on mitomycin C-treated SNL feeders (leukemia inhibitory factor-secreting STO cells) and maintained in Dulbecco modified Eagle medium containing 15% ES cell certified fetal bovine serum, 2 mM glutamine, 1 × 104 U/ml of penicillin, 1 × 104 μg/ml of streptomycin, and 100 μM mercaptoethanol.
Fluorescent Immunocytochemistry of Cultured THY1+ Germ Cells
Expression of POU3F1 and POU5F1 by cultured THY1+ germ cell clumps was conducted as described previously [6]. Primary antibodies were goat anti-human POU5F1 polyclonal (1:100 dilution; R&D Systems Inc., Minneapolis, MN) and goat anti-human POU3F1 polyclonal (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). Secondary detection included incubation with Alexa 488-conjugated rabbit anti-goat IgG polyclonal antibody (Invitrogen Inc., Carlsbad, CA).
Immunohistochemistry of Prepubertal and Adult Mouse Testis Cross-Sections
Expression of POU3F1 and POU5F1 in the male mouse germline was examined using immunohistochemistry analysis of testis cross-sections. Testes were excised and fixed in 4% paraformaldehyde overnight at 4°C. Samples were then dehydrated and embedded in paraffin, and 6-μm sections were created and adhered to glass slides. Antigen retrieval involved boiling sections in sodium citrate buffer (10 mM sodium citrate [pH 6.0]) for 15 min. Endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide for 10 min. Sections were then blocked in 10% normal rabbit serum, followed by incubation with goat anti-human POU5F1 polyclonal (1:100 dilution; R&D Systems Inc.) or goat anti-human POU3F1 polyclonal (1:100 dilution; Santa Cruz Biotechnology) primary antibody overnight at 4°C. On the next day, sections were washed three times in PBS, followed by incubation with biotinylated rabbit anti-goat polyclonal antibody (1:200 dilution) for 1 h at room temperature. Sections were again washed three times in PBS and stained with a streptavidin-horseradish peroxidase detection kit (Zymed Laboratories Inc.; Invitrogen, Carlsbad, CA). Hematoxylin counterstaining was conducted to label cell nuclei, and sections were examined using light microscopy. Digital images were captured with a Spot 2 MP Firewire Color Mosaic Digital Microscope Camera (Diagnostic Instruments Inc., Sterling Heights, MI).
Quantitative RT-PCR Analyses
Relative quantifications of gene expression in cultured THY1+ germ cells and ES cells were conducted using quantitative real-time PCR analysis. RNA samples were isolated using Trizol reagent (Invitrogen) and treated with DNase I to remove possible contaminating genomic DNA. Purity of RNA samples was determined based on spectrophotometric determination of the 260:280 ratio, and only samples with a value of 1.8 or higher were used for analysis. For each sample, 1 μg of mRNA was reverse transcribed using oligo(d)T priming and Moloney murine leukemia virus RT. Quality of resulting cDNA was determined by conventional PCR analysis for glyceraldehyde 3-phosphate dehydrogenase (Gapdh) expression and agarose gel electrophoresis. To determine relative expression of Pou3f1 and Pou5f1 gene expression, SYBR green assays were conducted using an ABI 7300 sequence detection system (ABI, Foster City, CA). To make quantitative comparisons, relative expression of specific genes of interest was normalized to that of ribosomal protein S2 (Rps2) as described previously [6]. To determine expression of Nodal, RNA was isolated from ES cells, cultured THY1+ germ cells, and freshly isolated THY1+ germ cells using Trizol reagent (Invitrogen), and 1 μg of RNA was reverse transcribed using oligo(d)T priming. Possible genomic DNA contamination was removed by treatment with DNase I, and PCR products were visualized by agarose gel electrophoresis. All primers were designed using Primer Express 3 software (ABI) and are listed in Table 1.
TABLE 1.
Sequences of primers used for RT-PCR analyses.
siRNA Treatments
Transient reduction of Pou3f1 and Pou5f1 expression in cultured THY1+ germ cells was achieved by transfection with siRNA oligonucleotides as described previously [5, 6]. Briefly, cultured THY1+ germ cell clumps were separated from feeder cells using gentle pipetting, which yields greater than 90% pure germ cell suspensions [5]. Single-cell suspensions were generated by digestion with trypsin-EDTA, and 2–3×105 cells were plated per well in a 12-well plate format. Cells were then transfected with 75 pmol of gene-specific or nontargeting control siRNA oligonucleotides using Lipofectamine 2000 (Invitrogen). For Pou3f1 siRNA, predesigned single-gene-specific and nontargeting control oligonucleotides were purchased from Ambion Inc. (Carlsbad, CA). For Pou5f1 siRNA, pooled gene-specific and nontargeting oligonucleotides were purchased from Dharmacon Inc. (Chicago, IL). For all transplantation experiments, cells were transfected for 18 h, followed by replacement of serum-free medium (mSFM; [1]).
SSC Cultures
Primary cultures of THY1+ germ cells were established from prepubertal 6-day-postpartum (dpp) Rosa donor males as described previously [8]. Briefly, THY1+ germ cells were isolated from donor testes by magnetic activated cell sorting using mouse anti-rat THY1 monoclonal antibody conjugated to magnetic microbeads (Miltenyi Biotec Inc., Auburn, CA). Cells were plated in a 12-well format on monolayers of SNL-STO feeder cells rendered mitotically inactive by treatment with mitomycin C. Cultures were maintained in mSFM and supplemented with 20 ng/ml of recombinant human GDNF (R&D Systems Inc.) and 1 ng/ml of recombinant human FGF2 (BD Biosciences, San Jose, CA). In these conditions, germ cells form clumps that consist of both SSCs and non-stem cell spermatogonia, and SSC self-renewal is supported for greater than 6 mo.
SSC Transplantation Assays
Stem cells are defined by their capacity to reestablish functionality of the tissue from which they derive. For SSCs, this ability is reestablishment of spermatogenesis after transplantation into a recipient testis [18, 19]. Thus, transplantation experiments were conducted to examine unequivocally the maintenance of SSCs in cultures of THY1+ germ cells as described previously [8]. Seven days after transfection with Pou3f1, Pou5f1, or control siRNA, total cultured cell populations were collected by trypsin-EDTA digestion, followed by filtering through a 40-μm cell strainer. Resulting single-cell suspensions were resuspended in mSFM at a standard concentration of 1 × 106 cells/ml, and 10 μl of cell suspension (10 000 cells) was microinjected into each recipient testis. For all experiments, three to four recipients (six to eight testes) were transplanted with each replicate cell suspension. Approximately 2 mo after transplantation, recipient testes were collected and incubated with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) to stain colonies of spermatogenesis derived from transplanted Rosa donor SSCs. Each donor colony is clonally derived from an individual SSC present in the injected donor cell suspension [20]. Thus, the number of colonies was counted to determine relative SSC numbers in transplanted cultured cell populations treated with different siRNAs using the following formula: SSC Number / 105 Thy1+ Cells Cultured = [Number of Donor-Derived Colonies of Spermatogenesis × (105 Total Cells Harvested / 105 Cells Transplanted)] × [1 / 105 Thy1+ Cells Originally Cultured].
Statistical Analysis
All data are presented as the mean ± SEM for three replicate experiments. Differences between means for all treatments were determined using the general linear model univariate ANOVA function of SPSS statistical software (Chicago, IL).
Western Blot Analyses
Cells were lysed at 4°C in RIPA buffer (1% Triton X-100, 50 mM Tris-HCl, 135 mM NaCl, 0.1% sodium deoxycholate, 2 mM EDTA, 50 mM NaF, 2 mM sodium orthovanadate, 10 μg/ml of aprotinin, 10 μg/ml of leupeptin, and 1 mM phenylmethylsulfonyl fluoride). Protein lysate (20 μg) was separated on 12% SDS-PAGE gels and blotted onto nitrocellulose membrane. Blots were blocked in 5% dry milk powder dissolved in PBS, followed by incubation with anti-POU3F1 (1:500, C-20; Santa Cruz Biotechnology), anti-POU5F1 (1:500, AF1759; R&D Systems Inc.), or anti-actin (1:5000; Sigma-Aldrich Inc., St. Louis, MO) primary antibodies. Blots were then incubated with horseradish peroxidase-conjugated secondary antibodies (1:2000) and developed with ECL substrate (Pierce; Rockford, IL).
RESULTS
Pou3f1, but Not Pou5f1, Gene Expression in Cultured THY1+ Germ Cells Is Regulated by GDNF
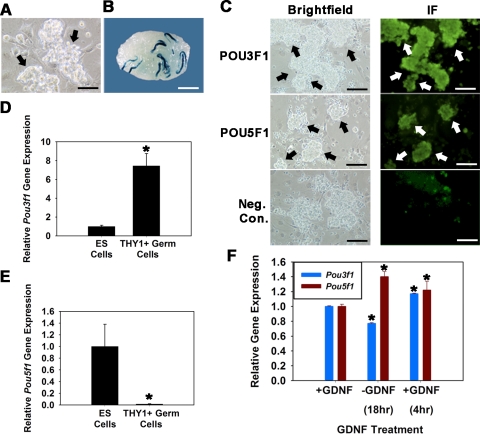
In a previous study [5], we used microarray analysis to examine GDNF-regulated gene expression in cultured THY1+ germ cells. Mining of that database revealed regulation of Pou3f1, but not Pou5f1, expression. Herein, we confirmed and expanded on those findings. Cultures of THY1+ germ cells were established from 6-dpp LacZ-expressing Rosa donor mice (Fig. 1A), and the presence of SSCs was confirmed by functional transplantation (Fig. 1B). Expression of both POU3F1 and POU5F1 proteins by cultured THY1+ germ cells was confirmed using fluorescent immunocytochemistry (Fig. 1C). In ES cells, the level of POU5F1 expression is critical for maintenance of pluripotency. In contrast, POU3F1 expression is reduced compared with POU5F1 in ES cells and is dispensable for pluripotency but has a major role in tissue-specific function [16]. Comparison of cultured THY1+ germ cells with ES cells revealed that the level of Pou3f1 gene expression in ES cells is 13.3% (n = 3 different cultures, P = 0.01) of that in THY1+ germ cells (Fig. 1D). In contrast, Pou5f1 gene expression in THY1+ germ cells is significantly lower, expressed at only 1.4% (n = 3 different cultures, P = 0.01) of that in ES cells (Fig. 1E). Next, the effect of GDNF exposure on expression of Pou3f1 and Pou5f1 was examined in cultured THY1+ germ cells. Upon withdrawal of GDNF from serum-free culture medium, Pou3f1 expression declined by 23% (n = 3 different cultures) and was up-regulated by 34% upon replacement of GDNF for 4 h (Fig. 1F). In comparison, expression of Pou5f1 increased by 29% upon GDNF withdrawal and was not up-regulated by GDNF stimulation. Because exposure to GDNF is essential for long-term maintenance of SSCs within THY1+ germ cell cultures, these results suggested that POU3F1 is an important intrinsic regulator of SSC survival and self-renewal.
FIG. 1.
Expression of POU3F1 and POU5F1 in cultured mouse THY1+ germ cells. A) Germ cell clumps (arrows) in established cultures of THY1+ germ cells from Rosa donor mouse pups. Bar = 100 μm. B) Recipient mouse testis transplanted with cultured THY1+ germ cells established from Rosa donor mouse pups. Each blue colony of donor spermatogenesis is derived from a single SSC present in the injected cell suspension. Donor spermatogenesis unequivocally demonstrates the presence of SSCs in the cultured cell population. Bar = 2 mm. C) Immunofluorescence analyses of POU3F1 and POU5F1 expression in cultured THY1+ germ cell clumps established from Rosa donor mouse pups. All germ cell clumps (arrows) stain for expression of both molecules. Bars = 100 μm. D and E) Relative Pou3f1 (D) and Pou5f1 (E) gene expression in cultured ES cells and THY1+ germ cells determined using quantitative real-time PCR analysis. Data are the mean ± SEM for three different replicate cultures, and the asterisk denotes significant difference between means. F) Effect of GDNF withdrawal and replacement on expression of Pou3f1 and Pou5f1 in cultured THY1+ germ cells established from Rosa donor mouse pups. Cultures were maintained with GDNF supplementation (+GDNF) and subjected to 18-h withdrawal of the growth factor (−GDNF), followed by replacement for 4 h (+GDNF). Removal of GDNF resulted in down-regulation of Pou3f1 gene expression (blue bars) but up-regulation of Pou5f1 gene expression (red bars). Data are the mean ± SEM for three different replicate cultures, and the asterisk denotes significant difference at P < 0.05 from +GDNF before withdrawal.
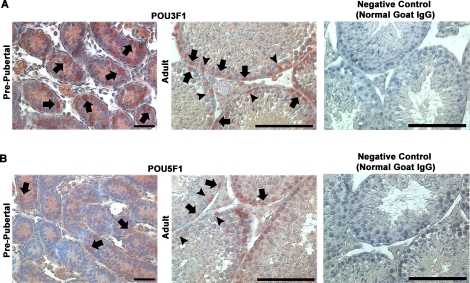
Expression of POU3F1 Is Localized to Spermatogonia in Both Prepubertal and Adult Mouse Testes
Previous investigations identified Pou3f1 transcript expression in the adult mouse testis [14]. However, expression was not localized to specific cell types within the seminiferous epithelium. To address this issue, we examined POU3F1 expression in cross-sections of prepubertal and adult mouse testes using immunohistochemistry. These analyses revealed that expression of POU3F1 is found among spermatogonia in both 6-dpp prepubertal pup testes and 2-mo-old adult mouse testes (Fig. 2A). Similarly, POU5F1 expression was also found in spermatogonia in both prepubertal and adult mouse testes (Fig. 2B). The pattern of POU3F1 and POU5F1 expression in the male germline was nearly identical. Surprisingly, both POU3F1 and POU5F1 staining was also found in Sertoli cell nuclei in adult mouse testes. These cells were clearly identifiable based on prominent nucleoli staining, which is a defining characteristic of Sertoli cells. This unexpected observation was not the result of nonspecific staining from immunohistochemistry procedures because similar staining was not found in negative controls incubated with normal IgG in place of primary antibody (Fig. 2). Clearly, expression of POU3F1 in the germline was restricted to spermatogonia. Collectively, these results demonstrate that (similar to POU5F1) POU3F1 is a spermatogonially expressed gene that may have an important function in regulating SSC functions.
FIG. 2.
Localization of POU3F1 and POU5F1 expression in the male mouse germline. Using immunohistochemistry, the expression of both POU3F1 (A) and POU5F1 (B) was examined in cross-sections of 6-dpp prepubertal pup and 2-mo-old adult mouse testes. Among germ cells, staining was observed in spermatogonia (arrows) in pup testes. In adults, expression was observed in the nuclei of both spermatogonia (arrows) and Sertoli cells (arrowheads). In the germline, POU3F1 and POU5F1 staining was only present in spermatogonia in testes of both pup and adult mice. Spermatogonia are identified based on a nuclear morphology that is flattened along the basement membrane, and Sertoli cells are identified based on distinctive staining of nucleoli. Negative control staining is of adult mouse testes in which primary antibodies against POU3F1 or POU5F1 were replaced by normal goat IgG. Bars = 50 μm.
Reduced Expression of Pou3f1, but Not Pou5f1, Impairs Maintenance of Mouse SSCs In Vitro
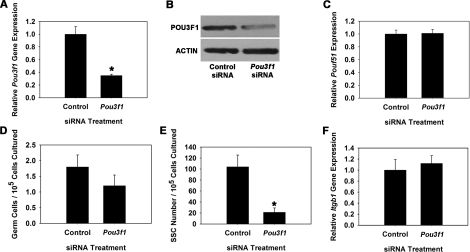
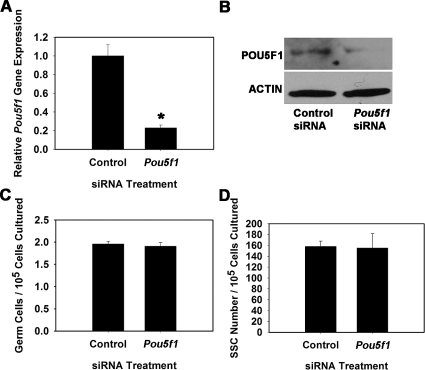
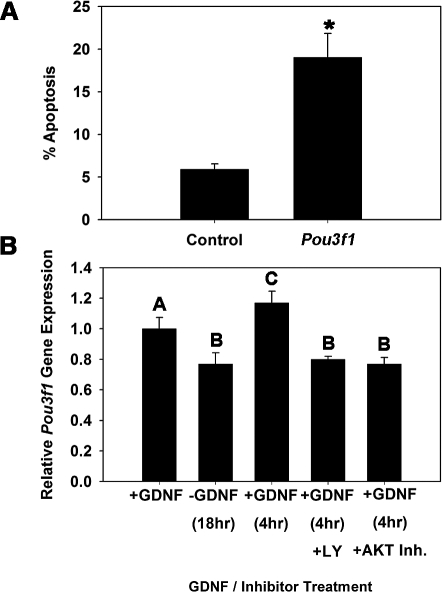
To explore whether POU3F1 has an important role in SSC function, we transiently reduced expression in cultured THY1+ germ cells using RNA interference. Cultures were then maintained for 7 days, the approximate equivalent of one SSC self-renewal cycle (5.6 days) in vitro [1], and transplantation analyses were conducted to unequivocally determine effects on SSC maintenance. For comparison, similar experiments were conducted to determine effects of impairing POU5F1 expression on SSC maintenance. Transfection of THY1+ germ cells with a single Pou3f1-targeting siRNA oligonucleotide significantly (P = 0.01) reduced transcript expression to only 35% (n = 3 different cultures) of that in cells treated with nontargeting control siRNA at 18 h after transfection (Fig. 3A). Western blot analysis confirmed that POU3F1 protein expression was also reduced (Fig. 3B). Additionally, expression of Pou5f1 did not change upon treatment with Pou3f1 siRNA, indicating that expression of these two POU domain factors is not linked within cultured mouse SSCs (Fig. 3C). Treatment of THY1+ germ cells with a single Pou5f1-targeting siRNA oligonucleotide was ineffective at significantly reducing expression (data not shown), likely due to the low expression level in THY1+ germ cells. Thus, a pool of siRNA oligonucleotides targeting Pou5f1 was utilized that significantly (P = 0.03) reduced expression to only 23% (n = 3 different cultures) of that in cells transfected with nontargeting control pooled siRNAs (Fig. 4A), and Western blot analysis confirmed reduction of POU5F1 protein levels (Fig. 4B). After approximately one self-renewal cycle of 7 days, the total number of germ cells was 67% in Pou3f1 siRNA-treated cultures compared with controls (Fig. 3D), but this difference was not significant (P = 0.27). Similarly, the total number of germ cells was not significantly different (P = 0.58) between Pou5f1- and control siRNA-treated cultures (Fig. 4C). However, transplantation analyses revealed that stem cell number was significantly (P = 0.02) reduced in Pou3f1 siRNA-treated cultures to only 20% (n = 3 different cultures) of that in control cultures (Fig. 3E). The possibility that impaired SSC homing contributed to these results was ruled out by determining that expression of β1-integrin (Itgb1), a molecule implicated as key to SSC homing after transplantation [21], was not reduced by Pou3f1 siRNA treatment (Fig. 3F). In contrast, examination of Pou5f1 siRNA-treated cultures using transplantation analyses revealed that SSC number was not different (P = 0.92) compared with control siRNA-treated cultures (Fig. 4D). Collectively, these results indicate that POU3F1, but not POU5F1, is an important intrinsic regulator of mouse SSC maintenance in vitro. The concentration of SSCs was significantly decreased by Pou3f1 siRNA treatment without significantly decreasing total germ cells, indicating a greater importance of POU3F1 for regulation of SSC self-renewal and survival than for proliferation and survival of non-stem cell spermatogonia also present in the cultured THY1+ germ cell population. The effect of Pou3f1 siRNA was three to four times greater on SSC number than on total germ cell number (Fig. 3, D vs. E).
FIG. 3.
Effect of reducing Pou3f1 gene expression on SSC maintenance in vitro. A) Relative Pou3f1 gene expression in THY1+ germ cell cultures at 18 h after treatment with nontargeting control or Pou3f1 siRNA. Treatment with Pou3f1 siRNA resulted in 65% ± 2% reduction of gene expression (n = 3 different cultures). B) Western blot analysis of POU3F1 protein expression at 18 h after treatment with nontargeting control or Pou3f1 siRNA. C) Relative gene Pou5f1 gene expression in THY1+ germ cell cultures at 18 h after treatment with nontargeting control or Pou3f1 siRNA. D) Effect of Pou3f1 siRNA treatment on maintenance of total germ cells in cultures of THY1+ spermatogonia at 7 days after transfection. E) Effect of Pou3f1 siRNA treatment on maintenance of SSCs at 7 days after transfection using functional transplantation as an assay. F) Effect of Pou3f1 siRNA treatment on expression of β1-integrin in cultured THY1+ germ cells determined by quantitative real-time PCR analysis. All data are the mean ± SEM for three different replicate cultures, and the asterisk denotes significant difference between means of treatments.
FIG. 4.
Effect of reducing Pou5f1 expression on SSC maintenance in vitro. A) Relative Pou5f1 gene expression in THY1+ germ cell cultures at 18 h after treatment with nontargeting control or Pou5f1 siRNA. Expression of Pou5f1 transcript was reduced by 77% ± 3% in Pou5f1 siRNA-treated cultures compared with those treated with nontargeting control siRNA (n = 3 different cultures). B) Western blot analysis of POU5F1 protein expression at 18 h after treatment with nontargeting control or Pou5f1 siRNA. C) Effect of Pou5f1 siRNA treatment on total germ cell maintenance in cultures of THY1+ spermatogonia at 7 days after transfection. D) Effect of Pou5f1 siRNA treatment on maintenance of SSCs at 7 days after transfection using functional transplantation as an assay. No significant difference was found between control and Pou5f1 siRNA treatments for either total germ cell numbers or SSC numbers. Data are the mean ± SEM for three different replicate cultures, and the asterisk denotes significant difference at P < 0.05.
GDNF-Induced Survival of Cultured THY1+ Germ Cells Involves Stimulation of Pou3f1 Expression via PIK3/AKT Signaling
Decline in SSC numbers after one self-renewal cycle in vitro could result from impaired self-renewal or apoptosis, which are independent mechanisms of SSC maintenance. The influence of GDNF on SSC functions promotes self-renewing proliferation, while also stimulating antiapoptotic pathways that support SSC survival. Stimulation of Pou3f1 expression by GDNF could contribute to either event. Thus, to further define the mechanistic role of POU3F1 in SSC maintenance, we examined cell survival in SSCs containing THY1+ germ cell cultures in response to impaired Pou3f1 expression (Fig. 5A). In control cultures treated with nontargeting siRNA, 5.9% (n = 3 different cultures) of the cells were apoptotic at 30 h after transfection. In comparison, the percentage of apoptotic cells was significantly (P = 0.01) higher in Pou3f1 siRNA-treated cultures, in which 19.0% (n = 3 different cultures) of the cells were apoptotic. In a previous study [6], we showed that GDNF activates the PIK3/AKT signaling pathway to promote survival of cultured THY1+ germ cells. To determine whether this same signaling mechanism controls Pou3f1 expression, we examined the effects of pharmacological inhibition of PIK3 or AKT signaling on GDNF up-regulation of Pou3f1 gene expression (Fig. 5B). Similar to previous experiments, Pou3f1 expression declined upon 18-h withdrawal of GDNF and was up-regulated at 4 h after GDNF replacement. Inhibition of either PIK3 or AKT signaling completely abrogated GDNF up-regulation of Pou3f1 gene expression. Collectively, these results demonstrate that the antiapoptotic effect of GDNF stimulation in SSCs involves activation of Pou3f1 expression, which supports SSC self-renewal and maintenance in vitro.
FIG. 5.
Impact of impairing Pou3f1 gene expression on survival of THY1+ germ cells in vitro. A) Effect of Pou3f1 siRNA treatment (30 h) on the percentage of apoptotic cells. The asterisk denotes significant difference between means of treatments. B) Examination of GDNF-regulated Pou3f1 expression through the PIK3/AKT signaling pathway using quantitative real-time PCR analysis. THY1+ germ cells containing SSCs were cultured in the presence of GDNF (+GDNF) and subjected to 18-h withdrawal of the growth factor (−GDNF), followed by replacement of GDNF for 4 h (+GDNF) in the presence of vehicle (dimethyl sulfoxide), the PIK3-specific inhibitor LY294002 (+LY [10 μM]), or AKT inhibitor IV (+AKT Inh. [20 nM]). Data are the mean ± SEM for three different replicate cultures, and bars with different letters are significantly different at P < 0.05. The inhibitors prevented the GDNF rebound at 4 h after an 18-h deprivation.
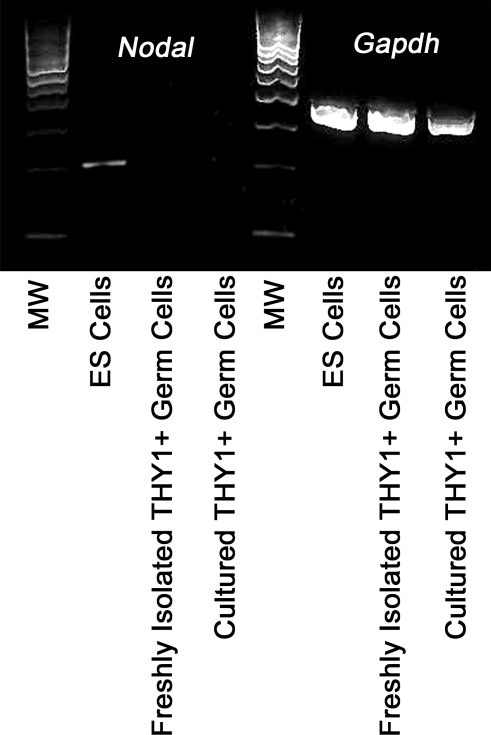
NODAL, a Putative Stimulator of POU5F1 Expression, Is Not Expressed by the SSCs Containing THY1+ Germ Cell Populations of Mouse Testes
In ES cells, expression of POU5F1 is regulated in part by the transforming growth factor-β-like molecule NODAL [22]. Recently, findings by He et al. [23] suggest that NODAL is also expressed by mouse SSCs and regulates their self-renewal via autocrine stimulation of POU5F1 expression. Because POU5F1 expression is low in cultured mouse SSCs (Fig. 1E) [1, 12, 23–25] and not essential for GDNF-induced self-renewal of SSCs in cultured THY1+ germ cells (Fig. 4C), we examined whether NODAL is expressed by these cells. Using RT-PCR analyses, Nodal gene expression was detected in ES cells but was absent in both cultured and freshly isolated THY1+ spermatogonial populations proven to contain SSCs by transplantation (Fig. 6). Thus, NODAL expression was readily detected in ES cells, where it has a role in self-renewal and pluripotency but could not be detected in SSC-enriched populations of germ cells. The low expression of POU5F1 and the absence of a role in promoting self-renewal of SSCs in cultured THY1+ germ cells in part may be due to the absence of expression of autocrine stimulators such as NODAL.
FIG. 6.
Expression of NODAL in the SSCs containing THY1+ spermatogonial population. Shown are results of RT-PCR analyses of Nodal gene expression in ES cells, freshly isolated THY1+ germ cells, and cultured THY1+ germ cells. Only ES cells were found to express NODAL, whereas the THY1+ germ cell population that contains SSCs does not express this autocrine regulator of POU5F1 expression. Two different THY1+ germ cell preparations and cultures were examined. MW, 100-bp-molecular weight marker.
DISCUSSION
POU domain transcription factors have diverse roles in developmental processes and stem cell functions. The SSCs are an adult stem cell population whose self-renewal and differentiation are required for continual male fertility, with fate decisions ultimately dependent on cell survival. The role of POU domain transcription factors in controlling these functions of SSCs is poorly understood. Because of rarity in the testis, studying SSCs in vivo is challenging. Fortunately, development of in vitro culture systems that support long-term maintenance of mouse SSCs has provided an invaluable tool to critically study pathways regulating SSC fate decisions and survival. To date, POU5F1 has been the only POU domain molecule examined for importance in SSC functions, and results of those investigations indicate an essential role in self-renewal [13]. Our previous large-scale microarray analyses revealed that Pou3f1, but not Pou5f1, gene expression is regulated by GDNF in cultured SSCs containing THY1+ germ cell populations [5]. In the present study, we further examine the role of POU3F1 in regulating SSC functions and provide a comparison with POU5F1.
Previous investigations identified Pou3f1 gene expression in mouse testes, but localization to specific cell populations was not described [14]. In the present study, immunolocalization analyses of cross-sections from testes of 6-dpp mice revealed that expression of POU3F1 protein is restricted to spermatogonia. This staining pattern was similar to that of POU5F1, which has been regarded as a germ cell-specific POU factor [12, 24, 25]. Surprisingly, staining of POU3F1 and POU5F1 was seen in nuclei of both spermatogonia and Sertoli cells in cross-sections of testes from adult mice. In transgenic mice harboring a POU5F1-GFP transgene, expression has been well characterized as germ cell specific at 7 dpp [24]; however, exact cell types expressing GFP in these mice during adulthood have not been described. Investigations by Buaas et al. [26] localized POU5F1-EGFP expression to seminiferous tubules of adult mice, but specific expression by germ cells was not reported. Our findings indicate that expression of both POU3F1 and POU5F1 is initially localized to spermatogonia specifically in neonatal mice, but in postpubertal spermatogenesis of adult mice, staining for both POU factors is also present in Sertoli cells. Expression of POU3F1 and POU5F1 was not seen in germline cells more differentiated than spermatogonia. The importance of this developmentally linked expression pattern of POU factors in mouse spermatogenesis is unknown. However, these findings suggest that POU5F1 may not be a specific marker of germ cells in adult mouse testes.
In the present study, expression of both POU3F1 and POU5F1 was identified and measured in cultured THY1+ germ cells from mice at 6 dpp. These primary cultures provide a source of SSCs proven by a functional ability for some of the cells to reestablish spermatogenesis upon transplantation. Compared with ES cells that express high levels of both molecules, cultured THY1+ germ cells were determined to express a low level of Pou5f1 but higher levels of Pou3f1. Previous findings have shown that high levels of Pou5f1 expression are necessary for maintaining pluripotency of ES cells [10], but expression is relatively low in adult stem cell populations, including testicular stem cells [1, 27–29]. While Pou5f1 expression has been identified in several adult stem cell populations, loss-of-function investigations suggest it is dispensable for regulation of fate decisions [30]. Activation of specific gene expression upon stimulation by GDNF is an essential regulatory mechanism of SSC self-renewal and survival [1]. Thus, GDNF stimulation can be considered a specific criterion for genes essential for both of these SSC fates. Our previous studies [5, 6] revealed that Pou3f1, but not Pou5f1, expression is regulated by GDNF stimulation in cultured THY1+ germ cells. This finding was confirmed in the present study, indicating that POU3F1 is a key regulator of SSC functions. In contrast, lack of GDNF regulation and low level of expression in cultured THY1+ germ cells suggest that POU5F1 does not have a key role in SSC functions.
Reduction of Pou5f1 expression by as much as 77% in the present study did not impair maintenance of SSCs in cultures of THY1+ germ cells from prepubertal mice. This finding indicates that POU5F1 is not an essential regulator of SSC fate decisions, contradicting findings by Dann et al. [13] showing that reduction of Pou5f1 expression impairs mouse SSC self-renewal in vitro. Several reasons could account for the discrepancy of findings between experiments by Dann et al. [13] and the present study. First, different culture conditions for maintaining primary SSC-containing germ cell populations were utilized. In the present study, THY1+ germ cells were maintained in serum-free conditions with GDNF and FGF2 supplementation only, whereas Dann et al. [13] utilized germline stem (GS) cell culture conditions [12]. While both cell populations contain germ cells capable of reestablishing spermatogenesis after transplantation, several different characteristics are evident. Dann et al. [13] reported that a major portion of cultured GS cell populations is c-KIT+, indicating differentiated non-SSC spermatogonia, whereas c-KIT expression is weak in cultured THY1+ germ cells [1]. Thus, reduction of POU5F1 expression in vastly different cultured germ cell populations could have different effects on SSC proliferation. Recently, Morimoto et al. [31] reported that c-KIT+ cells in GS cell cultures were capable of reestablishing spermatogenesis at a low frequency when transplanted into recipient mouse testes. Unfortunately, studies by Dann et al. [13] and Morimoto et al. [31] cannot be compared directly because the donor ages used for generating GS cultures were different. Morimoto et al. [31] utilized donor mouse pups at 0 dpp, when the germ cell population consists of a heterogeneous mix of c-KIT+ and c-KIT− gonocytes that have not transformed into spermatogonia. In contrast, Dann et al. [13] conducted studies on GS cells established from donors at 9–10 dpp, a cell line they labeled DGC1; at this age, the germ cell population is vastly different, consisting of the full range of spermatogonia, but not gonocytes. The c-KIT+ cells in GS cell cultures derived from gonocytes are likely different from c-KIT+ cells in cultures derived from spermatogonia. Thus, it is difficult to compare the c-KIT+ cells in GS cell cultures established by Dann et al. [13] and Morimoto et al. [31].
A second difference between the work by Dann et al. [13] and the present study is the method used for reducing expression of POU5F1. In the present study, Pou5f1 gene expression was transiently reduced by transfection with siRNA oligonucleotides, whereas stable reduction of Pou5f1 expression by lentiviral transduction of a short hairpin RNA (shRNA) transgene was used by Dann et al. [13]. In both studies, impairment of SSC maintenance in vitro was assayed by functional transplantation into recipient testes. This functional assay requires that SSCs translocate from the lumen of seminiferous tubules to the basement membrane, essentially in the reverse of normal spermatogenesis. Stable reduction of specific gene expression by lentiviral shRNA transduction may impact SSC migration to the basement membrane upon transplantation [21]. This possibility cannot be eliminated as a cause of impaired SSC colonization following transplantation of cultured GS cells expressing Pou5f1 shRNA. Thus, interpretation of results by Dann et al. [13] in regard to SSC self-renewal could be confounded by effects on SSC migration after transplant caused by Pou5f1 shRNA transduction. Finally, long-term knockdown of POU5F1 with shRNA may cause later effects on SSCs and other germ cells that result in the inability to differentiate and form colonies of spermatogenesis in recipient testes following transplantation.
Recent findings by He et al. [23] indicate that NODAL is expressed by mouse SSCs and acts in an autocrine manner to regulate self-renewal by stimulating POU5F1 expression. Unfortunately, those analyses were conducted on heterogeneous germ cell populations from prepubertal mouse testes with undetermined stem cell content and an immortalized cell line with unproven stem cell phenotype. Thus, it is difficult to interpret findings in regard to the impact of NODAL on SSCs directly. Previous investigations utilizing microarray analyses to examine enriched gene expression in THY1+ germ cells with proven SSC content from transplantation analyses found that NODAL expression is absent [32]. Similarly, microarray-based gene expression investigations by Shima et al. [33] did not detect NODAL expression in type A spermatogonia from prepubertal mouse testes. In the present study, we could not detect Nodal gene expression by either cultured or freshly isolated SSCs from prepubertal mouse testes, confirming the previous microarray results. However, Nodal expression was clearly detected in ES cells, confirming accuracy of the reagents and procedures utilized. This lack of NODAL expression by germ cell populations with proven SSC content and phenotype could explain the relatively low expression of POU5F1 in these cells compared with ES cells that abundantly express NODAL and require POU5F1 for self-renewal. This finding highlights the distinct difference between roles of OCT transcription factors in regulating self-renewal of stem cells from an embryonic and an adult tissue-specific lineage.
Extrinsic stimulation by GDNF is a major driver of SSC self-renewal and survival [34]. Several studies have examined the intrinsic pathways activated by GDNF within mouse spermatogonia to promote SSC self-renewal [5–7, 23, 35]; however, the pathways influencing SSC survival are poorly understood. Previous studies [6, 35] showed that GDNF activation of the PIK3/AKT signaling pathway controls SSC survival, promoting long-term in vitro maintenance. Thus, one criterion for an intrinsic regulator of SSC survival is up-regulation by GDNF via PIK3/AKT signaling. Results of the present study determined that Pou3f1 gene expression satisfies this criterion in cultured THY1+ spermatogonia. Further analysis confirmed this hypothesis by revealing that reduction of Pou3f1 expression with siRNA treatment induced apoptosis in cultured THY1+ spermatogonia, resulting in impaired SSC maintenance. In vivo, POU3F1 is expressed by all proliferating spermatogonia (Fig. 2), as are receptors for GDNF, RET proto-oncogene, and GDNF family receptor alpha 1 (GFRA1), suggesting it is a general regulator of germ cell survival. However, transplantation analyses revealed that impaired Pou3f1 expression had a greater effect on maintenance of SSCs (4.9-fold difference from controls) in vitro than it did on total spermatogonia (1.5-fold difference from controls) in vitro. This finding indicates that POU3F1 has a more profound impact on maintenance of SSCs than it does on other proliferating spermatogonia. Collectively, results of the present study demonstrate that POU3F1 is an intrinsic regulator of GDNF-induced survival and self-renewal of mouse SSCs. While POU5F1 is the essential POU domain transcription factor for maintenance of ES cells, results of our study indicate that this role is replaced by POU3F1 in adult SSCs.
Acknowledgments
The authors thank Dr. A. Bradley for donation of ES cells, and C. Freeman and R. Naroznowski for assistance with animal maintenance.
Footnotes
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, grants HD044445 (R.L.B.), HD052728 (R.L.B.), and HD058137 (J.M.O.). Additionally, research was supported by the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation (R.L.B.) and by start-up funds from The Pennsylvania State University (J.M.O.).
REFERENCES
- Kubota H, Avarbock MR, Brinster RL.Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 2004; 101: 16489–16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DL.Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci U S A 2005; 102: 17430–17435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu BY, Kubota H, Avarbock MR, Brinster RL.Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc Natl Acad Sci U S A 2005; 102: 14302–14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Muneto T, Lee J, Takenaka M, Chuma S, Nakatsuji N, Horiuchi T, Shinohara T.Long-term culture of male germline stem cells from hamster testes. Biol Reprod 2008; 78: 611–617. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL.Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A 2006; 103: 9524–9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Brinster RL.Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem 2007; 282: 25842–25851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Jian J, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M.Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells 2008; 26: 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL.Spermatogonial stem cells. Methods Enzymol 2006; 419: 259–282. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H, Smith A.Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998; 95: 379–391. [DOI] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG.Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000; 24: 372–376. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005; 122: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, Kuwana T, Kehler J, Abe K, Schöler HR, Suda T.Identification and characterization of stem cells in prepubertal spermatogenesis in mice small star, filled. Dev Biol 2003; 258: 209–225. [DOI] [PubMed] [Google Scholar]
- Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH.Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells 2008; 26: 2928–2937. [DOI] [PubMed] [Google Scholar]
- Meijer D, Graus A, Kraay R, Langeveld A, Mulder MP, Grosveld G.The octamer binding factor Pou3f1: cDNA cloning and expression in early embryonic cells. Nucleic Acids Res 1990; 18: 7357–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Treacy MN, Simmons DM, Ingraham HA, Swanson LW, Rosenfeld MG.Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature 1989; 340: 35–41. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Rohdewohld H, Neuman T, Gruss P, Scholer HR.Oct-6: a POU transcription factor expressed in embryonal stem cells and in the developing brain. EMBO J 1990; 9: 3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazvini M, Mandemakers W, Jaegle M, Piirsoo M, Driegen S, Koutsourakis M, Smit X, Grosveld F, Meijer D.A cell type-specific allele of the POU gene Oct-6 reveals Schwann cell autonomous function in nerve development and regeneration. EMBO J 2002; 21: 4612–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR.Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A 1994; 91: 11303–11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW.Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A 1994; 91: 11298–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Miki H, Ogonuki N, Takehashi M, Morimoto T, Ogura A, Shinohara T.Clonal origin of germ cell colonies after spermatogonial transplantation in mice. Biol Reprod 2006; 75: 68–74. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, Raducanu A, Nakatsuji N, Fässler R, Shinohara T.Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell 2008; 3: 533–542. [DOI] [PubMed] [Google Scholar]
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ.Nodal signaling in the epiblast patterns the early mouse embryo. Nature 2001; 411: 965–969. [DOI] [PubMed] [Google Scholar]
- He Z, Jiang J, Kokkinaki M, Dym M.Nodal signaling via an autocrine pathway promotes proliferation of mouse spermatogonial stem/progenitor cells through Smad2/3 and Oct-4 activation. Stem Cells 2009; 27: 2580–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler HR, Hatzopoulos AK, Balling R, Suzuki N, Gruss P.A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J 1989; 8: 2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimizu T, Sugiyama N, De Felice M, Yeom YI, Ohbo K, Masuko K, Obinata M, Abe K, Schöler HR, Matsui Y.Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice. Dev Growth Differ 1999; 41: 675–684. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE.Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 2004; 36: 647–652. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Iwano T, Lee J, Kazuki Y, Inoue K, Miki H, Takehashi M, Toyokuni S, Shinkai Y, Oshimura M, Ishino F, et al. Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development 2005; 132: 4155–4163. [DOI] [PubMed] [Google Scholar]
- Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW, Yancopoulos GD, Murphy A, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature 2007; 449: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T.Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod 2003; 69: 612–616. [DOI] [PubMed] [Google Scholar]
- Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, Scholer HR, Tomilin A, Jaenisch R.Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell 2007; 1: 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto H, Kanatsu-Shinohara M, Takashima S, Chuma S, Nakatsuji N, Takehashi M, Shinohara T.Phenotypic plasticity of mouse spermatogonial stem cells. PLoS One 2009; 4: e7909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Oatley MJ, Avarbock MR, Brinster RL.Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development 2009; 136: 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima JE, McLean DJ, McCarrey JR, Griswold MD.The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol Reprod 2004; 71: 319–330. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL.Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol 2008; 24: 263–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T.Akt mediates self-renewal division of mouse spermatogonial stem cells. Development 2007; 134: 1853–1859. [DOI] [PubMed] [Google Scholar]