Abstract
WEE1 homolog 2 (WEE2, also known as WEE1B) is a newly identified member of the WEE kinase family that is conserved from yeast to humans. The aim of the present study was to determine the spatiotemporal expression pattern and the function of WEE2 during oocyte maturation in a nonhuman primate species, the rhesus macaque. Among 11 macaque tissues examined, WEE2 transcript is predominantly expressed in the ovary and only weakly detectable in the testis. Within the ovary, WEE2 mRNA is exclusively localized in the oocyte and appears to accumulate during folliculogenesis, reaching the highest level in preovulatory follicles. Microinjection of a full-length WEE2-GFP (green fluorescent protein) fusion mRNA indicates a specific nuclear localization of WEE2 protein in both growing and fully grown germinal vesicle (GV)-intact oocytes. Taking the long double-stranded RNA-mediated RNA interference approach, we found that down-regulation of WEE2 led to meiotic resumption in a subset of GV oocytes even in the presence of a phosphodiesterase 3 inhibitor. On the other hand, overexpression of WEE2 delays the reentry of oocytes into meiosis in both mice and monkeys. These findings suggest that WEE2 is a conserved oocyte-specific meiosis inhibitor that functions downstream of cAMP in nonhuman primates.
Keywords: gamete biology, meiosis, meiotic resumption, oocyte, oocyte development, ovary, rhesus macaques, WEE family, WEE2 (WEE1B)
WEE2 acts as an oocyte-specific meiosis inhibitor downstream of cAMP in rhesus macaque monkeys.
INTRODUCTION
In most mammalian species, growing oocytes are arrested at prophase of meiosis I during folliculogenesis. Fully grown oocytes acquire the ability to undergo meiotic reduction in response to the midcycle luteinizing hormone (LH) surge, the onset of which is marked by breakdown of the germinal vesicle (the nucleus; GVBD) in the oocyte. The oocyte completes the first meiosis and immediately enters the second meiotic cell cycle without DNA replication, where it arrests again at metaphase. This process, known as oocyte maturation, is critical for the production of a fertilizable egg in females. Because LH receptors are not present in the oocyte [1], the function of LH likely is mediated via molecules produced by granulosa cells within the follicle. These locally produced molecules act directly on the oocyte and activate a cascade of signaling pathways that eventually lead to the activation of an intraoocyte protein complex, namely M-phase Promoting Factor (MPF) [2]. Despite recent progress in lower species, intraoocyte factors essential for meiotic resumption in primates are still not well characterized.
The MPF consists of a catalytic subunit, CDC2 (cell division cycle 2, also known as cyclin-dependent kinase 1 [CDK1]), and a regulatory subunit, cyclin B [3]. The complex is preassembled but remains inactive in competent oocytes until the midcycle LH surge [4]. High intraoocyte cAMP levels are essential for maintaining the inactive state of MPF [5–7], likely through protein kinase A (PKA)-dependent phosphorylation of proteins [8–12]. The drastic decrease of cAMP triggered by either the endogenous LH surge or disassociation of the oocyte from the follicular environment deactivates PKA and, eventually, leads to the activation of MPF. To our knowledge, no evidence shows that either CDC2 or cyclin B is a direct substrate of PKA, so the activation of MPF must be mediated by other oocyte-expressed factors. Indeed, previous studies have suggested that PKA may directly phosphorylate and regulate the activity of CDC25 (cell division cycle 25) phosphatases [8, 9] and WEE kinases [13–15] to form a bidirectional regulatory loop for MPF activity. In particular, when cAMP levels decrease, PKA deactivates and phosphorylations on CDC25 phosphatases and WEE kinases are removed; as a result, activated CDC25 phosphatases translocate to the nucleus, where they dephosphorylate CDC2 and, thus, activate MPF [12]. At the same time, deactivated WEE kinases allow the inhibitory phosphates to be removed from CDC2 by CDC25 phosphatases [16–18].
In mouse oocytes, it has been demonstrated that CDC25B is an essential phosphatase required for GVBD and also a likely target for PKA [8, 19]. However, the functions of WEE family kinases are not fully understood. Three WEE family members have been identified to date: WEE1 (WEE1 homolog), MYT1 (myelin transcription factor 1), and WEE2 (WEE1 homolog 2, previously known as WEE1B). Whereas both WEE1 and MYT1 are well documented as important mitosis regulators in multiple cell types of many species [20], studies on WEE2 are very limited. Human WEE2 was first identified as a testis-abundant gene and appeared to be of maternal origin during mouse embryogenesis [21]. Later studies elucidated that WEE2 played an important role in maintaining meiosis arrest in the mouse oocyte, because in vivo and in vitro knockdown of Wee2 mRNA resulted in a leaky meiotic resumption under conditions that would otherwise arrest the oocyte at the germinal vesicle (GV)-intact stage [22]. The current study reports the identification and characterization of WEE2 in a nonhuman primate species, the rhesus macaque. Functions of WEE2 during oocyte maturation are also determined by long double-stranded (ds) RNA-mediated RNA interference (RNAi) and recombinant WEE2 mRNA microinjection methodologies.
MATERIALS AND METHODS
Animals
Macaque tissues used for RNA isolation, protein extraction, and paraffin embedding were collected from adult rhesus monkeys between the ages of 5 and 15 yr through a tissue distribution program provided by the Division of Animal Resources at the Oregon National Primate Research Center (ONPRC). All animal protocols and procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Oregon Health & Science University and were conducted in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals. The general care and housing of rhesus monkeys at the ONPRC has been described previously [23].
Oocyte and Sperm Collection, Fertilization, and Embryo Culture
Growing oocytes were collected from follicles with diameters of less than 2.5 mm [24] that were mechanically dissected out from monkey ovaries. The granulosa cell-enclosed oocytes were subjected to microinjection in TALP/Hepes medium [25] and cultured in HECM-9 medium [26] for up to two days after injection. For rhesus monkey fully grown and mature oocyte collection, controlled ovarian stimulation cycles and laparoscopic follicular aspirations were performed as reported previously [27]. GV-intact oocytes were collected during controlled ovarian stimulation cycles on Day 8 without an ovulatory stimulus (human chorionic gonadotropin) [28]. GV oocytes used for microinjection were collected in TALP/Hepes medium containing 10 μM ORG 9935 (a phosphodiesterase 3 [PDE3] inhibitor; Schering-Plough Corp.) [29] and maintained at 37°C until use. Follicular aspiration was conducted on Day 9 or Day 10 (24–36 h after human chorionic gonadotropin) to obtain metaphase I (MI) and metaphase II (MII) oocytes.
To produce fertilized eggs and embryos for study, monkey semen was collected by the Assisted Reproductive Technologies (ART) core at ONPRC using an IACUC-approved protocol [25]. After being washed, counted, and assessed for motility, the sperm were activated in TALP/Hepes medium containing 0.02% caffeine and 0.05% cAMP [30]. Intracytoplasmic sperm injection was performed by injection of one spermatozoon into the cytoplasm of an MII oocyte [31]. The objective criteria for normal fertilization were the formation of the male and female pronuclei and the presence of two polar bodies upon examination the next morning. Fertilized eggs were cultured in HECM-9 medium until the 4-cell stage and then transferred to HECM-9 medium containing 5% fetal bovine serum until the blastocyst stage [32]. Embryo culture media were changed every 48 h, and incubations were maintained at 37°C with 5% CO2 in humidified air. In general, the ART core at ONPRC can obtain a fertilization rate of greater than 90% with intracytoplasmic sperm injection, and approximately 60% of the fertilized eggs will develop to the blastocyst stage in vitro [31]. These in vitro-produced embryos have resulted in live births after embryo transfer, and no significant difference has been observed between monkey infants produced by ART and those produced by natural mating [32]. Thus, the quality of the fertilized eggs and preimplantation embryos is comparable to those produced in vivo.
Mouse oocytes were obtained from female 129B6 mice at 19–25 days of age. The mice were injected with 5 IU of equine chorionic gonadotropin intraperitoneally, and GV-intact oocytes were collected in vitro 44–48 h later through ovarian puncture. Mouse oocytes were collected in MEMα/Hepes medium (Invitrogen) containing 0.2 mM isobutylmethylxanthine (IBMX; Sigma-Aldrich), a nonspecific phosphodiesterases inhibitor, and then maintained at 37°C until use. After injection, mouse oocytes were cultured in KSOM medium (Millipore) with or without IBMX.
RNA Isolation, RT-PCR, Quantitative PCR, and In Situ Hybridization
Extraction of RNA from monkey oocytes and embryos was performed with the Absolutely RNA Nanoprep Kit (Stratagene) and from other tissues using TRIzol reagent (Invitrogen) according to the manufacturers' instructions. Total RNA isolated from the macaque tissues and cells were treated with RNase-free DNase (Promega) and reverse-transcribed into cDNA with random hexamers and Superscript III Reverse Transcriptase (Invitrogen). PCR primers used for WEE2 and PPIA (peptidylprolyl isomerase A, also known as cyclophilin A) are listed in Table 1.
TABLE 1.
Primers used for PCR amplification.
Quantitative PCR (Q-PCR) primers and TaqMan probe were designed using PrimerExpress software (Applied Biosystems) and were purchased from Invitrogen and Applied Biosystems, respectively. The primer and probe sequences for WEE2 are shown in Table 1. Amplifications were conducted in a 10-μl final volume containing 250 nmol/L of TaqMan WEE2 probe, 300 nmol/L of WEE2 forward and reverse primers, 250 nmol/L of TaqMan 18S probe, 100 nmol/L of forward and reverse 18S primers, 1× TaqMan Universal PCR master mix (Applied Biosystems), and oocyte/early embryo cDNA. The Q-PCR reactions were conducted in an ABI PRISM 7500 Fast Real-Time PCR System (Applied Biosystems). The internal standard curve, used for relative mRNA quantification, was generated from five twofold dilutions of pooled monkey oocyte/early embryo cDNA. The threshold cycle (CT) values for unknown samples were used to extrapolate the amount of RNA equivalents from the internal standard curve. The RNA equivalent values were then normalized to 18S RNA equivalent values derived from the same internal standard curve.
Macaque WEE2 gene-specific amplicon obtained by RT-PCR as described above was cloned into the pGEMT-vector (Promega). The [α-35S]UTP-labeled antisense and sense probes were generated by the Riboprobe T7/SP6 combination systems (Promega). Hybridization of the RNA probes was carried out on paraffin-embedded ovary and testis sections (thickness, 5 μm) as previously described [33, 34].
Preparation of Mouse and Monkey WEE2 Long dsRNA and mRNA
A 541-bp PCR product, corresponding to 995-1535 bp of the macaque WEE2 mRNA sequence (GenBank accession no. NM_001105546), was amplified from a macaque ovary cDNA pool. A 629-bp green fluorescent protein (GFP) cDNA fragment was also amplified from a plasmid template, pEGFP-N1 (Clontech), and used as a nontargeting control, because the GFP gene does not exist in the macaque genome. Each primer (Table 1) was synthesized with and without a 27-bp T7 promoter sequence tagged at its 5′-end. The PCR products were confirmed by sequencing and purified by phenol/chloroform extraction and ethanol precipitation. Sense and antisense single-stranded (ss) RNAs were synthesized separately using MEGAscript T7 kit (Applied Biosystems-Ambion) according to the manufacturer's manual and mixed at the end of the in vitro transcription reaction. To generate dsRNA, the ssRNA mixture was heated at 70°C for 15 min, gradually cooled at room temperature to allow linearization, and then treated with DNase I and RNase A (Applied Biosystems-Ambion) to remove the DNA template and redundant ssRNA. The purified dsRNA was dissolved in RNase-free water, and the concentration was determined with a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific).
Full-length mouse (ms) and monkey (mk) WEE2 cDNA coding regions were amplified by Phusion High-Fidelity PCR reagents (New England Biolabs) from mouse and monkey ovary cDNA pools (GenBank accessions numbers and primer sequences are listed in Table 1), respectively, and cloned into the pMDL2 plasmid [35]. The pMDL2-ms(mk)WEE2 constructs were linearized by a unique restriction enzyme site downstream of an enhanced GFP-coding region and purified by phenol/chloroform extraction. The ms(mk)WEE2-GFP fusion mRNA was synthesized with mMESSAGE mMACHINE High Yield Capped RNA Transcription Kit (Applied Biosystems-Ambion) and dissolved in RNase-free water at approximately 2 μg/μl.
Because the pMDL2 vector does not contain a translational start codon, GFP protein cannot be directly expressed from the enhanced GFP-coding region on the vector. We PCR amplified and cloned a nearly full-length Zar1 (zygote arrest 1) cDNA sequence (corresponding to 28–1101 bp of GenBank accession no. NM_174877) into the pMDL2 vector to generate a msZar1-GFP fusion mRNA using the same strategy described above. Mouse Zar1 is a maternal effect gene and has no known effect on oocyte maturation [34]. Therefore, the msZar1-GFP fusion mRNA was injected into GV oocytes at approximately 2 μg/μl as a negative control.
Oocyte Microinjection and Culture
Injection pipettes were made from sterile borosilicate glass with filament (BF100-78-10) using a P-87 micropipette puller (Sutter Instrument Co.) and angled at approximately 60° with a microforge (Narishige Co.). Oocyte microinjections were performed on an Olympus IX70 inverted microscope with a heated stage equipped with Transferman NK2 micromanipulators and CellTram Air/Oil pressure control systems (Eppendorf Co.). The dsRNA or mRNA solution at 0.5–1 μl was back-loaded to the injection needle using a pipette fitted with an Eppendorf microloader tip. Microinjections were performed in 10-μl droplets of PDE inhibitor-containing MEMα/Hepes (for the mouse oocyte) or TALP/Hepes (for the monkey oocyte) medium covered by light mineral oil. Approximately 5–10 pl of mRNA solution were injected into each mouse oocyte, and no more than 5% of the total cellular volume of dsRNA or mRNA solution was injected into the monkey oocytes. Individual dsRNA-injected monkey GV oocytes were maintained in 50-μl droplets of HECM-9 medium with 10 μM ORG9935 at 37°C under a humidified atmosphere of 5% CO2 for up to 4 days. Control and WEE2 mRNA-injected mouse and monkey GV oocytes were cultured in IBMX-containing KSOM or ORG9935-containing HECM9 medium, respectively, overnight before being washed and transferred to corresponding medium without PDE inhibitors. The presence and absence of an intact nucleus (GV) in the oocyte was used as an indicator for oocyte meiotic status in both species.
Protein Extraction and Western Blot Analysis
Fresh or flash-frozen macaque tissues were homogenized in RIPA buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM ethylenediaminetetra-acetic acid, 1% NP-40, and 0.1% SDS) containing 1× protease inhibitor cocktail (Roche Applied Science). For each sample, 50 μg of total protein in 1× Laemmli sample buffer (Bio-Rad Laboratories) was heated at 95°C for 5 min before being loaded onto a 4–20% polyacrylamide Tris-HCl gel (Bio-Rad Laboratories) and electrophoresed in 1× Tris/glycine/SDS buffer at 100 V for 1 h. The protein was then transferred onto a Protran nitrocellulose membrane (Whatman) and sequentially blotted with StartingBlock T20 (TBS) Blocking Buffer (Pierce), rabbit anti-WEE2 (a gift from Dr. Marco Conti at University of California, San Francisco) at a 1:800 dilution, and horse radish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin G (Thermo Fisher Scientific) at a 1:25 000 dilution. After incubation with an enhanced chemiluminescence substrate (Thermo Fisher Scientific), the signal was visualized on a Kodak Biomax Light Film. The same membrane was also reblotted with HRP-conjugated anti-β-ACTIN (Abcam) at a 1:8000 dilution for an internal loading control. To verify the efficiency of the WEE2 knockdown, six monkey oocytes from each group (uninjected, dsGFP-injected, and dsWEE2-injected) were washed in PBS and collected into 1× Laemmli sample buffer. The oocyte lysate was directly used for Western blot analysis as described above, except that SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) was used in the final incubation and the membrane was reblotted with HRP-conjugated anti-β-ACTIN without stripping.
Statistics
Results are presented as the mean ± SEM. Three replicates were performed for each microinjection experiment, and all data were analyzed using the Student t-test. Differences in the data were considered to be significant at P < 0.05.
RESULTS
Expression of WEE2 in Rhesus Macaques
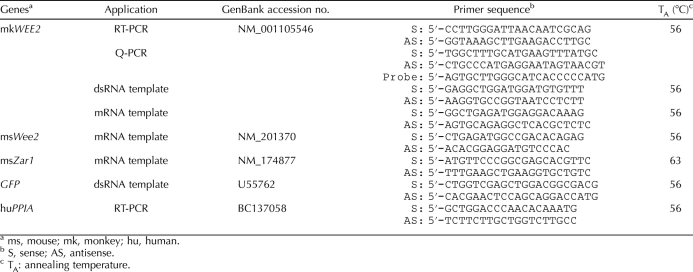
The macaque WEE2 mRNA sequence was used as a template to design gene-specific primers (Table 1). To test the tissue distribution of WEE2, RT-PCR was performed in cDNA derived from 11 rhesus macaque tissues. As shown in Figure 1A, WEE2 transcripts were highly abundant in the ovary and weakly detectable in the testis but were not amplified in any of the somatic tissues examined.
FIG. 1.
Spatial and temporal localization of WEE2 transcript in rhesus macaques. A) Distribution of WEE2 in the macaque tissues. PPIA was used as an internal control. Br, brain; He, heart; Hy, hypothalamus; Ki, kidney; Li, liver; Lu, lung; Pit, pituitary; Ov, ovary; Ovi, oviduct; Te, testis; Ut, uterus. B) In situ hybridization of WEE2 in paraffin-embedded macaque ovary and testis sections. AF, antral follicle; BF, bright field; DF, dark field; PF, primary follicle; PrF, primordial follicle; SF, secondary follicle; ST, seminiferous tubule. Original magnification ×200. C) Detection of WEE2 mRNA during oocyte maturation and preimplantation embryo development by RT-PCR (top) and Q-PCR (bottom). PPIA was used as an internal loading control. Bl, blastocysts; GV, germinal vesicle-intact oocytes; MI, metaphase I oocytes; MII, metaphase II oocytes; Mr, morula; Zy, zygotes; 2-C, 2-cell embryo; 4-C, 4-cell embryo; 8-C, 8-cell embryo. Q-PCR data are presented as the mean ± SEM of three independent experiments.
Using the aforementioned WEE2 PCR amplicon as a probe, we performed in situ hybridization to detect the cellular localization of WEE2 mRNA in the macaque ovary and testis (Fig. 1B). WEE2 transcript is exclusively localized in the oocyte within the ovary, and the earliest stage that WEE2 becomes detectable is in primordial follicles. Although in situ hybridization is not considered to be an accurate quantitative methodology, it is apparent that WEE2 mRNA accumulates throughout folliculogenesis and reaches the highest level in preovulatory antral follicles. Consistent with the RT-PCR result, faint signals of WEE2 mRNA were also visible in germ cells within seminiferous tubules in the testis by in situ hybridization (Fig. 1B), suggesting a low-level expression of WEE2 in the macaque male gonad. To illustrate the temporal distribution of WEE2 after ovulation, RT-PCR and Q-PCR were also carried out in macaque oocytes and preimplantation embryos (Fig. 1C). The expression of WEE2 persists in maturing (GV) and mature (MI and MII) ooctyes, decreases in fertilized eggs (zygotes), and eventually disappears after the 8-cell embryonic stage.
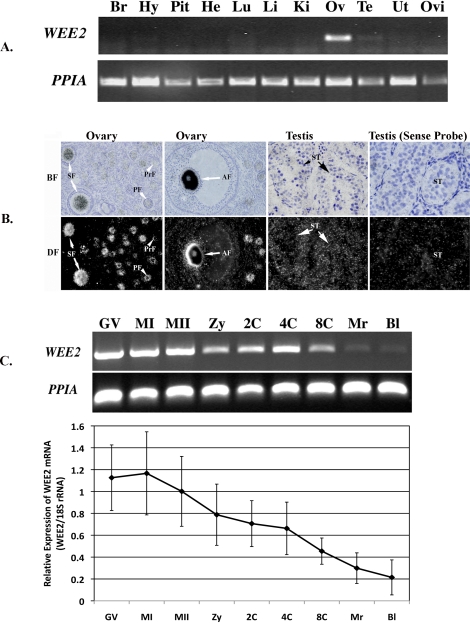
Using an antibody generated against the N-terminal of mouse WEE2 protein, we detected a band at approximately 65 kDa in both the monkey and mouse ovary (Fig. 2A). The size of the detected protein is close to the predicted molecular weight of monkey and mouse WEE2 (i.e., 62.9 and 62.2 kDa, respectively). Surprisingly, a specific band was also detected in the total protein freshly isolated from the adult testis despite low-level expression of WEE2 transcript in this tissue. Monkey brain and spleen proteins were used as negative somatic tissue controls, because no expression of WEE2 mRNA was detected in these tissues by RT-PCR.
FIG. 2.
Tissue and cellular localization of macaque WEE2 protein. A) Western blot in the mouse ovary and selected monkey tissues. An approximately 65-kDa band was specifically detected in the ovary from both species as well as in the monkey testis. β-ACTIN (42 kDa) was used as an internal loading control. Monkey brain and spleen samples were run on the same gel but not on lanes adjacent to the testis. Br, brain; Mk, monkey; Ms, mouse; MW, molecular weight marker; Ov, ovary; Sp, spleen; Te, testis. B) Distribution of GFP-tagged full-length macaque WEE2 protein in growing oocytes isolated from secondary follicles, fully grown GV, and MI oocytes. Arrowheads denote the nucleus in the oocyte. BF, bright field; DF, dark field. Original magnification ×100 for Growing Oocytes and ×150 for all other panels.
No specific staining was detected when the same antibody was used for immunohistochemistry in the ovary and testis. To determine the subcellular localization of WEE2 protein in the oocyte, we generated a WEE2-GFP fusion mRNA in vitro. Once injected into growing and fully grown GV-intact oocytes, the GFP-tagged WEE2 mRNA begins to translate within 2 h, and the green fluorescence lasts for more than 4 days in the macaque oocyte (data not shown). As shown in Figure 2B, WEE2-GFP fusion protein is specifically localized in the nucleus of GV-intact oocytes at different sizes and diffuses into the whole cytoplasm after GVBD.
Functional Analysis of WEE2 During Oocyte Maturation
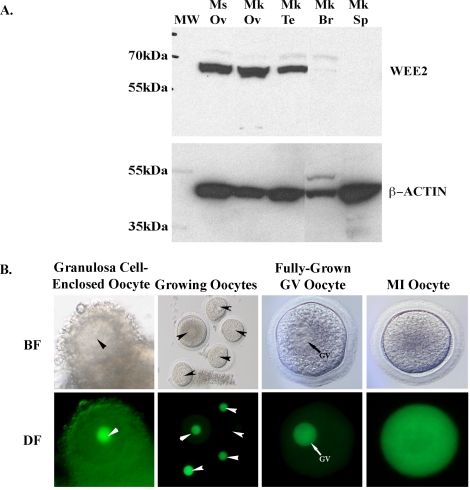
To test the function of WEE2 during oocyte maturation in rhesus macaques, we performed WEE2 knockdown using dsRNA-mediated RNAi methodology. To track the meiotic state of each oocyte, both GFP and WEE2 dsRNA-injected oocytes were cultured in individual droplet of medium and examined every 24 h. Thirty-seven uninjected macaque oocytes (from three different monkeys) were also included in the experiment to obtain a general GVBD rate in normal, untreated oocytes. Whereas only a few GFP dsRNA-injected oocytes (4/44, 8.75% ± 7.20%) escaped from the GV arrest state in PDE3 inhibitor-containing medium during in vitro culture for up to 4 days, a significant subset of WEE2 dsRNA-injected oocytes underwent GVBD after 96 h (21/55, 38.47% ± 5.89%) (Fig. 3A). The GVBD rate in dsGFP-injected oocytes was similar to that in uninjected oocytes (2/37, 4.42% ± 5.19%). Although GVBD can be observed in dsWEE2-injected monkey oocytes after 48-h culture, the difference between the WEE2 knockdown oocytes and controls (uninjected and dsGFP-injected) is not statistically significant at either 48 h (Fig. 3B) or 72 h (data not shown). Indeed, effective WEE2 knockdown (>80%) at the protein level was verified in WEE2 dsRNA-injected oocytes at 96 h post-dsRNA injection by Western blot analysis (Fig. 3C).
FIG. 3.
WEE2 knockdown in macaque oocytes. A) Meiosis resumption measured by GVBD in GFP (dsGFP, control) and WEE2 (dsWEE2) dsRNA-injected oocytes. Four representative oocytes were selected from each group, and the images were taken at the time of injection (0 h) and 96 h later. Original magnification ×150. B) Comparison of GVBD rate in WEE2 knockdown oocytes. Data are presented as the mean ± SEM. *P < 0.01 vs. uninjected and GFP dsRNA-injected oocytes at 96 h. C) WEE2 protein expression in uninjected (Uninj.) and injected monkey oocytes. Lysate of six oocytes was loaded on each lane. β-ACTIN was used as an internal loading control.
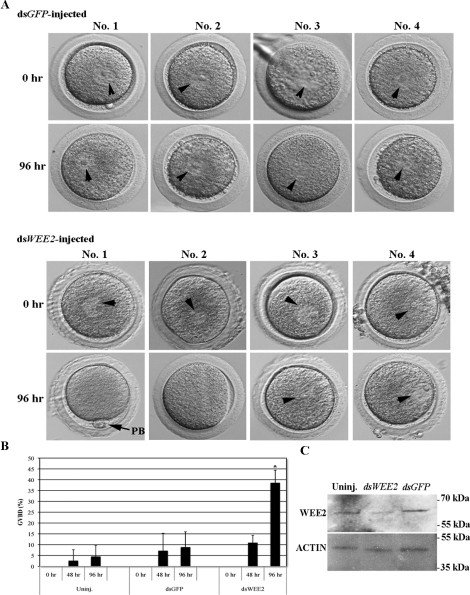
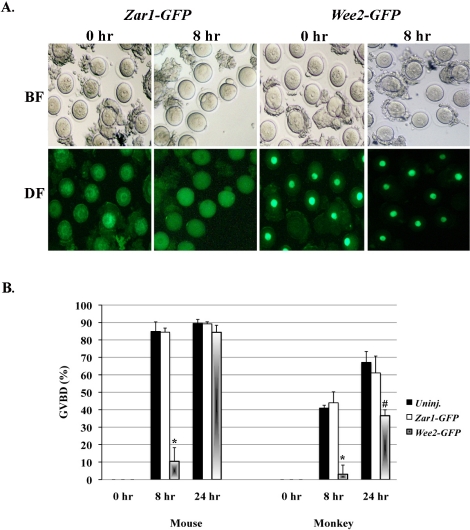
Because down-regulation of WEE2 caused the release of more than one third of oocytes from the meiotic arrest state in the presence of a PDE3 inhibitor, we next asked what would happen if WEE2 protein is overexpressed in GV-stage oocytes. A full-length WEE2-coding mRNA tagged with a GFP-coding sequence was synthesized and injected into fully grown GV oocytes derived from mice and rhesus monkeys. The mRNA-injected oocytes were maintained in PDE3 inhibitor-containing medium for a period of time (8–14 h) to allow protein synthesis from the injected mRNA. GV-intact oocytes were washed and transferred to medium without PDE inhibitors, with this time point designated as 0 h. Zar1 is an oocyte-specific maternal effect gene; previous studies in mice [34] and our preliminary test in both mouse and monkey oocytes (unpublished data) showed no effect of ZAR1 on oocyte maturation. Thus, msZar1-GFP fusion mRNA was used as a negative control. Fully grown oocytes undergo spontaneous meiotic resumption upon being released from the follicular environment because of a decline of intraoocyte cAMP levels. This process, however, is delayed in Wee2-GFP mRNA-injected oocytes from both the mouse and monkey (Fig. 4).
FIG. 4.
Overexpression of WEE2 in mouse and monkey oocytes. A) Progress of oocyte maturation in Zar1-GFP (control) and Wee2-GFP mRNA-injected mouse oocytes. The oocytes were maintained in 0.2 mM IBMX-containing KSOM medium overnight after injection and then washed and cultured in IBMX-null KSOM medium for up to 24 h. The time of oocytes transfer to IBMX-free medium is designated as 0 h. The images were taken after 8-h culture in IBMX-null medium. BF, bright field; DF, dark field. Original magnification ×100. B) Comparison of GVBD in control and Wee2 mRNA-injected mouse and monkey oocytes. *P < 0.01 vs. uninjected (Uninj.) and Zar1-GFP mRNA-injected at 8 h of culture, #P < 0.05 vs. uninjected monkey oocytes at 24 h of culture. Data are presented as the mean ± SEM.
In the mouse, most uninjected (77/89, 84.89% ± 5.45%) and Zar1-GFP mRNA-injected (126/149, 84.42% ± 2.39%) oocytes progressed to the MI or MII stage within 8 h of culture in PDE inhibitor-null medium, whereas only 10.43% ± 7.86% (10/75) of the Wee2-GFP mRNA-injected oocytes underwent GVBD (P < 0.01) (Fig. 4A). At 24 h of culture, no significant difference was observed in oocyte maturation rate between groups, at least in the mouse. Although only a relatively small number (33) of monkey GV oocytes were injected with mkWEE2-GFP mRNA, only one GVBD (3.0% ± 5.25%) was observed in these WEE2-overexpressed oocytes after 8 h of culture in PDE inhibitor-null medium. In contrast, 40.95% ± 1.65% (9/22) of uninjected and 43.98% ± 6.26% (13/29) of msZar1-GFP mRNA-injected monkey oocytes entered MI or MII stage during the same time period (Fig. 4B) (P < 0.01). Interestingly, at 24 h of culture, whereas 67.14% ± 6.22% (15/22) of uninjected and 61.11% ± 9.62% (18/29) of msZar1-GFP mRNA-injected oocytes underwent GVBD, only 36.57% ± 3.34% (12/33) of mkWEE2-GFP mRNA-injected oocytes re-entered meiosis. This number is significantly lower (P < 0.05) than those in control groups, implying a possible difference in oocyte maturation between the mouse and primate models.
DISCUSSION
The present study examines the spatiotemporal distribution of WEE2 in rhesus macaque monkeys and demonstrates that WEE2 is a meiosis inhibitor specific to the oocyte. The female germ cell-predominant expression pattern of WEE2 in the macaque is consistent with a previous report in the mouse, except that no signal was detected in the mouse testis by Northern blot analysis [22]. This difference may be simply attributed to the highly sensitive nature of RT-PCR methodology applied in the present study. Notably, WEE2 was reportedly the most abundant in the human testis [21]; our data also showed that both WEE2 transcript and protein were detectable in the macaque testis by in situ hybridization and Western blot analysis, implying a possible species-specific expression pattern of WEE2. The temporal presence and decay of WEE2 transcript during the postovulatory period coincides with the transition from a transcriptionally quiescent oocyte to an active embryo [36, 37], indicating an exclusive origin of WEE2 from the maternal genome in rhesus macaques. Unlike CDC25B, which is a major phosphatase required for the activation of MPF in the mouse oocyte and only relocates to the nucleus from the cytoplasm before GVBD [9], WEE2 protein is consistently localized in the nucleus during oocyte growth. The coexistence of WEE2 and CDC25B in the oocyte during the periovulatory period implies a possible counteraction between the two proteins during oocyte maturation. Overall, the spatial and temporal expression pattern of WEE2 suggests a role of the protein in later development of the oocyte in nonhuman primates.
The long dsRNA-induced gene knockdown technique has been widely applied in oocytes and early embryos derived from different species, including the mouse, bovine, and monkey [38–40]. In contrast to somatic cells, the interferon pathway, which can be triggered by dsRNA longer than 30 bp and subsequently induces cell death, does not seem to exist in oocytes [41]. The RNAi data showing that down-regulation of WEE2 can overcome the inhibitory effect of high intraoocyte cAMP levels on meiotic resumption suggest a downstream role of WEE2 relative to cAMP and PKA in the macaque oocyte. This phenotype in macaque oocytes supports previous observations in mouse oocytes of a leaky meiotic resumption in approximately 25–30% of WEE2 knocked-down oocytes derived from either wild-type or PDE3A-deficient mice [22]. A notable difference in our findings is that the onset of GVBD in macaque oocytes appears to require a relatively longer duration post-dsRNA injection than mouse oocytes in vitro (96 h vs. 24 h). The timing for phenotypic observation after specific gene knockdown is determined by the stability of preexisting target protein, because dsRNA diminishes gene expression only at the mRNA level. As mentioned before, green fluorescence emitted from WEE2-GFP fusion protein lasts approximately 4 days in the macaque oocyte, so this may represent a natural turnover rate of WEE2 protein. Other possible reasons leading to the timing difference in oocyte GVBD after WEE2 knockdown could be because different regions of WEE2 mRNA were targeted or that different strategies were applied to induce RNAi (long dsRNA vs. small interfering RNA). The WEE2 knockdown effect observed in the present study appears to be specific, because the expression of β-ACTIN is not altered and no distinguishable decrease of WEE2 protein is observed in dsGFP-injected oocytes.
The partial meiotic resumption observed in WEE2 knocked-down oocytes confirms that WEE2 is not the only factor required for maintenance of the inactive state of MPF. Other oocyte-expressed phosphatases and kinases are likely to compensate the role of WEE2. Also, our data corroborates a previous report [22] demonstrating that WEE2 is a relatively stable protein. Although the WEE2 protein level was remarkably reduced at 96 h post-RNAi, the remaining WEE2 protein likely remains functional and contributes to meiotic arrest in some oocytes. Previous research in mice and frogs [15, 16, 22] suggested that WEE2 might be a direct substrate for PKA, and PKA phosphorylation could increase the activity of WEE2. If this is the case, a decrease of intraoocyte cAMP will diminish the activity of WEE2, which eventually will be overwhelmed by the action of activated phosphatases (e.g., CDC25B). This could explain why overexpressed WEE2 protein only blocked meiotic resumption for a short period of time. Another interesting finding is that the effect of WEE2 overexpression seems to last longer in monkey oocytes than in mouse oocytes. The phenomenon may be related to baseline levels of cAMP, the relative activity of phosphodiesterases in the mouse and monkey oocyte, or different mechanisms regulating WEE2 or MPF activity in the rhesus macaque oocyte. More in-depth mechanistic studies are required to solve the puzzle in primates. Coincidently, while the current manuscript was under preparation, two publications from Naito's group [42, 43] reported that WEE2 is a key inhibitory factor for CDC2 during porcine oocyte maturation, supporting a conserved role of WEE2 in the oocyte of mammalian species. Interestingly, it appears that the cooperation of MYT1, WEE2, and CDC25 is required for the regulation of MPF activity in the mouse oocyte [44], whereas MYT1 does not seem to participate in meiotic arrest in the porcine oocyte [42, 43], further revealing a potential species-specific difference in meiotic regulation.
In summary, our results demonstrate that WEE2 functions as a meiosis inhibitor downstream of cAMP and PKA in the monkey oocyte. Further analysis of phosphorylation sites and key functional elements on WEE2 protein, as well as interrelationships of WEE2 with other oocyte-expressed cell-cycle regulators and signaling pathways, may eventually unravel the molecular machinery driving the function of WEE2 and oocyte maturation.
Acknowledgments
The authors thank Dr. Marco Conti from University of California, San Francisco for providing anti-WEE2 polyclonal antibodies. The authors also thank the following core facilities at ONPRC: the ART core for assisting in monkey oocyte and early embryo collection, the Molecular and Cellular Biology core for sequencing, and the Imaging and Morphology core for tissue processing and photography.
Footnotes
Supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement (U54HD55477, Richard L. Stouffer, Oregon National Primate Research Center) as part of the Specialized Cooperative Centers Program in Contraceptive Development Research. C.B.H. was a postdoctoral fellow supported by a Reproductive Biology training grant (T32HD007133, Judy L. Cameron, Oregon National Primate Research Center). M.C.P. was supported by a Fogarty International Center grant (TW/HD-00668, P. Michael Conn, Oregon National Primate Research Center). X.W. was supported, in part, by a start-up fund provided by Oregon National Primate Research Center, Oregon Health & Science University.
REFERENCES
- Amsterdam A, Koch Y, Lieberman ME, Lindner HR.Distribution of binding sites for human chorionic gonadotropin in the preovulatory follicle of the rat. J Cell Biol 1975; 67: 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekel N.Cellular, biochemical and molecular mechanisms regulating oocyte maturation. Mol Cell Endocrinol 2005; 234: 19–25. [DOI] [PubMed] [Google Scholar]
- Morgan DO.Principles of CDK regulation. Nature 1995; 374: 131–134. [DOI] [PubMed] [Google Scholar]
- Chesnel F, Eppig JJ.Synthesis and accumulation of p34cdc2 and cyclin B in mouse oocytes during acquisition of competence to resume meiosis. Mol Reprod Dev 1995; 40: 503–508. [DOI] [PubMed] [Google Scholar]
- Cho WK, Stern S, Biggers JD.Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J Exp Zool 1974; 187: 383–386. [DOI] [PubMed] [Google Scholar]
- Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V.Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest 2004; 114: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari S, Horner K, Mehlmann LM, Conti M.Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest. Dev Biol 2008; 316: 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirino G, Wescott MP, Donovan PJ.Protein kinase A regulates resumption of meiosis by phosphorylation of Cdc25B in mammalian oocytes. Cell Cycle 2009; 8: 665–670. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Z, Xu XY, Li XS, Yu M, Yu AM, Zong ZH, Yu BZ.Protein kinase A modulates Cdc25B activity during meiotic resumption of mouse oocytes. Dev Dyn 2008; 237: 3777–3786. [DOI] [PubMed] [Google Scholar]
- Kimura N, Hoshino Y, Totsukawa K, Sato E.Cellular and molecular events during oocyte maturation in mammals: molecules of cumulus-oocyte complex matrix and signalling pathways regulating meiotic progression. Soc Reprod Fertil Suppl 2007; 63: 327–342. [PubMed] [Google Scholar]
- Kovo M, Kandli-Cohen M, Ben-Haim M, Galiani D, Carr DW, Dekel N.An active protein kinase A (PKA) is involved in meiotic arrest of rat growing oocytes. Reproduction 2006; 132: 33–43. [DOI] [PubMed] [Google Scholar]
- Dekel N.Protein phosphorylation/dephosphorylation in the meiotic cell cycle of mammalian oocytes. Rev Reprod 1996; 1: 82–88. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Sagata N.Mechanism for inactivation of the mitotic inhibitory kinase Wee1 at M phase. Proc Natl Acad Sci U S A 2007; 104: 3753–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows AE, Sceurman BK, Kosinski ME, Richie CT, Sadler PL, Schumacher JM, Golden A.The C. elegans Myt1 ortholog is required for the proper timing of oocyte maturation. Development 2006; 133: 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Conti M.New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate. Cell Cycle 2006; 5: 227–231. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nakajo N, Sagata N.The existence of two distinct Wee1 isoforms in Xenopus: implications for the developmental regulation of the cell cycle. EMBO J 2002; 21: 2472–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher RN, Holman PS, Fattaey A.Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity. J Biol Chem 1997; 272: 22300–22306. [DOI] [PubMed] [Google Scholar]
- Parker LL, Piwnica-Worms H.Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 1992; 257: 1955–1957. [DOI] [PubMed] [Google Scholar]
- Lincoln AJ, Wickramasinghe D, Stein P, Schultz RM, Palko ME, De Miguel MP, Tessarollo L, Donovan PJ.Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet 2002; 30: 446–449. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Leise WF., III.Measurement of Wee kinase activity. Methods Mol Biol 2005; 296: 299–328. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Ando H, Watanabe N, Kitamura K, Ito K, Okayama H, Miyamoto T, Agui T, Sasaki M.Identification and characterization of human Wee1B, a new member of the Wee1 family of Cdk-inhibitory kinases. Genes Cells 2000; 5: 839–847. [DOI] [PubMed] [Google Scholar]
- Han SJ, Chen R, Paronetto MP, Conti M.Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol 2005; 15: 1670–1676. [DOI] [PubMed] [Google Scholar]
- Lanzendorf SE, Zelinski-Wooten MB, Stouffer RL, Wolf DP.Maturity at collection and the developmental potential of rhesus monkey oocytes. Biol Reprod 1990; 42: 703–711. [DOI] [PubMed] [Google Scholar]
- Alak BM, Wolf DP.Rhesus monkey oocyte maturation and fertilization in vitro: roles of the menstrual cycle phase and of exogenous gonadotropins. Biol Reprod 1994; 51: 879–887. [DOI] [PubMed] [Google Scholar]
- Macklon NS, Stouffer RL, Giudice LC, Fauser BC.The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev 2006; 27: 170–207. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Paprocki AM, VandeVoort CA.Causes of developmental failure of in vitro matured rhesus monkey oocytes: impairments in embryonic genome activation. Hum Reprod 2003; 18: 826–833. [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Zelinski-Wooten MB.Overriding follicle selection in controlled ovarian stimulation protocols: quality vs. quantity. Reprod Biol Endocrinol 2004; 2: 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Patel B, McMenamin M, Moran E, Paprocki AM, Kihara M, Schramm RD, Latham KE.Effects of follicle size and oocyte maturation conditions on maternal messenger RNA regulation and gene expression in rhesus monkey oocytes and embryos. Biol Reprod 2005; 72: 890–897. [DOI] [PubMed] [Google Scholar]
- Jensen JT, Zelinski-Wooten MB, Schwinof KM, Vance JE, Stouffer RL.The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation during gonadotropin-stimulated ovarian cycles in rhesus macaques. Contraception 2005; 71: 68–73. [DOI] [PubMed] [Google Scholar]
- Boatman DE, Bavister BD.Stimulation of rhesus monkey sperm capacitation by cyclic nucleotide mediators. J Reprod Fertil 1984; 71: 357–366. [DOI] [PubMed] [Google Scholar]
- Wolf DP.Assisted reproductive technologies in rhesus macaques. Reprod Biol Endocrinol 2004; 2: 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DP, Thormahlen S, Ramsey C, Yeoman RR, Fanton J, Mitalipov S.Use of assisted reproductive technologies in the propagation of rhesus macaque offspring. Biol Reprod 2004; 71: 486–493. [DOI] [PubMed] [Google Scholar]
- Wu X, Chen L, Brown CA, Yan C, Matzuk MM.Interrelationship of growth differentiation factor 9 and inhibin in early folliculogenesis and ovarian tumorigenesis in mice. Mol Endocrinol 2004; 18: 1509–1519. [DOI] [PubMed] [Google Scholar]
- Wu X, Viveiros MM, Eppig JJ, Bai Y, Fitzpatrick SL, Matzuk MM.Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nat Genet 2003; 33: 187–191. [DOI] [PubMed] [Google Scholar]
- Homer HA, McDougall A, Levasseur M, Murdoch AP, Herbert M.RNA interference in meiosis I human oocytes: towards an understanding of human aneuploidy. Mol Hum Reprod 2005; 11: 397–404. [DOI] [PubMed] [Google Scholar]
- Braude P, Bolton V, Moore S.Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 1988; 332: 459–461. [DOI] [PubMed] [Google Scholar]
- Zheng P, Patel B, McMenamin M, Paprocki AM, Schramm RD, Nagl NG, Jr, Wilsker D, Wang X, Moran E, Latham KE.Expression of genes encoding chromatin regulatory factors in developing rhesus monkey oocytes and preimplantation stage embryos: possible roles in genome activation. Biol Reprod 2004; 70: 1419–1427. [DOI] [PubMed] [Google Scholar]
- Wu X.Maternal depletion of NLRP5 blocks early embryogenesis in rhesus macaque monkeys (Macaca mulatta). Hum Reprod 2009; 24: 415–424. [DOI] [PubMed] [Google Scholar]
- Paradis F, Vigneault C, Robert C, Sirard MA.RNA interference as a tool to study gene function in bovine oocytes. Mol Reprod Dev 2005; 70: 111–121. [DOI] [PubMed] [Google Scholar]
- Svoboda P.Long dsRNA and silent genes strike back:RNAi in mouse oocytes and early embryos. Cytogenet Genome Res 2004; 105: 422–434. [DOI] [PubMed] [Google Scholar]
- Stein P, Zeng F, Pan H, Schultz RM.Absence of non-specific effects of RNA interference triggered by long double-stranded RNA in mouse oocytes. Dev Biol 2005; 286: 464–471. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Shimaoka T, Kano K, Naito K.Insufficient amount of Cdc2 and continuous activation of Wee1 B are the cause of meiotic failure in porcine growing oocytes. J Reprod Dev 2009; 55: 553–557. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Nishimura T, Kano K, Naito K.Critical effect of pigWee1B on the regulation of meiotic resumption in porcine immature oocytes. Cell Cycle 2009; 8: 2375–2384. [DOI] [PubMed] [Google Scholar]
- Oh JS, Han SJ, Conti M.Wee1B, Myt1, and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption. J Cell Biol 2010; 188: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]