Abstract
Five experiments were conducted to test the hypothesis that psychosocial stress interferes with the estrous cycle of sheep. In experiment 1, ewes were repeatedly isolated during the follicular phase. Timing, amplitude, and duration of the preovulatory luteinizing hormone (LH) surge were not affected. In experiment 2, follicular-phase ewes were subjected twice to a “layered stress” paradigm consisting of sequential, hourly application of isolation, restraint, blindfold, and predator cues. This reduced the LH pulse amplitude but did not affect the LH surge. In experiment 3, different acute stressors were given sequentially within the follicular phase: food denial plus unfamiliar noises and forced exercise, layered stress, exercise around midnight, and transportation. This, too, did not affect the LH surge. In experiment 4, variable acute psychosocial stress was given every 1–2 days for two entire estrous cycles; this did not disrupt any parameter of the cycle monitored. Lastly, experiment 5 examined whether the psychosocial stress paradigms of experiment 4 would disrupt the cycle and estrous behavior if sheep were metabolically stressed by chronic food restriction. Thirty percent of the food-restricted ewes exhibited deterioration of estrous cycle parameters followed by cessation of cycles and failure to express estrous behavior. However, disruption was not more evident in ewes that also encountered psychosocial stress. Collectively, these findings indicate the estrous cycle of sheep is remarkably resistant to disruption by acute bouts of psychosocial stress applied intermittently during either a single follicular phase or repeatedly over two estrous cycles.
Keywords: cortisol, estrous cycle, follicular phase, food restriction, LH surge, luteinizing hormone, ovulatory cycle, stress
Repeated exposure to acute psychosocial stress does not interfere with the preovulatory LH surge or other estrous cycle parameters in the ewe.
INTRODUCTION
Disruptive effects of stress on gonadotropin secretion and reproductive function are well established [1–5]. Severe perturbations to homeostasis, such as immune/inflammatory stress, interfere with the ovulatory cycle in species ranging from rodents to ruminants to primates [6–9]. Less invasive disturbances, such as psychosocial stress, suppress gonadotropin secretion, but little definitive evidence exists that, by themselves, these disturbances interfere with the ovarian cycle. Given the pervasive nature of psychosocial stress in today's society, it is important to understand the extent to which this type of stress impacts reproductive fitness as well as the underlying mechanisms. Functional hypothalamic amenorrhea, a common menstrual cycle disorder in humans, has been attributed to psychosocial stress [10–12], but direct evidence that this reflects a stress response is lacking. More definitive evidence in monkeys indicates extended psychosocial stress combined with other stressors can interfere with reproductive hormone secretion and disrupt the menstrual cycle [13, 14]. In sheep, the preovulatory luteinizing hormone (LH) surge can be delayed by truck transport [15], but to our knowledge, no evidence is available that this type of psychosocial stress disrupts the estrous cycle.
We have determined that psychosocial stress inhibits pulsatile LH secretion in ovariectomized sheep by reducing both gonadotropin-releasing hormone (GnRH) pulse amplitude [16] and pituitary responsiveness to GnRH [17]. Although the suppression of pulsatile GnRH secretion is independent of cortisol action [16], inhibition of pituitary responsiveness is caused by the concurrent rise in plasma cortisol [17]. To gain insight into the impact of psychosocial stress on reproductive function and the mediatory role of cortisol and other factors in this response, we aimed to develop a model whereby psychosocial stress interferes with the estrous cycle of sheep. To this end, we conducted a series of experiments to test effects of short-term and prolonged exposure to acute bouts of various psychosocial stressors previously found to stimulate glucocorticoid secretion in sheep. The present report describes our finding that the estrous cycle of the ewe is surprisingly resistant to disruption by psychosocial stress.
MATERIALS AND METHODS
Five experiments were conducted in the breeding seasons of 5 yr (September–January, 2002–2007) on mature Suffolk ewes maintained under standard husbandry conditions at the Sheep Research Facility near Ann Arbor, Michigan. The ewes were obtained from multiple suppliers and, thus, were of diverse genetic stock. Ewes were fed hay and alfalfa pellets and had free access to water and mineral licks. All expressed estrous cycles before use, and none were previously exposed to a known stressor. To enable groups of ewes to be studied simultaneously, the follicular phase of the cycle was synchronized using two intravaginal progesterone-releasing devices (Controlled Intravaginal Drug Release Devices [CIDRs]; DEC International) as described elsewhere [18]. CIDRs maintain a luteal-phase plasma progesterone concentration (2–4 ng/ml) and remain in place for 14 days (duration of the luteal phase). Upon CIDR removal, the follicular phase is initiated, and the preovulatory LH surge occurs approximately 48 h later. Except as noted, ewes were not penned with rams during experiments, and blood was sampled via indwelling jugular cannulae. Procedures were approved by the Committee for the Use and Care of Animals at the University of Michigan.
Psychosocial Stress Models
Nine psychosocial stress paradigms were applied variously across experiments. Pilot studies indicated that each enhanced cortisol secretion.
Isolation.
Animals were individually moved from group housing to separate rooms to eliminate visual contact with other sheep (auditory cues still possible).
Layered stress.
Ewes were exposed hourly to four cumulative stressors: isolation, restraint by confinement in a pen (0.5 × 1.2 m), blindfold to remove visual cues, and predator threat by the sound of a barking dog played on a compact disc (CD).
Food denial.
Animals were penned adjacent to flock mates being fed at the normal time. Experimental ewes were not fed until after completion of the stress (2 h).
Noise/exercise.
Animals were forced to walk around a room (5 × 6 m) for 15–20 min, followed by 5–10 min of rest during which time random, unfamiliar noises were played via CD (e.g., helicopter, jackhammer, and exotic animal noises). This was repeated for 1–2 h.
Circadian stress.
Ewes were awakened around midnight and forced to walk around a room (5 × 6 m) for 15–20 min, followed by 5–10 min of rest. This was repeated for 1–2 h.
Transport.
Animals were transported in a livestock trailer on a variety of road surfaces and speeds for approximately 1.5 h. After a 30- to 45-min rest period, transport resumed for 1 h.
Mock shear.
Ewes were restrained upright on the rump, and electric shears were lightly moved around the body, removing small amounts of wool from the midsection, head, and fore and hind legs for 5 min. After a 1-h rest period, the process was repeated.
Barking dog.
Two live dogs were brought into the room where ewes were penned to prevent physical contact. The dogs barked intermittently for 5 min and were then removed. The stress was repeated 45 min later.
Blindfold/barking-dog CD.
Ewes were blindfolded, and a CD of a barking dog was played for 5 min. This was repeated every 20 min for 1–2 h.
Experimental Designs
Five experiments were performed to test if psychosocial stress disrupts the estrous cycle. Because the outcome of each experiment dictated the design of subsequent ones, the rationale, purpose, and design of each experiment are presented in Results.
Assays
Luteinizing hormone was measured in duplicate aliquots of plasma (5–200 μl) using a modification [19] of a previously described radioimmunoassay [20, 21] and is expressed in terms of NIH-LH-S12. Intra- and interassay coefficients of variation were 5.0% and 6.6%, respectively, and assay sensitivity averaged 0.6 ng/ml (37 assays). Plasma cortisol was determined in duplicate aliquots (50 μl) using the Coat-a-Count cortisol kit (Siemans Healthcare Diagnostics, Inc.) validated for use in sheep [22]. Intra- and interassay coefficients of variation were 7.3% and 8.0%, respectively. Assay sensitivity averaged 0.7 ng/ml (32 assays). Progesterone was measured in duplicate 100-μl aliquots of plasma using the Coat-a-Count progesterone kit (Siemans Healthcare Diagnostics, Inc.) previously validated for use in sheep [23]. Intra- and interassay coefficients of variation were 8.2% and 9.3%, respectively, and assay sensitivity averaged 0.2 ng/ml (62 assays). Glucose was assayed in duplicate 10-μl plasma aliquots by a glucose oxidase-based colorimetric assay (Modified Trinder; Pointe Scientific, Inc.) validated for use in sheep [24].
Data Analysis
The LH surge was defined as a rise in plasma LH concentration exceeding 2 SD above the presurge baseline and maintained for at least 4 h. Latent period to the surge was the interval from progesterone withdrawal (CIDR removal) to LH surge peak; the peak was defined as surge amplitude. LH surge duration was defined as the interval from the surge onset (i.e., when the LH value exceeded 2 SD of the presurge baseline) to the time that LH fell to 10% of the surge peak. Hormone concentrations were log-transformed before analysis to normalize variation across a broad range of values. LH pulses were identified by the Cluster pulse-detection algorithm [25]. As in our previous studies [26], peak and nadir cluster sizes were set at one and two, and the t-statistic used to determine significant increases or decreases in hormone concentration was 2.6. LH pulse amplitude was defined as the difference between the peak and preceding nadir. Frequency was calculated as number of pulses per sampling period. Further details of statistical analyses are presented in Results. Significance was defined as P < 0.05.
RESULTS
Experiment 1: Does Repeated Isolation Disrupt the Preovulatory LH Surge?
The follicular phase of the estrous cycle was synchronized in two groups of ewes: nonstress control (n = 6) and repeated isolation stress (n = 6). Controls were housed separately as a group to avoid exposure to the stress group. Stressed ewes were housed together before CIDR removal (0 h) and individually moved to isolation rooms for 6 h on three occasions during a single follicular phase: 12–18, 24–30, and 36–42 h after CIDR removal. These times represent the early and midfollicular phase and the immediate presurge period, respectively (LH surge peak expected ∼48 h after CIDR removal). After each isolation, ewes were returned to their common room. Blood was sampled from both groups every 30 min from 2 h before to 2 h after each isolation to assay cortisol and then every 2 h thereafter until 84 h to monitor the LH surge. Previous work indicates that isolation inhibits pulsatile LH secretion in ovariectomized ewes (Karsch, unpublished data), but to our knowledge, effects on the preovulatory LH surge have not been described.
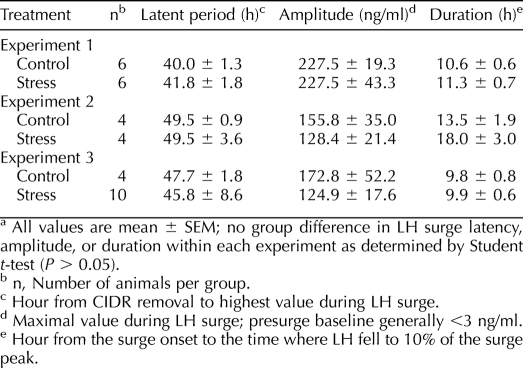
In control ewes, plasma cortisol remained low throughout sampling (mean ± SEM, 11.0 ± 1.8 ng/ml). In stressed ewes, cortisol increased from 13.5 ± 2.2 ng/ml before stress to 35.3 ± 4.1, 29.7 ± 4.0, and 35.0 ± 5.9 ng/ml during the three respective bouts of isolation (P < 0.02; prestress vs. stress as determined by repeated-measures ANOVA). Peak values during stress (37.5 ± 1.0 ng/ml) averaged approximately threefold over basal levels. All ewes exhibited the preovulatory LH surge. Timing, amplitude, and duration of the surge did not differ between control and stress ewes as determined by Student t-test (Table 1, experiment 1).
TABLE 1.
Effect of psychosocial stress on LH surge parameters in experiments 1, 2, and 3.a
Experiment 2: Does the Layered Stress Paradigm Disrupt the Preovulatory LH Surge?
Because experiment 1 revealed repeated exposure to the same stressor did not affect the incidence, timing, amplitude, or duration of the preovulatory LH surge, we hypothesized that variable psychosocial stress would be disruptive. Thus, we applied the layered stress paradigm in which novel psychosocial stressors are sequentially added over a 4-h period (see Materials and Methods). This paradigm stimulates cortisol and inhibits GnRH and LH pulse amplitude in ovariectomized ewes [16, 17]. In addition to monitoring cortisol and the LH surge, LH pulses were examined before the surge.
The follicular phase of the estrous cycle was synchronized in two groups of ewes: nonstress control (n = 4) and stress (n = 4). Starting 12 h after CIDR removal, jugular blood for LH pulse analysis was sampled at 6-min intervals for 14 h; cortisol was assayed at 30-min intervals. Ewes in both groups were maintained under calm conditions for the first 4 h of sampling. At that point (i.e., 16 h after CIDR removal), the stress group was exposed to the 4-h layered stress paradigm; controls remained in calm conditions throughout. Stressed ewes were then returned to a common room for 2 h and subjected to a second 4-h bout of layered stress (22–26 h after CIDR removal, 6-min sampling continued). Blood was then sampled every 3 h until 72 h after CIDR removal to assess the LH surge.
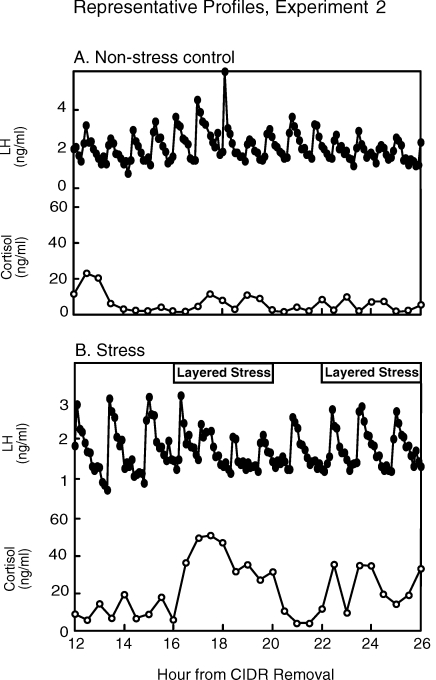
Representative LH and cortisol profiles are shown in Figure 1. Cortisol remained at basal levels throughout sampling in nonstressed controls (10.2 ± 2.0 ng/ml). Repeated-measures ANOVA revealed mean plasma cortisol concentrations increased during the first exposure to the layered stress (prestress, 8.7 ± 1.8 ng/ml; stress, 35.9 ± 4.7 ng/ml; treatment × time interaction, P < 0.05). During the second stress exposure, mean cortisol tended to increase; values rose to only 17.6 ± 3.6 ng/ml (P = 0.08 compared to prestress values). Peak cortisol values during the second bout of stress were 43% less than peak values during the first exposure (second bout, 28.0 ± 5.6 ng/ml; first bout, 49.3 ± 8.6 ng/ml; P < 0.02, paired t-test).
FIG. 1.
LH and cortisol profiles for one representative ewe kept under nonstress conditions (A) or exposed to the layered stress paradigm (B) on two occasions in experiment 2. The layered stress paradigm is depicted at the top in B and consisted of sequential hourly application of isolation, restraint, blindfold, and predator cues.
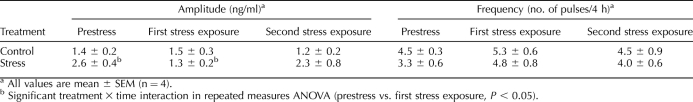
In controls, LH pulse frequency and amplitude did not change significantly during the frequent sampling period (Fig. 1 and Table 2). In stressed ewes, mean LH pulse amplitude decreased by 50% during the first stress period compared to prestress values (P < 0.05, repeated-measures ANOVA). The second bout of stress, however, did not alter the amplitude of LH pulses compared to prestress values (P > 0.05). No change in LH pulse frequency was detected in either group. Importantly, all ewes expressed the LH surge, and no difference was observed in surge timing, amplitude, or duration as determined by Student t-test (Table 1, experiment 2).
TABLE 2.
Effect of psychosocial stress on LH pulse parameters in experiment 2.
Experiment 3: Does Repeated, Variable Psychosocial Stress Disrupt the LH Surge?
In experiments 1 and 2, neither repeated isolation nor the layered stress affected timing, amplitude, or duration of the preovulatory LH surge. However, either the same stressor was repeatedly used (experiment 1), which might have caused habituation, or the stress encompassed only a limited (possibly insufficient) portion of the follicular phase (experiment 2). We thus hypothesized that repeated, variable stress during a major portion of the follicular phase would interfere with the LH surge.
The follicular phase was synchronized in two groups: stress (n = 10) and nonstress control (n = 4). Stress ewes were exposed to five different stressors between 15 and 42 h after CIDR removal (0 h) as follows: 1) food denial combined with noise/exercise (15–18 h), 2) layered stress (22–26 h), 3) circadian stress (31–32 h), 4) transport (38–41 h), and 5) mock shear (41–42 h). Blood was sampled every 20 min during stress to monitor cortisol and every 3 h from 26 to 72 h to assess the LH surge. Previous work suggests transport by itself, at the time used here, delays the preovulatory LH surge [15].
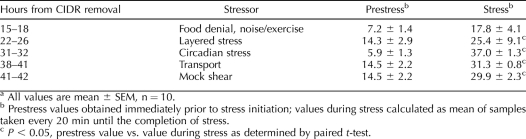
Each stress except food denial significantly increased mean plasma cortisol levels compared to immediate prestress values (paired t-test) (Table 3). Although cortisol declined toward the initial prestress level between stresses, values immediately before the next stress generally remained elevated; for example, values before the last stress were twofold greater than the initial prestress value (14.5 ± 2.2 vs. 7.2 ± 1.4 ng/ml, respectively; P < 0.05 by paired t-test). Despite the sustained cortisol response, sequential exposure to the five different stressors did not influence the occurrence, timing, amplitude, or duration of the LH surge (Table 1, experiment 3).
TABLE 3.
Cortisol values (ng/ml) for experiment 3.a
Experiment 4: Does Extended Psychosocial Stress Disrupt the Estrous Cycle?
Results to this point suggest repeated exposure to acute psychosocial stress during a single follicular phase does not disrupt the preovulatory LH surge, although such stress did increase glucocorticoid secretion and, as shown for the layered stress in experiment 2, inhibit pulsatile LH secretion. Work in monkeys indicates more prolonged psychosocial stress combined with other stressors could interfere with menstrual cycles [13, 14]. Here, we tested if prolonged exposure to acute bouts of psychosocial stress over the course of more than two cycles disrupts the estrous cycle of ewes. To reduce effects of habituation (i.e., desensitization during prolonged exposure to continous stress), the stressors were applied in random order.
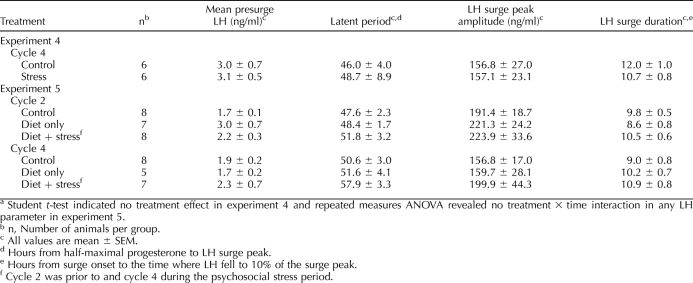
Estrous cycles of 12 ewes were synchronized using CIDRs during the early breeding season (September). Ewes were randomly allocated to two groups: nonstress control (n = 6) and stress (n = 6). Daily blood samples were taken via jugular venipuncture to monitor progesterone for five consecutive estrous cycles (October–December). Both groups were kept under nonstress conditions for cycles 1 and 2 to establish basal estrous cycle parameters. Beginning in the follicular phase of cycle 3, the stress group was acutely subjected to six different stressors in random sequence every 1–2 days for 39 days (exceeding two cycles): barking dog, circadian stress, layered stress, mock shear, transport, and noise/exercise. Between acute stress sessions, ewes were isolated as much as facilities and space would allow (∼50% of the time). Nonstress controls remained in calm conditions. Blood for cortisol assay was sampled before and immediately after each acute stress. During the follicular phase of cycle 4 (second stress cycle), samples were taken at 4-h intervals to assess presurge and surge LH secretion (4-h sampling in each ewe began when progesterone fell to at least 50% of the peak value as determined by daily progesterone assays). Thereafter, daily sampling continued under nonstress conditions in cycle 5 to assess carryover effects, because observations in monkeys suggest that stress effects may manifest in the subsequent cycle [8, 13].
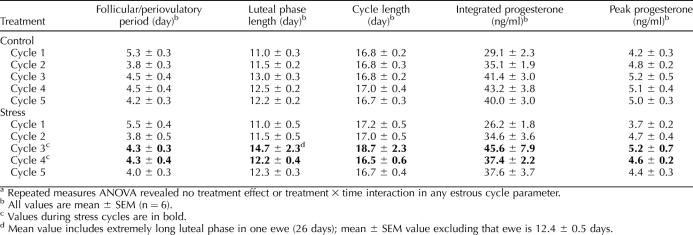
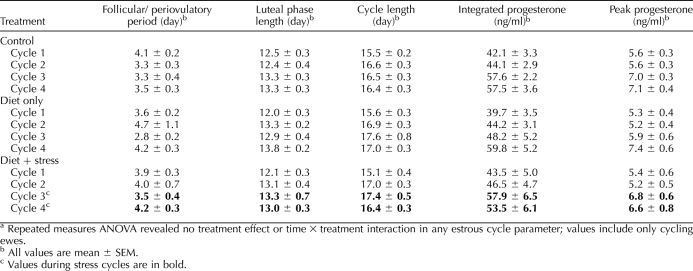
Nine estrous cycle parameters were examined: 1) length of follicular phase/periovulatory period: days plasma progesterone concentration was less than 0.5 ng/ml; 2) luteal-phase length: days plasma progesterone was 0.5 ng/ml or greater; 3) cycle length: sum of parameters 1 and 2; 4) integrated luteal-phase progesterone: sum of all values within a given luteal phase; 5) peak luteal-phase progesterone: average of three highest contiguous values within a given luteal phase; 6) presurge LH value (cycle 4): mean plasma LH values from the time progesterone fell to half-maximal to the onset of the LH surge; 7) latency to LH peak (cycle 4): time from half-maximal progesterone to apex of surge; 8) LH surge amplitude: maximal value during surge; and 9) LH surge duration.
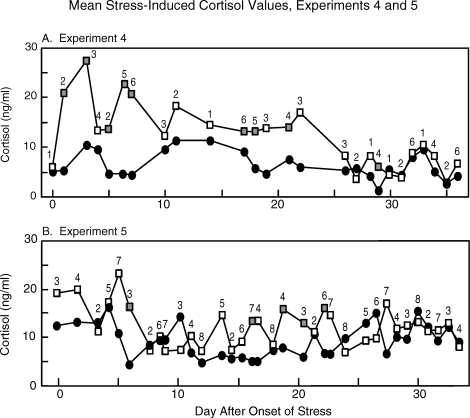
Plasma cortisol (weekly samples) remained low throughout the experiment in nonstress controls (9.4 ± 0.4 ng/ml) and in stressed ewes during nonstress cycles (8.8 ± 0.9 ng/ml; cycles 1, 2, and 5). Initially, cortisol was increased by acute stresses, but responses became progressively dampened until no elevation in cortisol was detected. In this regard, cortisol increased significantly following 8 of 14 stress occasions during the first 3 wk of stress but on only 1 of 12 subsequent stress occasions (Fig. 2A). It should be noted, however, that peak cortisol responses likely were missed, because the cortisol rise was monitored just after each stress session.
FIG. 2.
Mean prestress (closed circles) and immediate poststress (open and shaded squares) plasma cortisol concentrations in stress ewes in experiment 4 (A) and experiment 5 (B). The numbers above the poststress values depict the stressor used: 1 = barking dog, 2 = circadian stress, 3 = layered stress, 4 = mock shear, 5 = transport, 6 = noise/exercise, 7 = isolation, and 8 = blindfold/barking dog CD. Poststress values indicated by shaded squares denote a significant difference between pre- and poststress values as determined by paired t-test (P < 0.05, n = 6 ewes in experiment 4 and n = 8 ewes in experiment 5).
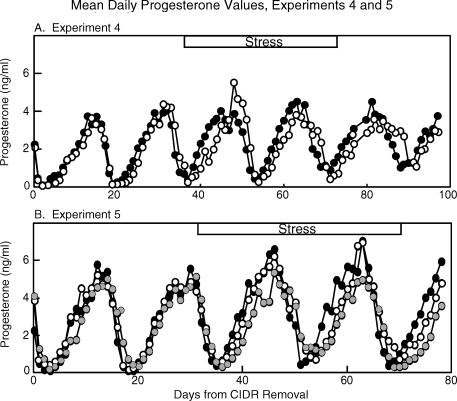
Overall, the mean plasma progesterone profile was similar in stressed and control ewes (Fig. 3A). Repeated-measures ANOVA revealed no effect of stress on any estrous cycle parameter examined (no treatment effects or treatment × time interactions). Length of the cycle and its stages as well as progesterone values were consistent within groups and similar between groups across the five estrous cycles (Table 4). Furthermore, presurge LH concentrations and LH surge parameters (cycle 4) were not altered by stress (Table 5, experiment 4). Of note, in the first stress cycle, one ewe exhibited a prolonged luteal phase compared to the mean for rest of the group (26 vs. 12.4 ± 0.5 d for other ewes); progesterone values for this ewe were not included in Figure 3A.
FIG. 3.
Mean daily plasma progesterone values in experiment 4 (A) for nonstress control (closed circles) and stress ewes (open circles), and in experiment 5 (B) for nonstress control (closed circles), diet-only (gray circles), and diet-plus-stress (open circles) ewes. The period of stress is depicted at the top of each panel. In experiment 4 (A), values from one stress ewe that expressed an abnormal luteal phase in cycle 3 were excluded. In experiment 5 (B), values were excluded once ewes ceased to express estrous cycles. In experiment 4 (A), n = 5–6 ewes/group, and in experiment 5 (B), n = 5–8 ewes/group.
TABLE 4.
Effect of psychosocial stress on estrous cycle parameters in experiment 4.a
TABLE 5.
Presurge LH values and LH surge parameters for experiments 4 and 5.a
Experiment 5: Does Extended Psychosocial Stress Disrupt the Estrous Cycle and Sexual Behavior of Nutritionally Restricted Ewes?
Experiment 4 did not reveal any deleterious effects of stress on the estrous cycle, with the possible exception of a lengthened luteal phase in one ewe during the first stress cycle, as mentioned above. This ewe was leaner and appeared to be in poorer body condition than the others. While conducting experiment 4, we became aware of a study in monkeys indicating that psychosocial stress alone had no effect on the menstrual cycle but was disruptive when combined with the metabolic stress of diet plus exercise [14]. Collectively, these observations led to the hypothesis tested here: repeated, acute variable psychosocial stress would disrupt the estrous cycle in nutritionally compromised ewes.
Beginning 5 mo before monitoring reproductive endpoints, 16 ewes were fed a restricted-calorie diet designed to reduce body weight by 25% when estrous cycles began at onset of the breeding season (September). Daily caloric intake was initially lowered by reducing the standard diet by 25%, after which food restriction was individually adjusted to attain and maintain the targeted weight loss. Ewes were weighed weekly before daily feeding and were monitored daily for adverse health effects (e.g., lethargy, emaciation, and lack of social interaction). At the start of the breeding season, food-restricted ewes were allocated to two groups balanced for body weight: diet only (n = 8) and diet plus psychosocial stress (n = 8). Control ewes (n = 8) were normally fed and nonstressed.
This experiment was conducted in the same manner as experiment 4 except carryover effects of stress were not monitored in cycle 5, because experiment 4 indicated no such effects. Instead, reproductive behavior was monitored in cycle 5, because recent evidence indicates psychosocial stress interferes with certain aspects of sexual behavior (proceptivity and attractiveness of ewe to ram) in ovariectomized ewes treated with hormones to induce estrus [27]. Also, blood was sampled at 3-h intervals in the follicular phases of both cycle 2 (prestress) and cycle 4 (stress) to obtain better resolution of presurge and surge LH secretion than in the previous experiment and to allow comparison within individuals before and during stress. Additional samples were collected before feeding at the start of cycle 1 and the end of cycles 3 and 5 to assay glucose.
To facilitate monitoring of reproductive behavior, CIDRs were inserted at the onset of the luteal phase in cycle 5 and left in place for 14 days after cycle 5. Beginning 20 h after CIDR removal, ewes in the diet-plus-stress group were individually moved from isolation rooms every 6 h to a pen containing a vasectomized ram and then observed for the onset of estrus, which was defined as the first immobilization that allowed the ram to mount. Control ewes and ewes in the diet-only group were walked from an adjacent pen into the pen containing the ram for behavioral testing. After 3 min of observation, ewes were removed from the test area to avoid further interaction with the ram. Ewes in the diet-plus-stress group were isolated between observations. Once ewes expressed receptive behavior, they were removed from the study. Observations continued until 68 h after CIDR removal (6 h after onset of receptivity in the last control ewe).
Metabolic indicators.
Ewes in the restricted-diet group lost 22% of their initial body weight (start, 80.0 ± 4.8 kg; end, 62.3 ± 3.5 kg). Ewes in the diet-plus-stress group lost 24% of their initial body weight (start, 76.9 ± 4.5 kg; end, 57.7 ± 2.2 kg). Weight loss ranged from 13% to 32%. Weights of normally fed controls did not change (start, 69.2 ± 2.4 kg; end, 70.6 ± 1.7 kg). Blood glucose levels were within the normal prefeeding range in sheep [28] for all groups at each time monitored (∼50 mg/dl).
Cortisol.
Plasma cortisol (weekly samples) in control and diet-only ewes remained low and unchanged throughout the experiment (7.1 ± 1.0 and 9.6 ± 1.2 ng/ml, respectively; P > 0.1, repeated-measures ANOVA). In diet-plus-stress ewes, cortisol was also low (9.4 ± 0.6 ng/ml) during nonstress cycles (cycles 1 and 2). During stress cycles (cycles 3 and 4), cortisol increased following 5 of 34 acute stress sessions (paired t-test of prestress vs. immediate poststress values) (Fig. 2B). However, as in experiment 4, maximal cortisol responses likely were missed, because samples were not taken during the actual stress periods. Unlike experiment 4, significant cortisol responses were not seen primarily in the initial stress periods.
Cycle parameters.
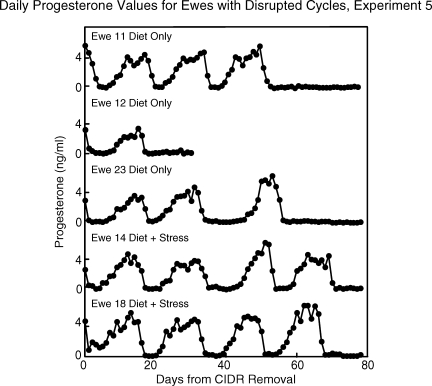
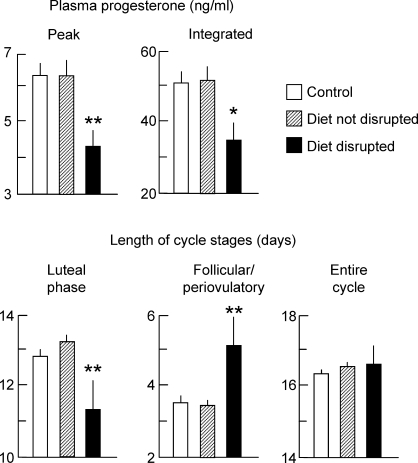
All ewes began to express estrous cycles at the start of the breeding season. Mean daily progesterone profiles were similar between control and experimental ewes that continued to express estrous cycles (Fig. 3B). In these ewes, repeated-measures ANOVA revealed no group differences for any estrous cycle parameter monitored (Table 6), including presurge LH values and LH surge parameters (Table 5, experiment 5). Notably, 5 of the 16 nutritionally compromised ewes either became anovulatory, as judged by lack of a luteal phase rise in progesterone, or had severely disrupted cycles, as judged by a follicular phase/periovulatory period duration exceeding twice the upper 95% confidence interval of control ewes (Fig. 4). Three of these ewes were in the diet-only group, and two were in the diet-plus-stress group. One of these ewes (ewe 12, diet-only group) became anovulatory after cycle 1 and was not monitored beyond the first missed cycle. Before cycles became severely disrupted or ceased altogether in these ewes, cycle characteristics began to deteriorate as judged by a 32% decrease in both the peak and integrated plasma progesterone values in the luteal phase, a shortening of the luteal phase, and a lengthening of the follicular phase (although total cycle length did not change) (Fig. 5). Average weight loss in animals that had disrupted cycles (18.6%) was no greater than that of the other nutritionally compromised ewes (23.9%). No control ewes stopped expressing cycles during the course of the study.
TABLE 6.
Effect of psychosocial stress on estrous cycle parameters in experiment 5.a
FIG. 4.
Mean daily plasma progesterone profiles of three diet-only and two diet-plus-stress ewes that ceased to express estrous cycles during experiment 5. Sampling ended in ewe 12 (diet only) after the first missed estrous cycle.
FIG. 5.
Mean estrous cycle parameters in controls (open bars; n = 8), diet-restricted animals (with or without psychosocial stress) that continued to cycle (hashed bars; n = 11), and diet-restricted animals that subsequently ceased to cycle (closed bars; n = 5) in experiment 5. Once an animal displayed disrupted cyclicity, values were excluded from the analysis (ANOVA). *P < 0.02, **P < 0.01 compared to control and diet-restricted animals that continued to have normal cycles.
Sexual behavior.
No significant differences were found in time of estrous onset (first mount by the ram) among control, diet-only, and diet-plus-stress groups as determined by repeated-measures ANOVA (43.3 ± 3.4, 48.2 ± 6.1, and 54.0 ± 4.5 h, respectively, after CIDR removal). However, when values in the diet-only and diet-plus-stress groups were combined (no significant difference between these groups), the latency to estrus was significantly increased compared to normally fed controls (51.4 ± 3.0 vs. 43.3 ± 3.4 h, respectively, after CIDR removal; P < 0.05, Student t-test). Four ewes had not entered estrus (two diet-only ewes, two diet-plus-stress ewes) by the end of the observation period (68 h) and were not included in the analysis. These were the same four ewes that either had severely disrupted estrous cycles or ceased to express cycles based on progesterone profiles (behavior was not monitored in the other ewe that stopped cycling [ewe 12]).
DISCUSSION
In the present study, we aimed to develop a model in which psychosocial stress disrupts the estrous cycle of sheep, a model that could be used to examine mechanisms and mediators of stress-induced suppression of reproductive activity. Neither repeated exposure to various acute psychosocial stressors during a single follicular phase (experiments 1–3) nor repeated exposure to such stressors over the course of two estrous cycles (experiments 4 and 5) altered the incidence, timing, amplitude, or duration of the preovulatory LH surge. In all five experiments collectively, the overall mean latent period to the LH peak among 33 stressed ewes were remarkably similar to that of 28 nonstressed controls (49 vs. 47 h in stressed and control ewes, respectively). Likewise, overall mean peak of the LH surge for all experiments was similar (166 and 174 ng/ml in stressed and control ewes, respectively). Thus, an effect of our stress paradigms on LH surge characteristics, if any, was negligible and not detectible by our sampling intervals (2–4 h across the five experiments).
The lack of an effect of psychosocial stress on the LH surge was unexpected based on earlier findings that one of the stressors employed, transport shortly before onset of the preovulatory LH surge, delayed the LH surge of the ewe [15]. Furthermore, our stress paradigms elicited other neuroendocrine stress responses, such as enhanced cortisol and reduced pulsatile LH secretion, both of which could negatively impact the estrous cycle and the LH surge. For example, exogenous cortisol can disrupt the follicular phase of the cycle [29, 30] and delay both the spontaneous [29, 30] and estradiol-induced [31, 32] LH surge of the ewe. These disruptive effects of cortisol, however, were observed with plasma cortisol increments considerably higher and more prolonged than those induced by stress in the present study. Therefore, the lack of effect of our psychosocial stress paradigms on the LH surge could have resulted, at least in part, from an insufficient magnitude or duration of the stress-induced increment in plasma cortisol.
In addition to lack of an effect on the LH surge, repeated exposure to various random psychosocial stressors over the course of two estrous cycles did not interfere with expression of the estrous cycle or the duration of its follicular and luteal phases, nor did it impair function of the corpus luteum as monitored by plasma progesterone concentrations (experiment 4). Although this suggests lack of an effect on mechanisms that underlie generation of the estrous cycle, it remains possible that the stress paradigms employed here could interfere with reproductive endpoints that were not monitored, such as ovulation rate, percentage of ewes that ovulate or of ovulated eggs that can be fertilized, implantation, and so on. Further work would be needed to address this possibility.
Repeated exposure to variable, acute psychosocial stressors also failed to interfere with the estrous cycle when combined with the metabolic stress of caloric restriction (experiment 5). Although estrous cycles and sexual behavior were disrupted in two of eight ewes receiving the combined stress, this cannot be attributed to psychosocial stress, because the cycle and behavior were similarly disrupted in three of the eight metabolically stressed ewes not subjected to psychosocial stress. Lack of an effect of psychosocial stress was unexpected based on studies with rhesus monkeys in which chronic psychosocial stress combined with other stress types, including metabolic stress, interfered with the menstrual cycle and reproductive hormone secretion [13, 14]. Nevertheless, work in pigs suggests that repeated exposure to acute stress, as employed in the present study, is also insufficient to disrupt the cycle [33]. The possibility that duration of exposure to stress might account for the differing results is considered below.
The cessation of estrous cycles in nearly one third of the food-restricted animals in experiment 5 (both groups combined) is noteworthy, because ewes in our flock rarely stop expressing estrous cycles once they commence at the onset of the breeding season. For example, cycles persisted throughout the observation period spanning five estrous cycles in all 14 control ewes of experiments 4 and 5 combined. Of interest, cessation of cycles was preceded by inadequate corpus luteum function (reduced progesterone secretion and short luteal phase) and a prolonged follicular phase. These observations are consistent with the stress-induced disturbances of menstrual cycle characteristics reported in rhesus monkeys [13, 14, 34] and with the conclusion that inadequate secretory function of the corpus luteum is an early stage of stress-induced derangement of the ovulatory cycle [13, 34]. In addition, the metabolically stressed ewes in the present study exhibited a prolonged latent period to onset of sexual receptivity (both groups combined).
Further work is needed to determine how metabolic stress interferes with corpus luteum function and disrupts the estrous cycle and sexual behavior. Long-term caloric restriction of sheep was previously reported to inhibit pulsatile LH secretion, which would be expected to negatively impact the estrous cycle, but this was seen only with severe weight loss and deterioration of body condition (e.g., 40% weight loss) [35, 36]. In the present study, weight loss averaged considerably less, and it was no greater in ewes that stopped cycling (18.6%) than in those that continued to cycle (23.9%). Although we did not characterize LH pulses in food-restricted ewes, the presurge plasma LH concentration assessed by 3-hourly sampling, which reflects LH release during a time when LH is primarily pulsatile, was not significantly lowered by reduced caloric intake. It would be useful to determine if the food-restriction paradigm used here alters LH pulse frequency and/or amplitude as well as other aspects of estrous cycle regulation (e.g., FSH secretion, follicular development and estradiol secretion, or responsiveness of behavioral centers or the LH surge-generating mechanism to the stimulatory effects of estradiol).
Our present findings raise an important question. How can we account for the finding that metabolic stress interfered with the estrous cycle in some ewes whereas our psychosocial stress paradigms were uniformly ineffective in this regard? We forward three explanations that, alone or in combination, might explain this result: 1) desensitization to psychosocial stress over time (i.e., habituation) [37, 38], 2) the nature or severity of the stressor, and 3) duration of the stress. Initial evidence for habituation was obtained in experiment 2, in which the layered stress paradigm was applied twice during the follicular phase; significant stimulation of cortisol and inhibition of LH pulses were seen only during first exposure to the stressor. Although we attempted to minimize habituation in subsequent experiments by using differing psychosocial stressors in random sequence, desensitization was still suggested by progressively declining (experiment 4) or minimal (experiment 5) cortisol responses, but it should be emphasized that cortisol was not monitored at the expected time of peak responses in those experiments.
Based on the cortisol response, however, no sign of habituation was observed in experiment 1, yet repeated isolation did not interfere with the LH surge. This points to our other explanations for the lack of cycle disruption: nature/severity and duration of the stress. Regarding the nature or severity of the stress, it is noteworthy that another stress type, acute immune/inflammatory stress modeled by a 26-h infusion of endotoxin, disrupted the follicular phase of sheep by inhibiting pulsatile LH and estradiol secretion and by delaying or preventing the LH surge and estrous behavior [7]. Endotoxin infusion also stimulated cortisol secretion for at least 26 h. That our psychosocial stress paradigms were not as severe is suggested by a smaller plasma cortisol elevation (<40 ng/ml) compared to that observed with endotoxin (>100 ng/ml) [7], and the cortisol rise was not sustained through the stress period. Thus, the psychosocial stressors used here might have been relatively weak compared to endotoxin and insufficient to disrupt the estrous cycle.
The lack of an effect on the estrous cycle might also have been caused by the duration of the stress. In all experiments, we applied psychosocial stress acutely and intermittently. Although the stress period spanned two estrous cycles in experiments 4 and 5, the duration of any individual stress session during that period might not have been sufficient to elicit disruptive effects, and the intermittent nature of the stress might have allowed the ewes to recover from one bout of stress before the next one was applied. In contrast, the metabolic stress paradigm, which disrupted the cycle in some ewes, was continuous and initiated 5 mo before reproductive endpoints were monitored, and it continued for several more months during the observation period. It may well be that the estrous cycle of the ewe would be disrupted only in response to chronic psychosocial stress.
Despite the potential importance of duration, we are not aware of any definitive evidence that psychosocial stress by itself interferes with the cycle. Even with the aforementioned studies in primates, in which chronic psychosocial stress was disruptive, its efficacy alone either was not tested [13] or psychosocial stress had to be combined with other stress types to disrupt the cycle [14]. Perhaps the drive to reproduce is so strong that compensatory mechanisms, such as habituation, diminish the ability of psychosocial stress to interfere with cyclicity. This might be especially true in seasonal breeders, such as sheep, in which the physiologic drive and selection pressure to take advantage of the window of opportunity to reproduce is so intense that perturbations caused by the type of stressors used here are insufficient to interfere with ovulation. Seasonal changes in both reproductive neuroendocrine and glucocorticoid responses to stress have been observed [39–42], but their relevance to estrous cycle disruption has not been investigated.
In conclusion, the estrous cycle of the ewe appears to be remarkably resistant to disruption by acute bouts of psychosocial stress, whether applied intermittently during a single follicular phase or repeatedly over the course of several estrous cycles. This contrasts to the disruptive influence of a more severe stress type, immune/inflammatory stress [7], or chronic metabolic stress caused by caloric restriction (experiment 5). Because of an increasing awareness of the consequences of psychosocial stress on a myriad of physiological processes, including reproduction, our findings encourage further work to address why the psychosocial stress models employed in the present study failed to interfere with cyclicity as well as to explore whether chronic psychological stress interferes with the estrous cycle in this species.
Acknowledgments
We thank Doug Doop and Gary McCalla for their expert animal care and Morton Brown, Alison Cooper, Jennifer Davis, Kari Dugger, Loren Heller, James Lee, Christopher McCrum, Sarah Musil, Andrew Pytiak, Harold Render, Dustin Robinson, Juho Won, and Britta Wunderlich for their assistance in planning, conducting, and interpreting the experiments. We are also grateful to Drs. Gordon D. Niswender and Leo E. Richert, Jr., for supplying LH assay reagents.
Footnotes
Supported by NSF Grant IOB0520597 and NIH Grants HD30773, HD051360.
REFERENCES
- Rivier C, Rivest S.Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod 1991; 45: 523–532. [DOI] [PubMed] [Google Scholar]
- Ferin M.Neuropeptides, the stress response, and the hypothalamo-pituitary-gonadal axis in the female rhesus monkey. Ann N Y Acad Sci 1993; 697: 106–116. [DOI] [PubMed] [Google Scholar]
- Dobson H, Smith RF.What is stress, and how does it affect reproduction? Anim Reprod Sci 2000; 60–61: 743–752. [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI, Clarke IJ.Effects of stress on reproduction in nonrodent mammals: the role of glucocorticoids and sex differences. Rev Reprod 2000; 5: 105–113. [DOI] [PubMed] [Google Scholar]
- Breen KM, Karsch FJ.New insights regarding glucocorticoids, stress and gonadotropin suppression. Front Neuroendocrinol 2006; 27: 233–245. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Rivest S.Effect of immune and metabolic challenges on the luteinizing hormone-releasing hormone neural system in cycling female rats: an evaluation at the transcriptional level. Endocrinology 1997; 138: 1374–1384. [DOI] [PubMed] [Google Scholar]
- Battaglia DF, Krasa HB, Padmanabhan V, Viguié C, Karsch FJ.Endocrine alterations that underlie endotoxin-induced disruption of the follicular phase in ewes. Biol Reprod 2000; 62: 45–53. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Barth A, Zhu J, Ferin M.Stress and the menstrual cycle: relevance of cycle quality in the short- and long-term response to a 5-day endotoxin challenge during the follicular phase in the rhesus monkey. J Clin Endocrinol Metab 1998; 83: 2454–2460. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Ferin M.Stress and the menstrual cycle: short- and long-term response to a 5-day endotoxin challenge during the luteal phase in the rhesus monkey. J Clin Endocrinol Metab 1999; 84: 623–626. [DOI] [PubMed] [Google Scholar]
- Reifenstein EC., JrPsychogenic or “hypothalamic” amenorrhea. Med Clin North Am 1946; 30: 1103–1114. [DOI] [PubMed] [Google Scholar]
- Berga SL, Girton LG.The psychoneuroendocrinology of functional hypothalamic amenorrhea. Psychiatr Clin North Am 1989; 12: 105–116. [PubMed] [Google Scholar]
- Marcus MD, Loucks TL, Berga SL.Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril 2001; 76: 310–316. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Ferin M.Inadequate luteal function is the initial clinical cyclic defect in a 12-day stress model that includes a psychogenic component in the rhesus monkey. J Clin Endocrinol Metab 2002; 87: 2232–2237. [DOI] [PubMed] [Google Scholar]
- Williams NI, Berga SL, Cameron JL.Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocinol Metab 2007; 293: E270–E276. [DOI] [PubMed] [Google Scholar]
- Dobson H, Tebble JE, Phogat JB.Smith RF. Effect of transport on pulsatile and surge secretion of LH in ewes in the breeding season. J Reprod Fertil 1999; 116: 1–8. [DOI] [PubMed] [Google Scholar]
- Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FK.Psychosocial stress inhibits gonadotropin-releasing hormone pulses independent of cortisol action on the type II glucocorticoid receptor. Endocrinology 2009; 150: 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen KM, Oakley AE, Pytiak AV, Tilbook AJ, Wagenmaker ER, Karsch FJ.Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress? Endocrinology 2007; 148: 1882–1890. [DOI] [PubMed] [Google Scholar]
- Van Cleeff J, Karsch FJ, Padmanabhan V.Characterization of endocrine events during the periestrous period in sheep after estrous synchronization with controlled internal drug release (CIDR) device. Domest Anim Endocinol 1998; 15: 23–34. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Karsch FJ, Foster DL.A new concept for control of the estrous cycle of the ewe based on the temporal relationships between luteinizing hormone, estradiol and progesterone in peripheral serum and evidence that progesterone inhibits tonic LH secretion. Endocrinology 1977; 101: 807–817. [DOI] [PubMed] [Google Scholar]
- Niswender GD, Midgley AR, Jr, Reichert LE., JrRadioimmunologic studies with murine, ovine and porcine luteinizing hormone. Rosenborg E.Gonadotropins Los Altos, CA:Geron-X;1968: 299–306. [Google Scholar]
- Niswender GD, Reichert LE, Jr, Midgley AR, Jr, Nalbandov AV.Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology 1969; 84: 1166–1173. [DOI] [PubMed] [Google Scholar]
- Battaglia DF, Bowen JM, Krasa HB, Thrun LA, Viguié C, Karsch FJ.Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: a simultaneous view from hypophyseal portal and peripheral blood. Endocrinology 1997; 138: 4273–4281. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ.Evidence for short or ultrashort loop negative feedback of gonadotropin-releasing hormone secretion. Neuroendocrinology 1995; 62: 248–258. [DOI] [PubMed] [Google Scholar]
- Hileman SM, Schillo KK, Boling JA, Estienne MJ.Effects of age on fasting-induced changes in insulin, glucose, urea nitrogen, and free fatty acids in sera of sheep. Proc Soc Exp Biol Med 1990; 194: 21–25. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML.Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol 1986; 250: E486–E493. [DOI] [PubMed] [Google Scholar]
- Debus N, Breen KM, Barrell GK, Billings HJ, Brown M, Young EA, Karsch FJ.Does cortisol mediate endotoxin-induced inhibition of pulsatile luteinizing hormone and gonadotropin-releasing hormone secretion? Endocrinology 2002; 143: 3748–3758. [DOI] [PubMed] [Google Scholar]
- Pierce BN, Hemsworth PH, Rivalland ET, Wagenmaker ER, Morrissey AD, Papargiris MM, Clarke IJ, Karsch FJ, Turner AI, Tilbrook AJ.Psychosocial stress suppresses attractivity, proceptivity and pulsatile LH secretion in the ewe. Horm Behav 2008; 54: 424–434. [DOI] [PubMed] [Google Scholar]
- McCandless EL, Dye JA.Physiological changes in intermediary metabolism of various species of ruminants incident to functional development of rumen. Am J Physiol 1950; 162: 434–446. [DOI] [PubMed] [Google Scholar]
- Macfarlane MS, Breen KM, Sakurai H, Adams BM, Adams TE.Effect of duration of infusion of stress-like concentrations of cortisol on follicular development and the preovulatory surge of LH in sheep. Anim Reprod Sci 2000; 63: 167–175. [DOI] [PubMed] [Google Scholar]
- Breen KM, Billings HJ, Wagenmaker ER, Wessinger EW, Karsch FJ.Endocrine basis for disruptive effects of cortisol on preovulatory events. Endocrinology 2005; 146: 2107–2115. [DOI] [PubMed] [Google Scholar]
- Wagenmaker ER, Breen KM, Oakley AE, Pierce BN, Tilbrook AJ, Turner AI, Karsch FJ.Cortisol interferes with the estradiol-induced surge of luteinizing hormone in the ewe. Biol Reprod 2009; 80: 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce BN, Clarke IJ, Turner AI, Rivalland ET, Tilbrook AJ.Cortisol disrupts the ability of estradiol-17beta to induce the LH surge in ovariectomized ewes. Domest Anim Endocrinol 2009; 36: 202–208. [DOI] [PubMed] [Google Scholar]
- Turner AI, Hemsworth PH, Tilbrook AJ.Susceptibility of reproduction in female pigs to impairment by stress or elevation of cortisol. Domest Anim Endocrinol 2005; 29: 398–410. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Centeno ML, Cameron JL.Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol 2008; 38: 199–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatman WR, Judkins MB, Dunn TG, Moss GE.Luteinizing hormone in nutrient-restricted ovariectomized ewes. J Anim Sci 1990; 68: 1097–1102. [DOI] [PubMed] [Google Scholar]
- Henry BA, Goding JW, Tilbrook AJ, Dunshea FR, Blache D, Clarke IJ.Leptin-mediated effects of undernutrition or fasting on luteinizing hormone and growth hormone secretion in ovariectomized ewes depend on the duration of metabolic perturbation. J Neuroendocrinol 2004; 16: 244–255. [DOI] [PubMed] [Google Scholar]
- Pitman DL, Ottenweller JE, Natelson BH.Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol Behav 1988; 43: 47–55. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T.Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology 2001; 26: 443–459. [DOI] [PubMed] [Google Scholar]
- Dobson H, Tebble JE, Ozturk M, Smith RF.Effects of transport on pulsatile LH release in ovariectomized ewes with or without prior steroid exposure at different times of year. J Reprod Fertil 1999; 117: 213–222. [DOI] [PubMed] [Google Scholar]
- Kenagy GJ, Place NJ.Seasonal changes in plasma glucocorticoids of free-living yellow-pine chipmunks: effects of reproduction and capture and handling. Gen Comp Endocrinol 2000; 117: 189–199. [DOI] [PubMed] [Google Scholar]
- Stackpole CA, Turner AI, Clarke IJ, Lambert GW, Tilbrook AJ.Seasonal differences in the effect of isolation and restraint stress on the luteinizing hormone response to gonadotropin-releasing hormone in hypothalamopituitary disconnected, gonadectomized rams and ewes. Biol Reprod 2003; 69: 1158–1164. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Adelson JD, Nelson RJ.Short days increase hypothalamic-pituitary-adrenal axis responsiveness. Endocrinology 2007; 148: 3402–3409. [DOI] [PubMed] [Google Scholar]