Abstract
The development of blastomeres separated from two-cell stage murine embryos has been compared. Blastomeres were removed from the zona pellucida (ZP) and cultured individually; the twin embryos were compared during their progression to blastocyst in terms of development rate, cell number, morphology, conformation at the four-cell stage, and CDX2 and POU5F1 (also known as OCT4) expression. In general, twin embryos, whether obtained from superovulated or normally bred dams, displayed comparable cell numbers as they advanced. They formed morulae and blastocysts more or less synchronously with each other and with control embryos, although possessing about half of the latter's cell number. Despite this apparent synchrony, the majority of twin blastocysts differed in terms of their relative complements of POU5F1+/CDX2− cells, which represent inner cell mass (ICM), and POU5F1+/CDX2+ cells, which identify trophectoderm (TE). Many, but not all, exhibited a disproportionately small ICM. By contrast, demiembryos retained within their ZP and created by randomly damaging one of the two blastomeres in two-cell stage embryos exhibited a more normal ratio of ICM to TE cells at blastocyst and significantly less variance in ICM cell number. One possible explanation is that ZP-free demiembryos only infrequently adopt the same conformation as their partners, including the favorable tetrahedral form, at the four-cell stage, suggesting that such embryos exhibit a high degree of plasticity with regard to the orientation of their first two cleavage planes and that a significant number likely deviate from paths that provide an optimal geometric progression to blastocyst. These data could explain the difficulty of creating monozygotic twins from two-cell stage embryos.
Keywords: CDX2, early development, embryo, embryo bisection, inner cell mass, ovulation, POU5F1, trophectoderm, trophoblast
Blastomeres separated at the two-cell stage progress in relative synchrony, but the resulting twin blastocysts frequently differ in their ratio of inner cell mass to trophectoderm and likely potential for generating fetuses and hence monozygotic twins.
INTRODUCTION
The production of monozygotic twins by splitting two-cell stage embryos into their component blastomeres and then allowing the resulting demiembryos to develop to adults could be extremely useful in attempts to understand why human twins can differ so much in phenotype despite their near [1] or complete genetic identity. In particular, such twin animals could be used to evaluate the extent to which phenotypic discordance over time is linked to epigenetic change, such as alterations in DNA methylation, brought about by environmental and other influences [2–5]. Twins produced from the very earliest blastomeres would be the most desirable in this regard since initial epigenetic differences would presumably be minimal, and the embryos, if transferred to different dams, could be used to test the effects of contrasting maternal diets on the epigenome.
Although twin bovine [6–8], ovine [9], caprine [10, 11], equine [12], porcine [13], and rhesus monkey [14] young have been created by dividing multicellular, preimplantation embryos into two halves, producing twin offspring from single blastomeres appears to have been more challenging, with only a few cases reported [15, 16]. Possibly because of the availability of inbred strains, less attention has been paid to creating twins in the mouse than in commercially important farm species such as cattle, although twin production by bisection of murine morulae has been successful [17–19]. It has long been clear that separated two-cell stage murine blastomeres develop efficiently into blastocysts in vitro [20] and provide fetuses [17, 21], but there are only a few reports indicating that it is possible to produce twins from such cells [22–25] and none that employed blastomeres from embryos at later stages of development unless the isolated cells were given support by aggregation with tetraploid embryos [26, 27]. In general, it appears that twinning from two-cell stage embryos, although difficult, is best achieved by embedding the blastomeres individually in a protective matrix such as agar, allowing the demiembryos to develop to the blastocyst stage, and finally transferring the fully developed blastocyst to appropriately synchronized surrogate mothers [24, 28]. In contrast, naked blastomeres not placed in a protective gel, although able to form blastocysts, developed to term extremely poorly after the transfer [24]. Moreover, placing single blastomeres into empty zonae seemed to be less effective than embedding embryos in a gel. This result is particularly surprising in view of the fact that a single undamaged blastomere cohabiting a zona with a twin rendered incapable of dividing by poking a needle into its nucleus can readily progress to blastocyst and provide a fetus despite having only about half the normal number of cells when the blastocyst forms [29]. Experiments on embryo splitting also have relevance to an ongoing argument about whether the first two blastomeres that form when a zygote divides are to some extent prepatterned and have biased fates [30–35]. The production of viable fetuses and live young from single blastomeres derived from two-cell stage murine embryos is clearly evidence that both cells are totipotent and has been cited as evidence that the blastomeres are most likely not differentially prepatterned [36].
In the present paper, we have readdressed the issue as to whether sister blastomeres derived from two-cell stage mouse embryos are equivalent in terms of developmental potential. In addition, we have compared the development of such singletons with that of single blastomeres occupying a zona in which their partner had been damaged to prevent further cell division. Finally, we examined the effect of superovulation on the successful production of twin blastocysts from two-cell stage sister blastomeres. To accomplish these comparisons, we first determined whether the single viable blastomeres progressed to morulae and blastocysts at the same rate as each other and controls and assessed whether each contributed equivalently to trophectoderm (TE) and inner cell mass (ICM). Our results provide a possible explanation for why it has proven difficult to derive monozygotic twins from separated two-cell stage blastomeres.
MATERIALS AND METHODS
Embryo Collection and Culture
Embryos were derived from CF-1 females by either superovulation (S embryos) or natural breeding (N embryos). Superovulation was achieved by an intraperitoneal injection of pregnant mare serum gonadotropin (5 international units [IU], Calbiochem, San Diego, CA) at 1300 h, 46 h ahead of human chorionic gonadotropin injection (10 IU, Calbiochem) at 1100 h on the day of breeding. CF-1 stud males were introduced to the female mice at 1700 h, and females were selected for retrieval of two-cell stage embryos between 1000 and 1100 h the day following the appearance of copulatory plugs, that is, ∼36 h postcoitus (pc). Alternatively, two-cell stage embryos were recovered from females bred without superovulation at this same time. Oviducts were flushed in CZB-Hepes buffer [37] lacking glucose (mCZB-Hepes) to collect the embryos. Embryos were cultured in KSOM-AA (potassium simplex-optimized medium with amino acids, Chemicon International, Philipsburg, NJ) at 37°C under 5% CO2 and 100% humidity (84 and 108 h pc for morula and blastocyst, respectively). All the experiments with mice were conducted in accordance with the National Research Council publication Guide for Care and Use of Laboratory Research Involving Animals under Protocols 1835 and 4073, and were approved by the University of Missouri's Animal Care and Use Committee.
Isolation of Blastomeres from Two-Cell Stage Embryos
Micromanipulation of embryos was performed under an inverted microscope (Nikon, DIAPHOT-300) equipped with a standard micromanipulation system (Leitz, Model M). Two-cell stage embryos recovered at 36 h pc were transferred in 2 μl of Ca2+- and Mg2+-free PBS supplemented with 0.1% polyvinyl alcohol (PBS-PVA), which were covered with mineral oil. An embryo was secured by using a holding pipette and a slit made in the zona pellucida (ZP) close to the side of one blastomere by means of a sharp glass needle. The blunt tip of a glass rod was then used to put pressure on the ZP-enclosed embryo, thereby allowing the blastomere closest to the slit to be squeezed out, generally without apparent damage. The second blastomere was recovered in a similar manner. Although the two blastomeres were physically indistinguishable, they were arbitrarily assigned a number and cultured individually in 2 μl of KSOM-AA. Some embryo pairs were harvested at 84 h, others atpb=2pt 108 h.
Production of Half-Ablated (Demi) Embryos
Two-cell stage embryos recovered at 36 h pc were transferred in 2 μl of mCZB-Hepes covered with mineral oil to the stage of an inverted microscope. The embryo was secured by using a holding pipette, and one blastomere was damaged by inserting a glass needle repeatedly into its nucleus. The choice of blastomeres for destruction was arbitrary, and no attempt was made to remove the compromised blastomere, which remained intact throughout developmental progression, from the ZP.
Immunocytochemistry
Although sister embryos were cultured individually, they were always processed together as a pair from fixation to mounting on the same slide. Embryos were fixed with 4% paraformaldehyde in PBS containing 0.1% PVA for 20 min at room temperature, treated with 0.1% Triton X-100 and 5% goat serum in PBS for 10 min at room temperature, and placed in a 1:1000 dilution of a rabbit polyclonal antibody raised against the N terminal region (amino acids 1–138) of recombinant POU5F1 [38] and 1:100 dilution of mouse monoclonal antibody to CDX2 (Biogenex, San Ramon, CA) in 1% (w/v) bovine serum albumin in PBS (BSA-PBS) overnight at 4°C. After washing with BSA-PBS, the specimens were incubated with secondary antibodies, Alexa Fluor 568 goat anti-rabbit IgG and Alexa Fluor 488 goat anti-mouse IgG (Invitrogen, Carlsbad, CA) at a 1:400 dilution in BSA-PBS for 2 h at room temperature. To visualize the nuclei, the embryos were counterstained with 4′,6-diamidino-phenylindole (DAPI, Invitrogen) diluted 1:600 with BSA-PBS. The half-ablated (demi) embryos obtained by blastomere destruction at the two-cell stage and nonmanipulated control CF-1 embryos were processed in the same manner except that the ZP was removed by acidic Tyrode solution [35] after fixation with paraformaldehyde. After mounting the specimens on slides, antigen distribution was examined under a Zeiss LSM 510 two photon confocal system.
Data Analysis
The data were obtained from embryos derived from mice that had either been superovulated (S, nine replicates) or bred naturally (N, five replicates). The differences in embryo development noted between S and N embryos and also within pairs at 60 and 108 h pc were analyzed by a chi-square test, with P < 0.05 considered significant. For the experiments of differential competence of embryos derived from two-cell blastomere splitting, cells were classified as either POU5F1+/CDX2+ (positive for both antigens), POU5F1+/CDX2−, or POU5F1−/CDX2−. The latter were invariably cells in mitosis and were not included further in the analysis as they could not be classified. The fraction of POU5F1+/CDX2− cells in an embryo was calculated as the number of POU5F1+/CDX2− cells divided by the sum of the POU5F1+/CDX2− and POU5F1+/CDX2+ cells.
Differences between sister pairs in total cell number, number of POU5F1+/CDX2− and POU5F1+/CDX2+ cells, and the fraction of POU5F1+/CDX2− cells were assessed for significance by analysis of variance (version 9.1, SAS Institute Inc., Cary, NC), with P < 0.05 considered significant. The analysis of variance among groups of embryos was also analyzed by using the PROC MIXED procedure in SAS, and tests for homogeneity among all variances were determined by the likelihood ratio test. Test of difference between variances was then performed with an f-test. If the variances differed, for example, between demiembryos produced by splitting versus those produced by ablation, the mean differences were tested by using the Satterth adjustment in the MIXED procedure. If the variances were equal, a Fisher least-significant difference test was used.
RESULTS
Developmental Progression of Isolated Two-Cell Blastomeres
The success rates for embryo splitting, assessed by the ability to obtain two, intact, apparently undamaged blastomeres, was significantly lower (chi-square test, P < 0.05) for embryos obtained after superovulation (S embryos; 80 pairs produced from 180 embryos in nine replicates; average success rate 44.4%) than for embryos from naturally bred dams (N embryos; 68 pairs from 82 embryos in five replicates; average success rate 83%). The likely explanation for this difference is that the volume of the perivitelline space was visibly less for S embryos than for N embryos [35]. This tighter fit made it much more difficult to introduce a slit into the ZP without rupturing one of the blastomeres.
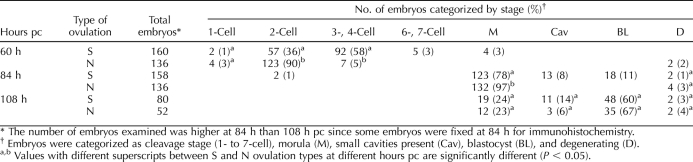
At 24 h after blastomere splitting (60 h pc), 96% of the isolated blastomeres had cleaved at least once (Fig. 1, A1 and B1). In the case of N embryos, about 90% were observed to be at the two-cell stage (Table 1), whereas a majority (64%) of the S embryos had undergone further cell divisions (P < 0.05). At 84 h pc, 97% of the N embryos had formed structures that resembled small, compacted morulae except that they were generally not spherical and the underlying conformation of the blastomeres could still be discerned (Fig. 1, A3 and B3). Consistent with the data at 60 h, the S embryos were still more advanced at 84 h than the N embryos (P < 0.05). Only 78% were at the morula stage, while at least 19% had begun to cavitate and to form blastocysts (Table 1).
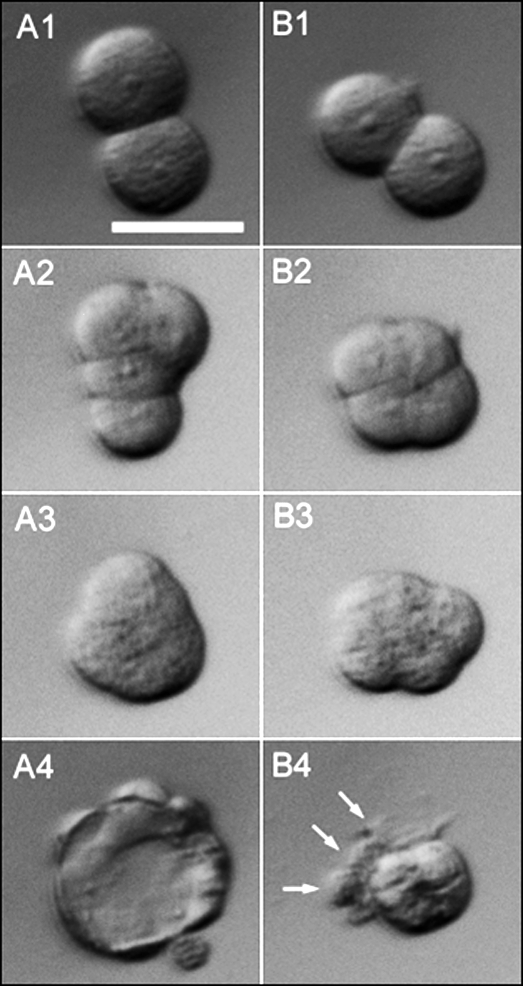
FIG. 1.
Embryo development after splitting two-cell stage embryos into individual blastomeres. Two-cell blastomeres were bisected microsurgically from a single two-cell stage embryo retrieved at 24 h pc and removed from the enclosing ZP. The two sister embryos were cultured individually as demiembryo A (A1–A4) and demiembryo B (B1–B4). At 60 h pc (24 h after bisection), both sisters had cleaved once (A1 and B1). By 72 h pc, the embryos were at the four-cell stage (A2 and B2) and showed signs of early compaction but with different conformations (see also Fig. 3). By 84 h, both embryos (A3 and B3) were around the eight-cell stage and had compacted fully even though they did not have the usual close-to-spherical shape of control morulae. Instead, the contour of the embryos was irregular, and the conformation of individual blastomeres was visible. By 108 h pc, one sister had formed a blastocyst (A4) whereas the other (B4) possessed a compacted cluster of cells and three outer cells (arrowed), one of which possessed a large vacuole. The B4 abnormal blastocyst would be classified as Cav in Tables 1 and 2. Bar = 50 μm.
TABLE 1.
Development of bisected 2-cell blastomeres originating from superovulation (S) or natural breeding (N).
FIG. 2.
Kinds of cavity observed in embryos at 108 h pc after development from bisected two-cell stage embryos. At 108 h pc, embryos derived from two-cell blastomere displayed a range of morphologies although the majority had advanced to form normal appearing blastocysts, often similar to the expanded blastocyst shown here (D) and in Figure 1A4. Some embryos with few cells displayed more than one vacuole (A), single blastomeres with an apparent vacuole (B), or a single small cavity (C). Embryos resembling A to C were categorized as Cav in Tables 1 and 2. Bar = 50 μm.
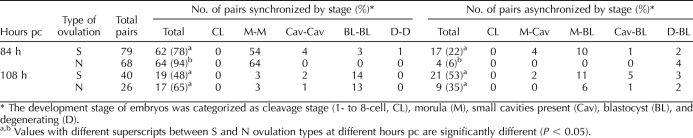
By 108 h, a few embryos from each group were still morulae, while most (60% S and 67% N) had formed blastocysts (Table 1) with a relatively normal morphology (Figs. 1A4 and 2D). A minority of the embryos from both the S and N groups had acquired small cavities, had abnormal morphologies, and generally consisted of only a few cells (Figs. 1B4 and 2, A–C), features that have been observed by others [39]. These embryos are categorized as Cav in Tables 1 and 2. However, 83 out of 132 embryos from the 66 pairs of twin blastomeres that survived (Tables 1 and 2) had cavitated to form blastocysts. Synchrony between sister embryos in terms of their morphological development was still evident at 108 h when a total of 27 pairs of S and N twin embryos out of 66 (Table 2) consisted of identifiable blastocysts. Six pairs were classified as morulae. Seventeen others comprised blastocyst-morula pairs, while in the remaining 16 pairs at least one embryo had developed poorly. In summary, sister embryos show relatively high synchrony in their development.
TABLE 2.
Comparison of embryo development between pairs of sister blastomeres originating from superovulation (S) or natural breeding (N).
Conformation of Embryos at the Four-Cell Stage
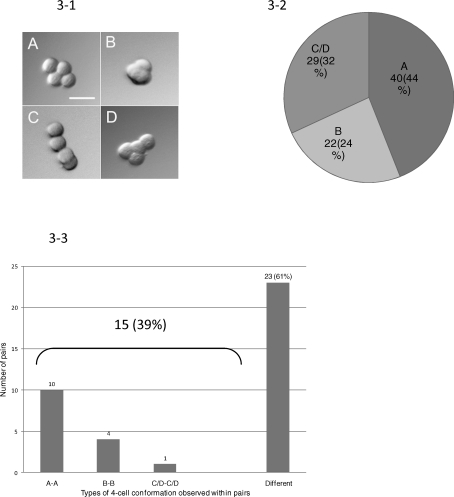
Suzuki and coworkers [40] noted that many zona-free control mouse embryos and demiembryos produced by splitting at the two-cell stage assumed unusual conformations when they were at the four-cell stage. We made similar observations with the CF-1 strain of mouse (Fig. 3-1). Sister embryos, following splitting, were examined at 60 and 72 h with regard to their relative conformations at the four-cell stage. Of the embryos that were observed at the four-cell stage and for which conformation data could be inferred, 40:91 (44%) were planar (Fig. 3-1A), 22:91 (24%) were tetrahedral (Fig. 3-1B), while 29:91 (32%) were in one of the open chain conformations (Fig. 3-1, C and D), which we combined into one category (Fig. 3-2) because it was often difficult to make the distinction between the two in our retrospective examination of images. It was possible to obtain comparative data on sister embryos for 38 pairs. In 23:38 pairs (61%), sister embryos had not adopted the same conformation at the four-cell stage (Fig. 3-3). For example, only four pairs of embryos showed the tetrahedral B/B conformation, while 10 of 38 exhibited the A/A planar conformation. The situation where both sister embryos were in one of the open conformations was rare (1:38). Together, these data suggest, that although sister embryos tend to progress to morula and blastocyst at comparable speeds and have comparable numbers of cells (see below), they are, more often than not, morphologically dissimilar at the four-cell stage.
FIG. 3.
The main kinds of conformations adopted by zona-free, four-cell stage, murine embryos after development from blastomeres derived from bisected two-cell stage embryos. 3-1) These images of typical four-cell stage embryos were observed at 72 h pc and resemble the four conformations reported by others [40] for four-cell stage, zona-free mouse embryos. Image A represents an embryo with a flat conformation; B, although compacting, has a tetrahedral conformation; C and D have an open conformation never seen in control embryos enclosed in a ZP. Because the conformations were classified retrospectively from stored images, it was often difficult to distinguish the two classes with the open configuration, that is, type C from type D, because of their orientations in the images, particularly after compaction had begun. Bar = 50 μm. 3-2) The proportion of zona-free demiembryos that assumed the different conformations A–D shown in 3-1. 3-3) Types of four-cell conformations observed within pairs of demiembryos.
Relative Equality in Cell Number Between Twin Embryos
Confocal microscopy performed on demiembryos immunostained for POU5F1 and CDX2 and counterstained with DAPI allowed the total number of cells to be assessed in each embryo (Table 3). Control, nonmanipulated embryos provided a basis for comparison. Thus, nonmanipulated CF-1 embryos cultured in KSOM-AA medium averaged about 19 cells at 84 h pc when they were compacted morulae (data not shown) and approximately 64 cells at 108 h pc, when they were predominantly expanded blastocysts (group D in Table 3).
TABLE 3.
Cell numbers at 108 h pc in blastocysts derived from zona-free bisected 2-cell stage embryos (A & B); zona enclosed, demiembryos derived by ablation of one blastomere at the 2-cell stage (C); and control, nonmanipulated embryos (D).
The N demiembryos formed following embryo splitting had fewer cells than S demiembryos when cultured 84 h (N embryos 7.0 ± 2.3; S embryos 13.4 ± 4.5). Both had fewer cells than nonmanipulated CF-1 control embryos cultured for the same time (18.8 ± 6.8). However, by 108 h, when the majority of both were blastocysts, N and S demiembryos did not differ significantly in cell number (groups A and B in Table 3). With a few exceptions, therefore, sister blastomeres not only progressed to blastocyst at similar rates (Table 2), but had similar numbers of cells at 108 h (Table 3), whether or not they were derived from S or N dams.
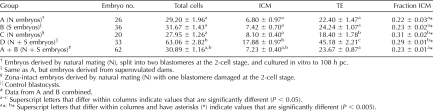
An analysis of the data for individual embryo pairs revealed that the two partners were generally well synchronized in terms of cell number (Fig. 4). For example, at 84 h when almost all embryos had formed compacted morula-like structures, 27 of 56 pairs, that is, 48%, of sister blastomeres had identical numbers of cells (Fig. 4, A and B). In 29 pairs, the cell numbers differed, but these disparities within pairs were generally small. Even at 108 h, when the demiembryos averaged about 31 cells (group A + B, Table 3), 13 of the 52 pairs (25%) analyzed had an equal number of cells (Fig. 4, D and C). Of the remainder, one sister outgained the other in cell number by more than one-third in only 14 pairs, and in six of these cases, one sister had developed poorly and was possibly damaged at separation, consisting of only a few cells. We conclude that one two-cell stage blastomere generally did not possess a proliferative advantage over the other when they were cultured separately.
FIG. 4.
Numbers of cells in demiembryo pairs at 84 and 108 h pc. Embryos were derived by bisection at the two-cell stage and twin partners cultured separately for either 84 h (A and B) or 108 h (C and D). Bisected embryos were derived either from normally bred dams (N; A and C) or superovulated dams (S; B and D). At 84 h when the embryos were mainly morulae and at 108 h when most were blastocysts, groups of embryos were fixed, immunostained for CDX2 and POU5F1, and counterstained with DAPI. The total number of cells in each embryo was then counted by using confocal microscopy to provide optical sections through the entire structure [35]. The numbers on the x-axis for each bar graph refers to a pair of demiembryos that were arbitrarily designated demiembryo 1 and demiembryo 2. The plots illustrate the relative similarity in cell number within twin pairs of embryos at both morula and blastocyst.
A Comparison of POU5F1/CDX2 Staining in Sister Embryos Derived after Two-Cell Blastomere Splitting
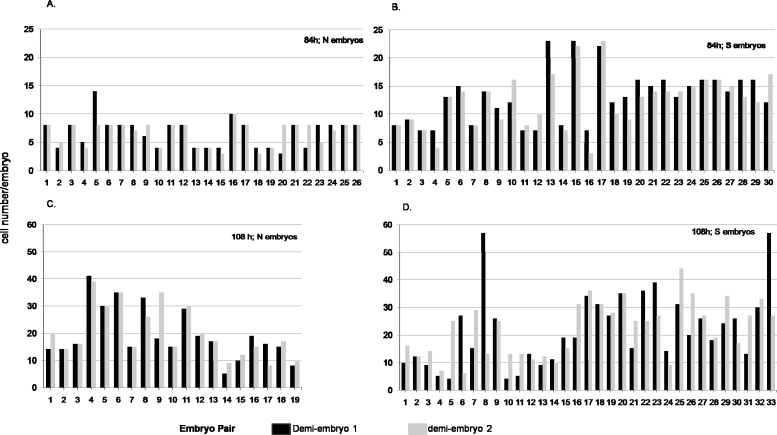
In blastocysts, POU5F1+/CDX2+ cells constitute TE, and POU5F1+/CDX2− cells constitute ICM. Therefore, it is possible to assess the relative size of ICM from the fraction of POU5F1+/CDX2− cells present and whether this fraction differed in sister pairs of demiembryos. Figure 5A provides the numbers of ICM and TE cells from 31 pairs of embryos in which each twin possessed 15 cells or greater at 108 h. In Figure 5A, the data were combined from the S and N groups because the two did not differ in mean cell number or in the variances observed in relative numbers of POU5F1+/CDX2− and POU5F1+/CDX2+ cells. The data are arranged so that sister embryos are adjacent to each other in the histograms, for example, 1a and 1b in Figure 5A constitute an embryo pair. The relative numbers of POU5F1+/CDX2− cells and POU5F1+/CDX2+ cells in sister embryos were similar in only about half the pairs (Fig. 5A). Moreover, the fraction of cells that constituted the ICM was often, but not always, smaller in these zona-free demiembryos than it was in zona-intact, nonmanipulated control embryos (Fig. 5B and Table 3). The ICM in these zona-free demiembryos was also generally smaller than in the zona-enclosed demiembryos (discussed below). Some examples of discrepancies in relative ICM size among twin embryos are illustrated in Figure 6. Figure 6A shows a pair of expanded blastocysts in which one (the embryo on the lower left) has an ICM consisting of nine cells, whereas the ICM of its twin (the smaller embryo on the upper right) has five cells. In Figure 6B, the ICMs of both expanded blastocysts were very small.
FIG. 5.
The number of ICM (POU5F1+/CDX2−; black bars) and TE (POU5F1+/CDX2+; grey bars) cells in cultured embryos at 108 h pc. A) Values for 31 pairs of twin embryos derived by bisection at the two-cell stage of development. In each case, both partners (a and b) had 15 or more cells and at least 27 had a well-defined blastocoel cavity. The data are derived from 13 N embryo pairs and 18 S embryo pairs. B) Values for 33 control (nonmanipulated) CF-1 embryos. C) Values from 20 zona-enclosed demiembryos in which the partner blastomere had been damaged at the two-cell stage of development to prevent it from dividing. In all the treatments, dividing cells that failed to stain for either antigen are not included in the analyses.
FIG. 6.
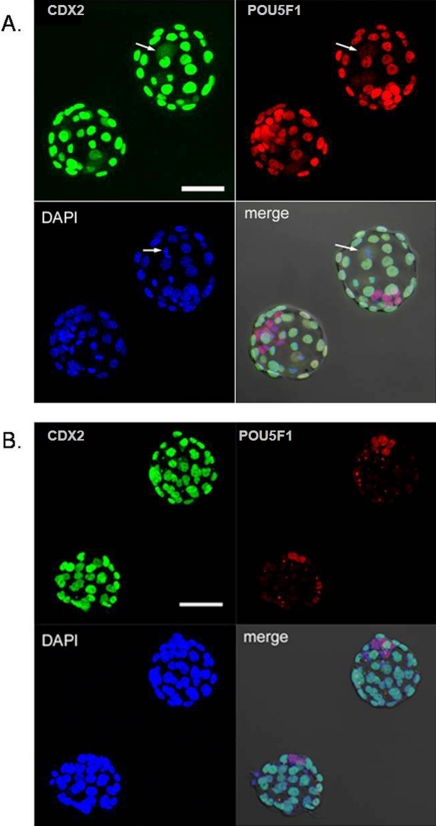
Expression of POU5F1 and CDX2 in sister blastocysts at 108 h pc after development from bisected two-cell stage embryos. A) These are typical examples of sister blastocysts at 108 h pc and show projection images (derived from optical sections obtained at 3 μm intervals) for CDX2 (green, upper left), POU5F1 (red, upper right), DAPI staining (blue, lower left), and a merged image of the three projection images and a differential interference contrast image (lower right) of two sister blastocysts derived from the same two-cell stage embryo after blastomere separation. The blastocyst on the upper right of each panel has 5 POU5F1+/CDX2− positive cells (ICM) and 28 POU5F1+/CDX2+ positive cells (TE). The arrows in the panels show a cell at mitotic metaphase within that blastocyst; the arrow in the DAPI image shows the alignment of chromosomes within that cell, while the arrows in the POU5F1 and CDX2 images show diffuse, cytoplasmic staining for POU5F1 and CDX2, respectively, within that cell. Because it is impossible to judge the nuclear association of the POU5F1 and CDX2 signals, the cells in mitosis were eliminated from further assessment of ICM/TE cell numbers. The blastocyst on the lower left of each panel has 9 POU5F1+/CDX2− nuclei (ICM) and 29 POU5F1+/CDX2+ nuclei (TE). The fraction of POU5F1+/CDX2− cells for the upper embryo is 0.15 (5/33) and for the lower embryo 0.24 (9/38). Bar = 50 μm. B) This panel shows a more extreme case in which the ICM is unusually small in both twins. The blastocyst on the upper right has 4 POU5F1+/CDX2− nuclei (ICM) and 33 CDX2+ nuclei (TE) in which only very weak POU5F1 staining can be detected, probably because the blastocyst is well expanded. The blastocyst on the lower left has 2 POU5F1+/CDX2− nuclei (ICM) and 29 POU5F1+/CDX2+ nuclei (TE). Bar = 50 μm.
Development of Zona-Enclosed Demiembryos to Blastocyst
The goal here was to compare whether single blastomeres not removed from their zonae and confined to the space they would normally occupy develop similarly to blastomeres removed from their zonae. The demiembryos were created by damaging one blastomere in the two-cell stage N embryos. Although the damaged cell failed to divide any further, it persisted, along with its undamaged partner, until the latter formed a blastocyst (data not shown). We treated 46 CF-1 two-cell stage embryos in this manner, of which 29 (63%) reached the blastocyst stage at 108 h pc. The formation of blastocysts from these demiembryos occurred only slightly less efficiently than with nonmanipulated control embryos (34:40 or 85% reached blastocyst). Experiments with a second mouse strain (NIH Swiss) provided almost identical results (data not shown). As expected, the total number of cells at the blastocyst stage was again significantly reduced (P < 0.05, Table 3) compared with controls, but were quite similar to that of CF-1 demiembryos produced by splitting and cultured without a ZP (Table 3). Again, comparable results were observed with the NIH Swiss strain (data not shown).
In contrast, the fraction of ICM cells in the zona-enclosed embryos in which only one blastomere had been allowed to progress to blastocyst was normal and comparable to that of the nonmanipulated controls (0.31 vs. 0.29, respectively, Table 3). This fractional value was significantly higher (P < 0.001) than that of the ICM fraction of the zona-free demiembryos produced by splitting the blastomeres at the two-cell stage (N embryos, 0.22; S embryos, 0.23), largely because the number of TE cells was significantly reduced while the number of ICM cells tended to be increased in the zona-enclosed embryos (group C, Table 3).
Comparison of Variances in Numbers of ICM Cells from Zona-Intact and Zona-Free Demiembryos
Although both zona-free and zona-enclosed demiembryos had similar numbers of cells at blastocyst (groups A and B, Table 3), a comparison of the distribution of cells between ICM and TE suggests that there was considerably less variability among the zona-enclosed group (Fig. 5C) than among those that were zona-free and generated by splitting at the two-cell stage (Fig. 5A). This impression was confirmed by a statistical test for differences among variances for the cell numbers derived from Table 3 and shown for individual embryos in Figure 5. The variances in total cell number were not different (32.1 for zona-enclosed vs. 64.5 for zona-free), whereas for ICM, they differed significantly (3.3 vs. 15.3; P < 0.05). For TE, the variances were not significantly different (27.4 vs. 36.2). Such a difference in variance in ICM cell numbers held true whether or not the zona-free demiembryos were considered as a group, that is, S and N combined, or separately. These analyses indicate that variability in ICM number is high in demiembryos that develop without an enclosing ZP and that a significant proportion of these embryos set aside an unusually small fraction of their cells to the ICM. This conclusion is also evident when examining the histograms shown in Figure 5A.
DISCUSSION
The main goal of this paper was to determine whether or not separated blastomeres derived from two-cell stage murine embryos showed dissimilar patterns of development. A secondary goal, which will be discussed first, was to test whether demiembryos derived from S dams developed differently from those obtained after normal breeding (N dams) because there are conflicting reports as to whether superovulation has deleterious effects on the oocyte and possibly subsequent development of the zygote [41–44]. Finally, we wished to determine whether demiembryos derived by embryo splitting and cultured without a ZP differed in their development from zona-enclosed demiembryos produced by ablating one blastomere at the two-cell stage.
Our observations on the effects of ovulation protocol in CF-1 mice provided three useful pieces of information. First, it was significantly more difficult to isolate competent two-cell stage blastomeres from S embryos than from N embryos, although the explanation for this difference would seem to be quite trivial. The tighter packing of the blastomeres within the ZP of CF-1 S embryos, as inferred by us [35] and others [32, 45], probably renders them more prone to damage when the slit is introduced. Whether these packing differences are due to oocyte shape or consistency or altered properties arising from the hormonal regimens experienced by the oocytes in their final stages of maturation is unclear. A second observation was that blastomeres from S dams, if isolated without damage, are equally as competent to progress in development as blastomeres derived from N dams. In fact, the S embryos advanced more rapidly than N embryos to the four-cell stage (Table 1) and still possessed more cells at the 84-h pc stage (Fig. 4, A and B), perhaps because they had been fertilized earlier and many had undergone a fourth round of cell cleavage. For reasons that are unclear, this head start of S embryos was lost as they progressed from morulae to blastocysts. The third observation was that S and N demiembryos were similar in their proportion of TE and ICM cells (Table 3). Our results suggest that under the conditions used here, superovulation is appropriate for producing two-cell embryos for splitting or other kinds of manipulation.
Consistent with the data of others [20, 22, 24, 25, 46, 47], bisecting two-cell stage embryos and separating them from their ZP did not interfere with the competency of the individual blastomeres to develop into more advanced structures. They advanced to morulae and finally to blastocysts at about the same speed as control embryos, despite having about half the normal number of cells. In addition, the embryos from twin pairs of blastomeres retained a considerable degree of similarity, both in terms of cell number and rate of progression to morulae and blastocysts during their development. Some degree of asynchrony was anticipated because the blastomeres of intact two-cell stage embryos tend to divide slightly out of phase [46, 48, 49], but this initial advantage was clearly insufficient to cause the progeny of one blastomere to outnumber those of the other in any significant manner. Where a major imbalance did occur, it may have been due to damage to one of the blastomeres during the process of separation. Very similar rates of development were also observed in experiments in which one blastomere of a two-cell embryo was damaged to prevent it from dividing, while the other was allowed to progress in its development (Tables 3), albeit within the confines of the original ZP, again confirming data reported by others [39, 50, 51]. Together, these experiments support the notion that the zygotic clock, that is, the time after fertilization, rather than number of cells in the embryo determines when differentiation begins and are consistent with the notion that blastomeres from two-cell stage embryos are developmentally equivalent in their potential.
In blastocysts, CDX2 is a marker of trophectoderm and is absent from the pluripotent cells of the ICM [35, 45, 52–55]. Accordingly, dual staining for CDX2 and POU5F1 makes it possible to assess the numbers of ICM and TE cells in a blastocyst (Fig. 5 and Table 3). Likewise, in control morulae, CDX2 is largely localized to polarized outer cells destined to become TE, while POU5F1+/CDX2− cells tend to be confined to the inner cell population. Since morulae derived from bisected two-cell stage embryos were smaller than control embryos and often consisted of eight or fewer cells at 84 h pc, it was perhaps no surprise that they contained a higher proportion of POU5F1+/CDX2+ cells than control morulae (data not shown) because most or all of the component blastomeres would have some surface exposure to the medium [56]. However, the twin blastocysts frequently differed markedly in the fraction of POU5F1+/CDX2− cells they contained, that is, one of the twins often had a larger ICM than the other (Figs. 5 and 6). The overall imbalance of TE to ICM in twin blastocysts (Fig. 5A and Table 3) and the often disproportionately small ICM in one or both embryos may explain why the production of monozygotic twin progeny in the mouse has proven so difficult. Even though singletons have been produced much more easily than twins from embryos split at the two-cell stage, pup numbers have still been low. We suspect, for example, that the two expanded blastocysts shown in Figure 6B, each of which had over 30 cells, would have a poor chance of providing fetuses in view of the few ICM cells present in either embryo. It is well established that blastocysts with a small ICM rarely, if ever, develop to form fetuses [50]. By contrast, demiembryos generated after damaging one member of a sister pair at the two-cell stage, although possessing similar numbers of cells as the zona-free demiembryos, provided a more balanced ratio of ICM to TE cells in their blastocysts (Fig. 5C and Table 3) and demonstrated a significantly reduced variance in ICM numbers. One explanation for these differences between the two kinds of demiembryos is that the continued presence of the large damaged blastomere helps preserve the constraints on cell packing as its dividing twin advances in its development and ensures a more optimal allocation of cells to the ICM.
The basis for the imbalance of POU5F1+/CDX2− to POU5F1+/CDX2+ cells in sister embryos that develop independently outside the ZP could indicate either subtle biochemical differences or some form of genetic mosaicism [57, 58] existing between blastomeres at the two-cell stage that causes subsequent bias in the division planes. If either of these explanations were valid, however, a greater variance in ICM number in the zona-enclosed, demiembryos produced by partner ablation would also have been anticipated. An alternative, and to us, more likely explanation is that the release of the blastomeres from the confines of the ZP causes the differential patterning at later stages of development. Suzuki et al. [40] observed four main types of conformation among four-cell stage embryos after they had been freed from their ZP at the zygote stage. Similar structures were seen at the four-cell stage among zona-free demiembryos that, like ours, had been bisected at the two-cell stage of development (Fig. 3). Significantly, the open chain forms (C and D) shown in Figure 3-1 are never encountered in zona-intact embryos, where physical restraints and available space presumably disallow an open architecture and where tetrahedral four-cell stage embryos tend to predominate. Comparable observations have been made on zona-confined and zona-free half-embryos [59, 60]. In our experiments, the majority of sister embryos at the four-cell stage differed from each other in the manner that their component blastomeres were arranged. As shown conclusively by others [40], four-cell embryos in open conformations, particularly form C (Fig. 3-1), give rise to significantly more blastocysts with a small ICM and poorer survival after transfer to surrogate dams than embryos with compacting, tetrahedral forms (Fig. 3-1B). Accordingly, it may be inferred that sister half-embryos that adopt contrasting conformations at the four-cell stage of development, as they mainly do, will likely differ in their relative numbers of ICM and TE cells when they reach the blastocyst stage.
Finally, we must ask why monozygotic twin embryos should adopt such widely different conformations at the four-cell stage when they are provided with the opportunity to divide in the absence of a restraining ZP. A major function of the ZP, in addition to protection, may be to pack the blastomeres into a form that is most likely to provide blastocysts with the highest developmental potential [40]. Encapsulating zona free embryos in alginate, for example, may serve a similar purpose [61], providing a possible explanation for why twinning is more successful when the separated blastomeres are embedded in a stiff matrix [24]. Once the physical restrictions of tight packing within a zona are relaxed, cleavage planes and hence lineage allocations probably become less predictable. Although they may start out with equivalent potential, isolated two-cell stage sister blastomeres do not necessarily traverse an identical geometrical path to blastocyst. Accordingly, their ability to provide a blastocyst with a balanced ratio of TE to ICM and to give rise to a pup may also differ. These considerations may have relevance if splitting early stage embryos is considered for clinical application in human reproductive medicine [62, 63].
Acknowledgments
We thank Norma McCormack for editing the paper and Steven Smith for technical assistance. Steve Smith, Dr. Laura Schulz, Dr. Cheryl S. Rosenfeld, and Dr. Jiude Mao provided valuable discussion and critical input throughout the study.
Footnotes
Supported by NIH Grant R01 HD21896-23.
REFERENCES
- Cheung VG, Bruzel A, Burdick JT, Morley M, Devlin JL, Spielman RS.Monozygotic twins reveal germline contribution to allelic expression differences. Am J Hum Genet 2008; 82: 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque FN, Gottesman II, Wong AH.Not really identical: epigenetic differences in monozygotic twins and implications for twin studies in psychiatry. Am J Med Genet C Semin Med Genet 2009; 151C: 136–141. [DOI] [PubMed] [Google Scholar]
- Whitelaw NC, Whitelaw E.How lifetimes shape epigenotype within and across generations. Hum Mol Genet 2006; 15(spec. no. 2):R131–R137. [DOI] [PubMed] [Google Scholar]
- Kaminsky ZA, Tang T, Wang SC, Ptak C, Oh GH, Wong AH, Feldcamp LA, Virtanen C, Halfvarson J, Tysk C, McRae AF, Visscher PM, et al. DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet 2009; 41: 240–245. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A 2005; 102: 10604–10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seike N, Saeki K, Utaka K, Sakai M, Takakura R, Nagao Y, Kanagawa H.Production of bovine identical twins via transfer of demiembryos without zonae pellucidae. Theriogenology 1989; 32: 211–220. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Elsden RP, Seidel GE., Jr.Pregnancy rates with bisected bovine embryos. Theriogenology 1984; 22: 521–531. [DOI] [PubMed] [Google Scholar]
- Heyman Y, Vignon X, Chesne P, Le Bourhis D, Marchal J, Renard JP.Cloning in cattle: from embryo splitting to somatic nuclear transfer. Reprod Nutr Dev 1998; 38: 595–603. [DOI] [PubMed] [Google Scholar]
- Willadsen SM, Godke RA.A simple procedure for the production of identical sheep twins. Vet Rec 1984; 114: 240–243. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y, Tokunaga T, Okubo Y, Sugie T.Beneficial effect of agar for the frozen storage of bisected embryos. Theriogenology 1987; 28: 317–322. [DOI] [PubMed] [Google Scholar]
- Oppenheim SM, Moyer AL, Bondurant RH, Rowe JD, Anderson GB.Successful pregnancy in goats carrying their genetically identical conceptus. Theriogenology 2000; 54: 629–639. [DOI] [PubMed] [Google Scholar]
- Allen WR, Pashen RL.Production of monozygotic (identical) horse twins by embryo micromanipulation. J Reprod Fertil 1984; 71: 607–613. [DOI] [PubMed] [Google Scholar]
- Reichelt B, Niemann H.Generation of identical twin piglets following bisection of embryos at the morula and blastocyst stage. J Reprod Fertil 1994; 100: 163–172. [DOI] [PubMed] [Google Scholar]
- Mitalipov SM, Yeoman RR, Kuo HC, Wolf DP.Monozygotic twinning in rhesus monkeys by manipulation of in vitro-derived embryos. Biol Reprod 2002; 66: 1449–1455. [DOI] [PubMed] [Google Scholar]
- Johnson WH, Loskutoff NM, Plante Y, Betteridge KJ.Production of four identical calves by the separation of blastomeres from an in vitro derived four-cell embryo. Vet Rec 1995; 137: 15–16. [DOI] [PubMed] [Google Scholar]
- Willadsen SM.The viability of early cleavage stages containing half the normal number of blastomeres in the sheep. J Reprod Fertil 1980; 59: 357–362. [DOI] [PubMed] [Google Scholar]
- Nagai J, Davis G, Nonaka K, Sasada H.Growth and reproduction of mice developed from bisected embryos. Theriogenology 1989; 32: 475–483. [DOI] [PubMed] [Google Scholar]
- Nagashima H, Matsui K, Sawasaki T, Kano Y.Production of monozygotic mouse twins from microsurgically bisected morulae. J Reprod Fertil 1984; 70: 357–362. [DOI] [PubMed] [Google Scholar]
- Moustafa LA, Hahn J.Experimental production of identical twins in the mouse. Dtsch Tierarztl Wochenschr 1978; 85: 242–244. [In German] [PubMed] [Google Scholar]
- Fiser PS, MacPherson JW.Development of embryonic structures from isolated mouse blastomeres. Can J Anim Sci 1976; 56: 33–36. [Google Scholar]
- Tarkowski AK.Experiments on the development of isolated blastomers of mouse eggs. Nature 1959; 184: 1286–1287. [DOI] [PubMed] [Google Scholar]
- Sotomaru Y, Kato Y, Tsunoda Y.Production of monozygotic twins after freezing and thawing of bisected mouse embryos. Cryobiology 1998; 37: 139–145. [DOI] [PubMed] [Google Scholar]
- Togashi M, Suzuki H, Miyai T, Okamoto MT.Production of monozygotic twins by splitting of 2-cell stage embryos in mice. Jpn J Anim Reprod 1987; 33: 51–57. [Google Scholar]
- Tsunoda Y, McLaren A.Effect of various procedures on the viability of mouse embryos containing half the normal number of blastomeres. J Reprod Fertil 1983; 69: 315–322. [DOI] [PubMed] [Google Scholar]
- Wang M, Kato Y, Tsunoda Y.Effects of several factors on the monozygotic twin production in the mouse. J Reprod Dev 1997; 43: 91–95. [Google Scholar]
- Valer Carstea B, Catunda Lemos AP, Ilie ED, Varga L, Bodo S, Kovacs A, Bosze Z, Gocza E.Production of identical mouse twins and a triplet with predicted gender. Cloning Stem Cells 2007; 9: 247–256. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK, Ozdzenski W, Czolowska R.Identical triplets and twins developed from isolated blastomeres of 8- and 16-cell mouse embryos supported with tetraploid blastomeres. Int J Dev Biol 2005; 49: 825–832. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y, Yasui T, Shioda Y, Nakamura K, Uchida T, Sugie T.Full-term development of mouse blastomere nuclei transplanted into enucleated two-cell embryos. J Exp Zool 1987; 242: 147–151. [DOI] [PubMed] [Google Scholar]
- Papaioannou VE, Mkandawire J, Biggers JD.Development and phenotypic variability of genetically identical half mouse embryos. Development 1989; 106: 817–827. [DOI] [PubMed] [Google Scholar]
- Gardner RL.The case for prepatterning in the mouse. Birth Defects Res C Embryo Today 2005; 75: 142–150. [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M.The first cell-fate decisions in the mouse embryo: destiny is a matter of both chance and choice. Curr Opin Genet Dev 2006; 16: 406–412. [DOI] [PubMed] [Google Scholar]
- Motosugi N, Bauer T, Polanski Z, Solter D, Hiiragi T.Polarity of the mouse embryo is established at blastocyst and is not prepatterned. Genes Dev 2005; 19: 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvet-Vallee S, Vinot S, Maro B.Mitotic spindles and cleavage planes are oriented randomly in the two-cell mouse embryo. Curr Biol 2005; 15: 464–469. [DOI] [PubMed] [Google Scholar]
- Hiiragi T, Solter D.Mechanism of first cleavage specification in the mouse egg: is our body plan set at day 0? Cell Cycle 2005; 4: 661–664. [DOI] [PubMed] [Google Scholar]
- Katayama M, Roberts RM.The effect of superovulation on the contributions of individual balstomeres from 2-cell stage CF1 mouse embryos to the blastocyst. Int J Dev Biol 2010; 54: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiiragi T, Alarcon VB, Fujimori T, Louvet-Vallee S, Maleszewski M, Marikawa Y, Maro B, Solter D.Where do we stand now? Mouse early embryo patterning meeting in Freiburg, Germany (2005). Int J Dev Biol 2006; 50: 581–586. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I.An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil 1989; 86: 679–688. [DOI] [PubMed] [Google Scholar]
- Ezashi T, Das P, Roberts RM.Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A 2005; 102: 4783–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulicke T, Autenried P.Potential of two-cell mouse embryos to develop to term despite partial damage after cryopreservation. Lab Anim 1995; 29: 320–326. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Togashi M, Adachi J, Toyoda Y.Developmental ability of zona-free mouse embryos is influenced by cell association at the 4-cell stage. Biol Reprod 1995; 53: 78–83. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Albertini DF.Assessment of oocyte quality following repeated gonadotropin stimulation in the mouse. Biol Reprod 2003; 68: 812–821. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ock SA, Chian RC.Effect of gonadotrophin stimulation on mouse oocyte quality and subsequent embryonic development in vitro. Reprod Biomed Online 2006; 12: 304–314. [DOI] [PubMed] [Google Scholar]
- Ertzeid G, Storeng R.The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod 2001; 16: 221–225. [DOI] [PubMed] [Google Scholar]
- Fortier AL, Lopes FL, Darricarrere N, Martel J, Trasler JM.Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Hum Mol Genet 2008; 17: 1653–1665. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Hiiragi T.Stochastic patterning in the mouse pre-implantation embryo. Development 2007; 134: 4219–4231. [DOI] [PubMed] [Google Scholar]
- Alarcon VB, Marikawa Y.Unbiased contribution of the first two blastomeres to mouse blastocyst development. Mol Reprod Dev 2005; 72: 354–361. [DOI] [PubMed] [Google Scholar]
- Illmensee K, Kaskar K, Zavos PM.Efficient blastomere biopsy for mouse embryo splitting for future applications in human assisted reproduction. Reprod Biomed Online 2005; 11: 716–725. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Mulnard JG, Graham CF.Cell division and cell allocation in early mouse development. J Embryol Exp Morphol 1978; 48: 37–51. [PubMed] [Google Scholar]
- Piotrowska K, Wianny F, Pedersen RA, Zernicka-Goetz M.Blastomeres arising from the first cleavage division have distinguishable fates in normal mouse development. Development 2001; 128: 3739–3748. [DOI] [PubMed] [Google Scholar]
- Papaioannou VE, Ebert KM.Mouse half embryos: viability and allocation of cells in the blastocyst. Dev Dyn 1995; 203: 393–398. [DOI] [PubMed] [Google Scholar]
- Rands GF.Size regulation in the mouse embryo. II. The development of half embryos. J Embryol Exp Morphol 1986; 98: 209–217. [PubMed] [Google Scholar]
- Ralston A, Rossant J.CDX2 acts downstream of cell polarization to cell-autonomously promote trophectoderm fate in the early mouse embryo. Dev Biol 2008; 313: 614–629. [DOI] [PubMed] [Google Scholar]
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J.CDX2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 2005; 132: 2093–2102. [DOI] [PubMed] [Google Scholar]
- Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, Rossant J.Interaction between Oct3/4 and CDX2 determines trophectoderm differentiation. Cell 2005; 123: 917–929. [DOI] [PubMed] [Google Scholar]
- Jedrusik A, Parfitt DE, Guo G, Skamagki M, Grabarek JB, Johnson MH, Robson P, Zernicka-Goetz M.Role of CDX2 and cell polarity in cell allocation and specification of trophectoderm and inner cell mass in the mouse embryo. Genes Dev 2008; 22: 2692–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K, Handyside AH.Cell allocation in twin half mouse embryos bisected at the 8-cell stage: implications for preimplantation diagnosis. Mol Reprod Dev 1993; 36: 16–22. [DOI] [PubMed] [Google Scholar]
- Bielanska M, Tan SL, Ao A.Chromosomal mosaicism throughout human preimplantation development in vitro: incidence, type, and relevance to embryo outcome. Hum Reprod 2002; 17: 413–419. [DOI] [PubMed] [Google Scholar]
- Marchetti F, Bishop J, Lowe X, Wyrobek AJ.Chromosomal mosaicism in mouse two-cell embryos after paternal exposure to acrylamide. Toxicol Sci 2009; 107: 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CF, Deussen ZA.Features of cell lineage in preimplantation mouse development. J Embryol Exp Morphol 1978; 48: 53–72. [PubMed] [Google Scholar]
- Graham CF, Lehtonen E.Formation and consequences of cell patterns in preimplantation mouse development. J Embryol Exp Morphol 1979; 49: 277–294. [PubMed] [Google Scholar]
- Elsheikh AS, Takahashi Y, Hishinuma M, Nour MS, Kanagawa H.Effect of encapsulation on development of mouse pronuclear stage embryos in vitro. Anim Reprod Sci 1997; 48: 317–324. [DOI] [PubMed] [Google Scholar]
- Illmensee K.Microsurgical cloning techniques for zygotes: a long-term dispute. Reprod Biomed Online 2009; 18: 7–8. [DOI] [PubMed] [Google Scholar]
- Illmensee K, Levanduski M, Vidali A, Husami N, Goudas VT.Human embryo twinning with applications in reproductive medicine. Fertil Steril 2010; 93: 423–427. [DOI] [PubMed] [Google Scholar]