Abstract
Islet-infiltrating lymphocytes of individual male and female non-obese diabetic (NOD) mice were examined with the purpose of determining the differences that lead to a predominance of diabetes in female versus males NOD mice. When normalized for the amount of islet lymphocytes recovered, the infiltrating lymphocytes of female NOD mice were indistinguishable from those of male NOD mice. The only observed difference was that islet inflammation progressed at an increased rate in female compared to male NOD mice. There was no difference in the composition of islet infiltrates in male and female NOD mice. Unexpectedly, the ratio of CD4+:CD8+ T cells was tightly controlled in the islets throughout diabetogenesis. The frequency of IL-4+ CD4+ T cells started high but quickly fell to 3% of the population which was maintained with increasing inflammation. A significant portion of the CD8+ T cells were islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) specific in both male and female NOD mice and this population was antigen experienced and increased at high levels of islet inflammation. Surprisingly, a large pool of antigen inexperienced naïve T cells was detected in the islets. We conclude the underlying immunological processes in both male and female NOD mice are similar while the rates differ and the presence of naïve T cell in the islets may contribute to epitope spreading.
Keywords: autoimmunity, FoxP3, IGRP, IL-4, naïve T-cell
INTRODUCTION
Type I diabetes (T1D) is caused by the immune destruction of the insulin-producing pancreatic β cells. Based on studies with the non-obese diabetic (NOD) mouse, the primary mediators of β cell destruction are CD4+ and CD8+ T cells 1–3. NOD mice spontaneously develop T1D, with 80% of females and 20% of males becoming overtly diabetic by 20 weeks of age4. The immunological basis for the disparity between the sexes, however, is not well characterized 5,6. Sex hormones influence the time of onset of T1D. Disease incidence is increased in castrated NOD males and administration of testosterone delays the onset of disease in NOD females7–10. In addition, several studies have examined the Th1/Th2 cytokine balance in male and female NOD mice and found that T cells were skewed to produce more Th1 cytokines in young females than in young males, although islet infiltrating cells from both male and female NOD mice can produce diabetes in NOD scid mice 11–13. Presently, the effect of the sex hormones on the immune system remains poorly understood and the balance of lymphocyte subsets in the islets in NOD males versus females over time has not been studied.
Susceptibility to diabetes is genetically linked in both NOD mice and humans to the MHC class II alleles. NOD mice deficient in β2-microglobulin and CD8+ T cells fail to develop diabetes. Thus, both CD4+ and CD8+ T cells are important for development of diabetes in NOD mice 14–25. Given the importance of T cells in T1D, there have been many attempts to understand their role in the progression of β cell autoimmunity 26,27. Unfortunately, these efforts have been hampered by the paucity of T cells that can be recovered from the islets. As a result, many studies have used pooled islets from several NOD mice, pancreatic lymph nodes (PLN), or spleen cells to study the progression and nature of the autoimmune response 12,28–31. While these studies have aided in elucidating the systemic immune response, a critical analysis of the T cell response in the islets from single individuals is still lacking.
Using multi-color flow cytometry we directly analyzed the islet-infiltrating lymphocytes of individual male and female NOD mice, then considered the data both as individual mice and in groups. Islets of female NOD mice were more heavily infiltrated than the islets of age matched males. Surprisingly, when considered as individuals and matched by the level of lymphocyte infiltration instead of age, the composition of islet infiltrates of male NOD mice resembles that of females. The number of both IL-4+ CD4+ T cells and Treg increased over time in the islets. In spite of this increase, the percentage of IL-4+ CD4+ T cells decreased. Interestingly in the midst of these changes, the ratio of CD4+:CD8+ T cells in the islets remained constant. Male NOD mice had antigen experienced IGRP reactive CD8+ T cells in their islets. These data indicate that male and female NOD mice progress towards diabetes with the same balance of lymphocytes, but at very different rates. A surprising finding of this study was the presence of significant levels of antigen inexperienced, apparently naïve, T cells in the islets of NOD mice.
METHODS
Mice
NOD/ShiLtJ mice and C.129-Il4tml Lky/J mice were purchased from Jackson Laboratories32. In our facility, female NOD mice become diabetic at a median age of 16 weeks, with 75% diabetic by 20 weeks of age indistinguishable from NOD/ShiLtJ mice at Jackson Labs(see Supplemental Fig. 1). In order to examine T cells with a Th2 phenotype, NOD IL-4 reporter mice (NOD.Cg-Il4tml Lky/Fre) were created by backcrossing the reporter gene from C.129-Il4tml Lky/J mice onto the NOD background using speed congenics 32,33. These NOD mice express eGFP when IL-4 mRNA is produced. Forty-three SNPs (Supplemental data Table 1) were used to distinguish between the BALB/c and NOD genomes. The IL-4 gene, located on chromosome 11 at base pair position 53 million, was flanked by SNPs at positions 33 million and 77 million; both were homozygous for NOD DNA. By backcross (BC)4 (N5), 41 of the 42 SNPs were homozygous for NOD markers (98.8% of the SNPs) and female mice in the N4 or BC3 generation became diabetic. The mice were backcrossed 3 more times without SNP selection to BC6 to assure homogeneity of the NOD genetic background. To select newly diabetic mice, blood sugar was monitored weekly using a FreeStyle Blood Glucose Meter and mice were considered diabetic after 2 consecutive blood glucose readings of over 250 mg/dL. Only mice with a blood sugar <150 mg/dL were used in this study, unless specifically designated as a diabetic mouse.
Isolation of Lymphocytes from Islets
Pancreata were isolated as previously described 34. Briefly, each pancreas was perfused with a 2 mg/ml solution of Collagenase P (Sigma), dissected and incubated at 37°C for 20 minutes. Islets were purified using a Ficoll PM 400 (Sigma) gradient, handpicked, counted and then cultured overnight in RPMI 1640 media containing 10% FBS and 4 ng/ml recombinant murine IL-2 (Invitrogen). Infiltrating lymphocytes were collected and filtered through a 40μm nylon filter. All islet-infiltrating cells from every mouse were analyzed by flow cytometry. Because the number of islets recovered is not uniform among mice, the “number of cells per islet” was determined by dividing the total number of cells in the sample by the number of cultured islets; therefore, a mouse that had 100 CD8+ T cells, 400 CD4+ T cells and 300 B cells recovered from 100 islets would be recorded as having 1 CD8+ T cell, 4 CD4+ T cells and 3 B cells per islet. Each sample constitutes all of the islet cells isolated from an individual mouse. An average of 103 islets was isolated per mouse. For further examples of how data was collected and treated, a chart of the raw data presented in Fig. 3 is included (Supplemental data Table 2). The data from each individual mouse was analyzed individually or alternatively combined with the data from mice of like sex and age to examine inter group differences.
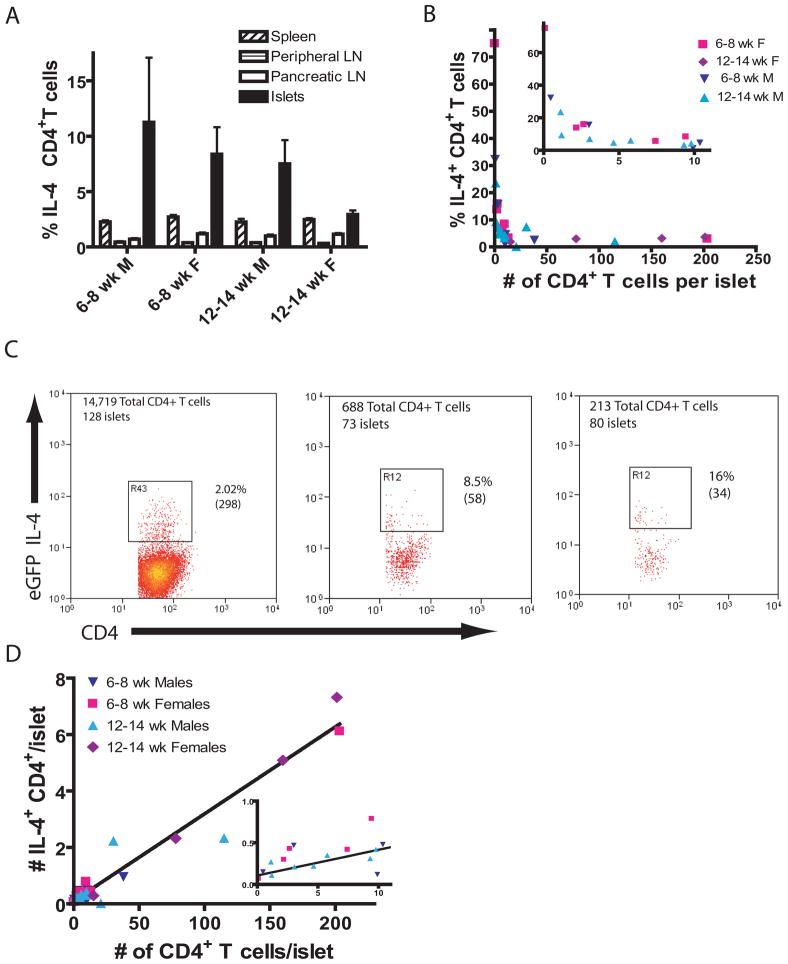
Figure 3.
The number and percentage of IL-4 capable CD4+ T cells change with age.
(A) The percentage of CD4+CD3+GFP+ T cells in the CD4+CD3+ T cell population. Islets had significantly more IL-4+ cells than other tissues in: 6–8 week males, p=0.006; 6–8 week females, p<0.0001; and 12–14 week males, p<0.0001; 12–14 week females, p=0.009 (Mann Whitney test). There was no significant difference in the percentage of IL-4+ cells in the islets with age and sex (Kruskal Wallis test).
(B) Only a small fraction of CD4+ T cells are IL-4+ after insulitis reaches 50 CD4+ T cells/islet. Inset show expanded scale near origin.
(C) These 3 panels show all of the islet-infiltrating CD3+CD4+ T cells isolated from a 12 week-old and two 6 week old female NOD-IL-4gfp mice. The total number of islets recovered from each mouse is given. The percentage of cells in the gate is shown and the number of cells is in parenthesis. See supplementary Fig. 2b for gating scheme.
(D) The fraction of IL-4 competent cells remains constant as insulitis increases (n = 26). The data fit a straight line with a slope of 0.0308+/−0.001542 and an intercept of 0.107+/–0.1093. R2=0.94 (6–8 week-old males N=5, 6–8 week-old females N=7, 12–14 week-old males N=10, 12–14 week-old females N=4). Inset show expanded scale near origin.
Raw data and calculations for the above graphs can be found in Supplemental data Table 2. A discussion of the relationships between X:Y ratios and the slope and Y-intercepts of straight lines can be found in Supplemental Fig. 3.
Flow Cytometry
Splenocytes and lymphocytes from NOD mice were examined using 9 color flow cytometry. Cells were stained with a cocktail of APC-Cy7-anti-CD3 (BD Biosciences, San Jose, CA), Pacific Orange-anti-CD4 (Invitrogen, Carlsbad, CA), PerCP-anti-CD8 (BD), Pacific Blue-anti-CD44 (Invitrogen), APC-anti-CD25 (eBioscience), PE-Texas Red-anti-CD62 (Invitrogen), PE-Cy7-anti-CD19 (BioLegends, San Diego, CA) and PE-ultra-avidin (Lenco, St. Louis, MO)-NRP-V7-Kd tetramer. Antibody concentrations used were determined by preliminary titrations. To assess FoxP3 expression, cells were fixed, permeablized and stained with PE-anti-FoxP3 antibody (eBioscience). Cells were analyzed on a Dako (Beckman-Coulter) Cyan-ADP(Colorado Springs, CO). “Minus one” controls were performed in each experiment to ensure that the fluorescence measured originated from the correct stain. Staining controls used syngeneic spleen cells.
Tetramer
H2Kd tetramers were prepared as previously described 35. Briefly, H2Kd, modified to contain a biotinylation sequence, and mouse β2 microglobulin were produced in E. coli and refolded with NRP-V7 peptide (KYNKANVFL from GenScript) in vitro, an islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) mimotope 36. Refolded NRP-V7-H2Kd monomer was purified by HPLC, biotinylated using Biotin Protein Ligase and assembled into tetramers by conjugation with UltraAvidin-R-PE (Leinco). In NOD mice most of the IGRP+ CD8+ T cells are also NRP-V7+. For convenience, NRP-V7 reactive cells are referred to as IGRP-specific.
RESULTS
Female NOD mice have more islet-infiltrating T cells than males at the same age
We examined the number of T cells infiltrating the islets. In both NOD males and females, the number of CD4+, CD8+ and B cells increased with age. In NOD males, there was an ~two-fold increase in the number of infiltrating cells, whereas a five-fold increase of CD4+, CD8+ and B cells was detected in NOD female mice between 6–8 and 12–14 weeks of age (Fig. 1A). This is consistent with previous reports that islets of NOD female mice are more heavily infiltrated than NOD male mice of the same age 5. However, it is of note that the number of infiltrating cells in males also increased with age, albeit more slowly than in females. 25–29 week-old male NOD mice were examined to determine if β cell autoimmunity continued to increase in males and levels of insulitis comparable to those seen in the 12–14 week-old females was achieved, or if insulitis in males was arrested at a certain point and did not progress. We found that 25–29 week-old male NOD mice had levels of islet lymphocytes similar to 12–14 week-old female NOD mice. By 25–29 weeks nearly all female NOD mice became overtly diabetic (Supplemental Fig. 1). We considered diabetic mice separately regardless of age and no diabetic mice were included in the analysis unless explicitly noted.
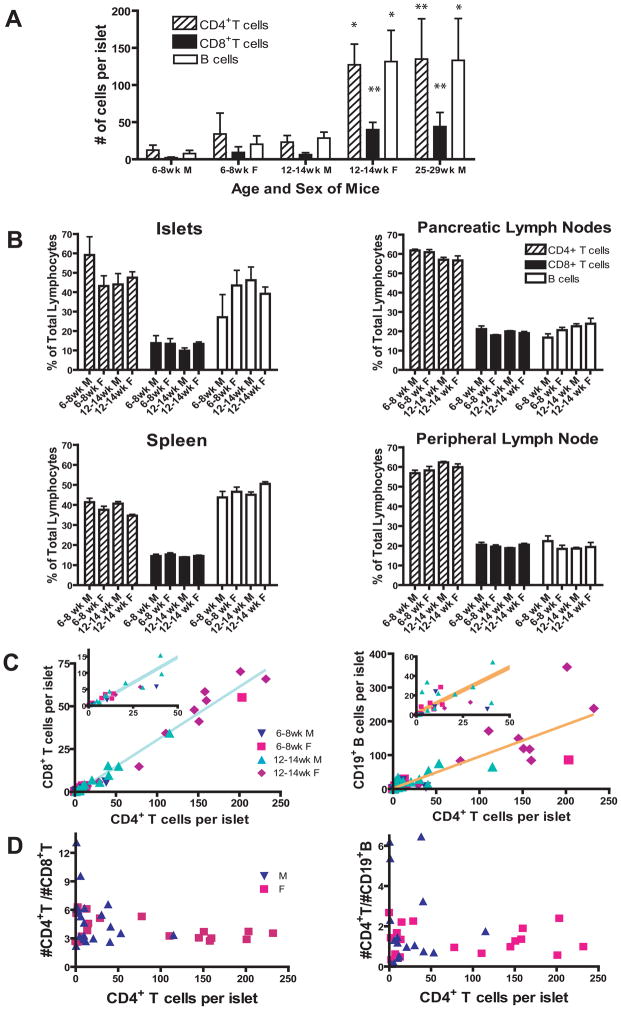
Figure 1.
Infiltrating T cells increase with age in a regulated fashion.
(A) The number of islet-infiltrating lymphocytes is higher in NOD females than males and increases with age. The total number of CD3+ CD4+ T cells, CD3+ CD8+ T cells or CD19+ B cells, as determined by flow cytometry (see supplementary Fig. 2a for gating scheme), was divided by the total number of islets isolated from individual NOD mice. In both male and female mice, the increase in infiltrating lymphocytes was significant (* p<0.05 and ** p<0.01 by Mann Whitney test).
(B) Islets contain a high percentage of B cells. CD4+ or CD8+ T cells and B cells were represented as a percent of the total lymphocytes. T cells are gated on CD3+ cells. (A and B; 6–8 week-old males N=10, 6–8 week-old females N=14, 12–14 week-old males N=22, 12–14 week-old females N=18, 25–29 week-old males N=6)
(C) There is a linear relationship between the number of CD4+ and CD8+ T cells per islet. The number of CD8+ T cells per islet was plotted against the number of CD4+ T cells per islet (n = 41, age 6 to 14 weeks). Data fit a straight line with a slope of 0.3107+/−0.0080 and a Y-intercept of −0.7292+/−0.695, r2=0.9738. Y=0.3107X − 0.7292, where Y= the number of CD8+ T cells per islet and X= the number of CD4+ T cells per islet. Females, 6–8 week, N=9, 12–14 week, N=10; males, 6–8 week, N=7, 12–14 week, N=15.
B cells per islet were plotted against the number of CD4+ T cells (n = 36). The data fit to a straight line with a slope of 0.9426, r2 of 0.7212. Females, 6–8 week, N=8, 12–14 week, N=10; males, 6–8 week, N=5, 12–14 week, N=13. Insets show expanded scale near origin.
(D) The ratio of CD4+:B cells varies more than the CD4+:CD8+ ratio. The ratio of CD4+:B cells varies from 3.2 to 0.17, a factor of 18, while the CD4+:CD8+ ratio varies from 9.6 to 2.2, a factor of 4.4. The data in C was recalculated to compare the CD8+:CD4+ T cell ratio and the B cell:CD4+ T cell ratio with the level of inflammation as determined by the number of CD4+ T cells/islet.
The composition of islet-infiltrating lymphocytes is similar in female and male NOD mice
We determined the composition of infiltrating cells over time in spleen, peripheral and PLN and the islets of NOD mice. Surprisingly, when grouped into 6–8 week and 12–14 week-old, males and females, there was no significant change in the percentage of CD4+, CD8+ or B cells over time in the spleen, peripheral lymph nodes, PLN or islets (Fig. 1B). Thus, while the number of infiltrating cells increased, the overall composition did not change.
Examination of individual NOD mice indicates CD4+ to CD8+ T cell ratios are tightly controlled in the islets
When individual NOD mice were examined, regardless of their sex or age, a linear relationship between the number of CD4+ and CD8+ T cells per islet was detected, indicating a strong association between the number of CD4+ and CD8+ T cells that is maintained in the islets during the progression of β cell autoimmunity in both males and females (Fig. 1C) (r2= 0.9735). The number of B cells also increased linearly with CD4+ T cells (r2 of 0.7212); however, the ratio of B cells to CD4+ T cells was less tightly regulated compared to CD8+ T cells (Fig. 1C and D).
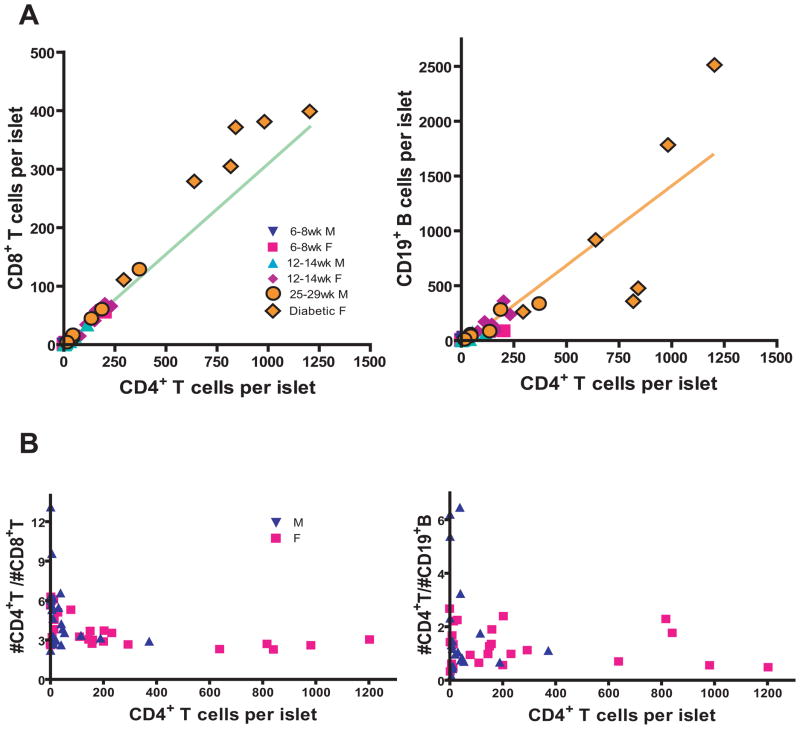
To further investigate the ratios of lymphocyte subsets as β cell autoimmunity progressed, we examined diabetic female NOD mice and 25–29 week-old male NOD mice. Diabetes is the consequence of β cell autoimmunity; however, loss of blood glucose homeostasis could alter the control mechanism. Therefore, islets were isolated from female NOD mice within 10 days of a blood glucose level >250 mg/dL. These mice had a CD4+:CD8+ T cell ratio of 2.7+/− 0.52, similar to non-diabetic female NOD mice (Fig 2). We then examined 25–29 week-old non-diabetic male NOD mice and found a statistically equivalent CD4+:CD8+ T cell ratio of 3.5+/− 0.77; again indistinguishable from younger non-diabetic females (Fig 2). Therefore at the onset of diabetes and in very old male NOD mice the number of infiltrating cells had greatly increased but the CD4+:CD8+ T cell and B cell:T cell ratios remained the same.
Figure 2.
The CD4+:CD8+ T cell ratio is maintained as insulitis progresses.
(A) Six diabetic female and six 25–29 week-old non-diabetic male NOD mice were plotted with the 42 or 36 individual NOD mice from Fig. 1C. The lines from Fig. 1C were extended to better allow comparison of the data.
(B) The data in A was recalculated to compare the ratio of CD8+ to CD4+ T cells or the ratio of B cells to CD4+ T cells with the level of inflammation as determined by the number of CD4+ T cells/islet.
A small but significant number of Th2 cells are detected in the islets of NOD mice
Th2 cell responses have been suggested to regulate at least some Th1 and Tc1 cell responses 37,38. We examined the expression of IL-4 secreting CD4+ T cells using NOD IL-4 reporter mice 32,39. The percentage of IL-4 producing CD4+ T cells was much higher in the islets of males and young females than in the spleen, peripheral lymph nodes and PLNs (Fig. 3A). The level of IL-4 producing CD4+ T cells in the spleen and periphery was the same in all mice regardless of sex and age. In contrast the percentage of IL-4 producing CD4+ T cells in the islets decreased with age in both males and females. When individual mice were examined, it became obvious that mice with low levels of infiltrates had higher levels of IL-4 producing CD4+ T cells (Fig. 3B & C). The percentage of IL-4 producing CD4+ T cells decreased before the number of CD4+ T cells reached 25 per islet, then maintained a constant level, consisting of ~3% of the CD4+ T cell population. Surprisingly when the number of IL-4 producing CD4+ T cells was directly compared to the total CD4+ T cells per islet, the number of IL-4 producing CD4+ T cells increased linearly with the increase of CD4+ T cell per islet, r2=0.94. The best fit line had a slope of 0.03 indicating an overall composition of 3% IL-4 competent cells in the CD4+ T cell population (Fig. 3D). At very low numbers of infiltrating CD4+ T cells, those cells are highly IL-4 producing. This was suggested because the best fit line does not pass through zero (see Supplemental Fig. 3). Close inspection of the raw data confirms that when very few cells (<50 CD4+ T cells/mouse) were recovered from the islets, they are 34% IL-4 competent. Overall our data demonstrates that the first CD4+ T cells to reach the islets had an increased percentage of IL-4 competent cells and while the IL-4 producing CD4+ T cells increased, they initially did so more slowly than the non-IL-4 producing CD4+ T cells and so were not able to influence the diabetegenic cells effectively.
Islet Tregs increase more slowly than effector T cells
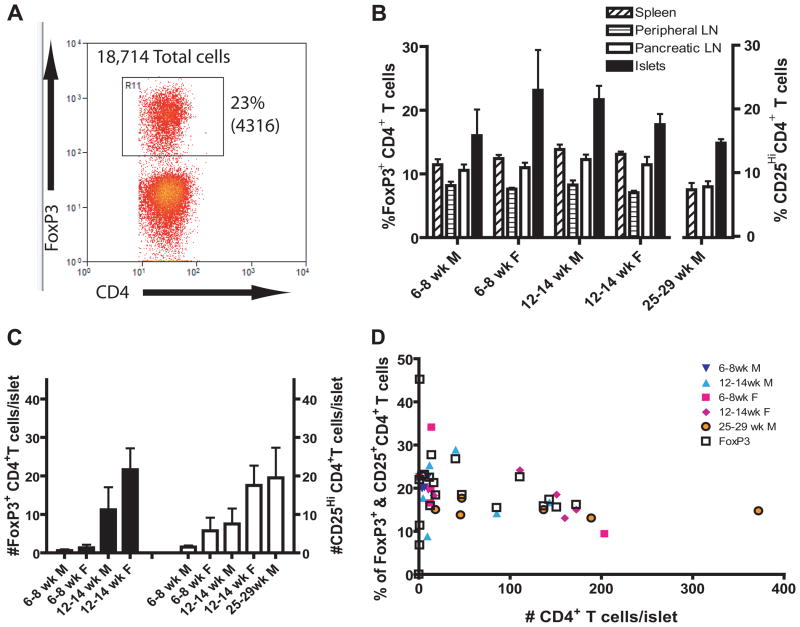
FoxP3+ Tregs are important in regulating autoimmunity. Strikingly, the frequency of FoxP3+ CD4+ T cells in the spleen, peripheral lymph nodes and PLNs was similar and stable at ~7–12% at all times examined. The highest level of Treg was in the islets where the frequency of Treg increased in NOD males between 6 and 12 weeks of age. The percentage decreased in NOD females, despite an increased number of FoxP3+ CD4+ T cells per islet over this same time (Fig. 4A, B and C). Thus, the drop in frequency in females was due to dilution in a greater number of FoxP3− CD4+ T cells. In individual NOD mice, the percentage of FoxP3+ CD4+ T cells was high in the early stages of β cell autoimmunity, with the exception of a few mice that had very few cells in the islets, but gradually decreased after 50 CD4+ T cells per islet (Fig. 4D). When the analysis was repeated using the CD25hi population as a surrogate marker for Treg, similar results were obtained (Fig. 4C). In 12–14 week-old male NOD mice, 20 percent of the islet-infiltrating CD4+ T cells were CD25hi, compared to only 13–15% were CD25hi in the islets of 25–29 week-old NOD male mice, indicating that Treg also decreased in males but at an older age. This suggests that Treg might be effective in young NOD females and older NOD males.
Figure 4.
The number of Treg increase with age. Treg were identified as CD4+CD3+FoxP3+ T cells.
(A) Dot plot shows the entire population of islet-infiltrating CD3+ T cells isolated from 148 islets recovered from one 12 week-old female NOD mouse. The percentage of cells in the gate is shown and the number of cells is in parenthesis. See supplementary Fig. 2a for gating scheme.
(B) Treg are expressed at the highest frequency in the islets. (6–8 week-old males N=4, 6–8 week-old females N=5, 12–14 week-old males N=4, 12–14 week-old females N=4, 25–29 week-old males N=6)
(C) The average number of islet-infiltrating CD4+CD3+FoxP3+ T cells per islet increases with age (black bars). The number of islet-infiltrating CD4+CD3+CD25hi T cells/islet is shown in white bars.
(D) The percentage of FoxP3+ or CD25hi cells in the CD4+CD3+ T cells pool gradually decreases with increasing insulitis (N = 29). In mice where CD25 was stained along side FoxP3, the CD25hi population coincided well.
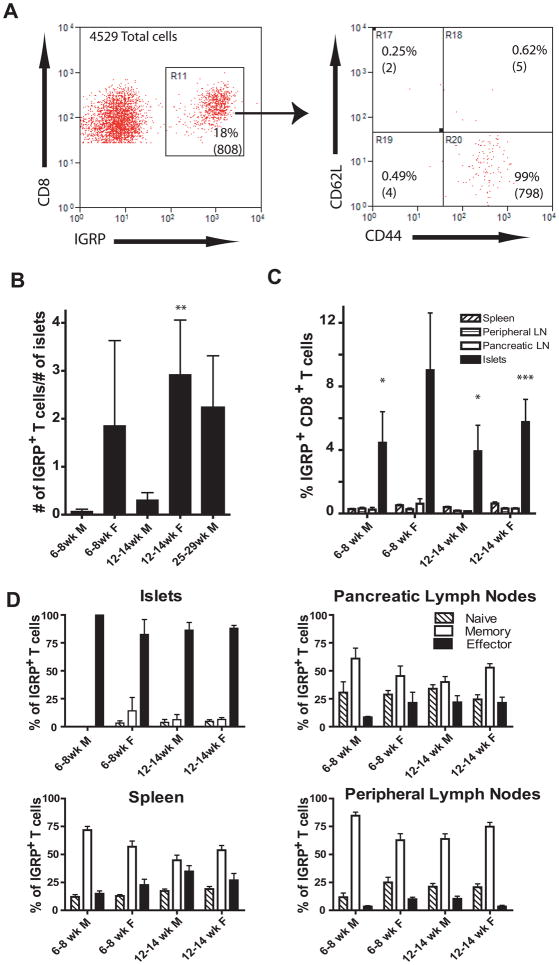
Antigen experienced IGRP-specific CD8+ T cells are found in the islets of both male and female NOD mice
IGRP is a known target of diabetogenic CD8+ T cells. A striking increase in the number, but not the percentage of IGRP-specific CD8+ T cells was detected in the islets (Fig. 5). The number of IGRP-specific CD8+ T cells in the islets of older NOD females increases 10-fold compared to age matched males (Mann Whitney test p= 0.004) (Fig. 5B). Nevertheless, 25–29 week-old male NOD mice had levels of IGRP-specific CD8+ T cells comparable to the 12–14 week-old females. Also the percentage of CD8+ T cells that were IGRP-specific was similar in NOD males and females (Fig. 5C). In contrast to the phenotype of IGRP-specific CD8+ T cells found in the periphery, nearly all of the IGRP-specific CD8+ T cells in the islets were effector phenotype, CD62Llo and CD44hi (Fig. 5A). In the periphery there was a significant fraction of naïve, CD62Lhi and CD44lo, or memory cells, CD62Lhi and CD44hi, but relatively few IGRP-specific CD8+ T cells with an effector phenotype (Fig. 5D). Thus the IGRP-specific CD8+ T cells in the islets of both male and female NOD mice are antigen experienced. This is consistent with previous research demonstrating islet-infiltrating lymphocytes can transfer diabetes to NOD scid recipients 11.
Figure 5.
IGRP-specific CD8+ T cells have an effector T cell phenotype in the islets
(A) The dot plots show the total islet-infiltrating CD3+CD8+ IGRP+ T cells isolated from 81 islets recovered from a 12 week-old female NOD mouse are antigen experienced. The percentage of cells in the gate is shown and the number of cells is in parenthesis. See supplementary Fig. 2b for gating scheme.
(B) Females have more IGRP-specific CD8+ T cells per islet than males of the same age but the percentage is similar between sexes. Twelve to fourteen week-old NOD female mice had significantly more IGRP-specific CD8+ T cells per islet than NOD males of the same age (p=0.004). IGRP-specific CD8+ T cells were identified using PE conjugated NRP-V7-Kd tetramers. To determine number of cells per islet, the total number of CD8+CD3+CD19− NRP-V7+ cells was divided by the number of islets isolated from individual mice. Therefore a mouse that had 100 IGRP+ T cells recovered from 100 islets would have 1 IGRP+ T cell per islet. An average of more than 100 islets was isolated from each mouse.
(C) Percentage of CD8+CD3+CD19− NRP-V7+ cells in the CD8+CD3+CD19− population was determined for spleen, inguinal lymph nodes, PLNs and islets. Islets had significantly more IGRP+ cells than other tissues in 6–8 week males (p=0.041) 12–14 week males (p=0.030) and 12–14 week females (p=0.0001). Six to eight week-old females were not significantly different (p=0.083) but this group contained two mice with no islet-infiltrating IGRP+ cells.
(D) IGRP-specific cells in the islets have an effector phenotype based on their expression of CD62L and CD44 (panel A).
(B–D, 6–8 week-old males N=5, 6–8 week-old females N=7, 12–14 week-old males N=11, 12–14 week-old females N=8, 25–29 week-old males N=3)
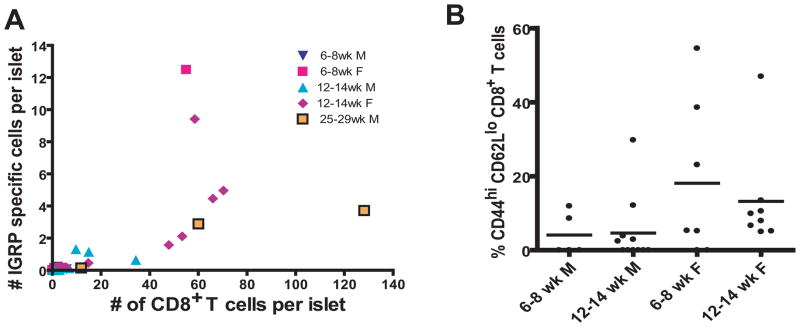
IGRP-specific CD8+ T cells represent a dominant specificity
We examined the relationship of CD8+ T cell number to the number of IGRP-specific CD8+ T cells. Interestingly, when the islets reached a critical level of inflammation (as defined by the number of CD8+ T cells/the number of islets from individual mice), the number of IGRP-specific CD8+ T cells increased faster than the total number of CD8+ T cells infiltrating the islets of NOD mice (Fig. 6A). The change in rate suggests that the total number of T cells increased at a constant rate, but IGRP reactive CD8+ T cells increased more rapidly in the islets. Indeed, this is consistent with expansion in situ the IGRP-specific CD8+ T cells made up a major subset of the effector CD8+ T cells in the young animals (Fig. 6B).
Figure 6.
IGRP-specific CD8+T cells do not increase linearly with increasing inflammation but do make up a substantial proportion of the effector CD8+ T cells pool.
(A) The number of CD8+CD3+CD19− NRP-V7+ cells per islet was plotted against the number of total CD4+ T cells per islet for 34 mice.
(B) Percent IGRP-specific effector CD8+ T cells (number of CD62LloCD44hi CD8+CD3+CD19− NRP-V7+ cells/number of CD62LloCD44hi CD8+CD3+CD19− cells x100) is shown. Krushal-Wallis test indicated means were not significantly different, p=0.066.
(A and B, 6–8 week-old males N=5, 6–8 week-old females N=7, 12–14 week-old males N=11, 12–14 week-old females N=8, 27 week-old males N=3)
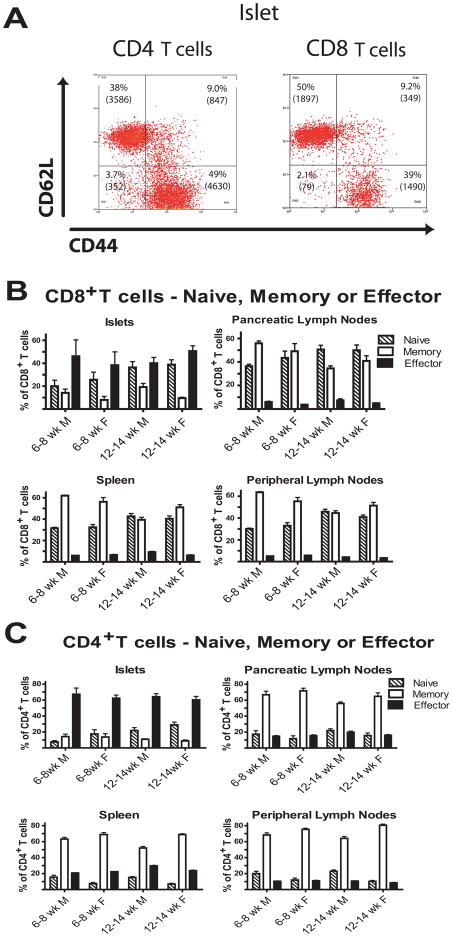
With increasing insulitis an increasing portion of the islet infiltrating T cells are naïve
While nearly all of the IGRP-specific CD8+ T cells in the islets were CD44hi and CD62Llo indicating they were antigen experienced and effector cells, a large portion of the CD8+ T cell pool in the islets was CD44lo and CD62Lhi indicating they are not antigen experienced and appear to have a naïve phenotype (Fig. 7A). The percent of CD44lo, CD62Lhi CD8+ T cells increased with age in both male and female NOD mice (Fig. 7B). An equivalent population was also observed in the CD4+ T cell pool; however, a smaller portion of the CD4+ T cell pool had a naïve phenotype in the islets (Fig. 7C). When individual mice were compared, naïve T cells increased with increasing insulitis (Fig. 8).
Fig. 7.
Islets from older female NOD mice have a substantial number of naïve and effector T cells.
(A) Representative gates for naïve (CD62Lhi CD44lo), memory (CD62Lhi CD44hi) and effector (CD62Llo CD44hi) T cells in 55 islets from a 12 week-old NOD female are shown. The percentage of cells in each gate is shown and the number of cells in each gate in the islets is in parenthesis.
(B and C) The percent of naïve CD4+ and CD8+ T cells in the islets increases with age while in lymphoid tissues the percent of naive, memory or effector phenotypes does not change. Cells were classified as in (A). There was a significant increase in naïve CD4+ T cells in the islets (p < 0.01) but not in other tissues. Other subsets did not change. (6–8 week-old males N=10, 6–8 week-old females N=14, 12–14 week-old males N=24, 12–14 week-old females N=18)
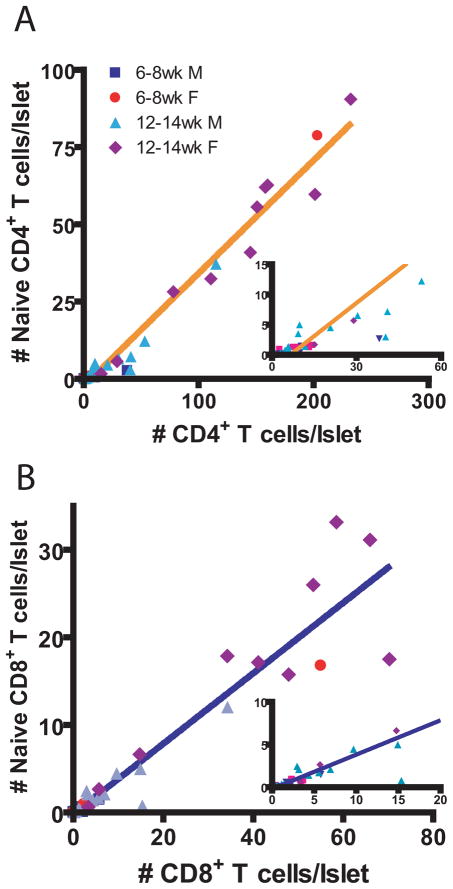
Fig. 8.
The number of naïve CD4+ and CD8+ T cells increases as insulitis increases. The number of naïve CD4+ or CD8+ T cells per islet was plotted against the number of total CD4+ or CD8+ T cells per islet (n = 42, age 6 to 14 weeks). (A) Naive versus total number of CD4+ T cells. Data fit a straight line with a slope of 0.3654+/−0.0104 and Y-intercept of −2.377+/−0.8384, r2 = 0.9686. Y = 0.3654 X 2.377 (B) Naive versus total number of CD8+ T cells. Data fit a straight line with a slope of 0.4023+/−0.0219 and Y-intercept of −0.1992+/−0.5453, r2 = 0.8937. Y = 0.4023 X − 0.1992 (6–8 week-old males N=7, 6–8 week-old females N=10, 12–14 week-old males N=15, 12–14 week-old females N=10)
We re-examined IL-4 competent T cells in the islets and found they were all CD44hi and CD62Llo, thus effector cells (data not shown). FoxP3+ CD4+ T cells were all CD44hi with some CD62Llo cells and some CD62Lhi cells, thus effector and memory cells (data not shown). Thus, the naïve phenotype cells in the islets were unlikely to be anti-islet CD8+ T cells, Th2 cells or Treg cells. This demonstrates an influx of peripheral naïve T cells.
Naïve T cells are not detected until the number of CD4+ T cells reaches 6.4 CD4+ T cells per islet indicating a threshold for inflammation is needed before naïve cells can enter (Fig. 8). A similar, but less pronounced, effect is seen for CD8+ T cells.
DISCUSSION
It has been documented that females have a higher incidence of autoimmune diseases both in humans and mice 40,41. NOD females are known to have a higher burden of islet infiltrates than males of the same age, correlating with an earlier onset of overt diabetes 4. The composition of islet-infiltrating lymphocytes in individual males and females has not previously been compared. We compared age-matched groups of male and female NOD mice and because of the disparity of islet inflammation between these two groups, we also compared the mice individually, based on their level of islet inflammation.
We found a surprisingly large percentage of B cells in the islets of both male and female NOD mice. This has been previously reported in female NOD mice but not in males 29,42. We report here that a large pool of B cells was present in the islets even as female NOD mice became diabetic and the tight relationship between the CD8+ and CD4+ T cells in the islets was maintained.
Despite the disparity in the level of inflammation, NOD males were similar to females in the tight control of the CD4+:CD8+ ratio and the CD4+ T cell:B cell ratio in the islets. Very old males attained levels of islet infiltrates similar to or greater than those seen in 12 week-old NOD females and with strictly controlled CD4+:CD8+ T cell ratios. This observation indicates that inflammation develops more slowly in males but in a similar manner. Indeed, in many NOD colonies the males can develop a significant incidence of diabetes43.
Progression of β cell autoimmunity with a fixed ratio of CD4+ and CD8+ T cells is puzzling. One possibility is that T cells in the islets are in equilibrium with the rest of the immune system, but this goes against the idea that entry into the islets is only accomplished by T cells that are activated in the PLN. That theory would require the lymph node to activate the appropriate ratios of CD4+ and CD8+ T cells. The tertiary lymphoid structure hypothesis posits that structures form in the islets that are similar to lymph nodes and in equilibrium with the rest of the body. If this is the case, a stringently controlled CD4+:CD8+ T cell ratio would be expected. However, a tightly controlled T cell:B cell ratio would also be expected and twice the level of B cells was found in the islets compared to the lymph nodes (Fig. 1B and D).
It has been shown that induction of IL-4+ CD4+ T cells can significantly delay the onset of diabetes and constitutive expression of IL-4 in the islet cells abrogates insulitis 37,38. Paradoxically, IL-4 knockout NOD mice have a normal onset of diabetes 44. We found that male NOD mice as a group had higher percentages of IL-4 competent CD4+ T cells in their islet-infiltrating CD4+ T cell pool than their female counterparts. However, when examined as a continuum of increasing inflammation, it was obvious that mice with very low levels of islet inflammation had high percentages of IL-4 competent cells in their infiltrating CD4+ T cell pool regardless of sex. In both male and female NOD mice, the contribution of IL-4+ expressing cells to the CD4+ T cell pool was considerably reduced early as diabetogenesis progressed, indicating that the differences were a result of the level of inflammation and not a characteristic of male or female. Bao et al. previously observed that, when stimulated in vitro, islet-infiltrating CD4+ T cells from 4 week-old male NOD mice produced more IL-4 than those from 4 week-old female NOD mice 10. Our results support this result, since low levels of inflammation were seen in very young male NOD mice, so the percentage of CD4+ T cells competent to express IL-4 can be quite high. The same study found that by 10 weeks the islet-infiltrating CD4+ T cells of male and female NOD mice produced equivalent amounts of IL-4 when stimulated in culture. Here we show that as the infiltrate in the islets increases, the percent of IL-4 competent T cells in the islets falls to 3% of the CD4+ T cells pool and is maintained at that level for males and females. This indicates that IL-4 competent CD4+ T cells are highly regulated in the islets during diabetogenesis. A novel finding of this study is the predictable composition of IL-4 competent CD4+ T cells, CD4+ T cells and CD8+ T cells in the islets as diabetogenesis progresses and the linear relationship between these components. It will be of great interest to know if treatments that cure diabetes in NOD mice and increase the percentage of IL-4 expressing T cells in the islets do so by decreasing the inflammation which results indirectly in an increase in IL-4 competent CD4+ T cells.
Tregs are also capable of suppressing inflammation. Given the decreased incidence of T1D in male NOD mice, one possibility was that males have higher levels of Treg. Contrary to these expectations, young female NOD mice had higher levels of Tregs in their islets than their male counterparts, although older female mice had slightly reduced levels of Tregs than their male counterparts. On the other hand, in very old male NOD mice the percentage of Treg had dropped substantially from the level of 12 week NOD males. When we examined the data on a continuum, levels of Tregs were highest early in disease and decreased slowly as inflammation continued to increase. These findings are in agreement with earlier work demonstrating that in NOD female mice FoxP3+ CD4+ T cells in the PLN and islets decline with age, while the FoxP3+ cells in the spleen do not 28,31. Again, changes in the frequency of FoxP3+ CD4+ T cells may be a result of the level of islet inflammation and not the sex of the NOD mice.
IGRP-specific CD8+ T cells are diabetogenic and have been used to predict the onset of diabetes 45,46. Accordingly, we anticipated male NOD mice might not have IGRP-specific CD8+ T cells. When grouped, female NOD mice did have much higher numbers of IGRP-specific CD8+ T cells. However, the number of IGRP+ T cells per islet in 27 week-old male NOD mice was comparable to that of the 12–14 week-old females. When we examined IGRP-specific T cells as percentage of the islet CD8+ T cell pool, female mice were not significantly different from male NOD mice. Next we examined the phenotype of the IGRP-specific CD8+ T cells to see if they were antigen experienced. As expected, the IGRP-specific CD8+ T cells in the islets of females were CD44hi CD62Llo effector cells, while those in the lymph nodes and spleen were CD62Lhi memory or naïve cells. The same distribution was also seen in males. Again, the only differences between the male and female populations of IGRP-specific CD8+ T cells were attributable to the total number of infiltrating cells. Enee et. al. recently reported similar results for male and female NOD mice expressing HLA-A2 as a trans gene, demonstrating that HLA-A2 had no effect of the Kd-restricted IGRP responses 47. These findings in males are similar to that reported here. Hence, there was no evidence of selective recruitment of IGRP-specific CD8+ T cells in female NOD mice.
One important observation from this study is the presence of large numbers of naïve T cells in the islets. It had been previously thought that T cells infiltrating the islets were largely if not exclusively activated, diabetogenic effectors. We found a large number of both naïve CD4+ and naïve CD8+ T cells. Thus the islets may correspond to the beginnings of “lymph node-like” structures seen in the pancreas of mice with advanced disease. Apparently the cells making up these lymphoid structures can sequester naïve T cells. We also observed a large pool of B cells infiltrating the islets, in agreement with others 29. B cells are required for the development of diabetes and have been shown to be required for epitope spreading 48. In mucosal tissue it has also been demonstrated that lymphotoxin produced by B cells can drive the formation of lymphoid structures 49. The present study indicates that large numbers of both B cells and naïve T cells are present in the islets in an increasingly inflammatory environment. The large population of naïve T cells may be involved in epitope spreading seen later where new autoantigen are recognized, and priming does not occur in the draining lymph node, but takes place in the lymphoid structures in the islets. Thus priming could occur in a more inflammatory environment.
The differences within the islets of male and female and young and old NOD mice did not appear to be discrete. Islet infiltrates in male NOD mice contained levels of CD8+ T cells, B cells, Th2, Treg, and IGRP reactive T cells comparable to those found in females at any set level of inflammation, as defined by the number of CD4+ T cells per islet. In addition, NOD male and female mice both had large pools of naïve T cells and B cells in their islets. Our data suggest that the processes are similar in male and female mice, but that the rates of progression differ. Indeed NOD/LtJ males ultimately developed diabetes at a 70% incidence, though not in the normally allotted 20 weeks 43. Our data also indicate diabetogenesis in the islets progresses with predictable levels of some lymphocytes. If the number of islets and number of CD4+ T cells harvested from the islets is known for an individual NOD mouse we offer formulas to predict the number of CD8+ T cells, IL-4 competent CD4+ T cells, naïve CD8+ T cells and naïve CD4+ T cells that are present.
In this manuscript we have focused on the immune system mediated aspects of diabetogenesis and the differences between males and females. We were able to show similar immune cells accumulating in islets, but at different rates. We argue that the differences between males and females are not due to qualitative differences in the autoimmune response. We also indentified large pools of apparently naïve CD4+ and CD8+ T cells, which in the presence of B cells in the islets, could contribute to epitope spreading.
Supplementary Material
Supplemental Figure 1
The incidence of diabetes in female NOD/LtJ mice from Jackson Labs in our animal facility.
Results are shown for 20 female NOD mice. Blood glucose of mice was monitored weekly and mice were considered diabetic when they had 2 consecutive blood sugar readings over 250 mg/dL. The median age of overt diabetes was 16 weeks, similar to Jax females, 15 weeks, plotted on the same graph 43.
Supplemental Figure 2
Dot plots of islet-infiltrating lymphocytes from individual NOD mice.
(A) Lymphocytes isolated from the islets of one individual 12 week-old female NOD mouse were analyzed by flow cytometry using the gating scheme shown above. A total of 16 378 CD4+ T cells, 5068 CD8+ T cells and 25 401 CD19+ B cells were analyzed from 148 islets isolated from mouse A. Therefore mouse A had 111 CD4+ T cells/islet, 34 CD8+ T cells/islet and 172 CD19+ B cells per islet.
(B) Lymphocytes were isolated from the islets of one individual 8 week-old female IL-4 reporter NOD mouse and analyzed by flow cytometry using the gating scheme shown above. A total of 16 472 CD4+ T cells, 4457 CD8+ T cells and 6899 CD19+ B cells were analyzed from 81 islets isolated from mouse B. Therefore mouse B had 203 CD4+ T cells/islet, 55 CD8+ T cells/islet and 85 CD19+ B cells per islet.
Supplemental Figure 3
A line describes a relationship between 2 variables. This can be written as Y=slope(X) + Y-intercept, where X and Y represent 2 different variables and the Y-intercept is the value of Y when X equals zero. In the present manuscript, the Y-intercept does not represent an actual data point; instead it is a theoretical extension of the predicted line. If the Y-intercept is zero, the ratio between the X and Y remains the same at all points and Y/X equals the slope. If the Y-intercept is greater than zero, the ratio, between X and Y, changes with different values of X. Y/X is larger for low values of X and approaches a constant value for large values of X. The line Y=0.03X+0.10 is graphed above in blue. This is the line that describes the relationship between IL-4 producing CD4+ T cells and CD4+ T cells, where Y = the number of IL-4 producing CD4+ T cells per islet and X = the number of CD4+ T cells. The predicted percent of Y in the X population, or 100(Y/X), is graphed in blue triangles. The resulting percentages resemble the measured percentages of IL-4 producing cells in the CD4+ T cells population (see Figure 3D). The line Y=0.03X+0.0 is graphed above in orange. The predicted percent of Y in the X population by this formula is graphed in orange diamonds and is always 3%.
Supplemental Table 2
Raw data and calculations for Figure 3. Islets were isolated from individual mice and counted. After overnight incubation at 37°C, the entire sample was analyzed by flow cytometry. The number of CD3+CD4+ T cells and GFP+CD3+CD4+ T cells was recorded for each sample. Calculations are indicated in the column heading.
Table 1.
SNPs used to cross IL-4 reporter gene from BALB/c to NOD background
| JAX SNP ID | refSNP(rs) number With NCBI hyperlink | New Chr pos (bp) Build 30 |
|---|---|---|
| 01-039504168-N | rs4222284 | 39 421 714 |
| 01-100109616-M | rs3695980 | 99 776 559 |
| 01-171257289-N | rs4222821 | 170 722 671 |
| 02-038092910-M | rs3709811 | 38 360 318 |
| 02-108953011-M | rs3657882 | 109 547 910 |
| 02-164961358-M | rs3697980 | 165 877 557 |
| 03-033933315-N | rs4223883 | 33 920 148 |
| 03-124103177-M | rs3720182 | 124 251 237 |
| 04-043070534-M | rs3725792 | 43 076 423 |
| 04-115172853-M | rs3696331 | 115 410 443 |
| 05-038394376-M | rs3716546 | 38 096 627 |
| 05-116192311-M | rs3661429 | 115 463 027 |
| 06-024909331-M | rs3665582 | 24 860 970 |
| 06-109051116-M | rs3715148 | 108 877 662 |
| 07-034063244-M | rs3710949 | 33 124 503 |
| 07-109117173-M | rs3670069 | 107 748 124 |
| 08-032986730-M | rs3653751 | 32 953 469 |
| 08-101099186-M | rs3726020 | 100 652 541 |
| 09-037841497-M | rs3705980 | 37 624 235 |
| 09-100909970-M | rs3658704 | 100 364 790 |
| 10-020086252-M | rs3667084 | 19 915 360 |
| 10-100345477-M | rs3710293 | 99 641 926 |
| 11-033040833-M | rs3723833 | 33 409 549 |
| 11-077198334-M | rs3658198 | 77 352 730 |
| 11-100359477-N | rs4229088 | 100 422 259 |
| 12-030450532-M | rs3669704 | 30 483 177 |
| 12-084289638-M | rs3707414 | 84 101 707 |
| 13-030913320-M | rs3654710 | 30 862 328 |
| 13-091042598-M | rs3675592 | 90 070 327 |
| 14-020426806-M | rs3682880 | 20 327 446 |
| 14-088110939-M | rs3684516 | 87 899 822 |
| 15-020953071-M | rs3714893 | 20 803 443 |
| 15-087100507-M | rs3690689 | 86 587 928 |
| 16-015729768-C | rs4165029 | 15 729 538 |
| 16-066862514-C | rs4197416 | 66 800 359 |
| 17-023385048-N | rs3023864 | 22 884 108 |
| 17-076128192-M | rs3674930 | 75 497 960 |
| 18-017475340-N | rs3090636 | 17 597 947 |
| 18-080151519-M | rs3656292 | 79 931 833 |
| 19-021081804-M | rs3692864 | 20 719 054 |
| 19-060030696-N | rs3023517 | 59 767 603 |
| X-049771621-M | rs3670652 | 50 829 113 |
Table 2.
| Age of mouse | # islets isolated | GFP+ CD4+ CD3+ T cells | GFP+ CD4+ CD3+ T cells/# islets | %CD4+ CD3+ T cell that are GFP+ | Total CD4+CD3+ T cells | Total CD4+CD3+T cells/# islets | |
|---|---|---|---|---|---|---|---|
| 6–8 weeks | males | ||||||
| 1 | 28 | 27 | 0.96 | 2.53% | 1066 | 38.07 | |
| 2 | 66 | 10 | 0.15 | 32.26% | 31 | 0.47 | |
| 3 | 68 | 32 | 0.47 | 15.61% | 205 | 3.01 | |
| 4 | 92 | 44 | 0.48 | 4.62% | 953 | 10.36 | |
| 5 | 137 | 17 | 0.12 | 1.25% | 1358 | 9.91 | |
| 6–8 weeks | females | ||||||
| 1 | 80 | 34 | 0.43 | 15.96% | 213 | 2.66 | |
| 2 | 83 | 25 | 0.30 | 13.81% | 181 | 2.18 | |
| 3 | 73 | 58 | 0.79 | 8.43% | 688 | 9.42 | |
| 4 | 81 | 496 | 6.12 | 3.01% | 16472 | 203.36 | |
| 5 | 145 | 64 | 0.44 | 3.36% | 1906 | 13.14 | |
| 6 | 98 | 41 | 0.42 | 5.63% | 728 | 7.43 | |
| 7 | 85 | 6 | 0.07 | 75.00% | 8 | 0.09 | |
| 12–14 weeks | males | ||||||
| 1 | 98 | 219 | 2.23 | 7.36% | 2977 | 30.38 | |
| 2 | 36 | 4 | 0.11 | 9.30% | 43 | 1.19 | |
| 3 | 45 | 12 | 0.27 | 23.53% | 51 | 1.13 | |
| 4 | 136 | 29 | 0.21 | 6.99% | 415 | 3.05 | |
| 5 | 128 | 298 | 2.33 | 2.02% | 14719 | 114.99 | |
| 6 | 112 | 47 | 0.42 | 4.28% | 1097 | 9.79 | |
| 7 | 78 | 27 | 0.35 | 6.00% | 450 | 5.77 | |
| 8 | 125 | 2 | 0.02 | 0.08% | 2591 | 20.73 | |
| 9 | 172 | 53 | 0.31 | 3.31% | 1601 | 9.31 | |
| 10 | 156 | 34 | 0.22 | 4.70% | 724 | 4.64 | |
| 12–14 weeks | females | ||||||
| 1 | 53 | 388 | 7.32 | 3.64% | 10661 | 201.15 | |
| 2 | 94 | 218 | 2.32 | 2.97% | 7331 | 77.99 | |
| 3 | 38 | 11 | 0.29 | 1.91% | 575 | 15.13 | |
| 4 | 164 | 835 | 5.09 | 3.18% | 26217 | 159.86 |
Acknowledgments
The expertise and assistance of Lucinda Hensley, Shaun Steel, Corey Morris and Michael Johnson was invaluable. This research is supported in part by National Institutes of Health Grants AI 0524354, AI052435-03S1, AI058014 and AI54843 and Grant 1-2008-24 from the Juvenile Diabetes Research Foundation. The work described in this article was carried out in accordance with the EC Directive 86/609/EEC for animal experiments and The Uniform Requirements for Manuscripts Submitted to Biomedical Journals.
References
- 1.Bendelac A, Carnaud C, Boitard C, Bach JF. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987;166(4):823–32. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller BJ, Appel MC, O’Neil JJ, Wicker LS. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988;140(1):52–8. [PubMed] [Google Scholar]
- 3.Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD. NON-Thy-1a donors. Diabetes. 1993;42(1):44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 4.Pozzilli P, Signore A, Williams AJK, Beales PE. NOD mouse coloies around the world-recent facts and figures. Immunology Today. 1993;14(5):193–196. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- 5.Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity. 1997;7(6):727–38. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5(6):601–4. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick F, Lepault F, Homo-Delarche F, Bach JF, Dardenne M. Influence of castration, alone or combined with thymectomy, on the development of diabetes in the nonobese diabetic mouse. Endocrinology. 1991;129(3):1382–90. doi: 10.1210/endo-129-3-1382. [DOI] [PubMed] [Google Scholar]
- 8.Fox HS. Androgen treatment prevents diabetes in nonobese diabetic mice. J Exp Med. 1992;175(5):1409–12. doi: 10.1084/jem.175.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toyoda H, Takei S, Formby B. Effect of 5-alpha dihydrotestosterone on T-cell proliferation of the female nonobese diabetic mouse. Proc Soc Exp Biol Med. 1996;213(3):287–93. doi: 10.3181/00379727-213-44060. [DOI] [PubMed] [Google Scholar]
- 10.Pearce RB, Formby B, Healy K, Peterson CM. Association of an androgen-responsive T cell phenotype with murine diabetes and Idd2. Autoimmunity. 1995;20(4):247–58. doi: 10.3109/08916939508995702. [DOI] [PubMed] [Google Scholar]
- 11.Ablamunits V, Elias D, Cohen IR. The pathogenicity of islet-infiltrating lymphocytes in the non-obese diabetic (NOD) mouse. Clin Exp Immunol. 1999;115(2):260–7. doi: 10.1046/j.1365-2249.1999.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao M, Yang Y, Jun HS, Yoon JW. Molecular mechanisms for gender differences in susceptibility to T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 2002;168(10):5369–75. doi: 10.4049/jimmunol.168.10.5369. [DOI] [PubMed] [Google Scholar]
- 13.Fox CJ, Danska JS. IL-4 expression at the onset of islet inflammation predicts nondestructive insulitis in nonobese diabetic mice. J Immunol. 1997;158(5):2414–24. [PubMed] [Google Scholar]
- 14.Maier LM, Wicker LS. Genetic susceptibility to type 1 diabetes. Curr Opin Immunol. 2005;17(6):601–8. doi: 10.1016/j.coi.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Cornall RJ, Prins JB, Todd JA, Pressey A, DeLarato NH, Wicker LS, Peterson LB. Type 1 diabetes in mice is linked to the interleukin-1 receptor and Lsh/Ity/Bcg genes on chromosome 1. Nature. 1991;353(6341):262–5. doi: 10.1038/353262a0. [DOI] [PubMed] [Google Scholar]
- 16.Wong FS, Visintin I, Wen L, Flavell RA, Janeway CA., Jr CD8 T cell clones from young nonobese diabetic (NOD) islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 cells. J Exp Med. 1996;183(1):67–76. doi: 10.1084/jem.183.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund T, O’Reilly L, Hutchings P, Kanagawa O, Simpson E, Gravely R, Chandler P, Dyson J, Picard JK, Edwards A, et al. Prevention of insulin-dependent diabetes mellitus in non-obese diabetic mice by transgenes encoding modified I-A beta-chain or normal I-E alpha-chain. Nature. 1990;345(6277):727–9. doi: 10.1038/345727a0. [DOI] [PubMed] [Google Scholar]
- 18.Nishimoto H, Kikutani H, Yamamura K, Kishimoto T. Prevention of autoimmune insulitis by expression of I-E molecules in NOD mice. Nature. 1987;328(6129):432–4. doi: 10.1038/328432a0. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in the initiation of insulin-dependent diabetes mellitus. Eur J Immunol. 1996;26(8):1762–9. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 20.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43(3):505–9. doi: 10.2337/diab.43.3.505. [DOI] [PubMed] [Google Scholar]
- 21.Katz J, Benoist C, Mathis D. Major histocompatibility complex class I molecules are required for the development of insulitis in non-obese diabetic mice. Eur J Immunol. 1993;23(12):3358–60. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 22.Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fischer PA, Podolin PL, Zijlstra M, Jaenisch R, Peterson LB. Beta 2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43(3):500–4. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 23.Utsugi T, Yoon JW, Park BJ, Imamura M, Averill N, Kawazu S, Santamaria P. Major histocompatibility complex class I-restricted infiltration and destruction of pancreatic islets by NOD mouse-derived beta-cell cytotoxic CD8+ T-cell clones in vivo. Diabetes. 1996;45(8):1121–31. doi: 10.2337/diab.45.8.1121. [DOI] [PubMed] [Google Scholar]
- 24.Nagata M, Santamaria P, Kawamura T, Utsugi T, Yoon JW. Evidence for the role of CD8+ cytotoxic T cells in the destruction of pancreatic beta-cells in nonobese diabetic mice. J Immunol. 1994;152(4):2042–50. [PubMed] [Google Scholar]
- 25.Kurts C, Carbone FR, Krummel MF, Koch KM, Miller JF, Heath WR. Signalling through CD30 protects against autoimmune diabetes mediated by CD8 T cells. Nature. 1999;398(6725):341–4. doi: 10.1038/18692. [DOI] [PubMed] [Google Scholar]
- 26.Adorini L, Gregori S, Harrison LC. Understanding autoimmune diabetes: insights from mouse models. Trends Mol Med. 2002;8(1):31–8. doi: 10.1016/s1471-4914(01)02193-1. [DOI] [PubMed] [Google Scholar]
- 27.Wucherpfennig KW, Eisenbarth GS. Type 1 diabetes. Nat Immunol. 2001;2(9):767–8. doi: 10.1038/ni0901-767. [DOI] [PubMed] [Google Scholar]
- 28.Mellanby RJ, Thomas D, Phillips JM, Cooke A. Diabetes in non-obese diabetic mice is not associated with quantitative changes in CD4+ CD25+ Foxp3+ regulatory T cells. Immunology. 2007;121(1):15–28. doi: 10.1111/j.1365-2567.2007.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serreze DV, Wasserfall C, Ottendorfer EW, Stalvey M, Pierce MA, Gauntt C, O’Donnell B, Flanagan JB, Campbell-Thompson M, Ellis TM, et al. Diabetes acceleration or prevention by a coxsackievirus B4 infection: critical requirements for both interleukin-4 and gamma interferon. J Virol. 2005;79(2):1045–52. doi: 10.1128/JVI.79.2.1045-1052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serreze DV, Chapman HD, Post CM, Johnson EA, Suarez-Pinzon WL, Rabinovitch A. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J Immunol. 2001;166(2):1352–9. doi: 10.4049/jimmunol.166.2.1352. [DOI] [PubMed] [Google Scholar]
- 31.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201(8):1333–46. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15(2):303–11. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 33.Wakeland E, Morel L, Achey K, Yui M, Longmate J. Speed congenics: a classic technique in the fast lane (relatively speaking) Immunol Today. 1997;18(10):472–7. doi: 10.1016/s0167-5699(97)01126-2. [DOI] [PubMed] [Google Scholar]
- 34.Wong CP, Li L, Frelinger JA, Tisch R. Early autoimmune destruction of islet grafts is associated with a restricted repertoire of IGRP-specific CD8+ T cells in diabetic nonobese diabetic mice. J Immunol. 2006;176(3):1637–44. doi: 10.4049/jimmunol.176.3.1637. [DOI] [PubMed] [Google Scholar]
- 35.Zhao R, Loftus DJ, Appella E, Collins EJ. Structural evidence of T cell xeno-reactivity in the absence of molecular mimicry. J Exp Med. 1999;189(2):359–70. doi: 10.1084/jem.189.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amrani A, Serra P, Yamanouchi J, Trudeau JD, Tan R, Elliott JF, Santamaria P. Expansion of the antigenic repertoire of a single T cell receptor upon T cell activation. J Immunol. 2001;167(2):655–66. doi: 10.4049/jimmunol.167.2.655. [DOI] [PubMed] [Google Scholar]
- 37.Gallichan WS, Balasa B, Davies JD, Sarvetnick N. Pancreatic IL-4 expression results in islet-reactive Th2 cells that inhibit diabetogenic lymphocytes in the nonobese diabetic mouse. J Immunol. 1999;163(3):1696–703. [PubMed] [Google Scholar]
- 38.Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 1996;184(3):1093–9. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohrs K, Harris DP, Lund FE, Mohrs M. Systemic dissemination and persistence of Th2 and type 2 cells in response to infection with a strictly enteric nematode parasite. J Immunol. 2005;175(8):5306–13. doi: 10.4049/jimmunol.175.8.5306. [DOI] [PubMed] [Google Scholar]
- 40.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2(3):119–25. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 41.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 42.Goldrath AW, Barber L, Chen KE, Alters SE. Differences in adhesion markers, activation markers, and TcR in islet infiltrating vs. peripheral lymphocytes in the NOD mouse. J Autoimmun. 1995;8(2):209–20. doi: 10.1006/jaut.1995.0016. [DOI] [PubMed] [Google Scholar]
- 43.http://type1diabetes.jax.org/incidence_studies/001976_-_NOD_Incidence_Study_2007.html
- 44.Wang B, Gonzalez A, Hoglund P, Katz JD, Benoist C, Mathis D. Interleukin-4 deficiency does not exacerbate disease in NOD mice. Diabetes. 1998;47(8):1207–11. doi: 10.2337/diab.47.8.1207. [DOI] [PubMed] [Google Scholar]
- 45.Trudeau JD, Kelly-Smith C, Verchere CB, Elliott JF, Dutz JP, Finegood DT, Santamaria P, Tan R. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J Clin Invest. 2003;111(2):217–23. doi: 10.1172/JCI16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, Serreze DV, Shabanowitz J, Hunt DF, Nathenson SG, et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A. 2003;100(14):8384–8. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enee E, Martinuzzi E, Blancou P, Bach JM, Mallone R, van Endert P. Equivalent specificity of peripheral blood and islet-infiltrating CD8+ T lymphocytes in spontaneously diabetic HLA-A2 transgenic NOD mice. J Immunol. 2008;180(8):5430–8. doi: 10.4049/jimmunol.180.8.5430. [DOI] [PubMed] [Google Scholar]
- 48.Tian J, Zekzer D, Lu Y, Dang H, Kaufman DL. B cells are crucial for determinant spreading of T cell autoimmunity among beta cell antigens in diabetes-prone nonobese diabetic mice. J Immunol. 2006;176(4):2654–61. doi: 10.4049/jimmunol.176.4.2654. [DOI] [PubMed] [Google Scholar]
- 49.McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174(9):5720–8. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1
The incidence of diabetes in female NOD/LtJ mice from Jackson Labs in our animal facility.
Results are shown for 20 female NOD mice. Blood glucose of mice was monitored weekly and mice were considered diabetic when they had 2 consecutive blood sugar readings over 250 mg/dL. The median age of overt diabetes was 16 weeks, similar to Jax females, 15 weeks, plotted on the same graph 43.
Supplemental Figure 2
Dot plots of islet-infiltrating lymphocytes from individual NOD mice.
(A) Lymphocytes isolated from the islets of one individual 12 week-old female NOD mouse were analyzed by flow cytometry using the gating scheme shown above. A total of 16 378 CD4+ T cells, 5068 CD8+ T cells and 25 401 CD19+ B cells were analyzed from 148 islets isolated from mouse A. Therefore mouse A had 111 CD4+ T cells/islet, 34 CD8+ T cells/islet and 172 CD19+ B cells per islet.
(B) Lymphocytes were isolated from the islets of one individual 8 week-old female IL-4 reporter NOD mouse and analyzed by flow cytometry using the gating scheme shown above. A total of 16 472 CD4+ T cells, 4457 CD8+ T cells and 6899 CD19+ B cells were analyzed from 81 islets isolated from mouse B. Therefore mouse B had 203 CD4+ T cells/islet, 55 CD8+ T cells/islet and 85 CD19+ B cells per islet.
Supplemental Figure 3
A line describes a relationship between 2 variables. This can be written as Y=slope(X) + Y-intercept, where X and Y represent 2 different variables and the Y-intercept is the value of Y when X equals zero. In the present manuscript, the Y-intercept does not represent an actual data point; instead it is a theoretical extension of the predicted line. If the Y-intercept is zero, the ratio between the X and Y remains the same at all points and Y/X equals the slope. If the Y-intercept is greater than zero, the ratio, between X and Y, changes with different values of X. Y/X is larger for low values of X and approaches a constant value for large values of X. The line Y=0.03X+0.10 is graphed above in blue. This is the line that describes the relationship between IL-4 producing CD4+ T cells and CD4+ T cells, where Y = the number of IL-4 producing CD4+ T cells per islet and X = the number of CD4+ T cells. The predicted percent of Y in the X population, or 100(Y/X), is graphed in blue triangles. The resulting percentages resemble the measured percentages of IL-4 producing cells in the CD4+ T cells population (see Figure 3D). The line Y=0.03X+0.0 is graphed above in orange. The predicted percent of Y in the X population by this formula is graphed in orange diamonds and is always 3%.
Supplemental Table 2
Raw data and calculations for Figure 3. Islets were isolated from individual mice and counted. After overnight incubation at 37°C, the entire sample was analyzed by flow cytometry. The number of CD3+CD4+ T cells and GFP+CD3+CD4+ T cells was recorded for each sample. Calculations are indicated in the column heading.