Abstract
Purpose
In vascular endothelial cells, low doses of ionizing radiation trigger the immediate activation of cytosolic phospholipase A2 (cPLA2). This event initiates pro-survival signaling which could be responsible for radioresistance of tumor vasculature. Thus, the development of radiosensitizers targeting these survival pathways may enhance tumor response to radiation therapy. Arachidonyltrifluoromethyl Ketone (AACOCF3), a specific cPLA2 inhibitor, was studied as a potential radiosensitizer.
Experimental Design
Vascular endothelial cells (3B11 and MPVEC) and lung tumor cells (LLC and H460) were treated with 1μM AACOCF3 for 30 minutes prior to irradiation. Treatment response was evaluated by clonogenic survival, activation of ERK1/2, tubule formation and migration assays. For in vivo experiments, mice with LLC or H460 tumors in the hind limbs were treated for 5 consecutive days with 10 mg/kg AACOCF3 administered daily 30 minutes prior to irradiation. Treatment response was assessed by tumor growth delay, Power Doppler Sonography and immunohistochemistry.
Results
In cell culture experiments, inhibition of cPLA2 with AACOCF3 prevented radiation-induced activation of ERK1/2 and decreased clonogenic survival of irradiated vascular endothelial cells but not the lung tumor cells. Treatment with AACOCF3 also attenuated tubule formation and migration in irradiated vascular endothelial cells. In both tumor mouse models, treatment with AACOCF3 prior to irradiation significantly suppressed tumor growth and decreased overall tumor blood flow and vascularity. Increased apoptosis in both tumor cells and tumor vascular endothelium was determined as a possible mechanism of the observed effect.
Conclusion
These findings identify cPLA2 as a novel molecular target for tumor sensitization to radiation therapy through the tumor vasculature.
Keywords: cytosolic phospholipase A2, radiosensitization, endothelium, lung cancer, microvasculature
Introduction
Despite advances in therapeutic regimens, local failure in lung cancer continues to be a problem for radiation therapy (1-3). Currently, the most common approach to improve the outcome of radiotherapy is to combine radiation with chemotherapeutic agents (4-8). However, many of the platinum-based chemotherapeutic agents used as standard treatment for lung cancer exhibit toxicity within normal tissues (9-13). Therefore, the development of non-toxic, yet effective molecular-targeted radiosensitizers is essential for improvement of the therapeutic ratio.
Understanding the response of the tumor microenvironment to ionizing radiation is important for the development of efficient radiosensitizing agents (14-16). Previous data has shown that the effectiveness of radiotherapy is limited by the response of the tumor vasculature (16, 17). Several studies have demonstrated that clinically relevant doses of ionizing radiation (2-5 Gy) elicit the activation of both Akt and ERK1/2 pro-survival signaling pathways in tumor endothelium (17-20). The result of such activation is increased radioresistance within the tumor blood vessels. Since ablation of the tumor vasculature enhances the treatment of cancer (21, 22), radiosensitizers that target these survival pathways could improve the outcome of lung cancer.
Upon irradiation, signal transduction is generated during the interaction of ionizing radiation with cellular membranes (23, 24). Our recent study demonstrated that ionizing radiation interacts with vascular endothelial cell membranes to produce a biologically active lysophospholipid. In response to 3 Gy, HUVEC exhibited immediate activation of cytosolic phospholipase A2 (cPLA2) (17). cPLA2 belongs to a superfamily of enzymes that specifically cleave the acyl ester bond of membrane phospholipids at the sn-2 position (25-28). As a result of this hydrolysis, both free fatty acid and lysophospholipids are generated. Radiation-induced activation of cPLA2 in vascular endothelial cells resulted in the increased production of lysophosphatidylcholine (LPC), a lipid-derived second messenger which triggered Akt and ERK1/2 phosphorylation (17). LPC activates a wide range of cell types within the vascular system and can regulate a variety of biological functions including cytokine synthesis, migration, and endothelial growth factor expression (29, 30). According to our data and others, LPC could induce signal transduction through the transactivation of VEGFR-2 (Flk-1/KDR). The result of this signaling cascade is an increase in endothelial cell proliferation and survival (17, 29). Such data suggests that cPLA2 may play a critical role in the tumor vascular response to ionizing radiation and could be used as a molecular target for tumor radiosensitization.
In previous studies, much of the data was generated using normal vascular endothelial cells. However, the genes expressed by tumor endothelial cells differ significantly from the genes expressed by HUVEC and HMVEC. In order to analyze the efficacy of cPLA2 inhibitors as radiosensitizers in vitro, we selected endothelial cells that are considered to be more clinically relevant to malignant disease (31). To test the hypothesis that cPLA2 inhibition promotes the radiosensitization of tumor blood vessels, we used the 3B11 murine vascular endothelial cell line as well as murine pulmonary microvascular endothelial cells (MPMEC) isolated directly from mouse lung tissue.
To inhibit cPLA2, we used Arachidonyltrifluoromethyl Ketone (AACOCF3). This potent cPLA2 inhibitor is a cell-permeable trifluoromethyl ketone analog of arachidonic acid (25). NMR studies have demonstrated that the carbon chain of AACOCF3 binds in a hydrophobic pocket of cPLA2 and the carbonyl group of AACOCF3 forms a covalent bond with serine 228 in the enzyme active site (25, 32, 33). This cPLA2 inhibitor has been used extensively to study the role of cPLA2 in platelet aggregation and inflammation-associated apoptosis (34-37). In the present study, however, we utilized AACOCF3 to elucidate the role of cPLA2 in the tumor vasculature response to ionizing radiation. We found that when combined with radiation, the inhibition of cPLA2 with AACOCF3 disrupts the biological functions of the tumor vasculature, enhances destruction of tumor blood vessels and suppresses tumor growth. Thus, cPLA2 contributes to vascular endothelial cell radioresistance and presents a potential molecular target for tumor sensitization to radiotherapy.
Materials and Methods
Cell cultures and treatment
The murine endothelial cell line, 3B11, was purchased from American Type Culture Collection (Manassas, VA). 3B11 cells were maintained in DMEM plus 10% fetal bovine serum and 1% penicillin/streptomycin (Life Technologies, Gaithersburg, MD) in a 37°C and 5% CO2 environment. Murine Pulmonary Microvascular Endothelial Cells (MPMEC) were maintained in EGM-2-MV (Cambrex, East Rutherford, NJ). Cells from passage 2 to 3 were used in this study. Mouse Lewis Lung Carcinoma (LLC) and human large cell lung cancer H460 cells were obtained from American Type Culture Collection. The LLC cell line was maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, H460 cells were cultured in RPMI 1640 (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. AACOCF3 was purchased from EMD Biosciences (San Diego, CA). Cells were pretreated with either vehicle (70% EtOH) or 1 μM AACOCF3 (in 70% EtOH) for 30 minutes prior to irradiation (3 Gy). A Mark I 137Cs irradiator (J.L. Shepherd and Associates, San Fernando, CA) was used to deliver radiation to the cells.
Mouse tumor models, treatment and tumor growth delay study
Institutional Animal Care and Use Committee guidelines were followed when handling and treating all mice used in this study (IACUC-approved protocols M/04/013 and M/07/358). 106 of LLC or H460 cells were implanted into the hind limbs of C57/BL6 mice and nude mice, respectively. Once tumors exceeded 2 mm in diameter, the mice were stratified into groups of 5-6 animals representing similar distributions of tumor sizes. Tumor-bearing animals were administered intraperitoneally with vehicle or 10 mg/kg AACOCF3 30 minutes prior to irradiation with 3 Gy (Therapax DXT 300 X-ray machine, Pantak Inc., East Haven, CT). Treatment was repeated for 5 consecutive days. Tumor size was monitored by external caliper measurements.
Isolation of mouse lung endothelial cells
Primary cultures of Murine Pulmonary Microvascular Endothelial Cells (MPMEC) were isolated as described previously (38). Briefly, 1-3 month old C57/BL6 mice were anesthetized, and the lung vasculature was perfused with PBS and 2 mM EDTA followed by 0.25% trypsin and 2 mM EDTA via the right ventricle. The heart and lungs were excised and incubated at 37°C for 20 min. The visceral pleura was removed, and the perfusion was repeated. Primary vascular endothelial cells were recovered and grown on tissue culture plates coated with 0.2% gelatin for 3 days in EGM-2-MV containing 5% FCS (Cambrex, East Rutherford, NJ).
Clonogenic survival
3B11, LLC, and H460 cells were plated and allowed to attach for 5 h. Cells were then treated with EtOH or 1 μM AACOCF3 for 30 minutes followed by irradiation with 0, 2, 4, or 6 Gy. Medium was changed after irradiation. After 1 week, plates were fixed with 70% EtOH and stained with 1% methylene blue. Colonies consisting of ≥ 50 cells were counted with a dissection microscope. Average survival fraction was calculated as (number of colonies/number of cells plated)/(number of colonies for corresponding control/number of cells plated) with SEM from three experiments.
Western immunoblot analysis
Total cell lysates from 3B11, LLC, and H460 were harvested 3 minutes after the beginning of irradiation. Total protein extraction was performed using M-PER kit (Pierce, Rockford, IL). Protein concentration was quantified using BCA Reagent (Pierce). Protein extracts (80 μg) were subjected to Western immunoblot analysis using antibodies for the detection of phospho-ERK1/2Thr202/Tyr204 and total ERK1/2 (both from Cell Signaling Technologies, Danvers, MA). Antibody to actin (Sigma) was used to evaluate protein loading in each lane. Immunoblots were developed using the Western Lightning Chemiluminescence Plus detection system (PerkinElmer, Wellesley, MA) according to the manufacturer’s protocol.
Matrigel-based tubule formation assay
In order to analyze capillary-like formation, 24-well plates were coated with Matrigel (300 μl per well, BD Biosciences, Bedford, MA) and incubated for 30 min at 37°C. Primary MPMEC or 3B11 cells were plated over solidified Matrigel and allowed to attach for 30 minutes prior to treatment. Cells were then treated with vehicle or 1 μM AACOCF3 for 30 minutes and irradiated with 3 Gy. Once tubules began to form from the control cells, digital microphotographs of the wells were obtained. Four randomly selected high power fields (HPF) per sample were counted, and tubule formation was quantified as the average number of tubule branches per HPF.
Endothelial cell migration assay
To assay cell migration, 3B11 or MPMEC were added to the top chamber of 24-well plates (100,000 cells/well) with 8 μm matrigel-coated inserts (Costar Corning, Corning, NY). Fresh medium (600 μl) was added to the bottom chamber, and cells were incubated for 30 minutes to allow attachment. Both chambers were then treated with vehicle or 1 μM AACOCF3 for 30 minutes prior to irradiation (3 Gy). Cells were incubated for 24 hours at 37°C in 5% CO2. After 24 hours, all remaining cells on the upper surface of the transwell insert filter were removed with a cotton swab. The insert filters were rinsed in PBS, fixed in 100% methanol, and stained with DAPI (2.5 ng/μl). Migrated cells in 6 randomly selected HPF from each sample were counted, and the average number of migrated cells per HPF was calculated.
Power Doppler Sonography analyses of tumor blood flow
C57/BL6 mice bearing LLC tumors received daily intraperitoneal injections with vehicle or 10 mg/kg AACOCF3. Tumors were irradiated with 3 Gy 30 minutes after inhibition. Treatment was repeated for 5 consecutive days. Twenty-four hours after the final treatment, tumor blood flow was analyzed by three-dimensional Power Doppler Sonography. Tumors were sonographically imaged with a VisualSonics VEVO 770 small animal high resolution ultrasound scanner equipped with a 40 MHz transducer and a mechanically scanned, single-element aperture (VisualSonics, Toronto, Canada). The focal zone of the transducer was set at 6 mm from the scanning surface. The scanning plane was perpendicular to the long axis of the lower extremity along the entire length of the tumor. Three-dimensional images in Power Doppler mode were acquired by computer-controlled translation of the transducer over the whole length of the tumor by acquiring 2D images every 100 μm. The following ultrasound scanner settings were used: Power Doppler transmission frequency-23 MHz, Power Gain 100%, wall filter 2.3 mm/s, scan speed 2.0 mm/s. Three-dimensional tumor volume was reconstructed using semi-automated segmentation of 10-12 parallel planes through the 2D images using the VisualSonics image analysis software. To determine blood flow, the average Vascular Index (%) was calculated as the ratio of color coded pixels (representing blood flow) to the total pixel volume of the tumor.
Histological analysis of tumor vascularity
Tumor-bearing mice were treated with daily intraperitoneal injections of vehicle or 10 mg/kg AACOCF3 30 minutes prior to irradiation with 3 Gy. Treatment was continued for 5 consecutive days. Twenty-four hours after the final treatment, tumors were resected, fixed in 10% formalin, and sectioned into 5 μm sections. Sections were incubated with 20 μg/ml Proteinase K for antigen retrieval and then hybridized overnight with rabbit anti-human von Willebrand Factor (vWF) antibody (1:100, DakoCytomation, Carpinteria, CA). Sections were subsequently incubated with Alexa Fluor 488-conjugated goat anti-rabbit antibody (1:500, Invitrogen Molecular Probes, Carlsbad, CA) for one hour at room temperature before imaging. Positive staining was observed by immunofluorescent microscopy, and vessels were counted (6 randomly selected HPF per sample). Vascularity was determined as the average number of stained vessels per HPF.
TUNEL staining and immunofluorescence staining for phospho-Akt
Mice bearing LLC tumors received daily intraperitoneal injections of vehicle or 10 mg/kg AACOCF3 30 minutes prior to irradiation with 3 Gy. Treatment was continued for 5 consecutive days. Twenty-four hours after the final treatment, tumors were harvested, fixed in 10% formalin, and sectioned into 5 μm sections. TUNEL staining was performed with the DeadEnd Colorimetric TUNEL System (Promega, Madison, WI) following the manufacturer’s instructions. Positive staining was observed by light microscopy. To detect Akt phosphorylation, sections were incubated with 20 μg/ml Proteinase K for antigen retrieval and then hybridized overnight at 4°C with antibodies for phospho-AktThr308/Ser473 (1:100, Cell Signaling Technologies, Danvers, MA, USA). Sections were subsequently incubated with Alexa Fluor 488-conjugated goat anti-rabbit antibody (1:500, Invitrogen Molecular Probes, Carlsbad, CA) for one hour at room temperature. Counterstaining was performed using DAPI (2.5 ng/μl), and positive staining was observed by immunofluorescence microscopy.
Tumor vascular window model and vascular length density analysis
The tumor window model experiment was described previously (39). Briefly, LLC tumor cells were injected into the dorsal skinfold of C57/BL6 mice. After approximately 2 weeks, sufficient vascular networks had developed within all of the mice. Mice were then treated with AACOCF3, 3 Gy, or AACOCF3 + 3 Gy. Prior to treatment, the window frame was marked with coordinates, which were used to photograph the same microscopic field each day. Blood vessel appearance was monitored at 24-hour increments over a period of 120 hours. Once the study was complete, color photographs were scanned into Photoshop and analyzed using ImagePro software. Within this software, vascular center lines were positioned along the entire length of the blood vessels. Vascular length density was then calculated by the software as total pixilation of blood vessel length per area of tissue.
Statistical analysis
Statistical tests were performed using SigmaPlot 8.0 data analysis software. Data are presented as group means ± standard error of the mean (SEM). An unpaired Student t test was used to evaluate the significance of the differences between 2 sets of data. A calculated p-value less than 0.05 was considered statistically significant.
Results
Inhibition of cPLA2 with AACOCF3 enhances cell death and prevents activation of pro-survival signaling in irradiated vascular endothelial cells
To determine whether treatment with AACOCF3 affects cellular viability in irradiated tumor cells as well as vascular endothelial cells, we performed clonogenic survival assays for 3B11, LLC, and H460 cell lines. The studides showed that treatment with AACOCF3 enhances radiation-induced cell death among 3B11 vascular endothelial cells (Fig. 1A). The most pronounced statistically significant increase in radiosensitization was observed at 2 Gy. At this dose, 3B11 cells exhibited a 30% decrease in survival in comparison to irradiated cells treated with vehicle alone (Fig. 1A, B). Treatment with AACOCF3 did not result in radiosensitization of LLC or H460 (Fig. 1A, B).
Fig. 1. Inhibition of cPLA2 with AACOCF3 enhances cell death and prevents activation of pro-survival signaling in irradiated vascular endothelial cells.
Clonogenic Survival. (A, B) 3B11, LLC, and H460 cells were plated and treated with 1 μM AACOCF3 for 30 min prior to irradiation. After 1 week, colonies consisting of ≥ 50 cells were counted and normalized for plating efficiency. A) Shown are average surviving fractions and SEM from three experiments; * p < 0.05. B) Shown is a bar graph of percent survival after exposure to 2 Gy; * p < 0.05. ERK phosphorylation. C) 3B11, LLC, and H460 cells were treated with 1 μM AACOCF3 for 30 min prior to irradiation with 3 Gy and lysed 3 min after the beginning of irradiation. Shown are the Western blot analyses with specific antibodies to phospho-ERK1/2, total ERK1/2, and actin.
Our previous studies have demonstrated that in irradiated HUVEC, activation of cPLA2 regulates ERK1/2 phosphorylation, one of the radiation-induced pro-survival kinases (17), To investigate whether the effects of AACOCF3 on 3B11 cell viability are due to the similar changes in radiation-induced pro-survival signaling, we performed western immunoblot analysis for ERK1/2 phosphorylation using total cell lysates of all three tested cell lines (Fig. 1C). At 3 minutes post-irradiation, AACOCF3 prevented the radiation-induced phosphorylation of ERK1/2 in vascular endothelial cells, but not in LLC or H460 tumor cells (Fig.1C). This suggests that in response to radiation, differential radiation-induced cPLA2-dependent activation of ERK1/2 is a key regulator of cell survival of vascular endothelial cells versus tumor cells.
Inhibition of cPLA2 attenuates tubule formation in irradiated vascular endothelial cells
To determine whether cPLA2 inhibition alters the ability of vascular endothelial cells to form capillary-like structures, 3B11 or MPMEC were treated with vehicle or 1 μM AACOCF3 for 30 minutes prior to irradiation. Tubule formation was analyzed in Matrigel-coated 24-well plates. When 3B11 cells were treated with either AACOCF3 or radiation alone, only a slight decrease in tubule formation was observed as compared to control cells (Fig. 2A, 47 vs. 53 and 40 vs. 53, respectively). However, a combined treatment of AACOCF3 followed by irradiation significantly decreased tubule formation as compared to control cells (Fig. 2A, 29 vs. 53). A similar response was observed in MPMEC, in which treatment with AACOCF3 prior to irradiation reduced the average number of tubules formed per HPF by 50% when compared to control cells (Fig. 2B).
Fig. 2. cPLA2 inhibitor AACOCF3 attenuates tubule formation and migration in irradiated vascular endothelial cells.
Tubule formation in A) 3B11 cells or B) primary MPMEC. Cells were cultured onto Matrigel in the absence (control) or presence of 1 μM AACOCF3 for 30 minutes and then irradiated with 3 Gy. A) Representative micrographs of capillary tubule formation taken 5 hours after treatment are shown. Tubule formation was quantified as the number of tubule branches per high power field (4 HPF per sample). Shown are bar graphs of the average tubule formation for 3B11 (A) and MPMEC (B) with SEM from three independent experiments; *, p < 0.05. Migration assay is shown for C) 3B11 cells or D) MPMEC cells were added to the top chamber of 24 well plates with 8 μm matrigel-coated inserts. Fresh medium was added to the bottom chamber, and both chambers were treated with vehicle (control) or 1 μM AACOCF3 for 30 minutes prior to irradiation with 3 Gy. After 24 hours, the insert chambers were stained with DAPI and migrated cells were counted (6 HPF per sample). Shown are representative micrographs of migrated 3B11 taken 24 hours after treatment (C) and bar graphs of the average number of migrated cells per HPF for 3B11 (C) and MPMEC (D) with SEM from three independent experiments; *, p < 0.05.
Inhibition of cPLA2 results in decreased migration in irradiated vascular endothelial cells
To determine whether cPLA2 inhibition affects the migratory ability of vascular endothelial cells, 3B11 or MPMEC were treated with vehicle or 1 μM AACOCF3 for 30 minutes prior to irradiation. Migration was assessed by a transwell filter migration assay. In the 3B11 cell line, no significant reduction in migration was observed in cells treated with either radiation or AACOCF3 alone (Fig. 2C). In contrast, treatment with AACOCF3 prior to irradiation reduced migration by 39% in comparison to cells treated with vehicle alone (Fig. 2C, 62 vs. 112). MPMEC produced comparable results in which treatment with AACOCF3 prior to irradiation significantly attenuated the number of migrated cells per HPF (Fig. 2D, 11 vs. 63).
Inhibition of cPLA2 represses tumor growth in irradiated mouse models
To determine the efficacy of cPLA2 inhibition in vivo, both syngeneic and heterogeneic mouse lung tumor models were used. Mouse LLC or human H460 tumor cells were injected subcutaneously into the right hindlimbs of 6-week old male C57/BL6 or nude mice, respectively. Tumor-bearing mice received daily intraperitoneal injections of vehicle or 10 mg/kg AACOCF3 30 minutes prior to irradiation. Treatment was repeated for 5 consecutive days and tumor volume was determined by external caliper measurements. In the LLC model, treatment with either radiation or drug alone failed to produce a significant delay in tumor growth (Fig. 3A, B, 2.6 days vs. 1.8 days, respectively.) Mice treated with a combined treatment of AACOCF3 and radiation, however, produced a statistically significant tumor growth delay of 12.2 days (Fig. 3A, B, p < 0.05). In the H460 tumor mouse model, inhibition of tumor growth was not observed in mice treated with AACOCF3 alone (Fig. 3C). Treatment with radiation resulted in a tumor growth delay of 5.5 days, but maximum tumor growth inhibition of 12.3 days was observed in mice treated with AACOCF3 plus radiation (Fig. 3C). Fig. 3D illustrates the differences in H460 tumor volumes among treatment groups 13 days post-injection. Although radiation resulted in decreased tumor size compared to untreated mice (Fig. 3D, 950 mm3 vs. 1447 mm3), a combined treatment of AACOCF3 and radiation enhanced tumor growth suppression by an additional 40% (Fig. 3D, 567 mm3).
Fig. 3. Inhibition of cPLA2 with AACOCF3 decreases tumor size in irradiated mouse models.
Using heterotopic tumor models of Lewis Lung Carcinoma (LLC) (A, B) or H460 large cell carcinoma of the lung (C, D), mice were treated intraperitoneally with vehicle (control) or 10 mg/kg AACOCF3 and tumors were irradiated 30 minutes later with 3 Gy. Treatment was repeated for 5 consecutive days. Tumor volumes were calculated using external caliper measurements. A) Shown are the mean LLC tumor volumes in each of the treatment groups (control, IR, AACOCF3, and AACOCF3 + IR). B) LLC; Shown is a bar graph of the average number of days taken to reach 700 mm3 in comparison to control with SEM from group of 4 to 6 mice; *, p = 0.02. C) H460; Shown is a bar graph of the average tumor volumes 13 days post-injection with SEM from group of 4 to 6 mice; *, p =0.05. D) H460; Shown is a bar graph of the average number of days taken to reach 1400 mm3 in comparison to control with SEM from group of 4 to 6 mice.
cPLA2 inhibition attenuates tumor blood flow and decreases vascularity in irradiated tumors
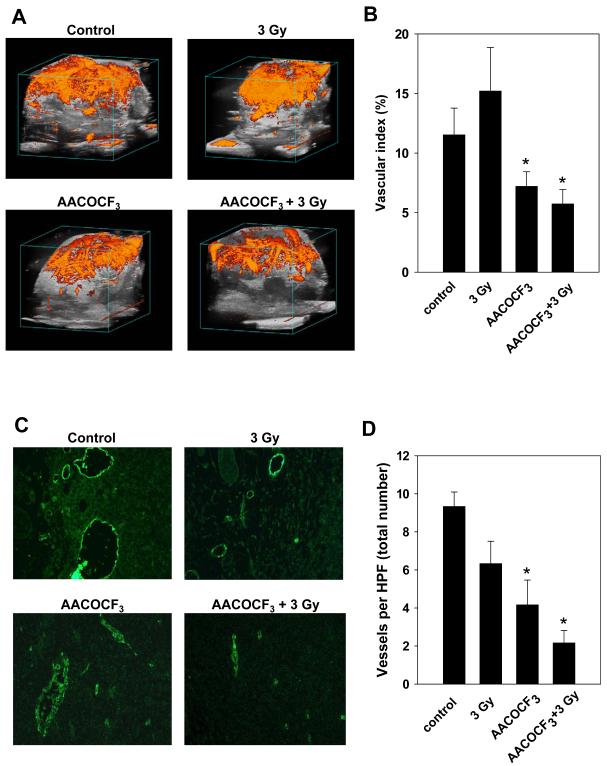
To determine if the cPLA2 inhibitor AACOCF3 impedes tumor blood flow, mice bearing LLC tumors were treated once daily for 5 consecutive days with either vehicle or 10 mg/kg AACOCF3 for 30 minutes followed by irradiation with 3 Gy. Tumor blood flow was analyzed by three-dimensional Power Doppler Sonography. As shown in Fig. 4A, B, the average Vascular Indices were 12% and 15% for untreated and irradiated tumors, respectively. Treatment with AACOCF3 resulted in a 1.6-fold decrease in tumor Vascular Index as compared to untreated mice (Fig. 4B, 7.5% vs. 12%). This decrease in tumor vasculature was further potentiated in mice that received a combined treatment of AACOCF3 and radiation (Fig. 4B, 2-fold, 6% vs. 12%).
Fig. 4. cPLA2 inhibitor AACOCF3 attenuates vascularity in irradiated tumors.
C57/BL6 mice with LLC tumors received intraperitoneal injections of vehicle or 10 mg/kg AACOCF3 30 minutes prior to irradiation with 3 Gy. Treatment was repeated for 5 consecutive days. Twenty-four hours after the final treatment, tumor blood flow was analyzed by three-dimensional Power Doppler sonography (A, B) or tumors were harvested, fixed in 10% formalin, sectioned into 5 μm sections and stained with anti-vWF antibody (C, D). A) Shown are representative images of tumor blood flow. B) Shown is a bar graph of the average Percent Vascular Index with SEM from group of 3 to 5 animals; *, p < 0.05. C) Shown are representative micrographs of vWF-stained vessels. D) Shown is the bar graph of the average number of stained vessels per HPF with SEM from group of 3 to 5 mice; *, p < 0.05.
To investigate whether inhibition of cPLA2 using AACOCF3 affects tumor vascularity, tumor-bearing mice were treated with daily intraperitoneal injections of vehicle or 10 mg/kg AACOCF3 30 minutes prior to irradiation with 3 Gy. Treatment was continued for 5 consecutive days. Twenty-four hours after the final treatment, tumors were resected, fixed, and sectioned into 5 μm sections. Sections were then stained with anti-vWF antibody. Staining was assessed by immunofluorescent microscopy. Fig. 4C, D, illustrates the effects of cPLA2 inhibition on vascularity within irradiated tumors. Radiation alone produced a slight reduction in vascularity in comparison to untreated mice (Fig. 4D, 6% vs. 10%). In addition, treatment with AACOCF3 resulted in a significant decrease in total blood vessel number (Fig. 4D, 4%). The most pronounced reduction in overall vascularity, however, was observed in mice treated with AACOCF3 followed by radiation (Fig. 4D, 2%).
Treatment with AACOCF3 results in apoptosis and inhibition of Akt phosphorylation within irradiated tumors
To determine the effects of AACOCF3 on cellular viability in vivo, we performed TUNEL staining on LLC tumor sections from treated mice and analyzed the sections for the presence of apoptosis (Fig. 5A). In tumor sections from untreated mice or mice treated with radiation alone, we did not find TUNEL-positive cells. Mice treated with the inhibitor alone exhibited TUNEL-positive staining within a small population of cells. The most pronounced effect was detected in tumors from mice treated with a combination of AACOCF3 and irradiation. Within these tumors, we observed a significant increase in apoptosis of the endothelial cells lining the tumor blood vessels (Fig. 5A, black arrows). The results also revealed elevated TUNEL-positive staining among those tumor cells located in close proximity to the blood vessels (Fig. 5A, red arrows).
Fig. 5. Treatment with AACOCF3 results in increased apoptosis and decreased Akt phosphorylation within irradiated tumor models.
C57/BL6 mice with LLC tumors were treated as described in Fig. 4. A) TUNEL staining and hematoxylin counterstaining were performed on 5 μm sections from LLC tumors. Shown are representative micrographs of tumor with blood vessels from treated mice White arrows indicate TUNEL-positive endothelial cells; red arrows indicate TUNEL-positive tumor cells. B) Phospho-Akt immunofluorescence staining (green) and DAPI counterstaining (blue) were performed on LLC tumor sections from treated mice. Shown are representative micrographs of positive staining for Akt phosphorylation in LLC tumors.
To determine whether AACOCF3 affects radiation-induced pro-survival signaling within irradiated tumors, we performed immunofluorescence staining for phospho-Akt on tumor sections from LLC-bearing mice (Fig. 5B). In tumors from untreated mice or mice treated with 3 Gy, we observed increased levels of Akt phosphorylation. In comparison, treatment with drug alone resulted in decreased phospho-Akt levels throughout the entire tumor. Inhibition of Akt phosphorylation was further potentiated in tumors from mice that received a combined treatment of 10 mg/kg AACOCF3 followed by irradiation with 3 Gy.
Tumor vascular window model and vascular length density analysis
To investigate the effects of cPLA2 inhibition on tumor vasculature radiosensitivity, we used a dorsal skinfold window model. LLC cells were implanted into the dorsal skinfold window in C57/BL6 mice, and a sufficient vascular network was allowed to develop. Vascular windows were then treated with the cPLA2 inhibitor AACOCF3 30 minutes prior to irradiation with 3 Gy. At 72 hours post treatment, the average Percent Vascular Length Densities were 71% and 42% for mice treated with drug alone or irradiation, respectively (Fig. 6). A combined treatment of AACOCF3 and radiation, however, resulted in statistically significant destruction of tumor blood vessels (Fig. 6, 26%). These radiosensitization effects persisted at 96 hours post treatment, in which vascular length density was further reduced by AACOCF3 followed by irradiation (Fig. 6B, 13%).
Fig. 6. Tumor vascular window model and vascular length density analysis.
Lewis Lung Carcinoma (LLC) cells were implanted into the dorsal skinfold window in C57/BL6 mice. A sufficient vascular network was allowed to develop, and windows were treated with AACOCF3 30 minutes prior to irradiation with 3 Gy. Color photographs were taken daily to monitor blood vessel appearance. A) Shown are representative micrographs of 40X magnification of LLC tumor vascular window models at 0 hr and 72 hr after treatment. B) Changes in the quantity of blood vessels over time were compared with that observed at 0 h. Shown is a bar graph of the percent vascular length density 72 and 96 hours after treatment of implanted tumors with SEM from group of 3 to 5 mice; *, p < 0.05.
Discussion
In the tumor vascular endothelium, clinically relevant low doses of radiation trigger the activation of pro-survival signaling pathways. This results in enhanced radioresistance of tumor vasculature which can significantly diminish overall cancer treatment success rates. Thus, pharmacologic agents and therapeutic strategies that confer radiosensitivity to tumor blood vessels could improve tumor control. Cytosolic phospholipase A2 (cPLA2) is a key component in the response of vascular endothelial cells to ionizing radiation. Radiation-induced activation of cPLA2 leads to the downstream phosphorylation of the pro-survival targets Akt and ERK1/2 (17). In our present study, we provide evidence that chemical inhibition of cPLA2 with AACOCF3 can increase the radiation-induced destruction of tumor blood vessels and may significantly improve the response of radioresistant lung tumors to radiation therapy.
Using a syngeneic lung tumor model of Lewis Lung Carcinoma, we demonstrated that a combined treatment of AACOCF3 and radiation inhibited tumor growth by 75% as compared to untreated tumors. To further verify the role of cPLA2 in tumor growth progression, we used a xenograft lung tumor model of H460 cells. Treatment with AACOCF3 plus radiation resulted in tumor growth suppression similar to that of the LLC tumor model. However, since H460 cells are more radiosensitive than LLC, the increase in radiosensitization from the combined treatment was not as pronounced. Therefore, these findings suggest that cPLA2 is a relevant molecular target for lung tumors that are highly resistant to radiation therapy.
Another strategy to evaluate the efficacy of cPLA2 inhibition in vivo is to quantitatively measure the effects on tumor blood flow (40). In the current study, we used Power Doppler Sonography to provide a non-invasive assessment of the efficacy of this antivascular therapy. This strategy is supported by prior studies of inhibition of molecular targets in the vascular endothelium (41, 42). We showed that cPLA2 inhibition with AACOCF3 reduced tumor blood flow in irradiated tumors. Thus, these data support the hypothesis that cPLA2 inhibition combined with radiation leads to a disruption of tumor blood vessel function. To assess whether the overall tumor blood vessel number was affected by cPLA2 inhibition, we used von Willebrand Factor (vWF) as a marker for vascularity. vWF is a glycoprotein that mediates platelet adhesion to sites of vascular injury, and is a well accepted immunohistochemical marker of endothelial cells (43-45). Our study revealed that tumors from mice treated with AACOCF3 followed by radiation exhibited a significant reduction in blood vessel number. Upon further immunohistochemical analysis, we found that this attenuation of blood flow and reduction in overall vascularity is associated with the induction of apoptosis within tumor vascular endothelial cells. In addition, immunofluorescence staining revealed that treatment with AACOCF3 followed by 3 Gy suppresses radiation-induced Akt phosphorylation in irradiated tumors. This observation along with increased apoptosis in tumor cells and tumor vasculature suggests the possibility of multiple mechanisms involved in the radiosensitizing effects of the cPLA2 inhibitor AACOCF3. Thus, future investigation of the host-tumor interaction may provide clarification of the molecular events responsible for the increased efficacy of cPLA2 inhibition in irradiated cancer.
The tumor vascular window model allows direct measurement of the vascular response to ionizing radiation in vivo (39). Our results revealed that, in the absence of irradiation, AACOCF3 alone had no significant effect on existing vasculature. When treated with AACOCF3 followed by radiation, however, enhanced destruction of tumor blood vessels was observed. Taken together, these data demonstrated that in irradiated mouse tumor models, AACOCF3 disrupts the biological functions of the tumor vasculature, enhances destruction of tumor blood vessels and suppresses tumor growth. Therefore, the cPLA2 inhibitor AACOCF3 is an effective radiosensitizer in mouse lung tumor models.
To provide further in vitro validation for our therapeutic strategy, we investigated the effects of cPLA2 inhibition in the 3B11 cell line as well as in primary vascular endothelial cells isolated directly from lung tissue. In selecting the appropriate cell-based models for the pre-clinical development of radiosensitizers, it is ideal to identify a murine vascular endothelial cell line that maintains the expression of traditional endothelial cell markers as well as markers found to be expressed by human tumor endothelial cells (31). 3B11 is a murine vascular endothelial cell line that expresses the mouse homologs for five tumor endothelial markers: mTEM1, mTEM3, mTEM5, mTEM7, and mTEM8. Like normal endothelial cells, 3B11 also expresses many of the murine homologs of standard endothelial cell markers including, sialomucin/CD34, GPIIIB/CD36, and VCAMI/CD106 (31). Thus, 3B11 has been identified as a relevant murine model for tumor vascular endothelial cells (31, 46, 47). In addition, we also used primary pulmonary vascular endothelial cells, the closest physiological cell culture model for lung cancer vasculature. Our cell culture experiments demonstrated that cPLA2 inhibition with AACOCF3 followed by radiation prevented radiation-induced activation of pro-survival signaling (ERK1/2) and significantly enhanced cell death. This effect was observed in vascular endothelial cells but not in lung tumor cell lines. This differential response suggests a fundamental regulatory role for radiation-induced cPLA2-dependent activation of pro-survival signal transduction pathways in the viability of irradiated vascular endothelial cells as compared to tumor cells. Additional functional assays revealed that treatment with AACOCF3 reduced migration and attenuated tubule formation in irradiated 3B11 and primary vascular endothelial cells. Taken together, these data indicate that cPLA2 inhibition confers radiosensitivity to tumor vasculature and may disrupt tumor blood vessel function.
These findings identify cPLA2 as a critical component of the tumor vascular response to ionizing radiation. In the present study, we found that cPLA2 inhibition prevents the formation of a functional tumor vascular network both in vivo and in cell culture; disrupts already formed tumor blood vessels and significantly delays tumor growth. Thus, cPLA2 inhibition with AACOCF3 enhances the radiation-induced destruction of tumor vasculature and may significantly improve lung tumor response to radiation therapy.
Statement of Translational Relevance.
In the tumor vascular endothelium, clinically relevant low doses of radiation trigger the immediate activation of cytosolic phospholipase A2 (cPLA2). This results in pro-survival signaling and enhanced radioresistance of tumor vasculature which can significantly diminish overall cancer treatment success rates. Since ablation of the tumor vasculature enhances the treatment of cancer, the development of pharmacologic agents that confer radiosensitivity to tumor blood vessels could improve tumor control. In our present study, we provide evidence that chemical inhibition of cPLA2 with AACOCF3 prevents the formation of a functional tumor vascular network, increases the radiation-induced destruction of tumor blood vessels and significantly delays growth of lung tumors, especially those exhibiting high levels of radioresistance. Thus, these findings identify cPLA2 as a critical component of the tumor vascular response to ionizing radiation and suggest that cPLA2 is a novel molecular target for tumor sensitization to radiation therapy.
Acknowledgments
Financial support: NIH/NCI, R01-CA125757 (DH, LG), R21-CA128456 (DH, LG), R01-CA112385 (DH), 2R01-CA089674 (DH, EY), R01-CA088076 (DH, LG), P50-CA090949 (DH); Elsa U. Pardee Foundation (EY, AL)
References
- 1.Wagner H., Jr. Postoperative adjuvant therapy for patients with resected non-small cell lung cancer: still controversial after all these years. Chest. 2000;117:110S–8S. doi: 10.1378/chest.117.4_suppl_1.110s. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, Machtay M, Kaiser LR, et al. Non-small cell lung cancer: prognostic factors in patients treated with surgery and postoperative radiation therapy. Radiology. 1999;213:845–52. doi: 10.1148/radiology.213.3.r99dc23845. [DOI] [PubMed] [Google Scholar]
- 3.Clamon G, Herndon J, Cooper R, Chang AY, Rosenman J, Green MR. Radiosensitization with carboplatin for patients with unresectable stage III non-small-cell lung cancer: a phase III trial of the Cancer and Leukemia Group B and the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:4–11. doi: 10.1200/JCO.1999.17.1.4. [DOI] [PubMed] [Google Scholar]
- 4.Dietz A, Boehm A, Mozet C, Wichmann G, Giannis A. Current aspects of targeted therapy in head and neck tumors. Eur Arch Otorhinolaryngol. 2008 doi: 10.1007/s00405-008-0697-6. [DOI] [PubMed] [Google Scholar]
- 5.Forastiere AA, Trotti A, Pfister DG, Grandis JR. Head and neck cancer: recent advances and new standards of care. Journal of Clinical Oncology. 2006;24:2603–5. doi: 10.1200/JCO.2006.07.1464. [DOI] [PubMed] [Google Scholar]
- 6.Iranzo V, Bremnes RM, Almendros P, et al. Induction chemotherapy followed by concurrent chemoradiation for patients with non-operable stage III non-small-cell lung cancer. Lung cancer (Amsterdam, Netherlands) 2008 doi: 10.1016/j.lungcan.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 7.McGinn CJ, Shewach DS, Lawrence TS. Radiosensitizing nucleosides. Journal of the National Cancer Institute. 1996;88:1193–203. doi: 10.1093/jnci/88.17.1193. [DOI] [PubMed] [Google Scholar]
- 8.Stratford IJ. Concepts and developments in radiosensitization of mammalian cells. International journal of radiation oncology, biology, physics. 1992;22:529–32. doi: 10.1016/0360-3016(92)90868-i. [DOI] [PubMed] [Google Scholar]
- 9.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Seminars in nephrology. 2003;23:460–4. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 10.Berns JS, Ford PA. Renal toxicities of antineoplastic drugs and bone marrow transplantation. Seminars in nephrology. 1997;17(1):54–66. [PubMed] [Google Scholar]
- 11.Goldstein RS, Mayor GH. Minireview. The nephrotoxicity of cisplatin. Life sciences. 1983;32:685–90. doi: 10.1016/0024-3205(83)90299-0. [DOI] [PubMed] [Google Scholar]
- 12.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem cells. 2000;18:10–8. doi: 10.1634/stemcells.18-1-10. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Li M, Rinehart JJ, Zhang R. Pretreatment with dexamethasone increases antitumor activity of carboplatin and gemcitabine in mice bearing human cancer xenografts: in vivo activity, pharmacokinetics, and clinical implications for cancer chemotherapy. Clin Cancer Res. 2004;10:1633–44. doi: 10.1158/1078-0432.ccr-0829-3. [DOI] [PubMed] [Google Scholar]
- 14.Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. Journal of Biological Chemistry. 1995;270:13333–40. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- 15.Shweiki D, Neeman M, Itin A, Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:768–72. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res. 2003;9:1957–71. [PubMed] [Google Scholar]
- 17.Yazlovitskaya EM, Linkous AG, Thotala DK, Cuneo KC, Hallahan DE. Cytosolic phospholipase A2 regulates viability of irradiated vascular endothelium. Cell death and differentiation. 2008;15:1641–53. doi: 10.1038/cdd.2008.93. [DOI] [PubMed] [Google Scholar]
- 18.Dent P, Yacoub A, Contessa J, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiation Research. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Zhan M, Han ZC. Phosphatidylinositide 3-kinase/AKT in radiation responses. Histology and histopathology. 2004;19:915–23. doi: 10.14670/HH-19.915. [DOI] [PubMed] [Google Scholar]
- 20.Zingg D, Riesterer O, Fabbro D, Glanzmann C, Bodis S, Pruschy M. Differential activation of the phosphatidylinositol 3′-kinase/Akt survival pathway by ionizing radiation in tumor and primary endothelial cells. Cancer Res. 2004;64:5398–406. doi: 10.1158/0008-5472.CAN-03-3369. [DOI] [PubMed] [Google Scholar]
- 21.Folkman J. Tumor angiogenesis: therapeutic implications. The New England journal of medicine. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 22.Strijbos MH, Gratama JW, Kraan J, Lamers CH, den Bakker MA, Sleijfer S. Circulating endothelial cells in oncology: pitfalls and promises. British journal of cancer. 2008;98:1731–5. doi: 10.1038/sj.bjc.6604383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haimovitz-Friedman A, Kan CC, Ehleiter D, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. The Journal of experimental medicine. 1994;180:525–35. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valerie K, Yacoub A, Hagan MP, et al. Radiation-induced cell signaling: inside-out and outside-in. Molecular cancer therapeutics. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 25.Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacological reviews. 2006;58:591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 26.Grewal S, Herbert SP, Ponnambalam S, Walker JH. Cytosolic phospholipase A2-alpha and cyclooxygenase-2 localize to intracellular membranes of EA.hy.926 endothelial cells that are distinct from the endoplasmic reticulum and the Golgi apparatus. The FEBS journal. 2005;272:1278–90. doi: 10.1111/j.1742-4658.2005.04565.x. [DOI] [PubMed] [Google Scholar]
- 27.Herbert SP, Odell AF, Ponnambalam S, Walker JH. The confluence-dependent interaction of cytosolic phospholipase A2-alpha with annexin A1 regulates endothelial cell prostaglandin E2 generation. The Journal of biological chemistry. 2007;282:34468–78. doi: 10.1074/jbc.M701541200. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi M, Rosenberg DW. Roles of cPLA2alpha and arachidonic acid in cancer. Biochimica et Biophysica Acta. 2006;1761:1335–43. doi: 10.1016/j.bbalip.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujita Y, Yoshizumi M, Izawa Y, et al. Transactivation of fetal liver kinase-1/kinase-insert domain-containing receptor by lysophosphatidylcholine induces vascular endothelial cell proliferation. Endocrinology. 2006;147:1377–85. doi: 10.1210/en.2005-0644. [DOI] [PubMed] [Google Scholar]
- 30.Marathe GK, Silva AR, de Castro Faria Neto HC, et al. Lysophosphatidylcholine and lyso-PAF display PAF-like activity derived from contaminating phospholipids. Journal of lipid research. 2001;42:1430–7. [PubMed] [Google Scholar]
- 31.Walter-Yohrling J, Morgenbesser S, Rouleau C, et al. Murine endothelial cell lines as models of tumor endothelial cells. Clin Cancer Res. 2004;10:2179–89. doi: 10.1158/1078-0432.ccr-03-1013. [DOI] [PubMed] [Google Scholar]
- 32.Street IP, Lin HK, Laliberte F, et al. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 1993;32:5935–40. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- 33.Trimble LA, Street IP, Perrier H, Tremblay NM, Weech PK, Bernstein MA. NMR structural studies of the tight complex between a trifluoromethyl ketone inhibitor and the 85-kDa human phospholipase A2. Biochemistry. 1993;32:12560–5. doi: 10.1021/bi00210a002. [DOI] [PubMed] [Google Scholar]
- 34.Duan L, Gan H, Arm J, Remold HG. Cytosolic phospholipase A2 participates with TNF-alpha in the induction of apoptosis of human macrophages infected with Mycobacterium tuberculosis H37Ra. J Immunol. 2001;166:7469–76. doi: 10.4049/jimmunol.166.12.7469. [DOI] [PubMed] [Google Scholar]
- 35.Fabisiak JP, Kagan VE, Tyurina YY, Tyurin VA, Lazo JS. Paraquat-induced phosphatidylserine oxidation and apoptosis are independent of activation of PLA2. The American journal of physiology. 1998;274:L793–802. doi: 10.1152/ajplung.1998.274.5.L793. [DOI] [PubMed] [Google Scholar]
- 36.Kirschnek S, Gulbins E. Phospholipase A2 functions in Pseudomonas aeruginosa-induced apoptosis. Infection and immunity. 2006;74:850–60. doi: 10.1128/IAI.74.2.850-860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calzada C, Vericel E, Mitel B, Coulon L, Lagarde M. 12(S)-Hydroperoxy-eicosatetraenoic acid increases arachidonic acid availability in collagen-primed platelets. Journal of lipid research. 2001;42:1467–73. [PubMed] [Google Scholar]
- 38.Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A. 2000;97:2202–7. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng L, Donnelly E, McMahon G, et al. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res. 2001;61:2413–9. [PubMed] [Google Scholar]
- 40.Goertz DE, Yu JL, Kerbel RS, Burns PN, Foster FS. High-frequency Doppler ultrasound monitors the effects of antivascular therapy on tumor blood flow. Cancer Res. 2002;62:6371–5. [PubMed] [Google Scholar]
- 41.Edwards E, Geng L, Tan J, Onishko H, Donnelly E, Hallahan DE. Phosphatidylinositol 3-kinase/Akt signaling in the response of vascular endothelium to ionizing radiation. Cancer Res. 2002;62:4671–7. [PubMed] [Google Scholar]
- 42.Tan J, Hallahan DE. Growth factor-independent activation of protein kinase B contributes to the inherent resistance of vascular endothelium to radiation-induced apoptotic response. Cancer Res. 2003;63:7663–7. [PubMed] [Google Scholar]
- 43.Alles JU, Bosslet K. Immunocytochemistry of angiosarcomas. A study of 19 cases with special emphasis on the applicability of endothelial cell specific markers to routinely prepared tissues. American journal of clinical pathology. 1988;89:463–71. doi: 10.1093/ajcp/89.4.463. [DOI] [PubMed] [Google Scholar]
- 44.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385–95. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto K, de Waard V, Fearns C, Loskutoff DJ. Tissue distribution and regulation of murine von Willebrand factor gene expression in vivo. Blood. 1998;92:2791–801. [PubMed] [Google Scholar]
- 46.Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–55. [PubMed] [Google Scholar]
- 47.St Croix B, Rago C, Velculescu V, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]