Abstract
Rationale
A large proportion of smokers consolidate their smoking patterns during young adulthood, and it is possible that the high rates of drinking found in this age group may facilitate the transition from nondaily to daily cigarette use.
Objective
The primary aim of this study was to examine how alcohol alters the subjective effects of smoking in heavy-drinking young adults (age 21–25) who are still at an experimental stage of smoking but show recent increases in their smoking behavior.
Methods
Using a within-subject design, we examined whether alcohol or the expectation of receiving alcohol increased either subjective responses to smoking or the amount smoked. Subjects participated in three sessions, in which they received alcohol (0.08 g/dL targeted blood alcohol level), a taste-masked placebo presented as alcohol, or a mixer beverage not presented as alcohol. Measures included positive and negative subjective reactivity (e.g., satisfaction, nausea, craving relief, and enjoyment of airway sensations) associated with smoking a single cigarette and subsequent ad lib smoking behavior.
Results
Both conditions in which the subjects expected to receive alcohol increased positive effects of smoking (satisfaction, calm, and taste), compared to the mixer beverage. Alcohol, compared to the placebo and mixer beverages, decreased negative effects (nausea) associated with smoking a cigarette and increased subsequent smoking.
Conclusions
This initial study has implications for understanding how alcohol and the expectation of alcohol improves the experience of smoking in nondaily smokers who are still at an experimental stage of smoking.
Keywords: Alcohol, Tobacco, Young adult, Subjective reactivity, Self-administration, Heavy drinker, Non-daily smoker
It is well established that alcohol consumption and tobacco use are highly correlated across levels of use and diagnostic categories in the general population (Dawson 2000; Grant et al. 2004; McKee et al. 2007) and in samples of young adults (Dierker et al. 2006; Jackson et al. 2003, 2005). Data obtained from the Harvard School of Public Health College Alcohol Study found that 98% of current college-aged smokers consume alcohol, and 59% of college-aged drinkers smoke (Weitzman and Chen 2005). Analyses of young adults (18–25) in the National Epidemiological Survey on Alcohol and Related Conditions demonstrated that 63% of daily smokers meet criteria for hazardous drinking, with this rate increasing to 72% in nondaily smokers (Harrison et al. 2008).
Longitudinal evidence from the National Longitudinal Study of Adolescent Health and the Adolescent Risk Study suggests that the co-occurrence of alcohol and tobacco use escalates during adolescence and reaches an asymptote by age 25 (Jackson et al. 2002). Moreover, these authors found that alcohol use strongly predicted tobacco use, more so than tobacco use predicted alcohol use. Additionally, both initiation and persistence of smoking were associated with prior drinking. Simon et al. (1995) demonstrated that alcohol use predicted post 1-year tobacco use in a large sample of adolescents. In college students, Sher et al. (1996) found reciprocal relationships between the development of alcohol and tobacco use disorders. Overall, these findings suggest that the concurrent use of alcohol and tobacco may facilitate the development of a tobacco use disorder.
Prevalence estimates collected over the prior decade find that approximately 30% of young adults are current smokers (Harrison et al. 2008; Johnston et al. 2001; Rigotti et al. 2000) and that young adults have the highest rates of smoking compared to older adults. Data from the 2007 National Health Interview survey shows that rates of current smoking among adults aged 18–24 were 20.7% higher than all adults. With regard to nondaily smoking, population studies have documented that 19–24% of current smokers are nondaily smokers (Hassmiller et al. 2003; Hennrikus et al. 1996), with younger age being significantly associated with this smoking pattern (Gilpin et al. 1997; Hassmiller et al. 2003; Hennrikus et al. 1996).
Research examining young adults has documented that nondaily smoking is most likely to occur in the context of alcohol use (Dierker et al. 2006; Nichter et al. 2006). We have found that young adult nondependent smokers report that 74% of all smoking episodes occurred while under the influence of alcohol (McKee et al. 2004). Interviews conducted with these smokers find that they are cognizant of the reasons why they co-use alcohol and tobacco and report that they experience increased pleasure from cigarettes and increased desire for cigarettes when drinking alcohol (McKee et al. 2004). When examining their episodic patterns of co-use, cigarette use was most likely to occur following heavy alcohol consumption (Harrison et al. 2009).
It is possible that alcohol may improve the subjective experience of cigarette smoking in heavy-drinking young adults who are still at an experimental stage of smoking, but this has yet to be investigated. A large proportion of smokers consolidate their smoking patterns during early adulthood, making this an important population to study. For example, Wechsler et al. (1998) reported that of college smokers, “11% had their first cigarette and 28% began to smoke regularly at or after the age of 19” (p. 1673). When examining adolescent to adult smoking trajectories, Chassin et al. (2000) characterized 39% (1,108/2,870) of smokers as late-stable smokers. These late-stable smokers had infrequent smoking during high school but increased to daily smoking over the course of early adulthood. It is possible that increased rates of drinking during early adulthood (Dawson et al. 2004) may be associated with this pattern of smoking behavior.
For the current study, we were interested in examining how alcohol might improve the subjective effects associated with cigarette smoking in heavy-drinking young adults (age 21–25) who are still at an experimental stage of smoking. The primary aim of this research was to investigate the effect of a high dose of alcohol, compared to a taste-masked placebo or a mixer beverage, on reactivity to tobacco and subsequent cigarette self-administration behavior. We hypothesized that alcohol (0.08 g/dL targeted blood alcohol level), compared to a placebo or a no-alcohol mixer beverage, would increase positive subjective effects (e.g., satisfaction) and decrease negative subject effects (e.g., nausea) associated with smoking a single cigarette. In young adult nondaily smokers, King et al. (2009) found that alcohol compared with placebo increased some positive effects of smoking. However, the King et al. (2009) study was designed to mask expectancy effects. It is possible that the expectation of alcohol consumption may be sufficient to alter subjective reactivity associated with cigarette smoking. Non-daily smoking has also been referred to as social smoking (Nichter et al. 2006; Shiffman et al. 2009) and may be associated with alcohol through nonpharmacological mechanisms. Thus, we included an expectancy manipulation. For the taste-masked placebo condition, subjects were told that the beverage contained alcohol, whereas the instructions for the alcohol and mixer conditions were consistent with the contents of the beverage. Secondary predictions included whether alcohol, compared to a placebo or mixer beverage, would increase ratings of tobacco and alcohol craving, the intensity of smoking behavior (i.e., greater puff volume, greater puff duration, shorter inter-puff interval, and greater depth of inhalation), and the number of cigarettes smoked. This initial study has implications for understanding how alcohol may facilitate the development of a tobacco use disorder and provide the basis for further investigations examining potential mechanisms for this effect.
Methods
Participants
Participants were eligible to enroll if they were 21–25 years of age and met criteria for experimental smoking, which was defined as having smoked less than 100 cigarettes in their lifetime (US DHHS 1990). To model individuals who may develop into the Chassin et al. (2000) group of “late-stable smokers,” we also required that participants had a recent escalation in their smoking behavior from “smoking less than once per month” to “smoking at least once per month but no more than four times per week” within the last 12 months. Heavy drinking was an inclusion criterion and was defined as consuming an average of at least 10 alcoholic drinks per week and drinking on at least 2 days per week with at least one heavy-drinking episode per week (5+ drinks for men, 4+ drinks for women; King et al. 2002). Participants also completed a physical exam during which urine drug screens, pregnancy test, EKG, and routine lab tests were conducted. Participants were excluded if they presented with current DSM-IV criteria for alcohol or nicotine dependence, or abuse or dependence of other substances, presented with current severe psychiatric disorders, currently seeking treatment for alcohol or tobacco use, had medical conditions that would contraindicate drinking or smoking, were positive for illicit substances (except cannabis), had used psychotropic drugs within the past 30 days, or were pregnant or nursing. A total of 19 participants completed the study (10 men and 9 women). Baseline characteristics are presented in Table 1. Overall, the sample was not nicotine dependent, drank heavily, smoked infrequently, and primarily smoked during alcohol consumption.
Table 1.
Baseline characteristics
| Variable | Mean (SD) or n, % | |
|---|---|---|
| Age | 22.21 (1.27) | |
| Gender | % Female | 9 (47%) |
| Race | Caucasian | 18, 95% |
| African American | 1, 5% | |
| Education | College | 17, 89% |
| High school | 2, 11% | |
| Baseline CO (ppm) | 6.68 (2.03) | |
| FTND score | 0.16 (0.37) | |
| Smoking days per month | 6.84 (5.01) | |
| Cigs per smoking day | 2.57 (1.40) | |
| Drinking days per week | 2.80 (0.79) | |
| Drinks per week | 16.53 (10.95) | |
| AUDIT score | 10.52 (3.89) | |
| % of smoking episodes that occur while drinking | 83% | |
CO carbon monoxide, FTND Fagerstrom Test for Nicotine Dependence, AUDIT Alcohol Use Disorder Identification Test
Procedure
Intake session
This study was approved by the Yale University Human Investigation Committee and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Written informed consent was obtained at the start of the intake session. During consent, subjects were informed that they would be instructed whether or not their beverage contained alcohol during the laboratory sessions. Participants provided demographic and drinking and smoking history information. The Structured Clinical Interview for DSM-IV (First et al. 1997) was used to exclude individuals who met diagnostic criteria for substance abuse or dependence. The Fagerström Test of Nicotine Dependence (Heatherton et al. 1991) was used to exclude for nicotine dependence (scores greater than 4 indicate dependence, range 0–10). The Timeline Followback (Sobell and Sobell 1993) was used to assess past 30-day drinking and smoking behavior. The Alcohol Use Disorders Identification Test was used to characterize alcohol consumption and related problems (Saunders et al. 1993; scores greater than 8 indicate harmful alcohol consumption, range 0–40). Breath carbon monoxide levels were assessed using a CO-meter (MC02 Monitor, MicroDirect, Auburn, ME, USA; values greater than 10 ppm indicate recent smoking), and breath alcohol levels were assessed with an Alco-Sensor III, Intoximeter (St. Louis, MO, USA). The absence of recent cocaine, opiate, benzodiazepine, barbiturate, or amphetamine was determined by urine drug test (JANT Pharmaceuticals, Encino, CA, USA).
Laboratory sessions
Participants completed three laboratory sessions (alcohol, placebo, and mixer) at the Yale Center for Clinical Investigation. Laboratory sessions started at 4:00 p.m. The three laboratory sessions were completed within a mean of 13.87 (SD=5.46) days. The order of the three sessions was randomly determined. For women, laboratory sessions were scheduled without regard to menstrual cycle phase (see Terner and de Wit 2006).
Participants were instructed to eat a regular lunch between 12:00 p.m. and 1:00 p.m. and not to eat anything else prior to the start of the laboratory session. They were instructed not to drink alcohol or smoke within 24 h of the session, which was confirmed by self-report and breath CO (<8 ppm) and breath alcohol readings. Baseline assessments of tobacco craving and alcohol craving were collected at the beginning of each laboratory session. They were provided with a small low-fat snack at 4:45 p.m. to standardize the time between food and alcohol consumption.
Alcohol beverage conditions
For the alcohol condition, participants consumed an alcoholic drink consisting of one part 80-proof liquor of the participant’s choosing to three parts mixer from a selection of equicaloric, noncaffeinated, noncarbonated drinks (McKee et al. 2006). The amount of alcohol in the drink was designed to raise blood alcohol levels to 0.08 g/dL based on a formula that takes into account gender, age, height, and weight of each subject (Watson 1989). This blood alcohol level was chosen on the basis of our prior work, which suggests that experimental smokers are likely to start to smoke when their drinking reaches a level consistent with a blood alcohol level (BAL) of 0.08 g/dL (Harrison et al. 2009). For the placebo condition, participants consumed a beverage consisting of four parts mixer with 1% alcohol (by volume; mean=2.72 ml, SD=1.07 ml). The 1% alcohol solution was floated on the top of the mixer as a taste mask (King et al. 2002). For the mixer condition, participants consumed a beverage consisting of four parts mixer.
Beverage administration
Beverages were divided into two equal drinks, with 5 min to consume each drink and a 5-min rest period in between each drink (King et al. 2002). Beverage administration started at 5:15 pm (time 0). During the alcohol and taste-masked placebo sessions, participants were informed that they would receive alcohol. During the mixer session, participants were informed that they would receive a nonalcoholic beverage. Beverage administration was double blinded. A second experimenter mixed the drinks and provided the participants the assigned information as to whether their beverage contained alcohol or not. This second experimenter also completed the breath alcohol measures, which were not disclosed to the primary experimenter or to the subject. Responses to alcohol were then assessed at +15 and +30 min (i.e., breath alcohol, tobacco craving, alcohol craving, and subjective effects of alcohol) in the order listed.
Smoking period
At +35 min from the start of alcohol consumption, participants smoked a single cigarette (regular size, filter tip, and of their preferred brand) with the smoking topography device. This timing was chosen so that the cigarette would be smoked during the ascending limb (and approaching the peak) of the blood alcohol curve. Prior studies with regular smokers demonstrated that tobacco craving peaks during the ascending limb (King and Epstein 2005). Our prior work with experimental smokers demonstrated that they are likely to start to smoke when they reach a peak BAL of 0.08 g/dL (Harrison et al. 2009). Responses to the single cigarette were assessed immediately (+45 min; CO level, breath alcohol, subjective effects of cigarettes, tobacco craving, alcohol craving, and subjective effects of alcohol).
Participants then started a 60-min ad lib smoking period at 6:15 p.m. They were provided with four cigarettes and instructed to “smoke as little or as much as they wished” using the smoking topography equipment. Assessments were repeated at +60 (immediately before the start of the ad lib period) and at +90 and +120 min. Subjects were discharged at 9:30 p.m., at which time all breath alcohol levels were below 0.02% (NIAAA 2005). Following the final laboratory session, participants were provided with normative information and moderate drinking guidelines (Miller et al. 1992) and were debriefed concerning the alcohol content of their three laboratory sessions.
Outcome measures
Subjective measures
The alcohol urge questionnaire (AUQ; Bohn et al. 1995) was used to assess alcohol craving. This eight-item measure was designed to assess an individual’s desire to drink alcohol right now. Tobacco craving was assessed with the Tiffany Questionnaire of Smoking Urges—Brief (QSU-B; Cox et al. 2001), which consists of 10 items to evaluate urges to smoke in response to positive (factor 1) or negative (factor 2) reinforcement. The biphasic alcohol effects scale (BAES; Martin et al. 1993) is a 14-item self-report, unipolar adjective rating scale used to measure the stimulant and sedative effects of alcohol. For the AUQ, QSU-B, and BAES, the response format was changed to a 100-point visual analogue scale (VAS) to increase the potential variability in responding. The alcohol effects scale is a locally developed instrument, which assessed subjective alcohol effects with a sum of five items (high, like, rush, feel good, and intoxicated). Participants indicated on a visual analogue scale (range 1–100) how much of an alcohol effect they were experiencing (McKee et al. 2006, 2008). This scale was adopted from others found in the literature (Schuckit 1984; drug effect questionnaire, White et al. 2006). The cigarette evaluation questionnaire is a 10-item self-report questionnaire used to assess subjective rewarding and aversive effects of smoking (satisfaction, psychological reward, nausea/dizziness, craving relief, and enjoyment of airway sensations; Westman et al. 1992).
Smoking topography
A Clinical Research Support System (CreSS from Plowshare Technologies) to assess smoking topography was used. Smoking topography measures of interest included puff frequency, puff volume, puff duration, inter-puff interval, depth of inhalation, and inter-cigarette interval. The device allowed for the assessment of smoking topography during ad lib periods involving multiple cigarettes.
Statistical analysis
The primary outcome was the subjective reactivity to the single cigarette. Analyses of variance were used to compare positive and negative reactivity to the single cigarette across beverage conditions. Similar analyses were conducted for the number of cigarettes smoked and smoking topography measures. Smoking topography data were cleaned with a utility program (PuffCleanUp, Plowshare Technologies), which combines or deletes data (i.e., false puffs), which can arise from movement artifacts. We used the recommended parameters for data cleaning. Smoking topography measures (number of puffs, puff duration, puff volume, peak puff volume, and inter-puff interval) were calculated for the single cigarette and for each cigarette smoked during the ad lib period.
Separate repeated measures multivariate analyses of variance were conducted to assess changes in tobacco and alcohol craving across time (+0, 15, 30, and 45 min; pre-drink, post-drink, pre-cigarette, and post-cigarette) by beverage conditions. Similar analyses were conducted to assess alcohol reactivity (stimulation, sedation, and subjective effects) following alcohol consumption (0, +15, and +30 min). We found no effect of gender on our primary outcomes.
Results
Subjective reactivity to single cigarette
After smoking the single cigarette, measures of smoking satisfaction [F(1,18)=13.65, p<0.01], good taste [F(1,18)=5.36, p<0.03], calmness [F(1,18)=7.59, p<0.01], and nausea [F(1,18)=11.68, p<0.003; see Fig. 1] differed across beverage conditions. Simple effects tested demonstrated that satisfaction, good taste, and calmness were greater in the alcohol and placebo conditions than in the mixer condition. Nausea was lower in the alcohol condition than in the placebo and mixer conditions. No effects of beverage condition were demonstrated for other positive (concentrate, awake, throat sensations, and craving reduction) or negative effects of smoking (dizzy, hunger, and irritable).
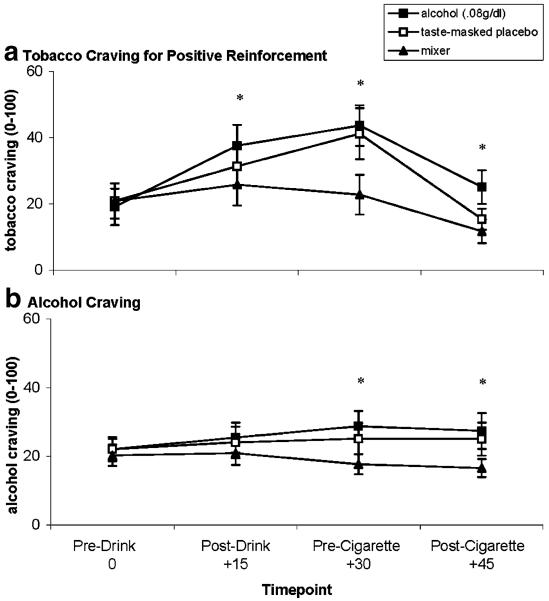
Fig. 1.
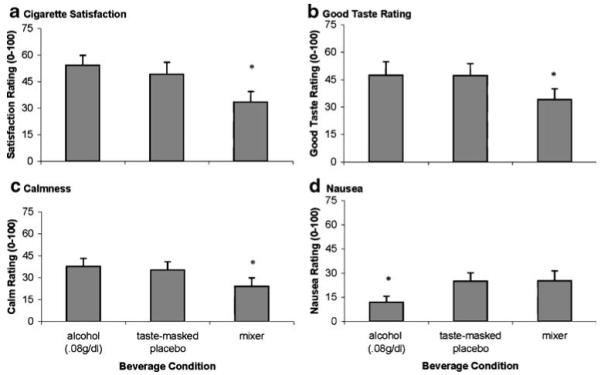
Mean subjective reactivity (+SE) to a single cigarette smoked after alcohol (ALC), taste-masked placebo (PLA), and mixer beverages (MIX). a Smoking satisfaction. b Good cigarette taste. c Calmness after smoking. d Nausea after smoking. For a, b, and c, asterisks indicate that planned contrasts showed ALC=PLA>MIX. For d, asterisk indicates ALC<PLA=MIX (p values<0.05)
Tobacco craving for positive and negative reinforcement
Ratings of tobacco craving for positive reinforcement varied over time and by beverage condition [F(1,18)=6.31, p<0.02; see Fig. 2a]. Simple effects testing within timepoint demonstrated that ratings of tobacco craving for positive reinforcement were greater following alcohol compared to placebo at +15 and +30 min following beverage consumption. At +30 min, tobacco craving was also greater in the placebo compared to the mixer condition. Following cigarette smoking (+45 min), tobacco craving for positive reinforcement was greater following the alcohol condition compared to placebo or mixer conditions. Levels of tobacco craving for negative reinforcement were low (all means <16.66 out of 100) and did not change across sessions or beverage conditions. This latter finding was expected, as participants were not dependent on nicotine and unlikely to smoke for withdrawal relief.
Fig. 2.
a Mean tobacco craving (+SE) for positive reinforcement and b mean alcohol craving (+SE) over time by alcohol (ALC), taste-masked placebo (PLA), and mixer (MIX) beverages. For a, asterisks indicate that planned contrasts at +15 ALC>MIX; +30 ALC=PLA>MIX; +45 ALC>PLA=MIX. For b, asterisks indicate that ALC>MIX (p values<0.05)
Alcohol craving
Alcohol craving varied by time and beverage condition [F(1,18)=4.50, p<0.05; see Fig. 2b]. Simple effects testing within timepoint demonstrated that ratings of alcohol craving were greater following alcohol compared to placebo or mixer beverages at +30 min following beverage consumption and +45 min following cigarette smoking.
Cigarettes smoked during ad lib period
Participants smoked more cigarettes during the alcohol condition (mean=1.16, SE=0.30) than during the placebo (mean=0.79, SE=0.24) and mixer conditions (mean=0.58, p<0.022) during the 60-min ad lib smoking period [overall F(1,18)=3.18, p<0.01]. This difference was due to the fact that subjects were most likely to smoke additional cigarettes during the alcohol condition. Fifty-eight percent of participants smoked after consuming an alcohol beverage, while only 42% and 32% smoked in the placebo and mixer conditions, respectively. Using McNemar tests for dependent proportions, the percentage of subjects who smoked additional cigarettes in the alcohol condition was greater than the placebo or the mixer condition (p values<0.05).
We also conducted supplementary analyses to determine if any of the significant differences observed on the subjective measures immediately following the single cigarette were associated with the number of cigarettes smoked during the ad lib period. When comparing the alcohol to the mixer condition (calculated as difference scores; alcohol minus mixer), there were significant associations with taste (r=0.50), calm (r=0.50), and tobacco craving for positive reinforcement (r=0.69). When comparing the placebo to the mixer condition, there were significant associations with satisfaction (r=0.49), taste (r=0.49), and tobacco craving for positive reinforcement (r=0.63). When comparing the alcohol to the placebo condition, there were no significant associations observed.
Smoking topography
No beverage condition differences were observed on any of the smoking topography measures during the single cigarette or during the ad lib period, p values>05.
Manipulation check for alcohol administration
Following the consumption of the beverage, peak breath alcohol levels for the alcohol session were 0.078% (SD=0.07). Thirty minutes following consumption of the beverage, ratings of alcohol effects (high, like, rush, feel good, and intoxicated) significantly differed by beverage condition and time [F(1,18)=22.01, p<0.001]. At +15 min, ratings of alcohol effects in the alcohol (mean=30.95, SE=3.06) and placebo condition (mean=29.72, SE=10.61) were greater than the mixer condition (mean=13.04, SE=3.23). By the +30-min timepoint, ratings of alcohol effects were greater in the alcohol condition (mean=31.27, SE=3.11), compared to the placebo (mean=16.22, SE=2.71) and the mixer condition (mean=13.07, SE=2.85). There were no significant effects of beverage condition found for ratings of stimulation (+30 min, mean=2.80, SE=0.31) or sedation (+30 min, mean=1.61, SE=0.23).
Following the last session, we asked subjects (n=14; five subjects were missing) whether they believed they had consumed alcohol or not for each of the three sessions. All subjects correctly guessed the alcohol content for the alcohol and mixer beverage conditions. For the placebo session, 71% guessed that they had consumed alcohol. In the 71% who believed that they had consumed alcohol, the pattern of the main findings for subjective reactivity and craving responses was not altered. However, ad lib smoking was reduced overall, with smoking in the alcohol condition (mean=0.50, SE=0.16) greater than the mixer condition (mean=0.20, SE=0.13) but not different from the placebo condition (mean=0.40, SE=0.16).
Discussion
This study was the first to examine how alcohol influenced subjective effects of smoking in heavy-drinking young adults who are experimental smokers with an escalating pattern of use. Contrary to our hypothesis, the expectation of alcohol consumption rather than actual alcohol consumption increased the positive subjective effects of smoking. During the alcohol and placebo conditions when alcohol was expected, ratings of smoking satisfaction, pleasurable taste, calmness, and tobacco craving were greater when compared to the mixer beverage. Additionally, when subjects expected to receive alcohol, tobacco craving for positive reinforcement and positive subjective effects of smoking (e.g., satisfaction, calm, and taste) were predictive of the amount smoked during the ad lib session. However, only alcohol consumption and not the expectation of alcohol consumption increased ad lib smoking and attenuated negative reactivity (i.e., nausea) associated with smoking. While King et al. (2009) included procedures to minimize expectancy effects, consistent findings were demonstrated. Following two standardized puffs from a cigarette, both the alcohol and taste-masked placebo beverages increased desire to smoke, satisfaction, enjoyable sensation, and enjoyable taste. However, other subjective effects (e.g., head rush) differed across alcohol and placebo conditions (King et al. 2009).
Our findings are supported by prior studies examining the effect of alcohol and placebo beverages on craving responses (King and Epstein 2005; Epstein et al. 2007; Sayette et al. 2005). The Sayette et al. (2005) study included an expectancy manipulation for the taste-masked placebo beverage and found that at 10-min post-consumption, both alcohol and placebo beverages increased tobacco craving in light daily smokers. The authors suggest that alcohol or the expectation of alcohol functioned as a cue for smoking prior to subjects being able to discern pharmacological differences between alcohol and placebo beverages. In the latter post-beverage timepoints, Sayette et al. (2005) found that tobacco craving was greater following the alcohol beverage relative to the placebo beverage. In our relatively inexperienced smokers, the expectation of alcohol consumption also functioned as a cue for smoking during the ascending limb by increasing tobacco craving for positive reinforcement during the alcohol and placebo conditions. Following smoking, tobacco craving decreased during both the alcohol and placebo conditions but remained greater following alcohol consumption; supporting the Sayette et al. (2005) finding that the pharmacological effects of alcohol may augment continued desire to smoke. Other studies, which have not manipulated instructions concerning beverage content, have found that alcohol but not taste-masked placebo beverages increased tobacco craving (King and Epstein 2005; Epstein et al. 2007).
Following alcohol consumption, heavy-drinking experimental smokers smoked more cigarettes, which was due to a greater likelihood of choosing to smoke additional cigarettes. Subjects were most likely to choose to smoke additional cigarettes (58%) following alcohol consumption, relative to the placebo (42%) and mixer conditions (32%). While alcohol increased the amount smoked, there were no differences in how each cigarette was smoked (i.e., number of puffs, puff duration, puff volume, peak puff volume, and inter-puff interval). These findings are in contrast to those demonstrated by King et al. (2009). In their sample of young adult heavy-drinking nondependent smokers, men but not women increased puff count, volume, and duration following alcohol consumption. However, King et al. (2009) calculated topography measures based on the total amount smoked during the ad lib period, rather than separately for each cigarette. When using cumulative topography measures, amount smoked is merged with how each cigarette was smoked; thus, it is unknown whether alcohol altered per cigarette indices of smoking topography. Additionally, subjects in the King et al. (2009) study were older and smoked more frequently in greater quantities than the subjects in the current study, which also may account for the differences in the smoking topography findings.
One clear difference that was observed between the alcohol and placebo conditions concerned the negative subjective effects (i.e., nausea) associated with smoking. A possible explanation for the expectancy-related variation could lie in the nature of the effects. Significant positive self-reported effects (e.g., calm, satisfaction) could be considered to be more cognitive, while the negative effects (nausea) may be more visceral and interoceptive. When manipulating pharmacological and expectancy effects associated with alcohol, self-reported rather than physiological effects generally demonstrate stronger expectancy effects (see McKay and Schare 1999 for review). However, it should be noted that nausea ratings were generally low (<26 out of 100), suggesting that this finding may be of limited clinical significance.
It is known that individuals who initially experience cigarette smoking as a pleasurable rather than a negative experience are most likely to develop a pattern of daily smoking (Ríos-Bedoya et al. 2009). If alcohol reduces negative subjective experiences and the expectation of alcohol improves positive subjective experiences associated with tobacco smoking, it is possible that these factors may facilitate the transition to regular daily smoking. Many smokers stabilize their smoking patterns in early adulthood (Chassin et al. 2000), and the current findings support the notion that alcohol may play a role in this trajectory by increasing the reinforcing value of smoking. It is thought that alcohol potentiates the reinforcing effects of tobacco and vice versa (Rose et al. 2004; Shiffman and Balabanis 1995). Microdialysis studies with nicotine-naive rats have found that alcohol and nicotine additively increase extracellular dopamine release in the nucleus accumbens, providing a possible explanation for this relationship (Tizabi et al. 2002).
This study has several limitations. The present work included a single dose of alcohol (0.08 g/dL targeted BAL), less than what the participants reported that they generally drank in a drinking episode. Future research could incorporate higher and lower doses of alcohol to explore dose-dependent effects on reactivity to smoking, as well as explore the full blood alcohol curve as the current study focused on ascending limb effects only. Interview-based findings have indicated that inexperienced smokers tend to drink to binge levels prior to smoking during a drinking episode (consistent with a 0.08 g/dL blood alcohol level) and then to continue to smoke intermittently during alcohol consumption (Harrison et al. 2009). Future work could also manipulate the method of nicotine delivery to determine whether alcohol and the expectation of alcohol improve reactivity to smoking tobacco more generally or to nicotine specifically. Studies have demonstrated that nicotine increases alcohol consumption in non-nicotine-dependent male smokers (Acheson et al. 2006; Barrett et al. 2006) and decreases consumption in non-nicotine-dependent female smokers (Acheson et al. 2006). While we did not find any effects of gender on our primary outcomes, this study was not powered to examine gender differences. Also, our sample was primarily Caucasian, and results may be limited in their generalizability to other racial or ethnic groups. Finally, as outlined in the methods section and similar to other investigations (Acheson et al. 2006; Fillmore et al. 2009; Perkins et al. 2004), we altered the response format of some of our measures, which may change the psychometric properties of these scales.
In our sample, we attempted to recruit individuals who may develop into Chassin’s et al. (2000) “late-stable smokers”, which had infrequent smoking during high school but increased to daily smoking over the course of early adulthood. Due to alcohol consumption, our minimum age was 21. We hypothesize that our findings would also generalize to younger samples, but this would have to be examined with other methodological approaches (e.g., event sampling). While we did demonstrate that positive and negative subjective reactions to smoking were altered either by alcohol or the expectation of alcohol consumption, use of a CReSS smoking topography system may have reduced smoking enjoyment (Blank et al. 2009). Also, subjective reactivity to smoking a cigarette was measured immediately after smoking the single cigarette. While the instructions requested that subjects respond to how the cigarette made them feel, it is possible that subjects misattributed the effects of alcohol to this tobacco-related questionnaire. Ideally, this same item set should have been administered with regard to alcohol effects just prior to smoking. There were also issues with the alcohol blinding. While we did demonstrate significant expectancy effects, only 71% believed they had consumed alcohol in the placebo condition. Asking subjects to discern the amount of alcohol in a drink would have been a more sensitive estimation of subjects’ abilities to discriminate the high-alcohol from the placebo beverage, given that the placebo contained a small amount of alcohol. Also, examining lower doses of alcohol (e.g., 0.04 g/kg) which can be more effectively blinded would further help to disentangle expectancy and pharmacological effects.
This preliminary study was unique in examining the effects of alcohol and alcohol expectancy on smoking reactivity in young adults who still at an experimental stage of smoking. The findings demonstrated that alcohol expectancy rather than alcohol influenced positive subjective effects of smoking, whereas alcohol rather than alcohol expectancy increased ad lib smoking and decreased negative subjective effects of smoking in relatively inexperienced young adult smokers who are heavy drinkers. These results support and extend longitudinal findings documenting that alcohol use facilitates the development of tobacco dependence (Jackson et al. 2002). Considering the role of alcohol in the development of nicotine dependence should lead to more effective prevention and treatment options.
Acknowledgments
Supported by the Alcoholic Beverage Medical Research Foundation, T32-AA015496, CTSA-UL1RR024139. The authors have no financial relationships with the sponsoring organizations. This experiment complies with the current laws of the USA.
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology. 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11:896–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Pitts SC, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood in a Midwestern community sample: multiple trajectories and their psychosocial correlates. Health Psychol. 2000;19:223–231. [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dawson DA. US low-risk drinking guidelines: an examination of four alternatives. Alcohol Clin Exp Res. 2000;24:1820–1829. [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS. Another look at heavy episodic drinking and alcohol use disorders among college and noncollege youth. J Stud Alcohol. 2004;65:477–488. doi: 10.15288/jsa.2004.65.477. [DOI] [PubMed] [Google Scholar]
- Dierker L, Lloyd-Richardson E, Stolar M, Flay B, Tiffany S, Collins L, Bailey S, Nichter M, Nichter M, Clayton R, The Tobacco Etiology Research Network (TERN) The proximal association between smoking and alcohol use among first year college students. Drug Alcohol Depend. 2006;81:1–9. doi: 10.1016/j.drugalcdep.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology. 2007;190:321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Osling EW, Martin CA, Kelly TH. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug Alc Depend. 2009;100:91–99. doi: 10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders. American Psychiatric Press; Washington: 1997. [Google Scholar]
- Gilpin E, Cavin SW, Pierce JP. Adult smokers who do not smoking daily. Addiction. 1997;92:473–480. [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Harrison ELR, Desai RA, McKee SA. Nondaily smoking and alcohol use, hazardous drinking, and alcohol diagnoses among young adults: findings from the NESARC. Alcohol Clin Exp Res. 2008;32:2081–2087. doi: 10.1111/j.1530-0277.2008.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison ELR, Hinson R, McKee SA. Experimenting and daily smokers: episodic patterns of alcohol and cigarette use. Addict Behav. 2009;34:484–486. doi: 10.1016/j.addbeh.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassmiller KM, Warner KE, Mendez D, Levy DT, Romano E. Nondaily smokers: who are they? Am J Public Health. 2003;93:1321–1327. doi: 10.2105/ajph.93.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerstrom Test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hennrikus DJ, Jeffery RW, Lando HA. Occasional smoking in a Minnesota working population. Am J Public Health. 1996;86:1260–1266. doi: 10.2105/ajph.86.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Cooper ML, Wood PK. Adolescent alcohol and tobacco use: onset, persistence, and trajectories of use across two samples. Addiction. 2002;97:517–531. doi: 10.1046/j.1360-0443.2002.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Wood PK, Bucholz KK. Alcohol and tobacco use disorders in a general population: short-term and long-term associations from the St. Louis ECA Study. Drug Alcohol Depend. 2003;71:239–253. doi: 10.1016/s0376-8716(03)00136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM, Sher KJ, Schulenberg JE. Conjoint developmental trajectories of young adult alcohol and tobacco use. J Abnorm Psychol. 2005;114:612–626. doi: 10.1037/0021-843X.114.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. Monitoring the future: National survey results on drug use, volume II: college students and adults ages 19–40. National Institute on Drug Abuse; Bethesda: 2001. NIH Publication No 01-4925. [Google Scholar]
- King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcohol Clin Exp Res. 2005;29:547–552. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- King AC, McNamara P, Conrad M, Cao D. Alcohol-induced increases in smoking behavior for nicotinized and denicotinized cigarettes in men and women. Psychopharm. 2009;207:107–117. doi: 10.1007/s00213-009-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McKay D, Schare ML. The effects of alcohol and alcohol expectancies on subjective reports and physiological reactivity: a meta-analysis. Addict Behav. 1999;24:633–647. doi: 10.1016/s0306-4603(99)00021-0. [DOI] [PubMed] [Google Scholar]
- McKee SA, Rounsaville D, Petrelli P, Hinson R. Subjective effects of smoking while drinking among college students. Nicotine Tob Res. 2004;6:111–117. doi: 10.1080/14622200310001656939. [DOI] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacol. 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG. Smoking status is a clinical indicator for alcohol misuse in US adults. Arch Int Med. 2007;167:716–721. doi: 10.1001/archinte.167.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, O’Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacol. 2008;196:189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Zwebwn A, DiClemente CC, Rychtarik RG. In: Motivational enhancement therapy manual: a clinical research guide for therapists treating individuals with alcohol abuse and dependence. Mattson ME, editor. Vol. 2. 1992. NIAAA Project MaTCH Monograph Series. [Google Scholar]
- [Accessed August 25, 2008];NIAAA guidelines (National Advisory Council on Alcohol Abuse and Alcoholism’s Recommended guidelines on ethyl alcohol administration) 2005 http://www.niaaa.nih.gov/Resources/ResearchResources/job22.htm#dependent.
- Nichter M, Nichter M, Loyd-Richardson EE, Flaherty B, Carkoglu A, Taylor N. Gendered dimensions of smoking among college students. J Adolescent Res. 2006;21:215–243. [Google Scholar]
- Perkins KA, Lerman C, Keenan J, Fonte C, Coddington S. Rate of nicotine onset from nicotine replacement therapy and acute responses in smokers. Nicotine Tob Res. 2004;6:501–507. doi: 10.1080/14622200410001696547. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Lee JE, Wechsler H. US college students’ use of tobacco products: results of a national survey. JAMA. 2000;284:699–705. doi: 10.1001/jama.284.6.699. [DOI] [PubMed] [Google Scholar]
- Ríos-Bedoya CF, Pomerleau CS, Neuman RJ, Pomerleau OF. Using MIMIC models to examine the relationship between current smoking and early smoking expectancies. Nicotine Tob Res. 2009;11:1035–1041. doi: 10.1093/ntr/ntp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine Tob Res. 2004;6:133–144. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Asland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT). WHO collaborative project on early detection of persons with harmful alcohol consumption. II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR. The effect of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychol Addict Behav. 2005;19:263–270. doi: 10.1037/0893-164X.19.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit M. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psych. 1984;41:879–885. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Gotham HJ, Erickson DJ, Wood PK. A prospective, high-risk study of the relationship between tobacco dependence and alcohol use disorders. Alcohol Clin Exp Res. 1996;20:485–490. doi: 10.1111/j.1530-0277.1996.tb01079.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis M. Associations between alcohol and tobacco. Alcohol and Tobacco: From Basic Science to Clinical Practice. 1995;30:17–36. [Google Scholar]
- Shiffman S, Kirchner TR, Ferguson SG, Scharf DM. Patterns of intermittent smoking: an analysis using Ecological Momentary Assessment. Add Behav. 2009;34:514–519. doi: 10.1016/j.addbeh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TR, Sussman S, Dent CW, Burton D, Flay BR. Prospective correlates of exclusive or combined adolescent use of cigarettes and smokeless tobacco: a replication-extension. Addict Behav. 1995;20:517–524. doi: 10.1016/0306-4603(95)00004-v. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Techniques to assess alcohol consumption. Humana; New Jersey: 1993. [Google Scholar]
- Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- US Department of Health and Human Services . The health benefits of smoking cessation. A report of the Surgeon General. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Public Health Service; Rockville, MD: 1990. DHHS Pub. No CDC 90–8416. [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: updating the fundamentals. In: Crow KE, Batt RD, editors. Human metabolism of alcohol. I. CRC; Boca Raton: 1989. pp. 41–56. [Google Scholar]
- Wechsler H, Rigotti NA, Gledhill-Hoyt J, Lee H. Increased levels of cigarette use among college students: a cause for national concern. JAMA. 1998;280:1673–1678. doi: 10.1001/jama.280.19.1673. [DOI] [PubMed] [Google Scholar]
- Weitzman ER, Chen Y. The co-occurrence of smoking and drinking among young adults in college: National survey results from the United States. Drug Alcohol Depend. 2005;80:377–386. doi: 10.1016/j.drugalcdep.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Westman EC, Levin ED, Rose JD. Smoking while wearing the nicotine patch; is smoking satisfying or harmful. Clin Res. 1992;40:871A. [Google Scholar]
- White TL, Lott DC, de Wit H. Personality and the subjective effects of acute amphetamine in healthy volunteers. Neuropsychopharmacology. 2006;31:1064–1074. doi: 10.1038/sj.npp.1300939. [DOI] [PubMed] [Google Scholar]