Abstract
Estrogen inhibition of oocyte maturation (OM) and the role of GPER (formerly known as GPR30) were investigated in zebrafish. Estradiol-17β (E2) and G-1, a GPER-selective agonist, bound to zebrafish oocyte membranes suggesting the presence of GPER which was confirmed by immunocytochemistry using a specific GPER antibody. Incubation of follicle-enclosed oocytes with an aromatase inhibitor, ATD, and enzymatic and manual removal of the ovarian follicle cell layers significantly increased spontaneous OM which was partially reversed by co-treatment with either 100 nM E2 or G-1. Incubation of denuded oocytes with the GPER antibody blocked the inhibitory effects of estrogens on OM, whereas microinjection of estrogen receptor alpha (ERα) antisense oligonucleotides into the oocytes was ineffective. The results suggest that endogenous estrogens produced by the follicle cells inhibit or delay spontaneous maturation of zebrafish oocytes and that this estrogen action is mediated through GPER. Treatment with E2 and G-1 also attenuated the stimulatory effect of the teleost maturation-inducing steroid, 17,20β-dihyroxy-4-pregnen-3-one (DHP), on OM. Moreover, E2 and G-1 down-regulated the expression of membrane progestin receptor alpha (mPRα), the intermediary in DHP induction of OM. Conversely DHP treatment caused a > 50% decline in GPER mRNA levels. The results suggest that estrogens and GPER are critical components of the endocrine system controlling the onset of OM in zebrafish. A model is proposed for the dual control of the onset of oocyte maturation in teleosts by estrogens and progestins acting through GPER and mPRα, respectively, at different stages of oocyte development.
Keywords: G protein-coupled estrogen receptor 1, GPER, G protein-coupled receptor 30, GPR30, oocyte maturation, aromatase inhibitor, defolliculation, meiotic arrest, estrogens
Introduction
17β-estradiol (E2) plays a critical role in vertebrate physiology, especially in the control and regulation of reproduction. The classic mechanism by which estrogens exert their biological effects is through binding and activation of the nuclear estrogen receptors, ERα and ERβ, resulting in alterations in gene transcription and protein synthesis over a time scale of several hours to days (Tsai and O’Malley, 1994; Hewitt and Korach, 2002). In addition, there is now substantial evidence that estrogens and other steroids can also exert nonclassical, rapid actions that do not involve gene transcription (nongenomic) and are initiated at the cell surface through binding to membrane estrogen receptors (Revelli et al., 1998; Norman et al., 2004). However, until recently the identities of the membrane receptors mediating many of these nonclassical estrogen actions were unknown. Rapid activation of the second messengers cAMP and MAPkinase by estrogens in SKBR3 human breast cancer cells that lack ERα and ERβ but express an orphan G protein coupled receptor, GPR30, was observed by Filardo and coworkers which led them to propose that GPR30 may be involved in estrogen signaling (Filardo et al., 2000). We subsequently showed that both wild type and recombinant human GPR30 display the localization, E2 binding and E2 signaling characteristics of a membrane estrogen receptor, and that E2 binding to GPR30 causes activation of a stimulatory G protein (Gs), resulting in stimulation of adenylyl cyclase (AC) activity (Thomas et al., 2005). The characteristics of GPR30 as a cell membrane estrogen receptor were confirmed in the same year by another research group (Revankar et al., 2005), and a variety of second messengers activated by estrogens through GPR30 have been identified in different cell models (Revankar et al., 2005; Hsieh et al., 2007; Wang et al., 2009). On the basis of a growing body of evidence that it functions as a membrane estrogen receptor, GPR30 has recently been renamed G protein-coupled estrogen receptor, GPER. GPER is widely expressed in mammalian tissues including human and hamster gonads (Owman et al., 1996; Wang et al., 2007), suggesting that it may have an important regulatory role in gonadal physiology, but its precise reproductive function(s) in vertebrate gonads remains unclear.
Recently, we demonstrated that Atlantic croaker GPER also has the characteristics of a membrane estrogen receptor coupled to a stimulatory G protein and is expressed on the plasma membranes of croaker oocytes (Pang et al., 2008). In addition, we discovered that estrogens exert an inhibitory influence on croaker oocyte maturation and that this effect is likely mediated through GPER (Pang et al., 2008). Moreover, preliminary results indicate that estrogens act via a similar mechanism to control the onset of oocyte maturation in zebrafish, which suggests this estrogen action is widespread in teleost fishes (Pang and Thomas, 2009). These findings are potentially of broad significance because they provide the first evidence that GPER has an important role in maintaining meiotic arrest in vertebrates. In addition, these studies have addressed a previously unexplored function of estrogens in teleosts in the control of oocyte maturation. However, several critical aspects of this estrogen action on fish oocytes remain unclear, such as the role of endogenous estrogens in regulating the onset of oocyte maturation, the pattern of GPER expression during oocyte development, the oocyte stages that are sensitive to estrogen inhibition of oocyte maturation, whether nuclear estrogen receptors are involved in this process, and how estrogens and maturation-inducing steroids may act in a coordinated fashion to regulate the onset of oocyte maturation.
The aim of the present study was to address these issues using zebrafish as an experimental model. Full-grown zebrafish ovarian follicles are larger and less fragile than those in croaker, enabling the follicle cell layers to be removed without damaging the oocytes and allowing microinjection of antisense oligo nucleotides into oocytes. Full-grown teleost oocytes cannot be fertilized because the first meiotic division has not been completed and is arrested at the G2/M border of prophase 1. When the environmental conditions are appropriate for ovulation and spawning a surge in luteinizing hormone (LH) secretion triggers the resumption of meiosis and other events comprising OM that enable the oocytes to be fertilized, including germinal vesicle migration (GVM) to the animal pole, germinal vesicle break-down (GVBD, nucleus disappears), formation of a micropyle through which sperm can enter the oocyte to fertilize it, and cleavage and solubilization of yolk proteins resulting in clearing of the ooplasm which becomes translucent. A small batch of 50–100 full-grown oocytes undergo OM each day in zebrafish which makes it an excellent model for examining the hormonal control of this process. The results show that GPER is localized to the oocyte plasma membrane and confirm its role as an intermediary in estrogen regulation of oocyte maturation. The pattern of GPER expression varies throughout oocyte development and is consistent with a physiological function at the end of the reproductive cycle. Hormonal regulation of GPER and membrane progestin receptor alpha (mPRα) by estrogens and the maturation-inducing steroid in this species were also investigated. Evidence is presented for dual control of oocyte maturation by estrogens and progestins through regulation of the expression of the two receptors mediating their actions, GPER and mPRα, and by activating their signaling pathways.
Materials and Methods
Chemicals
[2, 4, 6, 7-3H]-Estradiol-17β (84 Ci/mmol) was purchased from Amersham Pharmacia Biotechnology (Piscataway, NJ). Non-radioactive steroids were purchased from Sigma-Aldrich (St. Louis, MO) or Steraloids Inc. (Wilton, NH). All other chemical reagents were purchased from Sigma-Aldrich unless otherwise stated. G-1 was purchased from EMD chemicals (San Diego, CA). ICI182780 was purchased from Tocris Biosceinces (Ellisville, MO).
Animals
Mature zebrafish (Danio rerio) were purchased from a commercial supplier and stocked in the ratio of 5 females to 1 male in flow-through aquaria (~20 L) maintained at 25–27°C with a 14-hr light:10-h dark photoperiod. The fish were fed twice a day with commercial tropical fish food and supplemented with live brine shrimp once or twice a week.
Zebrafish oocyte maturation in vitro bioassay
Zebrafish ovarian follicles were isolated and incubated as described earlier (Pang and Thomas, 2009). Gravid female zebrafish were deeply anesthetized with 0.01% tricaine methanesulfonate solution for 2 min and humanely sacrificed by severing the spinal cord and blood supply using procedures approved by the University of Texas at Austin Animal Care and Use Committee. The ovaries were washed several times in 60% Leibovitz L-15 medium (Sigma-Aldrich) and the individual ovarian follicles were carefully separated with the aid of fine scapel blades without damaging the follicle cell layers. The diameters of the follicles were measured with an ocular micrometer under a dissecting microscope and several groups of follicles with different diameter ranges of 400–450 (early-vitellogenic), 450–550 μm (late vitellogenic) and 550–650 μm (full-grown), were selected and pooled for several experiments, whereas only the full-grown larger follicles (diameter: 550–650 μm) were used for all the other studies. The same-size follicles were randomly distributed into the wells of a 24-well plate (30–40 follicles/1 ml medium/well, 4 wells/treatment) and treated with various estrogens, 17, 20β-DHP, or an aromatase inhibitor (1, 4, 6-antrostatriene-3,17-dione, ATD, Sigma-Aldrich) dissolved in ethanol. ATD was selected for pharmacological inhibition of aromatase activity in this study because it has previously been shown to be effective in completely blocking aromatase activity in zebrafish ovarian tissue (Hinfray et al., 2006). The ovarian follicles were incubated for 6–16 hr and scored at the end of the incubation period for germinal vesicle breakdown (GVBD), an easily identifiable marker for oocyte maturation. Other oocyte maturation experiments were conducted with denuded oocytes, produced by two different procedures described below. All experiments were repeated three or more times to confirm the results.
Removal of follicle cells from ovarian follicles
The follicle cells (thecal cell and granulosa cell layers) were removed from the ovarian follicles either manually or by enzymatic digestion. The follicular cell layers of individual ovarian follicles were carefully removed manually with the aid of fine-tip forceps (Fontax #5) under the dissection microscope without injuring the oocytes. For enzymatic removal of follicle cells, the intact follicles were incubated in 60% L-15 medium containing collagenase (100 μg/ml) for 1 hr at room temperature with mild agitation, and repeated (10–20x) gentle pipetting of the follicles through a narrow pipette (1mm diameter) during the incubation. The resulting denuded oocytes were washed 3 times with fresh 60% L-15 medium before being transferred to wells for the in vitro oocyte maturation bioassay. Removal of follicle layers was confirmed by staining the denuded oocytes with DAPI (1 μg/ml) and observing them under a fluorescent microscope.
Preparation of zebrafish ovarian cryosections
Fresh zebrafish ovaries were washed three times with 60% Leibovitz L-15 medium and then equilibrated with ice-cold PBS solution. The tissues were then embedded in a small plastic tray with TFM embedding medium (TBS, Durham, NC) at −20°C for 30 min. The tissue blocks were either wrapped with paraffin film to prevent them from dehydrating and stored at −80°C for future use, or immediately cryosectioned with a cryostat. Ovarian tissue blocks were cryosectioned (thickness: 10–15 micron) at −20°C in the cryostat chamber and the sections were distributed onto pre-cooled, gelatin-coated glass slides. The slides were then warmed to room temperature and the sections were air-dried. All ovarian sections were examined under a microscope and those with good morphology were selected for immunohistochemical staining.
Immunohistochemistry of GPER in zebrafish ovaries
The air-dried ovarian cryosections were fixed in 100% ethanol at 4°C for 1 hr, followed by a 30-min incubation at 37°C in PBS to remove any remaining alcohol. The ovarian sections were then rinsed with PBS twice and blocked with 2% BSA (IgG free) in PBS for 1 hr at 4°C. Polyclonal antibodies raised against croaker GPER peptide (sequence: RDKLRLFIKQKASWC, identical to corresponding region of zebrafish GPER, Pang et al., 2008), alone or neutralized with antigen peptide, were added to the blocking solution (1:1000) and incubated with the tissue sections on the slides at 4°C overnight. After 3 washes with PBS, the slides were incubated with Alexafluor 488 goat anti rabbit IgG secondary antibody (1:2000. Invitrogen, Carlsbad, CA) in 2% BSA for 1 hr at room temperature. DAPI (Invitrogen, ~0.1μg/1ml) staining was used for nuclear visualization followed by 3 washes in PBS. The sections were wet-mounted with the Antifade Embedding Reagent (Invitrogen) under a cover glass. All the sections were examined with Nikon Eclipse E600 fluorescent microscope and the images were taken and processed with the Nikon imaging system.
Preparation of zebrafish ovarian and oocyte plasma membranes
Ovaries from sexually mature female zebrafish were pooled and intact ovarian follicles were separated and washed with 60% L-15 medium. The follicles were carefully homogenized with a 2-ml hand-held glass homogenizer in HAED buffer (25 mM Hepes, 10 mM NaCl, 1 mM dithioerythritol, 1 mM EDTA, pH 7.6) containing protease inhibitors. The homogenized tissue suspension was transferred to 1.5-ml centrifuge tubes and centrifuged at 1000 × g for 7 min to separate the yolk, and pellet the cell debris and nuclear fractions. The supernatant was then transferred to a new tube and centrifuged again at 20,000 × g for 20 min to pellet the plasma membranes. The ovarian membrane pellet was resuspended in 100–200 μl ice-cold HAED buffer containing protease inhibitor cocktail (Pierce, Rockford, IL) at a concentration of 1 mg/ml protein. The membrane preparations were analyzed immediately or stored at −80°C for future analysis.
Oocyte plasma membranes were prepared from denuded oocytes. The separated ovarian follicles were filtered with a 400–450 μm mesh to remove the small follicles, and then incubated with 3 ml of 1× trypsin/EDTA solution (Invitrogen) at room temperature for 1 hr with gentle shaking. The tissue suspension was pipetted several times during the enzyme digestion with a glass pipette to detach the ovarian follicle cells from the oocytes. The incubated ovarian follicles were washed several times with fresh 60% L-15 medium and settled by gravity suspension. The supernatant containing the separated follicle cells was removed and replaced with fresh medium several times until the supernatant became clear. Successful removal of follicle cells was confirmed by DAPI staining and examination under a fluorescence microscope. The oocyte plasma membranes were extracted following the general ovarian membrane extraction procedures described above.
Ovarian membrane estrogen receptor assay
Estrogen binding to ovarian membranes was assayed in a single point competition assay following a general estrogen membrane receptor binding protocol described previously (Loomis and Thomas, 2000; Thomas et al., 2005; Pang et al., 2008). Approximately 300 μl ovarian membrane suspension (0.5 mg/ml membrane protein) containing 4 nM of [3H]-estradiol 17-β ([3H]-E2) was incubated with 500~1000-fold excess non-radiolabeled E2 or G-1 or vehicle (total E2 binding) for 30 min at 4°C. At the end of the incubation period the membrane suspensions were filtered through presoaked GF/B glass fiber filters (Whatman) to separate the bound from free [3H]-E2, followed by three washes with cold buffer (5 ml each wash). The radioactivity on the filters was measured with a liquid scintillation counter (Beckman LS6000SC).
RT-PCR
Total zebrafish oocyte RNA was reverse transcribed into cDNA at 50°C for 1 hr in a total volume of 10 μl reaction solution containing 1× First Strand Buffer (Invitrogen), 10 mM dithiothreitol, 0.5 mM dNTP, 0.5 μg oligo-dT, and 100 U SuperScript III (Invitrogen). PCR was performed with 0.5 μl cDNA as a template in a 20 μl reaction mixture diluted from 2×PCR master mix (Promega, Madison WI). The primers for zebrafish GPER were designed according to the zebrafish GPER sequence (GenBank accession No. XM_688551). The sequences for the primers were: sense, 5′CAT CGG CCT GTT TCT CTC AT; antisense, 5′GTA GCA CAG GCC GAT AAT. After an initial denaturation for 4 min at 94°C, the cycling reaction was performed with a Thermal Cycler (Eppendorf, Hauppauge, NY) with the profile of 30 sec at 94°C, 30 sec at 55°C, and 1 min at 72°C for 35 cycles, followed by a 10-min extension at 72°C. The PCR mix (10 μl) was electrophoresed on an agarose gel containing ethidium bromide. The PCR for β-actin (primers: sense, 5′ GAG CAG GAG ATG GGA ACC; antisense, GAT GGA GTT GAA GGT GGT CT) was performed as an internal control for the RT-PCR.
Quantitative real-time RT-PCR
Real-time PCR was performed on a Mastercycler ep realplex (Eppendorf), with one-step Brilliant® II SYBR® Green QPCR Master Mix (Stratagene, La Jolla, CA) (25 μl) containing 0.0625 μl of Stratascript RT/RNase block enzyme mixture, 100 nM of sense and antisense primers and 0.5 μg of total RNA template. The primers were designed according to zebrafish GPER and mPRα (GenBank accession No. AY149121.1) sequences. GPER primers were: sense, 5′GGC ACC GAG CAG TTC AAT; antisense, 5′TGG TCA TCC TCT CCC TGT G. mPRα primers were: sense, 5′GCC TCC CTC ATT ATT TTA GTC; antisense, 5′ CCA AGA AGG CTG AGG CTG. Zebrafish β-actin gene was chosen as the control reference gene, the primers were: sense, 5′ GAA CGA CCA ACC TAA ACT CTC; antisense, 5′ GAG GAG GGC AAA GTG GTA A. The PCR was set up with the cycle profile of 50°C for 30 min for reverse transcription, 94°C for 10 min, followed by 40 cycles of 30 sec at 94°C, 1 min at 55°C, and 30 sec at 68°C. The melting curve was plotted after the amplification cycles with the profile of 95°C for 15 sec, 55°C for 15 sec, then ramping up to 95°C in 20 min and maintaining at that temperature for 15 sec to verify the specificity of the PCR product. The efficiencies of amplification for the two genes were calculated by the equation EFF=10(−1/slope)-1. Standard curves using serially diluted template were run for GPER, mPRα and β-actin. The primers had sufficient amplification efficiency (>95%), and the QPCR results were considered comparable. The ΔCt value of each RNA sample was calculated according to the QRT-PCR raw data (Ct values) and the mRNA level of the samples were compared with 2− ΔCt values.
Western blot analysis
The membrane proteins from zebrafish ovarian follicles with different diameters were extracted following the protocols mentioned above. The membrane samples (approximately 15–20 μg membrane protein per lane) were mixed with 5× reducing Western blot sample mix (Pierce) and incubated at room temperature for 20 min. The membrane proteins were then separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (Bio-rad). The blotted membrane was washed twice with TBS buffer followed by a 1 hr blocking step in 5% nonfat milk in TBST (50 mM Tris, 100 mM NaCl, 0.1% Tween 20 (pH 7.4)). The blot was then incubated overnight at 4°C with the Atlantic croaker GPER antisera (1:2000) raised against a synthetic 15-mer peptide (RDKLRLFIKQKASWC, corresponding to a peptide sequence of the C-terminus of croaker GPER which is identical in zebrafish). The zebrafish mPRα protein was detected using a polyclonal antibody generated against a peptide sequence in the N-terminal region of spotted seatrout mPRα which has 84% amino acid sequence identity with the corresponding region of zebrafish mPRα and has been used to detect mPRα in zebrafish oocyte membranes previously (Hanna et al., 2006). The blots were then washed 3 times in PBS and incubated with the HRP (horseradish peroxidase) conjugated goat anti rabbit IgG antibody (Cell Signaling, Beverly, MA) for 1 hr at room temperature. The blots were washed 3 times in PBS and then treated with enhanced chemiluminescence reagent (Pierce) and exposed to x-ray film to visualize the specific protein bands. To confirm the specificity of the immunoreaction, the GPER antibody was pre-incubated with the peptide antigen (5μg/μl) overnight at 4°C prior to use in Western blot analysis.
cAMP Measurement
The production of cAMP in zebrafish oocyte membrane preparations was measured as described previously (Thomas et al., 2005). Briefly, zebrafish oocyte membrane samples (2 mg/ml membrane protein) were incubated with 20 nM E2 or DHP, alone and in combination, or 100 nM other estrogens in 100 μl of cAMP assay buffer (20 mM KCl, 12 mM MgCl2, 3 mM EDTA, 2 mM ATP, 0.2 mM DTT, 10 mM creatine phosphate, 10 μg creatine kinase, and 20 mM Hepes, pH 7.5) at room temperature for 15 min. At the end of the incubation period the reaction mixtures were boiled for 10 min to terminate the reaction and then they were centrifuged at 12,000 × g for 5 min. The supernatant was collected and analyzed with a cAMP EIA kit (Cayman Chemical, Ann Arbour, MI) following the manufacturer’s instructions. Changes in cAMP concentrations in the oocytes after removal of the follicle cells or treatment with an aromatase inhibitor were also measured. At the end of the treatment period oocytes were washed 2 times and either processed immediately for measurement of cellular cAMP concentration following manufacturer’s instructions, or stored at −80°C for later measurement.
Microinjection of oocytes
The microinjection method published previously (Pang et al., 2008), was followed with minor modifications. The morpholino antisense oligonucleotide to zebrafish ERα (GenBank accession No. AF349412.1) mRNA (5′-AGG AAG GTT CCT CCA GGG CTT CTC T-3′) and the non-targeting control oligonucleotide (Gene Tools, Philomath, OR) were dissolved in Danieau buffer (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0mM HEPES (pH 7.6) in ultra-pure water) as suggested by the manufacturer and 1 nl was injected into oocytes at the late vitellogenic stage at a final concentration of 10 μM. The oocytes were then incubated in 1 ml 60% Leibovitz L-15 medium containing vehicle or 20 nM E2 in a 24-well plate at 28°C for 16 hr. The oocytes were scored at the end of incubation for oocyte maturation, and the success in blocking ERα mRNA synthesis in the injected oocytes was confirmed by RT-PCR (primer sequences: sense, 5′-GAA GGA GAA GCA GTA ATG AA. Antisense, 5′-GAG TAT GAT GGC TTT GAG AC). It was not possible to measure zebrafish ERα protein expression following this treatment because none of the commercially available ERα antibodies recognize any amino acid sequence in the zebrafish ER protein.
Data analysis
All the experimental data presented are means ± SEM of at least three observations and all experiments were repeated three or more times with separate batches of oocytes or tissues. Significant differences between paired treatment groups were analyzed by Student’s t test and multiple treatment groups by one-way ANOVA and Tukey’s (Sigma Stat, SPSS, Chicago, IL).
Results
Developmental changes in GPER mRNA and protein expression in zebrafish oocytes
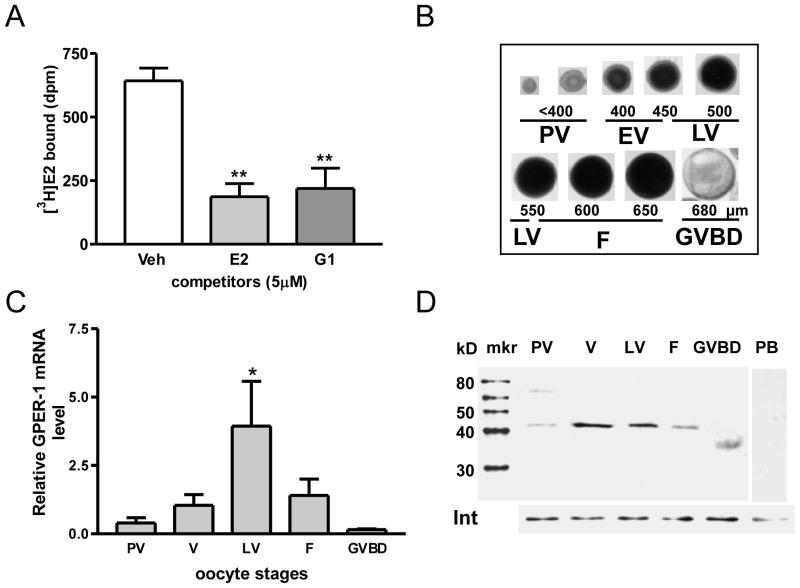
Both E2 and G-1 displaced [3H]-E2 binding to zebrafish ovarian membrane preparations in single point competition assays, indicating the presence of a specific E2 binding site on the plasma membrane that likely represents GPER because it recognizes G-1 (Fig. 1A). In order to examine the pattern of GPER expression during oocyte development, oocytes were separated on the basis of their diameters and appearance into the following developmental stages: pre-vitellogenic, early-vitellogenic, late-vitellogenic, full-grown, GVBD (Fig. 1B). Real-time RT-PCR showed that GPER mRNA increased during the early oocyte developmental stages to reach maximal levels in late-vitellogenic oocytes, over five-fold initial values (Fig. 1C). GPER mRNA expression had begun to decline by the time the oocytes were full-grown and was below initial developmental stage levels when they had completed GVBD (Fig. 1C). The GPER antibody detected single bands on Western blots of oocyte plasma membrane preparations (15 μg protein/lane) of the predicted molecular weight for zebrafish GPER, approximately 40KDa (Fig. 1D). Pre-incubation of the GPER antibody with the peptide antigen confirmed the specificity of the immunoreaction. Western blot analysis showed a similar developmental pattern of GPER protein expression as that observed for GPER mRNA. The GPER protein concentration was low in the plasma membranes of pre-vitellogenic stage oocytes and then reached its highest level in the early-vitellogenic and late vitellogenic stages. GPER protein concentrations had decreased in full-grown oocytes and became non-detectable in the oocytes that underwent GVBD (Fig 1D). The immunoreactive GPER band detected at the GVBD stage had a lower apparent molecular weight (~35kDa) which probably represents a partially degraded form of the GPER protein (Fig. 1D).
Fig. 1.
Estrogen binding to ovarian membranes and pattern of GPER mRNA and protein expression in zebrafish oocytes at different developmental stages. (A) Single-point competitive binding of [3H]-E2 to ovarian plasma membranes in the presence of 500-fold excess E2 and G-1 competitors. **, p<0.01 vs vehicle (Veh). (B) Appearance and diameters of zebrafish oocytes at different developmental stages. PV, pre-vitellogenic oocytes. EV, early-vitellogenic oocytes. LV, late-vitellogenic oocytes. F, full-grown oocytes. GVBD, oocytes undergoing germinal vesicle breakdown. (C) Quantitative RT-PCR measurement of GPER mRNA levels in the oocytes at different developmental stages. (D) Western blot analysis of GPER protein expression on the plasma membranes of oocytes at different development stages using a specific GPER antibody. mkr, protein size marker. Int, integrin loading control. PB, peptide block.
Localization GPER protein in zebrafish ovaries
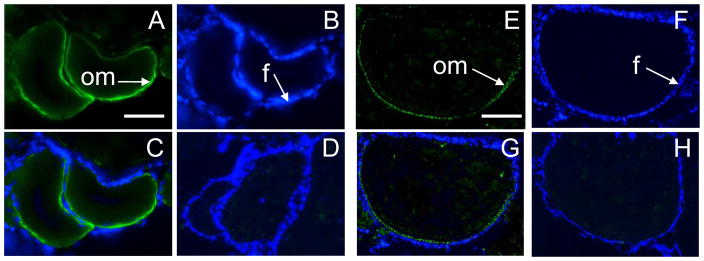
Strong green fluorescent staining was observed on the surface of both early-vitellogenic oocytes (Fig. 2A, C) and late-vitellogenic oocytes (Fig. 2E, G) in zebrafish ovarian tissue immunohistochemistry cryosections when probed with the specific GPER antibody, indicating the GPER protein is localized on or near the oocyte plasma membrane. No signal was detected after the antibodies had been neutralized by pre-incubating them with the antigen peptide (1–5 μg/μl antibody), confirming the specificity of the immunoreaction (Fig. 2D, H). In contrast, no green fluorescent staining was detected in the theca and granulosa follicle cell layers (Fig. 2C, G), visualized by DAPI staining of their nuclei (Fig. 2B, F), suggesting that GPER is not expressed in these cells. The finding that the GPER protein is expressed on the surface of zebrafish oocytes, but not on the ovarian follicular cells is in agreement with our previous RT-PCR data, which showed no GPER mRNA expression in zebrafish ovarian follicle cells (Pang and Thomas, 2009).
Fig. 2.
Localization of GPER in zebrafish ovarian tissue cryosection samples by immunohistochemistry using a specific GPER antibody. (A-D) Early-vitellogenic stage follicles. (E-H) Late-vitellogenic stage follicles. (A, E) Green fluorescent staining of zebrafish GPER on oocyte plasma membranes (om). (B, F) DAPI staining of nuclear DNA of follicular cells (f). (C, G) Merge. (D, H) Detection of GPER with the GPER antibody that had been neutralized with the antigen peptide and merged with DAPI stained images. Scale bar=100μm.
Effects of E2 and G-1 on GVBD in zebrafish oocytes
Until recently it was widely accepted that induction of OM in fish was largely dependent upon the stimulatory actions of maturation-inducing steroids such as DHP produced by the ovarian follicle in response to LH stimulation (Thomas et al., 2002). However, recent preliminary results indicate that removal of the ovarian follicle layers causes spontaneous OM in zebrafish in the absence of DHP stimulation (Pang and Thomas, 2008), suggesting the presence of both DHP-dependent and DHP-independent pathways regulating the onset of OM. Therefore, in the present study the effects of estrogen treatments on both DHP-dependent and spontaneous (DHP-independent) OM were investigated. Late-vitellogenic oocytes (diameter: 450–550 μm) and full-grown zebrafish oocytes (550–650 μm in diameter) were incubated in 60% L-15 medium with different concentrations of E2 for 6 hr. In the late-vitellogenic oocyte group, treatment with 10 and 100 nM E2 significantly inhibited spontaneous as well as DHP (17,20β-dihydroxy-4-pregnen-3-one, the maturation-inducing steroid in zebrafish) -induced oocyte GVBD; whereas in full-grown zebrafish oocytes, E2 treatment only significantly inhibited the DHP-induced GVBD, but had no effect on spontaneous GVBD (Fig. 3A). To test the sensitivity of late-vitellogenic oocytes to steroid hormones, we treated them with 5nM DHP in the presence or absence of 5nM E2 or G-1 for 6 hr. The results showed that DHP significantly increased oocyte GVBD to 70%; whereas co-culture of DHP with a low concentration of E2 (5 nM) or G-1 (5 nM) significantly decreased the GVBD compared to the DHP alone group (P<0.05) (Fig. 3B). The finding that the GPER agonist G-1 mimics the inhibitory actions of E2 is consistent with the conclusion of our previous studies that this estrogen action is mediated by GPER (Pang et al., 2008; Pang and Thomas, 2009). These results suggest that when GPER protein levels are the highest (LV stage) the oocytes are most sensitive to estrogen treatments and exogenous E2 inhibits both spontaneous as well as DHP-induced GVBD; whereas in the full-grown stage with lower GPER expression in the oocytes, exogenous E2 only causes inhibition of DHP-induced oocyte maturation.
Fig. 3.
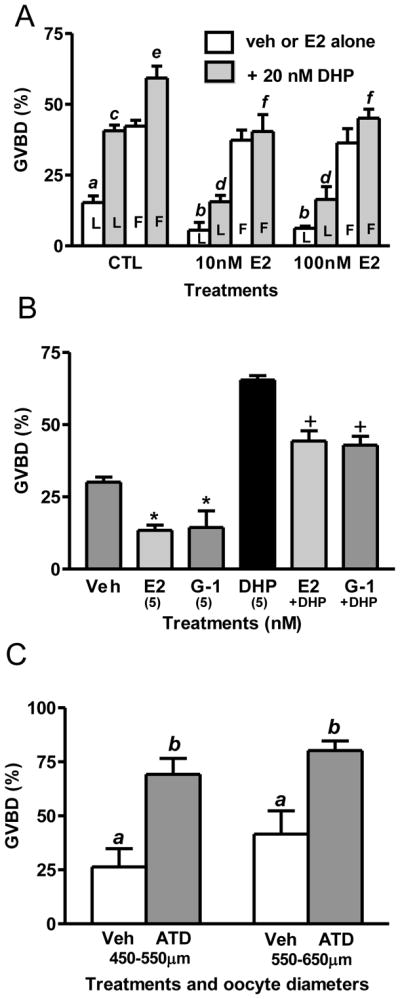
Effects of E2 and G-1 on spontaneous and DHP-induced oocyte GVBD. (A) Effects of 6 hr incubation with different doses of E2 with or without 20nM DHP on GVBD of late-vitellogenic (450–550 μm) and full-grown (550–650 μm) oocytes. ab, cd, ef, P<0.05. L, late-vitellogenic oocytes; F, full-grown oocytes. (B) Effects of 6 hr incubation with E2 and G-1 on spontaneous and DHP-induced GVBD of late-vitellogenic oocytes. E2, G-1: 5nM, *, +, P<0.05 compared to CTL and DHP, respectively. (C) Effects of 6 hr incubation with ATD (aromatase inhibitor) on spontaneous GVBD of oocytes with different diameters. ab, p<0.05. (N=3). Each treatment group was replicated 4 times (30–40 oocytes/replicate) in a single experiment. The combined results of three separate experiments with oocytes from different groups of donors are shown.
Our previous results showing that incubation of whole croaker or zebrafish ovarian follicles with the aromatase inhibitor ATD causes a marked increase in spontaneous maturation suggests that endogenous estrogens produced by the ovarian follicle cells exert a inhibitory influence on the resumption of meiotic maturation in these species (Pang et al., 2008; Pang and Thomas, 2009). In order to determine whether endogenous estrogens have inhibitory effects on GVBD of late-vitellogenic and full-grown zebrafish oocytes, both oocyte stages were co-incubated with ATD for 6 hr. The presence of the aromatase inhibitor in the incubation medium resulted in a significant increase in GVBD of both oocyte stages (Fig. 3C), indicating the presence of an inhibitory action of endogenous estrogens on the resumption of oocyte maturation throughout the later stages of oocyte development.
Effects of removal of ovarian follicle cells on GVBD in zebrafish oocytes
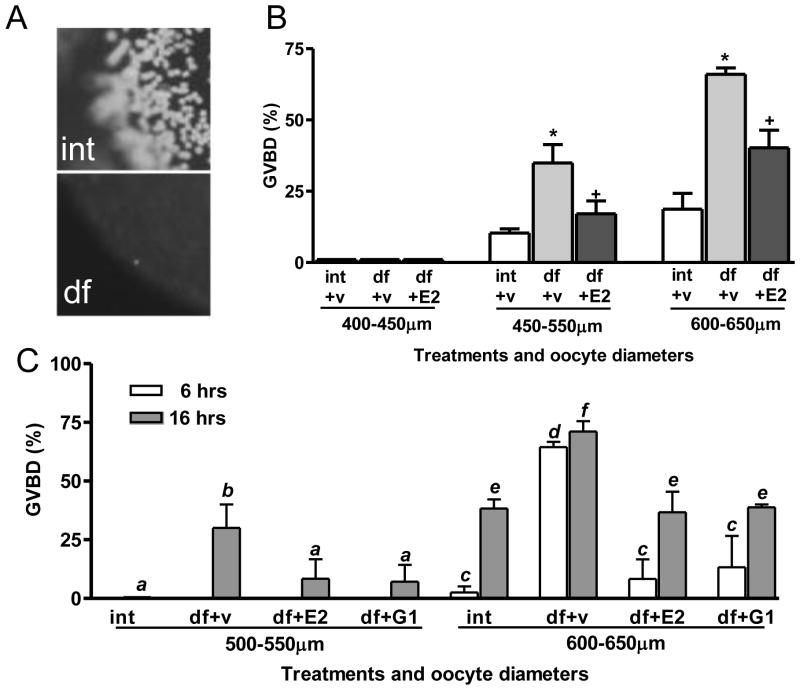
To establish that ovarian follicle cells can exert an inhibitory influence on oocyte maturation, the ovarian follicle layers were removed either enzymatically (collagenase treatment) or manually, and then the denuded oocytes were incubated in 60% L-15 medium in the GVBD bioassay. Ovarian follicles at various stages of development were treated with collagenase prior to the GVBD assay to determine during which stages the follicle cells have an inhibitory effect on oocyte maturation. Successful removal of ovarian follicular cell layers with collagenase treatment was confirmed by DAPI staining and observation under a fluorescent microscope (Fig. 4A). Removing the follicle layers of the largest full-grown oocytes, 600–650 μm in diameter, significantly increased the percent that underwent GVBD 6 hr later from approximately 20% to 70% (Fig. 4B), confirming an inhibitory influence of the ovarian follicle layer on oocyte maturation. Enzyme treatment of the 450–550 μm diameter (late-vitellogenic) ovarian follicles also resulted in a significant increase in GVBD but had no effect on oocytes smaller than 450 μm in diameter (early-vitellogenic, Fig. 4B). The addition of 100 nM E2 in the assay medium significantly attenuated the stimulatory effect of follicle cell removal by collagenase treatment on GVBD of the late-vitellogenic and full-grown oocytes (Fig. 4B).
Fig. 4.
Effects of enzymatic and manual removal of ovarian follicle cells (defolliculation) on oocyte maturation. (A) Intact ovarian follicles stained with DAPI, before and after collagenase treatment. int, intact follicle; df, defolliculated follicles. (B) Effects of E2 (100 nM) on the GVBD of intact (int) and enzymatically defolliculated (df) oocytes after 6 hr of incubation. (C) Effects of 100 nM E2 and G-1 on GVBD of manually defolliculated oocytes. ab, cd, ef: p<0.05. df, manually defolliculated oocytes. (N=3).
Manual removal of the ovarian follicle layers of later vitellogenic and full-grown oocytes resulted in a similar increase in GVBD to that observed after enzymatic treatment (Fig. 4C). No GVBD of intact follicle-enclosed oocytes (500–550 μm diameter) was observed after 6 and 16 hr incubation, whereas approximately 30% of the manually denuded oocytes had completed GVBD after 16 hr. The largest full-grown denuded oocytes, 600–650 μm in diameter, displayed a more rapid response to follicle cell removal, approximately 75% completing GVBD after 6 hr incubation, similar to the response to enzyme treatment (Fig. 4C). Treatment of denuded oocytes with 100 nM of E2 or G-1 significantly attenuated the increase in GVBD of both sizes of oocytes induced by follicle cell removal at the 6 hr and 16 hr time points, similar to the results obtained with oocytes denuded by enzymatic treatment (Fig. 4C).
The results from the studies with the aromatase inhibitor and with oocytes denuded by enzymatic digestion or manual removal of the follicle cells are consistent with an inhibitory influence of ovarian follicle cells on the resumption of oocyte maturation in zebrafish that is mediated, at least partly, through the follicular production of estrogens acting via GPER.
Effects of estrogens, an aromatase inhibitor and defolliculation of oocytes on cAMP levels
The results of a previous study with Atlantic croaker (Pang et al., 2008) show that E2 binding to recombinant GPER and to croaker oocyte plasma membranes activates a stimulatory G protein (Gs) resulting in increased cAMP production. Estrogen-induced increases in cAMP concentrations via GPER is a plausible mechanism by which estrogens could prevent the onset of oocyte maturation because it is well established that high levels of cAMP in fish oocytes, including croaker oocytes, are critical for maintaining meiotic arrest and that treatment with maturation inducing steroids causes a decrease in adenylyl cyclase activity (Jalabert and Finet, 1986; Pace and Thomas, 2005a). Therefore, to determine whether estrogens may be acting by the same mechanism to inhibit maturation of zebrafish oocytes, the effects of estrogens, GPER-specific agonists and DHP on cAMP accumulation, by zebrafish oocyte membranes were investigated. Treatment of oocyte membranes with 20 nM DHP caused a significant decrease in cAMP production compared to controls, whereas treatment with 20 nM E2 resulted in a significant increase in cAMP production (Fig. 5A). Similarly, treatment with the GPER agonists and nuclear ER antiestrogens tamoxifen (partial antagonist) and ICI182,780 (full antagonist) alone at a concentration of 100 nM also increased cAMP levels, consistent with previous reports of their GPER estrogen agonist activities on cAMP production in croaker ovaries and in HEK-293 cells transfected with human GPER (Pang et al., 2008; Thomas et al., 2005). Moreover, the addition of 20 nM E2 in combination with 20 nM DHP significantly increased membrane production of cAMP compared to that produced in response to DHP alone (Fig. 5A). In contrast, blocking endogenous estrogen production by treatment with an aromatase inhibitor or by removal of the follicle layers caused significant decreases in oocyte cAMP concentrations (Fig. 5B, C). All these results are consistent with a critical role for endogenous estrogens in maintaining oocyte meiotic arrest during the later stages of oocyte development in zebrafish through a GPER/Gs/adenylyl cyclase-dependent pathway.
Fig. 5.
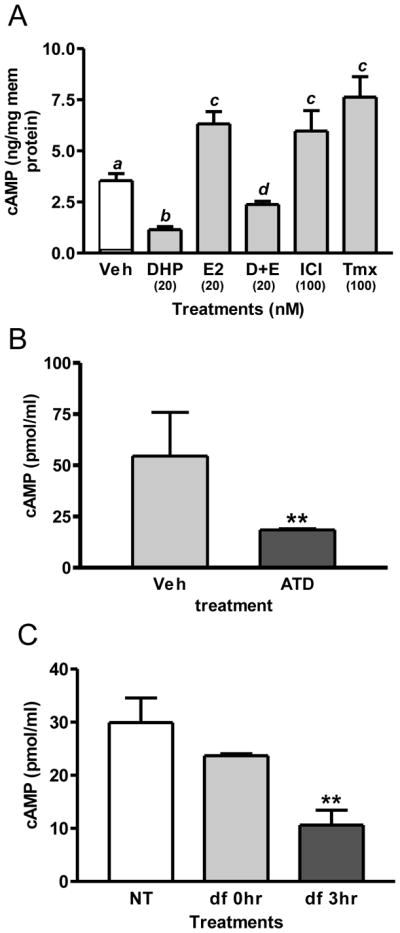
Effects of estrogen and aromatase inhibitor treatments and defolliculation of oocytes on cAMP levels. (A) Effects of treatment with estrogens and DHP on the production of cAMP by oocyte membranes. D+E, 20 nM DHP+E2. ICI, ICI182,780. Tmx, tamoxifen. ab, cd, ac: P<0.05. (B) Effects of 3 hr treatment with an aromatase inhibitor (ATD, 10μg/ml) on cAMP levels. **, P<0.01 compared to vehicle (Veh) control. (C) Effects of defolliculation (df) (collagenase, 100μg/ml, incubated for 30min) on cAMP levels after 3hr incubation. **, P<0.01 compared to no treatment control (NT) and 0 hr (df 0h). (N=3).
Identity of the estrogen receptor mediating estrogen regulation of oocyte maturation in zebrafish
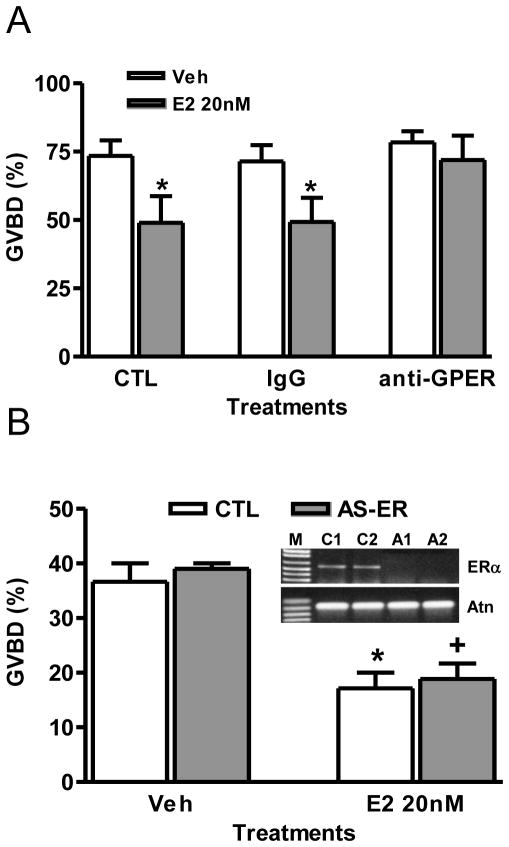
Both the present results with the GPER-specific agonist, G-1, and the results of earlier microinjection experiments with GPER antisense oligonucleotides (Pang et al., 2008), suggest GPER is involved in regulating the onset of oocyte maturation in zebrafish. To confirm this proposed role of GPER, the effects of an alternative approach to prevent GPER-dependent estrogen signaling, by incubating the denuded oocytes with a specific GPER antibody, on the inhibitory effects of estrogens were investigated in the GVBD bioassay. Incubation of collagenase-treated denuded oocytes with the specific GPER -polyclonal antibody (1:300) significantly attenuated the inhibitory effect of E2 on oocyte maturation, whereas incubation with control rabbit IgG was ineffective (Fig. 6A). Since the nuclear estrogen receptor alpha (ERα) is also expressed in zebrafish oocytes (see supplementary), we also examined the effects of microinjection of ERα antisense morpholino oligos into zebrafish oocytes on the inhibitory action of E2. Injection of ERα antisense oligos into oocytes markedly decreased ERα mRNA concentrations but did not alter the inhibitory effects of E2 (20 nM) on oocyte maturation, suggesting that ERα is not involved in this process (Fig. 6B). It is concluded from these studies that GPER is the sole estrogen receptor mediating the inhibitory actions of endogenous estrogens on GVBD of zebrafish oocytes.
Fig. 6.
Involvement of GPER in zebrafish oocyte maturation. (A) Effect of E2 on GVBD of defolliculated full-grown oocytes treated with IgG and fish GPER antibody (1:300). *, p<0.05 compared to Veh. (B) Effect of E2 (20 nM) on GVBD of LV oocytes (~550 μm) that had been micro-injected with control (CTL) and ERα antisense morpholino oligos. AS-ER, antisense ERα oligos. *, +, p<0.05 compared to vehicle (Veh). Gel image, RT-PCR detection of ERα mRNA levels in the micro-injected oocytes. M, DNA size marker. C1 and C2, control 1 and 2. A1 and A2, antisense oligos. Atn, actin control. (N=3).
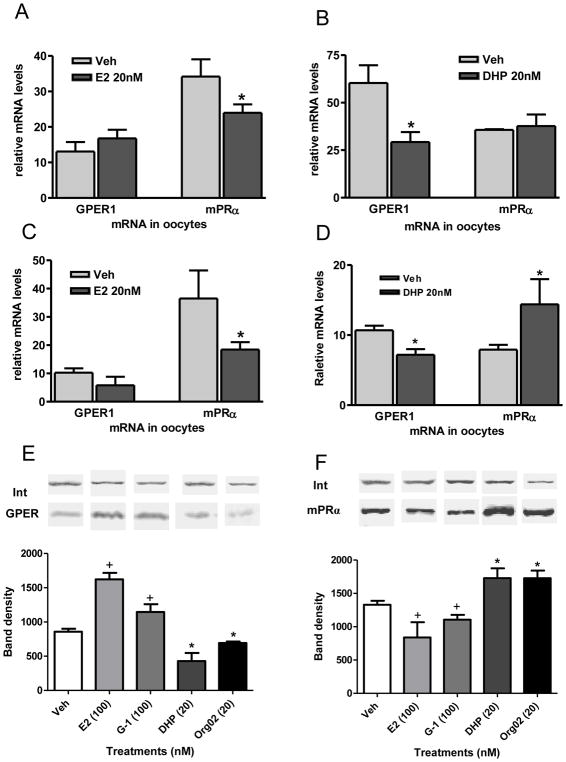
Estrogen and progestin regulation of GPER and membrane progestin receptor alpha concentrations in zebrafish oocytes
It is well established that the resumption of meiosis in fish oocytes is also regulated by progestin hormones that act through an intermediary, membrane progestin receptor alpha (mPRα), to induce oocyte maturation (Zhu et al., 2003; Tokumoto et al., 2006; Tubbs et al., 2009). In addition to their roles as ligands for GPER and mPRα, estrogens and progestins could potentially also regulate the onset of oocyte maturation by altering the expression of these two receptors. To test this possibility, the effects of 3 hr and 6 hr treatments in vitro with low physiological levels (20 nM) of E2 and DHP on GPER and mPRα mRNA levels in zebrafish oocytes were investigated. Treatment with E2 for 3 hr and 6 hr significantly down-regulated mPRα mRNA levels which was accompanied with decreased amounts of the mPRα protein on the oocyte plasma membranes after 4 hr treatment (Fig. 7A, C, F). Interestingly, the inhibitory effect of E2 on mPRα protein expression was mimicked by the GPER agonist, G-1 (Fig. 7F). Although GPER mRNA levels were unaffected at these time points after 20 nM E2 treatment, membrane concentrations of the GPER protein were significantly upregulated by 100 nM E2 and G-1 after 4 hr (Fig. 7E). Treatment with DHP had an opposite effect on mPRα mRNA concentrations, significantly increasing them after 6 hr incubation (Fig. 7D). Moreover, mPRα protein concentrations were elevated after treatment with DHP, an effect mimicked by the mPRα-selective agonist, Org 02-0 (Kelder et al., 2010, Fig. 7F). These progestin treatments also altered GPER mRNA and protein concentrations, causing significant decreases in mRNA after 3 hr and 6 hr incubations with DHP (Fig. 7B, D) and decreased protein levels after 4 hr treatments with DHP and Org 02-0 (Fig. 7E). Taken together the results suggest that estrogens may also exert an inhibitory influence on oocyte maturation and progestins a stimulatory one by altering the expression of GPER and mPRα. The results show that elevated estrogen concentrations upregulate GPER protein levels in oocyte membranes and downregulate mPRα levels, thereby promoting the maintenance of inhibitory pathways controlling the onset of oocyte maturation and suppressing the development of a stimulatory one. Conversely when progestin concentrations are elevated at the onset of oocyte maturation GPER expression is downregulated and mPRα upregulated, which would tend to suppress the inhibitory pathway and the stimulatory one, resulting in the resumption of meiotic maturation.
Fig. 7.
QRT-PCR detection of GPER1 and mPRα mRNA levels in full-grown oocytes which had been treated with 20nM E2 and DHP for 3 hr (A, B) and 6 hr (C, D). (E, F) Detection of cell membrane expression of GPER (E) and mPRα (F) proteins after 4 hr hormonal treatment by western blot analysis. Int, integrin loading control. *, +, P<0.05 compared to vehicle control. (N=3).
Discussion
The results of this study provide the first clear evidence in vertebrates that ovarian follicle cells exert a powerful inhibitory influence on spontaneous meiotic maturation of full-grown oocytes that is mediated, at least partly, through the production of estrogens. Removal of the ovarian follicle layers, either manually or by enzymatic digestion, resulted in significant increases in spontaneous maturation of both late vitellogenic and full-grown zebrafish oocytes that could be partially reversed by treatment with estrogens. This estrogen action appears to be nongenomic because it is not inhibited by treatment with actinomycin D (Peyton and Thomas, unpubl. obs). The finding that treatment of intact ovarian follicles with aromatase inhibitors caused a similar increase in spontaneous maturation of zebrafish oocytes further supports an inhibitory role of endogenous estrogens in regulating the resumption of oocyte maturation in this cyprinid fish species. We have recently demonstrated that treatment with aromatase inhibitors also increases spontaneous oocyte maturation in a distantly related perciform fish, Atlantic croaker, that can be reversed with estrogens (Pang et al., 2008). Interestingly, it was shown over 25 years ago that estrogens can inhibit hormone-induced oocyte maturation in salmonids (Jalabert, 1975; Jalabert and Fostier, 1984), but there are no reports of follow up studies to further explore these findings. Increased oocyte maturation has also been observed with Fundulus heteroclitus oocytes after removal of the ovarian follicular cell layers, suggesting the follicular layer also exerts an inhibitory influence in this species (Petrino et al., 2007). Taken together these results suggest inhibitory actions of estrogens on oocyte maturation are widespread amongst teleosts. Although a strong inhibitory influence of the ovarian follicle cell layers on the resumption of meiosis has also been demonstrated in several mammalian species, the identity of the inhibitory substance(s) produced by the follicle layers in mammals remains unclear (Sela-Abramovich et al., 2006; Motola et al., 2007).
Several lines of evidence from the present study indicate that these inhibitory actions of estrogens on zebrafish oocytes are mediated through the novel membrane estrogen receptor GPER. GPER is localized on the plasma membrane of zebrafish oocytes but is not expressed in ovarian follicle cells. The concentrations of both GPER mRNA and protein increase in zebrafish oocytes during oogenesis reaching maximal levels in late vitellogenic oocytes, consistent with an important inhibitory function in late stage oocytes capable of undergoing meiotic maturation. The finding that GPER transcript and protein levels begin to decrease in the largest (full-grown) oocytes that will undergo maturation within a few hours and are barely detectable by the time the oocytes have completed germinal vesicle breakdown also supports an inhibitory role of GPER in regulating the onset of oocyte maturation. Atlantic croaker oocytes also express GPER on the cell surface and have a similar developmental pattern of receptor expression (Pang et al., 2008), which suggests that GPER has the same physiological role in regulating oocyte maturation in other teleosts. Specific estrogen binding characteristic of GPER can be detected on both zebrafish and croaker oocyte membranes using the GPER-specific agonist, G-1. Moreover, G-1 and other GPER agonists increase cAMP production by oocyte membranes of both species, consistent with previous studies showing estrogen binding to GPER increases adenylyl cyclase activity through activation of a stimulatory G protein (Gs) (Thomas et al., 2005; Pang et al., 2008). Importantly, the results with zebrafish also show that G-1 acts as an estrogen agonist in the in vitro oocyte maturation bioassays, inhibiting maturation of both intact ovarian follicles and denuded oocytes. Furthermore, we have obtained clear evidence that the inhibitory actions of estrogens on maturation of zebrafish oocytes are blocked by preincubating them with the GPER antibody (Fig. 6A), or by down-regulating GPER expression by microinjecting the oocytes with GPER antisense oligonucleotides (Pang et al., 2008). In contrast, blocking synthesis of ER alpha (ER), which is also present in zebrafish oocytes, by microinjection with ER antisense oligonucleotides, did not abrogate the inhibitory effects of estradiol on oocyte maturation. Moreover, the ERalpha- and ERbeta-selective agonists, PPT (propylpyrazole triol) and DPN (diarylpropionitrile), were ineffective in inhibiting maturation of zebrafish oocytes (Thomas et al., in press). Collectively, these results suggest that the endogenous estrogens produced by ovarian follicle cells inhibit meiotic maturation of teleosts oocytes exclusively through GPER.
An action of endogenous estrogens to maintain high levels of cAMP in teleost oocytes through activation of the GPER/Gs/AC/cAMP signal transduction pathway is a plausible mechanism by which they could prevent oocyte maturation. Progestin induction of oocyte maturation in teleosts and amphibians is associated with a rapid decrease in oocyte cAMP concentrations (Sadler and Maller, 1981; Jalabert and Finet, 1986; DeManno and Goetz, 1987a; Pace and Thomas, 2005a), whereas drugs that increase cAMP levels inhibit oocyte maturation in response to progestin stimulation (Jalabert et al., 1976; DeManno and Goetz, 1987b). Similarly, our results with zebrafish and croaker show that estrogens attenuate both the progestin-induced decrease in cAMP levels and the induction of oocyte maturation. In addition, the inhibition of spontaneous oocyte maturation by exogenous estrogen treatments in zebrafish is associated with increases in AC activity, whereas eliminating endogenous estrogens by removing the ovarian follicle layers or by treatment with an aromatase inhibitor results in decreases in the production of cAMP or intraoocyte cAMP concentrations and increased oocyte maturation. All these results support an involvement of elevated cAMP levels and AC activity in the maintenance of oocyte meiotic arrest in teleosts by endogenous estrogens. However, it is likely that other signaling pathways are also involved in maintaining oocyte arrest. For example, our preliminary results (Peyton and Thomas, unpubl. obs) with inhibitors of membrane-associated matrix metalloproteinases (MMPs) and epidermal growth factor receptor (EGFR, ERB B1) and ERK1/2 activation suggest that βγ signaling through the stimulatory G protein also contributes to estrogen inhibition of zebrafish oocyte maturation. This signaling pathway is also probably mediated through GPER, because estrogens have previously been shown to activate MMPs resulting in release of EGF ligands and transactivation EGFR leading to activation of MAP kinase through GPER in breast cancer cells (Filardo et al., 2000; Filardo et al., 2002; Filardo and Thomas, 2005). Previously, several orphan GPCRs have also been implicated in inhibiting meiotic maturation of vertebrate oocytes through Gs. For example, there is clear evidence that an orphan GPCR, GPR3, is involved in maintaining oocyte meiotic arrest in zebrafish oocytes through activation of Gs (Kalinowski et al., 2004). Another orphan GPCR, GPR12, is also involved in preventing maturation of rodent oocytes through Gs, but it is not known whether it performs a similar function in zebrafish (Hinckley et al., 2005). In addition, a member of the TGF-β superfamily, BMP15, has been shown to inhibit hCG- and DHP-induced OM in zebrafish, possibly through down-regulation of mPRβ mRNA expression in the oocytes (Tan et al., 2009). Although these studies suggest that the maintenance of oocyte meiotic arrest in vertebrates is under complex regulatory control involving multiple receptors and signaling pathways, the interactions of GPER with other components of this system remain unclear.
An interesting finding of the present study is that GPER is also expressed in early vitellogenic oocytes that are not competent to undergo either spontaneous or progestin-induced maturation. Although we did not investigate GPER-dependent estrogen signaling in these early vitellogenic oocytes, it is likely that GPER functions as an estrogen receptor at this oocyte stage because estrogens are synthesized by the ovarian follicles of fish during vitellogenic oocyte growth (Wallace, 1985; Sakai et al., 1987). The coincidence of increased GPER expression in growing oocytes and ovarian estrogen production with the entire period when meiosis is arrested at prophase 1 suggests that the receptor also may be important for maintaining meiotic arrest of earlier stage maturationally-incompetent oocytes. However additional experiments will be required to test this hypothesis.
A detailed knowledge of the duration and mechanisms of inhibitory estrogen actions on oocyte maturation as well as possible interactions with the stimulatory hormonal mechanism in teleosts is critical for an overall understanding of the hormonal control of oocyte maturation in this vertebrate group. Oocyte maturation in fish and amphibians is induced by progestins produced by the ovarian follicle cells in response to the preovulatory increase in gonadotropin secretion (Thomas, 1994; Nagahama et al., 1995; Maller, 1998; Thomas et al., 2002). In teleosts the LH surge causes a switch in the steroidogenic pathway in ovarian follicles from the synthesis of estrogens and androgens to the production of the progestins 17,20β-dihydroxy-4-pregnen-3-one (DHP) and 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S), by altering the expression of steroidogenic enzymes involved in their synthesis (Nagahama, 1987; Trant and Thomas, 1989). The progestin in turn upregulates expression of a novel 7-transmembrane receptor, membrane progestin receptor alpha (mPRα) on fish oocyte plasma membranes (Zhu et al., 2003) and binds to it, causing activation of an inhibitory G protein and a decrease in cAMP levels through a decline in AC activity and an increase in phosphodiesterase activity, which in turn triggers the resumption of meiosis through inhibition of PKA activity (Pace and Thomas, 2005a,b). One of the most interesting findings of our studies with zebrafish and croaker is that estrogens acting through GPER inhibit or delay OM of full-grown oocytes in response to progestins, indicating this inhibitory mechanism is still present during an early stage of progestin-induced oocyte maturation. Moreover, the aromatase inhibitor experiments demonstrate that the mechanism is physiologically important during this stage, because endogenous estrogens maintain a strong inhibitory influence on full-grown maturationally-competent oocytes. We propose that maintenance of this inhibitory estrogen signal helps synchronize the maturation of the next large cohort of full-grown oocytes that will soon be ovulated and spawned. This is achieved by preventing precocious maturation of any of the oocytes within the cohort until the stimulatory progestin signal is strong enough to override this inhibition to induce maturation of all the competent oocytes. Synchronization of oocyte maturation and ovulation in teleosts is essential for the simultaneous release of the large number fully mature fertilizeable eggs in a single spawn (102–107 eggs) that is necessary for the reproductive success of teleosts.
The discovery that physiological concentrations of estrogens down-regulate mPRα mRNA and protein expression in zebrafish oocytes suggests that, in addition to inhibiting oocyte maturation by maintaining high intracellular levels of cAMP, endogenous estrogens also prevent oocyte maturation by inhibiting the progestin stimulatory oocyte maturation pathway through mPRα. Previous studies have shown that the development of oocyte maturational competence (i.e. the ability to undergo maturation in response to progestins) in croaker and other teleost species is dependent upon gonadotropin and progestin upregulation of mPRα protein concentrations on the oocyte surface (Thomas et al., 2001; Thomas et al., 2002; Zhu et al., 2003). Furthermore, the experiments with G-1 show that estrogen down-regulation of mPRα expression is mediated through GPER. On the other hand, estrogens acting through GPER were found to upregulate GPER protein expression in full-grown zebrafish oocytes, suggesting there is a third mechanism through which estrogens promote the inhibition of oocyte maturation. Interestingly, treatment of zebrafish oocytes with the progestin, DHP, in addition to upregulating mPRα concentrations, caused a decline in GPER mRNA and protein expression. Thus, there is reciprocal down-regulation of mPRα and GPER in full-grown zebrafish oocytes by estrogens and progestins, respectively, that is probably mediated through these receptors. To our knowledge, this is the first indication in any vertebrate model for direct interactions between two nonclassical steroid membrane receptors. Additional studies will be required, however, to determine the mechanisms and signaling pathways involved with these receptor interactions. The fact that the interactions involve the two novel 7-transmembrane receptors, GPER and mPRα, is intriguing because it is reminiscent of the multiple interactions between nuclear ERs and PRs in the regulation of vertebrate reproductive cycles (Selcer and Leavitt, 1988; Kirkland et al., 1992; Katzenellenbogen, 2000).
Conventional thinking would suggest that estrogens have no role in the regulation of oocyte maturation after the LH surge in teleosts because, as discussed previously, LH down-regulates the steroidogenic enzymes required for estrogen synthesis, upregulates those required to synthesize progestins, and the steroid precursors are shunted through alternative pathways to produce progestins. However, these changes occur over several hours because they involve changes in the rates of transcription and translation of steroidogenic enzyme genes. For example, production of 20β-S by croaker ovarian follicles in response to gonadotropin was only detected after priming with gonadotropin 24 hr previously (Patiño and Thomas, 1990). On the other hand, gonadotropin stimulation of steroid production through activation of StAR (steropidogenic acute regulatory protein) and involving steroidogenic enzymes already present in the ovarian follicle occurs over a shorter time scale, so that E2 and testosterone is synthesized during the initial 3–6 hr period of gonadotropin treatment in croaker in the absence of prior gonadotropin priming (Patiño and Thomas, 1990). Therefore, one would expect to find transient increases in estrogen production at the beginning of the periovulatory period in some teleost species prior to the increase in progestin synthesis, especially species that maintain significant estrogen production in post-vitellogenic follicles. In support of this increased production of E2 and T was observed by croaker ovarian follicles in response to gonadotropin treatment at the onset of oocyte maturation (Trant and Thomas, 1989). In addition, peaks in plasma E2 and T concentrations have been observed after gonadotropin treatment in white perch and striped bass that were at the germinal vesicle migration stage of oocyte maturation followed by declines in circulating E2 levels in later maturation stages (King et al., 1994; King et al., 1995). Similar increases in plasma E2 levels were observed in wild-caught white perch white bass, gilthead seabream and sea bass around the time of spawning (Kadmon et al., 1985; Prat et al., 1990; Berlinsky et al., 1995; Jackson and Sullivan, 1995). Similarly, in medaka ovarian production of E2 is increased 24–16 hr before spawning, around the time gonadotropin secretion increases (21–14 hr prior to spawning) and the oocytes become maturationally competent (Sakai et al., 1988). Collectively, these results indicate that there is a transitory peak in E2 production at the beginning of LH induction of oocyte maturation in at least some species of fish that maintains meiotic arrest during the initial phase of oocyte maturation until the stimulatory pathway mediated by progestins is strong enough to override this inhibitory mechanism.
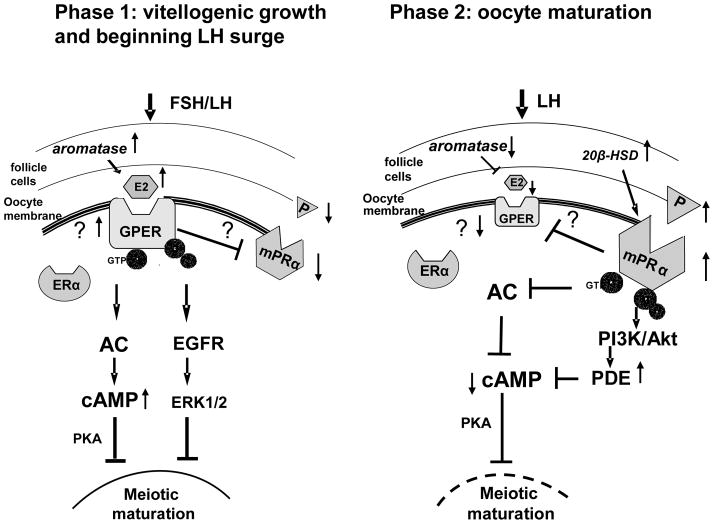
On the basis of these results, a model for the dual control of oocyte maturation in teleosts by estrogens and progestins during the two final phases of ovarian follicle and oocyte development is proposed (Fig. 8). During the first phase at the end of vitellogenic oocyte growth, and at the beginning of the LH surge in some species, the ovarian follicles produce large amounts of estrogens and minor quantities of progestins, resulting in activation of the inhibitory pathway regulating oocyte maturation, but not the stimulatory pathway. The estrogens act through GPER to activate a stimulatory G protein (Gs) resulting in stimulation of adenylyl cyclase activity and increases in cAMP production. The high levels of cAMP maintain meiotic arrest of fish oocytes, possibly through downstream signaling molecules such as protein kinase A. Preliminary evidence suggests that the βγ subunits of Gs also contribute to this inhibition through EGFR and ERK1/2. In addition, estrogens upregulate GPER expression to potentiate the inhibitory pathway and down-regulate mPRα expression to block the stimulatory one through activation of GPER via unknown pathways. As a result, the oocytes remain in meiotic arrest. In the second phase during oocyte maturation several hours later the steroidogenic pathway has switched to the production of progestins and estrogen production has declined. The increase in progestin concentrations causes upregulation of mPRα expression resulting in activation of the stimulatory pathway controlling oocyte maturation. At the same time progestins cause down-regulation of the GPER expression, which together with the reduction in estrogen levels, causes suppression of the inhibitory pathway. The results with the mPRα-specific agonist indicate that these effects are mediated through mPRα but the pathways have not been investigated. Previous studies have shown that progestin binding to mPRα results in activation of an inhibitory G protein (Gi) and decreases in adenylyl cyclase activity and cAMP levels leading to inhibition of protein kinase A, thereby releasing the oocyte from meiotic arrest and allowing oocyte maturation to proceed (Yoshikuni and Nagahama, 1994; Thomas et al., 2002; Pace and Thomas, 2005b). In croaker the βγ subunits of Gi are also involved in mediating the stimulation of oocyte maturation through activation of a PI3K/Akt-dependent pathway, resulting in upregulation of phosphodiesterase activity and increased degradation of cAMP (Pace and Thomas, 2005a). Additional experiments are currently underway to further test this model.
Fig. 8.
Proposed model of the dual control of the onset of oocyte maturation in teleosts by estrogens and progestins acting through GPER and mPRα, respectively, at different phases of oocyte development; phase 1: vitellogenesis and beginning of preovulatory LH surge, phase 2: oocyte maturation. ?, indicates the post-receptor signaling pathways involved in hormonal regulation of GPER and mPRα are currently unknown. Details of model are described in the “Discussion”.
The discovery of a powerful inhibitory action of endogenous estrogens on oocyte maturation in both a cyprinid (zebrafish) and perciform (croaker) fish, representing the two largest groups of teleost fishes, could have broad implications for finfish aquaculture and environmental toxicology. GPER is an attractive new target for developing novel hormonal treatments to induce oocyte maturation in candidate aquaculture finfish species that have been difficult to spawn in captivity. GPER is also a likely target on fish oocytes for interference by xenobiotic estrogenic compounds (xenoestrogens), because we have previously shown that xenoestrogens such as bisphenol A, nonylphenol and Kepone bind to human GPER with higher affinities than they do the nuclear ERs and acts as GPER agonists, stimulating cAMP production (Thomas and Dong, 2006). Our preliminary results showing that low concentrations of bisphenol A inhibit spontaneous maturation of both croaker and zebrafish oocytes are consistent with the existence in fish of this novel mechanism of endocrine disruption mediated through GPER.
Finally, studies comparing the functional characteristics of GPER in fishes and mammals have proven to be valuable for determining fundamental physiological functions of this G-protein coupled receptor that have been conserved during 200 million years of vertebrate evolution and for addressing some of the current controversies regarding the functions of GPER in mammals (Thomas et al., in press). For example, the recent proposed role of a variant truncated form of ERα, ER-α36, in mediating the nongenomic actions of estrogens in human cells expressing GPER (Kang et al., 2010), would need to be demonstrated for a homologous truncated variant ER protein in a fish model before it can be claimed to be an universal mechanism. To date no mRNAs have been detected in zebrafish genome databases with significant sequence homology to human ER-α36 other than the full-length ERs (α, β1 and β2) that could be used to investigate this possibility.
Supplementary Material
Acknowledgments
This research was funded by National Institutes of Health grant ESO 12961 to P.T. The assistance of Susan Lawson with animal care is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berlinsky DL, Jackson LF, Smith TIJ, Sullivan CV. The annual reproductive cycle of the white bass, Morone chrysops. J World Aquaculture Society. 1995;26:252–260. [Google Scholar]
- DeManno DA, Goetz FW. The effects of forskolin, cAMP, and cyanoketone on steroid-induced meiotic maturation of yellow perch (Perca flavescens) oocytes in vitro. Gen Comp Endocrinol. 1987a;66:233–243. doi: 10.1016/0016-6480(87)90272-3. [DOI] [PubMed] [Google Scholar]
- DeManno DA, Goetz FW. Steroid-induced final maturation in brook trout (Salvelinus fontinalis) oocytes in vitro: the effects of forskolin and phosphodiesterase inhibitors. Biol Reprod. 1987b;36:1321–1332. doi: 10.1095/biolreprod36.5.1321. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Hanna R, Pang Y, Thomas P, Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors a and b in transfected cells. J Endocrinol. 2006;190:247–260. doi: 10.1677/joe.1.06694. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS. Estrogen receptors: structure, mechanisms and function. Rev Endocr Metab Disord. 2002;3:193–200. doi: 10.1023/a:1020068224909. [DOI] [PubMed] [Google Scholar]
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol. 2005;287:249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Hinfray N, Palluel O, Turies C, Cousin C, Porcher JM, Brion F. Brain and gonadal aromatase as potential targets of endocrine disrupting chemicals in a model species, the zebrafish (Danio rerio) Envronmental Toxicology. 2006;21:332–7. doi: 10.1002/tox.20203. [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Yu HP, Frink M, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. G protein-coupled receptor 30-dependent protein kinase A pathway is critical in nongenomic effects of estrogen in attenuating liver injury after trauma-hemorrhage. Am J Pathol. 2007;170:1210–1218. doi: 10.2353/ajpath.2007.060883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LF, Sullivan CV. Reproduction of white perch: the annual gametogenic cycle. Trans Am Fish Soc. 1995;124:563–574. [Google Scholar]
- Jalabert B. Modulation of 17-alpha-hydroxy-20 beta-dihydroprogesterone or gonadotropic extract activity on the in vitro intrafollicular maturation of rainbow trout (Salmo gairdnerii) oocytes by various steroids lacking maturing activity. C R Acad Sci Hebd Seances Acad Sci D. 1975;281:811–814. [PubMed] [Google Scholar]
- Jalabert B, Bry C, Breton B, Campbell C. Effect of 17 alpha hydroxy-20 beta- dihydroprogesterone and progesterone on maturation and ovulation in vivo and on the plasma level of gonadotropic hormone t-GtH in rainbow trout Salmo gairdneri. C R Acad Sci Hebd Seances Acad Sci D. 1976;283:1205–1208. [PubMed] [Google Scholar]
- Jalabert B, Finet B. Regulation of oocyte maturation in the rainbow trout, Salmo gairdneri: role of cyclic AMP in the mechanism of action of the maturation inducing steroid (MIS), 17α hydroxy, 20β-dihydroprogesterone. Fish Physiol Biochem. 1986;2:65–74. doi: 10.1007/BF02264074. [DOI] [PubMed] [Google Scholar]
- Jalabert B, Fostier A. The modulatory effect in vitro of oestradiol-17beta, testosterone or cortisol on the output of 17 alpha-hydroxy-20beta-dihydroprogesterone by rainbow trout (Salmo gairdneri) ovarian follicles stimulated by the maturational gonadotropin s-GtH. Reprod Nutr Dev. 1984;24:127–136. [Google Scholar]
- Kadmon G, Yaron Z, Gordin H. Sequence of gonadal events and oestradiol levels in Sparus aurata (L.) under two photoperiod regimes. J Fish Biol. 1985;26:609–620. [Google Scholar]
- Kalinowski RR, Berlot CH, Jones TL, Ross LF, Jaffe LA, Mehlmann LM. Maintenance of meiotic prophase arrest in vertebrate oocytes by a Gs protein-mediated pathway. Dev Biol. 2004;267:1–13. doi: 10.1016/j.ydbio.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang Z. Involvement of estrogen receptor variant ER-α36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24 doi: 10.1210/me.2009-0317. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen BS. Mechanisms of action and cross-talk between estrogen receptor and progesterone receptor pathways. J Soc Gynecol Investig. 2000;7:S33–37. doi: 10.1016/s1071-5576(99)00058-1. [DOI] [PubMed] [Google Scholar]
- Kelder J, Azevedoa R, Pang Y, Vliega J, Dong J, Thomas P. Comparison between steroid binding to membrane progesterone receptor α (mPRα) and to nuclear progesterone receptor: Correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRα-specific agonists. Steroids. 2010;75:314–322. doi: 10.1016/j.steroids.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King VW, Berlinsky DL, Sullivan CV. Involvement of steroids in final maturation of white perch (Morone americana) and white bass (M. Chrysops); in vivo and in vitro studies. Fish Physiol Biochem. 1995;14:189–500. doi: 10.1007/BF00004349. [DOI] [PubMed] [Google Scholar]
- King WT, Thomas P, Harrell RM, Hodson RG, Sullivan CV. Plasma levels of gonadal steroids during final oocyte maturation of striped bass, Morone saxatilis L. Gen. Comp Endocrinol. 1994;95:178–191. doi: 10.1006/gcen.1994.1115. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Murthy L, Stancel GM. Progesterone inhibits the estrogen-induced expression of c-fos messenger ribonucleic acid in the uterus. Endocrinology. 1992;130:3223–3230. doi: 10.1210/endo.130.6.1375896. [DOI] [PubMed] [Google Scholar]
- Loomis AK, Thomas P. Effects of estrogens and xenoestrogens on androgen production by Atlantic croaker testes in vitro: evidence for a nongenomic action mediated by an estrogen membrane receptor. Biol Reprod. 2000;62:995–1004. doi: 10.1095/biolreprod62.4.995. [DOI] [PubMed] [Google Scholar]
- Maller JL. Recurring themes in oocyte maturation. Biol Cell. 1998;90:453–460. [PubMed] [Google Scholar]
- Motola S, Popliker M, Tsafriri A. Are steroids obligatory mediators of luteinizing hormone/human chorionic gonadotropin-triggered resumption of meiosis in mammals? Endocrinology. 2007;148:4458–4465. doi: 10.1210/en.2007-0445. [DOI] [PubMed] [Google Scholar]
- Nagahama Y. 17a, 20b-dihydroxy-4-pregnen-3-one: a teleost maturation-inducing hormone. Develop Growth Differ. 1987;29:1–12. doi: 10.1111/j.1440-169X.1987.00001.x. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Yoshikuni M, Yamashita M, Tokumoto T, Katsu Y. Regulation of oocyte growth and maturation in fish. Curr Top Dev Biol. 1995;30:103–145. doi: 10.1016/s0070-2153(08)60565-7. [DOI] [PubMed] [Google Scholar]
- Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- Owman C, Blay P, Nilsson C, Lolait SJ. Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt’s lymphoma and widely distributed in brain and peripheral tissues. Biochem Biophys Res Commun. 1996;228:285–292. doi: 10.1006/bbrc.1996.1654. [DOI] [PubMed] [Google Scholar]
- Pace MC, Thomas P. Steroid-induced oocyte maturation in Atlantic croaker (Micropogonias undulatus) is dependent on activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Biol Reprod. 2005a;73:988–996. doi: 10.1095/biolreprod.105.041400. [DOI] [PubMed] [Google Scholar]
- Pace MC, Thomas P. Activation of a pertussis toxin-sensitive, inhibitory G-protein is necessary for steroid-mediated oocyte maturation in spotted seatrout. Dev Biol. 2005b;285:70–79. doi: 10.1016/j.ydbio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Pang Y, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149:3410–3426. doi: 10.1210/en.2007-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Thomas P. Involvement of estradiol-17 beta and its membrane receptor, G protein coupled receptor 30 (GPR30) in regulation of oocyte maturation in zebrafish, Danio rerio. Gen Comp Endocrinol. 2009;161:58–61. doi: 10.1016/j.ygcen.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patiño R, Thomas P. Effects of gonadotropin on ovarian intrafollicular processes during the development of oocyte maturational competence in a teleost, the Atlantic croaker: evidence for two distinct stages of gonadotropin control of final oocyte maturation. Biol Reprod. 1990;43:818–827. doi: 10.1095/biolreprod43.5.818. [DOI] [PubMed] [Google Scholar]
- Petrino TR, Toussaint G, Lin YW. Role of inhibin and activin in the modulation of gonadotropin- and steroid-induced oocyte maturation in the teleost Fundulus heteroclitus. Reprod Biol Endocrinol. 2007;5:21. doi: 10.1186/1477-7827-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat F, Zanuy S, Carrillo M, de Mones A, Fostier A. Seasonal changes in plasma levels of gonadal steroids of sea bass, Dicentrarchus labrax L. Gen. Comp Endocrinol. 1990;78:361–373. doi: 10.1016/0016-6480(90)90026-i. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Revelli A, Massobrio M, Tesarik J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- Sadler SE, Maller JL. Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J Biol Chem. 1981;256:6368–6373. [PubMed] [Google Scholar]
- Sakai N, Iwamatsu T, Yamauchi K, Nagahama Y. Development of the steroidogenic capacity of medaka (Oryzias latipes) ovarian follicles during vitellogenesis and oocyte maturation. Gen Comp Endocrinol. 1987;66:333–342. doi: 10.1016/0016-6480(87)90242-5. [DOI] [PubMed] [Google Scholar]
- Sakai N, Iwamatsu T, Yamauchi K, Suzuki N, Nagahama Y. Influence of follicular development on steroid production in the medaka (Oryzias latipes) ovarian follicle in response to exogenous substrates. Gen Comp Endocrinol. 1988;71:516–523. doi: 10.1016/0016-6480(88)90282-1. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147:2280–2286. doi: 10.1210/en.2005-1011. [DOI] [PubMed] [Google Scholar]
- Selcer KW, Leavitt WW. Progesterone down-regulation of nuclear estrogen receptor: a fundamental mechanism in birds and mammals. Gen Comp Endocrinol. 1988;72:443–452. doi: 10.1016/0016-6480(88)90167-0. [DOI] [PubMed] [Google Scholar]
- Tan Q, Zagrodny A, Bernaudo S, Peng C. Regulation of membrane progestin receptors in the zebrafish ovary by gonadotropin, activin, TGF-beta and BMP-15. Mol Cell Endocrinol. 2009;312(1–2):72–9. doi: 10.1016/j.mce.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Thomas P. Hormonal control of final oocyte maturation in sciaenid fishes. In: KGDREPSST, editor. Nat Res. Council of Canada; Ottawa: 1994. pp. 619–625. [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:107–109. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pinter J, Das S. Upregulation of the maturation-inducing steroid membrane receptor in spotted seatrout ovaries by gonadotropin during oocyte maturation and its physiological significance. Biol Reprod. 2001;64:21–29. doi: 10.1095/biolreprod64.1.21. [DOI] [PubMed] [Google Scholar]
- Thomas P, Zhu Y, Pace M. Progestin membrane receptors involved in the meiotic maturation of teleost oocytes: a review with some new findings. Steroids. 2002;67:511–517. doi: 10.1016/s0039-128x(01)00180-5. [DOI] [PubMed] [Google Scholar]
- Thomas P, Alyea R, Pang Y, Peyton C, Dong J, Berg H. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1, GPER, in mammals and fish. Steroids. doi: 10.1016/j.steroids.2009.11.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumoto M, Nagahama Y, Thomas P, Tokumoto T. Cloning and identification of a membrane progestin receptor in goldfish ovaries and evidence it is an intermediary in oocyte meiotic maturation. Gen Comp Endocrinol. 2006;145:101–108. doi: 10.1016/j.ygcen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Trant JM, Thomas P. Changes in ovarian steroidogenesis in vitro associated with final maturation of Atlantic croaker oocytes. Gen Comp Endocrinol. 1989;75:405–412. doi: 10.1016/0016-6480(89)90175-5. [DOI] [PubMed] [Google Scholar]
- Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Tubbs C, Pace M, Thomas P. Expression and gonadotropin regulation of membrane progestin receptor alpha in Atlantic croaker (Micropogonias undulatus) gonads: Role in gamete maturation. Gen Comp Endocrinol. 2009 doi: 10.1016/j.ygcen.2009.06.017. (in press) [DOI] [PubMed] [Google Scholar]
- Wallace RA. Vitellogenesis and oocyte growth in nonmammalian vertebrates. Dev Biol. 1985;1:127–177. doi: 10.1007/978-1-4615-6814-8_3. [DOI] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Li Y, Kaler LJ, Proctor T, Vandenbark AA, Offner H. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J Immunol. 2009;182:3294–3303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Prossnitz ER, Roy SK. Expression of G protein-coupled receptor 30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology. 2007;148:4853–4864. doi: 10.1210/en.2007-0727. [DOI] [PubMed] [Google Scholar]
- Yoshikuni M, Nagahama Y. Involvement of an inhibitory G-protein in the signal transduction pathway of maturation-inducing hormone (17 alpha,20 beta-dihydroxy-4-pregnen-3-one) action in rainbow trout (Oncorhynchus mykiss) oocytes. Dev Biol. 1994;166:615–622. doi: 10.1006/dbio.1994.1341. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.