Abstract
Purpose
To use the EQ-5D instrument to evaluate the long-term health states of women with early stage breast cancer treated by breast-conserving surgery and radiation.
Materials and methods
1,050 women treated with conservative surgery and radiation with or without systemic therapy completed 2480 questionnaires during follow-up visits. The EQ-5D is a standardized and validated instrument for measuring quality of life outcomes. The descriptive system uses 5 dimensions of health with three possible levels of response that combine into 243 (35) possible unique health states that are each assigned a values-based index score from 0 to 1. The visual analog scale (VAS) rates health on a simple vertical line from 0 – 100. Higher scores correspond to better health status.
Results
The mean index scores were 0.89 (95% CI: 0.87-0.91) at 5 years, 0.9 (95% CI: 0.86-0.94) at 10 years, and 0.9 (95% CI: 0.83-1.0) at 15 years. There were no significant differences in health states between patients by age or compared with U.S. controls. There was a statistically significant positive correlation between the results of the VAS and descriptive system. Significant trends in health dimensions over 15 years were increased problems with self care and decreased problems with anxiety/depression, pain/discomfort, and performing usual activities.
Conclusions
This study of EQ-5D is unique and demonstrates very high quality of life in patients long-term after breast-conserving surgery and radiation. These health states are comparable to the adult female U.S. population. These data will provide valuable patient utility information for informing decision analyses investigating new treatments in women with breast cancer.
Keywords: Breast cancer, radiation therapy, EQ-5D, EuroQol, Health States
Introduction
Breast-conserving surgery and radiation are standard alternatives to mastectomy for eligible patients with Stage 0, I and II invasive breast cancer [1, 2]. Postoperative whole-breast radiation is associated with long-term local control on the order of 85-95% with equivalent survival outcomes as mastectomy [3-5]. Postoperative radiation for invasive breast cancer has also been associated with improved local control [4, 6] and overall survival [7] compared to breast-conserving surgery alone for most women. Highly selected women with favorable early stage disease, usually of advanced age or other serious comorbidities, may be offered the option of no radiation [1]. Decision-making about choosing one option over another for local treatment, particularly when survival outcomes are comparable, may often rest upon quality of life considerations.
Most studies examining quality of life after treatment of breast cancer patients have focused on comparisons between treatment by breast-conserving surgery or mastectomy [8]. Specific studies on women treated after breast-conserving surgery and postoperative radiation have been limited to focusing on the short-term time period from weeks to within the first year after radiation [9-14], with few studies examining quality of life with longer follow-up [15]. This type of data on quality of life is often of interest to individual patients and their treating physicians. For example, reports of high quality of life after postoperative radiation could lead to more women choosing or more oncologists recommending this treatment option. However, this type of data may also serve an important function from the perspective of medical economics. For these utility analyses to be possible, long-term control data on quality of life is needed after breast-conserving surgery and radiation.
The EQ-5D is a standardized and validated instrument for measuring health outcomes [16]. It is designed to be simple for patients to use and takes only a few minutes to complete. There are two components to the instrument. The first component is a descriptive system that is comprised of 5 descriptive dimensions of health with three possible levels of response (no problems, some problems, or extreme problems). The dimensions are mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. There are 243 (35) possible unique health states described by the combination of these 5 dimensions. Each unique health state is then able to be assigned a values-based index score from population samples. The second component is a visual analog scale (VAS) that rates health on a simple vertical line from 0 (worst imaginable health state) to 100 (best imaginable health state). The data on patient preferences and quality of life generated by the EQ-5D can be used for quality-adjusted survival analyses.
The purpose of this study is to use the EQ-5D instrument to evaluate the health states of women with early stage breast cancer long-term after treatment with conservative surgery and radiation.
Methods and Materials
The study population consists of 1,050 women with early stage breast cancer treated with breast-conserving surgery and radiation with or without systemic therapy. Inclusion criteria were American Joint Committee on Cancer stages 0, I or II breast cancer [17], radiation at Fox Chase Cancer Center, and completion of radiation therapy. Exclusion criteria for the study included male breast cancer, T3-T4 disease, stage IV disease, mastectomy, or patients treated without radiation. Patient demographics, tumor characteristics, and treatment-related information is entered prospectively into a database, and maintained and updated by a single data manager. The protocol for collection, storage and data retrieval is under compliance with the hospital Institutional Review Board and Health Insurance Privacy and Portability Act regulations.
All patients were treated with whole breast radiation (46-50 Gy), with or without regional nodal radiation, and a boost to the tumor bed (10-18 Gy). The total dose was generally determined by the extent of surgery and final margin status ranging from a median of 60 Gy with a negative margin to 64 Gy with a close margin to 66 Gy for a positive final margin. Whole breast radiation was conventional wedged photon tangents (n=676) earlier in the study period, while the majority of patients in later years received photon IMRT (n=374). Details of the conventional radiation treatment policy during this study period have been previously described [18]. Patients treated with either technique were placed in an alpha-cradle cast on a 10-20% wedged breast board for set-up reproducibility. All patients have been prescribed standard fractionation of 2 Gy per day to a total of 46-50 Gy over 4 ½-5 weeks. Conventionally treated patients were treated with breast tangents designed with either conventional fluoroscopic or CT-based simulation. Most patients were treated by a 6 MV linear accelerator, with higher energies with a beam spoiler used in patients with larger chest wall separations. IMRT patients undergo CT simulation to define the clinical target volume of the breast tissue and normal structures. The physician defines the clinical target volume (CTV) as the palpable breast tissue anterior to the chest wall to within 5 mm of the skin. A margin of 7 mm posteriorly and 1.5 cm superior and inferior are added to create a planning treatment volume (PTV). The IMRT technique used a combination of open and segmented tangential fields using volume-based inverse dose planning and step and shoot beam delivery. The primary tumor bed was boosted in 99% of patients for an additional 14 – 20 Gy. This boost was delivered with electrons in almost all patients. Treatment energy is dependent upon breast size and depth of the tumor bed from the skin surface.
During routine follow-up visits after completion of radiation, patients were asked to complete the EQ-5D questionnaire as part of a wider health assessment form. The EQ-5D was chosen for its ease of use, brief number of questions that can be completed rapidly suitable for busy outpatient clinics, and open access without added institutional or patient expense. Physician visits are typically 3 months after completion of radiation and then 6 month intervals for the 1st 5 years. Many patients then take the option of following annually after that. This study made no control for the interval between completing the questionnaire and completion of radiation — patients at all intervals of follow-up were included. The actuarial analysis accounted for the different intervals between treatment and completion of the questionnaire. There was also no means for controlling which patients continued with follow-up in the department of Radiation Oncology at all versus receiving their follow-up care elsewhere. There was no means for analyzing and reporting information on the non-responding patients who may have been eligible for study but chose not to participate in clinical outcomes research on the database protocol.
Statistics
The descriptive system for each questionnaire completed by the patient results in 243 (35) possible unique health states described by the combination of the 5 dimensions. Each unique health state is then able to be assigned a values-based index score from population samples from 0 – 1. EQ index score was averaged at each follow-up year and compared across demographic and tumor characteristics, such as age, adjuvant therapy, radiation therapy, and recurrence status. Change of the 5 descriptive health dimensions over time were studied by fitting linear regressions on percentages of patients reporting on each of the health dimensions Pearson correlation was used to check the association between EQ index score and visual analog scale (VAS).
Results
The characteristics of the study population are shown in Table 1. There were 2,480 questionnaires total completed. The number of entries per patient was 1 in 336 patients (32%), 2 in 306 patients (29%), 3 in 223 patients (21%), and 4 or more in remaining patients (18%). The mean length of follow-up between treatment and time of report was 3 years (range 0 – 24 years). There were 482 data points at 1 year, 171 data points at 5 years, 64 at 10 years, and 21 at 15 years. At the time of analysis of the questionnaire data, 1038 patients remained alive, with 20 of these having evidence of recurrent breast cancer. Twelve patients have died, 4 of breast cancer and 8 of other causes. There have been 27 local recurrences, 2 regional nodal recurrences, 3 local and regional recurrences, and 23 distant recurrences.
Table 1.
Patient characteristics
| Number of Patients | 1,050 |
|---|---|
| Years of Treatment | 8/82 – 8/07 |
| Age | |
| 18 – 44 | 140 (13%) |
| 45 – 64 | 595 (57%) |
| > 64 | 315 (30%) |
|
| |
| Tumor stage | |
| Tis | 192 (18%) |
| T1 | 714 (68%) |
| T2 | 141 (13%) |
|
| |
| Nodal Stage | |
| N0 | 644 (61%) |
| N1-3 positive | 174 (17%) |
| N4+ positive | 38 (4%) |
| NX | 194 (18%) |
|
| |
| Breast dose (Gy) | |
| Median | 46 |
| Range | 18 – 50.4 |
|
| |
| Total dose (Gy) | |
| Median | 60 |
| Range | 30 – 66 |
|
| |
| Radiation Technique | |
| Conventional | 676 (64%) |
| IMRT | 374 (36%) |
|
| |
| Systemic Therapy | |
| None | 353 (34%) |
| Chemotherapy only | 109 (10%) |
| Endocrine therapy only | 360 (34%) |
| Both | 228 (22%) |
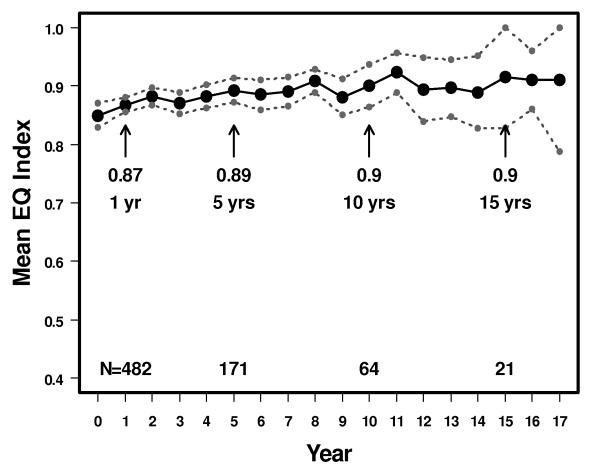
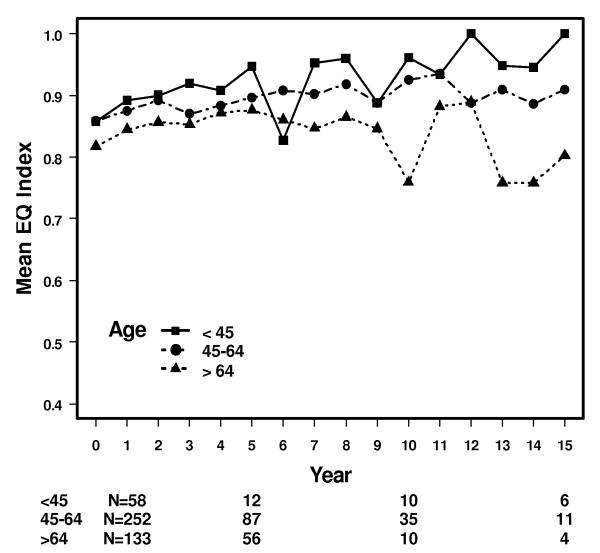
The mean descriptive index scores were 0.89 (95% CI: 0.87-0.91) at 5 years, 0.9 (95% CI: 0.86-0.94) at 10 years, and 0.9 (95% CI: 0.83-1.0) at 15 years (Figure 1). There were no significant differences in health states between patients by age. The mean by age at 5 and 10 years was 0.95 and 0.96 (ages 18–44), 0.9 and 0.93 (ages 45–64), and 0.88 and 0.76 (age >64) (Figure 2). For comparison, the reported mean index scores for the general U.S. female population is 0.86, and by age 0.91 (ages 18-44), 0.84 (ages 45-64) and 0.81 (age >64) [19].
Figure 1.
Mean descriptive index score in 1,050 patients with breast cancer treated by conservative surgery and radiation with or without systemic therapy. 95% upper and lower confidence intervals also shown. The vertical axis is shortened and does not extend from 0-1 to improve readability of the figure.
Figure 2.
Mean descriptive index scores by age for patients with breast cancer. The vertical axis is shortened and does not extend from 0-1 to improve readability of the figure.
There were no significant differences in mean index score by use of adjuvant systemic therapy when comparing women treated by chemotherapy only, tamoxifen only, both or neither (p > 0.05). There was no apparent difference in mean score by use of intensity modulated radiation therapy (IMRT) versus conventional radiation, although very few patients treated with IMRT had follow-up greater than 3 years. There were also no significant differences between patients with and without a recurrence, although the number of questionnaires from patients with recurrence (n=94) was small compared to those without recurrence (n=2386) dramatically limiting the power of the analysis to see such a difference.
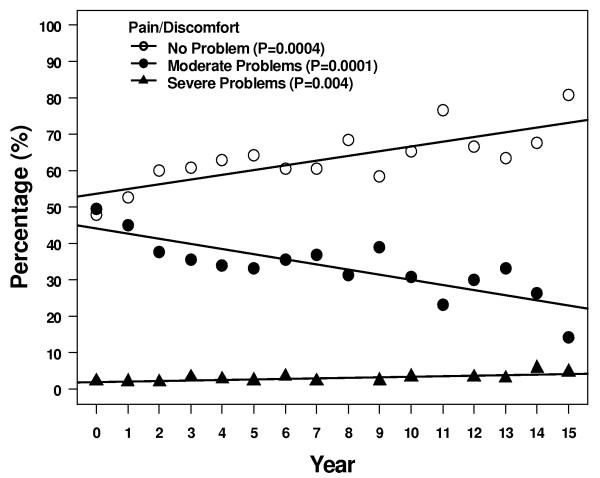
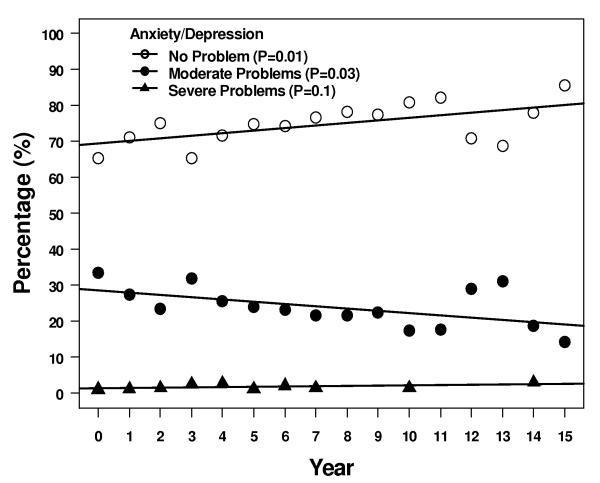
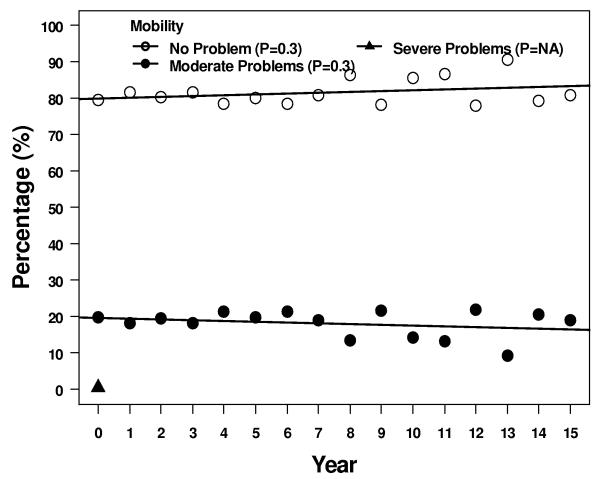
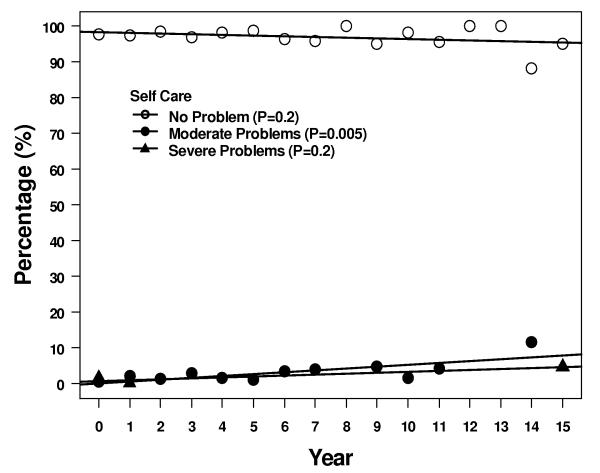
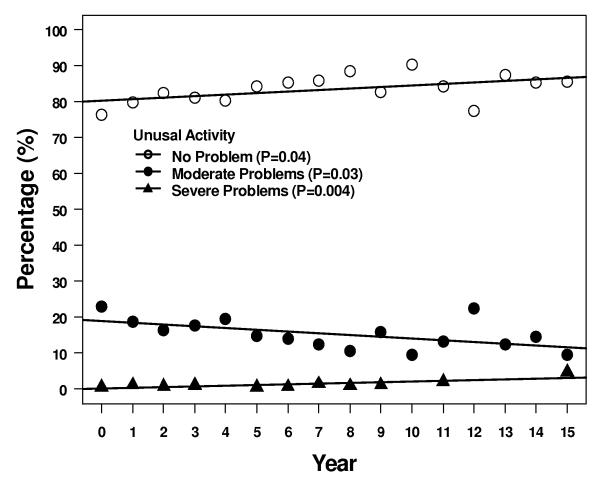
Four of the 5 descriptive health dimensions showed a significant trend of moving between levels 1 and 2 over 15 years - 1 scale trended up from 1 to 2, and 3 scales trended down from 2 to 1 (Figure 3). More patients reported some problems with self care, although most patients > 95% continued to report no problems. Over the same time period, fewer patients reported some problems with anxiety/depression, pain/discomfort, and usual activities at 15 years compared to immediately after treatment. Two of the 5 subscales, pain/discomfort and usual activity, had a significant trend in increasing reported level 3 but the absolute number of patients reporting this level of problems remained ≤ 5%.
Figure 3.
Results of the descriptive subscales. Figures show percentages of patients reporting no problems, some problems, or extreme problems during years 1 – 15. A = pain/discomfort, B = anxiety/depression, C = mobility, D = self care, and E = usual activities.
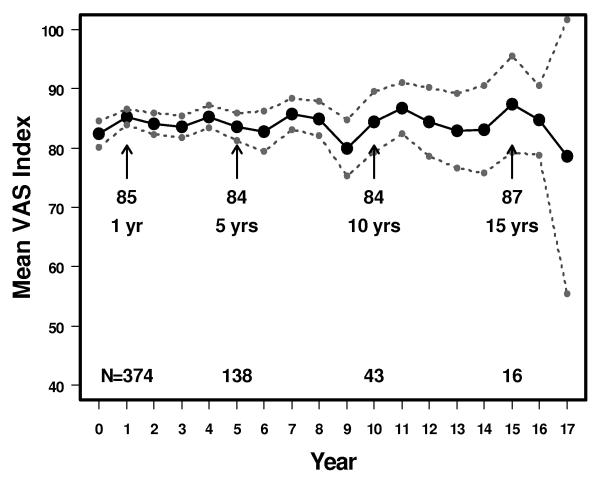
The mean scores for the VAS were 82 (95% CI: 80-85) at 0 years, 84 (95% CI: 81-86) at 5 years, and 84 (95% CI: 79-90) at 10 years (Figure 4). There was a statistically significant positive correlation between the results of the VAS and the Descriptive mean index score in each year from years 0 – 10. The correlation coefficient was 0.4 at 0 years (p<0.0001), 0.6 at 5 years (<0.0001) and 0.7 at 10 years (p<0.0001).
Figure 4.
Mean Visual Analog Scales scores in patients with breast cancer treated by conservative surgery and radiation with or without systemic therapy. 95% upper and lower confidence intervals also shown. The vertical axis is shortened and does not extend from 0-1 to improve readability of the figure.
Discussion
This study of EQ-5D is unique in demonstrating very high levels of overall quality of life in patients treated with breast-conserving surgery and radiation even up to 15 years after treatment. These mean health states are comparable to those reported for the general adult U.S. population. We found a strong correlation between the VAS mean scoring and the mean index score derived from the descriptive 5 question part of the EQ-5D. The VAS provides additional internal validity for the results of the descriptive system within our population. The index score from the descriptive system is derived by converting the unique individual health state (243 possible outcomes from the 5 separate questions) into a number from 0 – 1 based on a values based system of weighting each response. It is possible that different populations, such as the general population versus a cancer population, or a male population versus a female population, would assign different weighting to each of the possible health states. However, the VAS is a direct measurement of our patient’s own values judgement of their health state without this conversion process - yet was still in concordance with the descriptive mean index scores. The VAS scoring alone however would be more limited in studying what is affecting a patient’s health state compared to the descriptive system.
Our data is unable to distinguish whether effects observed over time may be attributable to breast-conserving surgery or radiation or both, since all patients received both treatments. At the immediate completion of treatment, Ganz et al reported quality of life in women after either mastectomy or breast-conserving surgery with or without radiation and chemotherapy [8]. They reported that women treated by lumpectomy had better physical functioning scores than women treated by mastectomy, but no difference in emotional well-being scores. Women’s emotional functioning was in general normal compared to a non-affected population of women. They found that there was no overall statistically significant difference in vitality scores between women treated with chemotherapy or not treated with chemotherapy. This was similar to our finding that women’s quality of life scores were not significantly different for women after radiation by use of systemic therapy. Using a scale from 1 – 10, women were found to have a high quality of life — the adjusted mean score after lumpectomy only was 7.7 and lumpectomy with chemotherapy 7.4.
Other studies have focused on the time period from weeks to months after radiation. There are data from a large clinical phase III trial randomized trial that the impact of radiation is non-significant on overall quality of life. Prescott et al reported a trial from Edinburgh of women aged 65 years or older randomized to receive postlumpectomy radiation or no radiation [9]. These women were selected for node negative, low-risk favorable early stage breast cancer. All were also treated with endocrine therapy. There was no difference in overall quality of life by the EQ-5D assessment. There were no recurrences within the first 15 months, so that the quality of life utilities were identical between treatments. Other clinical trials have reported either no significant difference in quality of life outcomes [10-12] or a modest decrease [13] in the short-term period of months after postlumpectomy radiation.
Lee et al reported quality of life of women treated with lumpectomy and radiation at baseline, completion, and 7 months after radiation [14]. The assessment tool was the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-30 and BR-23 questionairres. For the 61 women tested, there was no difference in quality of life scores between the three timepoints. The global health status scores for quality of life were high. The mean scores were 68, 71 and 78 at baseline, postradiation and at 7 months after treatment, respectively. Emotional functioning scores were also high (79 – 80 out of a possible 100). Fatigue was a strong predictor of quality of life, with mean scores from 23 – 33, but was back to baseline by the 7 month timepoint.
In a study of more long-term effects after radiation, Waljee et al reported quality of life in women treated with breast-conserving surgery over a four-year period by means of a questionnaire sent out at a single timepoint within a few years after treatment [15]. This differs from our methodology, where the EQ-5D was given to women at various times from the first follow-up visit after radiation to up to 15 years after treatment. However, despite using a different questionnaire, their findings were similar to our own results. On a scale of 0 – 100, they found that the mean quality of life of responding patients at the single timepoint was very high at 85.2. Compare this result to our finding of a mean of 84 from the EQ-5D VAS scale (also calibrated from 0 – 100) both 5 and 10 years after treatment. Their study also found that depressive symptoms occurred in 22.3% of women. We reported on the descriptive scale of the EQ-5D approximately 25 – 30% of women reported moderate problems with anxiety and depression, with a trend to decreasing incidence over 15 years of follow-up after treatment. This is consistent with the finding that anxiety and depression scores may not change or can actually improve during and up to 2 months after radiation [20].
While not directly measuring fatigue in this study, the descriptive subscales of performance of usual activities in this study showed that most patients (>85%) reported no problems on average by 15 years. Fewer patients reported some problems or extreme problems over time. Fatigue is reported in up to 34% of patients treated for breast cancer up to 10 years after diagnosis, with 21% of patients having persistent problems with fatigue over time [21]. Significant fatigue has been reported in as many as 43% of patients during radiation [22]. Fatigue generally increases during the weeks of radiation and reaches a plateau after week 4 [20, 22]. Two studies have shown that fatigue returns to baseline by 2 – 3 months after radiation [20, 23]. This is consistent with our observations. Only the subscale of ability to do self care activities showed a decrease in patients reporting no problems, but by 15 years the numbers reporting some problems (8%) and extreme problems (3.5%) was small. This is probably an observation effect of normal aging in some of our patients, given that 30% were aged 65 years or older at the time of radiation.
Decisions about coverage of new medical treatments, or allocating resources at the country or insurance payer level, requires cost and outcomes data. A cost-utility analysis is one method of outcomes research that factors in quality-adjusted survival with cost-effectiveness or cost-benefits when assessing a particular form of medical treatment [24]. Quality-adjusted survival, usually measured in quality-adjusted life-years (QALYs), uses patient preferences for health states. These types of analyses have been used to assess outcomes in early stage breast cancer [25-27]. These data could also be used to provide essential utility information for informing decision analyses investigating new treatments. Absent these outcomes data, new technologies and treatments have been placing an increasing burden on the health care system in the United States [28]. These analyses can be used to compare outcomes for radiation versus no radiation in selected subgroups of patients. Specifically with regards to breast cancer, data on utilities would be important in comparing new technology of IMRT to conventional radiation, or comparing new techniques of partial breast irradiation to whole breast irradiation. In the current study, with short-term follow-up in IMRT patients, we did not see a difference in quality of life outcomes for patients treated by IMRT compared to conventional radiation within 4 years from treatment.
There were many limitations to this study that need to be considered. A limitation of the study design is the restriction of the study population eligible to complete a questionnaire to patients returning to follow-up over time to our department. There were relatively few questionnaires at 10 years (64) and 15 years (21) which weaken the power of the study to detect differences in health outcomes compared to the early years of follow-up after treatment. The population was relatively early stage (18% in-situ, 79% node negative or clinically negative in the setting of DCIS) and therefore candidates for breast conservation in the first place. And patients exclusively following up for their care in Medical or Surgical oncology were not available for study inclusion. It may be expected that women with distant recurrence of disease may have been only seen by Medical Oncology from that point and not Radiation Oncology. There were relatively few women in this population completing questionnaires being followed in our department who experienced recurrence and subsequent death from disease. This study bias could not be overcome by contacting and collecting data on non-questionnaire completing patients. As a retrospective analysis of data collected in our department prospectively, there was no permission given by patients to contact them after treatment and/or mail them questionnaires if they did not return for follow-up in our department. Therefore, the conclusions of this study are applicable to the favorable group of women with early stage cancer, who are survivors of their breast cancer, and have absent other serious comorbidities and sufficient performance status for regular follow-up in an outpatient setting. However, the excellent outcomes in these patients that were comparable to the general population is an important finding that could be reassuring to the prospective radiation patient that recovery of quality of life in the years after treatment is the norm and not the exception.
Conclusion
Patient self-reported quality of life by the EQ-5D was high and remained stable for up to 15 years after treatment with breast-conserving surgery and radiation. There was good statistical correlation between patient-reported outcomes by either the VAS or descriptive system. These data will provide valuable patient utility information for informing decision analyses investigating new treatments in women with breast cancer.
Acknowledgment
The authors thank Cindy Rosser for her collection and management of the data for the study population.
Footnotes
CONFLICT OF INTEREST STATEMENT: NO CONFLICTS REPORTED
References
- 1. NCCN practice guidelines for breast cancer [PubMed]
- 2.NIH Consensus Conference Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. [PubMed] [Google Scholar]
- 3.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong J, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, Fisher ER, Wickerham DL, Deutsch M, Margolese R, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 6.Fisher ER, Dignam J, Tan-Chiu E, Costantino J, Fisher B, Paik S, Wolmark N. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17. Intraductal carcinoma. Cancer. 1999;86:429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative Group Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 8.Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, Bower JE, Belin TR. Quality of life at the end of primary treatment of breast cancer: First results from the moving beyond cancer randomized trial. J Natl Cancer Inst. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 9.Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, Goh TT, Lindley R, Cairns J. A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial. Health Technol Assess. 2007;11(31):1–149. iii–iv. doi: 10.3310/hta11310. [DOI] [PubMed] [Google Scholar]
- 10.Rayan G, Dawson LA, Bezjak A, Lau A, Fyles AW, Yi Q, Merante P, Vallis KA. Prospective comparison of breast pain in patients participating in a randomized trial of breast-conserving surgery and tamoxifen with or without radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:154–161. doi: 10.1016/s0360-3016(02)03826-9. [DOI] [PubMed] [Google Scholar]
- 11.Wengstrom Y, Haggmark C, Strander H, Forsberg C. Perceived symptoms and quality of life in women with breast cancer receiving radiation therapy. Eur J Oncol Nurs. 2000;4(2):78–88. doi: 10.1054/ejon.1999.0052. discussion 89-90. [DOI] [PubMed] [Google Scholar]
- 12.Back M, Ahern V, Delaney G, Graham P, Steigler A, Wratten C. Absence of adverse early quality of life outcomes of radiation therapy in breast conservation therapy for early breast cancer. Australas Radiol. 2005;49(1):39–43. doi: 10.1111/j.1440-1673.2005.01392.x. [DOI] [PubMed] [Google Scholar]
- 13.Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260–2266. [PubMed] [Google Scholar]
- 14.Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Quality of life of women treated with radiotherapy for breast cancer. Support Care Cancer. 2008;16:399–405. doi: 10.1007/s00520-007-0328-6. [DOI] [PubMed] [Google Scholar]
- 15.Waljee JF, Hu ES, Ubel PA, Smith DM, Newman LA, Alderman AK. Effect of esthetic outcome after breast-conserving surgery on psychosocial functioning and quality of life. J Clin Oncol. 2008;26:3331–3337. doi: 10.1200/JCO.2007.13.1375. [DOI] [PubMed] [Google Scholar]
- 16.EuroQol Group EuroQol - a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 17.American Joint Committee on Cancer . AJCC cancer staging manual. 5th edn Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- 18.Fowble B, Freedman G. Cancer of the Breast. In: Wang CC, editor. Clinical Radiation Oncology: indications, techniques and results. second edn Wiley-Liss, inc; 2000. [Google Scholar]
- 19.Luo N, Johnson JA, Shaw JW, Feeny D, Coons SJ. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Med Care. 2005;43(11):1078–1086. doi: 10.1097/01.mlr.0000182493.57090.c1. [DOI] [PubMed] [Google Scholar]
- 20.Geinitz H, Zimmermann FB, Stoll P, Thamm R, Kaffenberger W, Ansorg K, Keller M, Busch R, van Beuningen D, Molls M. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. Int J Radiat Oncol Biol Phys. 2001;51(3):691–698. doi: 10.1016/s0360-3016(01)01657-1. [DOI] [PubMed] [Google Scholar]
- 21.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4):751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 22.Wratten C, Kilmurray J, Nash S, Seldon M, Hamilton CS, O’Brien PC, Denham JW. Fatigue during breast radiotherapy and its relationship to biological factors. Int J Radiat Oncol Biol Phys. 2004;59(1):160–167. doi: 10.1016/j.ijrobp.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Irvine D, Vincent L, Graydon J, Bubela N. Fatigue in women with breast cancer receiving radiation therapy. Cancer Nursing. 1998;21:127–135. doi: 10.1097/00002820-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Konski A. Economic Analysis of Health Care Interventions. Seminars in Radiation Oncology. 2008;18(3):168–174. doi: 10.1016/j.semradonc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Konski A. Use of health plan data to estimate cost and outcomes of a breast cancer population. Int J Radiat Oncol Biol Phys. 1997;39:230. [Google Scholar]
- 26.Hayman JA, Hillner BE, Harris JR, Pierce LJ, Weeks JC. Cost-effectiveness of adding an electron-beam boost to tangential radiation therapy in patients with negative margins after conservative surgery for early-stage breast cancer. J Clin Oncol. 2000;18:287–295. doi: 10.1200/JCO.2000.18.2.287. [DOI] [PubMed] [Google Scholar]
- 27.Karnon J, Delea T, Barghout V. Cost utility analysis of early adjuvant letrozole or anastrozole versus tamoxifen in postmenopausal women with early invasive breast cancer: the UK perspective. Eur J Health Econ. 2008;9(2):171–183. doi: 10.1007/s10198-007-0058-1. [DOI] [PubMed] [Google Scholar]
- 28.Wallner PE, Konski A. A changing paradigm in the study and adoption of emerging health care technologies: coverage with evidence development. J Am Coll Radiol. 2008;5(11):1125–1129. doi: 10.1016/j.jacr.2008.06.008. [DOI] [PubMed] [Google Scholar]