Summary
Foetal exposure to antiandrogens alters androgen-sensitive development in male rodents, resulting in less male-typical behaviour. Foetal phthalate exposure is also associated with male reproductive development in humans, but neurodevelopmental outcomes have seldom been examined in relation to phthalate exposure. To assess play behaviour in relation to phthalate metabolite concentration in prenatal urine samples, we recontacted participants in the Study for Future Families whose phthalate metabolites had been measured in mid-pregnancy urine samples. Mothers completed a questionnaire including the Pre-School Activities Inventory, a validated instrument used to assess sexually dimorphic play behaviour. We examined play behaviour scores (masculine, feminine and composite) in relationship to (log10) phthalate metabolite concentrations in mother's urine separately for boys (N = 74) and girls (N = 71). Covariates (child's age, mother's age and education and parental attitude towards atypical play choices) were controlled using multivariate regression models. Concentrations of dibutyl phthalate metabolites, mono-n-butyl phthalate (MnBP) and mono-isobutyl phthalate (MiBP) and their sum, were associated with a decreased (less masculine) composite score in boys (regression coefficients −4.53,−3.61 and −4.20, p = 0.01, 0.07 and 0.04 for MnBP, MiBP and their sum respectively). Concentrations of two urinary metabolites of di(2-ethylhexyl) phthalate (DEHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) and mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and the sum of these DEHP metabolites plus mono(2-ethylhexyl) phthalate were associated with a decreased masculine score (regression coefficients −3.29,−2.94 and −3.18, p = 0.02, 0.04 and 0.04) for MEHHP, MEOHP and the sum respectively. No strong associations were seen between behaviour and urinary concentrations of any other phthalate metabolites in boys, or between girls’ scores and any metabolites. These data, although based on a small sample, suggest that prenatal exposure to antiandrogenic phthalates may be associated with less male-typical play behaviour in boys. Our findings suggest that these ubiquitous environmental chemicals have the potential to alter androgen-responsive brain development in humans.
Keywords: di(2-ethylhexyl) phthalate, dibutyl phthalate, play behaviour, prenatal exposure phthalates, Pre-School Activities Inventory, sex-dimorphism
Introduction
Phthalate esters are pervasive environmental chemicals. Although several of these are now banned for use in toys and some other products designed for young children (Kamrin, 2009), this legislation does not limit prenatal exposure. Moreover, phthalates are present in so many other products and manufactured in such quantity, that exposure is virtually universal (CDC, 2005). A large body of work in laboratories around the world has demonstrated that, in experimental animals, when exposure occurs during the period of foetal sexual differentiation, some phthalates, notably di(2-ethylhexyl) phthalate (DEHP) and dibutyl phthalate (DBP), inhibit the synthesis of testosterone by Leydig cells, thereby reducing foetal testosterone concentration (Welsh et al., 2008). As a result, male pups exhibit a cluster of altered androgen-dependent anatomical features that reflect disordered sex differentiation, including a reduced – that is, a less masculine – anogenital distance (AGD), impaired testicular descent and reduced genital size. This cluster of alterations has been referred to as the ‘phthalate syndrome’ (Foster, 2006). Some phthalate-related changes have also been identified in adult female rodents, but no significant changes have been reported in female neonates. In male rodents, the phthalate syndrome, which is initially identified neonatally, has been shown to have adverse consequences for later sexual development. We recently reported data demonstrating an association between prenatal exposure in humans, particularly to DEHP and its urinary metabolites, and a similar cluster of reproductive developmental outcomes in male infants (Swan et al., 2005; Swan, 2008). In addition, free serum testosterone in human male infants has been negatively correlated with levels of some phthalate metabolites in breast milk (Main et al., 2006). However, the long-term consequences of these findings for humans are uncertain.
In particular, the potential for these antiandrogens to influence the course of brain sexual differentiation has only recently been addressed (Engel et al., 2009). This question is rooted in our understanding of how gonadal hormones influence mammalian neural development. Testosterone exposure during early development produces a masculine neural phenotype by influencing cell survival, neural growth and neurochemical specification. In rodents, this process involves the enzyme aromatase, which, by a biological irony, converts testosterone into oestradiol, which then shapes the male structure. In rats, the critical programming window for genital tract development occurs in gestational days 18–21 (Welsh et al., 2008), a period that corresponds to a testosterone surge in the developing male. In humans, the testes begin to function at about week 8 of gestation and, while dates are uncertain, testosterone appears to be elevated in the male foetus from about weeks 8 to 24 of gestation (Reyes et al., 1973; Smail et al., 1981). The critical period for brain sexual differentiation is unknown and may not be the same as that for reproductive tract development. Whatever the critical period, testosterone is an essential mediator; if the antiandrogenic actions of phthalates reduce its secretion by the foetus, brain sexual differentiation may be altered.
Data from Swan et al. (2005) and Swan (2008) support the hypothesis that in humans, maternal exposure to phthalates, particularly DEHP, lowers foetal testosterone production and results in incomplete masculinization of the genital tract, resulting in a shortened AGD as well as incomplete testicular descent and smaller penile size. These data suggest that the same process might plausibly influence brain sexual differentiation and its expression in sexually dimorphic behaviours. Play behaviours offer themselves as a test of the hypothesis that phthalate exposures during gestation may alter brain sexual differentiation and its behavioural outcomes.
Young male and female humans, rats and non-human primates all show sex differences in play behaviours. Young male rats and non-human primates, for example, engage in more play-fighting or rough-and-tumble play than their female counterparts (Pellis, 2002; Wallen, 2005). Young male rhesus monkeys, like boys, also show distinct preferences for toys with wheels (Hassett et al., 2008) and vervet monkeys show sex differences in toy preferences similar to those shown previously in children (Alexander & Hines, 2002). Finally, and more central to our hypothesis, standardized inventories of sex differences in play behaviours have been constructed, such as the Pre-School Activities Inventory (PSAI; Golombok & Rust, 1993), which has been shown to be sensitive to early androgen exposure (Hines et al., 2002; Auyeung et al., 2009) and to reflect the endocrine-disrupting properties of dioxins and Polychlorinated Biphenyls (PCBs) (Vreugdenhil et al., 2002). We chose to use a slightly modified version of the PSAI (which we refer to as PSAI-M) to investigate changes in sex-typical play behaviours in a subsample of the population described in Swan et al. (2005) and Swan (2008).
Materials and methods
Study population
The Study for Future Families (SFF) is a multi-centre pregnancy cohort study in which women and their partners were recruited at prenatal clinics. Initial recruitment took place at clinics affiliated with university hospitals in Los Angeles, CA (Harbor-UCLA and Cedars-Sinai), Minneapolis, MN (University of Minnesota Health Center) and Columbia, MO (University Physicians) between September 1999 and December 2002 (Swan et al., 2003). Recruitment in Iowa City, IA (University of Iowa) was conducted during 2002–2005. Couples who were at least 18 years old, who spoke English or Spanish and whose pregnancy was conceived without medical treatment were eligible. If the couple agreed to participate, both partners completed questionnaires and gave a serum sample (at mean 28.6 weeks of pregnancy) and, for those recruited after September 2000, a urine sample.
In 2000, we initiated a follow-up study (SFFII) to measure genital parameters in human infants in relation to their mother's prenatal phthalate exposure. Eighty-five per cent of SFFI participants had agreed to be recontacted, and we invited these mothers to take part in SFFII if the pregnancy ended in a live birth, the baby was 2–36 months of age at the time of recontact, the mother lived within 50 miles of the clinic, and had provided a urine sample during pregnancy. In 2006, we initiated a second follow-up of SFF children with the goal of examining play behaviour in relation to prenatal phthalate metabolite concentration, results of which are reported here. For this study, children born during 2000–2003 who had a physical exam in SFFII and whose mothers had provided a urine sample were eligible. Human subject committees at all participating institutions approved SFF-I and SFF-II and all subjects signed informed consents for each study. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was limited and determined not to constitute engagement in human subjects’ research.
Sample collection and measurement of phthalate metabolites
Urine samples were collected during mid-pregnancy at the time of the mother's prenatal visit. Staff at the Division of Laboratory Sciences of the CDC, which had no access to subject data, carried out the analyses. The uri-nary concentrations of nine urinary phthalate metabolites were measured using an analytical approach that involves the enzymatic deconjugation of the metabolites from their glucuronidated form, automated on-line solid-phase extraction, separation with high performance liquid chromatography and detection by isotope-dilution tandem mass spectrometry (Silva et al., 2004). Isotopically labelled internal standards were used along with conjugated internal standards to increase precision and accuracy of the measurements. Along with the samples, each analytical run included calibration standards, reagent blanks and quality control materials of high and low concentration to monitor for accuracy and precision. Limits of detection (LOD) were in the low nanogram per millilitre (ng/mL) range. Most metabolite concentrations were above the LOD; those below the LOD were assigned the value LOD divided by the square root of 2, which has been recommended when, as in this case, the data are not highly skewed (Hornung & Reed, 1990).
Pre-School Activities Inventory
Parents were asked to complete the PSAI (Golombok & Rust, 1993) as well as a brief questionnaire that included questions on other relevant covariates (such as age, number and age of siblings, parental education and questions on parental attitudes towards sex-atypical toy choice). The PSAI is designed to discriminate play behaviour both within and between the sexes, and has been standardized on children in the UK, the Netherlands and the US (Golombok et al., 2008). It consists of 24 items (12 considered ‘feminine’ and 12 ‘masculine’) addressing three aspects of play behaviour: type of toys, activities and child characteristics. Answers are given on a 5-point, Likert-type scale ranging from ‘never’ to ‘very often’. A total score is computed based on the sum of scores for masculine items, minus the sum of scores for feminine items following Golombok & Rust (Golumbok & Rust 1993). A higher total (composite) score implies more male-typical play behaviour and a lower score implies more female-typical play behaviour. We also looked at the masculine and feminine subscale totals separately. For these subscales, a higher score on the feminine scale indicates more feminine play behaviour, whereas a higher score on the masculine scale indicates more masculine play behaviour.
Parental Attitude Scale
As the child's choice of a toy might depend on the availability of that toy in the household, or the parents’ views about the child's play with that toy, we attempted to assess the parental attitudes towards sex-atypical play. The mother was asked, ‘what would you do if you had a boy who preferred toys that girls usually play with?’ The five possible responses ranged from ‘strongly encourage’ (him to play in this way) to ‘strongly discourage’. She was also asked how she thought the father of a boy would respond to these questions (see Appendix). The five possible responses for each parents’ attitude were coded 1–5 and summed for each parent. This resulted in a score, which we called parental attitude-boys (PAB), reflecting the combined responses of both parents towards boys’ play, for which a value of 2 reflects the strongest encouragement of a boy to play with toys ‘girls usually play with’ and 10 the strongest discouragement of such play, whereas six indicates neutrality. A similar scale (PAG) was constructed for girls. PAB was included when modelling boys’ PSAI-M in relation to phthalate metabolite concentrations and PAG when modelling girls’. As these variables have not been used previously and remain to be validated, we also analysed our data without including them to assess their influence on our results.
Statistical analysis
Phthalate metabolite distributions were reviewed. As these distributions were extremely skewed, and because the relationships between metabolites concentrations and PSAI-M scores were markedly more linear on the logarithmic scale, metabolite concentrations were log10-transformed in all analyses. Prior to examining associations between phthalate metabolite concentrations and outcomes, we reviewed the data for extreme values (e.g. those that were more than 1.5 times the interquartile range above the 75th percentile, after log transformation) and excluded three subjects whose DEHP metabolite concentrations were extreme, and one whose MnBP concentration was extreme. One girl whose mother was missing all phthalate metabolite concentrations was also excluded. In addition, prior to examining associations between phthalate metabolite concentrations and outcomes, we conducted an item analysis of each of the 24 PSAI questions and examined their distribution in boys and girls. After examining unadjusted correlations between phtha-late metabolites and the PSAI-M scores, we conducted multiple regression analyses to examine the relationships between these variables. Covariates initially considered in these analyses were: creatinine concentration, sex and age of child, maternal age, parental education, number of the same and opposite sex siblings, ethnicity, clinic location and parental attitude. Covariates that altered the effect estimates for at least one metabolite-play behaviour score by at least 10% (maternal age, boy's age, mother's education, father's education, parental attitude and the interaction of mother's education and parental attitude) were retained in the model. Regression analyses were performed using Generalized Linear Models (SAS Institute Inc, 1999).
Results
As questionnaires were mailed to families 4–7 years after their initial enrollment in the study, many had moved and could no longer be contacted. Of the 334 eligible families to whom questionnaires were mailed, 128 were returned undeliverable, presumably because the family had moved. Of the 206 questionnaires that were not returned undeliverable, all but 56 were returned completed (72.8%). Mothers who returned completed questionnaires were somewhat more likely to be Caucasian (88% vs. 78.6%) and to have completed college (73.3% vs. 68.4%) than those who could not be contacted, or who failed to return a completed questionnaire.
We conducted an item analysis of the 24 questions (available upon request). Q5F (‘avoids taking risks’) had been classified as a ‘feminine’ question. However, the mean (2.6) and median (3.0) were equal in boys and girls in our sample. Similarly, the mean (3.2) and median (4.0) for Q5A (‘likes to explore new surroundings’), which was classified as a masculine question, were the same in boys and girls. As these items were not sex-dimorphic in our sample, they were dropped, reducing the total number of items to 22 and forming the modified instrument (PSAI-M), which we used in all analyses. As shown in Table 1, the mean and standard deviations of the composite scores obtained using PSAI-M in our population were in close agreement with those for several thousand children obtained using the original PSAI (Golombok et al., 2008).
Table 1.
Summary statistics for modified Pre-School Activities Inventory (PSAI) compared with published data on PSAIa
| PSAI-M (current study) | PSAI (Golombok et al., 2008) | |
|---|---|---|
| Boys | ||
| N | 74 | 2726 |
| Mean age (months) | 60 | 57 |
| Mean composite score (SD) | 65.9 (8.6) | 64.2 (8.8) |
| Girls | ||
| N | 71 | 2775 |
| Mean age (months) | 59 | 57 |
| Mean composite score (SD) | 31.4 (8.7) | 35.1 (9.4) |
PSAI-M excludes two items that were equally distributed in boys and girls in the current study.
None of the urinary concentrations of phthalate metabolites other than those of DEHP and DBP were associated with play behaviour scores in boys or girls in either univariate or initial multivariate analyses (p-values were between 0.23 and 0.99 for all metabolites other than those of DEHP and DBP; data not shown). Nor were the concentrations of any phthalate metabolites associated with play behaviour in girls. Therefore, the remaining analyses were limited to metabolites of DEHP: mono-(2-ethylhexyl) phthalate (MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) as well as their sum (denoted RDEHP), and metabolites of DBP: mono-n-butyl phthalate (MnBP) and mono-isobutyl phthalate (MiBP), as well as their sum (denoted ΣDBP) in relation to play behaviour scores in boys.
Summary statistics for the three PSAI-M scores and other covariates are shown in Table 2. Mean parental attitude scores (PAB and PAG) were close to neutral, with parents of girls somewhat more likely to discourage sex-atypical play (A value of 3 indicates the parent would neither encourage nor discourage such play. The sum of these, combining attitudes of both parents, was included in multivariate models, for which a value of 6 indicates neutrality).
Table 2.
Summary of Pre-School Activities Inventory (PSAI) scores and covariates
| Variable | Boys (N = 74)a Mean/SD/range | Girls (N = 71)a Mean/SD/range |
|---|---|---|
| PSAI-M Scores | ||
| Composite | 65.9/8.6/37.3–83.5 | 31.4/8.7/13.0–51.6 |
| Masculine | 40.8/5.9/26–53 | 27.9/5.7/16.0–43.0 |
| Feminine | 24.7/6.1/13–43 | 43.2/5.5/30.0–54.0 |
| Parental Attitude Scores | ||
| PAB (Boys’ play) | 5.6/1.5/2.0–8.0 | 5.9/1.6/ 2.0–10.0 |
| PAG (Girls’ play) | 5.0/0.6/3.0–9.0 | 6.9/1.3/3.0–10.0 |
| Mother's age (years) | 30.5/5.2/18.3–40.3 | 30.3/5.4/18.7–41.3 |
| Child's age (years) | 5.0/0.6/3.6–6.4 | 4.9/0.7/3.6–6.0 |
| Mother completed college (%) | 81.1 | 63.3 |
| Father completed college (%) | 64.9 | 63.3 |
Excludes four mothers with extreme phthalate metabolite values and one with missing phthalate values.
Phthalate metabolite concentrations in our sample (shown in Table 3), are consistent with those reported in a national sample (CDC, 2005). We initially included creatinine concentration in these models to adjust for urinary dilution, However, creatinine concentration was not retained in final models, as point estimates were close to zero (p-values = 0.52–0.90) and removing this variable had little influence on the effect estimates.
Table 3.
Mean and percentiles for DEHP and DBP metabolite concentration in prenatal urine by sex
| Metabolite (ng/mL) | Boys (N = 74)a |
Girls (N = 71)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phthalate | Mean | 25th | 50th | 75th | % > LOD | Mean | 25th | 50th | 75th | % > LOD | |
| DEHP | MEHP | 5.2 | 1.4 | 2.9 | 6.2 | 78.3 | 8.7 | 1.2 | 4.3 | 11.3 | 77.4 |
| MEHHP | 16.0 | 5.2 | 9.8 | 17.3 | 98.6 | 18.3 | 6.3 | 12.1 | 21.5 | 98.5 | |
| MEOHP | 14.3 | 4.7 | 9.0 | 17.9 | 94.5 | 15.5 | 5.4 | 10.8 | 20.7 | 97.1 | |
| ΣDEHP | 35.6 | 11.7 | 22.6 | 40.3 | 42.5 | 16.0 | 28.0 | 58.2 | |||
| DBP | MnBP | 19.4 | 6.9 | 12.5 | 28.3 | 97.2 | 23.0 | 9.0 | 18.0 | 32.3 | 98.5 |
| MiBP | 4.0 | 0.7 | 2.4 | 5.1 | 72.9 | 4.1 | 1.5 | 2.8 | 5.0 | 80.2 | |
| ΣDBP | 23.4 | 8.3 | 14.8 | 34.0 | 27.0 | 10.0 | 20.5 | 37.1 | |||
| Creatinine (mg/dL) | 79.3 | 33.0 | 59.0 | 124 | 94.3 | 51.7 | 76.4 | 130 | |||
LOD, limit of detection.
Excludes four samples with extreme phthalate metabolite concentration and one with missing phthalate concentrations.
The two metabolites of DBP, as well as their sum, were associated with a decreased (less masculine) composite score in boys. Regression coefficients were −4.53, −3.61 and −4.20 (p = 0.01, 0.07 and 0.04) for MiBP, MnBP and their sum (denoted ΣDBP) respectively (Table 4). Of the DBP metabolites, only MiBP was associated (positively) with the feminine score (coefficient 2.48, p = 0.07). The (weak) negative associations between DBP metabolites and the masculine score were unremarkable.
Table 4.
Regression coefficients (95% CI) for boys’ Pre-School Activities Inventory (PSAI) scores on concentration of (log10) phthalate metabolite concentration in prenatal urine
| Phthalate | Metabolite | Composite | Masculine | Feminine |
|---|---|---|---|---|
| DEHP | MEHP | –1.04 (–4.72 to 2.63) | –0.95 (–3.85 to 1.95) | –0.01 (–2.66 to 2.66) |
| MEHHP | –2.24 (–5.95 to 1.46) | –3.29 (–6.14 to –0.43) | –1.25 (–3.93 to 1.44) | |
| MEOHP | –2.44 (–6.10 to 1.22) | –2.94 (–5.78 to –0.10) | –0.72 (–3.39 to 1.95) | |
| ΣDEHP | –2.64 (–6.60 to 1.32) | –3.18 (–6.26 to –0.10) | –0.78 (–3.67 to 2.11) | |
| DBP | MnBP | –3.61 (–7.48 to 0.26) | –2.21 (–5.29 to 0.87) | 1.07 (–1.77 to 3.92) |
| MiBP | –4.53 (–8.12 to –0.94) | –1.65 (–4.57 to 1.28) | 2.48 (–0.16 to 3.92) | |
| ΣDBP | –4.20 (–8.18 to –0.23) | –2.32 (–5.50 to 0.86) | 1.50 (–1.43 to 4.43) |
Using Generalized Linear Models controlling for boy's age, mother's age, mother's education, parents’ attitude towards boy's play, and interaction of mother's education and attitude towards boy's play.
Concentrations of two urinary metabolites of DEHP, MEOHP and MEHHP, as well as the sum of concentrations of MEHHP, MEOHP and MEHP (denoted ΣDEHP), were associated with a decreased masculine score. Regression coefficients were −3.29, −2.94 and −3.18 (p = 0.02, 0.04 and 0.04 for MEHHP, MEOHP and ΣDEHP respectively), as seen in Table 5. Associations between DEHP metabolites and the composite and feminine scores were weak (all p-values >0.36).
Table 5.
Regression coefficients (95% CI) for girls’ Pre-School Activities Inventory (PSAI) scores on concentration of (log10) phthalate metabolite concentration in prenatal urine
| Phthalate | Metabolite | Composite | Masculine | Feminine |
|---|---|---|---|---|
| DEHP | MEHP | –0.01 (–3.37 to 3.37) | 0.07 (–2.15 to 2.29) | 0.07 (–2.26 to 2.40) |
| MEHHP | –1.08 (–5.13 to 2.97) | –0.56 (–3.23 to 2.11) | 0.42 (–2.38 to 3.23) | |
| MEOHP | –1.39 (–5.57 to 2.78) | –0.82 (–3.57 to 1.93) | 0.45 (–2.45 to 3.34) | |
| ΣDEHP | –0.81 (–4.94 to 3.31) | –0.42 (–3.14 to 2.30) | 0.32 (–2.53 to 3.17) | |
| DBP | MnBP | –1.07 (–5.46 to 3.32) | 0.21 (–2.69 to 3.10) | 1.18 (–1.85 to 4.20) |
| MiBP | 0.38 (–3.86 to 4.63) | 1.04 (–1.75 to 3.82) | 0.69 (–2.24 to 3.62) | |
| ΣDBP | –0.87 (–5.41 to 3.67) | 0.21 (–2.78 to 3.20) | 1.01 (–2.13 to 4.14) |
Using Generalized Linear Models controlling for boy's age, mother's age, mother's education, parents’ attitude towards boy's play, and interaction of mother's education and attitude towards boy's play.
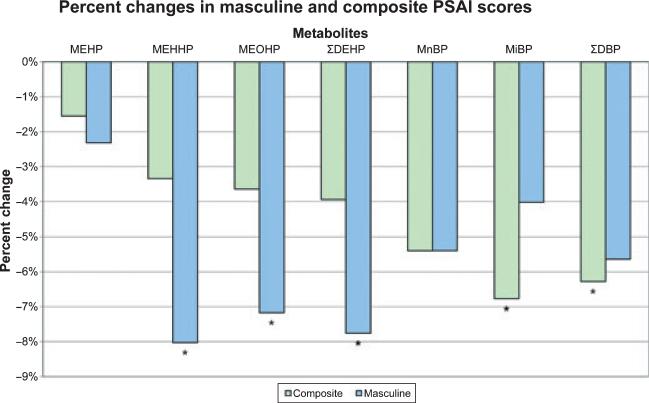
While not all associations between DEHP and DBP metabolite concentrations and play behaviour reached statistical significance at p = 0.05, all regression coefficients for the masculine and composite scores and these metabolites were negative for boys, suggesting that these metabolites are (to various degrees) associated with less masculine play behaviour. To express these in terms that are easier to interpret than the regression coefficients, we show the per cent change in the composite and masculine scores the model would predict, if the mothers’ metabolite concentration was increased from the 10th to the 90th percentile. These predicted changes are shown in Fig. 1. Those for which the significance probability of the underlying effect estimates reached p = 0.05 are starred.
Figure 1.
Percent change in PSAI-M score expected if the phthalate metabolite concentration in boy's mother's prenatal urine was increased from the 10th percentile to the 90th percentile. Stars indicate p-values of <0.05 for regression coefficients in multivariate model.
We reran these analyses excluding the Parental Attitude Scores (and their interaction with maternal education). We found that questions on parental attitude were important for some associations, but not all. Removing these questions resulted in changes to the effect estimates of between 0 and 12.5%. In particular, adding these parental attitude questions strengthened the associations between DEHP metabolites and the masculine score. On the other hand, when PAB is not included, the association between MiBP concentrations and boys’ feminine score is somewhat stronger (coefficient = 2.79, p-value = 0.048), implying more feminine play with higher DBP exposure. However, after adjusting for parental attitude this association is reduced somewhat (coefficient 2.48, p = 0.07), suggesting some negative confounding by PAB.
Discussion
Even with a relatively small pool of subjects, the message emerging from this study is consistent with the hypothesis that animated its design. Namely, that the antiandrogenic properties of some phthalate esters, documented in scores of papers on the developing male rodent reproductive tract, and more recently by studies in humans (Swan, et al., 2005; Swan, 2008) possess the potential to modify male behaviour, potentially reflecting changes to the developing brain. If replicated in a larger sample, it would be a finding with implications that extend far beyond the scope of children's play preferences, as noted below.
How large were the changes we observed? Given the non-intuitive nature of the outcome scores and the fact that they were modelled in relation to the logarithm (base 10) of the urinary metabolite concentrations, interpretation of the regression coefficients is not straightforward. As an example, consider the coefficient of −3.29 for (log10) MEHHP in relation to the masculine score as an example, which implies that a one-unit change in the log10 MEHHP metabolite concentration was associated with a decrease of 3.29 in the boys’ masculine score, conditional on fixed values of all other model covariates. The 10th and 90th percentiles of log10 MEHHP differ by 1.0, so the predicted change in the boys’ masculine score for an increase in MEHHP concentration from the 10th to the 90th percentile is a decrease of −3.29. If a boy had a typical (median) masculine score (40.5) and his mother's MEHHP metabolite concentration increased from the 10th to 90th percentile, his score would be expected to decrease to 37.5 (a decrease of 8%), which would bring his masculine score down to the 27th percentile. Other predicted decreases in boy's PSAI masculine and composite scores associated with unit increases in DEHP and DBP metabolite concentrations are shown in Fig. 1.
As noted in the introduction, there are critical periods for hormonal influences on brain development in mammals, beginning in utero. Sex differences in patterns of behaviour relate to differences in brain organization that develop under the influence of testosterone and its metabolites during this period, although societal expectations and contingencies, beyond the period of early development, also exert significant influences. It is understandable, therefore, that antiandrogenic chemicals, a class that includes phthalates, are capable of influencing the development of male-typical behaviour, much as they impair masculinization of genital structures. Two other antiandrogenic chemicals, vinclozolin (Hotchkiss et al., 2003; Colbert et al., 2005) and flutamide (Casto et al., 2003), also reduce male-typical play behaviour in male rats that are exposed prenatally.
Our subject population consisted of children 3.6–6.4 years of age. Many investigations of children's behaviours seem to accord sex differences a minor role, providing no standards for boys and girls separately. Many standard behavioural assessments do not provide information on sex differences in young children. For example, the Ages and Stages questionnaire (Squires et al., 1997) is standardized on the basis of age and not distinguished by sex. Norms for the Parents’ Evaluation of Developmental Status questionnaire (Brothers et al., 2008) are also not differentiated by sex (Brothers et al., 2008).
Sex differences in play preferences, however, are detectable early in development. Toy choices in girls and boys have been found to differ in infants as young as 12–13 months of age (Servin et al., 1999; Van De Beek et al., 2009), and may be manifest even earlier, as reflected in visual attention. For instance, Alexander et al. (2009) found that 3- to 8-month-old girls and boys showed different patterns of visual fixations to images of dolls and trucks. Similar sex differences in toy preferences have been seen in non-human primates (Alexander & Hines, 2002; Hassett et al., 2008). These differences may be based on perceptual features of the stimuli that arouse different responses in males and females governed by how the brain was organized by androgens during development. In a finding somewhat related to ours, gestational phthalate exposure was reported to be related to different patterns of response in male and female neonates on the Brazelton Neonatal Behavioural Assessment Scale (Engel et al., 2009).
Phthalates are endocrine-disrupting chemicals (EDCs). Sex-specific influences of environmental chemicals on endpoints such as reproductive tract anomalies are comparatively straightforward to investigate, which accounts for the substantial literature on such measures. A comprehensive evaluation of how development is altered by EDCs must include neurobehavioural endpoints. Here, appropriate tools for assessment are particularly crucial because they may reveal important effects whose nature (e.g. cognitive style, social behaviours such as play, temperament) makes them easy to overlook in conventional toxicity assessment.
We obtained only a single prenatal urine sample from each woman and most were obtained quite late in pregnancy (mean 28.3 weeks). Therefore, the phthalate metabolite concentrations reported here may not reflect exposure during the most sensitive developmental window. However, a recent study of the variability of phthalate metabolite concentrations in men of reproductive age found that a single urine sample was reasonably predictive of the subject's exposure to the parent phthalate over 3 months (Hauser et al., 2004). This may reflect habitual use of phthalate-containing household and consumer products.
Our analysis took a simple approach to the link between phthalate exposure and play behaviour by examining one phthalate at a time. As we hypothesized based on anti-androgenic activity reported in many rodent (as well as human) studies, metabolites of DEHP and DBP were most strongly associated with play behaviour in males, whereas other phthalate metabolites (MEP, MCPP, MMP and MBzP) were not, when examined individually. However, our environment, in contrast, exposes us to many phthalates, to a variety of other antiandrogenic agents, and to other exposures with unknown effects and interactions. Of our children examined at a mean age of 12 months, 81% had detectable concentrations of at least seven phthalate metabolites in their urine (Sathyanarayana et al., 2008). As shown by Rider et al. (2008), Sharpe et al. (1995) and Howdeshell et al. (2008), antiandrogens with diverse mechanisms of action, including phthalates, exert similar effects on male reproductive development in a dose-additive fashion. These findings suggest that exposure assessments should be based on exposure to multiple agents that act on common endpoints if we are not to underestimate their combined effects. Our own data indicate that, even where not statistically significant, associations for all five of the DEHP and DBP metabolites suggested less male-typical play behaviour. A more sophisticated model of joint action of these metabolites is warranted.
This study is the first to relate complex sexually dimorphic behaviour to phthalate exposure. Although our results are based on a relatively small sample, their internal consistency and their compatibility with current knowledge about how gonadal hormones mould sex differences in brain and behaviour support their plausibility. Their implications warrant extensive investigation.
Acknowledgements and grant information
This work was supported by grants from the U.S. Environmental Protection Agency and the National Institutes of Health (Environmental Health Sciences Center Grant ES01247, R21ES015509 and R01-ES09916 to the University of Rochester, MO1-RR00400 to the University of Minnesota, MO1-RR0425 to Harbor-UCLA Medical Center), and by grant 18018278 from the State of Iowa to the University of Iowa. We acknowledge the technical assistance of Antonia Calafat, Manori Silva, Ella Samandar, James Preau, Jack Reidy and others at the Centers for Disease Control and Prevention (CDC) in measuring the urinary concentrations of phthalate metabolites. We thank the health care providers and study participants at University Physicians Clinic, Columbia, MO; Fairview Riverside Women's Clinic, Minneapolis, MN; Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center; Cedars-Sinai Medical Center; and University of Iowa Hospitals and Clinics, and Jenny Carwile for her assistance in data collection and analysis. The authors declare they have no competing financial interests.
Appendix: Parental Attitude Questions
In the following four questions, ‘Encourage’ means ‘Encourage him (or her) to play in the way described in the question’ and ‘Discourage’ means ‘Discourage him (or her) from playing in the way described in the question’.
References
- Alexander GM, Hines M. Sex differences in response to children's toys in nonhuman primates (Cercopithecus aethiops sabaeus). Evolution and Human Behavior. 2002;23:467–479. [Google Scholar]
- Alexander GM, Wilcox T, Woods R. Sex differences in infants’ visual interest in toys. Archives of Sexual Behavior. 2009;38:427–433. doi: 10.1007/s10508-008-9430-1. [DOI] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, Hines M. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychological Science. 2009;20:144–148. doi: 10.1111/j.1467-9280.2009.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers KB, Glascoe FP, Robertshaw NS. PEDS: developmental milestones – an accurate brief tool for surveillance and screening. Clinical Pediatrics (Phila) 2008;47:271–279. doi: 10.1177/0009922807309419. [DOI] [PubMed] [Google Scholar]
- Casto JM, Warda OB, Bartke A. Play, copulation, anatomy, and testosterone in gonadally intact male rats prenatally exposed to flutamide. Physiology & Behavior. 2003;79:633–641. doi: 10.1016/s0031-9384(03)00120-3. [DOI] [PubMed] [Google Scholar]
- CDC . Third National Report on Human Exposure to Environmental Chemicals CDC. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Environmental Health, Division of Laboratory Sciences; Atlanta, GA: 2005. [Google Scholar]
- Colbert NKP, Cote JM, Concannon JB, Jurdak NA, Minott SB, Markowski VP. Perinatal exposure to low levels of the environmental antiandrogen vinclozolin alters sex-differentiated social play and sexual behaviors in the rat. Environmental Health Perspectives. 2005;13:700–707. doi: 10.1289/ehp.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, Wolff MS. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30:522–528. doi: 10.1016/j.neuro.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. International Journal of Andrology. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Golombok S, Rust J. The measurement of gender role behaviour in pre-school children: a research note. Journal of Child Psychology and Psychiatry. 1993;34:805–811. doi: 10.1111/j.1469-7610.1993.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Golombok S, Rust J, Zervoulis K, Croudace T, Golding J, Hines M. Developmental trajectories of sex-typed behavior in boys and girls: a longitudinal general population study of children aged 2.5–8 years. Child Development. 2008;79:1583–1593. doi: 10.1111/j.1467-8624.2008.01207.x. [DOI] [PubMed] [Google Scholar]
- Hassett JM, Siebert ER, Wallen K. Sex differences in rhesus monkey toy preferences parallel those of children. Hormones and Behavior. 2008;54:359–364. doi: 10.1016/j.yhbeh.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environmental Health Perspectives. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Golombok S, Rust J, Johnston KJ, Golding J. Testosterone during pregnancy and gender role behavior of pre-school children: a longitudinal, population study. Child Development. 2002;73:1678–1687. doi: 10.1111/1467-8624.00498. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondectable values. Applied Occupational and Environmental Hygiene. 1990;5:46–51. [Google Scholar]
- Hotchkiss AKO, Vandenbergh JG, Gray LE., Jr An environmental antiandrogen, vinclozolin, alters the organization of play behavior. Physiology and Behavior. 2003;79:151–156. doi: 10.1016/s0031-9384(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, Gray LE., Jr A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague Dawley rat in a cumulative, dose additive manner. Toxicological Sciences. 2008;105:153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Kamrin MA. Phthalate risks, phthalate regulation, and public health: a review. Journal of Toxicology and Environmental Health. Part B, Critical Reviews. 2009;12:157–174. doi: 10.1080/10937400902729226. [DOI] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environmental Health Perspectives. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis SM. Sex differences in play fighting revisited: traditional and nontraditional mechanisms of sexual differentiation in rats. Archives of Sexual Behavior. 2002;31:17–26. doi: 10.1023/a:1014070916047. [DOI] [PubMed] [Google Scholar]
- Reyes FI, Winter JS, Faiman C. Studies on human sexual development I. Fetal gonadal and adrenal sex steroids. Journal of Clinical Endocrinology and Metabolism. 1973;37:74–78. doi: 10.1210/jcem-37-1-74. [DOI] [PubMed] [Google Scholar]
- Rider CV, Furr J, Wilson VS, Gray LE., Jr A mixture of seven antiandrogens induces reproductive malformations in rats. International Journal of Andrology. 2008;31:249–262. doi: 10.1111/j.1365-2605.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute, Inc . The SAS System for Windows, version 8.0. SAS Institute, Inc; Cary, NC: 1999. [Google Scholar]
- Sathyanarayana S, Karr CJ, Lozano P, Brown E, Calafat AM, Liu F, Swan SH. Baby care products: possible sources of infant phthalate exposure. Pediatrics. 2008;121:e260–e268. doi: 10.1542/peds.2006-3766. [DOI] [PubMed] [Google Scholar]
- Servin A, Bohlin G, Berlin L. Sex differences in 1-, 3-, and 5 year old toy-choice in a structured play setting. Scandinavian Journal of Psychology. 1999;40:43–48. doi: 10.1111/1467-9450.00096. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Fisher JS, Millar MM, Jobling S, Sumpter JP. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environmental Health Perspectives. 1995;103:1136–1143. doi: 10.1289/ehp.951031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Slakman AR, Reidy JA, Preau JL, Jr, Herbert AR, Samandar E, Needham LL, Calafat AM. Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction. Journal of Chromatography B: Analytical Technologies in the Biomedical & Life Sciences. 2004;805:161–167. doi: 10.1016/j.jchromb.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Smail PJ, Reyes FI, Winter JSD, Faiman C. The fetal hormone environment and its effect on the morphogenesis of the genital system. In: Kogan SJ, Hafez ESE, editors. Pediatric Andrology. Martinus Jijhoff; The Hague: 1981. [Google Scholar]
- Squires J, Bricker D, Potter L. Revision of a parent-completed development screening tool: ages and Stages Questionnaires. Journal of Pediatric Psychology. 1997;22:313–328. doi: 10.1093/jpepsy/22.3.313. [DOI] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environmental Research. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, Hatch M, Redmon JB, Wang C, Overstreet JW, The Study for Future Families Research, G Geographic differences in semen quality of fertile US males. Environmental Health Perspectives. 2003;111:414–420. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental Health Perspectives. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Beek C, van Goozen SHM, Buitelaar JK, Cohen-Kettenis PT. Prenatal Sex Hormones (Maternal and Amniotic Fluid) and Gender-related Play Behavior in 13-month-old Infants. Archives of Sexual Behavior. 2009;38:6–15. doi: 10.1007/s10508-007-9291-z. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil HJI, Slijper FME, Mulder PGH, Weisglas-Kuperus N. Effects of perinatal exposure to PCBs and dioxins on play behavior in Dutch children at school age. Environmental Health Perspectives. 2002;110:A593–A598. doi: 10.1289/ehp.021100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Frontiers in Neuroendocrinology. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. The Journal of Clinical Investigations. 2008a;118:1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]