Abstract
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that mediates diverse dioxin toxicities. While the acute effects of activation of the AhR pathway by 2,3,7,8-tetrachlorodibenzo-p-dioxin have been a focus of past study, the role of this pathway in normal physiology and aging is unclear. The purpose of this study was to identify the portion of the reproductive axis (ovary vs. hypothalamus and pituitary gland (H-H axis)) and the stages of the reproductive lifespan (fetal and early postnatal life vs. adolescence and adulthood) that are particularly sensitive to the effects of TCDD during female reproductive aging. Adult pregnant Lewis rat dams were dosed with corn oil vehicle or TCDD (50 ng/kg-week by gavage) on days 14 and 21 of gestation and postnatal days 7 and 14 to provide in utero and lactational (IUL) exposure to pups. Female pups (n=96) were weaned on postnatal day 21 and dosed with TCDD or vehicle weekly. Half of the pups were used as donors for ovary transplantation while the remainder were recipients. Following ovary transplantation, rats (n=6-8 per group) received weekly TCDD or vehicle again until sacrifice at 8 months of age. Beginning at vaginal opening, reproductive cycles were monitored by vaginal cytology for 10 days each month. Blood samples were collected at 2200 h on proestrus to measure concentration of 17β-estradiol in serum. Real-time PCR was used to determine differences in Cyp1a1, Cyp19a1, Cyp17a1, LHR, FoxA2 and FoxJ1 genes expression between control and remaining groups. IUL exposure of the H-H axis plus adult exposure of the whole body to TCDD significantly delayed puberty in females rats. Data analysis revealed an accelerated onset of acyclicity by 5 months in all groups involving IUL exposure of the developing ovary to TCDD. 17β-estradiol was significantly decreased in animals receiving TCDD during IUL exposure of the H-H axis. CYP1a1 expression was markedly greater in the liver than in ovarian tissue and correlated with ongoing TCDD exposure. Aromatase, 17α-hydroxylase and LHR gene expressions were largely unchanged (or occasionally elevated) by TCDD. FoxA2 and FoxJ1 mRNAs were similarly of limited value mechanistically, although FoxJ1 was much higher in TTT females (receiving TCDD as donor, recipient and adult). This study reveals a particular sensitivity of the developing ovary to TCDD leading to early loss of reproductive function with age.
Keywords: aryl hydrocarbon receptor, TCDD, ovary, aging, rat
Introduction
Dioxin-like compounds are environmental contaminants of great concern produced as unwanted byproducts of industrial processes and combustion (Kulkarni et al., 2008). Some are resistant to chemical and biological degradation and bioaccumulate in animals and humans (Larsen, 2006). The most toxic dioxin is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). The toxicity of the TCDD is almost exclusively mediated through the aryl hydrocarbon receptor (AhR; Beischlag et al., 2008).
The AhR is a member of the basic helix-loop-helix (bHLH)-Per-ARNT-Sim (PAS) family of transcriptional factors that control a variety of developmental and physiological events (Pocar et al., 2005; Beischlag et al., 2008). Acute AhR pathway activation by TCDD causes female endocrine disruption, altered sexual behavior, diminished fertility, endometriosis-like symptoms, teratogenesis and abortion (Gray & Ostby, 1995; Birnbaum, 1998; Sharara et al., 1998; Petroff et al., 2001). However, little is known of the potential effects of chronic environmental exposure to AhR ligands on lifelong reproductive performance and aging of the reproductive system.
In our previous studies, chronic exposure to TCDD hastened reproductive aging in females in a reproductive phenotype, including declining estradiol synthesis and increasingly irregular reproductive cycles without depletion of follicular reserves (Franczak et al., 2006; Shi et al., 2007). Ovarian and hypothalamic dysfunction are central to most theories of reproductive senescence (Page et al., 1982; Steger & Peluso, 1987; vomSaal et al., 1994; Rubin, 2000); however, the role of the AhR pathway in the loss of reproductive function with age is unclear. The present in vivo study was intended to identify the portion of the reproductive axis (ovary vs. hypothalamus and pituitary gland) and the stages of the reproductive lifespan (fetal and early postnatal life vs. adolescence and adulthood) that are particularly sensitive to the effects of TCDD during female reproductive aging. This was attempted using an ovary transplantation model that allowed selective ovarian and hypothalamo-hypophyseal exposure to TCDD in female rats.
Materials and Methods
Animals
Inbred rats of a strain allowing transplantation without immunosuppression (Lewis Furth, Charles River Laboratories; [Pan et al., 2003]) were used in the experiment. Adult pregnant females (n=24) were purchased and housed under a 12:12 light:dark photoperiod with controlled temperature (23±2°C) and humidity. Rodent chow (Ralston Purina Co., St. Louis, MO) and water were provided ad libitum. All procedures were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Treatments of animals and ovary transplantation
Dams were dosed with corn oil vehicle or TCDD (50 ng/kg-week by gavage) on days 14 and 21 of gestation and postnatal days 7 and 14 to provide in utero and lactational (IUL) exposure to pups, as was done in our previous study (Shi et al., 2007). This dose of TCDD (50 ng/kg-week) mimic lifetime exposure of high risk populations to dioxin-like compounds (Geusau et al., 2001) and have been well characterized for other endpoints in previous chronic studies (Kociba et al., 1978). TCDD (CAS 1746-01-6; MW, 321.9; purity >99%) was obtained from Cambridge Isotope Laboratories, Inc. (Lenexa, KS). Female pups (n=96) were weaned on postnatal day 21 and dosed with TCDD or vehicle weekly. Half of the pups were used as donors for ovary transplantation while the remainder were recipients (Fig. 1; Table 1). All rats were anesthetized for ovary transfer (50 mg/kg ketamine and 8 mg/kg xylazine, IP). Donor animals were humanely sacrificed and ovaries collected using sterile technique. To facilitate intrabursal transplantation of intact ovaries, recipient animals were slightly older than donors (21d vs. 30d, respectively). Ovaries of recipient females were exteriorized by bilateral flank incisions, ovarian bursae fenestrated with fine scissors, and ovaries removed using blunt dissection with fine forceps. Transferred ovaries were placed into the bursal space, and edges of the bursal incision were apposed using gentle pressure with fine forceps. The transplantation model allowed us to examine whether the ovary or the hypothalamo-hypophyseal (H-H) axis is more sensitive to TCDD during the in utero-lactational (IUL) period vs. later life.
Fig. 1.
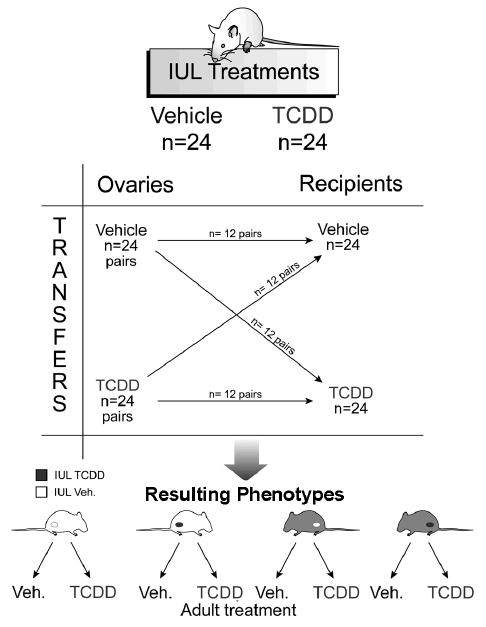
Experimental scheme for ovary transfer experiment. Adult pregnant dams were dosed with corn oil vehicle (V) or TCDD (50 ng/kg-week by gavage; T) on days 14 and 21 of gestation and postnatal days 7 and 14 to provide in utero and lactational (IUL) exposure to pups. Female pups (n=96) were weaned on postnatal day 21 and dosed with TCDD or vehicle weekly. Half of the pups were used as donors for ovary transplantation while the remainder were recipients. Following ovary transplantation, rats (n=6-8 per group) received weekly TCDD or vehicle again until sacrifice at 8 months of age. Animal groups were named by three-letter designations, with the first letter indicating IUL exposure for the donor animal, the second letter indicating IUL recipient treatment, and the third letter denoting post-transplantation treatment.
Tab. 1.
Names of treatment groups
| IUL Treatments | Treatment after transplantation | Final group name | |
|---|---|---|---|
| Ovary (DONOR) | H-H axis (RECIPIENT) | ||
| Vehicle | Vehicle | Vehicle | VVV |
| TCDD | VVT | ||
| Vehicle | TCDD | Vehicle | VTV |
| TCDD | VTT | ||
| TCDD | Vehicle | Vehicle | TVV |
| TCDD | TVT | ||
| TCDD | TCDD | Vehicle | TTV |
| TCDD | TTT | ||
IUL: in utero/lactational exposure, V: vehicle, T: TCDD, H-H axis: hypothalamo-hypohyseal axis
After transplantation surgery, pups (n=6-8 per group) received weekly TCDD or vehicle again until sacrifice at 8 months of age. Animal groups are named by three-letter designations, with the first letter indicating intrauterine and lactational exposure for the donor animal (IUL treatment denoting TCDD action through the ovary), the second letter indicating IUL recipient treatment (IUL treatment denoting TCDD action through the H-H axis), and the third letter denoting post-transplantation treatment (adulthood treatment) (Fig. 1, Table 1). For some endpoints (estrous cyclicity, serum estradiol), data for all animals with no IUL ovarian TCDD treatment (VVT, VTV, VTT groups), as well as for all animals with IUL ovarian treatment (TVV, TVT, TTV, TTT), were pooled. The VVV group constituted the control group. Procedural controls included naïve (n=6 per group) and sham-operated (n=6 per group) females treated with vehicle or TCDD.
All rats were monitored daily for vaginal opening. Beginning at vaginal opening, reproductive cycles were monitored by vaginal cytology for 10 days each month (Fugo & Butcher, 1971; Rubin, 2000). After TCDD-treated animals showed declining cyclicity at 6 months, all cycling rats were cannulated and blood samples were collected at 2200 h on proestrus. Anesthesia for cannulation was scheduled to prevent effects on the daily neural signal for the gonadotropin surge in the rat, as described previously (Greig & Weisz, 1973; Harms & Ojeda, 1974; Shi et al., 2007). Rats were sacrificed by decapitation, and liver and ovaries were removed at 8 months of age. Tissues were immediately frozen in liquid nitrogen and stored at -80°C or processed stained with hematoxylin and eosin for histology using a single midsaggital section for ovarian morphology.
Estradiol assay
Concentration of 17β-estradiol in serum samples were measured using Estradiol EIA Kit (Cayman Chemical Company), according to the manufacturer's recommendations. Sensitivity of the assay was 4.2 pg/ml and the intra-assay coefficient of variation was 10%.
Real-time PCR
Quantitative real-time PCR was used to determine mRNA expression of cytochrome P450, subfamily 1A, polypeptide 1 (Cyp1a1) in liver and ovaries and aromatase (Cyp19a1), 17α-hydroxylase (Cyp17a1), forkhead box A2 (FoxA2), forkhead box J1 (FoxJ1) and LH receptor (LHR) in ovaries. Cyp1a1 gene expression was measured as a biomarker indicating activation of the AhR in response to TCDD. Cyp19a1, Cyp17a1 and LHR genes were chosen based on their important role in ovarian steroidogenesis. Selection of FoxA2 and FoxJ1 was based on their role in cell survival and aging and previous upregulation observed in premature reproductive aging due to TCDD (Valdez et al., 2009).
Total RNA was extracted using the RNeasy®Mini Kit (Qiagen) following the manufacturer's recommendations and quantified using 260 nm/280 nm spectrophotometric assay. Two μg of total RNA was used for first strand cDNA synthesis with SuperScript III RNase Reverse Transcriptase (Invitrogen), according to manufacturer's protocol.
Pre-validated primers and probe sets, as well as TaqMan chemistry, were supplied by Applied Biosystems. Reactions were performed in 25 μl reaction mixture volumes, with a final reaction concentration of 1×TaqMan® Universal PCR Master Mix, 250 nM for the probe, and 900 nM for each primer. One μl of each cDNA sample was added to the master mixture.
Thermal cycling conditions were as follows: 15 min at 95°C for denaturing and 1 min at 60°C for annealing and extension. β-actin was used as a housekeeping gene to normalize samples for variation in RNA loading. Relative gene expression was calculated using ΔΔCt method. Each sample was run in duplicate.
Statistics
All data are presented as mean±SEM. Results of vaginal opening, 17β-estradiol serum concentration and genes expression were analyzed by two way analysis of variance with time and treatment as main effects. When significant main effects were found, individual means were compared using a Tukey test. Percentages of reproductive cyclicity with aging among groups were tested by chi-square analysis. A value of p<0.05 was considered significant.
Results
Effect of TCDD on vaginal opening
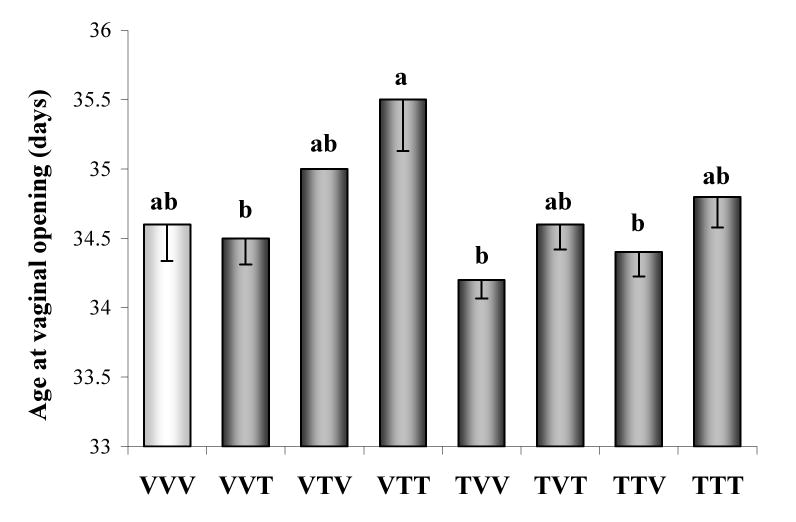
No differences in age at vaginal opening were found between the control and transplant groups (Fig. 2). However, vaginal opening was significantly delayed in VTT transplant group compared with VVT, TVV and TTV transplant groups. It seems that IUL exposure of the H-H axis plus adult exposure of the whole body to TCDD significantly delayed puberty in female rats.
Fig. 2.
Female age (days) at vaginal opening in Lewis Furth rats exposed to vehicle (V) or TCDD (50 ng/kg/week; T). The experimental design, depicted in Fig. 1, included ovarian transplantation performed after weaning and involved intrauterine and lactational (IUL) as well as postnatal exposure to vehicle or TCDD. Animal groups were named by three-letter designations, with the first letter indicating IUL exposure for the donor animal, the second letter indicating IUL recipient treatment, and the third letter denoting post-transplantation (adult) treatment. Bars with different letters denote a significant differences (p<0.05) among groups.
Effect of TCDD on vaginal cytology and ovarian follicular reserves
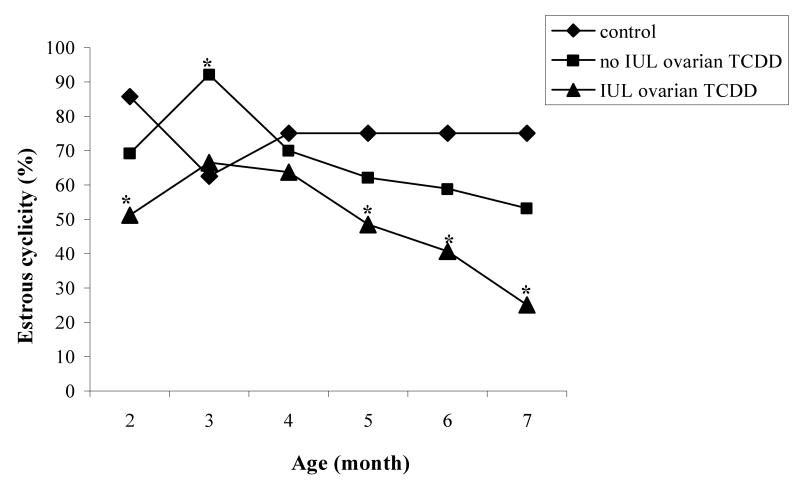
Transplantation itself resulted in prolonged reproductive cycles, with 5-6 day interestrous intervals rather than the 4-day cycles typical for Lewis Furth rats. This was associated with significant loss of ovarian follicular reserves in comparison to sham and naïve controls. Sham and naïve controls showed normal estrous cyclicity. Subsequent data analysis focused on the onset of acyclicity rather than regularity or irregularity in females bearing transplanted ovaries. Post-hoc analysis revealed an accelerated onset of acyclicity by 5 months in all groups involving IUL exposure of the developing ovary to TCDD (Fig. 3). In contrast, sham and naïve-operated female rats treated with TCDD showed an entry into acyclity by 8 months of age (data not shown). As in past studies [4], these doses of TCDD had no evident impact on total follicle numbers.
Fig. 3.
Percentage of females showing estrous cyclicity (%) following exposure to vehicle (V) or TCDD (50 ng/kg/week; T) performed according to experimental design depicted in Fig. 1. Control group constituted of animals prenatally and postnatally treated with vehicle (VVV). Females in utero and lactationally (IUL) treated with vehicle (VVT, VTV, VTT) were pooled into “no IUL TCDD” group and females IUL treated with TCDD (TVV, TVT, TTV, TTT) produced “IUL TCDD” group. Asterisks represent significant differences (p<0.05) among groups.
Serum 17β-estradiol
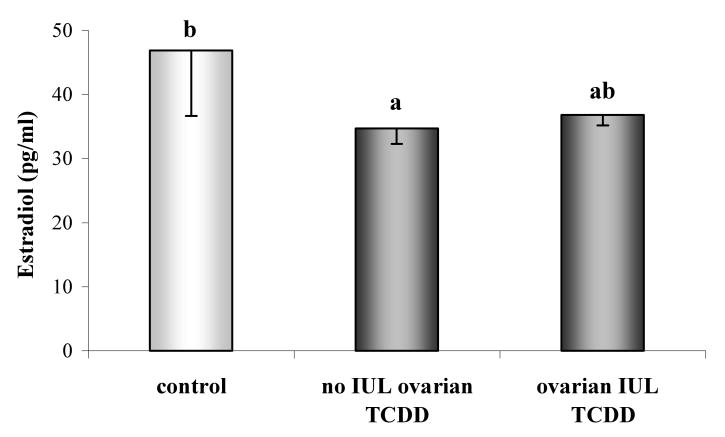
Compared to controls (VVV), proestrus serum concentration of 17β-estradiol was significantly decreased (p<0.05; Fig. 4) in females that were not exposed to TCDD during gestation and lactation (no IUL ovarian TCDD, i.e. in VVT, VTV and VTT groups). Only a tendency (p<0.10) in serum 17β-estradiol decrease was found in rats treated with TCDD (IUL ovarian TCDD: TVV, TVT, TTV, TTT) during these reproductive periods.
Fig. 4.
Proestrus serum concentration of 17β-estradiol (mean±SEM) in Lewis Furth females following exposure to vehicle (V) or TCDD (50 ng/kg/week; T) performed according to experimental design depicted in Fig. 1. Control group constituted of animals prenatally and postnatally treated with vehicle (VVV). Females in utero and lactationally (IUL) treated with vehicle (VVT, VTV, VTT) were pooled into “no IUL TCDD” group and females IUL treated with TCDD (TVV, TVT, TTV, TTT) produced “IUL TCDD” group. Bars with different letters denote a significant differences (p<0.05).
Gene expression
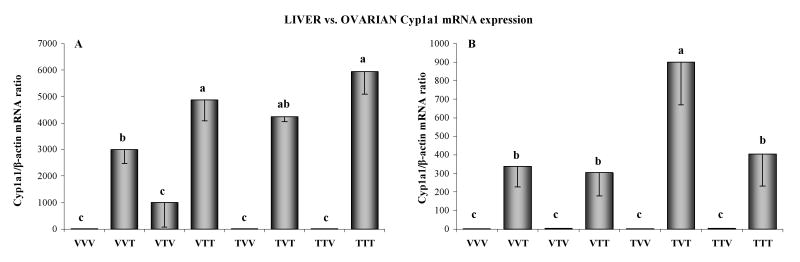
Ovarian and hepatic expression of Cyp1a1 gene was near the limit of detection in control rats (VVV) and rats with only IUL exposure to TCDD (VTV, TVV, TTV; Fig. 5). A significant (p<0.05) induction of Cyp1a1 mRNA in the liver and ovary was demonstrated in rats exposed to TCDD during adulthood (VVT, VTT, TVT, TTT). The most significant increase in hepatic Cyp1a1 gene expression was detected in animals with IUL exposure of the H-H axis followed by adult exposure to TCDD (VTT), as well as with IUL ovarian and H-H axis exposure, followed by adult exposure to TCDD (TTT) (Fig. 5A). Ovarian Cyp1a1 gene expression was the highest in animals with IUL exposure of the ovary and adult exposure to TCDD (TVT; Fig. 5B).
Fig. 5.
Hepatic (A) and ovarian (B) cytochrome P450, subfamily 1A, polypeptide 1 (Cyp1a1) gene expression measured by real-time PCR in female rats exposed to vehicle (V) or TCDD (50 ng/kg/week; T). The experimental design, depicted in Fig. 1, included ovarian transplantation performed after weaning and involved intrauterine and lactational (IUL) as well as postnatal exposure to vehicle or TCDD. Animal groups were named by three letter designations with the first letter indicating IUL exposure for the donor animal, the second letter indicating IUL recipient treatment and the third letter denoting post-transplantation (adult) treatment. Data were normalized by relative quantification with β-actin as housekeeping gene. Bars with different letters denote a significant differences (p<0.05) among groups.
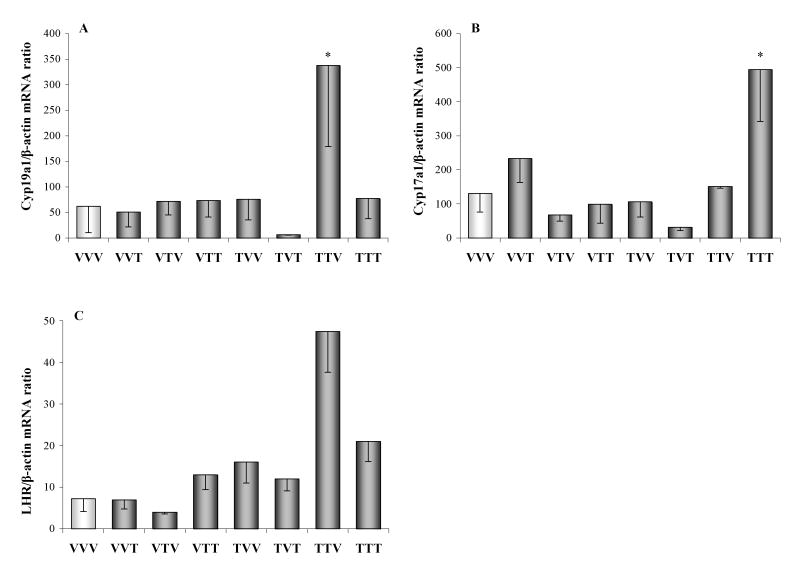
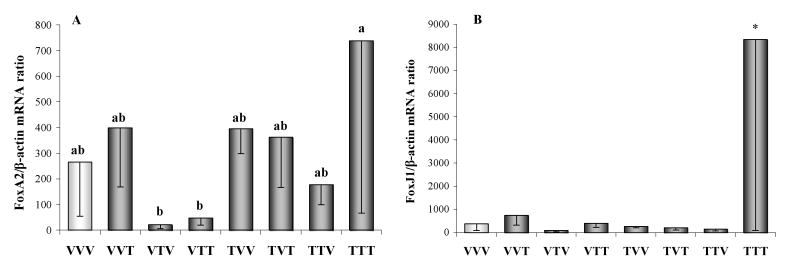
Compared with control rats, ovarian Cyp19a1 mRNA expression was significantly increased in females with IUL exposure of ovaries and H-H axis to TCDD (TTV; p<0.05; Fig. 6A). 17α-hydroxylase and LHR gene expression in the ovary did not differ among groups (Fig. 6B,C). Similarly, no differences between controls (VVV) and all remaining groups were found in ovarian FoxA2 mRNA expression (Fig. 7A). In contrast, ovarian FoxJ1 gene expression increased (p<0.05) in animals exposed to TCDD throughout the entire fetal and adult life (TTT) compared to controls (VVV; Fig. 7B).
Fig. 6.
Ovarian (A) aromatase (Cyp19a1), (B) 17-α hydroxylase (Cyp17a1), and (C) LH receptor (LHR) gene expression measured by real-time PCR in female rats exposed to vehicle (V) or TCDD (50 ng/kg/week; T). The experimental design, depicted in Fig. 1, included ovarian transplantation performed after weaning and involved intrauterine and lactational (IUL) as well as postnatal exposure to vehicle or TCDD. Animal groups were named by three letter designations with the first letter indicating IUL exposure for the donor animal, the second letter indicating IUL recipient treatment and the third letter denoting post-transplantation (adult) treatment. Data were normalized by relative quantification with β-actin as housekeeping gene. Asterisk denotes a significant difference (p<0.05) between control (VVV) and treated group.
Fig. 7.
Ovarian (A) forkhead box A2 (FoxA2) and (B) forkhead box J1 (FoxJ1) gene expression measured by real-time PCR in female rats exposed to vehicle (V) or TCDD (50 ng/kg/week; T). The experimental design, depicted in Fig. 1, included ovarian transplantation performed after weaning and involved intrauterine and lactational (IUL) as well as postnatal exposure to vehicle or TCDD. Animal groups were named by three letter designations with the first letter indicating IUL exposure for the donor animal, the second letter indicating IUL recipient treatment and the third letter denoting post-transplantation (adult) treatment. Data were normalized by relative quantification with β-actin as housekeeping gene. Bars with different letters denote a significant difference (p<0.05) among groups. Asterisk designates a significant difference (p<0.05) between control (VVV) and TTT group.
Discussion
The examination of the reproductive effects of toxicants such as TCDD in vivo is inherently complicated by simultaneous actions on the ovary and the neuroendocrine hypothalamus and pituitary gland (Hombach-Klonisch et al., 2005; Hutz et al., 2006). To complicate matters further, the actions of TCDD over time on the aging female reproductive system may merely be cumulative, or they may involve periods of particular sensitivity during transient programming or developmental steps (Hombach-Klonisch et al., 2005). In this ambitious experiment, we attempted to separate ovarian from extraovarian actions of TCDD in vivo using a transplantation approach in inbred Lewis Furth rats. This was combined with selective exposure to TCDD for the donor and recipient females in utero and lactationally, and also during subsequent adult life. Such an approach allowed observation of TCDD actions on the developing vs. adult ovary and the developing vs. adult extraovarian tissues (esp. hypothalamus and pituitary). The resulting data provide novel insights previously requiring in vitro models with their limitations.
Unsurprisingly, transplantation resulted in longer and less regular estrous cycles than observed in normal Lewis rats accompanied by modestly diminished follicular reserves. Our analysis, therefore, focused on the final loss of cyclicity with age rather than normality and length of the reproductive cycles, as has been typical of our past studies of aging and TCDD exposure (Franczak et al., 2006; Shi et al., 2007). Our data confirmed a preferential acceleration of acyclicity with age following IUL exposure of the ovary to TCDD. This was accompanied by a modest decline in serum estradiol in comparison with controls, as observed previously by our group (Franczak et al., 2006; Shi et al., 2007) and others (Chaffin et al., 1996; Salisbury & Marcinkiewicz, 2002). The decline in estradiol was similar in magnitude for TCDD exposure regardless of whether it involved the IUL ovarian TCDD associated with premature acyclicity, but was only statistically significant for groups with extraovarian developmental exposure to TCDD. Based on these data, in combination with our two preceding studies of TCDD and female reproductive aging (Franczak et al., 2006; Shi et al. 2007), it seems most likely that chronic TCDD exposure leads to accelerated loss of reproductive cyclicity through developmental ovarian mechanisms other than follicular depletion, while deficits in estradiol production may involve a combination of ovarian and extraovarian targets. This is perhaps overreaching for the current data set, however, and additional studies will be required to confirm this localization and its underlying mechanisms.
Gene expression was assessed for a number of markers of TCDD exposure and action. CYP1a1 expression was markedly greater in the liver than in ovarian tissue. CYP1a1 mRNA was correlated with ongoing TCDD exposure and was decreased in controls and groups receiving past rather than current treatment with the dioxin. Aromatase and 17α-hydroxylase expressions were largely unchanged (or occasionally elevated) by TCDD, failing to provide an explanation for declining estradiol with chronic TCDD seen in past studies (Franczak et al., 2006; Shi et al., 2007) and, to some extent, here. FoxA2 and J1 mRNAs were similarly of limited value mechanistically, although FoxJ1 was much higher in TTT females (receiving TCDD as donor, recipient and adult). This unique FoxJ1 expression pattern was unaccompanied by a parallel change in phenotype or steroid profile as examined here.
This study suggests a particular sensitivity of the developing ovary to TCDD in vivo. IUL ovarian exposure led to a premature loss of estrous cyclicity with age. Potential targets of such abnormal AhR activation through IUL exposure might include formation of the primordial follicular pool, establishment of the “gonadostat” or other aspects of ovarian and neuroendocrine interplay, and genetic or epigenetic aspects of control within the cumulus-oocyte-complex. Additionally, this work confirms the two salient features of premature reproductive senescence from chronic TCDD exposure in this model: 1) decreased serum estrogen and 2) loss of cyclicity (or normal cyclicity) with age. Future work needs to focus on developmental ovarian targets as determinants of long-term reproductive health in environments polluted with AhR ligands such as TCDD.
References
- 1.Beischlag TV, Morales JL, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Critical Reviews in Eukaryotic Gene Expression. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birnbaum L. Developmental effects of dioxins. In: Korach KS, editor. Reproductive and Developmental Toxicology. Marcel Dekker; New York: 1998. pp. 87–112. [Google Scholar]
- 3.Chaffin CL, Peterson RE, Hutz RJ. In utero and lactational exposure of female Holtzman rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: modulation of the estrogen signal. Biology of Reproduction. 1996;55:62–67. doi: 10.1095/biolreprod55.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Franczak A, Nynca A, Valdez KE, Mizinga KM, Petroff BK. Effects of acute and chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin on the transition to reproductive senescence in female Sprague-Dawley rats. Biology of Reproduction. 2006;74:125–130. doi: 10.1095/biolreprod.105.044396. [DOI] [PubMed] [Google Scholar]
- 5.Fugo NW, Butcher RL. Effects of prolonged estrous cycles on reproduction in aged rats. Fertility and Sterility. 1971;22:98–101. doi: 10.1016/s0015-0282(16)38044-x. [DOI] [PubMed] [Google Scholar]
- 6.Geusau A, Abraham K, Geissler K, Sator MO, Stingl G, Tschachler E. Severe 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) intoxication: clinical and laboratory effects. Environmental Health Perspectives. 2001;109:865–869. doi: 10.1289/ehp.01109865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray LE, Ostby JS. In utero 2,3,7,8-tetrachlorodibenzo-p-dioxin alters reproductive morphology and function in female rat offspring. Toxicology and Applied Pharmacology. 1995;133:285–294. doi: 10.1006/taap.1995.1153. [DOI] [PubMed] [Google Scholar]
- 8.Greig F, Weisz J. Preovulatory levels of luteinizing hormone, the critical period and ovulation in rats. Journal of Endocrinology. 1973;57:235–245. doi: 10.1677/joe.0.0570235. [DOI] [PubMed] [Google Scholar]
- 9.Harms PG, Ojeda SR. A rapid and simple procedure for chronic cannulation of the rat jugular vein. Journal of Applied Physiology. 1974;36:391–392. doi: 10.1152/jappl.1974.36.3.391. [DOI] [PubMed] [Google Scholar]
- 10.Hombach-Klonisch S, Pocar P, Kietz S, Klonisch T. Molecular actions of polyhalogenated arylhydrocarbons (PAHs) In female reproduction. Current Medicinal Chemistry. 2005;12:599–616. doi: 10.2174/0929867310504050599. [DOI] [PubMed] [Google Scholar]
- 11.Hutz RJ, Carvan MJ, III, Baldridge MG, Conley LK, Heiden TK. Environmental toxicants and effects on female reproductive function. Trends in Reproductive Biology. 2006;2:1–11. doi: 10.1901/jaba.2006.2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kociba RJ, Keyes DG, Beyer JE, Carreon RM, Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CN, Barnard SD, Hummel RA, Humiston CG. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicology and Applied Pharmacology. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni PS, Crespo JG, Afonso CAM. Dioxin sources and current remediation technologies – a review. Environmental International. 2008;34:139–153. doi: 10.1016/j.envint.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Larsen JC. Risk assessments of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and dioxin-like polychlorinated biphenyls in food. Molecular Nutrition and Food Research. 2006;50:885–896. doi: 10.1002/mnfr.200500247. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, VanderElst J, Vandenbroecke R, Dhont M. Early massive follicle loss and apoptosis in heterotopically grafted newborn mouse ovaries. Human Reproduction. 2002;17:605–611. doi: 10.1093/humrep/17.3.605. [DOI] [PubMed] [Google Scholar]
- 16.Page RD, Butcher RL. Follicular and plasma patterns of steroids in young and old rats during normal and prolonged estrous cycles. Biology of Reproduction. 1982;27:383–392. doi: 10.1095/biolreprod27.2.383. [DOI] [PubMed] [Google Scholar]
- 17.Pan F, Ebbs A, Wynn C, Erickson L, Jang MS, Crews G, Fisniku O, Kobayashi M, Paul LC, Benediktsson H, Jiang AH. FK778, a powerful new immunosuppressant, effectively reduces functional and histologic changes of chronic refection in reat renal allografts. Transplantation. 2003;75:1110–1114. doi: 10.1097/01.TP.0000063704.19149.E3. [DOI] [PubMed] [Google Scholar]
- 18.Petroff BK, Roby KF, Gao X, Son DS, Williams S, Johnson D, Rozman KK, Terranova PF. A review of mechanisms controlling ovulation with implications for the anovulatory effects of polychlorinated dibenzo-p-dioxin in rodents. Toxicology. 2001;148:91–107. doi: 10.1016/s0300-483x(00)00367-x. [DOI] [PubMed] [Google Scholar]
- 19.Pocar P, Fischer B, Klonisch T, Hombach-Klonisch S. Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction. 2005;129:379–389. doi: 10.1530/rep.1.00294. [DOI] [PubMed] [Google Scholar]
- 20.Rubin BS. Hypothalamic alterations and reproductive aging in female rats: evidence of altered luteinizing hormone-releasing hormone neuronal function. Biology of Reproduction. 2000;63:968–976. doi: 10.1095/biolreprod63.4.968. [DOI] [PubMed] [Google Scholar]
- 21.Salisbury TB, Marcinkiewicz JL. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and 2,3,4,7,8-pentachlorodibenzofuran reduces growth and disrupts reproductive parameters in female rats. Biology of Reproduction. 2002;66:1621–1626. doi: 10.1095/biolreprod66.6.1621. [DOI] [PubMed] [Google Scholar]
- 22.Sharara FI, Seifer DB, Flaws JA. Environmental toxicants and female reproduction. Fertility and Sterility. 1998;70:613–622. doi: 10.1016/s0015-0282(98)00253-2. [DOI] [PubMed] [Google Scholar]
- 23.Shi Z, Valdez KE, Ting AY, Franczak A, Gum SL, Petroff BK. Ovarian endocrine disruption underlies premature reproductive senescence following environmentally relevant chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biology of Reproduction. 2007;76:198–202. doi: 10.1095/biolreprod.106.053991. [DOI] [PubMed] [Google Scholar]
- 24.Smith CC, Cizza G, Gomez M, Greibler C, Gold PW, Sternberg EM. The estrous cycles and pituitary-ovarian function in Lewis and Fischer rats. Neuroimmunomodulation. 1994;1:231–235. doi: 10.1159/000097170. [DOI] [PubMed] [Google Scholar]
- 25.Steger RW, Peluso JJ. Sex hormones in the aging female. Endocrinology Metabolism Clinics of North America. 1987;16:1027–1043. [PubMed] [Google Scholar]
- 26.Valdez KE, Shi F, Ting A, Petroff BK. Effect of chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin in female rats on ovarian gene expression. Reproductive Toxicology. 2009;28:32–37. doi: 10.1016/j.reprotox.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.vomSaal S, Finch CE, Nelson JF. Natural history and mechamisms of reproductive aging in humans, laboratory rodents and other selected vertebrates. In: Knobil E, Neill JD, editors. The Physiology of Reproductive. Raven Press; New York: 1994. pp. 1213–1314. [Google Scholar]