Abstract
Background
Pregnancy may alter protein binding (PB) of highly bound protease inhibitors due to changes in plasma concentrations of albumin and α-1 acid glycoprotein (AAG). Small changes in PB can greatly impact the fraction of drug unbound (FU) exerting pharmacological effect. We report lopinavir (LPV) PB during third trimester (antepartum, AP) compared to ≥1.7 weeks postpartum (PP) to determine if FU changes compensate for reduced total concentrations reported previously.
Methods
P1026s enrolled women receiving LPV/ritonavir, soft gel capsules 400/100 mg or 533/133 mg twice daily. LPV FU, albumin and AAG were determined AP and PP.
Results
AP/PP samples were available from 29/25 women respectively with all but one woman receiving the same dose AP/PP. LPV FU was increased 18% AP vs. PP (mean 0.96 ± 0.16% AP vs. 0.82 ± 0.21% PP, P = 0.001). Mean protein concentrations were reduced AP (AAG = 477 mg/L; albumin = 3.28 mg/dL) vs. PP (AAG = 1007 mg/L; albumin = 3.85 mg/dL) (P<0.0001 for each comparison). AAG concentration correlated with LPV binding. Total LPV concentration did not correlate with LPV FU AP or PP. However, higher LPV concentration PP was associated with reduced PB and higher FU after adjustment for AAG.
Conclusions
LPV FU was higher and AAG lower AP vs. PP. The 18% increase in LPV FU AP is smaller than the reduction in total LPV concentration reported previously and is not of sufficient magnitude to eliminate the need for an increased dose during pregnancy.
Keywords: lopinavir, pharmacokinetics, plasma proteins, pregnancy, protein binding
Introduction
The current US Public Health Service (USPHS) Perinatal Guidelines recommend treatment with highly active antiretroviral (ARV) therapy (HAART) for most pregnant women for maternal control of HIV and prevention of mother-to-child transmission [1]. Lopinavir/ritonavir (LPV/r) is one of the most common boosted protease inhibitor (PI) combinations used by pregnant women in the United States and continues to be the first-line choice for PI therapy for HIV-1-infected pregnant women in many clinical centres.
Optimum dosing of PI-based regimens during pregnancy can be complicated by substantial changes in the pharmacokinetics of ARVs, which can be more pronounced during the third trimester of pregnancy. Alterations of gastrointestinal function during pregnancy may impair drug absorption. Drug distribution may increase due to increases in plasma volume, total body water and body fat stores. Pregnancy may affect drug metabolism including the induction of hepatic and gastrointestinal metabolic enzymes [2,3]. For example, cytochrome p450 (CYP) metabolism changes with mean increases of 35% reported for the activity of CYP3A4, the primary isozyme responsible for lopinavir (LPV) biotransformation [2]. Consistent with these changes, we previously reported a 28% decrease in LPV plasma exposure, as estimated by the area under the plasma concentration vs. time curve (AUC) during third-trimester pregnancy (antepartum, AP) compared to post-partum (PP) in 17 HIV-1-infected pregnant women receiving a standard LPV/r dose of 400/100 mg twice daily (bid) [4]. More recently, we have confirmed that increasing the LPV dose during pregnancy to 533/133 mg bid offsets the reduced exposure we previously observed [5].
Pregnancy may also be associated with a decrease in protein binding (PB) of drugs in plasma due to dilutional decreases in albumin and α-1 acid glycoprotein (AAG) concentrations and the displacement of drugs from binding sites by steroid and placental hormones [6–8]. LPV is highly bound to plasma proteins including albumin and AAG with binding of >99%. Pregnancy potentially alters this binding to clinically relevant proportions such that small changes in PB associated with pregnancy may cause large changes in the percentage of unbound drug (fraction unbound; FU). Unbound drug is the pharmacologically active component of total drug concentrations and the fraction of drug free to traverse membranes and exert therapeutic effect. An increase in LPV FU during pregnancy may partially offset the decrease in total drug concentrations observed with standard dosing [4].
Our primary objectives were to (a) measure the PB of LPV during the third trimester of pregnancy (AP) and PP, (b) determine FU of LPV AP and compare to PP estimates, (c) assess whether AAG or albumin concentration correlate with FU and (d) assess whether LPV total drug concentrations influence FU.
Methods
Study population and design
International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) Protocol 1026s (P1026s) is an ongoing, prospective, nonrandomized, unblinded, multicentre study of the pharmacokinetics of currently prescribed ARVs used by HIV-1-infected pregnant women. P1026s is a sub-study of P1025, a prospective cohort study of HIV-1-infected pregnant women receiving care at IMPAACT sites. This report describes only the PB results for those women who were prescribed LPV/r 133/33 mg soft gel capsules (SGC). Results on the pharmacokinetics of total LPV for these women have been published separately [4,5].
Eligibility criteria for the LPV/r arm of P1026s were: enrolment in P1025, age ≥13 years, initiation of LPV/r as part of clinical care before 35 weeks’ gestation and intent to continue the current regimen until at least 6 weeks PP. Exclusion criteria included concurrent use of medications known to interfere with absorption, metabolism or clearance of LPV or ritonavir (RTV), multiple gestation or clinical or laboratory toxicities that in the opinion of the site investigator would likely require a change in medications. This study was approved at local institutional review boards for all participating sites and informed consent was obtained from all subjects. P1026s enrolled two cohorts of women receiving LPV/r 133/33 mg SGC. Women in the first cohort received standard LPV/r dosing of three capsules orally bid, providing LPV 400 mg/RTV100 mg per dose. Women in the second cohort received four capsules, providing LPV 533/RTV 133 mg bid. Each participating subject’s primary care provider determined the choice of ARV medications used for each subject’s clinical management and remained responsible for her management throughout the study. Study participation was to continue until completion of PP pharmacokinetic sampling.
Pharmacokinetic evaluations of LPV occurred at >30 weeks’ gestation (AP) and ≥1.7 weeks PP. LPV exposure (of total drug) as measured by the AUC (previously published) [4,5] was estimated within 2 weeks of sample collection for each subject and compared to the estimated 10th percentile obtained from nonpregnant adults receiving the standard LPV/r dose. Results were provided to each subject’s primary care provider so that dose adjustment could be made if needed. For each pharmacokinetic determination, subjects were required to be on a consistent LPV/r dose for at least 2 weeks to assure steady-state conditions. Determination of LPV FU (as reported herein) was carried out on the same days as the pharmacokinetic evaluations [4,5].
Details relating to clinical and laboratory monitoring for subjects receiving LPV/r as part of P1026s have been described elsewhere [4,5]. Briefly, clinical evaluations and laboratory testing to evaluate drug effectiveness and toxicities were carried out as part of the parent study P1025 and as part of routine clinical care. The study team reviewed reported toxicities on monthly conference calls and each subject’s primary care provider remained responsible for toxicity management.
Pharmacokinetic sample collection
Blood samples were collected on two separate occasions for determination of LPV total drug exposure (AUC) and the FU: AP (>30–36 weeks’ gestation) and PP (≥1.7 weeks after delivery). Prior to each pharmacokinetic study day, adherence to LPV/r administration was addressed by instructing women to take their drugs at the same time as on the day of the pharmacokinetic evaluation for three preceding (consecutive) days and to record the exact time of drug administration for the last two doses preceding pharmacokinetic study dose administration. The study dose was administered as an observed dose after a standardized meal of approximately 850 kilocalories, with 55% of calories from fat. Blood samples for plasma determinations were collected immediately prior to the dose and at 2, 4, 6, 8, and 12 h post-dose via an indwelling peripheral venous catheter.
LPV analysis for total and percentage unbound drug
For estimation of LPV FU, plasma samples from each pharmacokinetic study day were pooled into low concentration samples (0 and 12 h post-dose) and high concentration samples (2 through 8 h post-dose). Pooling of samples was carried out to provide sufficient sample volume for FU determinations. Pooled specimens were analyzed for both total LPV concentration and the FU.
Total LPV concentrations for pooled specimens were quantified within the Pediatric Clinical Pharmacology Laboratory at the University of California, San Diego using a validated reverse-phase multiplex high performance liquid chromatography (HPLC) method as previously described [4,5]. Briefly, the method had a lower limit of quantitation (LOQ) adequate for quantitating drug in all collected samples (0.091 µg/mL) and had interassay coefficients of variation (CV) of <11% for the LOQ and all controls.
The PB method employed ultrafiltration (filter units were Micron YM-10 (10 000 MWCO from AMICON, Billercia, MA, USA) and radiolabelled drug (3H) purified and supplied by Abbott Laboratories, Abbott Park, IL, USA (specific activity 8.06 Ci/mmol, >99% purity). Pooled plasma samples were centrifuged to remove particulate material. Radiolabel was added to 1 mL of cleared plasma to give an initial concentration of approximately 30 ng/mL. The spiked plasma aliquots were equilibrated for 30 min at 37 °C before ultrafiltration. Spiked plasma (300 µL) was placed into the sample reservoir of the Micron centrifugal filter device and centrifuged for 1 h, at 22 °C, in a fixed head micro centrifuge at high speed, around 12 000 × g. Filters were processed in duplicate for each sample. Duplicate aliquots (100 µL) of each spiked plasma and ultrafiltrate sample (200 µL) were radioassayed directly in Cytoscint in a liquid scintillation counter. Since protein is necessary for appropriate filter functioning, we used an indirect method to assess binding to the filter. We attempted to block the filter units with PEG and tested plasma with 3H LPV. The results showed very low nonspecific binding. This is consistent with Abbott Laboratories’ findings of negligible nonspecific binding (T. Reisch, Metabolic Disease Research, Abbott Laboratories, personal communication).
Assay reproducibility was assessed prior to the start of the patient experiments. Six filters were processed with a high LPV spike (approximately 14 500 ng/mL) and five filters were processed using blank (no LPV) plasma. The %CV for the filtrate DPM (disintegrations per minute) was <5%. The experiment was repeated in the middle of the testing period and the %CV for filtrate (five filters) DPM was also <5%. Additionally the high control and blank plasma were processed in duplicate with each batch of subject samples. The mean %bound showed %CV of <0.1 (n = 8 testing dates).
FU was calculated according to the following formulas:
| (1) |
AAG and albumin measurements
AAG was determined using an FDA approved kit [Human AAG RID (Radial Immunodiffusion) Kit, The Binding Site Inc., San Diego, USA). The method was validated in the Pediatric Pharmacology Laboratory of the University of California, San Diego for heated EDTA plasma samples. The method has a calibration range of 190–1900 mg/mL. Samples with values lower than 190 mg/mL were repeated using double the amount of sample. Low, middle and high controls (n = 8 of each) showed precision and accuracy of <13.1% CV and within 3.5% deviation, respectively. Albumin was determined at the Clinical Laboratory Improvement Amendments (CLIA) certified clinical chemistry laboratories associated with the clinical study sites.
Pharmacokinetic and statistical analysis
The Wilcoxon signed rank test was used to compare LPV FU and other variables measured during the third trimester of pregnancy (AP) with the corresponding PP measurements. Linear regression was used to investigate the impact on LPV FU of total drug concentration, AAG, albumin concentration, LPV dose administered and the time of PP evaluations. Generalized estimating equations were used to account for the intra-subject correlations.
Results
AP and PP evaluations were carried out in 29 and 25 women, respectively for whom sufficient plasma was available. Of these women, all but one received the identical dose for both AP and PP study periods; 16 received the LPV/r 400/100 mg bid dose and 12 received the 533/133 mg bid dose. One subject received both LPV/r doses at differing points of the study.
Table 1 summarizes subject demographic and disease characteristics obtained at the time of AP pharmacokinetic sampling. Median age was 31.4 years ranging from 18.2 to 40.9 years, with the majority of women being either black (35%) or Hispanic (45%). Median gestational age was 33.9 weeks ranging from 30.4 to 37.4 weeks, and median time of PP PK evaluation since delivery was 3.4 weeks with a range of 1.7–12.9 weeks.
Table 1.
Demographic and disease characteristics at third trimester (AP) pharmacokinetic sampling*
| Characteristic n = 29 | Median (Min. Max.) Statistic |
|---|---|
| Age (years) | 31.4 (18.2, 40.9) |
| CD4 count (cells/µL)† | 446 (126, 1049) |
| Weight (kg) | 86.3 (64, 130) |
| HIV-1 RNA (copies/mL)† | 71 (<20, 41 853)‡ |
| Gestational age (weeks) | 33.9 (30.4, 37.4) |
| Time of postpartum pharmacokinetics since delivery (weeks) |
3.4 (1.7, 12.9) |
| Race/ethnicity | |
| White (nonHispanic) | 10% |
| Black (nonHispanic) | 35% |
| Hispanic | 45% |
| Asian Pacific Islander | 3% |
| Other | 7% |
Median (minimum, maximum) for continuous variables and percentages for categorical variables.
Closest measurement within 8 weeks from the third trimester (AP) pharmacokinetic evaluation.
27/28 (96.4%) subjects for whom data are available had viral load suppressed below 400 copies/mL.
Table 2 presents the values and percent difference AP vs. PP for AAG concentration, albumin concentration, and LPV FU. Both AAG and albumin were significantly lower during pregnancy compared to PP (P<0.0001). LPV FU was significantly higher during pregnancy compared to PP for the 0 + 12 h pooled samples and the 2 through 8 h pooled samples, analyzed separately or combined average FU (for both 0 + 12 h and 2 through 8h pooled samples) was 18% higher AP compared to PP (P = 0.001) (Table 2).
Table 2.
Mean values (SD) and percent change antepartum vs. postpartum for AAG concentration, albumin concentration and lopinavir FU
| AP (n = 29) | PP (n = 25) | Median % Difference AP vs. PP (95% CI) | P value | |
|---|---|---|---|---|
| AAG conc (mg/L)* | 477 (145) | 1007 (374) | ↓ 52% (44%, 59%) | <0.0001 |
| Albumin conc (mg/dL) | 3.28 (0.41) | 3.85 (0.49) | ↓ 15% (11%, 19%) | <0.0001 |
| LPV FU (%) (0 + 12 h) | 0.91 (0.14) | 0.78 (0.18) | ↑ 17% (6%, 29%) | 0.005 |
| LPV FU (%) (2–8 h) | 1.02 (0.22) | 0.87 (0.25) | ↑ 19% (6%, 33%) | 0.002 |
| LPV FU (%) (average) | 0.96 (0.16) | 0.82 (0.21) | ↑ 18% (7%, 30%) | 0.001 |
Mean (SD).
AP, antepartum; FU, fraction unbound; LPV, lopinavir; PP, postpartum.
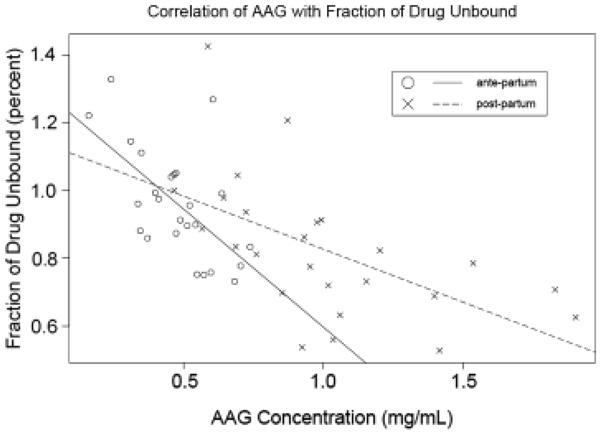
LPV FU decreased as a function of increasing AAG concentration in both the AP and PP periods (Fig. 1). At the AP pharmacokinetic evaluation, each 100 mg/L (or 0.1 mg/mL) increase in AAG was associated with a decrease in LPV FU of 0.07% (P<0.0001) and at the PP pharmacokinetic evaluation, each 100 mg/L increase in AAG was associated with a decrease of 0.05% in LPV FU (P<0.0001) after adjustment for total LPV concentrations. Total plasma LPV concentration alone was not significantly correlated with LPV FU during either the AP or PP pharmacokinetic visits. However, a higher total plasma LPV concentration PP was significantly associated with reduced LPV binding and higher FU (P<0.0001) after adjustment for AAG concentration. That is, each 1 µg/mL increase in total LPV concentration was associated with a 0.02% increase in LPV FU. Albumin was not significantly correlated with LPV FU for either of the pharmacokinetic study days.
Figure 1.
Correlation of α-1 acid glycoprotein (AAG) concentration (mg/mL) with fraction of drug unbound (percent).
Women receiving high dose LPV/r (533/133) did not have significantly different LPV FU compared to women receiving standard dose LPV/r (400/100). Likewise, the time that elapsed before PP evaluations did not appear to have an effect on LPV FU when comparing measurements ≤4 weeks to those >4 weeks PP, a break-point near the median time for PP visits (3.4 weeks) (data not shown).
Discussion
The antiviral effect of LPV depends on multiple factors that extend beyond the pharmacokinetics of total or unbound drug. These factors include processes affecting the penetration of drug into target cells and tissues (e.g. transport proteins) and how susceptible the virus is to the ARV. A primary goal of pharmacokinetic studies is to measure active drug as close to the site of pharmacological activity as is possible. Therefore PK studies that measure the percentage unbound (FU) of highly bound drugs (i.e. the fraction that is pharmacologically active and free to traverse membranes) may help investigators get one step closer to achieving this goal. However, it remains unclear if unbound drug exposure in plasma alone is a strong predictor of active drug exposure within cells and target tissues.
The pharmacokinetic exposure of LPV as estimated through the AUC of total drug is reduced 28% with standard LPV/r SGC dosing during pregnancy as previously reported. In addition, the 12 h trough concentration, a measurement sometimes employed in clinical practice, is reduced 56% during pregnancy [4]. Eighty percent of these women had pharmacokinetic parameters below the target 10th percentile of nonpregnant women in late pregnancy leading to the study of a second cohort of women receiving an increased dose of LPV 533/RTV 133 mg twice daily. Eighty-one percent (81%) of women receiving the increased dose had AUC estimates exceeding the target 10th percentile during third-trimester pregnancy [5].
To further characterize the effect of pregnancy on LPV pharmacokinetics we determined changes in the plasma proteins albumin and AAG and the LPV FU in nearly all women who had undergone pharmacokinetic analysis and for whom sufficient sample was available [4,5]. We observed decreases in AAG and albumin concentrations and an increase in LPV FU during pregnancy. The increase in LPV FU averaged 18%, which may partially mitigate a reduction in total LPV concentrations observed with standard dosing [4]. These data are consistent with a recent report in 10 HIV-infected women where LPV FU was altered by 38% [6].
In spite of the potential benefits of measuring active drug as close to the site of pharmacological activity as possible, few pharmacology studies have successfully done so, in part due to difficulty in quantitating the active drug component and in part due to difficulty in collecting samples close to the site of activity (i.e. peripheral blood mononuclear cells, cerebrospinal fluid, lymph node tissue) [7,8]. As stated, one step toward improving our understanding of ARV pharmacokinetics is to measure FU in plasma as FU is free to traverse biological membranes, penetrate target tissue and exert pharmacological effect [9]. Other factors also contribute to how well a drug reaches its site of action, including the degree to which a drug is influenced by transport proteins (e.g. P-glycoprotein) present on cellular membranes [9]. Changes in PB will be most clinically relevant for highly bound drugs, since small changes in binding of highly bound drugs can have substantial effects on FU [10]. For highly bound ARV drugs such as the PIs, clinical situations likely to impact FU include pregnancy, infancy, renal or liver disease or concomitant therapies that displace drugs from PB sites [11–13].
This is one of the first studies addressing the effect of pregnancy on PB of any ARV drug despite long-term knowledge that pregnancy causes substantial reductions in plasma protein concentrations. Albumin concentrations fall from 3.5 to a range of 2.5 to 3.0 mg/dL during the first half of pregnancy and steroid and placental hormones displace drugs from binding sites leading to an overall decrease in the binding capacity of albumin and an increase in FU [14]. For example, the FU of phenytoin, a drug that binds to albumin, increases during pregnancy [14]. Data describing changes in AAG with pregnancy are less definitive. Two studies report an overall decrease in AAG concentration over the course of pregnancy while others report no change [11,12]. PB during pregnancy also may be affected by increased concentrations of endogenous ligands (i.e. free fatty acids), that may compete for drug binding sites of albumin and AAG. LPV has been reported to bind to both albumin and AAG, as does the PI nelfinavir [15,16].
AAG and albumin concentrations in our subjects were significantly altered during pregnancy compared to PP (P<0.0001). This is expected due to the change in weight and fluid volume consistent with pregnancy. The weight gain in our subjects of up to 10–15 kg during the course of pregnancy is also expected, although mean total weight for our subjects is higher than weights expected for pregnant women in some international settings where women on average are of smaller stature. Weight gain alone is not expected to impact FU.
Albumin concentrations did not appear significantly to influence FU, but AAG concentrations had a significant effect, in that each 100 mg/L increase in AAG was associated with a decrease in LPV FU of 0.07 and 0.05% for third trimester and PP evaluations, respectively (P<0.0001 during both time periods, with adjustment for total LPV, at the PP evaluation). In contrast, LPV dose and time of PP evaluations did not have a significant effect on FU. However, higher LPV concentration during PP evaluations, after adjustment for AAG, was associated with reduced binding and higher FU.
Understanding the relevance of altered binding of highly bound drugs can be challenging. The most important impact of an increase or decrease in FU is on how one ‘interprets’ the measurement of total drug concentrations. Changes in FU rarely, in and of themselves, lead to a change in dosage recommendations. However, when interpreting total drug exposure (for highly bound drugs), free drug exposure should be considered as well, especially under conditions where FU may be altered (e.g. pregnancy). A classic example illustrating the importance of investigating FU changes is with phenytoin, a drug for which therapeutic drug monitoring (TDM) is employed. Patients with severe renal disease on average have a doubling of phenytoin FU when compared to patients with normal renal function [10]. Therefore, when TDM is used to optimize phenytoin therapy, the total drug concentrations targeted for adequate seizure control in renal patients are approximately 50% the concentrations targeted for patients with normal renal function. For example, a phenytoin concentration of 8 mcg/mL would represent an adequate concentration for a patient with severe kidney impairment while this same concentration may be deemed sub-therapeutic for an individual with normal kidney function.
The same could hold true for other highly bound drugs used to treat a distinct population. In the case of LPV use in pregnancy, the observed 18% relative increase in LPV unbound fraction during pregnancy (FU) should be considered when interpreting total drug measurements in pregnancy. However, the FU change of 18% we measured is smaller than the 28% reduction in AUC and the 56% reduction in 12 h trough concentration of total drug reported previously [4]. This earlier study demonstrated that an increased dose of LPV during pregnancy normalized total drug exposure and was well tolerated, yielding recommendations for this higher dose during third trimester and potentially second-trimester pregnancy with a return to standard dosing 2 weeks following delivery [5]. On balance, the 18% increase in LPV unbound fraction does offset some of the change seen with total drug exposure, but is not of sufficient magnitude to eliminate the need for an increased dose during pregnancy.
Acknowledgements
The P1026s team wishes to thank the volunteers participating in this study and the study coordinators at the participating sites. We thank Abbott Laboratories, Abbott Park, Illinois, for their support of this work.
The P1026s Team: Mark Mirochnick, MD; Alice M. Stek, MD; Edmund Capparelli, Pharm.D.; Brookie M. Best, Pharm.D.; Cheng-cheng Hu, PhD; Sandra K. Burchett, MD; Carol Elgie, BS; Diane T. Holland, MPhil; Beth Sheeran, MS, RD; Janne Schiffhauer, BS; Maureen Shannon, MS, CNM; James D. Connor, MD; Francesca Aweeka, Pharm.D.; Bradley W. Kosel, Pharm.D.; Kathleen A. Medvik, BS, MT; Elizabeth Smith, MD; Jennifer S. Read, MD.
University of Florida, Jacksonville, Florida; University of Miami Pediatric/Perinatal: Adolfo Gonzalez-Garcia, MD; Mary Jo O’Sullivan, MD; Gwendolyn B. Scott, MD; Liset Taybo, MD; Children’s Hospital of Boston, Boston, Massachusetts; Columbia IMPAACT CRS, New York, New York: Seydi Vazquez-Bonilla, RN; Alice Higgins, RN; Diane Tose, RN; Phil LaRusse; University California, San Diego Maternal, Child and Adolescent HIV CRS, San Diego, California: Andrew Hull, MD, Mary Caffery, RN, Linda Proctor, RN, CNM, Stephen A. Spector, MD; Baystate Medical Center, Springfield, Massachusetts: Barbara Stechenberg, MD, Eileen Theroux, RN, BSN, Maripat Toye, RN, MS; Boston Medical Center Pediatric HIV Program, Boston Massachusetts: Ann Marie Regan, NP, RN, Desiree Jones-Eaves, RN, Stephen Pelton MD, Meg Sullivan, MD; WNE Maternal Pediatric Adolescent AIDS CTU/CRS, Worcester, Massachusetts; Katherine Luzuriaga, MD Sharon Cormier, RN; New Jersey Medical School CRS, Newark, New Jersey; UCLA Los Angeles/Brazil AIDS Consortium (LABAC) CRS, Los Angeles, California: Yvonne J. Bryson, MD, Maryanne Dillon, RNC, NP, Audra Deveikis, MD, Susan Marks, RN; Children’s Hospital and Regional Medical Center, Seattle, Washington: Jane Hitti, MD, MPH, Ann Melvin, MD MPH, Michele Acker, PNP, Deb Goldman, ARNP, MPH; University of Colorado, Denver, Colorado: Adriana Weinberg, MD, Jill Davies, MD, Carol Salbenblatt, MSN, Suzanne Paul, FNP; SUNY Stony Brook, Stony Brook, New York: Sharon Nachman, MD, Denise Ferraro, RN, Jennifer Griffin, NP, Paul Ogburn, MD; Los Angeles County and USC Medical Center: Ana Melendrez, RN, Françoise Kramer, MD, LaShonda Spencer, MD, Andrea Kovacs, MD.
Sources of Funding: The project described was supported by Grant Number U01AI068632 and 1 U01 AI068616 from the National Institute of Allergy and Infectious Diseases (NIAID). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
This study was also supported by the General Clinical Research Center Units funded by the National Center for Research Resources (Grant M01 RR00533, 5 M01 RR01271), the Pediatric/Perinatal HIV Clinical Trials Network of the National Institute of Child Health and Human Development (Contract N01-HD-3-3365), and the Pediatric Pharmacology Research Unit Network of the National Institute for Child Health and Human Development (Grant U01-HD-031318-11).
References
- 1. [accessed 29 April 2009];USPHS Task Force Recommendations for the use of antiretroviral drugs in HIV-infected pregnant women for maternal health and interventions to reduce perinatal HIV transmission in the US. Available at http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf.
- 2.Tracy TS, Venkataramanan R, Glover DD, et al. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. Am J Obstet Gynecol. 2005;192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Krauer B, Krauer F, Hytten FE. Drug disposition and pharmacokinetics in the maternal-placental-fetal unit. Pharmacol Ther. 1980;10:301–328. doi: 10.1016/0163-7258(80)90085-6. [DOI] [PubMed] [Google Scholar]
- 4.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 5.Mirochnick M, Best BM, Stek AM, et al. Lopinavir exposure with an increased dose during pregnancy. JAIDS. 2008;49:485–491. doi: 10.1097/QAI.0b013e318186edd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiser J, Mawhinney S, Kinzie K, et al. Total and unbound lopinavir/ritonavir pharmacokinetics in a concentration-guided study of HIV-infected women throughout pregnancy and post-partum. 16th Conference on Retroviruses and Opportunistic Infections.; Montreal, Canada. 2009. Feb, [Poster #946] [Google Scholar]
- 7.Aweeka FT, Rosenkranz SL, Segal Y, et al. NIAID AIDS Clinical Trials Group. The impact of sex and contraceptive therapy on the plasma and intracellular pharmacokinetics of zidovudine. AIDS. 2006;20:1833–1841. doi: 10.1097/01.aids.0000244202.18629.36. [DOI] [PubMed] [Google Scholar]
- 8.McGee B, Smith N, Aweeka F. HIV pharmacology: barriers to the eradication of HIV from the CNS. HIV Clin Trials. 2006;7:142–153. doi: 10.1310/AW2H-TP5C-NP43-K6BY. [DOI] [PubMed] [Google Scholar]
- 9.Summerfield SG, Stevens AJ, Cutler L, et al. Improving the in vitro prediction of in vivo central nervous system penetration: integrating permeability, P-glycoprotein efflux, and free fractions in blood and brain. J Pharmacol Exp Ther. 2006;316:1282–1290. doi: 10.1124/jpet.105.092916. [DOI] [PubMed] [Google Scholar]
- 10.Liponi DF, Winter ME, Tozer TN. Renal function and therapeutic concentrations of phenytoin. Neurology. 1984;34:395–397. doi: 10.1212/wnl.34.3.395. [DOI] [PubMed] [Google Scholar]
- 11.Haram K, Augensen K, Elsayed S. Serum protein pattern in normal pregnancy with special reference to acute-phase reactants. Br J Obstet Gynaecol. 1983;90:139–145. doi: 10.1111/j.1471-0528.1983.tb08898.x. [DOI] [PubMed] [Google Scholar]
- 12.Wood M, Wood AJ. Changes in plasma drug binding and alpha 1-acid glycoprotein in mother and newborn infant. Clin Pharmacol Ther. 1981;29:522–526. doi: 10.1038/clpt.1981.73. [DOI] [PubMed] [Google Scholar]
- 13.Notarianni LJ. Plasma protein binding of drugs in pregnancy and in neonates. Clin Pharmacokinet. 1990;18:20–36. doi: 10.2165/00003088-199018010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Perucca E, Crema A. Plasma protein binding of drugs in pregnancy. Clin Pharmacokinet. 1982;7:336–352. doi: 10.2165/00003088-198207040-00004. [DOI] [PubMed] [Google Scholar]
- 15.Motoya T, Thevanayagam LN, Blaschke TF, et al. Characterization of nelfinavir binding to plasma proteins and the lack of drug displacement interactions. HIV Med. 2006;7:122–128. doi: 10.1111/j.1468-1293.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 16.Gulati A, Boudinot D, Gerk PM. Binding of lopinavir to human {alpha}1-acid glycoprotein and serum albumin. Drug Metab Dispos. 2009;37:1572–1575. doi: 10.1124/dmd.109.026708. [DOI] [PMC free article] [PubMed] [Google Scholar]