Abstract
Myostatin deficiency leads to both an increased rate of protein synthesis and skeletal muscle hypertrophy. However, the mechanisms involved in mediating these effects are not yet fully understood. Here, we demonstrate that genetic loss of myostatin leads to enhanced muscle expression of both PKB and mTOR/S6K signalling components, consistent with their elevated activity. This is associated with a reduction in the expression of PGC1α and COXIV, proteins which play important roles in maintaining mitochondrial function. Furthermore, we show that these changes in signalling and protein expression are largely independent of alterations in intramuscular amino acid content. Our findings, therefore, reveal potential new mechanisms and further contribute to our understanding of myostatin-regulated skeletal muscle growth and function.
Keywords: growth differentiation factor-8 (GDF-8), mTOR, p70S6K1, PKB/Akt
1. Introduction
Muscle atrophy, or a loss in muscle mass, can occur in response to a variety of different conditions or metabolic disorders such as aging, impaired respiratory function, cancer and diabetes [1-4]. Among the unfavourable effects that may arise from a reduction in skeletal muscle mass include abated physical strength and mobility, as well as the development of insulin resistance due to its role as a metabolically active tissue.
Myostatin, or growth differentiation factor-8 (GDF-8), is a member of the transforming growth factor-β (TGF-β) family of related proteins which acts to negatively regulate skeletal muscle growth. This inhibitory effect has been demonstrated mainly through its genetic loss or mutation in a number of different species including humans [5-8]. More specifically, myostatin regulates the number of muscle fibres by inhibiting myogenic proliferation and differentiation, effects which result from its binding and signalling through cell-bound activin type II receptors expressed in this tissue [9-14].
Exposure of differentiated myotubes to growth factors such as insulin-like growth factor-I (IGF-I) leads to a hypertrophic response, characterised by an increase in myotube diameter and elevated rate of protein synthesis [15,16]. Importantly, the induction of protein synthesis by activation of PKB accompanied by mammalian target of rapamycin (mTOR)-mediated signalling is sufficient to promote hypertrophy in skeletal muscle both in vitro and in vivo [16,17].
mTOR, a key integrator of nutrient and growth factor signals, regulates protein synthesis by phosphorylating both p70 S6 kinase (S6K) and translational initiation factor 4E-binding protein-1 (4E-BP1), respectively leading to their activation and inhibition. A number of studies have demonstrated that myostatin acts as a negative regulator of mTOR-directed signalling [11,13,18-21], consistent with its inhibitory effect on protein synthesis, although the mechanisms by which it does so are not fully understood. Here, we provide further insight into the myostatin-mediated regulation of skeletal muscle mass by showing that genetic loss of myostatin leads to the upregulation of PKB expression and that of mTOR/S6K signalling components, namely S6K and its downstream target ribosomal S6 protein (rpS6), concomitant with an observed increase in their phosphorylation. Furthermore, we demonstrate this response occurs largely in the absence of any significant change in intramuscular free amino acid content.
In addition, both PKB and mTOR have been implicated in the regulation of mitochondrial function. However, whereas active PKB promotes the downregulation in expression of peroxisome proliferator-activated receptor-gamma coactivator 1 α (PGC-1α), a transcription coactivator involved in regulating mitochondrial biogenesis [22], pharmacological inhibition of mTOR by rapamycin has been shown to suppress transcription of genes involved in mitochondrial oxidative function [23]. Therefore, we determined the expression of two key proteins important for mitochondrial oxidative function, namely PGC-1α and COX IV. We demonstrate here markedly reduced expression of both proteins under circumstances when PKB and mTOR/S6K signalling are simultaneously elevated in response to myostatin deficiency. Our observations are fully consistent with a switch towards an increased proportion of fast-twitch glycolytic type muscle fibres and also provide a possible explanation for the associated changes in protein synthesis, muscle mass and nutrient metabolism in skeletal muscle of myostatin deficient animals.
2. Materials and Methods
2.1 Materials
Antibodies against PKB, phospho-PKB-Ser473, phospho-p70S6K-Thr389, phospho-p44-42 MAPK-Thr202/Tyr204, p44-42 MAPK, phospho-S6-Ser240/244 and S6 were all from New England Biolabs (Beverley, MA). GAPDH antibody was from Sigma (Poole, UK). Antibody against COX IV was from Invitrogen (Madison, WI). PGC-1α antibody was purchased from Calbiochem (La Jolla, CA). HRP (horseradish peroxidase)-conjugated anti-(rabbit IgG) and anti-(mouse IgG) were obtained from New England Biolabs (Beverley, MA). All other chemicals were from Sigma-Aldrich unless otherwise stated.
2.2 Generation of Myostatin-deficient Mice
Animal studies were performed in accordance with the guidelines of the NIH Animal Care and Use Committee. Mice carrying a targeted mutation in the myostatin gene (MSTN-KO) [6] were produced from matings between heterozygotes that had been backcrossed 6 times into the C57BL/6 genetic background and genotyped as described [24]. Only male mice aged between 30 and 32 weeks were used to obtain all data presented.
2.3 Western Blot Analysis
Gastrocnemius muscle was isolated from mice, snap frozen in liquid nitrogen and homogenized in lysis buffer [50 mM Tris/HCl (pH 7.4), 0.27 M sucrose, 1 mM sodium orthovanodate, 1 mM EDTA, 1 mM EGTA, 10 mM sodium β-glycerophosphate, 50 mM NaF, 5 mM sodium pyrophosphate, 1% (v/v) Triton X-100, 0.1% 2-mercaptoethanol and protease inhibitors]. Cell lysates (40 µg) were subjected to SDS/PAGE on a 10% resolving gel and immunoblotted as previously described [25]. Immobilon-P membranes (Millipore, Bedford, MA) were probed with primary antibodies as indicated in the figure legends. Primary antibody detection was performed with the appropriate HRP (horseradish peroxidase)-conjugated anti-rabbit or anti-mouse IgG and resulting signals visualized using enhanced chemiluminescence by exposure to Konica Minolta X-ray autoradiographic film.
2.4 Amino Acid Analysis by HPLC
Muscle extracts were prepared for and analysed using HPLC as previously described [26]. Briefly, 20 mg of muscle tissue was homogenized in 12% PCA followed by derivatization using a mixture of ethanol, dH2O, TEA and phenylisothiocyanate (PITC) in a 7:1:1:1 ratio. The resulting phenylthiocarbamyl peptides were separated by a Hewlett Packard 1050 HPLC system (Minnesota, USA) using standard protocols. Comparison of retention times using amino acid standards was used to identify individual amino acids together with relative changes in peak size to measure their abundance.
2.5 Statistical Analysis
Statistical significance was determined by one way analysis of variance (ANOVA) using GraphPad Prism software. Data was considered statistically significant at P-values < 0.05.
3. Results and Discussion
3.1 Elevated levels of PKB and mTOR/S6K signalling components present in skeletal muscle of myostatin-deficient mice
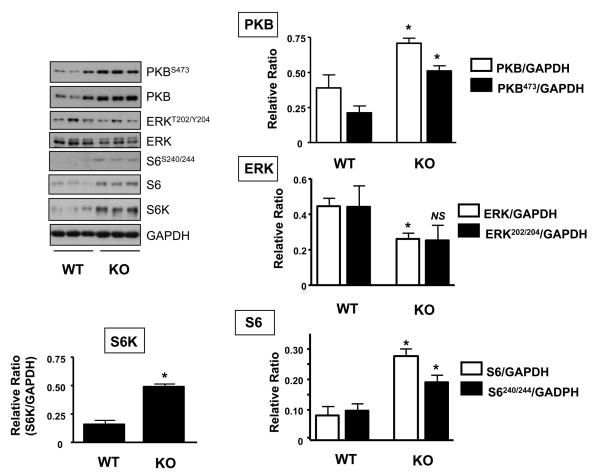
Activation of both the PI3K/PKB and mTOR/S6K pathways have been implicated as important signalling events involved in mediating increases in skeletal muscle mass, primarily by activating important downstream targets responsible for protein translation initiation and protein synthesis [15,27]. Previous studies have established that inhibiting myostatin function may lead to increased myofibrillar protein synthesis and skeletal muscle hypertrophy [5,6,28-30]. To investigate the mechanism(s) by which myostatin acts to regulate these processes, we assessed the expression and phosphorylation status of PKB and components of the mTOR/S6K pathway in myostatin-deficient mice (MSTN-KO). In comparison to wild type littermate control (WT) mice, PKB expression is significantly elevated by 1.8-fold along with a significant increase in PKB activity in the gastrocnemius muscle of MSTN-KO mice (Fig. 1). This is consistent with a previous study by Morissette et al. demonstrating a similar increase in PKB activity in accordance with elevated mRNA and protein levels of PKB in this same animal model [31]. However, in contrast to that study, we also observe a marked increase (both around 3-fold) in ribosomal protein S6 (rpS6) expression and that of its upstream kinase, S6K, thereby providing a likely explanation for the elevated levels of phosphorylated rpS6 at Ser240/244 in the MSTN-KOs (Fig. 1). It is unclear what may account for the observed differences in these studies, but it is plausible that differences in animal age as well as use of different muscle types may account for these contrasting findings. Our study used gastrocnemius muscle extracts from mice of 30-32 weeks of age, whereas Morissette et al. used quadriceps muscle from much younger animals (8-20 weeks of age). Therefore, further work will be required to establish whether distinct mechanisms underlie the development of hypertrophy with age in different muscle types.
Figure 1. Effect of myostatin deficiency on PKB, S6K and ERK1/2 protein expression and activity in skeletal muscle.
Gastrocnemius muscle was isolated from wild type control (WT) or homozygous myostatin knockout (MSTN-KO) mice and resulting tissue extracts immunoblotted using the indicated antibodies. Representative immunoblots and corresponding quantifications are shown. Each bar represents the mean ± S.E.M. for four individual animals. Asterisks indicate statistically significant differences versus wild type (WT) animals. *, P < 0.05; NS, not significant.
The upregulation of PKB/mTOR/S6K signalling in MSTN-KO mice is consistent with the reciprocal down-regulation of these proteins by myostatin and would, at least in part, explain the increase in protein synthesis and muscle hypertrophy observed in these animals. However, this increase in protein expression is not all-inclusive as, for instance, the levels of GAPDH remain unaltered whilst those of ERK1/2 actually significantly decrease (1.7-fold) in the MSTN-KOs (Fig. 1). The reduction in total ERK1/2 expression is also reflected by a lower level of its phosphorylation although the difference did not reach statistical significance in this case. This is a surprising observation given the role of ERK1/2 both as a key transcriptional regulator and in promoting cellular growth, whereby it has been shown to positively regulate protein synthesis [32,33]. Our data, therefore, suggests that inhibiting myostatin function does not promote growth through the elevation of ERK1/2 activity. This observation is, however, consistent with a previous study demonstrating that myostatin can activate rather than inhibit the Ras/Raf/ERK1/2-cascade in C2C12 myoblasts and, in doing so, implicates a role for this pathway in myostatin-mediated inhibition of muscle cell growth and differentiation [34]. Indeed, this may also be part of a counter-regulatory response to accelerated muscle growth. In addition to these changes in PKB/mTOR/S6K signalling components, we cannot exclude the possibility that autocrine production of upstream regulators such as muscle-derived growth factors may also be co-ordinately regulated. However, we observe no significant difference in the expression of mechano growth factor (MGF or IGF-1Eb in rodents) in muscle obtained from WT and MSTN-KO mice at the level of mRNA (data not shown), consistent with previous studies showing little change or even a slight decrease in IGF-1 expression with myostatin-deficiency [35-37]. On the other hand, these same studies demonstrate significant increases in IGF-2, a pro-myogenic growth factor, in response to loss of myostatin function [35-37]. Therefore, additional work will be required to determine the individual contribution of such upstream regulators upon cellular signalling and hypertrophy resulting from myostatin-deficiency.
What purpose may regulation of PKB/mTOR/S6K signalling by myostatin serve? Firstly, although induction of PKB/mTOR/S6K signalling promotes muscle hypertrophy, it has been shown that activation of PKB by either insulin or growth factors alone is not sufficient to induce a hypertrophic response even in the presence of amino acids [38]. In addition, inhibition of mTOR activity does not completely block hypertrophy in response to loss of myostatin function [30, 31]. This therefore suggests that PKB and mTOR/S6K may, at least to some extent, act synergistically to promote a full hypertrophic response and that the induction of both PKB and S6K/rpS6 expression and activity may be required to convey the MSTN-KO phenotype. Secondly, as well as being important for the modulation of protein synthesis through effects on protein translation, it has also recently been shown that S6K1 can directly phosphorylate Rictor, a component of mTOR complex-2 (mTORC2) [39]. Although the phosphorylation of Rictor by S6K1 does not lead to major changes in mTORC2-kinase activity, it can alter interactions of this complex with other proteins including 14-3-3 proteins. Therefore, upregulation of S6K expression and activity can itself act as an additional signalling input into mTORC2 by potentially altering its activity towards other target proteins that may be involved in the hypertrophic response.
With regards to PKB, it is now well established that it can induce protein synthesis by phosphorylating and inhibiting the tuberous sclerosis (TSC1/TCS2) complex, a negative regulator of mTOR [40]. In addition, PKB also phosphorylates PRAS40 (proline-rich Akt substrate-40) [41]. Although the exact function(s) of PRAS40 is still unknown, it has been shown to bind mTOR, via Raptor, leading to the suppression of mTOR activity [42,43]. Therefore, in both these cases, elevated PKB activity would further contribute towards induced mTOR/S6K signalling.
Furthermore, PKB also has an important role in the regulation of protein degradation. One such target of PKB in this process are the forkhead box O (FoxO) transcription factors. PKB phosphorylates FoxO proteins preventing their translocation into the nucleus, thereby suppressing their transcriptional activity towards genes encoding the E3 ubiquitin ligases MuRF1 and MAFbx, both of which mediate ubiquitin-mediated proteasomal degradation and are thought to be involved in the development of muscle atrophy [44]. In the case of MSTN-KO mice, the higher levels of PKB activity would be expected to suppress transcription of these atrogenes, although surprisingly, a previous study has shown that their levels either remain unaltered or are actually increased in myostatin-deficient mice, possibly representing a counter-regulatory response to an increase in muscle mass [31]. Consistent with this, although overexpressing myostatin causes skeletal muscle atrophy it does not appear to alter activity of the ubiquitin-proteasome pathway [19]. Likewise, exposing cultured myotubes to myostatin results in reduced protein synthesis but has no significant effect on the rate of protein degradation [10]. This therefore raises the issue as to whether manipulating myostatin function is itself sufficient to modulate proteasomal-mediated degradation, or whether there may be additional factors involved that it may act in conjunction with to mediate its effects. Also, we cannot exclude the possibility that myostatin acts to regulate other proteolytic mechanisms (e.g. lysosmal protein degradation) in vivo.
3.2 Myostatin modulation of amino acid composition in skeletal muscle
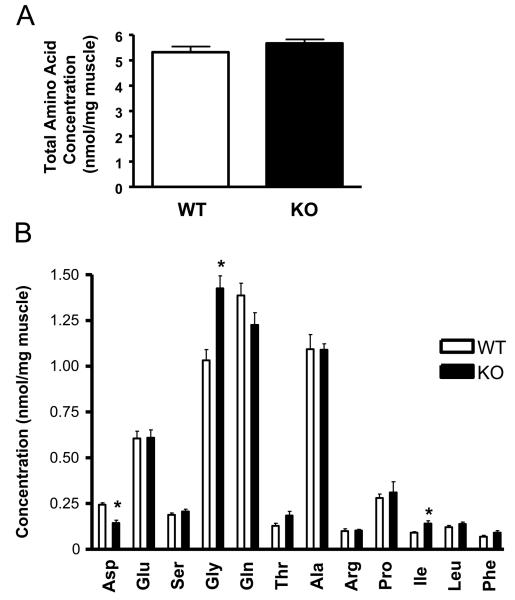
Due to the marked effects of myostatin deficiency upon muscle mass and signalling involved in regulating protein synthesis, we wanted to further explore the possibility that loss of myostatin function may also cause enhanced muscle growth by modulating amino acid composition within this tissue. To do this, gastrocnemius muscle was isolated from five wild type and five MSTN-KO non-fasted mice, and then subjected to HPLC analysis in order to compare the relative abundance of the 12 amino acids indicated in Fig. 2. By this analysis, despite the increase in mean gastrocnemius muscle mass (70% from 156 mg to 265 mg) and corresponding overall intramuscular amino acid pool size in MSTN-KOs, there was little difference in total intramuscular amino acid content between MSTN-KO and WT mice when expressed as an amount per mg of muscle tissue (Fig. 2A). This therefore suggests that enhanced muscle growth and changes in myocellular signalling observed in MSTN-KO mice occur independently of total intramuscular amino acid concentration.
Figure 2. Comparison of amino acid profiles of gastrocnemius muscle obtained from wild type and myostatin-deficient mice.
Gastrocnemius muscle isolated from individual wild type (WT) and myostatin deficient (KO) mice was prepared for and subjected to HPLC analysis in order to determine; (A) the total content of amino acids or (B) the concentration of the individual amino acids indicated. Data are presented as mean ± S.E.M. (n = 5 animals group). *, P < 0.05.
However, three out of the 12 amino acids analysed were found to have their concentrations significantly altered in response to myostatin deficiency, with levels of isoleucine increased by 56% and those of aspartate and glycine reduced and increased by around 40% respectively (Fig. 2B). Isoleucine, a branched amino acid, has previously been shown to activate S6K and regulators of mRNA translation in skeletal muscle [45,46]. Therefore, its elevated levels in MSTN-KO mice may contribute towards enhanced protein synthesis and the hypertrophic phenotype of these animals. In addition, administration of isoleucine has also been demonstrated to convey a hypoglycaemic effect involving increased glucose uptake into skeletal muscle – one of the phenotypic attributes of MSTN-KO mice [47,48]. The fact that glycine is selectively upregulated in MSTN-KO mice is surprising but a previous study has shown that administering glycine can counteract myofibrillar proteolysis by downregulating atrophic and proteolytic-related genes [49]. Therefore, it is possible that this increase in muscle glycine content may further augment the hypertrophic response by suppressing proteolysis.
3.3 Myostatin deficiency reduces oxidative capacity in skeletal muscle
The multifarious functions of skeletal muscle arise from differences in fibre type distribution which is primarily responsible for the metabolic diversity found in this tissue. This variation in metabolic makeup can be further altered under different pathophysiological conditions. For instance, factors such as physical inactivity and insulin resistance all coincide with an overall reduced oxidative capacity [50,51]. In contrast, increased physical activity, associated with improved insulin sensitivity, elevates expression of oxidative enzymes whilst reducing those with glycolytic activity [50-52]. Therefore, we wanted to determine whether myostatin deficiency alters the expression of peroxisome proliferator-activated receptor-gamma coactivator 1 α (PGC-1α), a key regulator of mitochondrial biogenesis, and of cytochrome c oxidase IV (COX IV), the terminal enzyme in the electron transport chain in mitochondria.
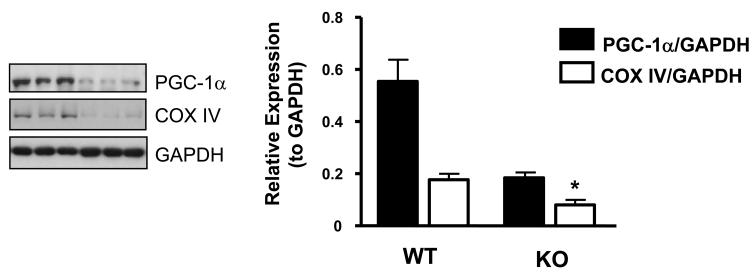
As shown in Fig. 3, the expression of both PGC-1α and COX IV is significantly reduced by 3 and 2.2-fold respectively in the gastrocnemius muscle of MSTN-KO compared to wild type mice, indicative of a reduction in oxidative capacity. Therefore, interestingly, our data suggests that loss of myostatin function leads to a fibre type distribution more characteristic of an insulin resistant state, despite myostatin-deficient mice displaying increased insulin sensitivity and resistance to diet-induced obesity [48,51,53]. However, our observations are consistent with previous work demonstrating that genetic loss of myostatin promotes a shift towards a fast glycolytic phenotype [36,54-56]. In addition, the downregulation of PGC-1α and COX IV expression is in accordance with reduced lipid oxidation in skeletal muscle of MSTN-KO mice [48,57,58]. Further work will be required to determine whether the loss in expression of these mitochondrial markers is simply due to the lower proportion of oxidative Type I fibres present or if more acute inhibition of myostatin signalling, perhaps in cultured myotubes, can mimic this response. Again, the question remains as to whether the loss of myostatin signalling is directly causing the fibre type switch or if this is part of a feedback response to accelerated muscle growth.
Figure 3. Effect of myostatin deficiency on PGC-1α and COX IV protein expression in skeletal muscle.
Gastrocnemius muscle was isolated from wild type control (WT) or homozygous myostatin knockout (KO) mice and resulting tissue extracts immunoblotted for PGC-1α and COX IV. Representative immunoblots and corresponding quantifications are shown. Each bar represents the mean ± S.E.M. for three individual animals. Asterisks indicate statistically significant differences versus wild type (WT) animals. *, P < 0.05.
In conclusion, maintenance of skeletal muscle mass and function is crucial for proper regulation of blood glucose levels and lipid metabolism. Importantly, myostatin plasma levels and expression in muscle have been shown to be elevated in obesity and with progressive aging [59-61]. In accordance with this, recent studies have shown that inhibition of myostatin signalling either by the development of pharmacological inhibitors or otherwise may prove to be beneficial in the treatment of metabolic disorders such as Type II diabetes or for increasing skeletal muscle mass in patients with muscle-wasting diseases [48,62,63]. The findings presented in this study demonstrate some degree of selectivity by which such a course of treatment may have upon cellular signalling, although further work will be required to fully elucidate the consequences of modulating myostatin function upon other signalling systems. Only then can a more comprehensive understanding of its downstream effects and therefore its viability as a potential therapeutic target be acquired.
Acknowledgements
This work was supported by the Intramural Research Program of the NIH, NIDDK, the BBSRC, European Commission (contract LSHM-CT-20004-005272), Diabetes Research & Wellness Foundation and Diabetes UK.
Abbreviations
- PKB
protein kinase B
- mTOR
mammalian target of rapamycin
- GDF-8
growth differentiation factor-8
- TGF-β
transforming growth factor -β
- 4E-BP1
eukaryotic translation initiation factor 4E binding protein 1
- rpS6
ribosomal protein S6
- COX IV
cytochrome c oxidase subunit IV
- S6K
p70 ribosomal protein S6 kinase
- TSC
tuberous sclerosis complex
- PRAS40
proline-rich Akt substrate
- OXPHOS
oxidative phosphorylation
- MuRF1
muscle RING finger protein-1
- MAFbx
muscle atrophy F-box
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–94. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 2.Baracos VE. Regulation of skeletal-muscle-protein turnover in cancer-associated cachexia. Nutrition. 2000;16:1015–8. doi: 10.1016/s0899-9007(00)00407-x. [DOI] [PubMed] [Google Scholar]
- 3.Hasselgren PO, Fischer JE. Muscle cachexia: current concepts of intracellular mechanisms and molecular regulation. Ann Surg. 2001;233:9–17. doi: 10.1097/00000658-200101000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch GS, Schertzer JD, Ryall JG. Therapeutic approaches for muscle wasting disorders. Pharmacol Ther. 2007;113:461–87. doi: 10.1016/j.pharmthera.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7:910–6. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- 6.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 7.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997;94:12457–61. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SJ. Sprinting without myostatin: a genetic determinant of athletic prowess. Trends Genet. 2007;23:475–7. doi: 10.1016/j.tig.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98:9306–11. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH, Jr., Kull FC, Jr., Gonzalez-Cadavid N. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab. 2001;280:E221–8. doi: 10.1152/ajpendo.2001.280.2.E221. [DOI] [PubMed] [Google Scholar]
- 11.Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002;277:49831–40. doi: 10.1074/jbc.M204291200. [DOI] [PubMed] [Google Scholar]
- 12.Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res. 2003;286:263–75. doi: 10.1016/s0014-4827(03)00074-0. [DOI] [PubMed] [Google Scholar]
- 13.Rios R, Fernandez-Nocelos S, Carneiro I, Arce VM, Devesa J. Differential response to exogenous and endogenous myostatin in myoblasts suggests that myostatin acts as an autocrine factor in vivo. Endocrinology. 2004;145:2795–803. doi: 10.1210/en.2003-1166. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci U S A. 2005;102:18117–22. doi: 10.1073/pnas.0505996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musaro A, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 16.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–13. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 17.Bodine SC, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 18.McFarlane C, et al. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol. 2006;209:501–14. doi: 10.1002/jcp.20757. [DOI] [PubMed] [Google Scholar]
- 19.Amirouche A, et al. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology. 2009;150:286–94. doi: 10.1210/en.2008-0959. [DOI] [PubMed] [Google Scholar]
- 20.Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, Sandri M. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol. 2009;296:C1248–57. doi: 10.1152/ajpcell.00104.2009. [DOI] [PubMed] [Google Scholar]
- 21.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–70. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 22.Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes. 2003;52:642–9. doi: 10.2337/diabetes.52.3.642. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–40. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 24.Manceau M, Gros J, Savage K, Thome V, McPherron A, Paterson B, Marcelle C. Myostatin promotes the terminal differentiation of embryonic muscle progenitors. Genes Dev. 2008;22:668–81. doi: 10.1101/gad.454408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajduch E, Alessi DR, Hemmings BA, Hundal HS. Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes. 1998;47:1006–13. doi: 10.2337/diabetes.47.7.1006. [DOI] [PubMed] [Google Scholar]
- 26.MacKenzie MG, Hamilton DL, Murray JT, Taylor PM, Baar K. mVps34 is activated following high-resistance contractions. J Physiol. 2009;587:253–60. doi: 10.1113/jphysiol.2008.159830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai KM, et al. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol. 2004;24:9295–304. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grobet L, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17:71–4. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- 29.Whittemore LA, et al. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300:965–71. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 30.Welle S, Burgess K, Mehta S. Stimulation of skeletal muscle myofibrillar protein synthesis, p70 S6 kinase phosphorylation, and ribosomal protein S6 phosphorylation by inhibition of myostatin in mature mice. Am J Physiol Endocrinol Metab. 2009;296:E567–72. doi: 10.1152/ajpendo.90862.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morissette MR, Cook SA, Buranasombati C, Rosenberg MA, Rosenzweig A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am J Physiol Cell Physiol. 2009;297:C1124–32. doi: 10.1152/ajpcell.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sale EM, Atkinson PP, Arnott CH, Chad JE, Sale GJ. Role of ERK1/ERK2 and p70S6K pathway in insulin signalling of protein synthesis. FEBS Lett. 1999;446:122–6. doi: 10.1016/s0014-5793(99)00193-3. [DOI] [PubMed] [Google Scholar]
- 33.Felton-Edkins ZA, Fairley JA, Graham EL, Johnston IM, White RJ, Scott PH. The mitogen-activated protein (MAP) kinase ERK induces tRNA synthesis by phosphorylating TFIIIB. EMBO J. 2003;22:2422–32. doi: 10.1093/emboj/cdg240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang W, Chen Y, Zhang Y, Wang X, Yang N, Zhu D. Extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase pathway is involved in myostatin-regulated differentiation repression. Cancer Res. 2006;66:1320–6. doi: 10.1158/0008-5472.CAN-05-3060. [DOI] [PubMed] [Google Scholar]
- 35.Kocamis H, Gahr SA, Batelli L, Hubbs AF, Killefer J. IGF-I, IGFII, and IGF-receptor-1 transcript and IGF-II protein expression in myostatin knockout mice tissues. Muscle Nerve. 2002;26:55–63. doi: 10.1002/mus.10160. [DOI] [PubMed] [Google Scholar]
- 36.Steelman CA, Recknor JC, Nettleton D, Reecy JM. Transcriptional profiling of myostatin-knockout mice implicates Wnt signaling in postnatal skeletal muscle growth and hypertrophy. FASEB J. 2006;20:580–2. doi: 10.1096/fj.05-5125fje. [DOI] [PubMed] [Google Scholar]
- 37.Gilson H, Schakman O, Combaret L, Lause P, Grobet L, Attaix D, Ketelslegers JM, Thissen JP. Myostatin gene deletion prevents glucocorticoid-induced muscle atrophy. Endocrinology. 2007;148:452–60. doi: 10.1210/en.2006-0539. [DOI] [PubMed] [Google Scholar]
- 38.Jaspers RT, van Beek-Harmsen BJ, Blankenstein MA, Goldspink G, Huijing PA, van der Laarse WJ. Hypertrophy of mature Xenopus muscle fibres in culture induced by synergy of albumin and insulin. Pflugers Arch. 2008;457:161–70. doi: 10.1007/s00424-008-0499-0. [DOI] [PubMed] [Google Scholar]
- 39.Treins C, Warne PH, Magnuson MA, Pende M, Downward J. Rictor is a novel target of p70 S6 kinase-1. Oncogene. 2010;29:1003–16. doi: 10.1038/onc.2009.401. [DOI] [PubMed] [Google Scholar]
- 40.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 41.Kovacina KS, Park GY, Bae SS, Guzzetta AW, Schaefer E, Birnbaum MJ, Roth RA. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem. 2003;278:10189–94. doi: 10.1074/jbc.M210837200. [DOI] [PubMed] [Google Scholar]
- 42.Thedieck K, Polak P, Kim ML, Molle KD, Cohen A, Jeno P, Arrieumerlou C, Hall MN. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS One. 2007;2:e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 44.Sandri M, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z, Jahn LA, Long W, Fryburg DA, Wei L, Barrett EJ. Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt this action. J Clin Endocrinol Metab. 2001;86:2136–43. doi: 10.1210/jcem.86.5.7481. [DOI] [PubMed] [Google Scholar]
- 46.Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab. 2004;287:E1–7. doi: 10.1152/ajpendo.00430.2003. [DOI] [PubMed] [Google Scholar]
- 47.Doi M, Yamaoka I, Nakayama M, Sugahara K, Yoshizawa F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2007;292:E1683–93. doi: 10.1152/ajpendo.00609.2006. [DOI] [PubMed] [Google Scholar]
- 48.Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One. 2009;4:e4937. doi: 10.1371/journal.pone.0004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakashima K, Yakabe Y, Ishida A, Katsumata M. Effects of orally administered glycine on myofibrillar proteolysis and expression of proteolytic-related genes of skeletal muscle in chicks. Amino Acids. 2008;35:451–6. doi: 10.1007/s00726-007-0573-5. [DOI] [PubMed] [Google Scholar]
- 50.Papa S. Mitochondrial oxidative phosphorylation changes in the life span. Molecular aspects and physiopathological implications. Biochim Biophys Acta. 1996;1276:87–105. doi: 10.1016/0005-2728(96)00077-1. [DOI] [PubMed] [Google Scholar]
- 51.Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol. 1997;83:166–71. doi: 10.1152/jappl.1997.83.1.166. [DOI] [PubMed] [Google Scholar]
- 52.Gollnick PD, Saltin B. Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin Physiol. 1982;2:1–12. doi: 10.1111/j.1475-097x.1982.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 53.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Girgenrath S, Song K, Whittemore LA. Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow-and fast-type skeletal muscle. Muscle Nerve. 2005;31:34–40. doi: 10.1002/mus.20175. [DOI] [PubMed] [Google Scholar]
- 55.Chelh I, Meunier B, Picard B, Reecy MJ, Chevalier C, Hocquette JF, Cassar-Malek I. Molecular profiles of Quadriceps muscle in myostatin-null mice reveal PI3K and apoptotic pathways as myostatin targets. BMC Genomics. 2009;10:196. doi: 10.1186/1471-2164-10-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McPherron AC, Huynh TV, Lee SJ. Redundancy of myostatin and growth/differentiation factor 11 function. BMC Dev Biol. 2009;9:24. doi: 10.1186/1471-213X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999;13:2051–60. doi: 10.1096/fasebj.13.14.2051. [DOI] [PubMed] [Google Scholar]
- 58.Houmard JA. Intramuscular lipid oxidation and obesity. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1111–6. doi: 10.1152/ajpregu.00396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milan G, Dalla Nora E, Pilon C, Pagano C, Granzotto M, Manco M, Mingrone G, Vettor R. Changes in muscle myostatin expression in obese subjects after weight loss. J Clin Endocrinol Metab. 2004;89:2724–7. doi: 10.1210/jc.2003-032047. [DOI] [PubMed] [Google Scholar]
- 60.Leger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11:163–175B. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- 61.Hittel DS, Berggren JR, Shearer J, Boyle K, Houmard JA. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes. 2009;58:30–8. doi: 10.2337/db08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradley L, Yaworsky PJ, Walsh FS. Myostatin as a therapeutic target for musculoskeletal disease. Cell Mol Life Sci. 2008;65:2119–24. doi: 10.1007/s00018-008-8077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akpan I, et al. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int J Obes (Lond) 2009;33:1265–73. doi: 10.1038/ijo.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]