Abstract
Testicular germ cell tumors (TGCT) are comprised of two histologic groups, seminomas and nonseminomas. We postulated that the possible divergent pathogeneses of these histologies may be partially explained by variable levels of net endogenous DNA damage. To test our hypothesis, we conducted a case-case analysis of 51 seminoma and 61 nonseminoma patients using data and specimens from the Familial Testicular Cancer study and the U.S. Radiologic Technologists cohort. A lymphoblastoid cell line was cultured for each patient and the alkaline comet assay was used to determine four parameters: tail DNA, tail length, comet distributed moment (CDM) and Olive tail moment (OTM). Odds ratios (OR) and 95% confidence intervals (95%CI) were estimated using logistic regression. Values for tail length, tail DNA, CDM and OTM were modeled as categorical variables using the 50th and 75th percentiles of the seminoma group. Tail DNA was significantly associated with nonseminoma compared to seminoma (OR50th percentile=3.31, 95%CI: 1.00, 10.98; OR75th percentile=3.71, 95%CI: 1.04, 13.20; p for trend=0.039). OTM exhibited similar, albeit statistically non-significant, risk estimates (OR50th percentile=2.27, 95%CI: 0.75, 6.87; OR75th percentile=2.40, 95%CI: 0.75, 7.71; p for trend=0.12) whereas tail length and CDM showed no association. In conclusion, the results for tail DNA and OTM indicate that net endogenous levels are higher in patients who develop nonseminoma compared with seminoma. This may partly explain the more aggressive biology and younger age-of-onset of this histologic subgroup compared with the relatively less aggressive, later-onset seminoma.
Keywords: comet assay, nonseminoma, DNA damage, seminoma, testicular neoplasms
INTRODUCTION
The etiopathogenesis of testicular germ cell tumors (TGCT) and its distinct histologic subtypes, seminoma and nonseminoma, largely remains to be elucidated. An increasing number of epidemiologic investigations are being undertaken to explore the causes of these tumors, a development piqued by the unexplained rise in incidence of these malignancies over the past 40 years in Western Europe and the United States (Bray, et al., 2006; Huyghe, et al., 2003; Purdue, et al., 2005). Although some risk factors have been consistently associated with risk of TGCT, specifically cryptorchidism, prior history of TGCT and family history of TGCT (McGlynn, 2001), much of the epidemiologic evidence remains weak and contradictory (Dieckmann & Pichlmeier, 2004). The current antithetical state of the TGCT literature may be partly attributable to the two main histologic groups having potentially divergent etiologies and natural histories (Aschim, et al., 2006; Coupland, et al., 1999).
Accumulation of cellular DNA damage, the failure to repair such and the loss of the ability to undergo apoptosis are hallmarks of all cancers. Net endogenous DNA damage, which is DNA damage accumulated in the absence of exogenous mutagen challenges, is the product of both the basal rate of DNA damage and the efficiency of DNA repair (Berwick & Vineis, 2000; Poulsen, et al., 1993). Single-strand DNA breaks and alkali-labile sites can be quantified using the alkaline comet assay (Singh, et al., 1988). Studies using the alkaline comet assay have suggested that higher levels of net endogenous DNA damage are associated with diverse cancers, including those of the lung (Jianlin, et al., 2006; Lou, et al., 2007), esophagus (Shao, et al., 2005), breast (Blasiak, et al., 2004; Sanchez, et al., 2004; Smith, et al., 2003), bladder (Schabath, et al., 2003) and ovary (Baltaci, et al., 2002).
Although testicular seminoma and nonseminoma are thought to arise from a common precursor (Oosterhuis & Looijenga, 2005), it is postulated that they have divergent pathogeneses, which may be explained, partially, by variable levels of net endogenous DNA damage. To assess our hypothesis, a case-case analysis of the two histologic subgroups of TGCT, seminoma and nonseminoma, was undertaken, using the alkaline comet assay to quantify net endogenous DNA damage.
MATERIALS & METHODS
Study Design
Testicular germ cell tumor cases for this analysis were identified from two existing studies at the National Cancer Institute: the Familial Testicular Cancer (FTC) study and the U.S. Radiologic Technologists (USRT) study. The focus of the family-based FTC study is the identification of TGCT susceptibility genes (Peters, et al., 2006). Families are eligible for enrollment if they have at least two confirmed cases of testicular cancer, or if they have a single bilateral case. For the current study, the FTC study provided samples from 72 family history positive unilateral TGCT cases, 6 family history positive bilateral cases and 18 family history negative bilateral cases. The USRT study is a cohort study of 143,517 radiologic technologists who were occupationally certified between 1926 and 1980 (Doody, et al., 1998). Incident cancers in the cohort, including sixteen TGCTs, were ascertained through 2005. All cases of TGCT were unilateral and all provided samples to the current study. The FTC and USRT studies were approved by the Institutional Review Board of the National Cancer Institute. Laboratory work conducted at Lawrence Livermore National Laboratory (LLNL) has been approved annually by the LLNL Institutional Review Board.
Comet Analysis
An Epstein Barr virus transformed lymphoblastoid cell line was prepared from peripheral blood lymphocytes obtained from each subject. DNA samples were identified by a unique ID code, and investigators were blinded to case-group status. The methods used have been described in detail (Sigurdson, et al., 2005). Briefly, cell lines were cultured in RPMI 1640 supplemented with 15% serum (Fetal Clone III, HyClone, Logan, Utah) and 2mM glutamine for 1 to 2 weeks prior to analysis. Viability was determined by Trypan blue dye exclusion. The alkaline comet assay was used to measure DNA damage in exponentially growing cells according to Singh et al. (1988) with slight modifications. Images of 50 cells on each of two slides were captured and comet parameters determined using Komet4.0©: Image Analysis and Data Capture software (Kinetic Imaging, Ltd., Merseyside, England). The image analysis software generates many comet parameters but only four were retained and analyzed: tail DNA, tail length, comet distributed moment (CDM) and Olive tail moment (OTM). These are the four most common comet parameters reported in the literature, two of which (tail DNA and OTM) have recently been endorsed as the most quantitative and robust for epidemiologic studies (Lee, et al., 2004). However, to-date there has been no systematic study of the relative merits or the underlying biological mechanisms associated with inter-individual variation in these parameters. “Tail DNA” is the percent of DNA (fluorescence) in the tail. “Tail length” is the length of the tail in μm. CDM is the moment of fluorescence of the whole comet and does not distinguish between head and tail. OTM, or tail moment, is the product of the percentage of DNA in the tail (tail DNA) and the distance between the means of the head and tail fluorescence distributions. Both CDM and OTM are expressed in arbitrary units. All four parameters describe the amount of endogenous DNA damage; high values are thought to correspond to an increased cellular DNA strand breakage and/or alkali-labile sites.
Statistical analysis
The geometric mean of tail length, tail DNA, CDM and OTM of 100 randomly selected cells per subject was used as a summary measure to reduce the influence of outliers. Normality of the subject-specific summary measures was assessed for each comet parameter by Kolmogorov–Smirnov tests and visual inspection of quantile–quantile plots. Comet values did not deviate from normality. Analysis of variance (ANOVA) was used to compare means of comet values stratified by various covariates, including date of cell culture, date of cell harvest, date of electrophoresis, date of image analysis, cell viability in culture, study from which sample originated, patient age at blood draw, age of TGCT diagnosis, history of cryptorchidism and TGCT laterality. Dates of cell culture, cell harvest, electrophoresis and image analysis were all highly correlated. If these variables were deemed to be confounding then the variable which produced the highest r2 was retained in the fully adjusted model. The association between comet values and histologic group was evaluated by calculating odds ratios (OR) and 95% confidence intervals using logistic regression. The cluster option was used to estimate the standard errors and variance-covariance matrix, in order to account for any intra-group correlation caused by a subset of the familial cases being related (i.e., more than one affected member of a multiple case family was permitted into the data set). Among the 24 bilateral TGCT cases, 8 men had discordant histology between the two affected testes. For analytic purposes, these individuals were coded as having nonseminoma based on the assumption that if endogenous DNA levels were different between the two histologic groups, they would more likely be higher in nonseminomas as they are more clinically aggressive (Andreyev, et al., 1993; Horwich, et al., 2006; Moller, 1993) and arise at earlier ages (Moller, 1993; Surveillance Epidemiology and End Results Program) than seminomas. Comet values for tail length, tail DNA, CDM and OTM were modeled as categorical variables using the 50th and 75th percentiles of the seminoma group as cut-points. All models were adjusted for the potential confounders listed above and were evaluated by comparing comet value ORs with and without each factor in the model. If a covariate altered the risk estimate by >10% it was considered a confounder and retained within the model. When applicable, tests for linear trend in risk according to the medians of each category of a given ordered categorical variable were conducted to evaluate possible dose-response relationships. To ascertain that any observed differences were not mediated by treatment (radiotherapy and chemotherapy), additional logistic regression analyses adjusting for these variables were undertaken. All statistical analyses were conducted with STATA 10 software (StataCorp, 2007). All tests were two sided, with p<0.05 defined as statistically significant. Coefficients of variation for the alkaline comet assay, as conducted at the LLNL using repeat samples, have been less than 15% (Sigurdson, et al., 2005).
RESULTS
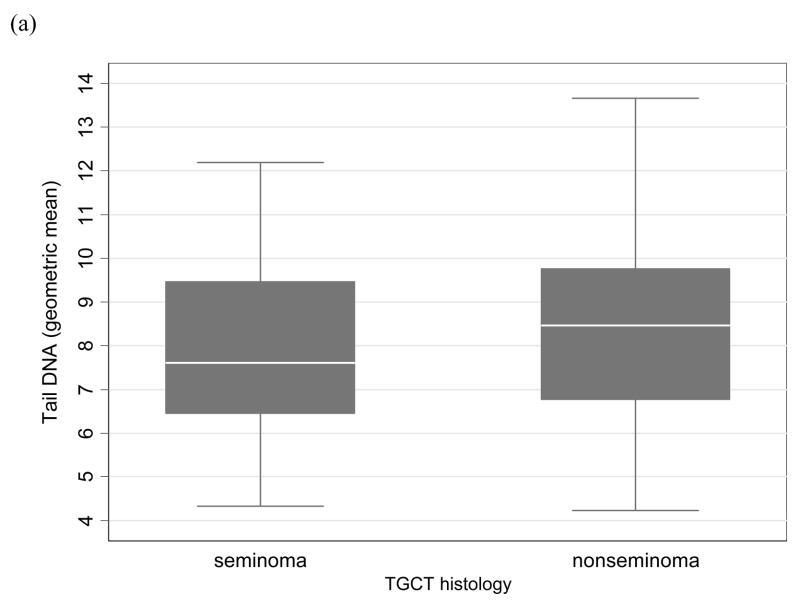
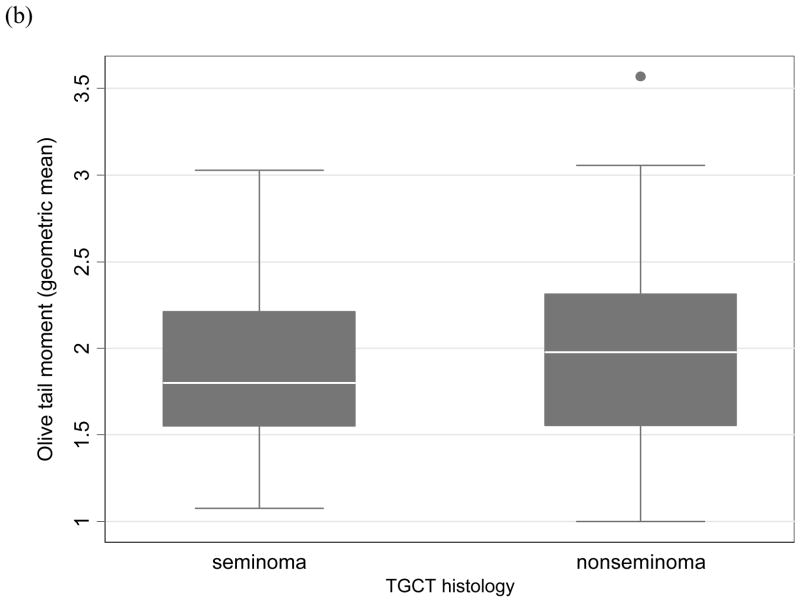
This case-case analysis included 112 TGCT patients (51 seminomas and 61 nonseminomas). Of the 112, there were 52 individual cases, 27 related case-pairs and 2 related case-triads. The overall mean age at TGCT diagnosis for all study participants was 31.2 years; the mean age at diagnosis for seminoma (34.1 years) was later than nonseminoma (28.7 years). Mean patient age at blood draw was 42.5 years, which was an average of 10 years post-diagnosis. Six of the 51 seminoma patients (11.8%) and four of the 61 nonseminoma patients (6.8%) had a history of cryptorchidism. The results of the logistic regression analyses are shown in the Table. Variables that affected a comet parameter’s risk estimate were included in the model. These variables were date of cell harvest for tail DNA and for OTM and date of cell electrophoresis for CDM. Other variables tested as potential confounders, such as patient age at blood draw, age at TGCT diagnosis and history of cryptorchidism, did not alter the risk estimates and thus were not retained in the final models. Tail DNA was observed to be significantly associated with nonseminoma versus seminoma (OR50th percentile=3.31, 95%CI: 1.00, 10.98; OR75th percentile=3.71, 95%CI: 1.04, 13.20). The trend across the tail DNA estimates was positively correlated with nonseminoma (p=0.039). Although the results for OTM did not reach statistical significance, the estimates and trend were similar to that observed for tail DNA (OR50th percentile=2.27, 95%CI: 0.75, 6.87; OR75th percentile=2.40, 95%CI: 0.75, 7.71; p for trend=0.12). Tail length and CDM were not associated with TGCT histology.These results did not differ when the 16 USRT or the eight bilateral seminoma/nonseminoma cases were excluded from the analysis. The Figure shows boxplots of tail DNA and OTM stratified by histology.
TABLE.
An analysis of comet parameters in cell lines from men diagnosed with nonseminoma compared to seminoma (referent group)
| Comet parameter | Nonseminoma vs. Seminoma
|
||||
|---|---|---|---|---|---|
| Seminoma (n) | Nonseminoma (n) | Odds ratio | 95% CI | p value | |
| Tail DNA† (%) | |||||
| 4.2 – 7.7 | 25 | 21 | 1.00 | Referent | |
| 7.8 – 9.4 | 13 | 19 | 3.31 | 1.00 – 10.98 | 0.050 |
| 9.5 – 13.7 | 13 | 21 | 3.71 | 1.04 – 13.20 | 0.043 |
| p for trend | 0.039 | ||||
| Tail length (μm) | |||||
| 12.1 – 32.1 | 25 | 29 | 1.00 | Referent | |
| 32.2 – 34.8 | 13 | 10 | 0.66 | 0.23 – 1.91 | 0.45 |
| 34.9 – 45.8 | 13 | 22 | 1.46 | 0.58 – 3.68 | 0.42 |
| p for trend | 0.64 | ||||
| Comet distributed moment‡ | |||||
| 14.6 – 20.1 | 25 | 30 | 1.00 | Referent | |
| 20.2 – 21.7 | 13 | 16 | 0.95 | 0.40 – 2.27 | 0.91 |
| 21.8 – 27.7 | 13 | 15 | 0.85 | 0.30 – 2.38 | 0.75 |
| p for trend | 0.75 | ||||
| Olive tail moment† | |||||
| 1.0 – 1.8 | 25 | 23 | 1.00 | Referent | |
| 1.9 – 2.2 | 13 | 18 | 2.27 | 0.75 – 6.87 | 0.15 |
| 2.3 – 3.6 | 13 | 20 | 2.40 | 0.75 – 7.71 | 0.14 |
| p for trend | 0.12 | ||||
Comet parameters were divided into three categories using the 50th and 75th percentile of the seminoma distribution.
adjusted for date of cell harvest
adjusted for date of electrophoresis
FIGURE. Boxplots of comet parameters by testicular germ cell tumor histology.
(a) tail DNA; (b) Olive tail moment. Each boxplot represents the geometric means of the respective comet parameter of 100 cells for each individual within the nonseminoma or seminoma group. The boxplots display the median (thick line), interquartile range (lower and upper box borders), the 5th and 95th percentiles (error bars), and extreme individual values (●).
In order to ensure that past treatment (radiotherapy and chemotherapy) did not confound our results, we also conducted analyses which adjusted for these variables (see supplementary table). All patients had undergone orchiectomy. Of the 41 seminoma patients, for which treatment data were available: 3 had received no chemotherapy or radiotherapy; 32, radiotherapy only; 5, chemotherapy only; and 1, both radiotherapy and chemotherapy. Of the 57 nonseminoma patients: 23 had received neither radiotherapy nor chemotherapy; 3, radiotherapy only; 27, chemotherapy only; and 4, both radiotherapy and chemotherapy. The small numbers in some of the treatment categories did not permit a high level of statistical power; this is partly due to the fact that treatment regimen is correlated with histology of testicular cancer, as is to be expected. The problem of inadequate statistical power was further exacerbated with the inclusion of three covariates in the logistic regression models. Therefore, risk estimates derived are inaccurate, as attested by very wide confidence intervals, and a cautious interpretation is warranted (see supplementary table).
DISCUSSION with CONCLUSIONS
To our knowledge, this is the first study to quantify net endogenous DNA damage in TGCT patients. The results for tail DNA and OTM, comet parameters recently endorsed as the most quantitative and robust for epidemiologic studies (Lee, et al., 2004), indicate that endogenous levels of single strand DNA breaks and alkali-labile sites are higher in men who develop nonseminoma compared with seminoma. This result may partly explain the more biologically aggressive nature (Andreyev, et al., 1993; Horwich, et al., 2006; Moller, 1993) and younger age at diagnosis (Moller, 1993; Surveillance Epidemiology and End Results Program) of nonseminoma compared with the relatively less aggressive, later onset seminoma. This postulate is consistent with the evidence that nonseminoma may derive from seminoma via transitional genetic re-programming (Oliver, 1990; Oosterhuis & Looijenga, 2003; Oosterhuis & Looijenga, 2005), a process that would theoretically be aided by increased genetic instability.
Quantitation of DNA damage in cultured lymphoblastoid cell lines, created using blood drawn on average 10 years post-diagnosis, is likely to represent an individual’s capacity to limit DNA damage and its associated health consequence. In theory, treatment regimens of radiotherapy and chemotherapy could influence the study’s results if treatment effects are manifested over a very long time period. However, after the effects of treatment had been taken into account, the risk estimates increased, a result which is counter-intuitive to the postulated effects of confounding by treatment (see supplementary table). Furthermore, previous associations between radiotherapy and/or chemotherapy and risk of (lymphoid) leukemia in testicular cancer survivors are tentative and inconsistent (Richiardi, et al., 2006; Robinson, et al., 2007; Travis, et al., 2005) while the alkali comet assay specifically quantifies strand breaks and alkali-labile sites, types of DNA damage whose repair is required for mitosis to proceed. These points provide further evidence that the results presented herein are unlikely to be affected by treatment regimen. Therefore, the increased levels of net endogenous DNA damage observed in patients who developed nonseminoma, relative to those who developed seminoma, most likely represent either a difference in the net rate of carcinogen metabolism and/or a difference in the ability to repair DNA. Moreover, the efficiency of these processes is likely to be representative of the core biology of these individuals over the whole life-time, thus even small differences could theoretically have large consequences for cancer risk.
The relationship between carcinogen metabolism and cancer risk is poorly understood, primarily due to the complexity of such metabolic interactions (Clapper, 2000). Most studies have focused on either phase I (e.g. cytochrome P450s (CYP)) or phase II (e.g. N-acetyltransferase, glutathione S-transferase) gene variants, but such variants have rarely been studied for their association with TGCT. One study has reported positive associations of CYP3A4 -392G and CYP3A5 6986G and an inverse association of CYP1A2 -163A with TGCT risk (Starr, et al., 2005). A second study observed low CYP1A2 activity to be associated with TGCT risk (Vistisen, et al., 2004). Both of these studies found risk was unaltered when stratified by histologic group.
Although the mitotically dividing cells of the testes may have a limited DNA damage response (Bartkova, et al., 2007), variation in DNA repair capacity could also explain the observed difference in levels of net endogenous DNA damage between TGCT histologies. Presently, only two studies have investigated DNA repair gene variants in relation to TGCT risk. The first study found no association of single nucleotide polymorphisms of XPD, ERCC1, XRCC3 and OGG1 with risk of TGCT or either histologic type (Laska, et al., 2005). However, this study only had 47% power to detect an OR of 2, an estimate which may be considered optimistic, especially given that a subsequently published genome-wide linkage study found no single major locus could account for familial aggregation of TGCT (Crockford, et al., 2006). The second study evaluated two polymorphisms of the XRCC1 gene and found that having at least one 399Gln allele increased TGCT risk (Tsuchiya, et al., 2006). This association was stronger when restricted to patients with pure seminoma or metastatic disease.
It has been perceived that seminoma and nonseminoma have a similar etiopathogenesis and the epidemiological evidence is certainly consistent with this notion, insofar that they arise through a common precursor lesion (carcinoma in situ) (Oosterhuis & Looijenga, 2005), have overlapping risk factors (Hardell, et al., 1998; Moller & Skakkebaek, 1997; Moss, et al., 1986; Pike, et al., 1987; Prener, et al., 1996; Prener, et al., 1992; Sabroe & Olsen, 1998; Weir, et al., 2000) and, in many countries, share similar incidence trends over time (Bray, et al., 2006). However, only a handful of studies investigating histologic differences have had a large number of cases (n>500) and many of the smaller studies may have lacked statistical power, increasing the chance of type two errors. Moreover, there is mounting epidemiologic evidence to suggest that a divergence occurs in the natural history of these histologies. Notable evidence includes cryptorchidism (Coupland, et al., 1999; Morrison, 1976; Prener, et al., 1996; Stone, et al., 1991), low birth weight (English, et al., 2003; Wanderas, et al., 1998) and low birth order (Richiardi, et al., 2004; Sabroe & Olsen, 1998) being predominantly associated with an increased risk of seminoma, while participation in specific sporting activities (Coupland, et al., 1999) and long gestational duration (Richiardi, et al., 2002) appear more protective against seminoma relative to nonseminoma. In addition, risk factors primarily associated with an increased risk of nonseminoma include testicular trauma (Coupland, et al., 1999; Stone, et al., 1991), history of at least one sexually transmitted disease (Coupland, et al., 1999), younger age at shaving initiation (McGlynn, et al., 2007) and short gestational duration (Richiardi, et al., 2002), while later age of puberty may have a stronger protective effect against nonseminoma than seminoma (Coupland, et al., 1999; Moller & Skakkebaek, 1996; Moss, et al., 1986). Although the literature is not congruent for any one of these histologic dissimilarities, the evidence is suggestive of a digression in the natural history and risk profile of these cancers, a hypothesis which is also indicated by divergent incidence rates for seminoma and nonseminoma in countries such as Italy (Bray, et al., 2006) and the U.S. (McGlynn, et al., 2003), and by the variable levels of net endogenous DNA damage reported here.
Strengths of this analysis include: its case-case design, which both analytically and statistically is the most legitimate design when assessing differences between cases, while it also mitigates concerns of reverse causality bias when using a cancer-free control group; and the use of lymphoblastoid cell lines, damage levels of which are expected to reflect the net effect of endogenous processes of metabolism and DNA repair and are unlikely to represent damage induced by exogenous exposures of the subjects, such as those pertaining to occupation, diet or other lifestyle variables, or damage inducing therapies. Any such DNA damage originating from exogenous exposures is likely to have been repaired, given the many cell generations that have passed between date of therapy, initial blood sampling, cell line production and our assays, while the estimates of the adjusted analyses also support this premise. The main limitation, meanwhile, is the assumption that basal damage in immortalized lymphoblastoid cell lines is a surrogate marker of the in vivo processes that contribute to endogenous DNA damage in testicular germ cells. It is important to realize that the use of freshly isolated lymphocytes in epidemiologic studies is fraught with logistical difficulties due to the time period of collection and the requirement to run all comet assays within a small time-window. This problem is exacerbated when working with a rare cancer, such as TGCT. Moreover, fresh lymphocytic material is likely to reflect recent exogenous exposures (in addition to endogenous processes), something which we did not want to quantitate, and use of such would have likely added noise to the results we ascertained. A second limitation is the inherent selection bias of the retrospective study design caused by death of potential participants, although the cure rate of TGCT cases is exceedingly high as evidenced by a five-year survival rate of >97% (Verdecchia, et al., 2007), a fact which may assuage such concerns. Lastly, it could be argued that single-strand breaks are not necessarily a detrimental finding; they in part represent intermediates of DNA repair, generally thought to be a beneficial process. However, it is likely that increased intermediates of DNA repair are the result of higher levels of DNA damage and/or slower repair, and increase the opportunity for carcinogenic mutations.
In summary, this study has found evidence for higher levels of net endogenous DNA damage in patients who developed nonseminoma relative to seminoma. Replication of this finding should promote future studies to focus on the mechanism(s) underlying these differences.
Supplementary Material
Acknowledgments
The authors wish to thank Diane Kampa from the University of Minnesota for subject recruitment and Laura Bowen of IMS for data management. The work was in part conducted under the auspices of the Lawrence Livermore National Laboratory, operated by Lawrence Livermore National Security, LLC, for the U.S. Department of Energy, National Nuclear Security Administration under Contract DE-AC52-07NA27344. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
FUNDING
Intramural Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Abbreviations
- CDM
comet distributed moment
- CI
confidence interval
- FTC
Familial Testicular Cancer study
- OR
odds ratio
- OTM
Olive tail moment
- USRT
U.S. Radiologic Technologists cohort
- TGCT
testicular germ cell tumor
References
- Andreyev HJN, Dearnaley DP, Horwich A. Testicular non-seminoma with high serum human chorionic gonadotrophin: The trophoblastic teratoma syndrome. Diagnostic Oncology. 1993;3:67–71. [Google Scholar]
- Aschim EL, Haugen TB, Tretli S, Daltveit AK, Grotmol T. Risk factors for testicular cancer--differences between pure non-seminoma and mixed seminoma/non-seminoma? International Journal of Andrology. 2006;29:458–467. doi: 10.1111/j.1365-2605.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- Baltaci V, Kayikcioglu F, Alpas I, Zeyneloglu H, Haberal A. Sister chromatid exchange rate and alkaline comet assay scores in patients with ovarian cancer. Gynecologic Oncology. 2002;84:62–66. doi: 10.1006/gyno.2001.6450. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Rajpert-De Meyts E, Skakkebaek NE, Lukas J, Bartek J. DNA damage response in human testes and testicular germ cell tumours: biology and implications for therapy. International Journal of Andrology. 2007 doi: 10.1111/j.1365-2605.2007.00772.x. [DOI] [PubMed] [Google Scholar]
- Berwick M, Vineis P. Markers of DNA repair and susceptibility to cancer in humans: an epidemiologic review. Journal of the National Cancer Institute. 2000;92:874–897. doi: 10.1093/jnci/92.11.874. [DOI] [PubMed] [Google Scholar]
- Blasiak J, Arabski M, Krupa R, Wozniak K, Rykala J, Kolacinska A, Morawiec Z, Drzewoski J, Zadrozny M. Basal, oxidative and alkylative DNA damage, DNA repair efficacy and mutagen sensitivity in breast cancer. Mutation Research. 2004;554:139–148. doi: 10.1016/j.mrfmmm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bray F, Richiardi L, Ekbom A, Forman D, Pukkala E, Cuninkova M, Moller H. Do testicular seminoma and nonseminoma share the same etiology? Evidence from an age-period-cohort analysis of incidence trends in eight European countries. Cancer Epidemiology, Biomarkers and Prevention. 2006;15:652–658. doi: 10.1158/1055-9965.EPI-05-0565. [DOI] [PubMed] [Google Scholar]
- Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Moller H. Trends in testicular cancer incidence and mortality in 22 European countries: continuing increases in incidence and declines in mortality. International Journal of Cancer. 2006;118:3099–3111. doi: 10.1002/ijc.21747. [DOI] [PubMed] [Google Scholar]
- Clapper ML. Genetic polymorphism and cancer risk. Current Oncology Reports. 2000;2:251–256. doi: 10.1007/s11912-000-0075-z. [DOI] [PubMed] [Google Scholar]
- Coupland CA, Chilvers CE, Davey G, Pike MC, Oliver RT, Forman D. Risk factors for testicular germ cell tumours by histological tumour type. United Kingdom Testicular Cancer Study Group. British Journal of Cancer. 1999;80:1859–1863. doi: 10.1038/sj.bjc.6690611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford GP, Linger R, Hockley S, Dudakia D, Johnson L, Huddart R, Tucker K, Friedlander M, Phillips KA, Hogg D, Jewett MAS, Lohynska R, Daugaard G, Richard S, Chompret A, Bonai?ti-Pellie C, Heidenreich A, Albers P, Olah E, Geczi L, Bodrogi I, Ormiston WJ, Daly PA, Guilford P, Fossa SD, Heimdal K, Tjulandin SA, Liubchenko L, Stoll H, Weber W, Forman D, Oliver T, Einhorn L, McMaster M, Kramer J, Greene MH, Weber BL, Nathanson KL, Cortessis V, Easton DF, Bishop DT, Stratton MR, Rapley EA. Genome-wide linkage screen for testicular germ cell tumour susceptibility loci. Human Molecular Genetics. 2006;15:443–451. doi: 10.1093/hmg/ddi459. [DOI] [PubMed] [Google Scholar]
- Dieckmann KP, Pichlmeier U. Clinical epidemiology of testicular germ cell tumors. World Journal of Urology. 2004;22:2–14. doi: 10.1007/s00345-004-0398-8. [DOI] [PubMed] [Google Scholar]
- Doody MM, Mandel JS, Lubin JH, Boice JD., Jr Mortality among United States radiologic technologists, 1926–90. Cancer Causes and Control. 1998;9:67–75. doi: 10.1023/a:1008801404245. [DOI] [PubMed] [Google Scholar]
- English PB, Goldberg DE, Wolff C, Smith D. Parental and birth characteristics in relation to testicular cancer risk among males born between 1960 and 1995 in California (United States) Cancer Causes and Control. 2003;14:815–825. doi: 10.1023/b:caco.0000003812.53344.48. [DOI] [PubMed] [Google Scholar]
- Hardell L, Nasman A, Ohlson CG, Fredrikson M. Case-control study on risk factors for testicular cancer. International Journal of Oncology. 1998;13:1299–1303. doi: 10.3892/ijo.13.6.1299. [DOI] [PubMed] [Google Scholar]
- Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet. 2006;367:754–765. doi: 10.1016/S0140-6736(06)68305-0. [DOI] [PubMed] [Google Scholar]
- Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. Journal of Urology. 2003;170:5–11. doi: 10.1097/01.ju.0000053866.68623.da. [DOI] [PubMed] [Google Scholar]
- Jianlin L, Jiliang H, Lifen J, Wei Z, Zhijian C, Shijie C, Shijie X. Variation of ATM protein expression in response to irradiation of lymphocytes in lung cancer patients and controls. Toxicology. 2006;224:138–146. doi: 10.1016/j.tox.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Laska MJ, Nexo BA, Vistisen K, Poulsen HE, Loft S, Vogel U. Polymorphisms in RAI and in genes of nucleotide and base excision repair are not associated with risk of testicular cancer. Cancer Letters. 2005;225:245–251. doi: 10.1016/j.canlet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Lee E, Oh E, Lee J, Sul D, Lee J. Use of the tail moment of the lymphocytes to evaluate DNA damage in human biomonitoring studies. Toxicological Sciences. 2004;81:121–132. doi: 10.1093/toxsci/kfh184. [DOI] [PubMed] [Google Scholar]
- Lou J, He J, Zheng W, Jin L, Chen Z, Chen S, Lin Y, Xu S. Investigating the genetic instability in the peripheral lymphocytes of 36 untreated lung cancer patients with comet assay and micronucleus assay. Mutation Research. 2007;617:104–110. doi: 10.1016/j.mrfmmm.2007.01.004. [DOI] [PubMed] [Google Scholar]
- McGlynn KA. Environmental and host factors in testicular germ cell tumors. Cancer Investigation. 2001;19:842–853. doi: 10.1081/cnv-100107746. [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;97:63–70. doi: 10.1002/cncr.11054. [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Sakoda LC, Rubertone MV, Sesterhenn IA, Lyu C, Graubard BI, Erickson RL. Body size, dairy consumption, puberty, and risk of testicular germ cell tumors. American Journal of Epidemiology. 2007;165:355–363. doi: 10.1093/aje/kwk019. [DOI] [PubMed] [Google Scholar]
- Moller H. Clues to the aetiology of testicular germ cell tumours from descriptive epidemiology. European Urology. 1993;23:8–13. doi: 10.1159/000474564. discussion 14–15. [DOI] [PubMed] [Google Scholar]
- Moller H, Skakkebaek NE. Risks of testicular cancer and cryptorchidism in relation to socio-economic status and related factors: case-control studies in Denmark. International Journal of Cancer. 1996;66:287–293. doi: 10.1002/(SICI)1097-0215(19960503)66:3<287::AID-IJC2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Moller H, Skakkebaek NE. Testicular cancer and cryptorchidism in relation to prenatal factors: case-control studies in Denmark. Cancer Causes and Control. 1997;8:904–912. doi: 10.1023/a:1018472530653. [DOI] [PubMed] [Google Scholar]
- Morrison AS. Cryptorchidism, hernia, and cancer of the testis. Journal of the National Cancer Institute. 1976;56:731–733. doi: 10.1093/jnci/56.4.731. [DOI] [PubMed] [Google Scholar]
- Moss AR, Osmond D, Bacchetti P, Torti FM, Gurgin V. Hormonal risk factors in testicular cancer. A case-control study. American Journal of Epidemiology. 1986;124:39–52. doi: 10.1093/oxfordjournals.aje.a114369. [DOI] [PubMed] [Google Scholar]
- Oliver RT. Clues from natural history and results of treatment supporting the monoclonal origin of germ cell tumours. Cancer Surveys. 1990;9:333–368. [PubMed] [Google Scholar]
- Oosterhuis JW, Looijenga LH. Current views on the pathogenesis of testicular germ cell tumours and perspectives for future research: highlights of the 5th Copenhagen Workshop on Carcinoma in situ and Cancer of the Testis. Acta Pathologica, Microbiologica, et Immunologica Scandinavica. 2003;111:280–289. doi: 10.1034/j.1600-0463.2003.1110131.x. [DOI] [PubMed] [Google Scholar]
- Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nature Reviews Cancer. 2005;5:210–222. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- Peters JA, Vadaparampil ST, Kramer J, Moser RP, Court LJ, Loud J, Greene MH. Familial testicular cancer: interest in genetic testing among high-risk family members. Genetics in Medicine. 2006;8:760–770. doi: 10.1097/01.gim.0000250506.15979.0c. [DOI] [PubMed] [Google Scholar]
- Pike MC, Chilvers CE, Bobrow LG. Classification of testicular cancer in incidence and mortality statistics. British Journal of Cancer. 1987;56:83–85. doi: 10.1038/bjc.1987.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen HE, Loft S, Wassermann K. Cancer risk related to genetic polymorphisms in carcinogen metabolism and DNA repair. Pharmacology and Toxicology. 1993;72 Suppl 1:93–103. doi: 10.1111/j.1600-0773.1993.tb01676.x. [DOI] [PubMed] [Google Scholar]
- Prener A, Engholm G, Jensen OM. Genital anomalies and risk for testicular cancer in Danish men. Epidemiology. 1996;7:14–19. doi: 10.1097/00001648-199601000-00004. [DOI] [PubMed] [Google Scholar]
- Prener A, Hsieh CC, Engholm G, Trichopoulos D, Jensen OM. Birth order and risk of testicular cancer. Cancer Causes and Control. 1992;3:265–272. doi: 10.1007/BF00124260. [DOI] [PubMed] [Google Scholar]
- Purdue MP, Devesa SS, Sigurdson AJ, McGlynn KA. International patterns and trends in testis cancer incidence. International Journal of Cancer. 2005;115:822–827. doi: 10.1002/ijc.20931. [DOI] [PubMed] [Google Scholar]
- Richiardi L, Akre O, Bellocco R, Ekbom A. Perinatal determinants of germ-cell testicular cancer in relation to histological subtypes. British Journal of Cancer. 2002;87:545–550. doi: 10.1038/sj.bjc.6600470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richiardi L, Akre O, Lambe M, Granath F, Montgomery SM, Ekbom A. Birth order, sibship size, and risk for germ-cell testicular cancer. Epidemiology. 2004;15:323–329. doi: 10.1097/01.ede.0000120043.45185.7e. [DOI] [PubMed] [Google Scholar]
- Richiardi L, Scelo G, Boffetta P, Hemminki K, Pukkala E, Olsen JH, Weiderpass E, Tracey E, Brewster DH, McBride ML, Kliewer EV, Tonita JM, Pompe-Kirn V, Kee-Seng C, Jonasson JG, Martos C, Brennan P. Second malignancies among survivors of germ-cell testicular cancer: a pooled analysis between 13 cancer registries. International Journal of Cancer. 2006;120:623–631. doi: 10.1002/ijc.22345. [DOI] [PubMed] [Google Scholar]
- Robinson D, Moller H, Horwich A. Mortality and incidence of second cancers following treatment for testicular cancer. British Journal of Cancer. 2007;96:529–533. doi: 10.1038/sj.bjc.6603589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabroe S, Olsen J. Perinatal correlates of specific histological types of testicular cancer in patients below 35 years of age: a case-cohort study based on midwives’ records in Denmark. International Journal of Cancer. 1998;78:140–143. doi: 10.1002/(sici)1097-0215(19981005)78:2<140::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Penarroja R, Gallegos F, Bravo JL, Rojas E, Benitez-Bribiesca L. DNA damage in peripheral lymphocytes of untreated breast cancer patients. Archives of Medical Research. 2004;35:480–483. doi: 10.1016/j.arcmed.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Schabath MB, Spitz MR, Grossman HB, Zhang K, Dinney CP, Zheng PJ, Wu X. Genetic instability in bladder cancer assessed by the comet assay. Journal of the National Cancer Institute. 2003;95:540–547. doi: 10.1093/jnci/95.7.540. [DOI] [PubMed] [Google Scholar]
- Shao L, Lin J, Huang M, Ajani JA, Wu X. Predictors of esophageal cancer risk: assessment of susceptibility to DNA damage using comet assay. Genes, Chromosomes and Cancer. 2005;44:415–422. doi: 10.1002/gcc.20254. [DOI] [PubMed] [Google Scholar]
- Sigurdson AJ, Hauptmann M, Alexander BH, Doody MM, Thomas CB, Struewing JP, Jones IM. DNA damage among thyroid cancer and multiple cancer cases, controls, and long-lived individuals. Mutation Research. 2005;586:173–188. doi: 10.1016/j.mrgentox.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Smith TR, Miller MS, Lohman KK, Case LD, Hu JJ. DNA damage and breast cancer risk. Carcinogenesis. 2003;24:883–889. doi: 10.1093/carcin/bgg037. [DOI] [PubMed] [Google Scholar]
- Starr JR, Chen C, Doody DR, Hsu L, Ricks S, Weiss NS, Schwartz SM. Risk of testicular germ cell cancer in relation to variation in maternal and offspring cytochrome p450 genes involved in catechol estrogen metabolism. Cancer Epidemiology, Biomarkers and Prevention. 2005;14:2183–2190. doi: 10.1158/1055-9965.EPI-04-0749. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 10. StataCorp LP; College Station, Texas: 2007. [Google Scholar]
- Stone JM, Cruickshank DG, Sandeman TF, Matthews JP. Laterality, maldescent, trauma and other clinical factors in the epidemiology of testis cancer in Victoria, Australia. British Journal of Cancer. 1991;64:132–138. doi: 10.1038/bjc.1991.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveillance Epidemiology and End Results Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2006 Sub (1973–2004) - Linked To County Attributes - Total U.S., 1969–2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission.
- Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, Hall P, Holowaty E, Andersen A, Pukkala E, Andersson M, Kaijser M, Gospodarowicz M, Joensuu T, Cohen RJ, Boice JD, Jr, Dores GM, Gilbert ES. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. Journal of the National Cancer Institute. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Mishina M, Narita S, Kumazawa T, Inoue T, Horikawa Y, Kakinuma H, Yuasa T, Matsuura S, Satoh S, Ogawa O, Habuchi T. Association of XRCC1 gene polymorphisms with the susceptibility and chromosomal aberration of testicular germ cell tumors. International Journal of Oncology. 2006;28:1217–1223. [PubMed] [Google Scholar]
- Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, Kunkler I. Recent cancer survival in Europe: a 2000–02 period analysis of EUROCARE-4 data. The Lancet Oncology. 2007;8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- Vistisen K, Loft S, Olsen JH, Vallentin S, Ottesen S, Hirsch FR, Poulsen HE. Low CYP1A2 activity associated with testicular cancer. Carcinogenesis. 2004;25:923–929. doi: 10.1093/carcin/bgh102. [DOI] [PubMed] [Google Scholar]
- Wanderas EH, Grotmol T, Fossa SD, Tretli S. Maternal health and pre-and perinatal characteristics in the etiology of testicular cancer: a prospective population-and register-based study on Norwegian males born between 1967 and 1995. Cancer Causes and Control. 1998;9:475–486. doi: 10.1023/a:1008857702380. [DOI] [PubMed] [Google Scholar]
- Weir HK, Marrett LD, Kreiger N, Darlington GA, Sugar L. Pre-natal and peri-natal exposures and risk of testicular germ-cell cancer. International Journal of Cancer. 2000;87:438–443. doi: 10.1002/1097-0215(20000801)87:3<438::aid-ijc20>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.