Abstract
During separation in a novel cage, guinea pig pups exhibit passive behavior that appears due to increased proinflammatory activity. To determine if separation also produces a febrile response, the present study used telemetry to provide continuous core temperature measurement of pups exposed to a novel cage for 3 hr while either alone or with their mother on two consecutive days. Separation from the mother increased core temperature, with the clearest effects occurring early during separation the second day. The increased temperature was not associated with an increase in locomotor activity. Further, passive behavior during isolation exhibited pronounced sensitization from the first to second day of separation. These results show that separation produces an increase in core temperature in our testing situation, and suggest that this increase represents true fever. The findings also provide further support for the hypothesis that maternal separation induces aspects of an acute phase response in guinea pig pups. The potential role of proinflammatory activity in promoting change across days in temperature and behavior is discussed.
Keywords: maternal separation, fever, core temperature, acute phase responses, sickness, proinflammatory, stress-induced sickness behavior, sensitization, attachment, guinea pigs
When an animal encounters a replicating pathogen (e.g., bacteria, fungus), cells of the immune system (e.g., monocytes, macrophages) secrete an array of peptide messengers, or cytokines, which orchestrate a systemic inflammatory reaction. This acute phase response [3] or just “sickness” consists of changes in both physiology [e.g., alterations in the relative production of various liver proteins, increased hypothalamic-pituitary-adrenal (HPA) activity] and behavior (e.g., a hunched posture, reduction in goal directed activity). A primary function of the acute phase response is to foster a centrally regulated increase in core temperature, i.e., fever, which tends to diminish pathogen replication while increasing the effectiveness of the host’s defenses [4, 13]. Many of the behaviors associated with sickness (e.g., hunched posture, shivering, piloerection) serve to promote fever [13, 30]. Over the last 10-15 years, it has become increasingly clear that stressors also can sometimes stimulate elements of an acute phase response [25]. For instance, electric shock has been found to stimulate proinflammatory activity, production of acute phase proteins, increases in core body temperature, and cytokine-dependent behavioral changes [10, 11, 24]. Indeed, stress-induced, like pathogen-induced, sickness appears to be mediated by proinflammatory cytokine activity [33, 34].
On the other hand, not all increases in core temperature during stress are necessarily aspects of an acute phase response or mediated by proinflammatory factors. Mild stressors, such as handling or exposure to a novel environment, can produce a rapid, transient rise in core body temperature that is sometimes referred to as “stress-induced hyperthermia”. While there is evidence that some of these increases in core temperature represent a true fever involving inflammatory signaling pathways [30], other cases appear to involve different underlying mechanisms. For instance, Vinkers et al [42] found an anti-inflammatory to readily reduce unambiguous fever but not core temperature elevations stimulated by restraint; and an anxiolytic to readily reduce temperature elevations to restraint, but not unambiguous fever. Similarly, Hashimoto et al [14] observed prenatal stress to produce differential effects on restraint-induced hyperthermia and unambiguous fever. Moreover, heat generated by increased locomotor activity or failure to dissipate heat to the environment (such as occurs during some forms of restraint) [26] can confound interpretation of temperature changes observed during stress.
One putative example of stress-activated elements of an acute phase response is seen during separation in guinea pig pups. When pups are isolated in a novel environment, they show a two-stage active/passive response [18] that is at least superficially similar to the two-stage “protest/despair” response of isolated primate infants [21, 27, 40]. Separated guinea pig pups initially vocalize at a high rate and may move about the enclosure; but after about an hour, they quiet and begin to exhibit a characteristic hunched or crouched stance together with prolonged eye-closure and extensive piloerection. These passive behaviors are similar to sickness behaviors observed in other species [13]. Several lines of evidence indicate that these behaviors of separated guinea pigs are, in fact, elements of a stress-induced acute phase response resulting from proinflammatory cytokine activity. Pups injected prior to separation with lipopolysacchride (LPS)—which elicits proinflammatory cytokine release and a robust acute phase response—display high levels of passive behavior immediately following separation, when pups typically are in the active behavior phase [17]. Moreover, the expression of passive behaviors during a prolonged (3-hr) separation can be attenuated with administration of various anti-inflammatory agents [20, 32, 36]. Increased expression of the proinflammatory cytokine, tumor necrosis factor-alpha, has also been observed in the spleens of animals separated for 3 hr [16]. In light of these findings, one might expect the separation procedure to induce a febrile response. An initial experiment obtained results consistent with this prediction. The core temperature of pups isolated in a novel environment was elevated above a pre-separation value at 1.5 hr, though by 3 hr, the elevation was no longer statistically significant [17].
The present experiment was designed to more-thoroughly examine the effect of separation on the core temperature response of guinea pig pups. In our earlier study [17], temperature was measured with a rectal probe, which limits the number of assessments that can be made (three in our case), and can produce variable readings due to struggling of the pup. Further, the disturbance involved in collecting the measurement is itself a potential temperature-elevating stressor [31]. In addition, because temperature effects were measured relative to a pre-separation baseline, it was not possible to determine the extent to which temperature change was due to handling and exposure to novelty as opposed to separation from the mother. In the present experiment, we used a remote sensing device to continuously provide temperature measurements during 3-hr separations without disturbance to the pups. To determine the effect of separation per se, the core temperature of pups placed into the novel cage alone was compared to that of pups tested in the cage with their mothers. Finally because stress-induced hyperthermia typically has been observed shortly following exposure to acute stressors [31, 41], but an elevation of core temperature together with other signs of an acute phase response can occur a day or more later, particularly it seems, when exposed to an additional stressor [11], we assessed temperature change during two, 3-hr separations initiated at a 24-hr interval. Measures of passive as well as active behaviors were made for comparison with temperature responses.
Methods
Animals
Albino guinea pigs (Cavia porcellus) of the Hartley strain were bred in our laboratory. Each mother and her litter were housed in opaque plastic cages (73 cm × 54 cm × 24 cm) with wire fronts and sawdust bedding. Water and guinea pig chow were available ad libitum. Lights were maintained on a 12:12 light: dark cycle, with lights on at 7:00 h. All procedures were approved by the Wright State University Animal Care and Use Committee. Pups were maintained with their mothers and any littermates for the duration of the experiments, being removed only for surgery, behavioral testing, and brief maintenance procedures (e.g., cage changes, weighing of pups). Separate groups of 10 pups (5 males, 5 females) were tested in each of two experimental conditions (Alone, With Mother). No more than one pup from a litter was assigned to either condition.
Surgery
A telemetry probe (PD4000 Emitter from Mini-Mitter Company, Bend, OR) was surgically implanted in the abdominal cavity of 11-16-day-old pups under aseptic conditions. Guinea pigs were anesthetized using isoflurane, with atropine (.05 mg/kg, i.p.) given to reduce secretory activity. A section of abdomen was shaved and swabbed with antiseptic solution, and small incisions (~ 1 cm) were then made through the skin and abdominal wall. The sterilized probe (23 cm × 8 cm dia) was inserted into the cavity and sutured to the inside of the abdominal wall. The incision through the wall was sutured shut and the overlying skin was closed using standard laboratory wound clips. Buprenorphine (0.015 mg/0.05 ml) was given immediately following surgery and again 24 h later for post-operative pain. Animals gained weight readily after surgery and locomotor activity appeared normal. At least 3 days intervened between surgery and the beginning of testing.
Test procedures
Testing was initiated at about 3-weeks of age (17-23 days). The precocial guinea pig appears to have completely competent thermoregulatory capabilities by about 8 days of age [7]. Guinea pig pups can be raised apart from their mothers from shortly after birth but, when left with her, typically do not wean until about Day 25 [22, 35]. Thus, pups tested in the present experiment were physically mature, had competent thermoregulatory abilities, and were in the late, preweaning period.
For testing, the pup was removed from its home cage and taken quietly in a carrying cage to a nearby testing room in which the pup was placed into a clear, empty plastic cage (47 cm × 24 cm × 20 cm) either alone or together with its mother (brought in a separate carrying cage). The test cage was placed on an energizer/receiver platform for remote telemetry (also from Mini-Mitter). To prevent escape, the cage was covered with a plastic lid that permitted ample passage of light. Temperature data were collected using Vital View (Mini-Mitter) software run by a remote computer. These data are processed by the software as mean values for each 3 min across the 3-hr test period. Movement of the pup was also detected with the telemetry system. Arbitrary units of activity were tallied in 3-min bins across the 3-hr test. The primary purpose of assessing activity was to provide an estimate of whether any observed changes in body temperature were associated with physical exertion. Other behavior measures were scored during Min 0-30, 60-90, and 150-180 by an observer behind 1-way glass. The passive behaviors of crouch (a characteristic crouched stance in which the feet are tucked beneath the body), eye-close (complete or near complete sustained closure of one or both eyes), and piloerection (over most of the body) were scored in a one-zero fashion each 1-min interval by trained observers (inter-observer reliability of at least 85%). The number of intervals in which the pup exhibited all three individual behaviors was also scored and designated as a “full passive” response. The number of occurrences of the primary behavior of the active stage, the whistle vocalization [5], was tallied on a hand counter. Vocalizations were detected with a microphone positioned over the test cage and broadcast via headphones to the observer. In the With Mother condition, the number of s that the pup was in physical contact with the mother also was scored. Test cages were cleaned with detergent after each test. Pups received two tests initiated at exactly a 24-hr interval. All testing began between 1000 and 1100 hr.
Data analysis
To further reduce temperature and activity count data before analysis, these measures were averaged across five, 3-min bins (i.e., time blocks of 15 min). Temperature and activity data then were evaluated with 4-way (Condition × Sex × Day × Time Block) analyses of variance (ANOVAs) with the last two factors treated as repeated measures. Because of heterogeneity of variance, activity data were subjected to a square root transformation prior to analysis. When sphericity was significant (p < 0.01) based on the Mauchly test, the p value was adjusted by the Huynh-Feldt method (degrees of freedom for original analysis are reported). To further assess a significant interaction for temperature data, simple interaction and main effects tests were conducted [44].
Because of large numbers of “zero” scores in particular cells, vocalizations and the four passive behavior measures were all assessed with non-parametric tests (Wilcoxon, matched-pairs, signed-ranks tests for within-subject comparisons and Mann-Whitney U tests for between subjects comparisons). Difficulties scoring eye-close in the With Mother condition (e.g., mother occluding view) led us to drop this measure as well as the “full passive” response (of which eye-close is a component) from the analyses for the With Mother condition. Preliminary analyses of sex differences for all measures that were analyzed with non-parametric tests revealed no effect of sex for any measure on either day. Therefore, male and female data were combined for all non-parametric analyses reported below. To assess the relation between response categories hypothesized to be mediated by proinflammatory activity in the Alone condition, Spearman-Rho correlation coefficients were calculated between temperature (mean and maximum 15-min block) and levels on each of the passive behavior measures on both days. In the With Mother condition, the sample size was reduced from 10 to 7 for crouch and full passive response and to 9 for other manually observed behaviors due to error. A significance level of p < 0.05 (2-tailed) was accepted throughout.
Results
Core Temperature
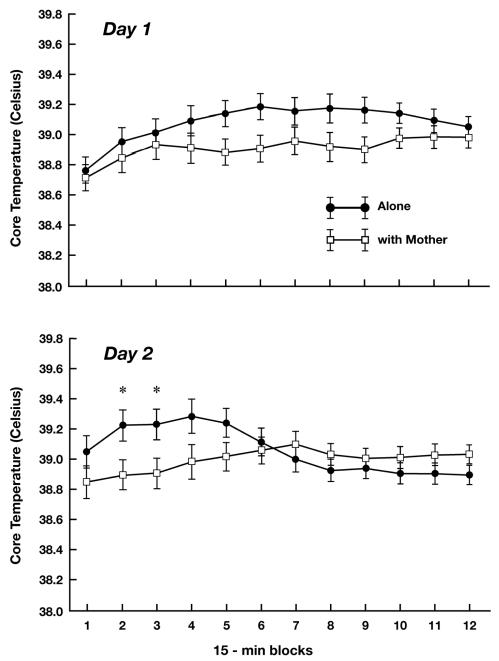
The ANOVA for core temperature yielded significant effects for Time Block, F (11, 176) = 4.21, p < 0.01, and Day X Time Block, F (11, 176) = 4.74, p < 0.01. However, both these effects were qualified by a significant three-way interaction of Condition X Day X Time Block, F(11, 176) = 5.62, p < 0.01. As can be seen in Figure 1, temperature values tended to be higher in the Alone condition than the With Mother condition during the middle portion of the test on Day 1 and during the early portion of the test on Day 2. To further examine the significant three-way interaction, simple interaction tests were conducted to determine if Condition interacted with Time Block at each day. On Day 1, this interaction was significant (p < 0.05), confirming that the temporal pattern of temperature change illustrated in Figure 1 (greatest difference between Alone and With Mother conditions during the middle time blocks) was statistically reliable. Nonetheless, when simple main effect tests were then conducted to compare the two conditions at each individual time block, no significant differences at any particular time blocks were obtained. For Day 2, the simple interaction effect again was significant (p < 0.01). In addition, simple main effect tests were significant for the second and third, 15-min blocks (p’s < 0.05). Thus, on Day 2 there was clear indication that being tested alone elevated core temperature more than did testing with the mother during Min 16-45.
Figure 1.
Mean (SE) core temperature of guinea pig pups tested alone and with their mother during 15-min time blocks of a 3-h test on two consecutive days. * p < 0.05
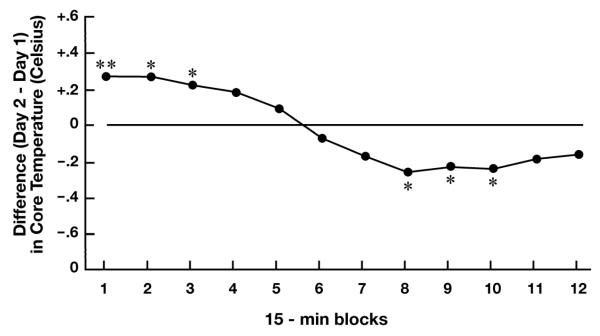
Additional simple interaction tests were conducted to determine if the temporal pattern of temperature change in a particular condition varied from Day 1 to Day 2. These tests were significant for the Alone (p < 0.01), but not the With Mother, condition. Simple main effect tests showed that pups tested alone had higher core temperature during the first (p < 0.01), second (p < 0.05) and third (p < 0.05) time blocks, and lower temperature during the eighth, ninth, and tenth (p’s < 0.05) time blocks on Day 2 relative to Day 1 (Fig. 2).
Figure 2.
Mean difference in core temperature of guinea pups tested alone on Day 2 relative to Day 1 (horizontal line). * p < 0.05; ** p < 0.01
Activity
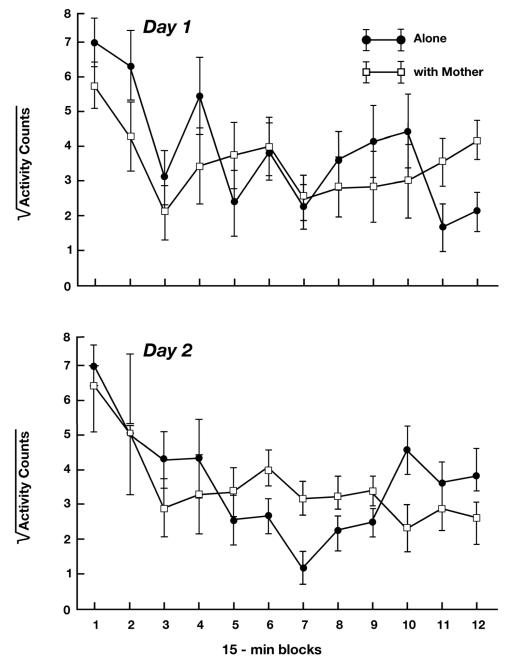
The ANOVA for the activity measure yielded significant effects for Time Block, F (11, 176) = 7.94, p < 0.01, Condition X Sex, F (1, 16) = 5.84, p < 0.05, and Condition X Day X Time Block, F (11, 176) = 1.87, p < 0.05. Because the primary purpose of assessing activity was for comparison with core temperature, and because activity, like temperature, exhibited a significant 3-way interaction of Condition X Day X Time Block, we omitted post-hoc tests for this measure, but plot the 3-way interaction in Figure 3 for comparison with core temperature (Fig. 1). As can be seen, the pattern of change over time in the activity of pups in the two conditions does not parallel the pattern seen for core temperature.
Figure 3.
Mean (SE) activity counts of guinea pig pups tested alone and with their mother during 15-min time blocks of a 3-h test on two consecutive days. Data were subjected to square root transformation to reduce heterogeneity of variance.
Other Behaviors
As expected, pups vocalized frequently on both days when tested alone. Although the median level of vocalizing in the Alone condition was higher on Day 2 than on Day 1, this difference was not statistically significant (p > 0.1, Table 1). The presence of the mother in the home cage greatly suppressed pup vocalizing on both days ( p’s < 0.01). When pups were with the mother in the home cage, they spent the great majority of the observation periods in physical contact with her (82.6% and 88.6% on Days 1 and 2, respectively).
Table 1.
Median (semi-interquartile range) of vocalizations in Alone and With Mother conditions
p < 0.01 vs With Mother
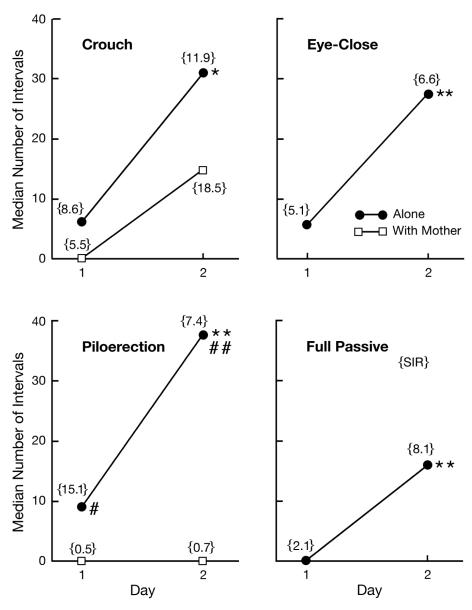
The most surprising finding the present study was the pattern across days in the passive behavior measures of pups tested alone. Crouch (p < 0.05), eye-close (p < 0.01), piloerection (p < 0.01), and full passive response (p < 0.01) all showed a significant, and at least 4-fold, increase from Day 1 to Day 2 (Fig. 4). The two measures of passive behavior for pups tested with their mothers (crouch, piloerection) were exhibited relatively infrequently. There was no significant difference between Day 1 and Day 2, though there was a tendency for crouching to increase (p = 0.08). Levels of piloerection were significantly greater in the Alone than in the With Mother condition on both Day 1 (p < 0.05) and Day 2 (p < 0.01). Correlation coefficients between measures of temperature and passive behavior in separated pups were all nonsignificant.
Figure 4.
Median number of 1-min intervals in which guinea pig pups exhibited crouching, eye closure, piloerection, or all three of these behaviors (full passive) during two, 3-h tests on consecutive days. All four behaviors were observed in pups tested alone; crouch and piloerection were observed in pups tested with their mothers. The semi-interquartile range {SIR} is provided as a measure of variability. * p < 0.05 vs Day 1; ** p < 0.01 vs Day 1; # p < 0.05 vs With Mother; ## p < 0.01 vs With Mother
Discussion
Separation from the mother increased the core body temperature of guinea pig pups. On Day 1, the temperature of separated pups was numerically higher than that of controls at each time block and the change in the relative difference between conditions over blocks was significant. On Day 2, clear elevations of core temperature attributable to separation were evident from Min 16-45. The pattern of temperature change was not paralleled by the pattern of change in motor activity. Further, separation increased both temperature and expression of passive behavior, which previous evidence indicates is mediated by proinflammatory factors [19, 32]. Temperature elevations due to separation were most distinct on Day 2, when passive behavior was greatest. These results, in conjunction with earlier findings that (1) stress-induced elements of an acute phase response can be detected a day following stressor exposure [11], and (2) that a 3-hr separation increases expression of tumor necrosis factor-alpha [16] in guinea pig pups, suggest that separation produced fever as an element of an acute phase response in the pups of this study.
The observation that the temperature elevation peaked at about 90 min of the first isolation is consistent with our earlier study using a rectal probe in which temperature increases over a pre-separation baseline were significant at 90, but not 180, min [17]. It should be pointed out, however, that in the present study all measurements were made following placement of the pups into the novel test cage so that comparison with a pre-separation baseline was not possible. As is clear in Figure 2, temperature was already plainly elevated in the first time bin on Day 2 relative to Day 1 (0.28° C increase). This increase could reflect increased basal temperature as a result of the initial separation experience, a more-rapid rise in temperature following onset of the second separation, or possibly an anticipatory response triggered by subtle environmental cues. In the With Mother condition, there was a tendency for temperature to rise relative to the 0-15 min time block on both days. This tendency could reflect the phenomena of stress-induced hyperthermia, which commonly is observed following mild stressors such as handling, and which may not depend on underlying proinflammatory activity [e.g., 31]. On the other hand, this tendency for temperature to increase during testing in the With Mother condition could have resulted from heat exchange between the mother and pup. Although pups of the age tested here exhibit mature thermoregulatory abilities [7], the presence of the mother nonetheless represents a potential confound for the study of stress-induced temperature change. However, it should be pointed out that even if heat exchange between mother and pup was contributing to core temperature measures in this condition, the effect would be an underestimation of the impact of the separation experience on core temperature increase (i.e., reduced the effect size for the separation variable) in the present study.
The sensitization of the immediate (i.e., first ~ 45 min) core temperature response in the Alone condition is unusual because it was produced by a single previous separation experience, and because there is a large body of literature showing that repeated exposure to the same stressor leads to habituation (such as was observed during the latter portion of the Alone test), not sensitization, of the core temperature response to stressors (See [2] for a review). The change in temperature across days in the Alone condition (and the more-pronounced difference between the Alone and With Mother conditions on Day 2) was accompanied by a striking sensitization of the passive behavioral responses. One interpretation of these results is that a central proinflammatory cascade evoked by maternal separation is the mechanism underlying the change across days in both the core temperature and passive behavioral responses.
As described above, the passive behavioral response on Day 1 appears to be mediated largely by proinflammatory activity [e.g., 32]. Further, there is a growing literature showing that cytokines play a role in stress sensitization. For instance, cytokine activation produces long-lasting increases in HPA responsiveness to novelty exposure or subsequent immune challenge in rats [1, 37], an effect that appears at least partially due to increased basal hypothalamic corticotropin-releasing factor (CRF) and vasopressin activity. Further, cytokine-induced sensitization of core body temperature 48 hr following LPS [9], and of sickness behavior as much as 4 weeks following cytokine administration [1] have also been observed. In short, there appears to be ample precedence in the literature for the notion that increased cytokine activity following maternal separation on Day 1 enhanced behavioral and core temperature responses to separation on Day 2. Two qualifications of this interpretation should be noted, however. First, all animals had experienced another stimulus that would be expected to produce a heightened inflammatory state: recent surgery to implant telemetry probes. It is possible that this procedure enhanced the effect of separation on behavioral measures and temperature in the present study. Second, the lack of correlation between levels of temperature and passive behavior suggest that the relation between them is more complex than a simple monotonic correspondence.
Evidence indicates that proinflammatory activity can contribute to at least some forms of depressive illness. Increased levels of proinflammatory cytokines and other signs of an acute phase response have been observed in depressed patients [23]. Use of a proinflammatory cytokine for chemotherapy often results in depressive symptoms [29, 34]. In laboratory animals, increased cytokine activity not only elicits depressive-like sickness behaviors (e.g., reduced goal-directed activity, social withdrawal, hunched posture), but sickness behavior can sometimes be reduced with antidepressants [39, 45]. Moreover, increased cytokine activity produces other physiological changes (e.g., increased HPA activity; reduced production of serotonin) thought to underlie depressive illness [28]. This evidence may be relevant in the present context because maternal separation and other forms of attachment disruption in humans are well known to be associated with an increased risk of depressive- and anxiety-related disorders in later life [6, 8, 43]. This association usually is hypothesized to depend on a sensitization of stress-related neurochemical (e.g., CRF) and neural (e.g., amygdala) activity due to the early life stressors [12, 38], which then enhances susceptibility to later psychopathology. Our finding that maternal separation produces a sensitization (albeit, one over only 24-hr) of passive behavior that appears mediated by proinflammatory activity, together with findings briefly described above indicating that cytokines can underlie longer-term sensitization of passive behavior and HPA activity in adult animals, suggests the possibility [15] that increased proinflammatory activity might be part of the means by which early attachment disruption promotes later depression.
Acknowledgements
The authors thank K. Andrew Miller, Allison Perkeybile, and Lillian Jones for assistance in this project. The research was funded by Grant MH068228 from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Anisman H, Merali Z, Hayley S. Sensitization associated with stressors and cytokine treatments. Brain Behav Immun. 2003;17:86–93. doi: 10.1016/s0889-1591(02)00100-9. [DOI] [PubMed] [Google Scholar]
- [2].Barnum CJ, Blandino P, Jr, Deak T. Adaptation in the corticosterone and hyperthermic responses to stress following repeated stressor exposure. J Neuroendocrinol. 2007;19:632–642. doi: 10.1111/j.1365-2826.2007.01571.x. [DOI] [PubMed] [Google Scholar]
- [3].Baumann H, Gauldie J. The acute phase responses. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- [4].Bernheim HA, Kluger MJ. Fever: effect of drug-induced antipyresis on survival. Science. 1976;193:237–239. doi: 10.1126/science.935867. [DOI] [PubMed] [Google Scholar]
- [5].Berryman JC. Guinea pig vocalizations: their structure, causation and function. Zeitschrift Tierpsychol. 1976;41:80–106. doi: 10.1111/j.1439-0310.1976.tb00471.x. [DOI] [PubMed] [Google Scholar]
- [6].Birtchnell J. Early parental death and psychiatric diagnosis. Soc. Psychiatry. 1972;7:202–210. [Google Scholar]
- [7].Blatteis CM. Postnatal development of pyrogenic sensitivity in guinea pigs. J Appl Physiol. 1975;39:251–257. doi: 10.1152/jappl.1975.39.2.251. [DOI] [PubMed] [Google Scholar]
- [8].Brown GW, Harris T, Copeland JR. Depression and loss. Brit. J. Psychiatry. 1977;130:1–18. doi: 10.1192/bjp.130.1.1. 1977. [DOI] [PubMed] [Google Scholar]
- [9].Deak T, Bellamy C, Bordner KA. Protracted increases in core body temperature and interleukin-1 following acute administration of lipopolysaccharide: implications for the stress response. Physiol Behav. 2005;85:296–307. doi: 10.1016/j.physbeh.2005.04.016. [DOI] [PubMed] [Google Scholar]
- [10].Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull. 2005;64:541–556. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- [11].Deak T, Meriwether JL, Fleshner M, Spencer R, Abouhamze A, Moldawer LL, Grahn RE, Watkins LR, Maier SF. Evidence that brief stress may induce the acute phase response in rats. Amer J Physiol. 1997;273:R1998–R2004. doi: 10.1152/ajpregu.1997.273.6.R1998. [DOI] [PubMed] [Google Scholar]
- [12].Gillespie CF, Nemeroff CB. Corticotropin-releasing factor and the psychobiology of early life stress. Cur Dir Psychol Sci. 2007;16:85–89. [Google Scholar]
- [13].Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- [14].Hashimoto M, Watanabe T, Fujioka T, Tan N, Yamashita H, Nakamura S. Modulating effects of prenatal stress on hyperthermia induced in adult rat offspring by restraint or LPS-induced stress. Physiol Behav. 2001;73:125–132. doi: 10.1016/s0031-9384(01)00473-5. [DOI] [PubMed] [Google Scholar]
- [15].Hennessy MB, Deak T, Schiml-Webb PA. Early attachment-figure separation and increased risk for later depression: potential mediation by proinflammatory processes. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2009.03.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hennessy MB, Deak T, Schiml-Webb, Barnum CJ. Immune influences on behavior and endocrine activity in early-experience and maternal separation paradigms. In: Czerbska MT, editor. Psychoendocrinology Research Trends. Nova Science Publishers; Hauppauge, NY: 2007. [Google Scholar]
- [17].Hennessy MB, Deak T, Schiml-Webb PA, Wilson SE, Greenlee TM, McCall E. Responses of guinea pig pups during isolation in a novel environment may represent stress-induced sickness behaviors. Physiol Behav. 2004;81:5–13. doi: 10.1016/j.physbeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- [18].Hennessy MB, Long SJ, Nigh CK, Williams MT, Nolan DJ. Effects of peripherally administered corticotropin-releasing factor (CRF) and a CRF antagonist: Does peripheral CRF activity mediate behavior of guinea pig pups during isolation? Behav Neurosci. 1995;109:1137–1145. doi: 10.1037//0735-7044.109.6.1137. [DOI] [PubMed] [Google Scholar]
- [19].Hennessy MB, Schiml-Webb PA, Deak T. Separation, sickness, and Depression: a new perspective on an old animal model. Cur Dir Psychol Sci. 2009;18:227–231. doi: 10.1111/j.1467-8721.2009.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hennessy MB, Schiml-Webb PA, Miller EE, Maken DS, Bullinger KL, Deak T. Anti-inflammatory agents attenuate the passive responses of guinea pig pups: evidence for stress-induced sickness behavior during maternal separation. Psychoneuroendocrinol. 2007;32:508–515. doi: 10.1016/j.psyneuen.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kaufman IC, Rosenblum LA. The reaction to separation in infant monkeys: anaclitic depression and conservation withdrawal. Psychosom Med. 1967;29:648–675. doi: 10.1097/00006842-196711000-00010. [DOI] [PubMed] [Google Scholar]
- [22].König B. Maternal activity budget during lactation in species of Caviidae (Cavia porcellus and Galea musteloides) Zeitschrift Tierpsychol. 1985;68:215–230. [Google Scholar]
- [23].Kronfol Z. Immune dysregulation in major depression: A critical review of existing evidence. Int J Neuropsychopharmacol. 2006;5:333–343. doi: 10.1017/S1461145702003024. [DOI] [PubMed] [Google Scholar]
- [24].Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695:279–282. doi: 10.1016/0006-8993(95)00930-o. [DOI] [PubMed] [Google Scholar]
- [25].Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:87–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- [26].McGivern RF, Zuloaga DG, Handa RJ. Sex differences in stress-induced hyperthermia in rats: restraint versus confinement. Physiol Behav. 2009;98:416–420. doi: 10.1016/j.physbeh.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mineka S, Suomi SJ. Social separation in monkeys. Psychol Bull. 1976;85:1376–1400. [PubMed] [Google Scholar]
- [28].Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11:198–209. doi: 10.1080/10253890701754068. [DOI] [PubMed] [Google Scholar]
- [29].Miyaoka H, Otsubo T, Kamijima K, Ishii M, Onuki M, Mitamura K. Depression from interferon therapy in patients with hepatitis C. Am J Psychiatry. 1999;156:1120. doi: 10.1176/ajp.156.7.1120. [DOI] [PubMed] [Google Scholar]
- [30].Oka T, Oka K, Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychom Med. 2001;63:476–486. doi: 10.1097/00006842-200105000-00018. [DOI] [PubMed] [Google Scholar]
- [31].Olivier B, Bouwknecht JA, Pattij T, Leahy C, van Oorschot R, Zethof TJJ. GABAA – benzodiazepine receptor complex ligands and stress-induced hyperthermia in singly housed mice. Pharmacol Biochem Behav. 2002;72:179–188. doi: 10.1016/s0091-3057(01)00759-6. [DOI] [PubMed] [Google Scholar]
- [32].Perkeybile AM, Schiml-Webb PA, O’Brien E, Deak T, Hennessy MB. Anti-inflammatory influences on behavioral, but not cortisol, responses during maternal separation. Psychoneuroendocrinol. 2009;34:1101–1108. doi: 10.1016/j.psyneuen.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Watkins LR, Maier SF, Rudy JW. Role of interleukin-1 beta in impairment of contextual fear condition caused by social isolation. Behav Brain Res. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- [34].Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogensis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schiml PA, Hennessy MB. Light-dark variation and changes across the lactational period in the behaviors of undisturbed mother and infant guinea pigs. J Comp Psychol. 1990;104:283–288. doi: 10.1037/0735-7036.104.3.283. [DOI] [PubMed] [Google Scholar]
- [36].Schiml-Webb PA, Deak T, Greenlee TM, Maken DS, Hennessy MB. Alpha-melanocyte stimulating hormone reduces putative stress-induced sickness behaviors in isolated guinea pig pups. Behav Brain Res. 2006;168:326–330. doi: 10.1016/j.bbr.2005.08.022. [DOI] [PubMed] [Google Scholar]
- [37].Schmidt ED, Aguilera G, Binnekade R, Tilders FJH. Single administration of interleukin-1 increased corticotropin releasing hormone and corticotropin releasing hormone-receptor mRNA in the hypothalamic paraventricular nucleus which paralleled long-lasting (weeks) sensitization to emotional stressors. Neurosci. 2003;116:275–283. doi: 10.1016/s0306-4522(02)00555-9. [DOI] [PubMed] [Google Scholar]
- [38].Schulkin J, McEwen BS, Gold PW. Allostasis, amygdala, and anticipatory angst. Neurosci Biobehav Rev. 1994;18:385–396. doi: 10.1016/0149-7634(94)90051-5. [DOI] [PubMed] [Google Scholar]
- [39].Shen Y, Connor TJ, Nolan Y, Kelly JP, Leonard BE. Differential effect of chronic antidepressant treatments on lipopolysaccharide-induced depressive-like behavioural symptoms in the rat. Life Sci. 1999;65:1773–1786. doi: 10.1016/s0024-3205(99)00430-0. [DOI] [PubMed] [Google Scholar]
- [40].Spitz RA. Anaclitic depression: an inquiry into the genesis of psychiatric conditions of early childhood. Psychoanal Study Child. 1946;2:313–342. [PubMed] [Google Scholar]
- [41].Vinkers CH, de Jong NM, Kalkman CJ, Westphal KGC, van Oorschot R, Olivier B, Korte SM, Groenink L. Stress-induced hyperthermia is reduced by rapid-acting anxiolytic drugs independent of injection stress in rats. Pharmacol Biochem Behav. 2009;93:413–418. doi: 10.1016/j.pbb.2009.05.017. [DOI] [PubMed] [Google Scholar]
- [42].Vinkers CH, Groenink L, van Bogaert MJV, Westphal KGC, Kalkman CJ, van Oorschot R, Oosting RS, Olivier B, Korte SM. Stress-induced hyperthermia and infection-induced fever: two of a kind? Physiol Behav. 2009;98:37–43. doi: 10.1016/j.physbeh.2009.04.004. [DOI] [PubMed] [Google Scholar]
- [43].Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- [44].Winer BJ. Statistical principles in experimental design. 2nd ed McGraw Hill; New York: 1971. [Google Scholar]
- [45].Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]