Abstract
High frequency spinal cord stimulation (HF-SCS) is a novel and more physiologic method of inspiratory muscle activation which involves stimulation of spinal cord pathways. In the present study, we determined if activation of the inspiratory intercostal muscles alone by this technique could be utilized to maintain artificial ventilation. In 7 anesthetized dogs, following C2 spinal cord section and bilateral phrenicotomy, trains of electrical stimulation (12 times/min) were applied at the T2 level. Eucapnea was maintained during an initial 5.5 hour period of continuous stimulation. During a subsequent 0.5 hour period, stimulus parameters were increased to induce hyperventilation resulting in a sustained fall in end-tidal PCO2 to 29.3 ± 0.4 mmHg. Single motor unit peak firing frequencies of the intercostal muscles during HF-SCS were similar to those occurring during spontaneous breathing. This technique holds promise as a method to restore ventilation in ventilator-dependent tetraplegics who do not have adequate phrenic nerve function for diaphragm pacing.
Keywords: diaphragm pacing, electrical stimulation, intercostal muscles
1. Introduction
Diaphragm pacing is a clinically useful method of providing artificial ventilatory support in patients with respiratory failure secondary to cervical spinal cord injury (Glenn, 1980; Glenn et al., 1980; Glenn et al., 1986; DiMarco, 2005; DiMarco et al., 2005a; DiMarco, 2006). Unfortunately, many patients with spinal cord injury have suffered damage to the phrenic motoneuron pools and/or phrenic nerves and therefore are not candidates for this technique (Glenn, 1980; Glenn et al., 1980). In previous animal studies (DiMarco et al., 1989), we demonstrated that artificial ventilation could be maintained for prolonged periods in anesthetized dogs utilizing ventral root stimulation with conventional stimulus frequencies (20 Hz). In subsequent clinical trials (DiMarco et al., 1994), however, ventilation could be maintained for only a few hours, most likely consequent to the onset of muscle fatigue. This may have occurred in part, as a consequence of the non-physiologic activation of motor units, which is characteristic of all peripheral nerve stimulation (Fournier and Sieck, 1988).
In the present study, we evaluated the utility of a new method of inspiratory muscle activation which requires the application of electrical stimulation of the spinal cord at high stimulus frequencies (300 Hz). When applied on the ventral surface of the spinal cord at the T2 level, this method results in activation of the inspiratory intercostal muscles and diaphragm with low stimulus currents (DiMarco and Kowalski, 2009). The method involves activation of the inspiratory motoneuron pools via spinal cord pathways resulting in more physiologic activation of the inspiratory muscles (DiMarco and Kowalski, 2009). This is reflected in the fact that both the diaphragm and intercostal muscles are activated at physiologic firing frequencies (5–5 Hz). Presumably, motor unit recruitment proceeds in an orderly fashion from slow to fast motor units (Henneman et al., 1965; Sieck, 1988). We also demonstrated that this technique is successful in maintaining artificial ventilation in anesthetized dogs for 6 hour periods without the development of system fatigue (DiMarco and Kowalski, 2009).
A substantial number of patients with ventilator-dependent spinal cord injury have damage to their phrenic nerves and/or motor units and therefore are not candidates for diaphragm pacing. The purpose of the present study therefore was to extend these prior observations to evaluate the utility of high frequency spinal cord stimulation (HF-SCS) of the intercostal muscles alone to provide complete ventilatory support. This method would require the repetitive activation of a greater portion of the intercostal motoneuron pool than that which occurs during spontaneous breathing (Sieck and Fournier, 1989). This evaluation was accomplished by assessing the effects of HF-SCS following bilateral cervical phrenicotomy.
2. Methods
Studies were performed in 7 mongrel dogs with a mean weight of 15.9 kg (range: 12.1 to 18.5 kg). All experiments were performed with the approval of the Institutional Animal Care and Use Committee of Case Western Reserve University. Animals were anesthetized with pentobarbital (25 mg/kg intravenously, given initially) followed by additional doses at 1–2 mg/kg, as needed to suppress any response to noxious stimuli. Studies were performed with the animals in the supine posture. Each dog was intubated with a large bore (10 mm ID) cuffed endotracheal tube which was sutured in the midcervical region. A femoral vein catheter was placed to administer fluids and supplemental anesthesia; a femoral arterial catheter was placed to monitor blood pressure (model PT300, Grass Instruments, Quincy, MA) and heart rate (P511 AC Amplifier, Grass Instruments). End-tidal PCO2 was monitored at the level of the trachea and oxygen saturation from the earlobe (Nellcor N-100, Hayward, CA). A heating blanket (Harvard Apparatus, Cambridge, MA) was used to maintain body temperature at 38 ± 0.5°C. Airway pressure was measured at functional residual capacity (FRC) following airway occlusion, with a pressure transducer (Validyne, MP45, Northridge, CA) connected to the airway opening. Tidal volume was recorded by electrical integration of the flow signal from a pneumotachograph, (Series 3700, Hans Rudolph, Kansas, City, MO).
Each of the cervical phrenic rootlets was severed bilaterally in all animals. A laminectomy was performed in the cervical region to gain access to the spinal cord which was sectioned at the C2 level to model patients with spinal cord injury. A hook forceps was lifted across the area of transection to verify complete section. A separate laminectomy was performed at the T4-T5 level for electrode placement. An 8 plate electrode with 4mm contact leads (Viasys NeuroCare, Madison, WI) was positioned under direct vision onto the ventral surface of the spinal cord and advanced to the T2 level. This location has been shown to result in maximal inspired volume generation based upon our previous studies (DiMarco et al., 1987; DiMarco and Kowalski, 2009). A separate ground electrode was implanted in the back musculature. A Grass square-wave pulse stimulator (model S88, Grass Instruments) equipped with a stimulus isolation unit (PSIU6, Grass Instruments) was used to provide electrical stimulation. Stimulus train duration was set at 1.2 s since a plateau in pressure and volume generation is usually achieved by this time; this duration is also in the range of stimulation applied during inspiratory muscle pacing.
Multiunit inspiratory muscle EMG recordings of the scalene, parasternal (2nd interspace), dorsal portion of the external intercostal muscle (3rd interspace, posterior axillary line) and costal diaphragm (via the 7th interspace) were obtained using bipolar teflon-coated stainless steel fine-wire electrodes, uninsulated at their terminal ~5 mm in each animal. Inspiratory muscle activation was also assessed by single motor unit (SMU) recordings (n=3). Teflon-coated stainless steel electrodes with an uninsulated portion of ~ 1 mm, to provide greater selectivity, were used to obtain SMU recordings. Multiunit EMG recordings were also obtained from the triceps brachii, extensor carpi radialis and flexor carpi ulnaris to assess potential activation of muscles of the forelimb (n=3). EMG potentials were amplified (1,000–10,000 times) and filtered (50 Hz to 5.0 kHz) (model BMI-830, Charles Ward Enterpises, Ardmore, PA). All parameters were monitored and stored on an eight-channel data-acquisition recorder (model Dash 8X, AstroMed, West Warwick, RI) for subsequent analysis (AstroView X, Data Review Software, AtroMed).
2.1. Airway Pressure measurements
Following C2 section, supramaximal stimulus amplitude (pulse width 0.2 ms) was determined in each animal as the level of current which resulted in maximal negative swings in airway pressure during airway occlusion (Pmax). Stimulus frequency was fixed at 300 Hz since this frequency was shown in previous studies to result in maximal negative airway pressure generation (DiMarco and Kowalski, 2009). The change in airway pressure during airway occlusion (utilizing submaximal stimulus amplitude selected for prolonged intercostal pacing) was also measured in each animal (ΔP). From this value, the fraction of maximal pressure (ΔP/Pmax) required during intercostal/accessory muscle pacing was determined. The product of ΔP/Pmax and duty cycle [inspiratory time (Ti)/total cycle duration (Ttot)] or pressure-time index was also calculated in each animal.
2.2. Protocol
Following bilateral phrenicotomy, multiunit and SMU activities of the scalene, parasternal and external intercostal muscles and inspired volume were recorded during spontaneous breathing. Subsequently, cervical spinal cord section was performed at the C2 level. HF-SCS was then applied over a range of stimulus amplitudes, at a fixed pulse width of 0.2 ms and stimulus frequency (300 Hz) to determine the stimulus amplitude (range: 0.9–1.2 mA) which resulted in inspired volumes which approximated those recorded during spontaneous breathing. Intercostal muscle pacing was then maintained utilizing these stimulus parameters with a stimulus train rate of 12/min, for 5.5 hours. During prolonged pacing, end-tidal PCO2, oxygen saturation, mean blood pressure and heart rate were continuously monitored. Inspired volume, minute ventilation and airway pressure generation were also monitored every 30 min. Multiunit inspiratory muscle EMGs were recorded during the initial period of chronic stimulation. SMU activities of the inspiratory muscles were also recorded during the initial period of stimulation and repeated during the final hour of stimulation. Forelimb muscle activation was also monitored during chronic stimulation.
Following 5.5 hours of chronic stimulation, stimulus amplitude was increased for an additional 30 min in each animal to induce hyperventilation as a further test of the capacity of the system to maintain artificial ventilation.
2.3. Data Analysis
SMU analysis included determination of mean peak discharge frequencies of the scalene, parasternal and external intercostal muscles. The magnitude of inspired volume and airway pressure generation, and peak SMU discharge frequency during spontaneous breathing and the first and last hour of chronic HF-SCS were compared. Comparisons were assessed, where applicable, using repeated-measures ANOVA and post hoc Newman-Keuls Tests. A p value < 0.05 was taken as statistically significant. Data are reported as means ± SE.
3. Results
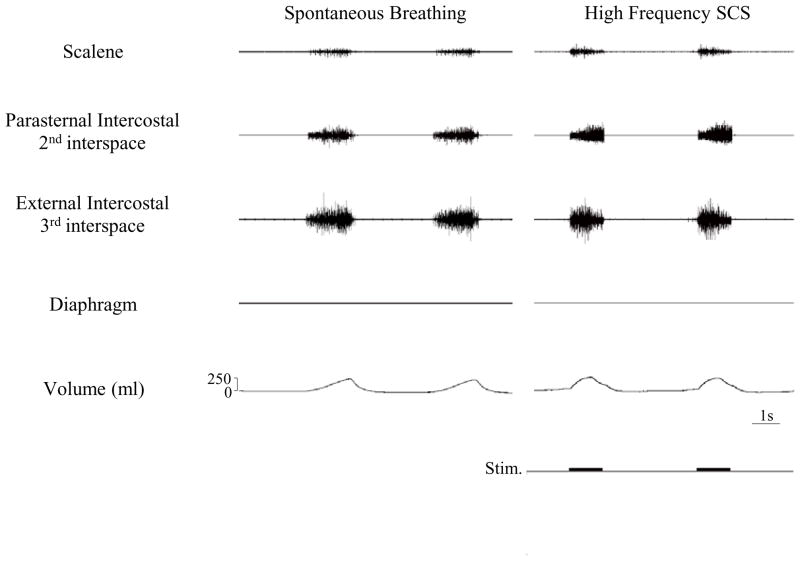
Multiunit electromyographic recordings of the scalene, parasternal, external intercostal and diaphragm muscles (post-phrenicotomy) are shown for one animal in Fig. 1. With adjustments in stimulus parameters to achieve inspired volumes of the same magnitude as those which occurred during spontaneous breathing, the scalene and each of the intercostal muscles were electrically active. HF-SCS resulted in an asynchronous EMG pattern which resembled that which occurred during spontaneous breathing. This asynchronous pattern in response to HF-SCS was observed in each animal. As expected, diaphragm EMG was electrically silent. During spontaneous breathing, each of the intercostal muscles was electrically active in all animals; scalene electrical activity, however, was detected in only 2 animals. Likewise, during HF-SCS, each of the intercostal muscles was electrically active while the scalene muscles were active in only the same 2 animals. While large burst activity was noted in the intercostal muscles, only relatively few motor units were detected in the scalene muscles during both spontaneous breathing and HF-SCS.
Figure 1.
Multiunit EMGs of the scalene, parasternal intercostal, external intercostal and diaphragm muscles during spontaneous breathing (left panel) and during HF-SCS (right panel). Stimulus parameters during HF-SCS were adjusted to approximate inspired volumes during spontaneous breathing. Consequent to phrenicotomy, diaphragm EMG was silent. As with spontaneous breathing, HF-SCS results in an asynchronous EMG pattern.
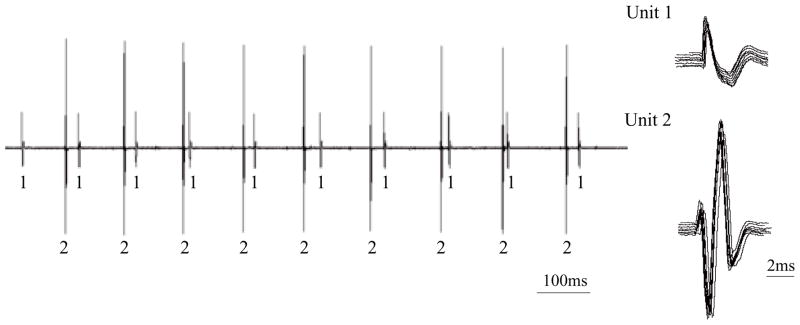
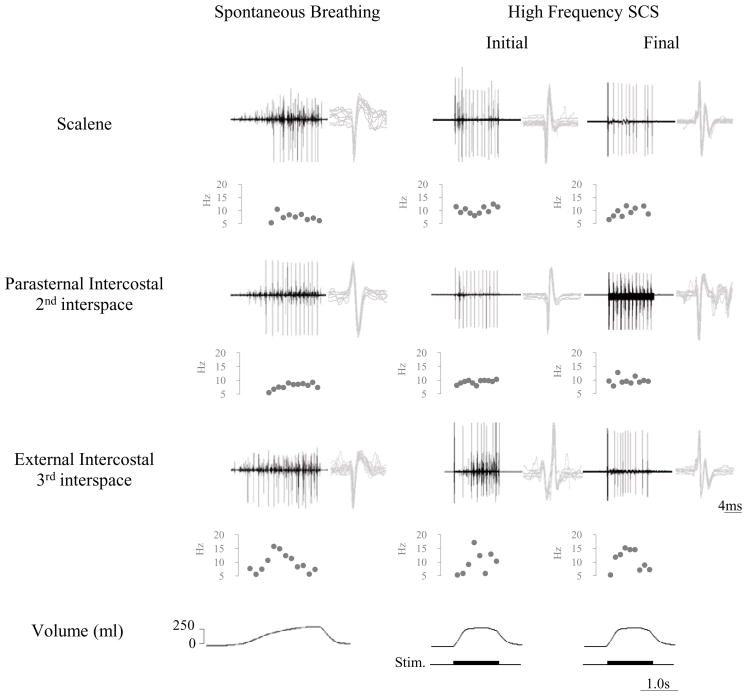
Characterization of SMUs in the parasternal muscle is shown for one animal during HF-SCS in Fig. 2. In this example, two separate motor units are readily identified during HF-SCS (300 Hz, 1.0 mA). Each of the SMUs is shown superimposed in the right portion of the figure, confirming their similar morphology. Firing frequencies were 8.8 and 9.7 Hz. Examples of single motor unit activities of the scalene, parasternal and external intercostal muscles during spontaneous breathing and HF-SCS are provided for one animal in Fig. 3. Raw tracings are displayed above a plot of instantaneous firing frequencies of a SMU from each muscle. As before, superimpositions of SMU recordings are also provided. The patterns observed during HF-SCS are qualitatively similar to those occurring during spontaneous breathing.
Figure 2.
Single motor unit (SMU) recordings from the parasternal intercostal muscle during HF-SCS in one animal. Two separate motor units are identified. Each of the SMUs is shown superimposed in the right panel, confirming their similar morphology. See text for further explanation.
Figure 3.
SMU activity from the scalene, parasternal and external intercostal muscles during spontaneous breathing (left panel) and during the initial and final hours of the 5.5 h period of chronic HF-SCS (right panel). EMG, instantaneous motor unit discharge frequency and volume are plotted. See text for further explanation
The mean peak firing frequencies of the scalene, parasternal and external intercostal muscles during spontaneous breathing and during the initial and final hour of chronic pacing are displayed in Table 1. These values were 8.3, 12.7 and 14.5 Hz during spontaneous breathing, respectively. During the initial hour of chronic pacing, peak firing frequencies were very similar at 9.9, 13.6 and 16.8 Hz, respectively (NS for each). Mean peak firing frequencies during the final hour of stimulation were 7.9, 13.4 and 15.4 Hz, respectively and also similar to those observed during the initial hour of chronic pacing (NS for each).
Table 1.
Mean Peak Firing Frequencies
| Inspiratory Muscle | Spontaneous Breathing | High Frequency SCS | |
|---|---|---|---|
| Initial | End | ||
| Scalene | 8.3 ± 0.8 Hz | 9.9 ± 1.4 Hz | 7.9 ± 1.3 Hz |
| Parasternal Intercostal 2nd Interspace | 12.7 ± 0.9 Hz | 13.6 ± 0.9 Hz | 13.4 ± 0.6 Hz |
| External Intercostal 3rd Interspace | 14.5 ± 1.1 Hz | 16.8 ± 0.7 Hz | 15.4 ± 0.7 Hz |
Mean pressure and time indices during chronic electrical stimulation are provided in Table 2. Mean duty cycle (Ti/Ttot) was 0.25 ± 0.01. The mean ratio of Δ P/Pmax was 0.53 ± 0.01. The mean product of these two variables or pressure-time index was 0.13 ± 0.01.
Table 2.
Mean Pressure and Time Indices Employed during Prolonged HF-SCS Following Bilateral Phrenicotomy
| Inspiratory Time (Ti) | Total Cycle Time (Ttot) | Duty Cycle (Ti/Ttot) | Paw during HF-SCS (ΔP) | Max Paw (Pmax) | ΔP/Pmax | Pressure-Time Index (Ti/Ttot × ΔP/Pmax) |
|---|---|---|---|---|---|---|
| 1.21 ± 0.01 s | 4.77 ± 0.10 s | 0.25 ± 0.01 | 9.43 ± 0.20 cmH2O | 17.71 ± 0.18 cmH2O | 0.53 ± 0.01 | 0.13 ± 0.01 |
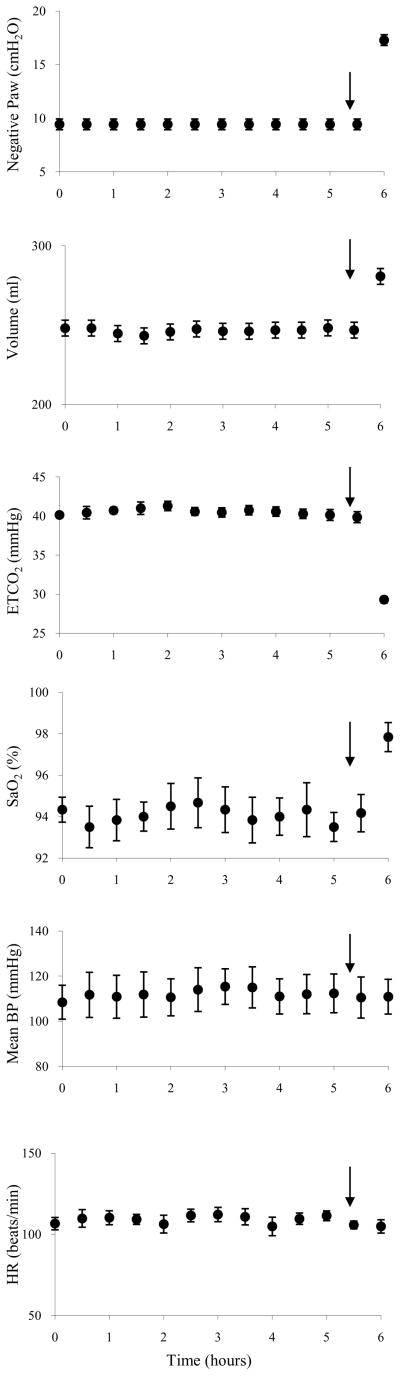
The mean effects of chronic HF-SCS applied over a 6 h period on airway pressure, inspired volume, end-tidal PCO2, oxygen saturation, blood pressure and heart rate, recorded every 30 minutes is provided in Fig. 4. Throughout the initial 5.5 h of chronic stimulation, airway pressure and inspired volume generation remained essentially unchanged. Mean airway pressure and inspired volume were 9.4 ± 0.2 cmH2O and 248 ± 4 ml during the initial hour and 9.4 ± 0.2 cmH2O and 247 ± 3 ml during the final hour, respectively (NS for each comparison). Mean gas tensions over the stimulation period for all animals are provided in Table 3. Over the 5.5 h period of stimulation, there were no significant changes in end-tidal PCO2 or oxygen saturation. In each animal, increases in stimulus parameters during the final 30 min of pacing resulted in a sustained decrease in end-tidal PCO2 to 29.3 ± 0.4 mmHg. Progressive increases in end-tidal PCO2 were not observed in any animal. Importantly, mean blood pressure and heart rate remained stable throughout the period of stimulation at 108 ± 8 mmHg and 107 ± 4 beats/min during the initial hour and 111 ± 9 mmHg and 106 ± 2 beats/min during the final hour of stimulation (NS for each comparison).
Figure 4.
Negative airway pressure (Paw) (following airway occlusion), inspired volume, end-tidal PCO2, arterial oxygen saturation (SaO2), mean blood pressure (BP), and heart rate (HR) are plotted every 30 min during continuous inspiratory muscle pacing with HF-SCS. Results are plotted as means ± SE. Airway pressure and inspired volume generation remained constant throughout the initial 5.5 h period of stimulation. End-tidal PCO2 and oxygen saturation were also maintained between 40.1 ± 0.3 and 39.9 ± 0.7 mmHg and 94.3 ± 0.6 and 94.2 ± 0.9%, respectively. Mean blood pressure and heart rate also remained stable throughout this period. After 5.5 h of pacing, stimulus parameters were increased, resulting in an increase in inspired volume and fall in PCO2 to 29.3 ± 0.4 mmHg, which was sustained over a 30 min period.
Table 3.
Mean Gas values
| Time | PetCO2 (mmHg) | SaO2(%) |
|---|---|---|
| 0 | 40.3 ± 0.3 | 94.3 ± 0.6 |
| 2 | 41.3 ± 0.6 | 94.5 ± 1.1 |
| 4 | 40.6 ± 0.6 | 94.0 ± 0.9 |
| 5.5 | 39.9 ± 0.8 | 94.2 ± 0.9 |
| 6 | 29.3 ± 0.4 | 97.8 ± 0.7 |
PetCO2, end-tidal PCO2; SaO2, arterial oxygen saturation.
During chronic HF-SCS, there was no observable limb motion. Moreover, triceps brachii, extensor carpi radialis and flexor carpi ulnaris EMGs were electrically silent.
4. Discussion
This study demonstrates that artificial ventilation can be sustained for prolonged periods by means of electrical activation of the intercostal muscles alone utilizing HF-SCS. During chronic inspiratory muscle pacing, there was no evidence of system fatigue as inspired volume, end-tidal PCO2 and oxygen saturation were maintained unchanged during the entire period of stimulation. As further evidence of system reserve, ventilation could be increased by adjustments in stimulus parameters resulting in a period of sustained hyperventilation for an additional 30 min. These results support the potential application of this technique to provide artificial ventilatory support in spinal cord injured patients dependent upon mechanical ventilation who are not candidates for diaphragm pacing.
4.1. Comparison to previous method of intercostal muscle pacing
In our previous study (DiMarco et al., 1987; DiMarco et al., 1989) evaluating intercostal muscle pacing in animals, electrical stimulation was also applied on the ventral surface of the spinal cord in the T2 region. With this technique however, electrical stimulation was applied at conventional stimulus frequencies (20 Hz) which required relatively high stimulus currents (3–4 mA) resulting in direct activation of the upper thoracic ventral roots (DiMarco et al., 1987; DiMarco et al., 1989). Consequently, this method is referred to as ventral root stimulation (VRS). While also successful in maintaining artificial ventilation for prolonged periods in animals, VRS was not successful in maintaining ventilatory support for more than a few hours in clinical trials (DiMarco et al., 1994). Despite the similar outcome results of the present animal study, it is likely that HF-SCS has the potential to achieve greater clinical success for several reasons.
First, VRS provides nonspecific stimulation, activating not only the intercostal muscles, but other muscles innervated by the upper thoracic roots (DiMarco et al., 1987; DiMarco et al., 1989), as well. As a result, this technique is associated with significant noninspiratory muscle contraction including forepaw contraction, and contraction of the muscles of the upper trunk (DiMarco et al., 1987; DiMarco et al., 1989). Similar noninspiratory muscle contraction was observed in clinical trials (DiMarco et al., 1994). Not surprisingly therefore, VRS is associated with significantly higher CO2 production and O2 consumption when compared to diaphragm pacing alone (DiMarco et al., 2004). For a given level of ventilation, intercostal pacing results in a significantly higher arterial PCO2 and lower SaO2 compared to diaphragm pacing (DiMarco et al., 2004). It is likely therefore that noninspiratory muscle contraction was a significant contributing factor to the lack of success in clinical trials of intercostal muscle pacing alone via VRS. In contrast, in our animal model, HF-SCS requires much lower stimulus currents (~ 1 mA) resulting in minimal ventral root stimulation, as evidenced by the lack of forepaw movement and absence of upper extremity EMG activity.
Second, HF-SCS results in a more physiologic pattern of muscle activation compared to VRS (DiMarco and Kowalski, 2009). Ventral root stimulation (DiMarco et al., 1989, DiMarco et al., 2005b), as with all peripheral nerve stimulation, results in synchronous activation of all motor axons in the electrical field of the stimulating electrode (Glenn, 1980; Glenn et al., 1980; Marsolais and Kobetic, 1983; Gregory and Bickel, 2005). This pattern of stimulation is reflected in the single large intercostal muscle EMG action potential which follows each stimulus spike (DiMarco et al., 1989). As with virtually all functional electrical stimulation techniques, this method results in a reversed order of motor unit recruitment: large diameter motor axons which innervate large, more fatigueable motor units, are more easily excited by imposed electric fields (Levy et al., 1990; Enoka, 2002; Gregory and Bickel, 2005). Smaller, fatigue- resistant motor units require higher stimulus levels and may not be fully activated, resulting in incomplete axonal stimulation and therefore incomplete muscle activation (Levy et al., 1990). HF-SCS, in contrast, results in an asynchronous pattern of EMG activation which resembles that occurring during spontaneous breathing (DiMarco and Kowalski, 2009). Moreover, peak SMU firing frequencies during HF-SCS were in the same range as those observed during spontaneous breathing (DiMarco and Kowalski, 2009). Of interest, these firing frequencies are also in the same range as that observed during spontaneous breathing in humans (Saboisky, et al., 2007) and in animals (Iscoe et al., 1976; Sieck et al., 1984). These findings indicate that the mechanism of intercostal muscle activation involves stimulation of spinal cord tracts that synapse with the inspiratory intercostal motoneuron pools (DiMarco and Kowalski, 2009). This method allows for processing of the stimulus within the motoneuron pools and the generation of a more physiologic signal. It is likely therefore that, consistent with Henneman’s size principle (Henneman et al., 1965; Berger, 1979; Dick et al., 1987; Hilaire et al., 1987; Sieck, 1988), HF-SCS results in an orderly pattern of motor unit recruitment with the early activation of the weakest and least fatigueable motor units with progression to stronger and more fatigueable units during inspiration. As with normal inspiration, this progression serves to maximize the range of force modulation and thereby inspired volume generation.
Finally, chronic intercostal muscle pacing via VRS, as with diaphragm pacing, is applied with relatively low stimulus frequencies of 20 Hz or less since chronic stimulation at higher frequencies may result in myopathic changes and muscle fatigue (Glenn, 1980; Glenn et al., 1980; Ciesielski et al., 1983). While low frequency stimulation increases the endurance characteristics of electrically stimulated muscles by conversion of the inspiratory muscles from a normally mixed fiber population to a uniform population of type I fibers, it also significantly reduces fiber diameter and thereby maximum force generation and secondarily maximum inspired volume generation (Pette and Vrbová, 1999). Fiber type conversion is not likely with HF-SCS since this method results in activation of the intercostal muscles at physiological firing frequencies. Histological characteristics and mechanical function therefore would be preserved.
4.2. Muscle activation and pressure development
Following bilateral phrenicotomy, the parasternal and external intercostal muscle groups were electrically active during HF-SCS and represented the major muscle groups responsible for inspired volume generation. Based upon previous studies (unpublished observations), these muscles were electrically active during HF-SCS from the first through fifth interspaces. The scalene muscles were only marginally active in a few animals and therefore did not participate in the observed responses. Lack of participation of this muscle group did not impact the results, however, since previous studies in dogs (DeTroyer and Kelly, 1984; DeTroyer et al, 1994;
DeTroyer et al., 2005) indicate that the scalene muscles do not have a significant respiratory function. It is not certain if the triangularis sterni or expiratory intercostal muscles were activated during HF-SCS, impeding the action of the inspiratory muscles and thereby resulting in negative work. This is unlikely to be a significant factor, however, since HF-SCS (with the phrenic nerves intact) results in inspired volumes which approximate the inspiratory capacity (DiMarco and Kowalski, 2009). Moreover, the expiratory intercostal muscles of the upper rib cage are quite thin and generate negligible opposing positive pressure (DiMarco et al., 1990; DiMarco et al., 1999a).
Previous studies (Bellemare and Grassino, 1982) have determined a critical tension-time index (product of the percent of maximal transdiaphragmatic pressure and duty cycle) for the human diaphragm. In spontaneously breathing humans, a tension-time index (TTdi) of less than 0.15 could be sustained for more than 45 min and was termed the critical TTdi. Values which exceeded this number were associated with fatigue (Bellemare and Grassino, 1982). The results of the present study also indicate that a tension time index of the intercostal muscles below 0.15 is not associated with the development of fatigue.
4.3. Study limitations
Despite the advantages of HF-SCS compared to VRS in terms of the pattern of intercostal muscle activation, there are several factors which may impact the success of any clinical application of this technique. With regard to the mechanism of intercostal muscle activation, we have speculated that the previously described intercostal to intercostal facilitatory reflex pathways may be mediating activation of the intercostal muscles via HF-SCS (Decima and von Euler, 1969; DiMarco and Kowalski, 2009). However, the specific spinal cord tracts mediating intercostal muscle activation via this technique are not known and may not exist in humans.
The novelty of this method of electrical activation of the inspiratory muscles presents unique potential sources of system fatigue which may involve the stimulated spinal cord tracts and/or synapses at the level of the motoneuron pools. However, the fact that the mean peak firing frequencies of the inspiratory muscles remained unchanged from the initial to the final hour of chronic stimulation suggests that fatigue at the level of the central nervous system was unlikely.
While the dog has been a useful model of translational research in other clinical applications including diaphragm pacing (DiMarco, 2005; DiMarco et al., 2005a; DiMarco et al., 2005b; Brown et al., 2006) and cough generation (DiMarco et al., 1995; DiMarco et al., 1999b; DiMarco et al., 2002; DiMarco et al., 2009a; DiMarco et al., 2009b), the difference in shape of the thorax between species may represent greater impediment to clinical translation. The human thorax is more compressed in the anteroposterior dimension (Woodburne, 1969), whereas the quadruped thorax is more compressed in the transverse dimension (Miller, et al., 1964). During inspiration there is much greater expansion of the anteroposterior dimension in the quadruped thorax. There is also no rotation at the chondrosternal junction in humans as there is in quadrupeds (DaSalvia et al., 1983). As a consequence, the intercostal muscles of the dog may have a greater mechanical advantage compared to humans.
4.4. Potential clinical application
Tetraplegics who have suffered injury to their phrenic motoneuron pools and/or phrenic nerves are not candidates for diaphragm pacing and therefore have no therapeutic options other than mechanical ventilation. The results of this study demonstrate that these patients may benefit from HF-SCS to provide artificial ventilatory support. This technique could also be applied in subjects with only a single phrenic nerve. In fact, combined intercostal and unilateral phrenic nerve stimulation utilizing conventional stimulus parameters has been successful in achieving ventilatory support (DiMarco et al., 2005b). Finally, since HF-SCS is a more physiological method of inspiratory muscle activation, this technique may provide a superior option for all patients with ventilator-dependent tetraplegia, even patients with intact phrenic nerve function. It is conceivable that the success rate in terms of achieving full-time ventilatory support may be higher with this method compared to conventional phrenic nerve pacing.
Acknowledgments
Special thanks to Ms. Dana Hromyak, RRT for providing technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellemare F, Grassino A. Effect of pressure and timing of contraction on human diaphragm fatigue. J Appl Physiol. 1982;53:1190–1195. doi: 10.1152/jappl.1982.53.5.1190. [DOI] [PubMed] [Google Scholar]
- Berger AJ. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol. 1979;42:76–90. doi: 10.1152/jn.1979.42.1.76. [DOI] [PubMed] [Google Scholar]
- Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–870. [PMC free article] [PubMed] [Google Scholar]
- Ciesielski TE, Fukuda Y, Glenn WW, Gorfien J, Jeffery K, Hogan JF. Response of the diaphragm muscle to electrical stimulation of the phrenic nerve. A histochemical and ultrastructural study. J Neurosurg. 1983;58:92–100. doi: 10.3171/jns.1983.58.1.0092. [DOI] [PubMed] [Google Scholar]
- Da Salvia KMC, Sayers BMA, Sears TA, Stagg DT. The changes in configuration of the rib cage and abdomen during breathing in the anesthetized cat. J Physiol (Lond) 1983;342:522–548. doi: 10.1113/jphysiol.1977.sp011779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decima EE, von Euler C. Excitability of phrenic motoneurones to afferent input from lower intercostal nerves in the spinal cat. Acta Physiol Scand. 1969;75:580–591. doi: 10.1111/j.1748-1716.1969.tb04413.x. [DOI] [PubMed] [Google Scholar]
- DeTroyer A, Cappello M, Brichant JM. Do canine scalene and sternomastoid muscles play a role in breathing? J Appl Physiol. 1994;76:242–252. doi: 10.1152/jappl.1994.76.1.242. [DOI] [PubMed] [Google Scholar]
- DeTroyer A, Kelly S. Action of the neck accessory muscles on the rib cage in dogs. J Appl Physiol. 1984;326:32. doi: 10.1152/jappl.1984.56.2.326. [DOI] [PubMed] [Google Scholar]
- DeTroyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev. 2005;85:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- Dick TE, Kong FJ, Berger AJ. Recruitment order of diaphragmatic motor units obeys Henneman’s size principle. In: Sieck GC, Gandevia SC, Cameron WE, editors. Respiratory Muscles and Their Neuromotor Control. Liss; New York: 1987. pp. 239–247. [Google Scholar]
- DiMarco AF. Restoration of respiratory muscle function following spinal cord injury: review of electrical and magnetic stimulation techniques. Respir Physiol Neurobiol. 2005;147:273–287. doi: 10.1016/j.resp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- DiMarco AF. Diaphragmatic pacing. In: Tobin MJ, editor. Principles and Practices of Mechanical Ventilation. 2. McGraw-Hill; New York: 2006. pp. 1263–1275. [Google Scholar]
- DiMarco AF, Altose MD, Cropp A, Durand D. Activation of intercostal muscles by electrical stimulation of the spinal cord. Am Rev Respir Dis. 1987;136:1385–1390. doi: 10.1164/ajrccm/136.6.1385. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Budzinska K, Supinski GS. Artificial ventilation by means of electrical activation of intercostal/accessory muscles alone in anesthetized dogs. Am Rev Respir Dis. 1989;139:961–967. doi: 10.1164/ajrccm/139.4.961. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Connors AF, Kowalski KE. Gas exchange during separate diaphragm and intercostal muscle breathing. J Appl Physiol. 2004;96:2120–2124. doi: 10.1152/japplphysiol.00628.2003. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. High-frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol. 2009;107:662–669. doi: 10.1152/japplphysiol.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a national institutes of health-sponsored clinical trial. Part I: Methodology and effectiveness of expiratory muscle activation. Arch Phys Med Rehabil. 2009a;90:717–725. doi: 10.1016/j.apmr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR, Frost FS, Creasey GH, Nemunaitis GA. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a national institutes of health-sponsored clinical trial. Part II: Clinical outcomes. Arch Phys Med Rehabil. 2009b;90:726–732. doi: 10.1016/j.apmr.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Supinski G, Romaniuk JR. Mechanism of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol. 2002;92:2341–2346. doi: 10.1152/japplphysiol.01231.2001. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Stefan SL, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest. 2005a;127:671–678. doi: 10.1378/chest.127.2.671. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Romaniuk JR, Kowalski KE, Supinski GS. Action of the intercostal muscles on the rib cage. Respir Physiol. 1990;82:295–306. doi: 10.1016/0034-5687(90)90099-k. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Romaniuk JR, Kowalski KE, Supinski G. Mechanical contribution of expiratory muscles to pressure generation during spinal cord stimulation. J Appl Physiol. 1999a;87:1433–1439. doi: 10.1152/jappl.1999.87.4.1433. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Romaniuk JR, Kowalski KE, Supinski G. Pattern of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol. 1999b;86:1881–1889. doi: 10.1152/jappl.1999.86.6.1881. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Romaniuk JR, Supinski G. Electrical activation of the expiratory muscles to restore cough. Am J Respir Crit Care Med. 1995;151:1466–1471. doi: 10.1164/ajrccm.151.5.7735601. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Supinski GS, Petro J, Takaoka Y. Evaluation of intercostal pacing to provide artificial ventilation in quadriplegics. Am J Respir Crit Care Med. 1994;150:934–940. doi: 10.1164/ajrccm.150.4.7921466. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Takaoka Y, Kowalski KE. Evaluation of intercostal and diaphragm pacing to provide artificial ventilation in tetraplegics. Arch Phys Med Rehabil. 2005b;86:1200–1207. doi: 10.1016/j.apmr.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Enoka RM. Activation order of motor axons in electrically evoked contractions. Muscle Nerve. 2002;25:763–764. doi: 10.1002/mus.10117. [DOI] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol. 1988;59:1055–1066. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- Glenn WW. The treatment of respiratory paralysis by diaphragm pacing. Ann Thorac Surg. 1980;30:106–109. doi: 10.1016/s0003-4975(10)61223-4. [DOI] [PubMed] [Google Scholar]
- Glenn WW, Hogan JF, Phelps ML. Ventilatory support of the quadriplegic patient with respiratory paralysis by diaphragm pacing. Surg Clin N Am. 1980;60:1055–1078. doi: 10.1016/s0039-6109(16)42233-4. [DOI] [PubMed] [Google Scholar]
- Glenn WWL, Phelps ML, Elefteriades JA, Dentz B, Hogan JF. Twenty years experience in phrenic nerve stimulation to pace the diaphragm. Pacing Clin Electrophysiol. 1986;9:780–784. doi: 10.1111/j.1540-8159.1986.tb06627.x. [DOI] [PubMed] [Google Scholar]
- Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther. 2005;85:358–364. [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Monteau R, Khatib M. Determination of recruitment order of phrenic motoneurons. In: Sieck GC, Gandevia SC, Cameron WE, editors. Respiratory Muscles and Their Neuromotor Control. Liss; New York: 1987. pp. 249–261. [Google Scholar]
- Iscoe S, Dankoff J, Migicovsky R, Polosa C. Recruitment and discharge frequency of phrenic motoneurones during inspiration. Respir Physiol. 1976;26:113–128. doi: 10.1016/0034-5687(76)90056-6. [DOI] [PubMed] [Google Scholar]
- Levy M, Mizrahi J, Susak Z. Recruitment, force and fatigue characteristics of quadriceps muscles of paraplegics isometrically activated by surface functional electrical stimulation. J Biomed Eng. 1990;12:150–156. doi: 10.1016/0141-5425(90)90136-b. [DOI] [PubMed] [Google Scholar]
- Marsolais EB, Kobetic R. Functional walking in paralyzed patients by means of electrical stimulation. Clin Orthop Relat Res. 1983;175:30–36. [PubMed] [Google Scholar]
- Miller ME, Christensen GC, Evans E. Anatomy of the Dog. WB Saunders; Philadelphia: 1964. [Google Scholar]
- Pette D, Vrbová G. What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve. 1999;22:666–677. doi: 10.1002/(sici)1097-4598(199906)22:6<666::aid-mus3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Gorman RB, DeTroyer A, Gandevia SC, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol. 2007;102:772–780. doi: 10.1152/japplphysiol.00683.2006. [DOI] [PubMed] [Google Scholar]
- Sieck GC. Diaphragm muscle: structural and functional organization. Clin Chest Med. 1988;9:195–210. [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaparhgm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Trelease RB, Harper RM. Sleep influences on diaphragmatic motor unit discharge. Exp Neurol. 1984;85:316–335. doi: 10.1016/0014-4886(84)90143-2. [DOI] [PubMed] [Google Scholar]
- Woodburne RT. Essentials of Human Anatomy. Oxford University Press; New York: 1969. [Google Scholar]