Abstract
Rationale
Modafinil is currently used as a treatment for daytime sleepiness.
Objectives
The objectives of this study were to explore the dopamine transporter (DAT)-related effects of modafinil on behavior and in vivo neurochemistry in rhesus monkeys (Macaca mulatta).
Methods
The effects of modafinil (3.0–10 mg/kg, i.v.) were evaluated on locomotor activity, reinstatement of cocaine-maintained behavior, extracellular dopamine levels in the caudate nucleus, and DAT occupancy in the dorsal striatum. Eight subjects were fitted with a collar-mounted activity monitor to evaluate sleep-activity cycles, with 4 days of baseline recording preceding an injection of saline or modafinil (3.0–10 mg/kg). The effects of modafinil (3.0–10 mg/kg) and cocaine (0.3 mg/kg) on reinstatement of behavior that was previously maintained under a second-order schedule of i.v. cocaine delivery were tested in a separate group of subjects (n=6). Finally, the effects of modafinil (3.0–10 mg/kg) on extracellular dopamine levels and DAT occupancy in vivo were characterized using microdialysis and positron emission tomography, respectively, in a within-subjects design (n=4).
Results
Modafinil significantly increased nighttime locomotor activity and reinstated cocaine-maintained behavior but did not affect daytime locomotor activity. Modafinil significantly increased striatal extracellular dopamine levels at a dose that resulted in DAT occupancy of 64.4% (putamen) and 60.2% (caudate).
Conclusion
The behavioral and in vivo dopaminergic effects of modafinil are consistent with the profile of a low potency DAT inhibitor and may indicate potential for abuse at high doses.
Keywords: Modafinil, Cocaine, Reinstatement, Nonhuman primate, PET, In vivo microdialysis, Dopamine, Imaging, Sleep
Introduction
The disruption of normal sleep patterns is a growing health concern. In order to maintain alertness in the face of sleep restrictions, many otherwise healthy individuals misuse over the counter and prescription drugs. For example, modafinil, a drug widely prescribed to treat narcolepsy and obstructive sleep apnea-associated somnolence (for review Minzenberg and Carter 2008) has been reported to selectively improve neuropsychological task performance in healthy volunteers (Turner et al. 2003), suggesting a possible nonmedical use by healthy individuals. Despite the current belief, based on studies in nondrug abusing individuals, that modafinil has low abuse liability (Myrick et al. 2004; O’Brien et al. 2006), its abuse-related effects warrant further evaluation.
Recent evidence indicates that modafinil can block dopamine transporters (DAT) and increase extracellular dopamine in the rat (Zolkowska et al. 2009) and in human brain (Volkow et al. 2009). This effect is known to be associated with the reinforcing effects of abused stimulants and thus may indicate that modafinil has significant abuse liability in vulnerable persons (Volkow et al. 2009). Given the well-documented importance of the DAT in the reinforcing effects of psychomotor stimulants (Ritz and Kuhar 1989; Howell and Wilcox 2001a; Howell and Kimmel 2008) and new preliminary evidence that modafinil can increase extracellular dopamine in humans at therapeutic doses (Volkow et al. 2009), a thorough preclinical examination of its DAT-related effects on behavior and in vivo neurochemistry was conducted in nonhuman primates.
The current study combined well-established behavioral measures, such as reinstatement of extinguished cocaine self-administration, with in vivo neurochemical assays to investigate the DAT-related effects of modafinil. Locomotor-stimulant effects were also evaluated. To determine if modafinil functioned as a DAT inhibitor within the dose range that produced significant behavioral effects, in vivo microdialysis and positron emission tomography (PET) imaging with the selective DAT ligand [18F]FECNT (Goodman et al. 2000) were performed. Extracellular dopamine levels were measured within the caudate nucleus and in vivo DAT occupancy was measured in both the caudate and the putamen. It was hypothesized that modafinil, through dopaminergic mechanisms, would reinstate cocaine self-administration in nonhuman primates. The results obtained provide important information about the mechanism of action of modafinil and show apparent DAT-related effects in nonhuman primates that may indicate the potential for abuse in humans.
Methods
Subjects
Eighteen (two male and 16 female) adult rhesus monkeys (Macaca mulatta) weighing 7–12 kg served as subjects. All subjects had a history of exposure to psychomotor stimulants, including cocaine. Each subject was individually housed within a primate colony and fed Purina monkey chow (Ralston Purina, St. Louis, MO, USA), fruits, and vegetables. Water was continuously available. The colony was maintained at an ambient temperature of 22±2°C at 45–50% humidity, and lights were set to a 12-h light/dark cycle. All procedures and studies strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication no. 85–23, revised 1985) and were approved by the Institutional Animal Care and Use Committee of Emory University.
Surgery
All animals used in self-administration and in vivo microdialysis experiments were surgically prepared with a chronic indwelling venous catheter under sterile conditions as previously described (Howell and Wilcox 2001b). Briefly, implantation was done under a combination of Telazol (tiletamine HCl and zolazepam HCL) and isoflurane (1.0–2.0%) anesthesia using aseptic techniques. One end of a silicone catheter was inserted into either the femoral or jugular vein and advanced into the vena cava. The distal end of the catheter was routed subcutaneously and attached to a vascular access port (Access Technologies, Skokie, IL, USA) in the intra-scapular region. Preoperative antibiotic (Rocephin) and postoperative analgesic (Banamine) were administered according to veterinary staff guidance. Catheters were flushed regularly with 1.5 mL of heparinized (100 U/mL) saline to maintain patency.
Drugs
Cocaine HCl (National Institute on Drug Abuse, Bethesda, MD, USA) was dissolved in 0.9% saline. Doses were calculated and are expressed as salts. Modafinil (Sigma, St. Louis, MO, USA) was dissolved in a warm mixture of 50% β-cyclodextrin and sterile water and allowed to cool to room temperature prior to injection. [18F]FECNT was synthesized and radiolabeled at the Yerkes Imaging Center (Goodman et al. 2000).
Locomotor activity and sleep–wake pattern
In order to quantify locomotor activity and sleep–wake patterns following modafinil administration, one male and seven female subjects were outfitted with Actiwatch (Mini Mitter, Bend, OR, USA) activity monitors for a period of 10 days. The Actiwatch consists of an omni-directional sensor that is sensitive to motion (recorded as activity counts) and has been previously shown to be a reliable, non-invasive method for activity monitoring (Mann et al. 2005). The monitors were programmed to record the total piezoelectric voltage generated over the preceding 15 s (i.e. epoch length=15 s). The devices record intensity, amount and duration of movement in all three planes by producing a voltage that is subsequently converted to an arbitrary count and data logged (for review see Mann et al. 2005). On the first day of the study, the Actiwatch sensor was attached to the subject’s collar while the subject was under ketamine (3.0–10 mg/kg, i.m.) anesthesia. Activity and sleep measurements for the subsequent 24 h were not included in the analysis in order to allow the subject to recover completely from anesthesia. Spontaneous baseline activity and sleep patterns were measured for the following 4 days (baseline recording). On the fifth day, a morning (~9 am) or an evening (~7 pm) injection of modafinil (3.0–10 mg/kg) or saline was administered i.v. via a saphenous catheter. All morning injections were given between 8 and 9 am, and all evening injections were given between 6 and 7 pm to reduce variability due to circadian activity patterns. Measurements were taken for an additional 7 days. This process was then repeated until all subjects had received each treatment (saline or modafinil) at each time point (morning or evening). The order in which this occurred was counterbalanced across subjects. The data were downloaded and analyzed with Actiware Sleep 3.4 (Mini Mitter Co. Inc., Bend, OR, USA). Activity counts were extracted and summed into hourly bins for each time point. The 4 days of baseline recording were averaged and the post-injection data normalized to the baseline average. Thus, data are presented as a percentage of the baseline activity level. The following sleep parameters were assessed: sleep efficiency (total sleep time percentage during the recording time), sleep latency (time elapsed between the start time of a given rest interval and the following sleep start time, in minutes), number (total number of continuous blocks with each epoch scored as sleep during the recording time), and duration (scored total sleep time divided by the number of sleep bouts for the given interval) of sleep bouts. “Sleep” was automatically determined from rest intervals (periods of data that contain periods of time when the subject activity is low and the subject is likely to be at rest).
Self-administration
Five female and one male subjects with previous experience of i.v. drug self-administration under a second-order schedule of drug delivery served as subjects (Kimmel et al. 2008). The apparatus and self-administration procedure were as previously described (Wilcox et al. 2005; Kirkland Henry et al. 2009). At the beginning of the test session, the behavioral chamber was illuminated with a red light which served as a discriminative stimulus. The second-order schedule consisted of a 10-min fixed interval (FI) during which completion of a fixed ratio (FR) 20 response requirement resulted in the 2-s illumination of a white light [FI 10 min (FR 20:S)]. Once the interval elapsed, completion of the FR requirement resulted in a change in the stimulus light from red to white for 15 s and a cocaine infusion (0.1 mg/kg in 2 ml infused over 7 s). This infusion was followed by a 60-s timeout; during which, stimulus lights were turned off and responding had no programmed consequences. At the end of the timeout, the red light was presented again to signal the initiation of the next interval component. Subjects had the opportunity to self-administer cocaine 5 days per week, and each 60-min session was comprised of five consecutive FI components.
Reinstatement
Cocaine-maintained behavior was extinguished by substituting saline for cocaine in the infusion syringe and omitting response-contingent presentations of the drug-paired stimulus (white light). Extinction sessions were otherwise identical to the procedures used during the maintenance of cocaine self-administration. Extinction sessions were conducted until response rates decreased to below 20% of baseline response rates, at which point extinction was considered to have been achieved. Reinstatement sessions were conducted by administering a noncontingent i.v. injection of saline, modafinil (3.0–10 mg/kg), or cocaine (0.3 mg/kg) 5 min prior to the start of the session. The injection volume for modafinil ranged from 5 to 20 ml (for the 3.0- and 10-mg/kg doses, respectively) due to limited solubility (7.0 mg/ml). All other noncontingent injections (i.e. saline and cocaine) were matched in volume to the modafinil injection. During reinstatement sessions, response-contingent presentations of the white light were restored but completion of the response requirement continued to result in the delivery of saline. Each reinstatement session was preceded by one or more extinction sessions to ensure that response rates were below 20% of baseline response rates.
In vivo microdialysis procedure
Four female subjects underwent aseptic stereotaxic surgery for the implantation of microdialysis guide cannula targeting the caudate nucleus, and microdialysis experiments were conducted as previously described (Czoty et al. 2000, 2002; Wilcox et al. 2005). Briefly, analytical probes (CMA 12) were inserted into guide cannulae that were designed to facilitate correct probe placement. The same four subjects were utilized in all microdialysis experiments. Microdialysis experiments were conducted no more frequently than once every 2 weeks in individual subjects. At the time of testing, each awake monkey was seated in a commercially available primate chair (Primate Products). Subjects were well adapted to the primate chair prior to the initiation of the study. Daily sessions lasted for approximately 4 h and were conducted within a ventilated, sound-attenuating chamber. The chair limited movement of the animals and facilitated connections between the implanted probes and appropriate perfusion equipment. A Lexan plate positioned perpendicular to the medial plane of the body, just above shoulder height, ensured that animals could not contact the probe area. A microinjection pump (CMA/102) located outside the chamber continuously delivered artificial cerebrospinal fluid (aCSF; Na2HPO4, 1.0 mM; NaCl, 150 mM; KCl, 3 mM; CaCl,1.3 mM; MgCl, 1.0 mM; and ascorbic acid, 0.15 mM) via FEP Teflon tubing to the probe for perfusion at a flow rate of 2.0 μl/min. Following a 1-h equilibrium period, 20-μl samples were collected every 10 min for the remainder of the session. All samples were collected outside of the test chamber to avoid environmental influences on neurochemistry. Modafinil (3.0–10 mg/kg, i.v.) and cocaine (0.3 mg/kg, i.v.) were administered to determine drug-induced increases in extracellular dopamine. A within-session control for tissue integrity was carried out at the end of each session by substitution of a potassium-enriched aCSF (KCl 54 mM and NaCl 103 mM) to induce voltage-dependent dopamine release. In all cases, the dopamine response to potassium-enriched aCSF was greater than 500%. High-pressure liquid chromatography (HPLC) and electrochemical detection were used to quantify extracellular levels of dopamine according to well-established analytical procedures (Czoty et al. 2000, 2002). The HPLC system consisted of a small-bore (3.2 mm× 150 mm, 3 μm) column (5 μm C18 stationary phase; Thermo Hypersil, Keystone Scientific Operations, Bellefonte, PA, USA), with a commercially available mobile phase (MD-TM, ESA, Inc., Chelmsford, MA, USA) delivered by an ESA 582 solvent delivery pump at a flow rate of 0.6 ml/min. Electrochemical analyses were performed with an ESA dual-channel analytical cell (model 5040; oxidation channel 220 mV, reduction channel −150 mV), guard cell (model 5020, potential=350 mV), and an ESA Coulchem II detector. A full range of dopamine standards (0.5–25 nM) was analyzed both before and after each set of samples to allow estimates of the concentration of dopamine in test samples and evaluate possible degradation over the course of the run. Chromatograms were generated and analyzed by EZChrom Elite software (version 3.1, Scientific Software, Pleasanton, CA, USA). For each session, the six samples prior to drug administration were averaged and normalized to 100%. Drug effects are expressed as a percent of this baseline.
PET neuroimaging
PET neuroimaging was performed at the Yerkes Imaging Center on a MicroPET Focus scanner (Siemens, Knoxville, TN, USA) in the same subjects used for microdialysis experiments. On the day of the PET study, subjects were anesthetized with Telazol (4 mg/kg, i.m.) and transported to the Yerkes Imaging Center. There, animals were intubated and anesthesia was maintained with 1.0–2.0% isoflurane. Subjects were positioned in the tomograph and connected to the physiological monitoring equipment. During image acquisition, vital signs were continuously monitored by a trained technician. A 10–20 min transmission scan was obtained for attenuation correction.
The selective DAT ligand (Goodman et al. 2000), 18F-2beta-carbomethoxy-3beta-(4-chlorophenyl)-8-(2-fluoroethyl)nortropane (18F-FECNT), was utilized to determine DAT binding potential. A slow bolus of approximately 5.0 mCi 18F-FECNT was injected over 5–6 min at a rate of 1 ml/min. Scanning began coincident with the start of radiotracer injection. The initial acquisition was a 28-frame dynamic sequence starting with 30-s scans and ending with 20-min scans for a total duration of 90 min. At 90 min, a single bolus dose (10 mg/kg, i.v.) of modafinil was injected, and a second dynamic sequence was acquired starting with 2-min frames and ending with 5-min frames. Hence, a dynamic PET sequence was obtained over the 2 h following injection of the radioligand. At the conclusion of the scan, subjects were returned to the colony.
A generalized reference tissue method was used to analyze the resulting occupancy data (Votaw et al. 2002). All images were reconstructed with measured attenuation correction, zoom factor 8, and Shepp–Logan reconstruction filter cutoff at 1 cycle/cm. The axial slice thickness was 3.375 mm. All images were decay corrected to the time of injection. Regions of interest for the caudate, putamen, and cerebellum were manually drawn on the summed images and used to obtain time–activity curves. The cerebellum, a region relatively devoid of DAT, served as the reference region to account for nonspecific binding. Five rate constants (R, k2, ka/3, kb/3, and k4) were used to fit a curve to the time–activity data for the caudate and putamen. Data were collected in two sections, with the assumption that drug infusion changed only the k3 (Bmax/kon) rate constant (i.e. modafinil competes with FECNT for binding at the DAT and thus decreases the apparent Bmax) from ka/3 (pre-drug infusion) to kb/3 (post-drug infusion). Accordingly, the k3/k4 ratio represents a measure of binding potential. The fraction of transporters occupied by the competing ligand was then estimated as 1−(kb/3/k4)(ka/3/k4). To calculate the expected curve, in the absence of modafinil infusion, these same parameters were used with the entire cerebellum reference curve to calculate the putamen or caudate curves to the end of the experiment. This model assumes rapid uptake of the competing ligand and correspondingly rapid occupancy of the target protein. However, the kinetics of modafinil and its low affinity for the DAT (Zolkowska et al. 2009) may violate this assumption. Accordingly, only the later time points corresponding to minutes 112.5–145 (22.5–55 min after modafinil injection) on the time–activity curves were used in calculating the kb values. Based on the microdialysis data, at these later time points, modafinil was expected to have achieved steady state occupancy, thus meeting the assumptions of the model and yielding a better fit of the parameters.
Statistics
All statistics were performed using GraphPad Prism 5.0. For self-administration studies, the primary dependent variable was response rates (responses/s). The effects of modafinil were compared to saline using Student’s t test. Locomotor, sleep–wake pattern, and reinstatement experiments were analyzed by repeated-measures ANOVA. In the presence of a main effect, a Duncan multiple comparisons post hoc test was conducted to determine which treatments were significantly different from baseline/saline levels. For microdialysis studies, dopamine levels were determined as nanomolar concentrations in dialysate unadjusted for probe recovery. The dopamine concentration for each 10-min sample was expressed as a percentage of the mean baseline value. Percent increase in extracellular dopamine relative to baseline was calculated as (extracellular dopamine level in the presence of the drug/baseline level of dopamine)*100 for each drug, dose, and combination. The peak dopamine responses for each set of microdialysis experiments were analyzed by one-way repeated-measures ANOVA to determine if there were significant increases in dopamine overflow due to modafinil or cocaine infusion compared to baseline. Mean baseline dopamine data were also analyzed by one-way repeated-measures ANOVA to identify any significant effect followed by Dunnett’s test. Significance was set at the p<0.05 level of confidence.
Results
Locomotor activity and sleep–wake pattern
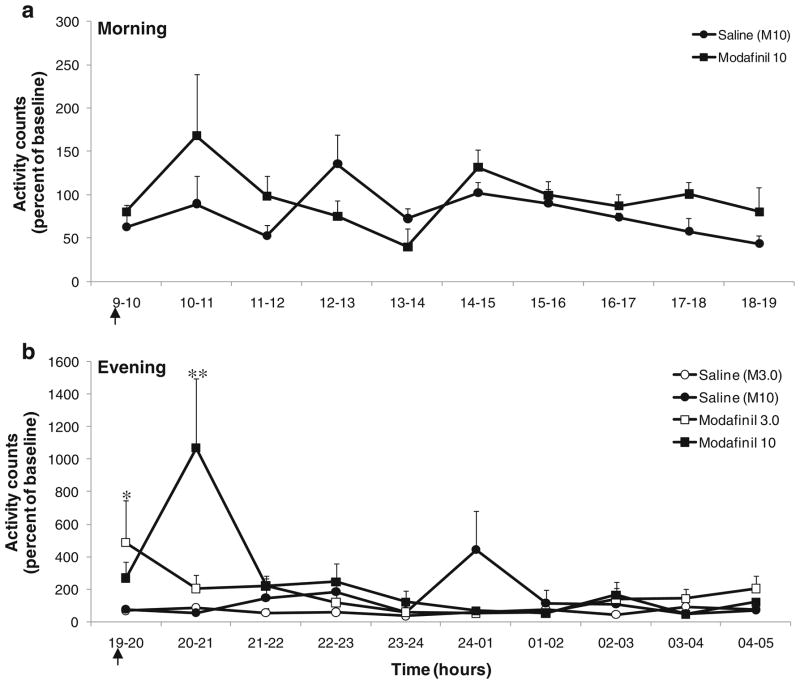
Locomotor activity: There was no significant difference in activity level following a morning injection of modafinil (10 mg/kg) as compared to saline (Fig. 1). However, the mean activity was significantly higher following modafinil (10 mg/kg) administration compared to modafinil (3.0 mg/kg) and saline (control) for 1 h following the evening injection (injection at 7 pm, peak activity at 8 pm; F(2,11) =2.92; p <0.001) as shown in Fig. 1. The mean activity values at 8 pm for the saline and modafinil (10 mg/kg) conditions were 57.6%±10.4 and 1,069.7%±425.9, respectively. Modafinil (3.0 mg/kg) also differed significantly from saline immediately following injection (7–8 pm time point; p<0.05).
Sleep–wake pattern: No significant effects were observed between the groups (modafinil vs. saline) for any sleep parameter across time points (baseline, injection day, and post-injection day) for morning or evening injections (data not shown).
Fig. 1.
Morning (a) and evening (b) locomotor activities after modafinil injection. Activity counts were extracted and summed into hourly bins. Abscissae: time expressed in hours and plotted on an absolute linear scale. Ordinates: activity counts expressed as a percentage of the baseline activity level. An * indicates a significant (p<0.05) difference from saline baseline only; ** indicates significant differences from modafinil (3.0 mg/kg) and saline (p<0.001). Arrows represent the time of injection. Each data point represents the group mean±SEM
Drug-induced reinstatement
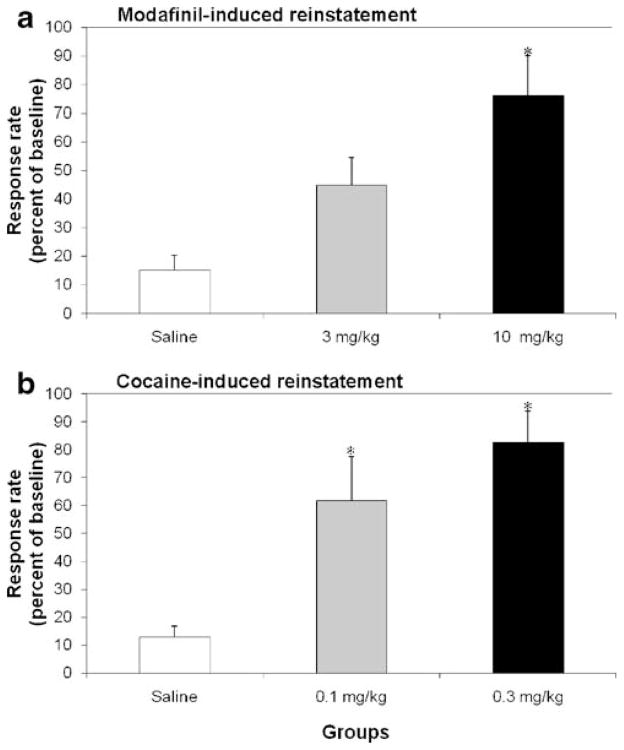
In reinstatement experiments, the lower dose of modafinil (3.0 mg/kg) induced increases in response rates greater than extinction criteria (i.e. more than 20% of baseline cocaine-maintained rates) but the effect did not reach statistical significance with respect to saline treatment (45% vs. 16%). However, the higher dose of modafinil (10 mg/kg) significantly reinstated cocaine-maintained responding compared with saline treatment (Fig. 2a; F(2,9) =8.06; p<0.01). The magnitude of modafinil-induced reinstatement was similar to that observed for cocaine-induced reinstatement at the doses tested (Fig. 2a). Both doses of cocaine significantly reinstated cocaine-maintained responding compared with saline treatment (p<0.05) as depicted in Fig. 2b.
Fig. 2.
Effects of modafinil (a) or cocaine (b) on reinstatement of cocaine-maintained behavior. Abscissae: dose of the respective drug. Ordinates: responses per second expressed as a percentage of the baseline response rate during cocaine self-administration. An * indicates a significant (p<0.05) difference from saline treatment. Each data point represents the group mean±SEM
In vivo microdialysis
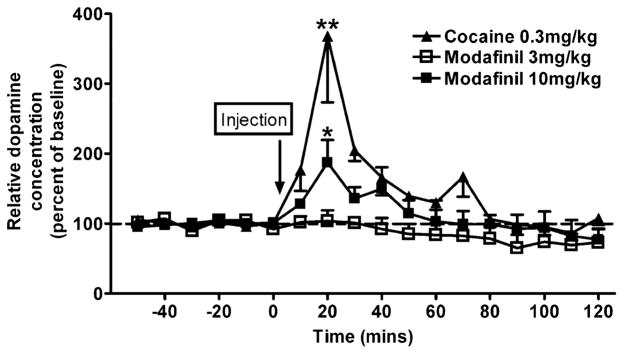
The lower dose (3.0 mg/kg) of modafinil had no significant effect on extracellular dopamine. However, the higher dose (10 mg/kg) increased extracellular dopamine in the caudate nucleus above baseline (5.04±1.49 nM uncorrected for probe recovery), with a significant peak effect at 20-min post-injection (Fig. 3; F(1,12) =2.16; p=0.02). Cocaine (0.3 mg/kg) also significantly increased extracellular dopamine above baseline (6.45±2.15 nM uncorrected for probe recovery), with a peak effect at 20-min post-injection.
Fig. 3.
Effects of modafinil or cocaine on extracellular dopamine in the caudate nucleus of awake rhesus monkeys (n=4). Abscissae: time expressed in minutes and plotted in reference to the drug injection. Ordinates: relative dopamine concentration expressed as a percentage of the basal level prior to drug administration. An * (p<0.05) or a ** (p<0.01) indicate a significant difference from baseline levels obtained during the 60 min prior to drug administration. Arrows represent the time of injection. Each data point represents the group mean±SEM
PET neuroimaging
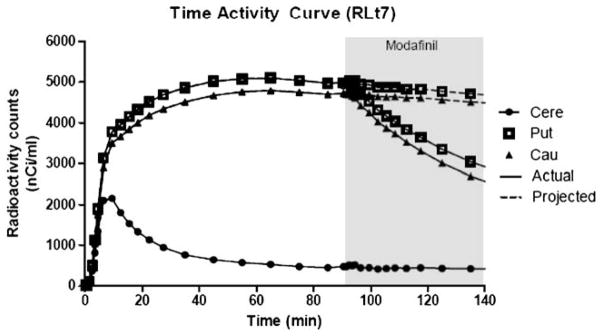
A single bolus injection of modafinil (10 mg/kg) resulted in average DAT occupancy of 60.2% in the caudate and 64.4% in the putamen (Table 1). Note that DAT occupancy values were very consistent across subjects. Representative time–activity curves are shown in Fig. 4.
Table 1.
Percentage of DAT occupancy by modafinil (10 mg/kg, i.v.) for individual subjects and the group mean (±SEM)
| Subjects | Putamen | Caudate |
|---|---|---|
| RLt | 69.0 | 63.3 |
| RJt | 62.3 | 62.0 |
| RHp | 66.1 | 59.0 |
| RNb | 60.3 | 56.5 |
| Mean (SEM) | 64.4 (1.9) | 60.2 (1.5) |
Fig. 4.
Representative time activity curves for [18F]FECNT in the caudate (cau), putamen (put) and cerebellum (cere) in rhesus monkey RLt-7. The displacement of [18F]FECNT binding, following administration of modafinil (10 mg/kg, i.v.) at 90 min, is shown with solid lines. The projected time activity curves, in the absence of modafinil administration, are shown with dotted lines. Abscissae: time expressed in minutes and plotted in reference to the injection of the radiotracer. Ordinates: radioactivity counts decay corrected and expressed on an absolute linear scale. Each data point represents the value obtained from a single representative subject
Discussion
The present study was designed to evaluate the DAT-related effects of modafinil in vivo in nonhuman primates. Our findings demonstrate that modafinil has a profile typical of psychomotor stimulants such as cocaine. Modafinil induced nocturnal locomotor-stimulant effects, as shown by significant increases in activity counts following evening administration. Modafinil also significantly reinstated previously extinguished cocaine-maintained responding. Finally, the same behaviorally active dose of modafinil significantly increased extracellular dopamine levels in the caudate and resulted in approximately 60% DAT occupancy in the caudate and the putamen, comparable to effects observed following doses of cocaine that reliably maintain self-administration (Votaw et al. 2002; Ito et al. 2002; Wilcox et al. 2002, 2005; Newman and Beardsley 2006).
Modafinil has been proposed as a potential medication for cocaine dependence. A limited number of clinical studies suggest that modafinil may improve clinical outcomes for the treatment of cocaine dependence, reducing self-reports of craving and cocaine-induced euphoria (Dackis et al. 2003, 2005; Hart et al. 2008; Anderson et al. 2009). Mechanistically, modafinil could reduce cocaine use in dependent patients by occupying the cocaine binding site on the DAT. Indeed, a variety of preclinical studies in nonhuman primates provide evidence that selective inhibitors of dopamine uptake may be useful pharmacotherapies in the treatment of cocaine abuse (Howell and Wilcox 2001a; Dutta et al. 2003; Lindsey et al. 2004; Howell et al. 2007). However, the use of selective DAT inhibitors as medications for cocaine abuse is limited by their potential for abuse. To this end, preclinical studies have shown that selective DAT inhibitors can function as reinforcers (Lindsey et al. 2004; Kimmel et al. 2007). Furthermore, studies have shown that under certain conditions, modafinil functions as a reinforcer in both humans (Stoops et al. 2005) and macaques (Gold and Balster 1996). In the present study, modafinil also reinstated previously extinguished cocaine-maintained responding at a dose that resulted in high levels of DAT occupancy and induced significant increases in extracellular dopamine. These data are supported by a previous study showing that modafinil has cocaine-like discriminative-stimulus effects in rodents (Gold and Balster 1996). Although this profile of effects suggests that modafinil may have some abuse liability in its own right (Gold and Balster 1996; Stoops et al. 2005; Volkow et al. 2009; Zolkowska et al. 2009; Bernardi et al. 2009), the abuse potential of modafinil in humans is likely limited by its low potency at the DAT compared to other CNS stimulants (Jasinski 2000; Stoops et al. 2005; O’Brien et al. 2006; see review of Castells et al. 2007 and reference therein). Furthermore, modafinil has poor solubility, which limits the routes of administration and amount that can be self-administered. Therefore, modafinil has remained viable as a medication for cocaine abuse.
The reinstatement paradigm has been used as a preclinical model of drug-induced craving (Grimm et al. 2001) and drug relapse in humans (Stewart and de Wit 1987). Moreover, conditions (such as stress) that are reported to provoke relapse in humans will also reinstate extinguished drug-maintained behavior in laboratory animals (Shaham et al. 2003). However, it has been argued that this procedure has not established predictive validity as many drugs that attenuate reinstatement elicit relapse (Katz and Higgins 2003). Accordingly, it is clear that more evidence is needed to make a solid argument regarding abuse potential based on modafinil-induced reinstatement. Modafinil has been reported to function as a reinforcer under an FR-30 schedule of self-administration in rhesus macaques (Gold and Balster 1996) and a modified progressive ratio schedule in humans (Stoops et al. 2005). However, it did not engender a conditioned place preference or function as a reinforcer under an FR-1 schedule of reinforcement in rodents (Deroche-Gamonet et al. 2002), suggesting that the reinforcing effects of modafinil may be evident over a limited range of conditions. Consistent with this notion, a recent study indicated that only in a specific genotype can modafinil increase vigor, subjective well-being, and maintain stable performance during the experimental session (Bodenmann et al. 2009). Modafinil self-administration experiments would have been the obvious choice to address abuse liability in the present study. However, poor solubility (7 mg/ml in 50% b-cyclodextrin) and prohibitive costs from commercial sources precluded the assessment of modafinil self-administration which requires repeated i.v. injections with multiple doses over extended periods.
In concert with previous studies (Madras et al. 2006; Zolkowska et al. 2009), the current results clearly indicate that modafinil binds to the DAT and increases dopamine levels. The findings of PET imaging experiments using [18F]-FECNT revealed that a behaviorally relevant dose of modafinil resulted in approximately 60% DAT occupancy and significantly increased extracellular dopamine levels to approximately 200% of baseline in the same animals. A similar relationship was observed between reinstatement effects and DAT occupancy for cocaine. A dose of cocaine that elicited reinstatement effects comparable to modafinil in the present study occupied 65–76% of DAT (Wilcox et al. 2002). Interestingly, the dose of modafinil that induced reinstatement effects and DAT occupancy comparable to cocaine was apparently less effective in increasing extra-cellular dopamine. While the difference was not statistically significant in our small sample, the apparent difference may be due to the involvement of other neurotransmitter systems in modafinil-induced reinstatement, such as the adrenergic system (Ballon and Feifel 2006). Madras et al. (2006) also found that modafinil (8.0 mg/kg) resulted in approximately 54% DAT occupancy in the striatum of baboons. Similarly, clinically relevant doses of modafinil significantly increased brain dopamine through blockade of DAT in the human brain (Volkow et al. 2009). Collectively, the current study in conjunction with previous reports demonstrates that modafinil can have significant effects on dopamine system function via DAT inhibition and supports a close relationship between DAT occupancy, increases in extracellular dopamine, and behavioral effects observed in previous studies with cocaine and other DAT inhibitors (Wilcox et al. 2002, 2005).
Our results suggest that modafinil can function as a locomotor stimulant, causing an increase in activity evident after evening administration. Similarly, Hermant et al. (1991) reported increases in nocturnal activity in rhesus monkeys after acute and chronic administration, indicating that modafinil may induce behavioral stimulation. These results are consistent with our findings in the reinstatement paradigm, where robust effects observed at the high dose were likely related to high DAT occupy and significant increases in dopamine. Interestingly, the low dose of modafinil had less pronounced but significant effects on locomotor activity while having no effect on extracellular dopamine. As previously mentioned, the disparity between behavioral effects and the magnitude of drug-induced increases in dopamine may be accounted for by other systems, such as the adrenergic system. Although there are no documented cases of modafinil abuse associated with its euphoric effects, there have been reports of improved neuropsychological task performance in healthy individuals (Turner et al. 2003). Such off-label use for its purported cognitive enhancing properties, especially in association with conditions of sleep deprivation, represents a high-risk behavior that should be monitored further and intervention efforts may be needed to curb this form of drug misuse (McCabe et al. 2005).
Acknowledgments
The authors express their gratitude to Lisa Neidert and Juliet Brown for capable technical assistance; Jon Nye and John Votaw for the help with PET analysis. We also thank Paul Chen for his aid in the conduct of the imaging procedures. The research was supported by USPHS Grants DA10344 (LLH), DA00517 (LLH), RR00165 (Yerkes National Primate Research Center), and Associacao Fundo de Incentivo a Psicofarmacologia (AFIP), FAPESP (98/14303) and CNPq (MLA, ST).
Footnotes
Conflict of interest The authors declared no conflict of interest.
Contributor Information
Monica L. Andersen, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd, Atlanta, GA 30329, USA. Department of Psychobiology, Universidade Federal de São Paulo (UNIFESP), São Paulo, SP, Brazil
Eileen Kessler, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd, Atlanta, GA 30329, USA.
Kevin S. Murnane, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd, Atlanta, GA 30329, USA
Jessica C. McClung, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd, Atlanta, GA 30329, USA
Sergio Tufik, Department of Psychobiology, Universidade Federal de São Paulo (UNIFESP), São Paulo, SP, Brazil.
Leonard L. Howell, Email: lhowell@emory.edu, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd, Atlanta, GA 30329, USA. Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, GA, USA
References
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, et al. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psych. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lewis JR, Lattal KM, Berger SP. Modafinil reinstates a cocaine conditioned place preference following extinction in rats. Behav Brain Res. 2009;204:250–253. doi: 10.1016/j.bbr.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenmann S, Xu S, Luhmann UF, Arand M, Berger W, Jung HH, Landolt HP. Pharmacogenetics of modafinil after sleep loss: catechol-O-methyltransferase genotype modulates waking functions but not recovery sleep. Clin Pharmacol Ther. 2009;85:296–304. doi: 10.1038/clpt.2008.222. [DOI] [PubMed] [Google Scholar]
- Castells X, Casas M, Vidal X, Bosch R, Roncero C, Ramos-Quiroga JA, Capellà D. Efficacy of central nervous system stimulant treatment for cocaine dependence: a systematic review and meta-analysis of randomized controlled clinical trials. Addiction. 2007;102:1871–1887. doi: 10.1111/j.1360-0443.2007.01943.x. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by micro-dialysis in awake squirrel monkeys. Psychopharmacology. 2000;148:299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, et al. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Darnaudéry M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV. Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology. 2002;161:387–395. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- Dutta AK, Zhang S, Kolhatkar R, Reith ME. Dopamine transporter as target for drug development of cocaine dependence medications. Eur J Pharmacol. 2003;479:93–106. doi: 10.1016/j.ejphar.2003.08.060. [DOI] [PubMed] [Google Scholar]
- Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology. 1996;126:286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing D, Votaw J, Ely TD, Lambert P, Owens MJ, Camp VM, Malveaux E, Hoffman JM. 18F-labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters. Nucl Med Biol. 2000;27:1–12. doi: 10.1016/s0969-8051(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation: incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Hermant JF, Rambert FA, Duteil J. Awakening properties of modafinil: effect on nocturnal activity in monkeys (Macaca mulatta) after acute and repeated administration. Psychopharmacology (Berl) 1991;103:28–32. doi: 10.1007/BF02244069. [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. The dopamine transporter and cocaine medication development: drug self-administration in nonhuman primates. J Pharmacol Exp Ther. 2001a;298:1–6. [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. Intravenous drug self-administration in nonhuman primates. In: Buccafusco JJ, editor. Methods of behavior analysis in neuroscience. CRC; Boca Raton: 2001b. pp. 91–110. [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;15:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, O’Connor JA, Carroll FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav. 2007;86:45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, Votaw JR, Mello NK, Carroll FI, Howell LL. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav. 2008;90:453–462. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland Henry P, Davis M, Howell LL. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology. 2009;205:237–247. doi: 10.1007/s00213-009-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, et al. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309:959–969. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. Pharmacol Exp Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Mann TM, Williams KE, Pearce PC, Scott EA. A novel method for activity monitoring in small non-human primates. Lab Anim. 2005;39:169–177. doi: 10.1258/0023677053739783. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. Erratum in: Addiction 100:573. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical, and post-marketing surveillance—a review of abuse liability issues. Ann Clin Psych. 2004;16:101–109. doi: 10.1080/10401230490453743. [DOI] [PubMed] [Google Scholar]
- Newman JL, Beardsley PM. Effects of memantine, haloperidol, and cocaine on primary and conditioned reinforcement associated with cocaine in rhesus monkeys. Psychopharmacology. 2006;185:142–149. doi: 10.1007/s00213-005-0282-2. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Dackis CA, Kampman K. Does modafinil produce euphoria? Am J Psychiatry. 2006;163:1109. doi: 10.1176/ajp.2006.163.6.1109. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther. 1989;248:1010–1017. [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H. Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag; New York: 1987. pp. 211–227. [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005;182:186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology. 2003;165:260–269. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votaw JR, Howell LL, Martarello L, Hoffman JM, Kilts CD, Lindsey KP, Goodman MM. Measurement of dopamine transporter occupancy for multiple injections of cocaine using a single injection of [F-18]FECNT. Synapse. 2002;44:203–210. doi: 10.1002/syn.10068. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, Howell LL. Self-administration of cocaine and the cocaine analog RTI-113: relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse. 2002;43:78–85. doi: 10.1002/syn.10018. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse. 2005;58:220–228. doi: 10.1002/syn.20199. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, et al. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]