Abstract
Background:
Tendon surface modification with a synthetic biopolymer, carbodiimide-derivatized hyaluronic acid and gelatin with the addition of lubricin (CHL), has been shown to reduce gliding resistance after tendon repair in an in vitro model. The purpose of the study was to investigate whether CHL would reduce adhesion formation and improve digital function after flexor tendon repair in a canine model in vivo.
Methods:
Sixty dogs were randomly assigned to either a biopolymer-treated group (n = 30) or an untreated control group (n = 30). The second and fifth flexor digitorum profundus tendons from each dog were lacerated fully at the zone-II area and then repaired. Passive synergistic motion therapy was started on the fifth postoperative day and continued until the dogs were killed on day 10, day 21, or day 42. The repaired tendons were evaluated for adhesions, normalized work of flexion, gliding resistance, repair strength, stiffness, and histological characteristics.
Results:
The normalized work of flexion of the repaired tendons treated with CHL was significantly lower than that of the non-CHL-treated repaired tendons at all time points (p < 0.05), and the prevalence of severe adhesions was also significantly decreased in the CHL-treated tendons at day 42 (p < 0.05). However, the repair failure strength and stiffness of the CHL-treated group were also significantly reduced compared with those of the control group at days 21 and 42 (p < 0.05) and the rate of tendon rupture was significantly higher in the treated group than in the control group at day 42 (p < 0.05).
Conclusions:
Treatment with the lubricin-containing gel CHL appears to be an effective means of decreasing postoperative flexor tendon adhesions, but it is also associated with some impairment of tendon healing. Future studies will be necessary to determine if the positive effects of CHL on adhesion formation can be maintained while reducing its adverse effect on the structural integrity of the repaired tendon.
Successful repair of lacerated flexor tendons, as indicated by the return of normal gliding function, remains a great challenge for the hand surgeon1-7. Clinical outcomes have been improved through the development of new suture material8,9, suture techniques10, and postoperative rehabilitation protocols11-15. Despite these advances, however, adhesion formation still occurs, resulting in restricted tendon gliding and reduced hand function16,17.
Recent studies have focused on improving tendon glide and reducing adhesions through the use of either low-friction suture materials and methods10,18 or physical adhesion barriers19, anti-adhesive reagents20, or tendon surface lubricants21-23. One such tendon lubricant is hyaluronic acid. Carbodiimide derivatization can be used to fix hyaluronic acid to the tendon surface, with use of gelatin as an intermediary. This compound, carbodiimide-derivatized hyaluronic-acid gelatin, has been used to decrease the gliding resistance of a repaired or grafted tendon24,25. Recently, carbodiimide-derivatized hyaluronic-acid gelatin has also been found to reduce adhesion formation in a flexor tendon graft model compared with that associated with untreated grafts26. Despite these advances, full restoration of normal digital function has not been achieved.
Lubricin, a mucinous glycoprotein responsible for the boundary lubrication of articular cartilage27,28, recently has been identified on the flexor tendon surface29. It has the same lubricating ability as normal synovial fluid in vitro. It also has considerable anti-adhesive properties30,31 and thus might be particularly attractive as an agent to reduce tendon adhesions postoperatively. Lubricin added to an extrasynovial tendon surface pretreated with carbodiimide-derivatized hyaluronic-acid gelatin further reduces the gliding resistance and was able to maintain a smooth tendon surface after 1000 cycles of simulated flexion/extension tendon motion in a canine model in vitro23. More recently, Taguchi et al. used the same surface-modification technology to improve the gliding ability of a repaired flexor tendon in a canine model in vitro32. However, to the best of our knowledge, lubricin-containing compounds have not been tested in vivo.
The purpose of this study was to investigate the effects of a novel compound containing lubricin, hyaluronic acid, and gelatin after flexor tendon repair in a canine model in vivo. We hypothesized that this compound would decrease postoperative adhesion formation and improve digital function without adverse effects.
Materials and Methods
Study Design
Sixty mixed-breed adult dogs weighing 20 to 25 kg were used. The study was approved by our Institutional Animal Care and Use Committee. Each dog had flexor tendon repairs in one paw, with the dogs randomly assigned to have the repaired tendons treated either with carbodiimide-derivatized hyaluronic-acid gelatin and lubricin (the CHL group, n = 30) or with no additional treatment (the control group, n = 30). The second and fifth flexor digitorum profundus tendons were fully lacerated and repaired in zone II. Rehabilitation with passive digital and wrist motion was initiated at postoperative day 5 and continued daily until the animals were killed at day 10 (n = 20), day 21 (n = 20), or day 42 (n = 20). The repaired tendons and the contralateral, untreated tendons were analyzed with gross evaluation of adhesions; with measurements of work of flexion to assess digital function, gliding resistance for surface assessment, and mechanical strength and stiffness; and with histological analysis to assess tendon healing.
Surgical Procedure and Surface Modification
The dogs were anesthetized with intravenous ketamine and diazepam. One randomly selected forelimb was shaved, scrubbed with povidone-iodine, and sterilely draped. The second and fifth flexor digitorum profundus tendons were approached through a lateral longitudinal incision in the digit. The flexor digitorum profundus tendons were completely lacerated 5 mm distal to the proximal digital flexor pulley and repaired with use of a two-strand modified Pennington technique10 with number-3-0 Ethibond sutures (Ethicon, Somerville, New Jersey). A simple running circumferential epitenon suture of 6-0 nylon (Ethicon) was used to reinforce the repair.
Following repair, the tendons in the treatment group were treated with carbodiimide-derivatized hyaluronic-acid gelatin and lubricin (CHL). First, a 95% solution of 1% sodium hyaluronate (1.5 × 106 molecular weight; Acros Organics, Geel, Belgium), 10% gelatin (Sigma Chemical, St. Louis, Missouri), 1% 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (Sigma Chemical), and 1% N-hydroxysuccinimide (NHS; Pierce Biotechnology, Rockford, Illinois), in 0.1M Mes (2-[N-morpholino]ethanesulfonic acid) buffer (Sigma Chemical), pH 6.0, was prepared as described by Tanaka et al.33. This compound was then used to coat the surface of the repaired tendon. After gelation of this compound (which took about five minutes), the excess gel was removed by moving the repaired tendon back and forth under the proximal pulley for five cycles. A volume of 0.2 mL of 260 μg/mL lubricin was applied to the surface of the CHL-treated tendon. In the control group, the surface of the repaired tendons was rinsed with saline solution only. Incisions were closed in layers (subcutaneous tissue and skin, without closure of the fibro-osseous sheath), and a sterile dressing was applied to the paw.
Following flexor tendon repair, a high radial neurectomy was performed through a lateral humeral incision. Denervation of the elbow and wrist extensors prevented the dogs from bearing weight on the operatively treated limb. This method has been shown to be well tolerated, safe, and reliable34,35 and avoids the need for a spica cast after surgery. A custom-made soft jacket was used to maintain the surgically treated paw under the chest. Intramuscular antibiotics were administered perioperatively. The animals were allowed immediate cage activity. The jacket was removed daily for wound care. Each dog was treated with a modified passive synergistic rehabilitation therapy protocol. This consisted of passive wrist extension with digital joint flexion and wrist flexion with digital joint extension for five minutes followed by passive metacarpophalangeal joint extension with proximal and distal interphalangeal joint flexion and metacarpophalangeal joint flexion with proximal and distal interphalangeal joint extension for another five minutes. The therapy was started on postoperative day 536 and continued twice a day, seven days per week, until the animals were killed.
Gross Evaluation of Adhesions
During dissection, the repaired tendons were grossly evaluated for adhesions between the tendon and its surrounding tissues, including the flexor sheath and tendon beds. The flexor sheath was opened through an area not included in the operation, away from the suture site, with care taken to avoid interfering with any adhesions between the tendon and sheath. With loupe magnification, adhesion formation was classified in each of two regions, between the tendon and sheath and between the tendon and bone, and as belonging in one of four categories: none (no adhesion observed between the tendon and its surrounding tissues), mild (adhesion involving <2 mm of the length between the tendon and the surrounding tissues that is easy to separate), moderate (adhesion of 2 to 4 mm between the tendon and the surrounding tissues that can be separated), or severe (adhesion of >4 mm between the tendon and the surrounding tissues that is difficult to separate). The adhesions were evaluated and graded by two of the investigators (C.Z. and R.L.K.), who reached a consensus regarding the final score in each region. The evaluation was not blinded. While this scoring method has been used previously for adhesion assessment35,37, we are unaware of any formal study of its reliability.
Any gap between tendon ends was measured with calipers (Mitutoyo, Tokyo, Japan) with a precision of ±0.02 mm. The gap size was recorded as small (1 to 3 mm) or large (>3 mm), on the basis of the work of Gelberman et al., which showed that gaps smaller than 3 mm were not associated with problems in tendon healing in a dog model38.
Measurement of Digital Work of Flexion
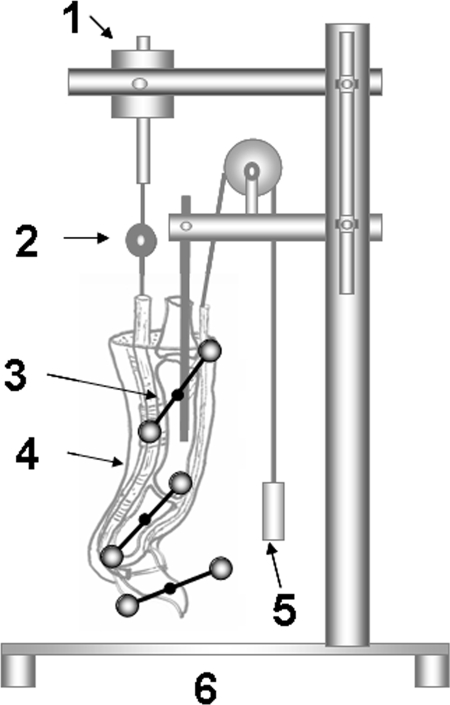
One of the two digits operated on in each paw was randomly assigned for evaluation of digital function by measuring the work of flexion26. The flexor digitorum profundus tendons were carefully exposed at the proximal metacarpal level, transected, and sutured to a cable connected to a load transducer. The repair site, surrounding tendon sheath, and overlying skin were all preserved. A Kirschner wire was inserted longitudinally through the metacarpal and the proximal phalanx to fix the metacarpophalangeal joint in extension. T-shaped pins, each containing two reflective markers, were fixed to the proximal, middle, and distal phalanges. The prepared digit was then mounted on the testing device by fixing the proximal Kirschner wire to a custom jig (Fig. 1). The actuator pulled the tendon proximally at a rate of 2 mm/sec, causing digital flexion. Digital motion was recorded by two orthogonal video cameras, and the marker motion was analyzed by motion analysis software (Motion Analysis Corporation, Santa Rosa, California). The change in angle between the distal phalangeal “T” and the middle phalangeal “T” determined the distal interphalangeal joint motion, and the change in angle between the middle phalangeal “T” and the proximal phalangeal “T” determined the proximal interphalangeal joint motion.
Fig. 1.
Work-of-flexion testing device. 1 = actuator; 2 = load transducer; 3 = T-shaped pin with a pair of reflective markers mounted on the distal, middle, and proximal phalanges; 4 = specimen; 5 = weight (50 g); and 6 = frame.
Work-of-flexion data were calculated from the area under the tendon displacement versus loading curve during digital flexion. The end point of digital flexion is difficult to determine on the basis of tendon excursion because of the possibility of tendon elongation, especially at the end of full flexion. The force/displacement data were truncated at the point where the distal interphalangeal angle reached 40° of flexion. A limit of 40° was chosen because it is less than, but close to, full flexion in this canine model. In order to precisely assess digital function, the work of flexion was divided by the total number of degrees of proximal and distal interphalangeal joint motion for normalization; this is termed normalized work of flexion and has been discussed previously36.
Measurement of Tendon Gliding Resistance
After measurement of the work of flexion, the repaired tendons were further dissected, with the proximal pulley kept intact. The repaired tendons were also evaluated at this point for gap size, with use of calipers to measure the distance, if any, between the repaired tendon ends. The gliding resistance between the tendon repair site and the proximal pulley was then measured with use of a custom tendon-pulley frictional testing device, as previously described39. Briefly, the specimen with the proximal pulley and repaired tendon was mounted on the device with the Kirschner wire that had been used to fix the metacarpophalangeal joint. The device consisted of one mechanical actuator with a linear potentiometer and load transducer that connected to the proximal end of the repaired tendon. The second load transducer connected to the distal end of the repaired tendon with a 500-g weight attached. The tendon was pulled proximally by the actuator against the weight at a rate of 2 mm/sec. The force differential between the proximal and distal tendon ends represents the gliding resistance.
Measurement of Repair Strength
To measure breaking strength, the repaired tendons were secured to a servohydraulic testing machine (MTS Systems, Eden Prairie, Minnesota) and distracted to failure at a rate of 20 mm/min. A differential variable reluctance transducer (MicroStrain, Williston, Vermont) was attached to the tendon through two barbed pins inserted perpendicularly into the tendon to measure gap formation during testing. The repair site was centered between the two pins. Tensile force, grip-to-grip displacement, and gap displacement measured with the transducer were collected at a rate of 20 Hz. The maximum breaking force was recorded. In addition, the repair stiffness was calculated from the slope of the linear region of the force versus gap-formation curve (as measured with the transducer) to measure the resistance to gap formation.
Histological Analysis
Two tendons in each group were evaluated histologically after they were sectioned longitudinally through the region of the repair site. The harvested tendons were fixed in formalin in a clamp that preserved their original length and prevented rotational distortion of the tendon during fixation. The specimens were then prepared for histological evaluation by the Mayo Histology Core Facility. Seven-micrometer-thick sections were cut from the tendons after the tendon was embedded in paraffin. The sections were stained with hematoxylin and eosin and then evaluated to note the presence of cells bridging the tendon repair site and the appearance of the tendon surface.
Statistical Analysis
One-way or two-way analysis of variance was used to analyze the differences in normalized work of flexion, gliding resistance, repair strength, and stiffness between the repaired tendons. The factors were CHL treatment (with or without) and three time points. With a sample size of ten, we had 80% power at a significance level of p < 0.05 to detect a 46% change in gliding resistance, a 37% change in ultimate strength, and a 44% change in normalized work of flexion. Effects of this magnitude were expected to be clinically relevant, and the sample sizes were set prior to the study.
The Fisher exact test was used to compare gap formation, rupture, and adhesion categories between the CHL-treated and non-CHL-treated repair specimens and also among the three time points. We had an 80% power at a significance level of p < 0.05 to detect an odds ratio of 6.4 or 2.9 when the sample size in each group was twenty or sixty, respectively. Effects of this magnitude were expected to be clinically relevant, and the sample sizes were set prior to the study. Any p value of <0.05 was reported as significant.
Source of Funding
This study was funded by a grant from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR44391).
Results
Gross Evaluation and Adhesion
Thirteen (22%) of the sixty repaired tendons in the CHL group ruptured, compared with two (3%) in the control (non-CHL-treated repair) group (p < 0.05) (Table I). The rupture rate of the CHL-treated tendons was significantly higher than that of the control tendons at day 42 (p < 0.05). Seventeen (28%) and fourteen (23%) of the repaired tendons in the CHL and control groups, respectively, had gap formation. There was no significant difference in the prevalences of either small or large gaps between the CHL-treated and control tendons.
TABLE I.
Gap Formation and Rupture by Treatment Group and Time
| Control Group |
CHL Group |
|||||||
| Day 10 (N = 20) | Day 21 (N = 20) | Day 42 (N = 20) | Total (N = 60) | Day 10 (N = 20) | Day 21 (N = 20) | Day 42 (N = 20) | Total (N = 60) | |
| 1-3 mm gap | 5 | 5 | 1 | 11 | 5 | 2 | 2 | 9 |
| >3 mm gap | 2 | 1 | 0 | 3 | 2 | 2 | 4 | 8 |
| Rupture | 1 | 1 | 0* | 2* | 3 | 3 | 7* | 13* |
| Total | 8 | 7 | 1 | 16 | 10 | 7 | 13 | 30 |
There was a significant difference between the CHL and control groups.
Five tendons from the control group and twelve tendons from the CHL group were excluded from adhesion scoring because of tendon rupture without adhesions, wound dehiscence, or infection. Sixteen (33%) of the forty-eight tendons in the CHL group had adhesions, compared with thirty-one (56%) of the fifty-five tendons in the control group (p < 0.05) (Table II). Three (6%) of the forty-eight tendons in the CHL group had severe adhesions, compared with eleven (20%) of the fifty-five tendons in the control group (p < 0.05). In the control group, there was an increasing trend for adhesion formation with time, with adhesions seen in eight of the tendons on day 10, eight on day 21, and fifteen on day 42, but these differences were not significant. There was a similar, although also not significant, trend in the CHL group, with adhesion formation in five tendons (31%) on day 10, in three on day 21, and in eight on day 42. However, in the control group, the proportion of tendons with severe adhesions at day 42 (seven of twenty) was significantly higher than the proportion of tendons at day 10 (one of eighteen) (p < 0.05) (Table II).
TABLE II.
Tendons in Different Adhesion Categories
| Control Group |
CHL Group |
|||||||
| Adhesion Category | Day 10 | Day 21 | Day 42 | Total | Day 10 | Day 21 | Day 42 | Total |
| None | 10 | 9 | 5 | 24 | 11 | 12 | 9 | 32 |
| Mild | 3 | 3 | 4 | 10 | 5 | 0 | 3 | 8 |
| Moderate | 4 | 2 | 4 | 10 | 0 | 2 | 3 | 5 |
| Severe | 1* | 3 | 7* | 11† | 0 | 1 | 2 | 3† |
| Total | 18 | 17 | 20 | 55‡ | 16 | 15 | 17 | 48‡ |
There was a significant difference between the time points.
There was a significant difference between the CHL and control groups.
Five tendons from the control group and twelve tendons from the CHL group were excluded from adhesion scoring because of tendon rupture without adhesions, wound dehiscence, or infection.
Normalized Work of Flexion
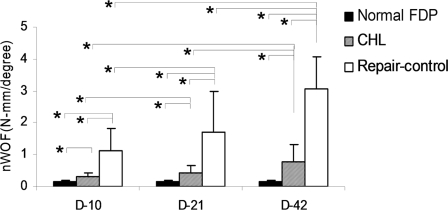
The value for the normalized work of flexion of the normal digit harvested from the untreated, contralateral paw was significantly lower than that in both repair groups (p < 0.05). The normalized work-of-flexion value of the CHL-treated tendons was significantly lower than that of the non-CHL-treated repaired tendons at all time points (p < 0.05). In both the CHL-treated and the non-CHL-treated repaired-tendon groups, the normalized work-of-flexion value at day 42 was significantly higher than that at day 21 and that at day 10 (p < 0.05). There was also a significant difference between day 21 and day 10 in these groups (Fig. 2).
Fig. 2.
The work of flexion normalized by the proximal interphalangeal and distal interphalangeal angle (nWOF) of repaired flexor digitorum profundus (FDP) tendons with or without CHL treatment and of the normal, contralateral digit at day 10, day 21, and day 42. An asterisk denotes a significant difference (p < 0.05) among the different treatments (normal, control, and CHL) at each time point or among the same treatments at different time points (days 10, 21, and 42). The error bars indicate one standard deviation.
Gliding Resistance, Repair Strength, and Histological Findings
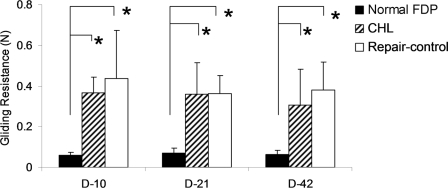
The gliding resistance of the repaired tendons in both the CHL-treated and the non-CHL-treated group was significantly higher than the gliding resistance of the normal contralateral flexor digitorum profundus tendon (p < 0.05). There was no significant difference in gliding resistance between the CHL and control groups at any time point (Fig. 3).
Fig. 3.
The gliding resistance of repaired flexor digitorum profundus (FDP) tendons with or without CHL treatment and of the normal, contralateral digit at day 10, day 21, and day 42. An asterisk denotes a significant difference (p < 0.05) among the different treatments (normal, control, and CHL) at each time point or among the same treatments at different time points (days 10, 21, and 42). The error bars indicate one standard deviation.
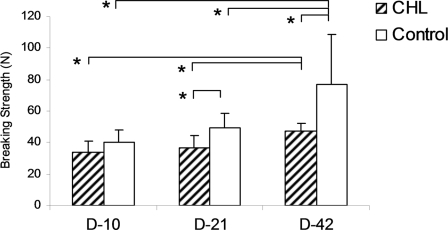
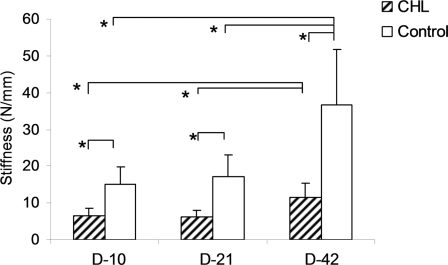
The value for maximum breaking strength in the CHL group was significantly lower than that in the control group at day 21 and day 42 (p < 0.05), but not at day 10. The value for stiffness in the CHL group was significantly lower than that in the control group at all time points (p < 0.05). In both the CHL and control groups, the maximum breaking strength and stiffness at day 42 were significantly greater than those at day 21 and day 10 (p < 0.05). However, there was not a significant difference between day 21 and day 10 (Figs. 4 and 5). No slippage of the differential variable reluctance transducer was observed during testing.
Fig. 4.
The breaking strength of the repaired tendons with or without CHL treatment at day 10, day 21, and day 42. An asterisk denotes a significant difference (p < 0.05) between the different treatments (control and CHL) at each time point or among the same treatments at different time points (days 10, 21, and 42). The error bars indicate one standard deviation.
Fig. 5.
The stiffness of the repaired tendons with or without CHL at day 10, day 21, and day 42. An asterisk denotes a significant difference (p < 0.05) between the different treatments (control and CHL) at each time point or among the same treatments at different time points (days 10, 21, and 42). The error bars indicate one standard deviation.
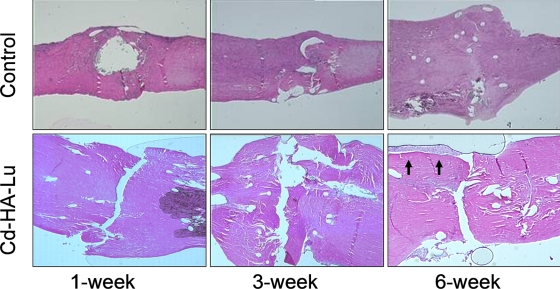
Histologically, the CHL-treated tendons displayed evidence of delayed healing, with large gaps visible between the repaired tendon ends, even at day 42. The repaired tendons that were not treated with CHL showed progressive healing, with cellular bridging of the repair site by day 42 (Fig. 6).
Fig. 6.
Representative histological sections showing delayed healing and gap formation at the repair site in the CHL-treated tendons (Cd-HA-Lu) (hematoxylin and eosin, ×40). Although epitenon cell proliferation can be observed on the tendon surface near the repair site on day 42 in the CHL-treated tendons, the cell layers are clearly separated from the tendon surface (black arrows). In contrast, the healing is more advanced in the corresponding non-CHL-treated repaired tendons.
Discussion
As we hypothesized, CHL effectively decreased adhesion formation after flexor tendon repair up to six weeks postoperatively, leading to improved digital function compared with that of the repaired tendon without CHL treatment. This beneficial effect may be due to several factors, including increased lubrication of the gliding surface, a smoother gliding surface, and the intrinsic anti-adhesive properties of CHL. Increased gliding ability has been shown to promote more effective postoperative rehabilitation18, and a smoother surface may decrease the potential for adhesions forming on tendon surfaces40. Recently, Chang et al. studied the anti-adhesive properties of lubricin with atomic force microscopy and demonstrated that lubricin develops strong repulsive interactions between two surfaces as a result of its amphiphilic and adsorbing properties to either hydrophobic or hydrophilic surfaces31. In addition to its physical properties, the biological anti-adhesive behavior of lubricin has been previously demonstrated by its prevention of cartilage repair of large defects30,41. Using a cartilage repair ex vivo model, Englert et al. demonstrated that lubricin has the desirable effect of preventing fusion of apposing surfaces of articulating cartilage but has the undesirable effect of inhibiting integrative cartilage repair30. To our knowledge, the current study is the first to show that these phenomena also occur in vivo in tendon repairs, with both the favorable result of reducing adhesions and the unfavorable result of inhibiting intrinsic and extrinsic tendon healing.
We measured two different aspects of tendon function in this study: gliding resistance (a proxy for friction) and work of flexion (a composite measure including not only friction but also adhesions, joint stiffness, and the weight of the digit). We believe that the gliding resistance measured in the current study was mainly affected by two factors, one favorable—namely, the lubricant attached onto the tendon surface—and one unfavorable—namely, the tendon surface roughness resulting from the laceration and sutures39. Although the gliding resistance of the repaired flexor tendon was decreased after CHL surface modification in vitro32, we did not observe this effect at ten days or longer in vivo. One possible explanation for the difference between the in vitro and in vivo results is lubricin degradation in vivo, which is supported by the observation that the half life of lubricin is about six days42. Also, in their in vitro study, Taguchi et al.32 determined the effect of lubricin on gliding resistance by comparing data from before and after surface modification of the same repaired tendons. This normalized comparison method eliminates variation due to the repair technique among different individual tendons and repairs, which could have a greater effect than CHL. In vivo, it is unfortunately not possible to measure the gliding resistance after repair but before application of the CHL, as the measurement can be obtained only after the digit is amputated from the original specimen. Because Taguchi et al. performed their evaluations within digits, their study had far greater power than ours, which examined differences between digits. While we could have matched their power by increasing the number of experimental animals, we judged the costs, both financial and in terms of animal use, not to be worth the potential benefits of increased statistical power.
We believe that the higher rupture rate and lower work-of-flexion values (a measure of adhesion breaking strength) can both be explained by the use of lubricin in our CHL formulation. Lubricin has both lubricating and anti-adhesive properties. When lubricin is applied to the tendon surface, these properties appear to effectively reduce adhesion formation. It also blocks any extrinsic source of tendon healing. Should some of the CHL enter the repair site, it could just as easily block adhesion of the healing tendon ends to each other—i.e., block the intrinsic tendon-healing mechanism. We believe that there was indeed such leakage into the repair site in some of our cases, despite our best efforts to exclude the CHL from the repair site itself. Clearly, this is a problem that must be addressed before a compound like CHL can be used clinically. If it is used as designed and if it is effective when so used, extrinsic healing is essentially blocked, leaving intrinsic healing as the only available repair method.
If the desired effect of CHL treatment is to block adhesions (and therefore extrinsic healing, which is mediated by adhesions), it becomes critically important that the intrinsic healing mechanism be maintained and, if possible, augmented. Enhancement of flexor tendon intrinsic healing combined with better protection of the lacerated tendon ends from CHL leakage would be one strategy for providing low-friction tendon junctures. Cell-based and growth-factor-based therapies have recently demonstrated encouraging results in terms of speeding tendon healing43,44. Gelatin is an appropriate medium to carry and deliver cells and/or growth factors to the laceration site45,46. A gelatin “patch” could also seal the repair site to prevent CHL intrusion. Additional studies are necessary to investigate this strategy and to determine if it would improve outcomes after flexor tendon repair with CHL surface modification.
This study had several other limitations. First, although we carried out histological evaluations, we did not perform immunohistochemistry staining to assess for residual lubricin on the tendon surfaces after the surgery. Second, we did not analyze other tendon biomarkers, such as type-I and III collagen, as we focused on the biomechanical evaluations. Finally, we did not compare CHL with other possible treatments and thus are unable to sort out the effects of hyaluronic acid and lubricin individually. However, a previous study of an in vivo tendon graft model by Tanaka et al. demonstrated that carbodiimide-derivatized hyaluronic acid had no adverse effects on either healing at the distal tendon-bone interface or graft cellularity47.
In conclusion, CHL surface treatment significantly decreased the prevalence of postoperative adhesions and improved digital function in our canine in vivo model of flexor tendon repair. However, these positive outcomes of CHL were achieved at the cost of impaired tendon healing, decreasing the repair strength and significantly increasing the rate of gapping and rupture of the repairs. Future research is needed to investigate potential methods of eliminating these adverse effects of CHL on tendon healing while preserving its anti-adhesive properties.
Footnotes
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR44391). Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1.Rosberg HE, Carlsson KS, Höjgård S, Lindgren B, Lundborg G, Dahlin LB. What determines the costs of repair and rehabilitation of flexor tendon injuries in zone II? A multiple regression analysis of data from southern Sweden. J Hand Surg Br. 2003;28:106-12 [DOI] [PubMed] [Google Scholar]
- 2.Strickland JW. Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg Am. 2000;25:214-35 [DOI] [PubMed] [Google Scholar]
- 3.Boyer MI, Harwood F, Ditsios K, Amiel D, Gelberman RH, Silva MJ. Two-portal repair of canine flexor tendon insertion site injuries: histologic and immunohistochemical characterization of healing during the early postoperative period. J Hand Surg Am. 2003;28:469-74 [DOI] [PubMed] [Google Scholar]
- 4.Amadio PC. What's new in hand surgery. J Bone Joint Surg Am. 2007;89:460-5 [DOI] [PubMed] [Google Scholar]
- 5.Ejeskär A. Flexor tendon repair in no man's land. II. Early versus late secondary tendon repair ad modum Kleinert. Scand J Plast Reconstr Surg. 1980;14:279-83 [DOI] [PubMed] [Google Scholar]
- 6.Strickland JW. Results of flexor tendon surgery in zone II. Hand Clin. 1985;1:167-79 [PubMed] [Google Scholar]
- 7.Silfverskiöld KL, May EJ. Gap formation after flexor tendon repair in zone II. Results with a new controlled motion programme. Scand J Plast Reconstr Surg Hand Surg. 1993;27:263-8 [PubMed] [Google Scholar]
- 8.Komatsu F, Mori R, Uchio Y. Optimum surgical suture material and methods to obtain high tensile strength at knots: problems of conventional knots and the reinforcement effect of adhesive agent. J Orthop Sci. 2006;11:70-4 [DOI] [PubMed] [Google Scholar]
- 9.Miller B, Dodds SD, deMars A, Zagoreas N, Waitayawinyu T, Trumble TE. Flexor tendon repairs: the impact of FiberWire on grasping and locking core sutures. J Hand Surg Am. 2007;32:591-6 [DOI] [PubMed] [Google Scholar]
- 10.Tanaka T, Amadio PC, Zhao C, Zobitz ME, Yang C, An KN. Gliding characteristics and gap formation for locking and grasping tendon repairs: a biomechanical study in a human cadaver model. J Hand Surg Am. 2004;29:6-14 [DOI] [PubMed] [Google Scholar]
- 11.Zhao C, Amadio PC, Tanaka T, Yang C, Ettema AM, Zobitz ME, An KN. Short-term assessment of optimal timing for postoperative rehabilitation after flexor digitorum profundus tendon repair in a canine model. J Hand Ther. 2005;18:322-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erhard L, Schultz FM, Zobitz ME, Zhao C, Amadio PC, An KN. Reproducible volar partial lacerations in flexor tendons: a new device for biomechanical studies. J Biomech. 2002;35:999-1002 [DOI] [PubMed] [Google Scholar]
- 13.Chow JA, Thomes LJ, Dovelle S, Monsivais J, Milnor WH, Jackson JP. Controlled motion rehabilitation after flexor tendon repair and grafting. A multi-centre study. J Bone Joint Surg Br. 1988;70:591-5 [DOI] [PubMed] [Google Scholar]
- 14.Silfverskiöld KL, May EJ. Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion. J Hand Surg Am. 1994;19:53-60 [DOI] [PubMed] [Google Scholar]
- 15.Vucekovich K, Gallardo G, Fiala K. Rehabilitation after flexor tendon repair, reconstruction, and tenolysis. Hand Clin. 2005;21:257-65 [DOI] [PubMed] [Google Scholar]
- 16.Tang JB. Clinical outcomes associated with flexor tendon repair. Hand Clin. 2005;21:199-210 [DOI] [PubMed] [Google Scholar]
- 17.Coats RW, 2nd, Echevarría-Oré JC, Mass DP. Acute flexor tendon repairs in zone II. Hand Clin. 2005;21:173-9 [DOI] [PubMed] [Google Scholar]
- 18.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma. 2001;51:917-21 [DOI] [PubMed] [Google Scholar]
- 19.Mentzel M, Hoss H, Keppler P, Ebinger T, Kinzl L, Wachter NJ. The effectiveness of ADCON-T/N, a new anti-adhesion barrier gel, in fresh divisions of the flexor tendons in Zone II. J Hand Surg Br. 2000;25:590-2 [DOI] [PubMed] [Google Scholar]
- 20.Moran SL, Ryan CK, Orlando GS, Pratt CE, Michalko KB. Effects of 5-fluorouracil on flexor tendon repair. J Hand Surg Am. 2000;25:242-51 [DOI] [PubMed] [Google Scholar]
- 21.Moro-oka T, Miura H, Mawatari T, Kawano T, Nakanishi Y, Higaki H, Iwamoto Y. Mixture of hyaluronic acid and phospholipid prevents adhesion formation on the injured flexor tendon in rabbits. J Orthop Res. 2000;18:835-40 [DOI] [PubMed] [Google Scholar]
- 22.Momose T, Amadio PC, Sun YL, Zhao C, Zobitz ME, Harrington JR, An KN. Surface modification of extrasynovial tendon by chemically modified hyaluronic acid coating. J Biomed Mater Res. 2002;59:219-24 [DOI] [PubMed] [Google Scholar]
- 23.Taguchi M, Sun YL, Zhao C, Zobitz ME, Cha CJ, Jay GD, An KN, Amadio PC. Lubricin surface modification improves extrasynovial tendon gliding in a canine model in vitro. J Bone Joint Surg Am. 2008;90:129-35 [DOI] [PubMed] [Google Scholar]
- 24.Yang C, Amadio PC, Sun YL, Zhao C, Zobitz ME, An KN. Tendon surface modification by chemically modified HA coating after flexor digitorum profundus tendon repair. J Biomed Mater Res. 2004;68:15-20 [DOI] [PubMed] [Google Scholar]
- 25.Sun YL, Yang C, Amadio PC, Zhao C, Zobitz ME, An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22:984-9 [DOI] [PubMed] [Google Scholar]
- 26.Zhao C, Sun YL, Amadio PC, Tanaka T, Ettema AM, An KN. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid. An in vivo canine model. J Bone Joint Surg Am. 2006;88:2181-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swann DA, Sotman S, Dixon M, Brooks C. The isolation and partial characterization of the major glycoprotein (LGP-I) from the articular lubricating fraction from bovine synovial fluid. Biochem. 1977;161:473-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jay GD. Characterization of a bovine synovial fluid lubricating factor. I. Chemical, surface activity and lubricating properties. Connect Tissue Res. 1992;28:71-88 [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Berger EJ, Zhao C, An KN, Amadio PC, Jay G. Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res. 2006;47:215-21 [DOI] [PubMed] [Google Scholar]
- 30.Englert C, McGowan KB, Klein TJ, Giurea A, Schumacher BL, Sah RL. Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis Rheum. 2005;52:1091-9 [DOI] [PubMed] [Google Scholar]
- 31.Chang DP, Abu-Lail NI, Guilak F, Jay GD, Zauscher S. Conformational mechanics, adsorption, and normal force interactions of lubricin and hyaluronic acid on model surfaces. Langmuir. 2008;24:1183-93 [DOI] [PubMed] [Google Scholar]
- 32.Taguchi M, Sun YL, Zhao C, Zobitz ME, Cha CJ, Jay GD, An KN, Amadio PC. Lubricin surface modification improves tendon gliding after tendon repair in a canine model in vitro. J Orthop Res. 2009;27:257-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka T, Sun YL, Zhao C, Zobitz ME, An KN, Amadio PC. Optimization of surface modifications of extrasynovial tendon to improve its gliding ability in a canine model in vitro. J Orthop Res. 2006;24:1555-61 [DOI] [PubMed] [Google Scholar]
- 34.Bishop AT, Cooney WP, 3rd, Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26:301-12 [DOI] [PubMed] [Google Scholar]
- 35.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. Effect of synergistic wrist motion on adhesion formation after repair of partial flexor digitorum profundus tendon lacerations in a canine model in vivo. J Bone Joint Surg Am. 2002;84:78-84 [PubMed] [Google Scholar]
- 36.Zhao C, Amadio PC, Paillard P, Tanaka T, Zobitz ME, Larson DR, An KN. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2004;86:320-7 [DOI] [PubMed] [Google Scholar]
- 37.Rothkopf DM, Webb S, Szabo RM, Gelberman RH, May JW., Jr An experimental model for the study of canine flexor tendon adhesions. J Hand Surg Am. 1991;16:694-700 [DOI] [PubMed] [Google Scholar]
- 38.Gelberman RH, Boyer MI, Brodt MD, Winters SC, Silva MJ. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am. 1999;81:975-82 [DOI] [PubMed] [Google Scholar]
- 39.Uchiyama S, Coert JH, Berglund L, Amadio PC, An KN. Method for the measurement of friction between tendon and pulley. J Orthop Res. 1995;13:83-9 [DOI] [PubMed] [Google Scholar]
- 40.Zhao C, Amadio PC, Momose T, Zobitz ME, Couvreur P, An KN. Remodeling of the gliding surface after flexor tendon repair in a canine model in vivo. J Orthop Res. 2002;20:857-62 [DOI] [PubMed] [Google Scholar]
- 41.Schaefer DB, Wendt D, Moretti M, Jakob M, Jay GD, Heberer M, Martin I. Lubricin reduces cartilage—cartilage integration. Biorheology. 2004;41:503-8 [PubMed] [Google Scholar]
- 42.Glasson SS, Rivera-Burmudez M, Tejada J, Mark L, Strassle B, Whiteside G, Resmini C, Blanchet T, Zollner R, Bendele A, Morris E, Flannery C. Intra-articular lubricin supplementation modifies disease progression and ameliorates pain in a rat model of osteoarthritis. Presented at the 55th Annual Meeting of the Orthopaedic Research Society; 2009 Feb 22-25; Las Vegas, NV. Poster no 1116 [Google Scholar]
- 43.Kryger GS, Chong AK, Costa M, Pham H, Bates SJ, Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg Am. 2007;32:597-605 [DOI] [PubMed] [Google Scholar]
- 44.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, Das R, Silva MJ. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg Am. 2007;32:373-9 [DOI] [PubMed] [Google Scholar]
- 45.Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B, Caplan AI. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267-77 [DOI] [PubMed] [Google Scholar]
- 46.Luo J, Mass DP, Phillips CS, He TC. The future of flexor tendon surgery. Hand Clin. 2005;21:267-73 [DOI] [PubMed] [Google Scholar]
- 47.Tanaka T, Zhao C, Sun YL, Zobitz ME, An KN, Amadio PC. The effect of carbodiimide-derivatized hyaluronic acid and gelatin surface modification on peroneus longus tendon graft in a short-term canine model in vivo. J Hand Surg Am. 2007;32:876-81 [DOI] [PubMed] [Google Scholar]