Abstract
Objective The goal of this study was to examine respiratory sinus arrhythmia (RSA), an indicator of parasympathetic nervous system-linked cardiac activity, and skin conductance level (SCL), a sympathetic indicator, as moderators of the link between child maltreatment and adolescent aggression. Method Participants were 234 maltreated (48.3% male) and 128 (57.8% male) comparison youth aged 9–16 years participating in wave 2 of a longitudinal study. Results Regression analyses suggest that among boys, high RSA may be protective against the effects of maltreatment on aggressive behavior. Among girls, the moderating effect of RSA was further moderated by SCL reactivity such that low levels of both baseline RSA and SCL reactivity, or conversely high levels of both baseline RSA and SCL reactivity, exacerbated the link between maltreatment and aggression. Conclusions High RSA may protect against the effects of maltreatment on aggressive behavior, though this effect may be moderated by SCL reactivity among girls.
Keywords: adolescents, behavior problems, child abuse and neglect.
Child maltreatment is associated with numerous behavior and emotional problems, including aggression, delinquency, and depression (Kendall-Tackett, Williams, & Finkelhor, 1993; Margolin & Gordis, 2000; Widom, 1989). However, not all maltreated youth exhibit problems. Individual factors may moderate outcomes (Flores, Cicchetti, & Rogosch, 2005; Margolin & Gordis, 2004). Much remains unknown about why some youth are more affected by maltreatment than are others. Certain lines of research suggest the importance of autonomic nervous system (ANS) variables in moderating the effects of stressful experiences (e.g., Katz & Gottman, 1995; Raine, 2005). The present study examines indicators of sympathetic (SNS) and parasympathetic (PNS) nervous system activity as moderators of the link between maltreatment and youth aggression.
Child Maltreatment and Aggressive Behavior
The link between maltreatment and aggressive behavior is well-established (e.g., Cullerton-Sen et al., 2008; George & Main, 1979; Margolin & Gordis, 2000; Widom, 1989). Maltreatment may disrupt affect regulation and may cause children to interpret ambiguous social cues as threatening and to respond to them aggressively (Cullerton-Sen et al., 2008). Traumatic abuse may cause anger and fear in relationships and a need to re-enact trauma with aggression (Haapasalo & Pokela, 1999).
Various types of maltreatment are linked with aggressive behavior. Links between physical abuse and aggressive behavior are well-documented (Dodge, Bates, & Pettit, 1990; Haskett & Kistner, 1991; Trickett, 1993). Physical abuse teaches that aggression is acceptable (Dodge, Pettit, & Bates, 1997) and may cause difficulties with emotion regulation (Repetti, Taylor, & Seeman, 2002). Sexual abuse can lead to hyper-reactivity to abuse-related cues and difficulty managing related emotions (Trickett, McBride-Chang, & Putnam, 1994) and is also linked with aggression (Einbender & Friedrich, 1989; Kendall-Tackett et al., 1993; Trickett & Gordis, 2004; Trickett et al., 1994). Though emotional abuse and neglect have received less attention, both are linked with aggression (Hildyard & Wolfe, 2002; Shaffer, Yates, & Egeland, 2009). Different types of maltreatment often co-occur (Trickett, Mennen, Kim, & Sang, 2009). Here we examine the overall effect of maltreatment on aggression.
Autonomic Nervous System Activity and Aggressive Behavior
SNS and PNS activity are related to aggressive behavior. SNS activity leads to excitation of the palmer eccrine sweat glands and thus increased conductivity in the surface of the skin. Low skin conductance level (SCL) is related to aggression, particularly among males (Fung et al., 2005; Lorber, 2004; McBurnett, Harris, Swanson, Pfiffner, & Tamm, 1993; Raine & Venables, 1984; van Goozen, Fairchild, Snoek, & Harold, 2007). Various theories explain this link. Low SCL may indicate fearlessness and poor inhibition of aggressive impulses (Raine, 2005; Shannon, Beauchaine, Brenner, Neuhaus, & Gatzke-Kopp, 2007; van Goozen & Fairchild, 2008; van Goozen et al., 2007). Central nervous system substrates of low tonic SCL activity may be aversive, leading to sensation-seeking behaviors, including aggression (Gatzke-Kopp, Raine, Loeber, Stouthamer-Loeber, & Steinhauer, 2002; van Goozen et al., 2007). Low SCL may also indicate impaired learning to avoid punishment (Raine, Lencz, Bihrle, LaCasse, & Colletti, 2000; Shannon, et al., 2007). Aggressive boys exhibit low SNS-linked cardiac activity in response to monetary reward, suggesting low reward sensitivity (Beauchaine, Hong, & Marsh, 2008). However, findings regarding low sympathetic indicators and aggressive behavior have not been completely consistent (Lorber, 2004). Although research with clinical populations suggests that low baseline SCL predicts aggression, high SCL reactivity to challenge has also been linked with aggressive behavior, particularly among non clinic samples (El-Sheikh, Erath, & Keller, 2007; Hubbard et al., 2002). Sex may also affect this relation. Beauchaine et al. (2008) found that higher skin conductance responding related to more aggression among girls. High SCL may reflect downstream effects of CNS processes related to fear, hostility, and aggression (Kagan, Reznick, & Snidman, 1987; van Goozen & Fairchild, 2008).
PNS activity also has been linked to aggression. PNS-linked cardiac activity can be measured by respiratory sinus arrhythmia (RSA), the variability of heart rate across the respiration cycle due to the influence of the vagus nerve on the sinoatrial node (Beauchaine, 2001). RSA relates positively to emotion regulation capacity and responsiveness and negatively to behavior problems (Beauchaine, 2001; Eisenberg et al., 1995; El-Sheikh et al., 2007). However, some findings have been inconsistent. For example, Dietrich, Riese, Sondeijker et al. (2007) found higher RSA to relate to externalizing problems. Sex may also affect this relation (e.g., Eisenberg et al., 1995). Beauchaine et al. (2008) report links between low RSA and aggression among boys but not girls. In addition to baseline RSA, researchers also have examined RSA reactivity to challenge. In response to stress, one would expect vagal withdrawal and lower RSA (Butler, Wilhelm, & Gross, 2006). Butler et al. (2006) report that increased RSA during social interaction relates to emotional suppression. Some studies suggest that higher RSA reactivity to a challenge task relates to lower levels of behavior problems (El-Sheikh, Harger, & Whitson, 2001; Hastings et al., 2008), though findings are inconsistent (El-Sheikh & Whitson, 2006).
ANS Measures as Moderators of the Effects of Maltreatment on Aggressive Behavior
SNS and PNS activity may moderate effects of maltreatment on aggressive behavior. Raine (2005) suggests that low SNS, as indicated by SCL, combined with abusive parenting may increase the risk of aggression, whereas higher SCL may be protective. Erath, El-Sheikh, and Cummings (2009) found that at values of lower SCL reactivity, the relation between harsh parenting and externalizing problems was heightened; this moderating relation was particularly consistent among boys. The combination of maltreatment with low SCL may be risky for children because low SCL may indicate low trait anxiety and inhibition (e.g., Kagan et al., 1987), which when combined with both traumatic experiences and aggressive parental models, would lead to low inhibition of aggressive behavior (Raine, 2005). On the other hand, high SCL reactivity has also been found to be a vulnerability factor for the effects of marital conflict on externalizing behavior (El-Sheikh et al., 2007). High SCL reactivity may indicate sensitization and thus increased defensiveness to the stress of interpersonal conflict. Drawing from these findings, either very low or very high SCL and SCL reactivity may increase the link between maltreatment and aggression.
PNS-linked cardiac activity, as indicated by RSA, may also interact with psychosocial factors to account for aggression. High RSA appears to buffer against the effects of marital conflict and other parenting problems (e.g., Blandon, Calkins, Keane, & O'B;rien, 2008). Katz and Gottman (1995) found that high vagal tone buffers the effects of marital conflict on child behavior problems. El-Sheikh (2005) found that low RSA exacerbates the link between parents’ problem drinking and children’s behavior problems. Results appear more consistent for baseline RSA than for RSA reactivity to challenge. El-Sheikh et al. (2001) found that high baseline RSA buffers the effects of marital conflict on boys’ and girls’ problems, but that higher change in RSA to challenge was protective only for boys. However, El-Sheikh and Whitson (2006) found that RSA suppression during challenge buffers the effects of marital conflict on internalizing problems in boys but increased the effect of marital conflict on girls. If high baseline RSA indexes emotion regulation and empathic ability, then high RSA may protect against the development of aggression in the face of maltreatment. Low RSA combined with maltreatment may carry risk due to the combination of trauma, poor affect regulation and empathy, poor social skills, and rigid and aggressive patterns of emotion expression (Beauchaine, 2001).
Multi-systems Approach
Beyond two-way interactions between environmental stressors and ANS functioning, examining interactions between both branches of the ANS together with maltreatment may shed light on moderating factors. Bauer, Quas, and Boyce (2002) argue that the pattern of activity across systems may be relevant to the development of emotional and behavior problems. To date, few researchers have examined the joint role of PNS and SNS activity in behavior problems among children exposed to family violence and maltreatment. An exception is the work of El-Sheikh et al. (2009), who examined how marital conflict interacts with SCL and RSA to predict behavior problems. Consistent with work by Bernston, Cacioppo, and Quigley (1991) in their conceptualization of autonomic space, these authors note that the SNS and the PNS have generally opposing actions on organ systems, such that the SNS engages the organism for fight or flight and involves increased heart rate and respiration, whereas the PNS corresponds to slower heart rate and respiration and lower overall emotional arousal. Bernston et al. (1991) argue that the PNS and SNS function along two separate dimensions and thus can change in reciprocal fashion such that the effects of sympathetic or parasympathetic activation are maximized. Alternatively, high co-activation of both of ANS and PNS simultaneously would result in a mixture of opposing effects on organ systems. El-Sheikh et al. (2009) found reciprocal, coordinated activation of the SNS and PNS (higher RSA/lower SCL or higher SCL/lower RSA) to buffer the effects of marital conflict on children’s problems, whereas co-activation and co-inhibition exacerbated the effects.
Drawing from this work and extrapolating to maltreatment, RSA and SCL may interact jointly with maltreatment to account for aggression. Youth who exhibit relatively high RSA or SCL may be protected against the negative effects of maltreatment on aggressive behavior. These youth may be better able to engage appropriately with stressful situations, to inhibit aggressive responses, to regulate difficult emotions and express them appropriately, and to experience empathy for the potential target of their own aggression (van Goozen & Fairchild, 2008). Low baseline levels of both RSA and SCL may reflect poor emotion regulation and low inhibition of aggression and may exacerbate negative effects of maltreatment (Beauchaine, 2001; Raine, 2005). Baseline RSA and SCL and RSA and SCL reactivity may combine in different ways to buffer or exacerbate effects of maltreatment. For example low or high reactivity in both SCL and RSA may suggest poor coordination in responses across the systems (El-Sheikh et al., 2009). Low resting RSA and high SCL reactivity may suggest poor ability to regulate emotion combined with hyperreactivity to stressors, potentially leading to behavior problems. Low baseline RSA with low SCL reactivity may suggest low emotion regulation combined with low ability to learn through positive and negative reinforcement.
The Present Study: Maltreatment, ANS Measures and Aggressive Behavior
Here we examine SCL and RSA as moderators of the link between maltreatment and youth aggression. First we examine whether maltreated youth differ from comparison youth in baseline RSA and SCL and RSA and SCL reactivity. Researchers examining the psychobiological effects of trauma have found increased reactivity in SNS indicators to trauma-associated stimuli (Casada, Amdur, Larsen, & Liberzon, 1998), potentially due to increased activity in the HPA axis and catecholamine systems (Charney, Deutch, Krystal, Southwick, & Davis, 1993; De Bellis et al., 1999; De Bellis & Putnam, 1994; Putnam & Trickett, 1997; Southwick & Friedman, 2001). Next we examine main and interactive effects of maltreatment, SCL and RSA in accounting for adolescent aggression.
We hypothesize that maltreatment will be associated with higher aggression scores; that maltreated youth will have lower baseline SCL and RSA and higher SCL and RSA change in response to the conflict clips; and that SCL and RSA will moderate the effect of maltreatment on aggressive behavior. Consistent with work by Beauchaine (2001), Katz and Gottman (1995), and El-Sheikh et al. (2009), we expect that higher RSA will buffer against the link between maltreatment and aggressive behavior. Furthermore, we expect that this effect will be moderated by SCL measures such that high SCL combined with high RSA and low SCL combined with low RSA will exacerbate the link between maltreatment and aggression, whereas high RSA combined with low SCL and low RSA combined with high SCL will be protective. Although we base this hypothesis on the concept of autonomic space (Berntson et al., 1991), we acknowledge that our measures of PNS and SNS activity differ from Bernston et al.’s conceptualization in that our measures of SNS and PNS are derived from two different organs (electrodermal and cardiac activity) instead of reflecting co-activation or co-inhibition of dually activated target organs such as the heart (Beauchaine, 2009). Based on sex differences observed in the literature (e.g., Eisenberg et al., 1995; El-Sheikh & Whitson, 2006; Erath et al., 2009), we analyze data separately for boys versus girls.
Methods
Participants
Participants included 362 youth from a longitudinal study examining effects of child maltreatment. The current study focuses on data from the second of a three-wave longitudinal study, when participants were 9–16 years old. Of the 454 participants in wave 1, 55 youth did not return for wave 2, and 37 were excluded due to equipment malfunction or other issues (e.g., child fell asleep during session). No significant differences on demographics emerged between cases included versus excluded from the analysis, except for total yearly household income before taxes, reported in categories. Youth excluded from analysis were more likely than those included to have a family income of <$15,000 (χ2 = 15.050, df = 6, p < .05).
We recruited maltreated youth from selected zip codes in Los Angeles County, based on having recent reports of child maltreatment to the Los Angeles County Department of Family and Children’s Services (DCFS). Participants needed to meet the following inclusion criteria: a report to DCFS within the past two months; age 9–12 years during wave 1; African American, Latino/a or Caucasian due to the aims of the larger study to examine effects of maltreatment in these ethnic groups; and live in 1 of the 11 selected zip codes. We recruited the comparison group from schools and neighborhoods from the same or demographically comparable census blocks. Comparison group youth had no history of involvement with DCFS. We obtained the names and contact information for these families from a local marketing firm. Of families we recruited, 77% of DCFS families and 50% of comparison families agreed.
Average age at wave 2 was 12.1 years (range 9.5 to 16.1; SD 1.21). Ethnic composition was 10.8% Caucasian, 37.6% African American, 39.0% Hispanic or Latino/a, and 12.7% of mixed racial background. Approximately half (51.7%) of the participants were male. Maltreated and comparison youth did not differ on ethnicity. However, maltreated youth were younger than were comparison youth (t = 2.25, df = 360, p < .05). Maltreated youth were less likely than were comparison youth to have biological or adoptive parents as their caretakers (χ2 = 52.62, df = 1, p < .001). Parents/guardians of maltreated youth were less likely than were comparison parents to have a high school diploma (χ2 = 35.15, df = 4, p < .001). Parents/guardians of maltreated youth were more likely than comparison parents to earn <$15,000 per year (χ2 = 41.69, df = 3, p < .001).
Based on information in the participants’ DCFS case records, we coded the maltreatment experiences of these youth (for a detailed report, see Trickett et al., 2009). Of the maltreated sample, 72% of youth had been neglected, 23% had been sexually abused, 50% had been physically abused, and 50% had been emotionally abused. Forty-seven percent of the maltreated youth had experienced multiple forms of abuse.
Procedures
All procedures were conducted with the approval of the university IRBs. As part of a larger protocol, we measured SCL, heart rate, and respiration during a baseline period and while participants viewed video clips depicting intense parent-child conflict. The clips appeared in the following sequence; 3 min of baseline, two non-conflict clips (3 min each) and four conflict clips (90 s to 3 min each). All participants viewed the clips in the same order. We examined second minute of the baseline because it allowed 1 min for these measures to stabilize, and because during the third minute of baseline, we measured blood pressure (not examined here), which affected some participants’ SCL. Video clips were a mixture of nationally known, studio-created films and films created by students at the University of Southern California. A bioamplifier (James Long Company, Caroga Lake, NY) recorded psychophysiological activity continuously while participants viewed the videos.
After consent procedures, we led the adolescent into a room with the bioamplifier, a TV monitor and DVD player. The adolescent self-applied three disposable ECG electrodes, two axillary (one per side) and one as a ground just above the navel. To collect SCL, we attached Ag/AgCl electrodes, filled with isotonic citrate salt electrode gel with gel contact area limited to a 1 cm diameter circle by double-sided adhesive collars, to the volar surfaces of the distal phalanges of the participant’s non dominant hand. We placed a respiration bellows around the participant’s torso.
Measures
Aggression was measured using parent/guardian report on the 23-item Reactive-Proactive Aggression Questionnaire (Raine, et al., 2006). The proactive subscale includes 12 items (e.g., “fight others to show who is on top,” “damage or break things for fun”). The reactive subscale includes 11 items (e.g., “yell at others when they annoy me,” “damage things when I am mad”). Items have three response choices: never (0), sometimes (1) or often (2) and measure a mix of physical and verbal aggression, hostility and anger. Raine et al. (2006) report alpha coefficients of .81–.87 for the subscales, and .89–.91 for the whole scale. Subscales were highly correlated, r = .71; we therefore analyzed only the Total Aggression scale (α = .91).
SNS activity was indicated by SCL baseline and reactivity (SCL and SCL-R). To collect tonic SCL, the bioamplifier used a 500 mV, 30 Hz sinusoidal excitation waveform. The bioamplifier yielded a skin conductance level output of 10 μS/V. The A/D converter had a 16-bit resolution and a ± 2.5 V input range. Data were digitized at 1 kHz. We measured SCL-R to the conflict clips as average SCL during presentation of the first two conflict clips minus the average SCL during the second minute of baseline.
PNS-linked cardiac activity was measured by RSA measured during second minute of baseline. ECG data were sampled and digitized at 1 kHz. R-wave times were extracted from the ECG channel and edited manually via ECGRWAVE software (James Long Company, Caroga Lake, NY, USA). The strain gauge respiration bellows connected to the bioamplifier for transduction, amplification, and digitization. The RSA program (James Long Company) calculated RSA as the difference between the minimum interbeat interval (IBI) during inspiration and the maximum IBI during expiration (in seconds). This program computes the difference in IBI twice for each respiration cycle, once for inspiration and once for expiration, assigning times of inspiration and expiration as the midpoints of each and calculating the arrhythmia. Grossman, van Beek, and Wientjes (1990) found the peak-to-valley method to be comparable to spectral analysis. We measured RSA reactivity (RSA-R) to the conflict clips as average RSA during presentation of the first two conflict clips minus the average RSA during second minute of baseline.
Results
Descriptive Information and Effects of Maltreatment on Aggression, RSA and SCL
Descriptive data for all study variables by maltreatment status appear in Table 1. Because baseline RSA and SCL were substantially skewed, we log transformed these data for all analyses, though Table 1 displays raw means for interpretability. Two-way group (maltreatment vs. comparison) by sex ANCOVAs examined the effects of maltreatment status and sex on aggression, baseline RSA, RSA-R, baseline SCL, and SCL-R, controlling for age, parent’s education, income, and ethnicity (African-American vs. Caucasian and Latino) due to lower SCL reported among African-American samples (Anderson & McNeilly, 1991). SCL-R analyses controlled for baseline SCL, and RSA-R analyses controlled for baseline RSA. A significant main effect of maltreatment emerged on aggression, such that maltreated youth had higher aggression scores than did comparison youth. No sex or maltreatment × sex interaction effect was significant on aggression. A main effect of sex emerged on baseline RSA, such that girls’ baseline RSA was higher than was boys’ RSA. Girls also had higher SCL-R than did boys. Repeated measures ANCOVAs controlling for child age revealed that among both boys and girls, SCL increased from baseline to the conflict clips (p < .05) but RSA did not increase.
Table I.
Means and SDs of Study Variables by Gender and Maltreatment Status
| Maltreated |
Comparison |
||||||
|---|---|---|---|---|---|---|---|
| Males (n = 113) | Females (n = 121) | Males (n = 74) | Females (n = 54) | F (maltreatment) | F (sex) | F (maltreatment × sex) | |
| Aggression | 10.81 (8.43) | 10.76 (8.36) | 8.65 (5.40) | 5.82 (4.77) | 14.59** | 3.02 | 3.75 |
| Baseline RSA (s) | .10 (.06) | .11 (.10) | .10 (.09) | .15 (.34) | .07 | 5.10* | .00 |
| Baseline SCL (μs) | 13.30 (6.21) | 13.12 (6.15) | 14.2 (6.6) | 13.27 (6.04) | .29 | .41 | .13 |
| RSA reactivity (s) | .03 (.33) | .28 (3.03) | −.01 (.09) | −.01 (.09) | .14 a | .47a | .15a |
| SCL reactivity (μs) | 3.53 (3.81) | 1.94 (2.79) | 3.46 (4.15) | 2.39 (3.13) | .16a | 12.49a** | 1.77a |
Note: Except where indicated with superscript, degrees of freedom for F-statistics are (1, 355). SCL = Skin conductance level. RSA = Respiratory sinus arrhythmia.
adf = (1, 353).
*p < .05. **p < .01.
Correlations among ANS Variables and Aggression
Correlations (see Table 2) examined relations among aggression, baseline RSA, RSA-R, baseline SCL, and SCL-R. Correlations between baseline physiological measures and aggression are bivariate, as are correlations between baseline RSA and RSA-R and between baseline SCL and SCL-R. All other correlations involving RSA-R control for baseline RSA, and all other correlations involving SCL-R control for baseline SCL. Among boys, baseline RSA was negatively correlated with aggression. Among girls, RSA and SCL measures were not significantly related to aggression. Baseline RSA and SCL-R were negatively correlated with each other among both boys and girls. Among girls, baseline SCL was positively correlated with SCL-R.
Table II.
Intercorrelations among aggression and psychophysiological measures (n = 187 boys and 175 girls)
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1. Total aggression | – | –.14 | –.14 | –.08 | .04 |
| 2. Baseline RSA | –.19* | – | –.03 | –.01 | –.19* |
| 3. Baseline SCL | .03 | .00 | – | –.15 | .29** |
| 4. RSA Reactivity | –.04 | –.11 | –.02 | – | .08 |
| 5. SCL-Reactivity | .09 | –.15* | –.00 | –.00 | – |
Note: Boys’ correlations appear below the diagonal. Girls’ correlations appear above the diagonal. RSA = Respiratory sinus arrhythmia. SCL = Skin conductance level. With the exception of analyses of correlations between baseline RSA and RSA reactivity and baseline SCL and SCL reactivity, analyses involving SCL Reactivity control for baseline SCL, and analyses involving RSA reactivity control for baseline RSA.
*p < .05. **p < .01.
Regression analyses Predicting Aggression
We examined the effects of maltreatment status (–1 = comparison, +1 = maltreated), baseline SCL, SCL-R, and baseline RSA in explaining variance in aggression. We conducted two equations. One examined maltreatment, baseline RSA, baseline SCL, and their two and three way interactions. The other equation examined maltreatment, baseline RSA, SCL-R, and their two and three way interactions. We were unable to examine equations involving RSA reactivity and its interactions with maltreatment and SCL due to high multicollinearity between RSA reactivity and its interactions with maltreatment. We centered all main effect predictors and multiplied centered scores to calculate interaction effects. We conducted analyses separately for boys versus girls. We entered all predictors simultaneously. Regression analyses, which appear in Table 3, controlled for age, ethnicity (–1 = Caucasian/Latino/a, +1 = African-American), parent/guardian education, and income. Covariates were non-significant in accounting for boys’ and girls’ aggression. Equations examining interactions with SCL-R also included baseline SCL entered simultaneously.
Table III.
Regression equations accounting for aggression from maltreatment, RSA, SCL-R, and their interactions (n = 187 boys and 175 girls)
| Boys |
Girls |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| β | t | F | R2 | β | t | F | R2 | ||
| Baseline RSA and Baseline SCL | |||||||||
| Total Equationa | 2.60 | .10 | 4.26** | .15 | |||||
| Maltreatment | .14 | 1.80 | .31 | 3.90** | |||||
| Baseline RSA | –.21 | –2.87** | –.11 | –1.50 | |||||
| Baseline SCL | .04 | .47 | –.09 | –1.12 | |||||
| Maltreatment × Baseline RSA | –.19 | –2.47* | –.13 | –1.75 | |||||
| Maltreatment × Baseline SCL | .05 | .65 | –.07 | –.92 | |||||
| RSA Baseline × Baseline SCL | .08 | .98 | .08 | 1.12 | |||||
| Maltreatment × Baseline RSA × Baseline SCL | .01 | .18 | .08 | 1.13 | |||||
| Baseline RSA and SCL Reactivity | |||||||||
| Total equationb | 2.63* | .11 | 4.59** | .18 | |||||
| Baseline SCL | .03 | .43 | –.19 | –2.41* | |||||
| Maltreatment | .14 | 1.75 | .32 | 4.07** | |||||
| Baseline RSA | –.19 | –2.57* | –.11 | –1.49 | |||||
| SCL Reactivity | .05 | .56 | .15 | 1.93 | |||||
| Maltreatment × Baseline RSA | –.16 | –2.11* | –.18 | –2.40* | |||||
| Maltreatment × SCL Reactivity | .14 | 1.74 | .02 | .24 | |||||
| Baseline RSA × SCL Reactivity | –.04 | –.54 | –.01 | –.14 | |||||
| Maltreatment × Baseline RSA × SCL Reactivity | –.10 | –1.19 | .17 | 2.26* | |||||
Note: RSA = Respiratory sinus arrhythmia. SCL = Skin conductance level.
*p < .05. **p < .01.
aFor F-tests, df = 7,170 boys and 7,159 for girls
bFor F-tests, df = 8,169 boys and 8,156 girls.
Boys’ Aggression
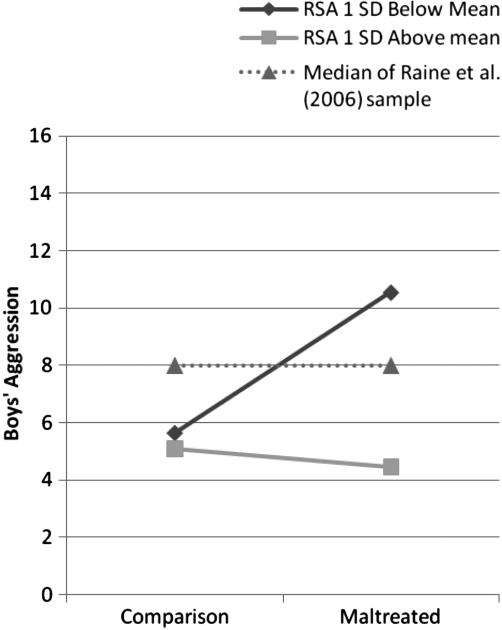
The first model examined the main and interactive effects of maltreatment, baseline RSA and baseline SCL on aggression. The overall equation was not significant, and thus individual predictors will not be discussed. The second model tested main and interactive effects of maltreatment, baseline RSA and SCL-R. The total equation was significant, accounting for 11% of the variability in aggression. Baseline RSA was negatively related to aggression, and the two-way interaction between RSA and maltreatment was significant. We probed this interaction according to procedures described by Aiken and West (1991). Figure 1 displays the relation between maltreatment and aggression at values of RSA 1 SD above and below the mean. At values of RSA 1 SD below the mean, the relation between maltreatment and aggression was significant and positive (b = 2.45, t = 4.47, p < .001); at values of RSA 1 SD above the mean, the relation was null (b = –.32, t = –.36, p = .72).
Figure 1.
Plot of the two-way interaction effect between maltreatment and baseline RSA in accounting for boys’ aggression.
Girls’ Aggression
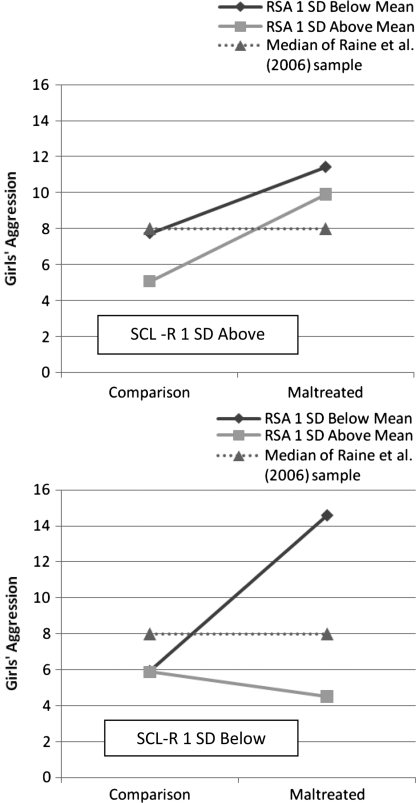
The first model examined maltreatment, baseline RSA, baseline SCL, and their interactions. The overall F-value was significant, with predictors accounting for 15% of the variance in aggression. The only significant predictor was maltreatment. The second equation examined maltreatment, baseline RSA, SCL-R and their interactions and also included baseline SCL. The total equation was significant, accounting for 18% of the variance. The main effect of maltreatment, the two-way maltreatment × RSA interaction, and the three-way maltreatment × baseline RSA × SCL-R interaction were significant. We probed the three-way interaction as the highest order effect. Tests of simple slopes (see Figure 2) reveal that at values of baseline RSA 1 SD below the mean, the relation between maltreatment and aggression was significant at values of SCL-R 1 SD below the mean (b = 5.76, t = 3.78, p < .001), but at values of baseline RSA 1 SD below the mean and SCL-R 1 SD above the mean, the relation between maltreatment and aggression was null (b = 2.46, t = 1.91, p = .058). In addition, at baseline RSA 1 SD above the mean and SCL-R 1 SD above the mean, the relation between maltreatment and aggression was also significant (b = 3.22, t = 2.29, p = .024), but at values of baseline RSA 1 SD above the mean and SCL-R 1 SD below the mean the relation between maltreatment and aggression was not significant (b = –.90, t = –.67, p = .50).
Figure 2.
Plots of the three-way interaction effect between maltreatment, baseline RSA, and SCL reactivity in accounting for girls’ aggression.
Discussion
The present data are consistent with ANS measures as moderators of the effects of maltreatment on youth aggression. Among boys, maltreatment positively related to aggression at lower but not higher values of RSA. Among girls, the moderating effect of baseline RSA on the link between maltreatment and aggression was further moderated by SCL reactivity. The relation between maltreatment and aggression was significant at relatively low RSA only when SCL reactivity was also relatively low. The relation between maltreatment and aggression was also significant at relatively high baseline RSA when SCL reactivity was also relatively high. In other words, at values of both baseline RSA and SCL reactivity being relatively low or relatively high, the relation between maltreatment and aggression was positive and significant. At values of low baseline RSA and high SCL reactivity or high baseline RSA and low SCL reactivity, the relation between maltreatment and aggression was not significant.
Overall, the data from this study suggest that among boys, high RSA may be protective against the effects of maltreatment on aggression. These data are consistent with other studies on the buffering effects of high RSA on psychosocial stress variables. For example, Katz and Gottman (1995) and El-Sheikh et al. (2001), found that high vagal tone buffers against the negative effects of marital conflict on the development of behavior problems. Our findings are also consistent with work by El-Sheikh (2005), who reports that high RSA buffers the effect of parent drinking on children’s behavior problems. We did not find three-way interactions between RSA, SCL, and maltreatment among boys. The lack of a significant three-way interaction differs from findings reported by El-Sheikh et al. (2009), though their studies examined boys and girls together and marital conflict rather than child maltreatment.
Among girls, the significant three-way interaction suggests that the moderating effect of RSA was further moderated by SCL reactivity. Low RSA exacerbated the effect of maltreatment on aggression only at low values of SCL reactivity. At low RSA and high SCL reactivity, the link between maltreatment and aggression was non-significant. Low RSA and SCL reactivity may represent a vulnerability to the effects of maltreatment on aggression, and high RSA and SCL reactivity may also carry some risk. These results are consistent with findings reported by El-Sheikh et al. (2009) and can be understood in terms of the theory of autonomic space (Berntson et al., 1991). Low baseline RSA combined with low SCL reactivity may reflect a lack of engagement of appropriate stress response and emotional regulation systems and may increase the negative effects of child maltreatment. The pattern of relatively high SCL reactivity combined with high baseline RSA may also reflect problematic or contradictory patterning of these systems (El-Sheikh et al., 2009) consisting of high reactivity to negative environmental experiences and thus a stronger link between maltreatment and aggression. In contrast, high baseline RSA coupled with low SCL reactivity and low baseline RSA coupled with high SCL reactivity may buffer against the effects of maltreatment on aggression in girls. El-Sheikh et al. interpret this combination as reciprocal SNS or PNS activation that suggests appropriate coordination between SNS and PNS. However, as we were unable to examine interactions with RSA reactivity due to multicollinearity issues, the terms “co-activation” and “co-inhibition” do not precisely apply to our models, as RSA is at baseline and SCL is in response to the videos. Moreover, we measure SNS and PNS effects on different organ systems.
Our findings are consistent with previous findings regarding sex effects in relations between SCL and RSA with aggression. The protective effect of RSA on boys’ aggression is consistent with much of the literature (Beauchaine et al., 2008). Among girls, the link between high SCL reactivity in the context of maltreatment and high RSA is consistent with results of Beauchaine et al. (2008), who found higher electrodermal activity to relate to girls’ aggression.
We found no main effect for maltreatment on any SNS or PNS indicators. We were surprised that maltreated youth did not have larger SCL responses to the videos, which would have suggested sensitization to conflict stimuli. One possible explanation is that the videos depicted milder conflict than those experienced by the maltreated youth. Another possibility is that the clips depicted conflict more intense than the comparison youths’ experiences, causing a stronger reaction among some of these participants and washing out group effects. Youth in both groups did demonstrate increased SCL from baseline to SCL during the conflict clips.
SCL reactivity did not relate to aggressive behavior as a main effect or in two-way interactions with maltreatment. This result is inconsistent with findings by Erath et al. (2009), who found that low SCL reactivity exacerbated the link between harsh parenting and children’s externalizing behaviors. One possible reason for the discrepancy is participants’ developmental stage. The relation between aggression and SCL reactivity is unclear among adolescents (Lorber, 2004). Erath et al. (2009) studied a younger sample than the present study. In addition, although SCL reactivity was not significant as a main effect, it interacted with maltreatment and baseline RSA to account for aggressive behavior in girls. RSA reactivity did not relate to aggressive behavior. Some researchers have found higher vagal suppression to be protective against behavior problems (El-Sheikh & Whitson, 2006), whereas others have found higher change in RSA to challenge to be protective (Hastings et al., 2008).
Our study has limitations. Our maltreatment classification is coarse. Further work should examine particular types of maltreatment. We targeted youth from certain ethnic backgrounds due to aims of the larger study and thus cannot generalize to other ethnicities. The cross-sectional design precludes knowing whether the maltreatment truly preceded the aggression. We cannot deduce causality as we cannot manipulate maltreatment experimentally. We were not able to examine interaction effects with RSA reactivity due to multicollinearity. Thus, the significant interactions involved baseline RSA and SCL reactivity, and we have limited information regarding coordination in the two systems to the same events. Moreover, because an electrodermal measure indexed SNS, and a cardiac measure indexed PNS, we did not study co-activation and co-inhibition according to autonomic balance theory as conceptualized by (Berntson et al., 1991), because they refer to organs dually innervated by SNS and PNS (Beauchaine, 2009). Our electrophysiological measures also do not purely index SNS and PNS; they are also influenced by other factors. Participants also may vary in their emotional reactions to the videos. In addition, baselines may have been too short for reliable assessments of RSA and SCL. The physiological measures also have large variability that may not be captured by the means. Some of the effect sizes are small, and these factors may have contributed.
Despite these limitations, this study contributes to knowledge about moderators of the effect of maltreatment on youth aggression. The multi-systems approach sheds light on patterns of PNS and SNS activity that may buffer or exacerbate effects of maltreatment. Understanding the role of these physiological systems in the effects of maltreatment may help identify youth who are particularly at risk. Research regarding how to maximize adaptive function of these systems may help protect against the negative effects of maltreatment.
Funding
National Institutes of Health grants K23 HD041428 and R01 HD039129 and the ABMRF/Foundation for Alcohol Research.
Conflict of interest: None declared.
Acknowledgments
The authors thank their colleagues at the USC Young Adolescent Project and the participating families. We also thank James Long for consultation regarding electrophysiological measurement.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publications, Inc; 1991. [Google Scholar]
- Anderson NB, McNeilly M. Age, gender, and ethnicity as variables in psychophysiological assessment: Sociodemographics in context. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1991;3:376–384. [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children's; behavior: advantages of a multisystem approach. Journal of Developmental Behavior Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray's; motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Some difficulties in interpreting psychophysiological research with children. Monographs of the Society for Research in Child Development. 2009;74:80–88. doi: 10.1111/j.1540-5834.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Hong J, Marsh P. Sex differences in autonomic correlates of conduct problems and aggression. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:788–796. doi: 10.1097/CHI.0b013e318172ef4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, O'B;rien M. Individual differences in trajectories of emotion regulation processes: the effects of maternal depressive symptomatology and children's; physiological regulation. Developmental Psychology. 2008;44:1110–1123. doi: 10.1037/0012-1649.44.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Casada JH, Amdur R, Larsen R, Liberzon I. Psychophysiologic responsivity in posttraumatic stress disorder: generalized hyperresponsiveness versus trauma specificity. Biological Psychiatry. 1998;44:1037–1044. doi: 10.1016/s0006-3223(98)00182-6. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Archives of General Psychiatry. 1993;50:295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- Cullerton-Sen C, Cassidy AR, Murray-Close D, Cicchetti D, Crick NR, Rogosch FA. Childhood maltreatment and the development of relational and physical aggression: the importance of a gender-informed approach. Child Development. 2008;79:1736–1751. doi: 10.1111/j.1467-8624.2008.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, et al. A.E. Bennett Research Award. Developmental traumatology. Part I: Biological stress systems. Biological Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Putnam FW. The psychology of childhood maltreatment. Child and Adolescent Psychiatric Clinics of North America. 1994;3:663–678. [Google Scholar]
- Dietrich A, Riese H, Sondeijker FE, Greaves-Lord K, van Roon AM, Ormel J, et al. Externalizing and internalizing problems in relation to autonomic function: a population-based study in preadolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:378–386. doi: 10.1097/CHI.0b013e31802b91ea. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990;250:1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Pettit GS, Bates JE. How the experience of early physical abuse leads children to become chronically aggressive. In: Cicchetti D, Toth SL, editors. Developmental perspectives on trauma: Theory, research, and intervention. Rochester, NY: University of Rochester Press; 1997. pp. 263–288. [Google Scholar]
- Einbender AJ, Friedrich WN. Psychological functioning and behavior of sexually abused girls. Journal of Consulting and Clinical Psychology. 1989;57:155–157. doi: 10.1037//0022-006x.57.1.155. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children's; social functioning: a longitudinal study. Child Development. 1995;66:1360–1384. [PubMed] [Google Scholar]
- El-Sheikh M. Does poor vagal tone exacerbate child maladjustment in the context of parental problem drinking? A longitudinal examination. Journal of Abnormal Psychology. 2005;114:735–741. doi: 10.1037/0021-843X.114.4.735. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Keller PS. Children's; sleep and adjustment: the moderating role of vagal regulation. Journal of Sleep Research. 2007;16:396–405. doi: 10.1111/j.1365-2869.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children's; adjustment and physical health: the moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Marital conflict and children's; externalizing behavior: interactions between parasympathetic and sympathetic nervous system activity. Monographs of the Society for Research in Child Development. 2009;74:vii, 1–79. doi: 10.1111/j.1540-5834.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: Vagal regulation as a protective factor. Journal of Family Psychology. 2006;20:30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Erath SA, El-Sheikh M, Mark Cummings E. Harsh parenting and child externalizing behavior: skin conductance level reactivity as a moderator. Child Development. 2009;80:578–592. doi: 10.1111/j.1467-8624.2009.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores E, Cicchetti D, Rogosch FA. Predictors of resilience in maltreated and nonmaltreated Latino children. Developmental Psychology. 2005;41:338–351. doi: 10.1037/0012-1649.41.2.338. [DOI] [PubMed] [Google Scholar]
- Fung MT, Raine A, Loeber R, Lynam DR, Steinhauer SR, Venables PH, et al. Reduced electrodermal activity in psychopathy-prone adolescents. Journal of Abnormal Psychology. 2005;114:187–196. doi: 10.1037/0021-843X.114.2.187. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Raine A, Loeber R, Stouthamer-Loeber M, Steinhauer SR. Serious delinquent behavior, sensation seeking, and electrodermal arousal. Journal of Abnormal Child Psychology. 2002;30:477–486. doi: 10.1023/a:1019816930615. [DOI] [PubMed] [Google Scholar]
- George C, Main M. Social interactions of young abused children: approach, avoidance, and aggression. Child Development. 1979;50:306–318. [PubMed] [Google Scholar]
- Grossman P, van Beek J, Wientjes C. A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology. 1990;27:702–714. doi: 10.1111/j.1469-8986.1990.tb03198.x. [DOI] [PubMed] [Google Scholar]
- Haapasalo J, Pokela E. Child-rearing and child abuse antecedents of criminality. Aggression and Violent Behavior. 1999;4:107–127. [Google Scholar]
- Haskett ME, Kistner JA. Social interactions and peer perceptions of young physically abused children. Child Development. 1991;62:979–990. doi: 10.1111/j.1467-8624.1991.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Nuselovici JN, Utendale WT, Coutya J, McShane KE, Sullivan C. Applying the polyvagal theory to children's; emotion regulation: Social context, socialization, and adjustment. Biological Psychology. 2008;79:299–306. doi: 10.1016/j.biopsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Hildyard KL, Wolfe DA. Child neglect: developmental issues and outcomes. Child Abuse and Neglect. 2002;26:679–695. doi: 10.1016/s0145-2134(02)00341-1. [DOI] [PubMed] [Google Scholar]
- Hubbard JA, Smithmyer CM, Ramsden SR, Parker EH, Flanagan KD, Dearing KF, et al. Observational, physiological, and self-report measures of children's; anger: Relations to reactive versus proactive aggression. Child Development. 2002;73:1101–1118. doi: 10.1111/1467-8624.00460. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–92. [Google Scholar]
- Kendall-Tackett KA, Williams LM, Finkelhor D. Impact of sexual abuse on children: A review and synthesis of recent empirical studies. Psychological Bulletin. 1993;113:164–180. doi: 10.1037/0033-2909.113.1.164. [DOI] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Margolin G, Gordis EB. The effects of family and community violence on children. Annual Review of Psychology. 2000;51:445–479. doi: 10.1146/annurev.psych.51.1.445. [DOI] [PubMed] [Google Scholar]
- Margolin G, Gordis EB. Children's; exposure to violence in the family and community. Current Directions in Psychological Science. 2004;13:152–155. [Google Scholar]
- McBurnett K, Harris SM, Swanson JM, Pfiffner LJ, Tamm L, et al. Neuropsychological and psychophysiological differentiation of inattention/overactivity and aggression/defiance symptom groups. Journal of Clinical Child Psychology. 1993;22:165–171. [Google Scholar]
- Putnam FW, Trickett PK. Psychobiological effects of sexual abuse. A longitudinal study. Annals of the New York Academy of Sciences. 1997;821:150–159. doi: 10.1111/j.1749-6632.1997.tb48276.x. [DOI] [PubMed] [Google Scholar]
- Raine A. The interaction of biological and social measures in the explanation of antisocial and violent behavior. In: Stoff DM, Susman EJ, editors. Developmental psychobiology of aggression. New York, NY: Cambridge University Press; 2005. pp. 13–42. [Google Scholar]
- Raine A, Dodge K, Loeber R, Gatzke-Kopp L, Lynam D, Reynolds C, et al. The reactive-proactive aggression questionnaire: Differential correlates of reactive and proactive aggression in adolescent boys. Aggressive Behavior. 2006;32:159–171. doi: 10.1002/ab.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH. Electrodermal nonresponding, antisocial behavior, and schizoid tendencies in adolescents. Psychophysiology. 1984;21:424–433. doi: 10.1111/j.1469-8986.1984.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Shaffer A, Yates TM, Egeland BR. The relation of emotional maltreatment to early adolescent competence: developmental processes in a prospective study. Child Abuse and Neglect. 2009;33:36–44. doi: 10.1016/j.chiabu.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick S, Friedman MJ. Neurobiological models of posttraumatic stress disorder. In: Gerrity E, Keane TM, Tuma F, editors. The mental health consequences of torture. Dordrecht, Netherlands: Kluwer Academic Publishers; 2001. pp. 73–87. [Google Scholar]
- Trickett PK. Maladaptive development of school-aged, physically abused children: Relationships with the child-rearing context. Journal of Family Psychology. 1993;7:134–147. [Google Scholar]
- Trickett PK, Gordis EB. Aggression and antisocial behavior in sexually abused females. In: Putallaz M, Bierman KL, editors. Aggression, antisocial behavior, and violence among girls: A developmental perspective. New York, NY: Guilford Publications; 2004. pp. 162–185. [Google Scholar]
- Trickett PK, McBride-Chang C, Putnam FW. The classroom performance and behavior of sexually abused females. Development and Psychopathology. 1994;6:183–194. [Google Scholar]
- Trickett PK, Mennen FE, Kim K, Sang J. Emotional abuse in a sample of multiply maltreated, urban young adolescents: Issues of definition and identification. Child Abuse and Neglect. 2009;33:27–35. doi: 10.1016/j.chiabu.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Goozen SH, Fairchild G. How can the study of biological processes help design new interventions for children with severe antisocial behavior? Development and Psychopathology. 2008;20:941–973. doi: 10.1017/S095457940800045X. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- Widom CS. Does violence beget violence? A critical examination of the literature. Psychological Bulletin. 1989;106:3–28. doi: 10.1037/0033-2909.106.1.3. [DOI] [PubMed] [Google Scholar]