Abstract
Purpose
To investigate the potential of human corneal stromal stem cells to assume a keratocyte phenotype and to organize extracellular matrix (ECM) in vitro similar to corneal stromal tissue.
Methods
Human corneal stromal stem cells (hCSSC) were isolated as side population cells by flow cytometry. Cloned hCSSC were cultured as free-floating pellets in serum-free media for 3 weeks. Gene expression was examined using gene array, quantitative RT-PCR, immunostaining, and immunoblotting. Transmission electron microscopy showed collagen fibril size and alignment.
Results
Pellet cultures of hCSSC in serum-free media upregulated the expression of keratocyte-specific genes and secreted substantial ECM containing characteristic stromal components: keratocan, keratan sulfate, collagen I, collagen V, and collagen VI. Abundant connexin 43 and cadherin 11 in pellets demonstrated cell-cell junctions typical of keratocytes in vivo. Electron microscopy of the pellet cultures revealed abundant fibrillar collagen, some of which was aligned in parallel arrays similar to those of stromal lamellae. Gene array identified expression in pellets of several genes highly expressed by keratocytes. Transcripts for these keratocyte genes—FLJ30046, KERA, ALDH3A1, CXADR, PTGDS, PDK4, MTAC2D1, F13A1—were increased by as much as 100-fold in pellets compared with hCSSC. Simultaneously, expression of stem cell genes BMI1, KIT, NOTCH1, SIX2, PAX6, ABCG2, SPAG10, and OSIL was reduced by a similar factor in pellets compared with hCSSC.
Conclusions
Scaffolding-free pellet culture of hCSSC induces keratocyte gene expression patterns in these cells and secretion of an organized stroma-like ECM. These cells offer a novel potential for corneal bioengineering.
The cornea is the outermost surface of the eye and the portal for light entry into the visual system. The strength and transparency of this organ rely on the highly organized extracellular matrix (ECM) of the corneal stroma, a tissue elaborated by unique neural crest-derived cells known as keratocytes. The stromal tissue is composed of 20 to 70 acellular collagenous lamellae of heterotypic types I and V fibrils with small uniform diameters, tightly packed in parallel orientation.1 This fibrillar collagen is embedded in a matrix of glycoproteins and proteoglycans that includes type VI nonfibrillar collagen and a unique family of keratan sulfate-containing proteoglycans essential for corneal transparency. The keratocytes, sandwiched between stromal lamellae, are the source of the molecular components of the stromal ECM and are largely quiescent in adult mammals. During wound healing, keratocytes become mitotic and motile, undergoing alteration of their ECM phenotype, depositing opaque scar tissue capable of causing permanent disruption of visual acuity. Keratoplasty using donated human tissue is currently the only effective procedure to correct visual impairment resulting from stromal scarring.
We recently identified a small population of cells in human corneal stroma with properties of adult stem cells.2 As do other adult stem cells, these cells express mRNA and protein for the multidrug transporter ABCG2 and can be expanded in culture through a number of population doublings without loss of this expression. These cells also express PAX6, a gene product present in embryonic and developing stromal cells but not in adult keratocytes.2 Like mesenchymal stem cells, corneal stromal stem cells (hCSSC) exhibit clonal growth and a potential for differentiation into multiple distinct tissue types.2 They express gene products characteristic of chondrocytes and neural cells when placed in culture environments known to elicit these phenotypes from other adult stem cell populations.2 As they differentiate, hCSSC lose ABCG2 and PAX6 expression. These stem cell markers were not regained when the differentiated cells were returned to stem cell maintenance culture conditions.2
The hCSSC expressed keratocan mRNA and protein as well as high molecular weight keratan sulfate when cultured in serum-free medium containing FGF2 and insulin. Keratocan glycanated with keratan sulfate is a unique product of the corneal stroma and is lost during in vitro expansion of cultured keratocytes.3,4 Secretion of this proteoglycan by cells derived from passaged hCSSC represents the first demonstration of induction of this keratocyte-specific molecule by passaged human cells in culture. Expression of these unique keratocyte products suggests hCSSC can adopt a keratocyte phenotype; however, in monolayer culture, these cells do not accumulate and organize the highly specialized ECM produced by stromal keratocytes in vivo. In the present study we examined the ability of three-dimensional culture conditions to induce elaboration of an organized stromal matrix by hCSSC. We also used gene array analyses to provide a more complete assessment of the gene expression phenotype of human keratocytes. These analyses yielded a panel of 18 genes providing an improved molecular definition of keratocyte phenotype. We found that culture of the hCSSC as a cell pellet in serum-free medium in the absence of rigid scaffolding or substratum induced an expression pattern of genes similar to that of keratocytes. Matrix expression and collagen organization in these pellets exhibited aspects of those seen in corneal stroma.
Materials and Methods
Materials
Antibodies used included anti-keratocan peptide antibody,3 J36 monoclonal to keratan sulfate,5 anti-collagen V (Chemicon, Temecula, CA), anti-collagen VI (Chemicon), connexin 43 (Zymed, South San Francisco, CA), and cadherin 11 (R&D Systems, Minneapolis, MN) for immunostaining. Secondary antibodies for Western blotting, peroxidase-labeled anti-mouse and anti-rabbit IgG, were from Santa Cruz Biotechnology (Santa Cruz, CA). For fluorescent staining, Alexa Fluor 488 anti-mouse IgG, anti-rabbit IgG, and nuclear dye TO-PRO-3 were obtained from Molecular Probes (Eugene, OR).
Side Population Cell Sorting
As previously described,2 hCSSC were isolated as a side population sorted on a high-speed cell sorter (MoFlo; DakoCytomation, Fort Collins, CO). They were cloned by limiting dilution and maintained in stem cell growth medium (SCGM) and split 1:3 by trypsinization when subconfluent.2
Pellet and Fibrin Gel Culture
For pellet culture, 2 × 105 cloned passage-20 hCSSC were collected in a conical-bottom 15-mL tube, centrifuged at 1500 rpm (400g) for 5 minutes to form a pellet. The pellets were cultured in SCGM for 3 days and then transferred into keratocyte differentiation medium (KDM; advanced MEM [Invitrogen] with 10 ng/mL fibroblast growth factor 2 [FGF2] and 0.1 mM ascorbic acid-2-phosphate), which was changed every 3 days for up to 3 weeks. For fibrin gel culture, 33 μL suspension of 12 × 106 cells/mL cloned passage 20 hCSSC were seeded into a fibrin gel, consisting of 134 μL of 5 mg/mL human fibrinogen (Sigma) and 33 μL of 100 U/mL bovine thrombin (Sigma). The gel formed in a cell culture incubator (37°C, 5% CO2) for 1 hour, and then SCGM containing 1 mg/mL ε-amino-N-caproic acid (Sigma) was added for 3 days. The medium was replaced with KGM containing ε-amino-N-caproic acid at 3 days and was changed at 3-day intervals. Corneal fibroblasts were produced from unfractionated stromal cells by growth in DMEM/F-12 containing 10% fetal bovine serum. After five passages, fibroblasts were used as control for pellet culture and fibrin gel culture. They were cultured under the same conditions as stem cells. Media were collected for Western blot to detect the expression of keratocan and keratan sulfate. Cells from the same cultures were lysed to make RNA for quantitative RT-PCR or were fixed for immunostaining.
Quantitative RT-PCR
Cell pellets and cells in fibrin gels were stored in a stabilizing reagent (RNAlater; Ambion, Austin, TX) for 1 day and then were homogenized. RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA). RNA was treated with DNAse I (Ambion) and was concentrated by alcohol precipitation. RNA (400 ng) was transcribed to cDNA in a 100-μL reaction containing 1× PCR II buffer (Roche Applied Science, Indianapolis, IN), 5 mM MgCl2, 200 μM dNTP mixture (Roche), 2.5 μM random hexamers (Invitrogen), 0.4 U RNase inhibitor, and 125 U SuperScript II reverse transcriptase (Invitrogen). Quantitative PCR of cDNA was performed using assays containing fluorescent hybridization probes (TaqMan; Applied Biosystems, Foster City, CA) or with direct dye binding (SYBR Green; Applied Biosystems) according to the manufacturer's instructions. Primers for SYBR assays designed using online software (Primer 3; http://frodo.wi.mit.edu/), had the sequences shown in Table 1. Catalog numbers of TaqMan assays are shown in Table 2. Reactions were carried out on triplicate samples for 40 cycles of 15 seconds at 95°C and 1 minute at 60°C after initial incubation at 95°C for 10 minutes. Reaction volume was 25 μL. For TaqMan assays, reactions contained 1× Universal PCR Master Mix (Applied Biosystems), 1× gene mix, and 2.5 μL cDNA. For SYBR dye-based assays, the reactions contained 1× PCR buffer (Applied Biosystems), 3 mM Mg2+, 200 μM dATP, dCTP, dGTP, and 400 μM dUTP, 0.025 U/mL AmpliTaq Gold polymerase, 2.5 μL cDNA and forward and reverse primers at optimized concentrations. A dissociation curve for each SYBR-based reaction was generated on a real-time thermocycler (Gene-Amp ABI Prism 7700 Sequence Detection System; Applied Biosystems) to confirm the absence of nonspecific amplification. Amplification of 18S rRNA was performed for each cDNA (in triplicate) for normalization of RNA content. A negative control lacking cDNA was also included in each assay. Relative mRNA abundance was calculated as the Ct for amplification of a gene-specific cDNA minus the average Ct for 18S expressed as a power of 2 (2–ΔCt). Three individual gene-specific values thus calculated were averaged to obtain mean ± SD.
Table 1.
Primers and Probes for Reverse Transcription–Polymerase Chain Reaction
| Gene | Primer Sequence |
|---|---|
| Keratocan | Forward: ATCTGCAGCACCTTCACCTT |
| NM_007035 | Reverse: CATTGGAATTGGTGGTTTGA |
| ALDH3A1 | Forward: CATTGGCACCTGGAACTACC |
| NM_000691 | Reverse: GGCTTGAGGACCACTGAGTT |
| Probe: CTTCAACCTCACCATCCAGCCCAT | |
| SPAG10 | Forward: CCCTTGGAGACGCAGTATGT |
| Y11718 | Reverse: GGGATGGTGTTGTCCTTCAG |
| Probe: AGCCCATCATCTGCCACCGG | |
| OSIL | Forward: GGAGGACGTGGAGACCATAC |
| NM_003900 | Reverse: CAGAAAGCCATTTGGCAAGA |
| Probe: TCCCTGCAGGATTACACCAGCA | |
| BMI1 | Forward: GCCTTAACGCTGTGTGTATGAC |
| NM_004427 | Reverse: ACCTGGAGATGCCAGAGAGTAG |
| CD166 | Forward: GTCTGCTCTTCTGCCTCTTGAT |
| NM_001627 | Reverse: TCATATTTCCATTTGCCAAACA |
| KIT | Forward: CGAGTTGGCCCTAGACTTAGAA |
| NM_000222 | Reverse: CTTTGTGATCCGACCATGAGTA |
| NOTCH1 | Forward: AGTCTCTGCAGTGCTGGAAGTA |
| NM_017617 | Reverse: CTTGCAGTACTGGTCGTACAGG |
| SIX2 | Forward: TGTTGGTTCTTGTTTGGGATTT |
| NM_016932 | Reverse: TTCCCTTCTGTGGTTCAAGACT |
Table 2.
Identification of Differentially Expressed hCSSC Genes
| Gene Symbol | Gene Name | RT-PCR Assay (ABI) | NCBI Reference |
|---|---|---|---|
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Hs99999905_m1 | NM_002046 |
| FLJ30046 | Hypothetical protein of SLAIN1 | Hs00287931_m1 | NM_144595, |
| CXADR | Coxsackie virus and adenovirus receptor | Hs00154661_m1 | NM_001338 |
| PTGDS | Prostaglandin D2 synthase | Hs00168748_m1 | NM_000954 |
| PDK4 | Pyruvate dehydrogenase kinase, isoenzyme 4 | Hs00176875_m1 | NM_002612 |
| KERA | Keratocan | Hs00559942_m1 | NM_007035 |
| EGR2 | Early growth response 2 | Hs00166165_m1 | NM_000399 |
| MTAC2D1 | Membrane targeting (tandem) C2 domain containing-1 | Hs00541117_m1 | NM_152332 |
| F13A1 | Coagulation factor XIII, A1 polypeptide | Hs00173388_m1 | NM_000129 |
| PAX6 | Paired homeobox gene 6 | Hs00240871_m1 | NM_000280 |
| ABCG2 | ATP-binding cassette G2 | Hs00184979_m1 | NM_004827 |
| SPAG10 | Sperm-associated antigen 10 | Custom design | Y11718.1 |
| OSIL | Sequestosome 1, p62 | Custom design | NM_003900 |
Gene Array
Total RNA was isolated from cultured cells processed and analyzed using the appropriate Affymetrix (Santa Clara, CA) products (cited here as catalog numbers). RNA internal standards (ABI catalog no. 900433) were added to the samples, and the mRNA component of the total RNA was reverse transcribed (ABI catalog no.900431) in the presence of a T7-(dT)24 primer. The resultant cDNA was extracted with an ABI kit (catalog no. 900371) and was transcribed in vitro in the presence of biotin-labeled ribonucleotides (ABI catalog no.900449). Biotinylated RNA (20 μg) was extracted and fragmented for 35 minutes at 94°C (ABI catalog no. 900371). Each sample was hybridized overnight (U133 Plus 2.0 GeneChip; ABI catalog no.900466). The chips were then washed, developed, and scanned (Agilent ChipScanner; Affymetrix). Raw data were processed and analyzed (Affymetrix GeneChip Operating System [GCOS] version 1.0), and derived data were copied into spreadsheets. Expression levels for each chip were normalized to the mean level for all chips. Differences greater than 10-fold in gene expression between hCSSC and keratocytes were used to select 8 to 10 genes for use as molecular markers. Primers for RT-PCR were designed for these and quantitative RT-PCR (qRT-PCR) carried out as described.
Immunoblotting
Proteoglycans were recovered from culture media by ion exchange chromatography on microcolumns (SPEC-NH2; Ansys Diagnostics, Lake Forest, CA), as described previously.3 Proteoglycans were digested with a mixture of keratanase II and endo-β-galactosidase.6 Digested and undigested samples were run on a 4%–20% SDS-PAGE gel, transferred to polyvinylidene difluoride membrane, and subjected to immunoblotting with antibody against keratocan3 and antibody J36 against keratan sulfate.5
Histology
Pellets and fibrin gels were rinsed briefly in phosphate-buffered saline (PBS), fixed for 12 to 15 minutes in 3% paraformaldehyde in PBS at room temperature, rinsed in PBS, embedded in optimal cutting temperature cutting compound (OCT), frozen, and stored at –20°C until they were cut into 8-μm sections on a cryostat. Sections were hydrated in PBS before staining. Nonspecific binding was blocked with 10% heat-inactivated goat serum. Sections were incubated for 1 hour at room temperature with primary antibodies. After two washes, secondary antibodies and nuclear counterstain (To-Pro-3; Invitrogen) were added at the same time, followed by incubation for 1 hour at room temperature. The samples were photographed using a confocal microscope with a 20× oil objective (Bio-Rad Laboratories, Hercules, CA).
Transmission Electron Microscopy
Pellets were rinsed briefly in PBS and were fixed for 2 hours in 2.5% glutaraldehyde, 4% paraformaldehyde, 0.1 M cacodylate buffer, and 8 mM CaCl2 on ice. Samples were postfixed with 1% osmium tetroxide, and they were en block stained with ethanolic uranyl acetate (2% uranyl acetate/50% ethanol). After dehydration with a graded ethanol series followed by propylene oxide, the tissues were infiltrated and embedded in a mixture of embedding medium (Polybed 812; Poly-sciences, Warrington, PA), nadic methyl anhydride, dodecenyl succinic anhydride, and DMP-30 (Polysciences). Sections were stained with 2% aqueous uranyl acetate and 1% phosphotungstic acid, pH 3.2. The sections were examined and photographed at 80 kV using a transmission electron microscope (Tecnai 12; FEI Company, Hillsboro, OR) equipped with a digital camera (Ultrascan US1000 2 K; Gatan Digital Imaging; Pleasanton, CA).7
Results
Three-Dimensional Culture of hCSSC
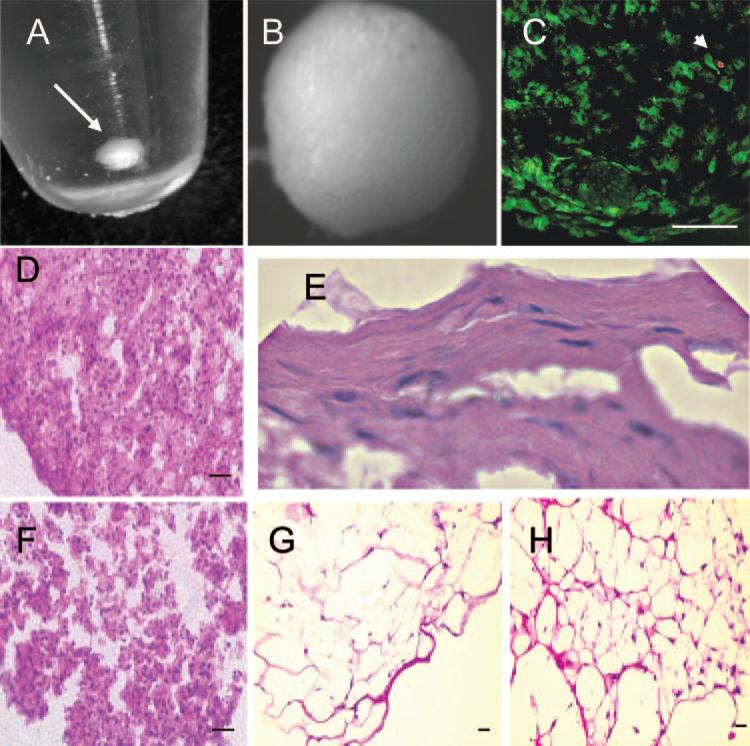
We recently showed that hCSSC express unique molecular markers of corneal keratocytes, keratocan, and keratan sulfate when cultured in a serum-free medium supplemented with insulin, ascorbate, and FGF2.2 These cultures, however, did not deposit ECM or form multiple cell layers. In the present study, we tested the hypothesis that three-dimensional culture of hCSSC induces a more complete differentiation of the hCSSC to keratocytes, including deposition of the specialized ECM found in the corneal stroma. We examined two culture models. In one, cells were embedded in fibrin gel. The second consisted of culture in a scaffolding-free pellet. Both these culture formats have been reported as effective in inducing enhanced matrix response in chondrocytes.8–10 Pellet cultures were initiated by centrifugation of 2 × 105 hCSSC in a 15-mL conical polystyrene tube (Fig. 1), after which cells were maintained in differentiation medium for various lengths of time. Initial observations found the cells formed smooth spheres (Fig. 1B) filled with viable cells (Fig. 1C). The diameters of the hCSSC pellets increased visibly over a period of several weeks in culture (not shown). For comparison, hCSSC were cultured in fibrin gel cultures using 2 × 105 cells/100 μL gel. Stromal fibroblasts were used as controls for both culture systems.
Figure 1.
Three-dimensional cultures of human corneal stromal stem cells. (A) Formation of a free-floating pellet after centrifugation of 2 × 105 hCSSC in a 15-mL conical polysty-rene tube (arrow). (B) Pellet has formed a smooth sphere after 1 week of culture. (C) Identification of viable cells after staining with calcein AM (green) and dead cells using propidium iodide (red, arrowhead) after 3 weeks of culture. (D) H&E staining of hCSSC cultured 3 weeks as a pellet. (E) Flattened cells near the periphery of an hCSSC pellet. (F) H&E of a pellet formed by fibroblasts cultured for 3 weeks. (G) Stained section of hCSSC cultured in a fibrin gel for 3 weeks. (H) Fibroblasts cultured in fibrin gel. Scale bars, 50 μm.
Analysis of the two types of three-dimensional cultures was carried out after 3 weeks’ incubation under serum-free conditions. Pellets formed by hCSSC were observed to be a highly cohesive tissue-like mass resisting physical and enzymatic dissociation by trypsin (not shown). The fibroblast pellets, on the other hand, formed a much less cohesive aggregate that tended to dissociate spontaneously even after fixation. Fibrin gels populated by both cell types were soft and fragile. Cells in the two types of culture were compared using hematoxylin and eosin (H&E) staining. Pellet cultures were dense and highly cellular. hCSSC had elaborated obvious ECM between the cells (Fig. 1D), whereas fibroblast pellets (Fig. 1F) were looser and had less matrix. In some hCSSC pellets, the cells near the edges of the spheres had flattened and more abundant matrix (Fig. 1E). Cells embedded in fibrin gels, by comparison, were more sparsely distributed and had extended cellular processes. H&E staining of the intercellular regions in the populated gels was not significantly different from fibrin gels formed without cells, suggesting little ECM deposition in these cultures (Figs. 1G, 1H).
Analysis of ECM Expression
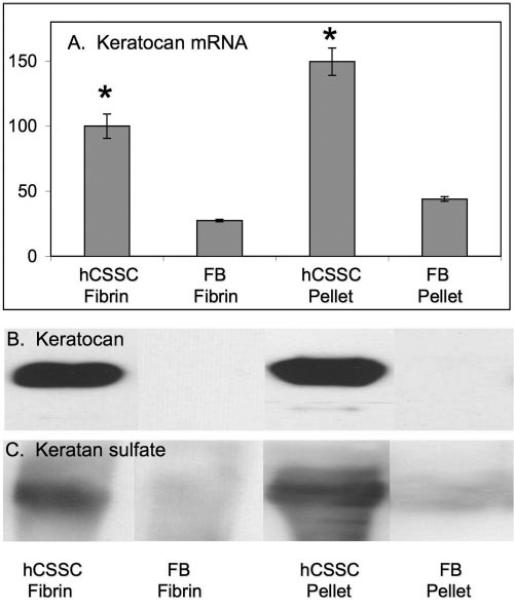
As an initial screen for keratocyte differentiation, expression of keratocan and keratan sulfate was assessed. These matrix molecules are uniquely abundant in the corneal stroma and are not expressed by hCSSC in stem cell growth media.2 Figure 2 shows quantitative real-time PCR (qRT-PCR) detection of keratocan mRNA pools (Fig. 2A). This transcript was significantly upregulated (P < 0.001) in the differentiated hCSSC in both fibrin and pellet cultures compared with fibroblasts. Keratocan expression was more elevated in pellets than in fibrin gels (P < 0.01). In proteoglycans isolated from culture media, keratocan and keratan sulfate were readily detected by immunoblotting (Figs. 2B, 2C). hCSSC cultured in fibrin gels and as pellets showed strong expression of keratocan and keratan sulfate, whereas fibroblasts had little expression of these markers. Because of the more abundant and cohesive connective tissue and the higher expression of keratocan and keratan sulfate, we conducted subsequent analyses only on pellet cultures.
Figure 2.
Keratocan and keratan sulfate expression in fibrin gel and pellet cultures. (A) Relative abundance of keratocan mRNA assayed by qRT-PCR. (B) Immunoblotting for keratocan. (C) Immunoblotting for keratan sulfate in proteoglycans isolated from culture media. hCSSC cultured in fibrin gel (hCSSC-Fibrin) and as pellets (hCSSC-Pellet) were compared with fibroblasts cultured in fibrin gel (FB-Fibrin) and as pellets (FB-Pellet). Error bars show SD of triplicate analyses. *Significant difference (P < 0.01) between fibroblasts and hCSSC in the same culture conditions.
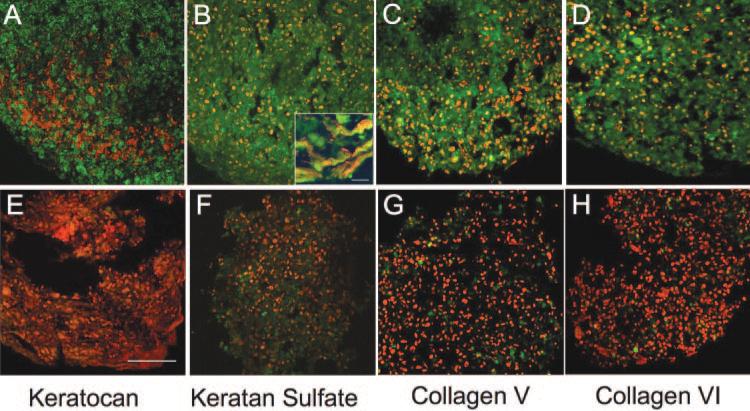
In monolayer cultures, keratan sulfate and keratocan are secreted almost exclusively into culture medium.4 The abundant matrix in the three-dimensional cultures suggested the possibility that these molecules were incorporated into this matrix. Figure 3 examined the retention of these markers in the matrix and the presence of collagens abundant in the stroma, specifically collagen types V and VI. Type 1 collagen was abundant in pellets of both fibroblasts and hCSSC (not shown). In pellet cultures with hCSSC, keratocan (Fig. 3A), keratan sulfate (Fig. 3B), collagen V (Fig. 3C), and collagen VI (Fig. 3D) were all readily detected. The Figure 3B inset shows keratan sulfate to be cell associated but also present in the ECM of the hCSSC pellet. Fibroblasts cultured under similar conditions (Figs. 3E–H) showed negative or very weak staining for each of these matrix components.
Figure 3.
Immunofluorescent staining of pellet ECM. Cryosections of 3-week pellets generated by hCSSC (A–D) and by fibroblasts (E–H) were stained for keratocan (A, E), keratan sulfate (B, F), collagen V (C, G), and collagen VI (D, H) (all green). Nuclei are counterstained in red. All are shown at the same magnification. Scale bar, 50 μm. (B, inset) Keratan sulfate (green) in the extracellular regions between cells counterstained with DiO (red). Scale bar, 5 μm.
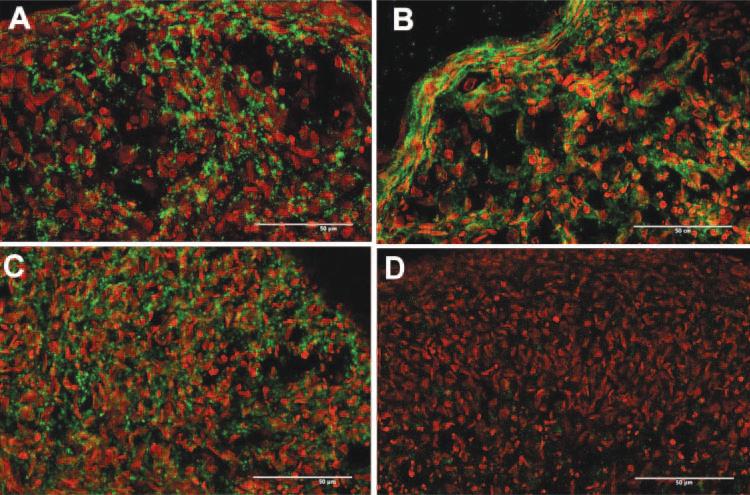
Immunofluorescent staining for connexin 43 showed this protein at cell-cell junctions throughout the cultures of pellets formed by both fibroblasts and hCSSC (Figs. 4A, 4C). The staining in hCSSC pellets appeared more intense. Cadherin 11 is a mesenchymal cell-junctional molecule present in keratocytes.11 Cadherin 11 was found throughout the hCSSC pellet but was only weakly detected in the fibroblast pellet (Figs. 4B, 4D). In the hCSSC pellet, cadherin 11 surrounded cells, suggesting its localization in cell– cell junctions. The staining was distinctly stronger in the regions of flattened cells near the periphery of the pellets (Fig. 4B).
Figure 4.
Cadherin 11 and connexin 43 in pellet cultures. Cryosections of pellets formed from hCSSC (A, B) or from fibroblasts (C, D) were stained for connexin 43 (A, C) and for cadherin 11 (B, D). Immunostaining is shown in green, and nuclei are counterstained in red. Scale bars, 50 μm.
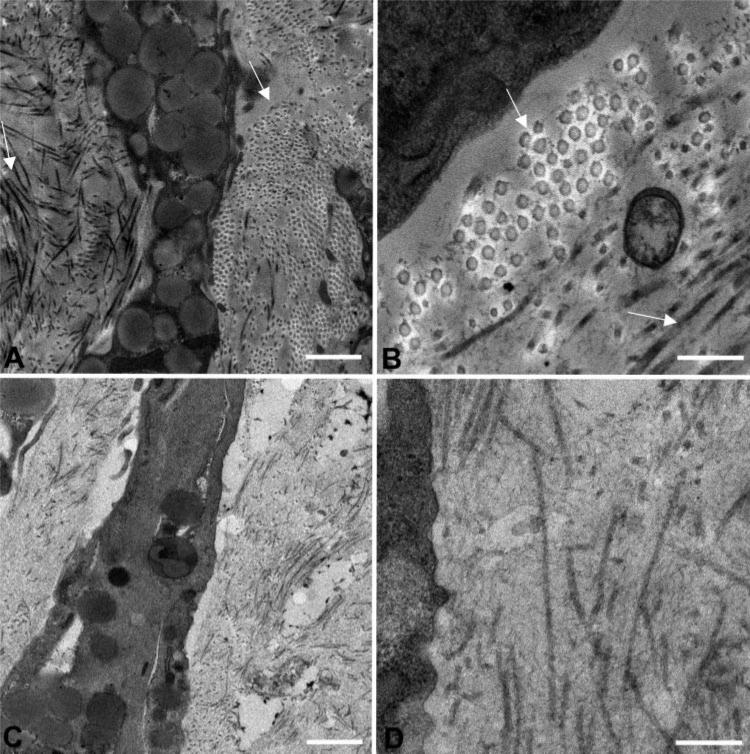
Electron Microscopy of Pellet Cultures
The ECM in the corneal stroma comprises lamellae formed of flattened bundles of collagen fibrils oriented in a parallel manner.12 Within each lamella, collagen fibrils are tightly packed and parallel and have small, uniform diameters. Electron micrographs of the pellet cultures in Figure 5 revealed abundant matrix containing fibrillar collagen deposited by the differentiating hCSSC. Regions of the culture were observed (arrows) in which collagen fibrils were clearly aligned in parallel arrays. These regions occurred most frequently near the periphery of the spheres but constituted a minority of the total matrix (Figs. 5A, 5B). As a comparison, the intercellular spaces of the fibro-blast pellet cultures showed few and relatively unorganized collagen fibrils (Figs. 5C, 5D). These results are consistent with the immunostaining results showing little collagen V (a fibrillar collagen) stained in fibroblast pellets (Fig. 3G).
Figure 5.
Transmission electron micrographs of the pellet cultures. (A, B) Morphology of hCSSC pellets. In the peripheral area, the collagen fibrils aligned in parallel arrays (arrows). (C, D) Similar sections from pellet cultures of fibroblasts. Scale bars, 1 μm (A, C); 300 nm (B, D).
Gene Expression
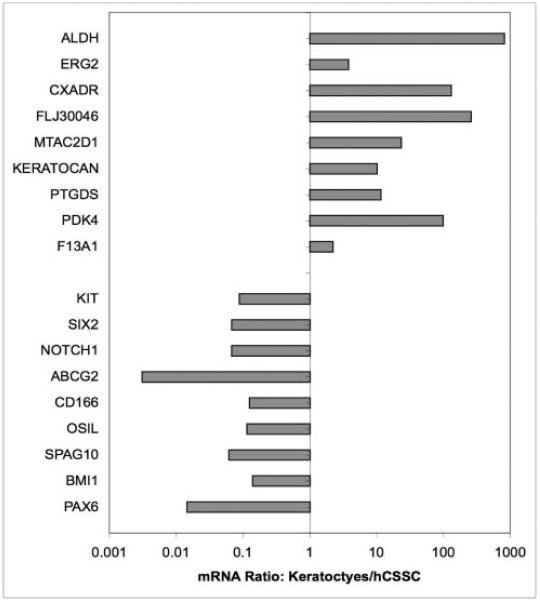
No individual gene products serve as generic markers for the stem cells derived from diverse adult tissues, but we have demonstrated that corneal stromal stem cells express ABCG2 and PAX6, whereas keratocytes have greatly reduced expression of these two gene products.2,13 Conversely, keratocytes but not hCSSC express keratocan14 and aldehyde dehydrogenase 3A1 (ALDH).15 We took advantage of the availability of pure populations of corneal stromal stem cells and keratocytes to discover genes useful in distinguishing these two cell types using gene array technology. mRNA from cultured primary keratocytes, fibroblasts, and hCSSC were compared (Affymetrix) during screening for 47,000 transcripts. Full results of this analysis will be published elsewhere, but initial screens for differentially expressed genes revealed more than 20 transcripts that differed by at least 10-fold between the two cell types. Using qRT-PCR, this differential expression was confirmed for 18 of these genes. As shown in Figure 6, seven transcripts (FLJ30046, CXADR, PTGDS, PDK4, F13A1, MTAC2D1, ERG) in addition to keratocan and ALDH were upregulated in keratocytes by 10- to 1000-fold when compared with hCSSC. Conversely, seven transcripts (KIT, SIX2, NOTCH1, CD166, OSIL, SPAG10, BMI1) in addition to ABCG2 and PAX6 were more abundant in hCSSC than in keratocytes. Not all these transcripts are well defined in terms of protein product or function; nevertheless, this list of 18 transcripts provides an expanded molecular definition of phenotypic differences in corneal stromal stem cells and keratocytes.
Figure 6.
Differential gene expression profiles of hCSSC and keratocytes. mRNA abundance relative to 18s ribosomal RNA was determined by qRT-PCR for a panel of 18 genes using cDNA from primary human keratocytes cultured in protein-free DME-F12 medium and from hCSSC in monolayer cultures in SCGM. Ratios of abundance of each transcript in the two cell types are expressed on a log scale.
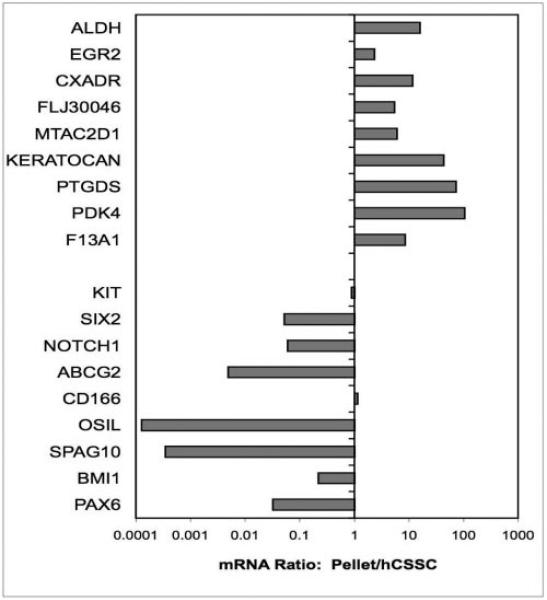
We applied this definition to a comparison of the hCSSC as monolayers and in pellet cultures. As shown in Figure 7, the ratio of the 18 target genes showed that all nine of the transcripts representing keratocyte markers were upregulated when hCSSC were cultured as pellets, many greater than 10-fold. Similarly seven of the nine hCSSC marker genes were downregulated in pellet cultures, most of these also by 10-fold. This transcription snapshot adds additional support to the conclusion that culture of hCSSC as a substratum-free pellet induces a transition of these cells to a full keratocyte phenotype.
Figure 7.
Changes in gene expression by hCSSC in pellet culture. mRNA abundance was compared from hCSSC cultured for 3 weeks as a pellet and from hCSSC in monolayer cultures in SCGM, as described in Figure 6. Ratios of abundance of each transcript between the cells cultured under different conditions are expressed on a log scale. Keratocyte marker genes are upregulated and stem cell genes are downregulated in pellets.
Discussion
Our data indicate that hCSSC, cultured as scaffolding-free pellets, differentiate into cells expressing a gene profile similar to that of human keratocytes and deposit tissue-like ECM with a composition and structure similar to that of the corneal stroma. After hCSSC were cultured as pellets for 3 weeks in a serum-free medium, they remained viable and had secreted keratocan, keratan sulfate, collagen V, and collagen VI, major components of corneal stroma ECM. By comparison, unfractionated stromal cells expanded in serum-containing media (fibroblasts) exhibited little expression and secretion of these matrix components when cultured under the same conditions. These results extended our previous findings2 that hCSSC can express the keratocyte marker keratocan, identified 18 new marker genes, and, importantly, showed deposition of stromal-like ECM. Accumulation of an organized ECM by corneal stromal cells in vitro is novel and has significant implications for corneal tissue engineering.
Primary quiescent keratocytes express differentiated products such as keratocan and keratan sulfate in vitro, but expansion of these cultures typically leads to loss of the differentiated phenotype.4 A recent report showed monkey keratocytes to maintain the expression of keratocan after multiple passages in a low-calcium medium16; however, with human cells, we were unable to maintain a full keratocyte phenotype using this medium (data not shown) and therefore adopted an approach using stem cells. Adult or “mesenchymal” stem cells are found in many non–self-renewing tissues and there are now multiple reports of such cells in the corneal stroma. In 2005, mouse stromal stem cells were identified and isolated using a sphere-forming assay.17 Shortly thereafter, we reported that cloned cells from bovine stroma spontaneously formed aggregates that detached from the substratum and continued growing as free-floating spheroids.13 In both cases these spheroids appeared to arise as a result of clonal growth of stromal stem or progenitor cells, analogous to the aggregates formed by neural stem cells (neurospheres). In the present study, stem cells were isolated from adult human stroma using FACS followed by formal cloning.2 These reports identifying stromal stem cells from three different species, each using a different method of isolation, provide strong support for the concept of a resident population of stem cells in the corneal stroma.
hCSSC do not exhibit a keratocyte phenotype; rather, they express gene products typical of adult mesenchymal stem cells. hCSSC can be expanded extensively in culture without loss of the stem cell character and after expansion, when transferred into serum-free medium containing insulin and FGF2 express keratocyte markers.2 This approach allows the generation of large numbers of keratocyte-like cells in vitro without the need to subject differentiated keratocytes to multiple cell divisions.
Using such differentiated stem cells, we were able to demonstrate, for the first time, formation of a matrix containing keratocan, keratan sulfate, collagen V, and collagen VI in vitro. The matrix was also rich in collagen I, the major fibrillar collagen in the stroma (data not shown). As shown in Figure 5, isolated regions of this matrix showed parallel alignment of collagen fibrils, suggestive of the lamellar orientation that characterizes the complex organization of corneal stroma. The pellet matrix does not approach the level of ultrastructural organization found in stroma in vivo, but the abundant matrix and the regions with some level of organization suggest that hCSSC retain the potential to organize a fully differentiated tissue. The fact that fibroblasts from the cornea do not retain the ability to secrete and organize stromal matrix suggests that this activity is dependent on embryonic or progenitor phenotype. Differentiated adult keratocytes may not, in fact, retain this ability. A key to matrix organization appears to be culture without substratum or scaffolding. Little matrix is deposited by the stem cells cultured in monolayers using the same culture medium.2 Similarly, deposition of matrix was less dense when cells were cultured in a fibrin gel. We observed an even greater lack of matrix deposition when the cells were embedded in conventional collagen gels (data not shown). The mechanism involved may relate to the increased cell-cell interactions occurring in pellets. Abundant cadherin-containing and connexin-containing junctions were observed in the pellet cultures (Fig. 4). Of these two junctions, cadherin junctions were more abundant in the hCSSC pellets than fibroblasts and were most evident in the periphery, where the more highly differentiated tissue was located (Fig. 4A). These results suggest a role for cell-cell interactions in directing secretion and organization of the matrix produced by the differentiating hCSSC. Canonical Wnt signaling, a process in which cadherin-bound beta catenin plays a role in cell division, has been shown to control the stemness and differentiation of adult stem cells in vitro and in vivo.18,19 The correlation of cadherin-containing cell-cell junctions at the sites of hCSSC differentiation to keratocytes suggests of a role for Wnt signaling in determining the differentiated state of the hCSSC.
Our work used gene array data to identify a panel of 18 genes useful in defining phenotypic differences between keratocytes and stromal stem cells. We used freshly isolated quiescent human keratocytes not exposed to serum or growth factors to identify gene expression patterns representing the differentiated state of these cells. Several of the genes identified for keratocytes were already known (keratocan, PTGDS, ALDH),2,4,15,20,21 and some were known but not in the context of the cornea. CXADR, for example, is known as the receptor for Coxsackie and Adenovirus but also appears to be an adapter protein involved in cell-cell junctions.22 PDK4 is a kinase involved in early steps of glucose metabolism and whose expression is controlled by retinoic acid.23 It has not previously been reported as upregulated in the cornea. The transcripts FLJ30046 and MTAC2D1 represent hypothetical proteins of unknown functions. It was fortuitous to observe that in the hCSSC array, a number of genes associated with embryonic development and stem cells were detected. SIX2, PAX6, and NOTCH1 are all expressed during neural crest/corneal development.24–26 BMI1, ABCG2, and KIT have all been associated previously with adult stem cells.27–30 SPAG10 is a transcript similar to lactadherin (MFGE8), a cell-associated protein involved in clearing apoptotic cells.31 OSIL (p62, sequestosome 1) is a phosphoprotein that has been linked to neural survival and differentiation.32 Even without understanding the functions these expressed genes play in maintenance of the unique phenotype of the cells, the marked change in expression patterns of the 18 genes provides a convincing argument that the hCSSC, even after more than 20 passages in vitro, switch from a stem-cell like phenotype to a keratocytes phenotype when they are cultured in a substratum-free pellet in serum-free conditions.
Taken together, our results make a strong argument that hCSSC represent adult precursors of keratocytes in vivo. The findings also point out the importance of the three-dimensional cellular environment in determining cell fate. In this work, we have also provided a more accurate means of defining the keratocyte phenotype, an assay that will serve in future studies to reveal the signaling processes involved in keratocyte differentiation and will help in designing means of developing bio-engineered corneal stroma tissue.
Acknowledgments
Supported by National Institutes of Health Grants EY016415, EY09368, EY03263, and P30-EY08098; Research to Prevent Blindness; and the Eye and Ear Foundation of Pittsburgh. JLF is a Jules and Doris Stein Research to Prevent Blindness Professor.
Footnotes
Disclosure: Y. Du, None; N. SundarRaj, None; M.L. Funder-burgh, None; S.A. Harvey, None; D.E. Birk, None; J.L. Funder-burgh, None
References
- 1.Funderburgh JL. Corneal proteoglycans. In: Iozzo R, editor. Proteoglycans: Structure, Biology, and Molecular Interactions. Marcel Dekker, Inc; New York: 2000. pp. 237–273. [Google Scholar]
- 2.Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long CJ, Roth MR, Tasheva ES, et al. Fibroblast growth factor-2 promotes keratan sulfate proteoglycan expression by keratocytes in vitro. J Biol Chem. 2000;275:13918–13923. doi: 10.1074/jbc.275.18.13918. [DOI] [PubMed] [Google Scholar]
- 5.Edward DP, Yue BY, Sugar J, et al. Heterogeneity in macular corneal dystrophy. Arch Ophthalmol. 1988;106:1579–1583. doi: 10.1001/archopht.1988.01060140747049. [DOI] [PubMed] [Google Scholar]
- 6.Funderburgh JL. Keratan sulfate: structure, biosynthesis, and function. Glycobiology. 2000;10:951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- 7.Hahn RA, Birk DE. beta-D xyloside alters dermatan sulfate proteoglycan synthesis and the organization of the developing avian corneal stroma. Development. 1992;115:383–393. doi: 10.1242/dev.115.2.383. [DOI] [PubMed] [Google Scholar]
- 8.Eyrich D, Brandl F, Appel B, et al. Long-term stable fibrin gels for cartilage engineering. Biomaterials. 2007;28:55–65. doi: 10.1016/j.biomaterials.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Wong MW, Qin L, Tai JK, Lee SK, Leung KS, Chan KM. Engineered allogeneic chondrocyte pellet for reconstruction of fibrocartilage zone at bone-tendon junction—a preliminary histological observation. J Biomed Mater Res B Appl Biomater. 2004;70:362–367. doi: 10.1002/jbm.b.30049. [DOI] [PubMed] [Google Scholar]
- 10.Xu C, Oyajobi BO, Frazer A, Kozaci LD, Russell RG, Hollander AP. Effects of growth factors and interleukin-1 alpha on proteoglycan and type II collagen turnover in bovine nasal and articular chondrocyte pellet cultures. Endocrinology. 1996;137:3557–3565. doi: 10.1210/endo.137.8.8754787. [DOI] [PubMed] [Google Scholar]
- 11.Masur SK, Conors RJ, Jr, Cheung JK, Antohi S. Matrix adhesion characteristics of corneal myofibroblasts. Invest Ophthalmol Vis Sci. 1999;40:904–910. [PubMed] [Google Scholar]
- 12.Foster CS, Azar DT, Dohlman CH. Smolin and Thoft's The Cornea: Scientific Foundations and Clinical Practice. 4th ed. Lippincott Williams & Wilkins; Philadelphia: 2004. [Google Scholar]
- 13.Funderburgh ML, Du Y, Mann MM, SundarRaj N, Funderburgh JL. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005;19:1371–1373. doi: 10.1096/fj.04-2770fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corpuz LM, Funderburgh JL, Funderburgh ML, Bottomley GS, Prakash S, Conrad GW. Molecular cloning and tissue distribution of keratocan: bovine corneal keratan sulfate proteoglycan 37A. J Biol Chem. 1996;271:9759–9763. doi: 10.1074/jbc.271.16.9759. [DOI] [PubMed] [Google Scholar]
- 15.Pei Y, Reins RY, McDermott AM. Aldehyde dehydrogenase (ALDH) 3A1 expression by the human keratocyte and its repair phenotypes. Exp Eye Res. 2006;83:1063–1073. doi: 10.1016/j.exer.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Kawakita T, Espana EM, He H, et al. Preservation and expansion of the primate keratocyte phenotype by downregulating TGF-beta signaling in a low-calcium, serum-free medium. Invest Ophthalmol Vis Sci. 2006;47:1918–1927. doi: 10.1167/iovs.05-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida S, Shimmura S, Shimazaki J, Shinozaki N, Tsubota K. Serum-free spheroid culture of mouse corneal keratocytes. Invest Ophthalmol Vis Sci. 2005;46:1653–1658. doi: 10.1167/iovs.04-1405. [DOI] [PubMed] [Google Scholar]
- 18.Cho KH, Baek S, Sung MH. Wnt pathway mutations selected by optimal beta-catenin signaling for tumorigenesis. FEBS Lett. 2006;580:3665–3670. doi: 10.1016/j.febslet.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 19.Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93:1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 20.Stramer BM, Cook JR, Fini ME, Taylor A, Obin M. Induction of the ubiquitin-proteasome pathway during the keratocyte transition to the repair fibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:1698–1706. [PubMed] [Google Scholar]
- 21.Berryhill BL, Kader R, Kane B, Birk DE, Feng J, Hassell JR. Partial restoration of the keratocyte phenotype to bovine keratocytes made fibroblastic by serum. Invest Ophthalmol Vis Sci. 2002;43:3416–3421. [PubMed] [Google Scholar]
- 22.Raschperger E, Thyberg J, Pettersson S, Philipson L, Fuxe J, Pettersson RF. The coxsackie- and adenovirus receptor (CAR) is an in vivo marker for epithelial tight junctions, with a potential role in regulating permeability and tissue homeostasis. Exp Cell Res. 2006;312:1566–1580. doi: 10.1016/j.yexcr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Kwon HS, Huang B, Ho Jeoung N, Wu P, Steussy CN, Harris RA. Retinoic acids and trichostatin A (TSA), a histone deacetylase inhibitor, induce human pyruvate dehydrogenase kinase 4 (PDK4) gene expression. Biochim Biophys Acta. 2006;1759:141–151. doi: 10.1016/j.bbaexp.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Boucher CA, Winchester CL, Hamilton GM, Winter AD, Johnson KJ, Bailey ME. Structure, mapping and expression of the human gene encoding the homeodomain protein, SIX2. Gene. 2000;247:145–151. doi: 10.1016/s0378-1119(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 25.Kanakubo S, Nomura T, Yamamura K, Miyazaki J, Tamai M, Osumi N. Abnormal migration and distribution of neural crest cells in Pax6 heterozygous mutant eye, a model for human eye diseases. Genes Cells. 2006;11:919–933. doi: 10.1111/j.1365-2443.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- 26.Kubu CJ, Orimoto K, Morrison SJ, Weinmaster G, Anderson DJ, Verdi JM. Developmental changes in Notch1 and numb expression mediated by local cell-cell interactions underlie progressively increasing delta sensitivity in neural crest stem cells. Dev Biol. 2002;244:199–214. doi: 10.1006/dbio.2002.0568. [DOI] [PubMed] [Google Scholar]
- 27.Lin T, Islam O, Heese K. ABC transporters, neural stem cells and neurogenesis—a different perspective. Cell Res. 2006;16:857–871. doi: 10.1038/sj.cr.7310107. [DOI] [PubMed] [Google Scholar]
- 28.Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113:175–179. doi: 10.1172/JCI20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt-Ott KM, Chen X, Paragas N, Levinson RS, Mendelsohn CL, Barasch J. c-Kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol. 2006;299:238–249. doi: 10.1016/j.ydbio.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Gagari E, Rand MK, Tayari L, et al. Expression of stem cell factor and its receptor, c-kit, in human oral mesenchymal cells. Eur J Oral Sci. 2006;114:409–415. doi: 10.1111/j.1600-0722.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 31.Shi J, Shi Y, Waehrens LN, Rasmussen JT, Heegaard CW, Gilbert GE. Lactadherin detects early phosphatidylserine exposure on immortalized leukemia cells undergoing programmed cell death. Cytometry A. 2006;69:1193–1201. doi: 10.1002/cyto.a.20345. [DOI] [PubMed] [Google Scholar]
- 32.Joung I, Kim HJ, Kwon YK. p62 modulates Akt activity via association with PKCzeta in neuronal survival and differentiation. Biochem Biophys Res Commun. 2005;334:654–660. doi: 10.1016/j.bbrc.2005.06.138. [DOI] [PubMed] [Google Scholar]