Abstract
The brain is dependent on glucose as a primary energy substrate, but is capable of utilizing ketones such as β-hydroxybutyrate (βHB) and acetoacetate (AcAc), as occurs with fasting, prolonged starvation or chronic feeding of a high fat/low carbohydrate diet (ketogenic diet). In this study, the local cerebral metabolic rate of glucose consumption (CMRglu; μM/min/100g) was calculated in the cortex and cerebellum of control and ketotic rats using Patlak analysis. Rats were imaged on a rodent PET scanner and MRI was performed on a 7-Tesla Bruker scanner for registration with the PET images. Plasma glucose and βHB concentrations were measured and 90-minute dynamic PET scans were started simultaneously with bolus injection of 2-Deoxy-2[18F]Fluoro-D-Glucose (FDG). The blood radioactivity concentration was automatically sampled from the tail vein for 3 min following injection and manual periodic blood samples were taken. The calculated local CMRGlu decreased with increasing plasma BHB concentration in the cerebellum (CMRGlu = −4.07*[BHB] + 61.4, r² = 0.3) and in the frontal cortex (CMRGlu = −3.93*[BHB] + 42.7, r² = 0.5). These data indicate that, under conditions of ketosis, glucose consumption is decreased in the cortex and cerebellum by about 10% per each mM of plasma ketone bodies.
1. INTRODUCTION
Neurodegeneration after oxidative stress limits the recovery of tissue response and appears to be caused by impaired glycolysis. If indeed there is a defect in glucose metabolism it might be beneficial to supplement energy metabolism with an alternate substrate. It was suggested that brain can supplement glucose as the principal energy substrate with ketone bodies1–3 without altering oxygen consumption4,5. Classic studies of ketosis induced by fasting or starvation in humans showed that brain function was maintained which was attributed to the utilization (oxidation) of ketone bodies as alternate energy substrates to glucose by the brain6. Rats that have been fasted for 2–3 days showed no difference in cerebral blood flow (CBF) or CMRO27.
One mechanism by which ketosis might be beneficial is through the metabolic step where ketones enter the TCA cycle at the level of citrate bypassing glycolysis, the step after pyruvate dehydrogenase complex where the enzyme activity is often impaired. Through feed-back regulation, ketones are known to down regulate glycolytic rates at various levels such as citrate, phosphofructokinase and/or hexokinase. In addition, particularly in brain, ketones are a carbon source for glutamate (anaplerosis) and thus help to balance glutamate/glutamine homeostasis through stabilization of energy metabolism in astrocyte following recovery from a hypoxic/ischemic event.
Based on our experiments and evidence in the literature, we have developed the hypothesis that ketones are effective against pathology associated with altered glucose metabolism, the rationale being that ketosis helps to regulate glucose metabolism. In this study, the effects of ketosis on the local cerebral metabolic rate of glucose consumption (CMRglu) were investigated in an in vivo rat model of ketosis using positron emission tomography (PET) with 2-[18F] fluoro-2-deoxy-D-glucose (FDG).
2. MATERIALS AND METHODS
Animal preparation and Diets
All procedures were performed in strict accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. Adult male Wistar rats (final weights: 175–250 g) were allowed to acclimate in the CWRU animal facility for one week before experiments. All rats were housed two-three in a cage, maintained on a 12:12 light-dark cycle with standard/ketogenic rat chow and water available ad libitum.
Diet Protocols
The ketogenic (KG) diet was purchased from Research Diets (New Brunswick, NJ, U.S.A.) and standard (STD) diet, Teklab 8664, was provided by the CWRU animal facility (see table 1). The KG diet protocol was chosen for its proven effectiveness for inducing moderate and stable ketosis in young adult rats and for its palatability8–10.
Table 1.
Diet composition
| Fat (%) | Protein (%) | Carbohydrate (%) | |
|---|---|---|---|
| Ketogenic (KG) | 89.5 | 10.4 | 0.1 |
| Standard (STD) | 27.5 | 20.0 | 52.6 |
Surgical Procedures
On the day of the experiment, anesthesia was induced by gas mixture (3% isoflurane in O2) and maintained with 1–2% isoflurane in O2 through a nasal cone. Ketogenic and control rats underwent the same procedures. Cannulae were placed in: (i) a ventral tail artery using polyethylene tubing (PE-50, 0.023″ i.d., 0.038″ o.d.), plasma glucose, lactate and ketone concentrations (ii) an external jugular vein into the right atrium using a silastic catheter (0.025″ i.d., 0.047″ o.d.) for FDG injection.. Lidocaine (1%) was infiltrated on the site. About 2cm incision was then made on the area to be cannulated and the tail artery was isolated. An intramedic(R) polyethylene tubing, size PE 50 with an I.D. of 0.58mm and O.D. of 0.965mm was then advanced into the artery. Two sutures were used to secure the catheter in place while the other end of the catheter was connected through a tubing to a Blood Pressure Analyzer for continuous recording and monitoring of the animal’s blood pressure. The neck area was also infiltrated with 1% lidocaine solution. An incision was made and the external jugular vein was isolated and catheterized with a silastic tubing (0.025″ i.d., .047″ o.d.) which was advanced into the right atrium. The catheter was secured and the incision wound closed with one or two sutures. The other end of the catheter was used for administration of the radioactive indicator to the animal. A small blood sample taken by tail stick from the KG group on the day of surgery was tested using a MediSense Precision Xtra ketone meter to document ketonemia (BHB > 1.0 mM).
MicroPET analysis
The animal was placed on a bed apparatus and moved into a 7-Tesla Bruker scanner for magnetic resonance imaging (MRI) to get anatomical reference for PET imaging. After MRI, the apparatus was moved into the gantry of a Concord Microsystems R4 microPET (Siemens, Knoxville, TN). A transmission scan was performed with a cobalt (57Co) point source for attenuation correction. A 90-minute dynamic PET scan was started simultaneously with bolus injection of 10 MBq/100 g of FDG. Plasma glucose, lactate and BHB concentrations were directly measured. The blood radioactivity concentration was automatically sampled from the tail artery for 3 min using a blood acquisition monitor (BAM) and manual blood samples were taken at 3.5, 4, 4.5, 5, 7, 10, 15, 30, 45, 60, 75 and 90 min. The emission data were rebinned into 17 time frames (3 × 20 sec, 2 × 1 min, 1 × 2 min, 6 × 5 min, 6 × 10 min). ROIs were manually defined on the frontal cerebral cortex and the cerebellum using the high resolution MRI images and mapped onto dynamic PET images. Patlak analysis was finally applied to extract the CMRGlu in both tissue types, assuming a lumped constant (LC) of 0.70511,12.
3. RESULTS
The diet conditions did not affect the physiological parameters body weight, plasma BHB, plasma glucose and plasma lactate (Table 2). All rats in each of the groups gained weight consistently over the experimental period. Weight gains did not show any significant differences between diet groups as well as with plasma lactate and glucose. BHB levels in blood were significantly higher in KG compared to the STD diet group.
Table 2.
Physiological Parameters (mean ± standard deviation).
| STD (n=6) | KG (n=10) | |
|---|---|---|
| Blood BHB, mM | 0.9 ± 0.6 | 3.78 ± 1.1* |
| Plasma glucose, mM | 10.73 ± 3.78 | 10.63 ± 1.55 |
| Plasma lactate, mM | 1.123 ±0.46 | 0.80 ± 0.06 |
| Weights, g | 267.56 ± 59.11 | 252.71 ± 47.45 |
p < 0.05
The CMRglu calculated with Patlak analysis was plotted as a function of the measured blood BHB concentration (Figure 1). These data showed that CMRglu decreases with increasing ketosis in brain of rats that were either short-term fasted or fed a KG diet for three weeks, (the blood [BHB] “0’ value represents non-ketotic rats). The calculated CMRglu linearly decreased with increasing blood ketone concentration in the frontal cortex (CMRGlu = −3.9* [BHB] + 42.7; R² = 0.484) (data not shown) and in the cerebellum (CMRGlu = −4.1*[BHB] + 61.4; R² = 0.30). A linear relationship between the CMRGlu in the frontal cortex and cerebellum was also observed (R² = 0.881). Most striking is that the data show a 10% decrease in CMRglu for every 1mM increase in blood ketone (BHB) levels. These data are consistent with our previous studies that, in combination with increased ketosis and increased transport of ketone bodies at the blood brain barrier, there are more ketones available for brain metabolism10
Figure 1.
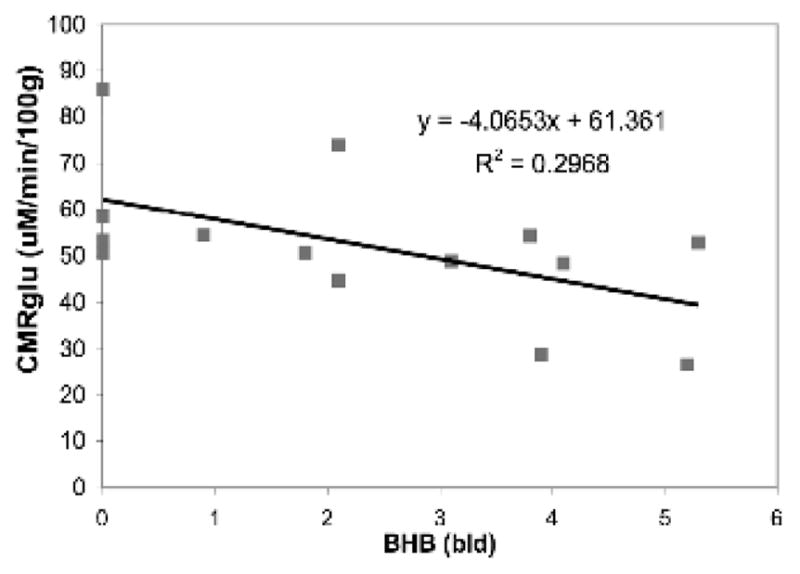
Cerebellum CMRglu.
Each rat was scanned on the PET scanner for 90 minutes post-injection and the list-mode data was rebinned in 17 timeframes. The PET and MRI images were registered and ROIs were drawn on the cerebellum and cortex (Figure 2 ). The micro PET data were used to detect upregulation of glycolysis and it provided insight into fates of glucose uptake and metabolism (CMRglu) during acute and chronic ketosis in rat brain. Pet scans using 18FDG, were used to examine the relationship between glucose uptake (CMRglu) and ketosis (ketone plasma levels). Our results showed differences in 18FDG uptake in ketotic vs non-ketotic rat brain.
Figure 2.
MicroPET images of rat brain.
4. DISCUSSION
From these data, we estimated that in the non-ketotic state with a blood concentration of 0.04 mM BHB and a cerebral blood flow of 1.0 ml/g/min, an extraction fraction of 8%10 is expected to result in a ketone influx rate of about 3 nmol/g/min. For ketone bodies to replace at least 10% of glucose oxidation an increase in blood ketone concentration and an up regulation of monocarboxylate transporters is required. The utilization of ketones has been shown to be proportional to the uptake3,13. Thus, for a 1.0 mM blood BHB, a 12% extraction fraction and a cerebral blood flow of 1.0 ml/g/min a ketone influx of 120 nmol/g/min can be expected. Glucose consumption rates in the anesthetized rat cerebral cortex are approximately 500 nmol/g/min, thus there would be sufficient ketone flux to replace 10% of the glucose oxidation.
It is hypothesized that ketone bodies play a neuroprotective role through an improvement in metabolic efficiency, by sparing glucose, and the degradation of muscle-derived amino acids for substrates15. During hypoxia, ketone bodies have been shown to be neuroprotective16,17 by depressing glucose uptake and CMRglu possibly due to metabolic bocks as a result of oxidative damage. Ketone bodies are thought to stabilize the lactate/pyruvate ratio and bypass the metabolic blocks associated with oxidative stress induced impairment of glucose metabolism.
Acknowledgments
The authors would like to thank Deb Sim, Dean Fang and John Richey for their technical support with the imaging work. This research has been supported by the National Institutes of Health, R01-NS38632 and P50 GM066309.
References
- 1.Hawkins RA, Biebuyck JF. Ketone bodies are selectively used by individual brain regions. Science. 1979;205:325–327. doi: 10.1126/science.451608. [DOI] [PubMed] [Google Scholar]
- 2.Hasselbalch SG, Madsen PL, Hageman LP, Olsen KS, Justesen N, Holm S, Paulson OB. Changes in cerebral blood flow and carbohydrate metabolism during acute hyperketonemia. Am J Physiol. 1996;270:E746–E751. doi: 10.1152/ajpendo.1996.270.5.E746. [DOI] [PubMed] [Google Scholar]
- 3.Balasse EO, Fery F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev. 1989;5:247–270. doi: 10.1002/dmr.5610050304. [DOI] [PubMed] [Google Scholar]
- 4.Corddry DH, Rapoport SI, London ED. No effect of hyperketonemia on local cerebral glucose utilization in conscious rats. J Neurochem. 1982;38:1637–1641. doi: 10.1111/j.1471-4159.1982.tb06644.x. [DOI] [PubMed] [Google Scholar]
- 5.Ruderman NB, Ross PS, Berger M, Goodman MN. Regulation of glucose and ketone-body metabolism in brain of anaesthetized rats. Biochem J. 1974;138:1–10. doi: 10.1042/bj1380001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF., jr Brain metabolism during fasting. J Clin Invest. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlquist G, Persson B. The rate of cerebral utilization of glucose, ketone bodies, and oxygen: a comparative in vivo study of infant and adult rats. Pediatr Res. 1976;10:910–917. doi: 10.1203/00006450-197611000-00002. [DOI] [PubMed] [Google Scholar]
- 8.al Mudallal AS, Levin BE, Lust WD, Harik SI. Effects of unbalanced diets on cerebral glucose metabolism in the adult rat. Neurol. 1995;45:2261–2265. doi: 10.1212/wnl.45.12.2261. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mudallal AS, LaManna JC, Lust WD, Harik SI. Diet-induced ketosis does not cause cerebral acidosis. Epilepsia. 1996;37:258–261. doi: 10.1111/j.1528-1157.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 10.Puchowicz MA, Xu K, Sun X, Ivy A, Emancipator D, LaManna JC. Diet-induced ketosis increases capillary density without altered blood flow in rat brain. Am J Physiol Endocrinol Metab. 2007 doi: 10.1152/ajpendo.00512.2006. [DOI] [PubMed] [Google Scholar]
- 11.Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–1531. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins RA, Mans AW, Davis DW. Regional ketone body utilization by rat brain in starvation and diabetes. Am J Physiol. 1986;250:E169–E178. doi: 10.1152/ajpendo.1986.250.2.E169. [DOI] [PubMed] [Google Scholar]
- 14.Puchowicz MA, Emancipator DS, Xu K, Magness DL, Ndubuizu OI, Lust WD, LaManna JC. Adaptation to chronic hypoxia during diet-induced ketosis. Adv Exp Med Biol. 2005;566:51–57. doi: 10.1007/0-387-26206-7_8. [DOI] [PubMed] [Google Scholar]
- 15.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Chang ASY, D’Alecy LG. Hypoxia and beta-hydroxybutyrate acutely reduce glucose extraction by the brain in anesthetized dogs. Can J Physiol Pharmacol. 1993;71:465–472. doi: 10.1139/y93-068. [DOI] [PubMed] [Google Scholar]
- 17.Masuda R, Monahan JW, Kashiwaya Y. D-beta-hydroxybutyrate is neuroprotective against hypoxia in serum-free hippocampal primary cultures. J Neurosci Res. 2005;80:501–509. doi: 10.1002/jnr.20464. [DOI] [PubMed] [Google Scholar]