Abstract
OBJECTIVE
Changes in glucose metabolism occurring during counterregulation are, in part, mediated by increased plasma free fatty acids (FFAs), as a result of hypoglycemia-activated lipolysis. However, it is not known whether FFA plays a role in the development of posthypoglycemic insulin resistance as well.
RESEARCH DESIGN AND METHODS
We conducted a series of studies in eight healthy volunteers using acipimox, an inhibitor of lipolysis. Insulin action was measured during a 2-h hyperinsulinemic-euglycemic clamp (plasma glucose [PG] 5.1 mmo/l) from 5:00 p.m. to 7:00 p.m. or after a 3-h morning hyperinsulinemic-glucose clamp (from 10 a.m. to 1:00 p.m.), either euglycemic (study 1) or hypoglycemic (PG 3.2 mmol/l, studies 2–4), during which FFA levels were allowed to increase (study 2), were suppressed by acipimox (study 3), or were replaced by infusing lipids (study 4). [6,6-2H2]-Glucose was infused to measure glucose fluxes.
RESULTS
Plasma adrenaline, norepinephrine, growth hormone, and cortisol levels were unchanged (P > 0.2). Glucose infusion rates (GIRs) during the euglycemic clamp were reduced by morning hypoglycemia in study 2 versus study 1 (16.8 ± 2.3 vs. 34.1 ± 2.2 μmol/kg/min, respectively, P < 0.001). The effect was largely removed by blockade of lipolysis during hypoglycemia in study 3 (28.9 ± 2.6 μmol/kg/min, P > 0.2 vs. study 1) and largely reproduced by replacement of FFA in study 4 (22.3 ± 2.8 μmol/kg/min, P < 0.03 vs. study 1). Compared with study 2, blockade of lipolysis in study 3 decreased endogenous glucose production (2 ± 0.3 vs. 0.85 ± 0.1 μmol/kg/min, P < 0.05) and increased glucose utilization (16.9 ± 1.85 vs. 28.5 ± 2.7 μmol/kg/min, P < 0.05). In study 4, GIR fell by ∼23% (22.3 ± 2.8 μmol/kg/min, vs. study 3, P = 0.058), indicating a role of acipimox per se on insulin action.
CONCLUSION
Lipolysis induced by hypoglycemia counterregulation largely mediates posthypoglycemic insulin resistance in healthy subjects, with an estimated overall contribution of ∼39%.
Physiological responses to insulin-induced hypoglycemia in humans are well established (1,2). Timely increments in secretion of counterregulatory hormones and specific symptom appearance prevent further fall in plasma glucose concentration (3). Counterregulatory hormones are all similarly critical in defense against hypoglycemia (3). These “anti-insulin” responses last several hours after a hypoglycemic episode ends (4). This condition of posthypoglycemic insulin resistance translating into postmeal hyperglycemia was described first by Somogyi (5) after his observation that overtreatment with evening regular insulin can result in hyperglycemia the following morning (6). In the clinical setting, this process can contribute to the instability of the metabolic control in patients with diabetes (7). With intermediate- and long-acting insulin as well as continuous subcutaneous insulin infusion currently available, fasting hyperglycemia after nocturnal hypoglycemia is either infrequent (7) or modest (8). Posthypoglycemic insulin resistance nevertheless results in significant postmeal hyperglycemia (9).
Mintz et al. (10) and Oakley et al. (11) described reduced rebound hyperglycemia after hypoglycemia in hypophysectomized patients, proposing a role for growth hormone and cortisol in the pathogenesis of posthypoglycemic insulin resistance, whereas Popp et al. (12) documented impaired glucose recovery from acute hypoglycemia induced by an intravenous insulin bolus after β-adrenergic blockade, suggesting an involvement of catecholamines, at least in the acute phase. In the mid-1980s, Bolli et al. (13) provided evidence that posthypoglycemic hyperglycemia in patients with type 1 diabetes is the result of counterregulatory hormonal response to hypoglycemia in concert with prevalent plasma insulin concentration, and that all of the hormones but glucagon may play a role. Long-lasting posthypoglycemic insulin resistance (up to 7–8 h) is induced in its early phase primarily by epinephrine response and in its late phase by growth hormone and cortisol (14–19). The mechanisms by which the counterregulatory hormones adrenaline, growth hormone, and cortisol induce posthypoglycemic insulin resistance are attributed to increased endogenous glucose production (liver and kidney) and suppressed glucose utilization (peripheral tissues, mainly muscles). However, it is possible that other mechanisms, i.e., indirect mechanisms, may also contribute. Indeed, earlier observations (20,21) indicate that activation of lipolysis, i.e., an increase in plasma free fatty acids (FFAs) and glycerol, plays a critical role in mediating the effects of catecholamines and other lipolytic, counterregulatory hormones in the defense against acute, insulin-induced hypoglycemia. It is conceivable that the same mechanisms continue to operate immediately after hypoglycemia and contribute to insulin resistance in subsequent hours.
The present series of studies was undertaken 1) to establish whether increased availability of FFA substrate after lipolysis and/or lipid oxidation in response to acute, insulin-induced hypoglycemia plays a role in the pathogenesis of posthypoglycemic insulin resistance, and if so, 2) to quantitate its contribution, and 3) to determine whether its effects are mediated by the liver or peripheral tissues, or both.
RESEARCH DESIGN AND METHODS
Subjects.
The study was carried out according to the Declaration of Helsinki after obtaining written informed consent from all subjects. Eight healthy volunteers (three women and five men) with no family history of diabetes or other endocrine diseases and who were not taking any medications participated in the study, which had been approved by the local ethics committee. Their mean (± SE) age was 28 ± 1.8 years and their mean BMI (kg/m2) was 22.8 ± 0.7. All subjects were studied on four different occasions, in random order, with an interval between studies of at least 2 weeks.
Protocol.
On all occasions, subjects presented to the Clinical Research Center of the Department of Internal Medicine, Endocrinology and Metabolism, University of Perugia at 6:00 a.m. after an overnight fast of 10 h. They were placed on bed rest and maintained a supine position until the end of the experiments at 7:00 p.m. To obtain arterialized-venous blood samples, a dorsal vein of a hand was cannulated retrogradely with a 18-gauge catheter needle, and the hand was maintained at 65°C in a thermoregulated Plexiglas box (22). An antecubital vein of the contralateral arm was cannulated with an 18-gauge catheter needle for infusions. Insulin, stable isotope–labeled tracers, and variable glucose (20% solution) were infused in all studies, whereas a heparin and lipid solution was infused only in study 4. Potassium chloride at the rate of 5 mEq/h was also infused along with saline in all clamp studies to prevent hypokalemia. All infusions were performed with separate syringe pumps (Harvard Apparatus, Inc., The Ealing Co., South Natick, MA). Both forearm venous lines were kept patent by saline solution infused at a rate of 30 ml/h. At 7:00 a.m., a primed 16-μmol/kg sterile, pyrogen-free constant infusion (0.22 μmol/kg/min) of [6,6-2H2]-glucose (Cambridge Isotopes Laboratories, Cambridge, MA) was started and maintained throughout to determine glucose kinetics as previously described (23,24). Three hours were allowed for isotopic equilibration, after which baseline blood samples were taken. Euglycemic and hypoglycemic clamps were achieved by a variable rate of infusion of 20% glucose enriched to 2.5% with [6,6-2H2]-glucose, to avoid non–steady-state errors in measurement of glucose turnover (25) and to maintain a blood glucose concentration at euglycemia (5.5 mmol/l) and hypoglycemia (3.2 mmol/l), respectively.
Lipid and carbohydrate oxidation expenditure were measured in all subjects by indirect calorimetry (26) for a 30-min period at baseline (−30 to 0 min) and during the last 30 min of each hour throughout. At 45 min before beginning experiments, a transparent plastic ventilated hood was placed over the subject's head and made airtight around the neck. Air flow and O2 and CO2 concentrations in the expired and inspired air were measured by a computerized continuous open-circuit system (Deltatrac; Datex Instruments, Helsinki, Finland) (27) that has a precision of 2.5% for oxygen consumption and 1.0% for carbon dioxide production. Protein oxidation was estimated from urinary excretion of urea.
Subjects underwent either a 3-h hyperinsulinemic-euglycemic (study 1), or hypoglycemic clamp (studies 2–4) in the morning between 10:00 a.m. and 1:00 p.m., (time segment 0–180 min [t1]). In studies 3–4, acipimox, an inhibitor of lipolysis, was given to suppress lipolysis. In study 4, to quantify the effects of acipimox per se on glucose metabolism, a lipid emulsion and heparin were infused to reproduce plasma FFA and glycerol concentrations similar to those of study 2 with spontaneous hypoglycemic activation of lipolysis. In t1, regular insulin (Eli Lilly Italia SpA), diluted to 1 unit/ml in 100 ml of saline solution containing 2 ml of the subject's blood, was infused at the rate of 1 mU/kg/min. Glucose was infused at variable rate to maintain euglycemia in study 1, whereas hypoglycemia (3.2 ± 0.1 mmol/l) was allowed to occur in studies 2–4. Acipimox 250 mg (5-methyl-pyrazene-carboxylic acid 4-oxide, Olbetam; Pfizer Italia srl, Latina, Italy) was given orally at 0 and 180 min to inhibit lipolysis in studies 3–4. To establish whether acipimox had effects other than antilipolysis, a triglyceride emulsion of 10% Intralipid (Fresenius Kabi, Verona, Italy; 10% soybean oil, 1.2% egg yolk phospholipids, and 2.25% glycerol) and heparin (Normoparin, heparin sodium; Farmaceutici Caber SpA, Ferrara, Italy) was infused at a variable rate (up to 1 ml/min and 0.2 units/kg/min for Intralipid and heparin, respectively) in study 4 to reproduce the increase in FFAs and glycerol observed in study 2. At 420 min, lipid/heparin infusion was halved (lipids 0.5 ml/kg/min and heparin 0.1 unit/kg/min), and at 480 min the heparin infusion was further reduced to 0.05 units/kg/min. Intralipid and heparin infusion rates were chosen based on pilot experiments as well as experience from previous studies in our laboratory (20).
At 1:00 p.m. (180 min) insulin infusion was stopped and euglycemia was recovered with variable glucose infusion in the time segment 180–420 min (t2) in all studies. At 4:00 p.m. (360 min), another capsule of acipimox 250 mg was given to maintain suppression of lipolysis in studies 3–4. Between 5:00 and 7:00 p.m. (time segment 420–540 min [t3]) subjects underwent a 2-h hyperinsulinemic-euglycemic clamp to measure insulin sensitivity. Insulin infusion was started again at 420 min in t3 at the constant rate of 1 mU/kg/min together with glucose infused at a variable rate to maintain euglycemia throughout. After collection of the final samples at 540 min, the subjects were fed. Finally, when plasma glucose was stable, intravenous lines were removed and the subjects discharged.
Analyses.
Arterialized blood samples were taken before beginning the isotope infusion to determine background glucose enrichments. To determine glucose concentrations and kinetics, arterialized blood samples were taken every 10 min during the last 30 min of the basal period and every 20 min during the insulin clamps. All blood samples were drawn into tubes containing EDTA and centrifuged. Plasma was stored at −80°C. Glucose enrichment was determined on its penta-acetate (penta-O-acetyl-β-d-glucopyranose) derivative by gas chromatography–mass spectrometry (gas chromatography HP 5890 II, mass spectrometry HP 5972A; Hewlett-Packard, Palo Alto, CA) in electron impact ionization mode monitoring the ions 200 and 202 for the unlabeled and d-[6,6-2H2]glucose, respectively (24). To maintain euglycemia and hypoglycemia, arterialized blood glucose was measured every 3–7 min (Beckman Glucose Analyzer II; Beckman Instruments, Fullerton, CA). Blood samples were collected at 30-min intervals and assayed for alanine (28), insulin (20), glucagon (20), cortisol (20), growth hormone (20), adrenaline and norepinephrine (20), FFA (Wako NEFA C test kit; Wako Chemicas, Neuss, Germany), 3-β-OH-butyrate (28), and glycerol (28). For FFA determination, blood (2 ml) was collected in tubes containing 50 μl of the lipoproteinlipase inhibitor diethyl-p-nitrophenyl-phosphate (Paraoxon; Sigma Chemical, St. Louis, MO) diluted to 0.04% in diethyl ether (29). Urine was collected from the onset to the end of each study period to determine nitrogen excretion using the Kjeldahl method (30).
Calculations.
Oxidation rates for carbohydrate and fat were calculated from indirect calorimetric measurements by averaging the data over the 30 min of measurements during each hour (31). Nonoxidative glucose utilization was calculated by subtracting the rate of glucose oxidation from the total rate of glucose uptake (31). Protein oxidation rate was measured from urinary nitrogen excretion before and during insulin infusion adjusted for changes in serum urea during insulin infusion (32).
Tracer-to-tracee ratio for glucose was calculated as the ratio between the master peak (M) and the enriched peak (M+2) after subtracting the background enrichment. The calculations were based on a steady-state assumption. For glucose, the total rate of appearance (Ra) and disappearance (Rd) was calculated as follows (μmol/kg/min): Ra = (Ftotal/Eglucose) − GIR and Rd = (Ftotal/Eglucose). Ftotal is the total infusion rate of glucose tracer (μmol/kg/min). Eglucose is the enrichment of glucose in plasma (tracer-to-tracee ratio). Glucose infusion rate (GIR) is the exogenous glucose administered during the clamp.
Statistics.
Data in text are given as means ± SE. Statistical analysis was performed by using mixed-model repeated-measures ANOVA, with Huynh-Feldt adjustment for nonsphericity. Post hoc comparisons (Newman-Keuls test) were carried out to pinpoint specific differences on significant interaction terms. P < 0.05 was considered to indicate statistically significant difference. A sample size of eight was chosen based on the calculation that it achieves 88% power to detect a difference of 6.6 μmol/kg/min between study 3 (lipolysis blocked by acipimox) and study 4 (lipolysis blocked by acipimox and plasma FFAs replaced) with an SD of 6.0 μmol/kg/min and a significance level (alpha) of 0.05 using a two-sided one-sample t test. We conducted the statistical analyses using NCSS/PASS 2007 software (Kaysville, UT).
RESULTS
Plasma glucose and insulin concentrations and rates of glucose infusion.
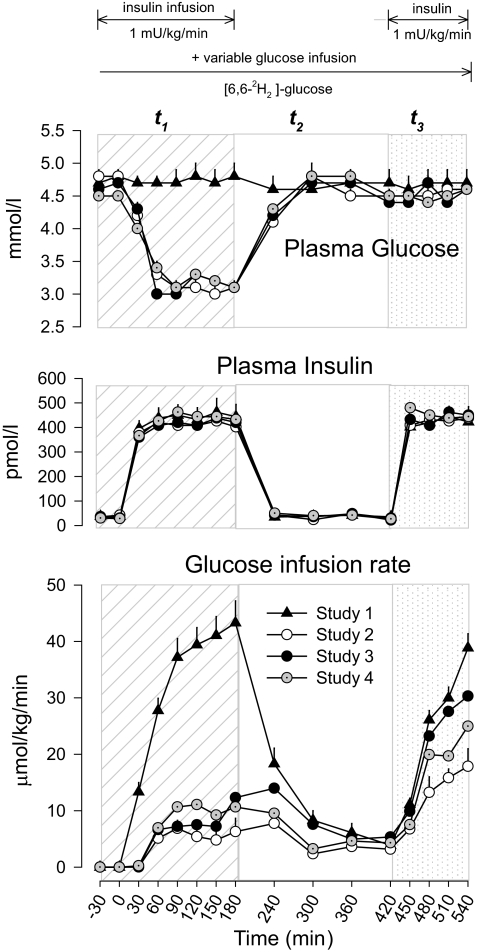
In t1, plasma glucose was maintained at baseline euglycemia in study 1 (hyperinsulinemic-euglycemic clamp; Fig. 1). In studies 2–4, plasma glucose was allowed to decrease to a nadir of 3.2 ± 0.1 mmol/l between 30 and 180 min (P > 0.2 between studies 2 and 4). Thereafter, plasma glucose increased to euglycemic levels of study 1 by 300 min and remained euglycemic until the end of the study (540 min).
FIG. 1.
Plasma glucose, insulin concentrations, and rates of glucose infusion in study 1 (euglycemia), study 2 (hypoglycemia), study 3 (hypoglycemia + acipimox), and study 4 (hypoglycemia + acipimox + heparin + intralipid). The diagonal area depicts t1 (0–180 min, euglycemia or hypoglycemia), the white area depicts t2 (180–420 min, euglycemia or recovery to hypoglycemia), and the dotted area depicts t3 (420–540 min, euglycemic clamp) of each study.
Plasma insulin was not different in the four studies.
Hypoglycemia in study 2 resulted in lower glucose infusion rates required to maintain euglycemia between 300 and 420 min of t2 compared with euglycemic study 1 (3.1 ± 1.3 vs. 6.1 ± 1.5 μmol/kg/min, P = 0.037). However, when lipolysis was blocked by acipimox in study 3, the rate of glucose infusion increased to values similar to those of study 1 (5.9 ± 6.1 vs. 12 ± 2.2 μmol/kg/min, P > 0.2). Finally, when FFAs and glycerol were replaced in study 4, the rate of glucose infusion was again reduced to values similar to those of study 2 (4.1 ± 1.9 vs. 3.1 ± 1.3 μmol/kg/min, P = 0.124). In the hyperinsulinemic-euglycemic clamp of t3 (420–540 min), the rate of glucose infusion required to maintain euglycemia was reduced by morning hypoglycemia in study 2 (510–540 min, 16.8 ± 2.3 vs. 34.1 ± 2.2 μmol/kg/min, study 2 vs. study 1, respectively, P < 0.001), but the effect was largely removed by blockade of lipolysis during hypoglycemia in study 3 (28.9 ± 2.6 μmol/kg/min, P > 0.2 vs. study 1), and largely reproduced by replacement of FFAs and glycerol in study 4 (22.3 ± 2.8 μmol/kg/min, P < 0.03 vs. study 1) (Fig. 1).
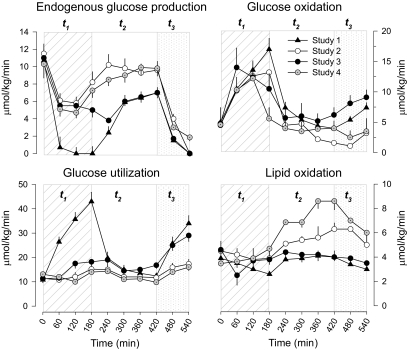
Rates of endogenous glucose production, glucose utilization, and glucose and lipid oxidation.
Hypoglycemia in study 2 compared with study 1 euglycemia resulted in increase in endogenous glucose production, suppression of utilization and oxidation of glucose, and stimulation of lipid oxidation in t2 (Fig. 2). In particular, endogenous glucose production and lipid oxidation were significantly greater (9.0 ± 0.9 vs. 5.9 ± 0.3 μmol/kg/min, P = 0.01, 5.5 ± 0.5 vs. 4.0 ± 0.4 μmol/kg/min, P = 0.07, study 2 vs. 1, respectively). Blockade of lipolysis (study 3) largely reversed these effects, which were reproduced by replacement of FFAs and glycerol (study 4). In addition, in t3, the morning hypoglycemia of study 2 compared with the euglycemic study 1, respectively resulted in lower suppression of endogenous glucose production (2 ± 0.3 vs. 0.1 ± 0.1 μmol/kg/min), lower oxidation (4.2 + 0.45 vs. 6.95 + 0.7 μmol/kg/min, P < 0.01) and utilization (16.9 ± 1.85 vs. 31.9 ± 3.15 μmol/kg/min, P = 0.001) of glucose, and less suppression of lipid oxidation (5 ± 0.45 vs. 3.1 ± 0.3 μmol/kg/min, P = 0.01). However, blockade of lipolysis (study 3) largely reversed all these changes (endogenous glucose production 0.85 ± 0.1 μmol/kg/min, glucose oxidation 8.8 ± 1.05 μmol/kg/min, glucose utilization 28.5 ± 2.7 μmol/kg/min, lipid oxidation 2.9 ± 0.25 μmol/kg/min). Finally, replacement of FFAs and glycerol largely reproduced the effect observed in study 2 (Fig. 2).
FIG. 2.
Glucose utilization, endogenous glucose production, glucose oxidation, and lipid oxidation in study 1 (euglycemia), study 2 (hypoglycemia), study 3 (hypoglycemia + acipimox), and study 4 (hypoglycemia + acipimox + heparin + intralipid). The diagonal area depicts t1 (0–180 min, euglycemia or hypoglycemia), the white area depicts t2 (180–420 min, euglycemia or recovery to hypoglycemia), and the dotted area depicts t3 (420–540 min, euglycemic clamp) of each study.
In t3, nonoxidative rates of glucose utilization (total glucose utilization rates minus glucose oxidation rates from indirect calorimetry) were 78, 75, 69, and 86% in studies 1, 2, 3, and 4, respectively, of the overall glucose utilization. The replacement of FFA levels in study 4 with concomitant administration of acipimox significantly increased nonoxidative rates of glucose utilization compared with study 3 (P = 0.02).
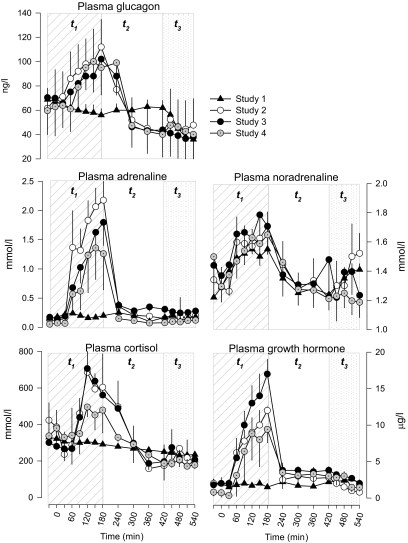
Plasma counterregulatory hormones concentrations.
Baseline plasma concentrations of all counterregulatory hormones were not different in studies 1–4 (Fig. 3). Plasma glucagon decreased slightly in the euglycemic time segment t1 of study 1. In contrast, in the hypoglycemia studies 2–4, plasma glucagon increased at 180 min and then returned to baseline values. Plasma adrenaline did not change in study 1, whereas it increased similarly in studies 2–4 by 180 min and, subsequently, returned to baseline values by 240 min. Plasma norepinephrine concentrations increased slightly between 30 and 180 min and were not different in all four studies. Plasma growth hormone did not change (baseline 2.0 ± 0.3 μg/dl) in the euglycemic study 1. In studies 2–4, plasma growth hormone peaked at 180 min (12 ± 0.3, 17 ± 2, 9.4 ± 1.8 μg/dl, respectively, P < 0.01 vs. study 1). There was a trend of a greater response of plasma growth hormone in study 3 compared with study 2 (P = 0.07) and study 4 (P = 0.08). Plasma cortisol decreased slightly in the euglycemic study 1, whereas it increased similarly in studies 2–4 (P < 0.05 vs. study 1) at 180 min. Afterward, plasma cortisol decreased to baseline values by 300 min.
FIG. 3.
Plasma counterregulatory hormones glucagon, adrenaline, norepinephrine, cortisol, and growth hormone in study 1 (euglycemia), study 2 (hypoglycemia), study 3 (hypoglycemia + acipimox), and study 4 (hypoglycemia + acipimox + heparin + intralipid). The diagonal area depicts t1 (0–180 min, euglycemia or hypoglycemia), the white area depicts t2 (180–420 min, euglycemia or recovery to hypoglycemia), and the dotted area depicts t3 (420–540 min, euglycemic clamp) of each study.
In t3, plasma concentrations of counterregulatory hormones glucagon, adrenaline, norepinephrine, growth hormone, and cortisol were not different among the four studies.
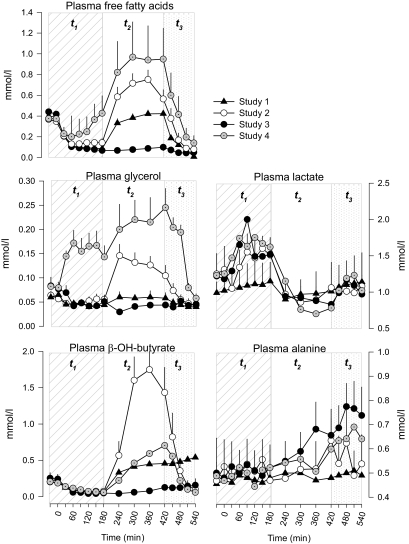
Plasma FFA, glycerol, β-hydroxybutyrate, lactate, and alanine concentrations.
In t1, plasma FFAs decreased after initiation of insulin infusion from an averaged baseline of 0.40 ± 0.03 to a nadir of 0.09 ± 0.02 mmol/l at 180 min with no differences in studies 1–3 (Fig. 4). After discontinuing insulin infusion (180 min), plasma FFAs returned to values similar to those of baseline by 420 min of t2. In the same segment of t2, plasma FFA levels were at all times less suppressed in study 2 compared with studies 1 and 3 (P < 0.05). Finally, replacement of FFAs and glycerol in study 4 reproduced plasma FFA concentrations similar to those of study 2 and greater than those of studies 1 and 3 (P < 0.05).
FIG. 4.
Plasma nonglucose substrates free fatty acids, glycerol, β-OH-butyrate, lactate, and alanine in study 1 (euglycemia), study 2 (hypoglycemia), study 3 (hypoglycemia + acipimox), and study 4 (hypoglycemia + acipimox + heparin + intralipid). The diagonal area depicts t1 (0–180 min, euglycemia or hypoglycemia), the white area depicts t2 (180–420 min, euglycemia or recovery to hypoglycemia), and the dotted area depicts t3 (420–540 min, euglycemic clamp) of each study.
After an initial suppression, plasma glycerol concentrations increased by 240 min (150 ± 23 mmol/l) in study 2. In studies 1 and 3, plasma glycerol concentrations were suppressed throughout, whereas in study 4 exogenous lipid emulsion produced plasma concentrations in the range of those of study 2. Plasma β-hydroxybutyrate followed a pattern similar to that of plasma FFA in all studies, although it was higher in study 2 compared with study 4.
In the euglycemic study 1, plasma lactate (baseline 1.0 ± 0.2 mmol/l) did not change. In studies 2–4, plasma lactate baseline values were similar to those of study 1, however plasma concentrations increased and peaked at 180 min (1.5 ± 0.24, 1.6 ± 0.17, and 1.5 ± 0.16 mmol/l, studies 2, 3, and 4, respectively); afterward, they decreased to baseline values by 240 min.
Plasma alanine concentrations did not change significantly from baseline in all studies. In t3, baseline (420 min) plasma FFA, glycerol, and β-hydroxybutyrate concentrations were significantly higher in studies 2 and 4 than studies 1 and 3. However, after insulin infusion, plasma FFA, glycerol, and β-hydroxybutyrate concentrations decreased in study 2 to concentrations similar to those of studies 1 and 3. In study 4, these metabolites followed the same pattern observed in studies 1–3. Plasma lactate concentrations increased slightly and similarly in all studies. Plasma alanine did not change, although levels tended to be higher in studies 3–4 (P = 0.081) (Fig. 4).
Effect of acipimox per se on insulin action.
Mean values of GIR and rates of endogenous glucose production and glucose utilization calculated during the last 30 min of the euglycemic clamp in t3 allow estimation of the likely contribution of acipimox per se, independent of the decrease in circulating FFA levels, on insulin action. In study 3, inhibition of lipolysis by acipimox determined an increase in GIR, paralleled by a similar increment of glucose disappearance, of ∼72% compared with study 2 (28.9 ± 2.6 vs. 16.8 ± 2.3 μmol/kg/min, respectively, P = 0.009). When plasma FFA and glycerol levels were replaced by infusing lipids and lipolysis was still blocked by acipimox in study 4, GIR was still higher by ∼ 33% compared with study 2 (22.3 ± 2.8 μmol/kg/min, vs. study 2, P = 0.026). This indicates a role of acipimox per se on insulin action and quantifies its relative contribution to the effects observed in study 3. Accordingly, the overall contribution of lipolysis to posthypoglycemic insulin resistance was estimated to be ∼39%.
DISCUSSION
The results indicate the following: First, the counterregulatory hormonal response to hypoglycemia contributes to reduced insulin action up to 9 h after an acute hypoglycemic episode. Second, this posthypoglycemic insulin resistance is generated by the counterregulatory hormones that act in part indirectly by activating lipolysis (contribution of 39%), and in part directly, i.e., by lipolysis-independent mechanisms (contribution of ∼60%). Third, the mechanisms of posthypoglycemic insulin resistance induced by lipolysis include increase in endogenous glucose production, suppression of peripheral utilization and oxidation of glucose, and increase lipid oxidation. Thus, the adipose tissue plays a pivotal role in determining and sustaining posthypoglycemic insulin resistance in humans.
In a late phase of hypoglycemia, a large part of the anti-insulin effects of counterregulatory hormones, mainly catecholamines, on production and utilization of glucose is not direct but is mediated by stimulation of lipolysis (20,21). The novel finding of the present study is that hypoglycemia-induced lipolysis also exerts long-lasting effects by blunting insulin action after restoration of euglycemia.
Administration of acipimox (study 3) suppressed lipolysis (as reflected by plasma FFA and glycerol concentrations) and markedly reduced lipid oxidation. This was associated with suppression of endogenous glucose production by 42%, increased glucose utilization by 64%, and increased glucose oxidation by 100%.
The mechanisms of regulation of glucose metabolism by fatty acids is not completely understood. The initial hypothesis of competition between FFAs and glucose for oxidation has been proposed by Randle et al. based on studies in vitro (33). Increased availability and oxidation of free fatty acids would increase levels of acetyl-CoA and citrate. The former inhibits pyruvate dehydrogenase, which in turn decreases glucose oxidation. The latter inhibits phosphofructokinase, which in turn decreases glycolysis and glucose utilization. Other studies in humans have demonstrated that the overall effect of elevation of levels of free fatty acids is increased lipid oxidation and suppressed oxidation and utilization of glucose (34), thus creating a condition of free fatty acid–induced insulin resistance (35). However, in addition to the hypothesis of Randle et al., it is likely FFAs interfere with insulin-stimulated glucose transport activity in muscles and induce insulin resistance by altering insulin signaling through insulin receptor substrate-1–associated phosphatidylinositol 3-kinase, resulting in decreased insulin-stimulated glucose transport activity (36).
It is likely that both glycerol and FFA contributed to the increase in the rate of hepatic glucose production observed in study 4 compared with study 3 (20). However, the design of the present experiments does not allow us to distinguish between the relative contribution of glycerol and FFAs to the increased hepatic glucose production.
All counterregulatory hormones increased in response to hypoglycemia (studies 1–3). However, with the exception of growth hormone (GH), which tended to be greater when lipolysis was blocked (study 3) compared with when lipolysis was allowed to occur (study 2) or FFA levels and glycerol were replaced (study 4), their levels were similar in studies 1–3. This effect on GH can be related to the lack of suppressive action of lower FFA levels on growth hormone secretion in study 3 (37).
From our study it is not possible to define the relative role of the individual counterregulatory hormones in the posthypoglycemic insulin resistance. However, because counterregulatory hormones were not affected by blockade of lipolysis, with the exception of GH, one might be tempted to speculate that, in addition to catecholamines (12), GH might direct, to some extent, the phenomenon of free fatty acid–induced posthypoglycemic insulin resistance. Indeed, earlier evidence points toward a critical role of GH and cortisol in insulin resistance after insulin-induced hypoglycemia (17,35). The elevation of norepinephrine in study 1 in which there was no hypoglycemia has to be considered as a response to insulin per se during the hyperinsulinemic-euglycemic clamp in t1 and t3. In fact, hyperinsulinemia per se stimulates sympathetic neural activity including norepinephrine elevation (38).
It is interesting to note that in one study the suppressive effect of acipimox on FFA and glycerol levels did inhibit recovery from hypoglycemia in a model of acute hypoglycemia induced by a 30-min insulin infusion in healthy subjects (39). However, the study did not examine the same effect in a model of more clinical prolonged hypoglycemia. It is possible that increased FFA and glycerol levels to hypoglycemia decrease insulin response during subsequent hypoglycemia. In fact, whereas antecedent hypoglycemia blunts neuroendocrine (and symptomatic) responses to subsequent hypoglycemia (4), Davis and Tate have shown that FFA levels and glucose infusion rates during subsequent afternoon hypoglycemia were higher and lower, respectively, after antecedent morning hypoglycemia compared with antecedent morning euglycemia, resulting in greater insulin resistance compensating for diminished neuroendocrine responses (40). More recently, it has been shown that insulin resistance can last up to 18 h after two brief episodes of antecedent hypoglycemia (4). In contrast, posthypoglycemic insulin resistance after antecedent hypoglycemia has not been studied in type 1 diabetic subjects. However, in those individuals, antecedent hypoglycemia is the major cause of hypoglycemia-associated autonomic failure syndrome, which, by reducing both symptoms of and physiological defense against developing hypoglycemia, favors severe hypoglycemia (41).
Hypoglycemia can be common also in people with type 2 diabetes, particularly under intensive glucose treatment (42,43). Whether the phenomenon of posthypoglycemic insulin resistance, demonstrated in healthy subjects in our present study, operates in people with type 2 diabetes who are already insulin resistant to some degree, thus contributing to worsened posthypoglycemia (hyper)glycemia, is not known.
Acipimox, a nicotinic acid analog and a potent inhibitor of lipolysis (44), is an established therapy for dyslipidemia. The antilipolytic action of acipimox is mediated through suppression of intracellular cAMP levels, with the subsequent decrease in cAMP-dependent protein kinase activity, leading to the reduced activity of hormone-sensitive lipase (45). Compared with nicotinic acid, acipimox has fewer side effects (light flushing) and a longer duration of action (46). In addition, by lowering circulating FFAs, acute administration of acipimox has been shown to improve insulin sensitivity in lean (47) and obese subjects and people with type 2 diabetes (48,49). The results of the present study are in line with earlier reports (50) indicating that acipimox improves insulin sensitivity by decreasing circulating FFA levels. In addition, our data show that acipimox also directly enhances insulin sensitivity. In fact, in study 3, GIR required to maintain euglycemia during the clamp (t3) was greater than in study 4, in which acipimox was given as in study 3 and plasma FFA levels were replaced by infusion of lipids, but higher than in study 2. If acipimox had no effects on insulin sensitivity, GIR would have approximately matched rates observed in study 2. In addition, glucose oxidation was not affected by acipimox, suggesting that the increase in GIR stimulated by acipimox (∼33%) must be accounted for by glucose storage as glycogen (nonoxidative glucose utilization).
Although our study reports the new finding that lipolysis induced by hypoglycemia counterregulation mediates in part posthypoglycemic insulin resistance, it has limitations. First, the study sample size was limited. It was, however, adequately powered to examine the issue and conducted under carefully controlled conditions. Second, although acipimox has been extensively adopted in metabolic studies (21,37,49,50), its use to investigate the role of lipolysis in posthypoglycemic insulin resistance may have exerted enhancing effects on in vivo insulin action independent of lipolysis and plasma FFA levels. We attempted to overcome this problem by planning study 4 to correct for the direct effects of acipimox on insulin action. Third, the results of our study have been obtained in healthy subjects and may not be immediately extrapolated to subjects with diabetes until specific studies are performed. Despite these limitations, the present study speaks to a major role of lipolysis in the pathogenesis of posthypoglycemic insulin resistance.
In conclusion, the present study demonstrates that the activation of lipolysis by counterregulatory hormones in response to hypoglycemia (indirect effects) accounts for ∼39% of the total effect on late posthypoglycemic insulin resistance. It is tempting to speculate that the late posthypoglycemic insulin resistance originates as a defensive mechanism to protect against recurrence of hypoglycemia after recent, antecedent hypoglycemia episode by limiting peripheral utilization and oxidation of glucose, thus increasing its availability for the brain. However, in subjects with type 1 diabetes, and long-term type 2 diabetes (in which pancreatic B-cell function is either totally or largely lost), posthypoglycemic insulin resistance may result in significant hyperglycemia especially after a meal, and interfere with day-long blood glucose control (7). In addition, in insulin-resistant type 2 diabetic patients, the reduced insulin action after hypoglycemia may exaggerate the preexisting insulin resistance and aggravate the cardiovascular risk.
ACKNOWLEDGMENT
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, Durrant J, Cryer P, Gerich J: Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol 1991; 260: E67–E74 [DOI] [PubMed] [Google Scholar]
- 2.Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Ciofetta M, Modarelli F, Di Vincenzo A, Annibale B, Lepore M, Lalli C: Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologia 1994; 37: 797–807 [DOI] [PubMed] [Google Scholar]
- 3.Bolli GB, Fanelli CG: Physiology of glucose counterregulation to hypoglycemia. Endocrinol Metab Clin North Am 1999; 28: 467–493, v [DOI] [PubMed] [Google Scholar]
- 4.Heller SR, Cryer PE: Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 1991; 40: 223–226 [DOI] [PubMed] [Google Scholar]
- 5.Somogyi M: Effect of insulin hypoglycemia on alimentary hyperglycemia. J Biol Chem 1951; 193: 859–871 [PubMed] [Google Scholar]
- 6.Somogyi M: Exacerbation of diabetes by excess insulin action. Am J Med 1959; 169–191 [DOI] [PubMed] [Google Scholar]
- 7.Perriello G, De Feo P, Torlone E, Calcinaro F, Ventura MM, Basta G, Santeusanio F, Brunetti P, Gerich JE, Bolli GB: The effect of asymptomatic nocturnal hypoglycemia on glycemic control in diabetes mellitus. N Engl J Med 1988; 319: 1233–1239 [DOI] [PubMed] [Google Scholar]
- 8.Havlin CE, Cryer PE: Nocturnal hypoglycemia does not commonly result in major morning hyperglycemia in patients with diabetes mellitus. Diabetes Care 1987; 10: 141–147 [DOI] [PubMed] [Google Scholar]
- 9.Frier BM, Corrall RJ, Ashby JP, Baird JD: Attenuation of the pancreatic beta cell response to a meal following hypoglycaemia in man. Diabetologia 1980; 18: 297–300 [DOI] [PubMed] [Google Scholar]
- 10.Mintz DH, Finster JL, Taylor AL, Fefer A: Hormonal genesis of glucose intolerance following hypoglycemia. Am J Med 1968; 45: 187–197 [DOI] [PubMed] [Google Scholar]
- 11.Oakley NW, Jacobs HS, Turner RC, Williams J, Aquino Cdos S, Nabarro JD: The effect of hypoglycaemia on oral glucose tolerance in normal subjects and patients with pituitary and adrenal disorders. Clin Sci 1970; 39: 663–674 [DOI] [PubMed] [Google Scholar]
- 12.Popp DA, Shah SD, Cryer PE: Role of epinephrine-mediated β-adrenergic mechanisms in hypoglycemic glucose counterregulation and posthypoglycemic hyperglycemia in insulin-dependent diabetes mellitus. J Clin Invest 1982; 69: 316–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolli GB, Gottesman IS, Campbell PJ, Haymond MW, Cryer PE, Gerich JE: Glucose counterregulation and waning of insulin in the Somogyi phenomenon (posthypoglycemic hyperglycemia). N Engl J Med 1984; 311: 1214–1219 [DOI] [PubMed] [Google Scholar]
- 14.Attvall S, Eriksson BM, Fowelin J, von Schenck H, Lager I, Smith U: Early posthypoglycemic insulin resistance in man is mainly an effect of beta-adrenergic stimulation. J Clin Invest 1987; 80: 437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attvall S, Fowelin J, von Schenck H, Lager I, Smith U: Insulin resistance in type 1 (insulin-dependent) diabetes following hypoglycaemia–evidence for the importance of beta-adrenergic stimulation. Diabetologia 1987; 30: 691–697 [DOI] [PubMed] [Google Scholar]
- 16.Fowelin J, Attvall S, von Schenck H, Smith U, Lager I: Characterization of the late posthypoglycemic insulin resistance in insulin-dependent diabetes mellitus. Metabolism 1990; 39: 823–826 [DOI] [PubMed] [Google Scholar]
- 17.Fowelin J, Attvall S, Von Schenck H, Smith U, Lager I: Combined effect of growth hormone and cortisol on late posthypoglycemic insulin resistance in humans. Diabetes 1989; 38: 1357–1364 [DOI] [PubMed] [Google Scholar]
- 18.Kollind M, Adamson U, Lins PE: Studies of insulin resistance following hypoglycemia in insulin-dependent diabetes mellitus. Acta Med Scand 1988; 223: 153–157 [DOI] [PubMed] [Google Scholar]
- 19.Kollind M, Adamson U, Lins PE, Curstedt T: Importance of growth hormone for blood glucose regulation following insulin-induced nocturnal hypoglycemia in insulin-dependent diabetes mellitus. Acta Med Scand 1988; 223: 159–164 [DOI] [PubMed] [Google Scholar]
- 20.Fanelli CG, De Feo P, Porcellati F, Perriello G, Torlone E, Santeusanio F, Brunetti P, Bolli GB: Adrenergic mechanisms contribute to the late phase of hypoglycemic glucose counterregulation in humans by stimulating lipolysis. J Clin Invest 1992; 89: 2005–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanelli C, Calderone S, Epifano L, De Vincenzo A, Modarelli F, Pampanelli S, Perriello G, De Feo P, Brunetti P, Gerich JE: Demonstration of a critical role for free fatty acids in mediating counterregulatory stimulation of gluconeogenesis and suppression of glucose utilization in humans. J Clin Invest 1993; 92: 1617–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M: Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol 1976; 41: 565–573 [DOI] [PubMed] [Google Scholar]
- 23.Tserng KY, Kalhan SC: Calculation of substrate turnover rate in stable isotope tracer studies. Am J Physiol 1983; 245: E308–E311 [DOI] [PubMed] [Google Scholar]
- 24.Wolfe RR: Radioactive and Stable Isotope Tracers in Biomedicine: principles and practice of kinetic analysis. New York, Wiley-Liss, 1992, p. 425–426 [Google Scholar]
- 25.Gastaldelli A, Coggan AR, Wolfe RR: Assessment of methods for improving tracer estimation of non-steady-state rate of appearance. J Appl Physiol 1999; 87: 1813–1822 [DOI] [PubMed] [Google Scholar]
- 26.Jequier E: Direct and indirect calorimetry in man. In Substrate and Energy Metabolism. Garrow JS, Halliday D: Eds. London, Wiley & Son, Ltd., 1985, p. 82–92 [Google Scholar]
- 27.Meriläinen PT: Metabolic monitor. Int J Clin Monit Comput 1987; 4: 167–177 [DOI] [PubMed] [Google Scholar]
- 28.Lowry O, Passoneau J: Typical fluorometric procedures for metabolite assays. In A Flexible System for Enzimatic Analysis Lowry O, Passoneau J: Eds. New York, Academic Press, Inc., 1972, p. 89–92 [Google Scholar]
- 29.Hawk PB: Kjedal method. In Practical Physiology Chemistry Hawk P, Oser B, Summerson W: Eds. Philadelphia, Blakiston; 1947, p. 814–822 [Google Scholar]
- 30.Miles J, Glasscock R, Aikens J, Gerich J, Haymond M: A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res 1983; 24: 96–99 [PubMed] [Google Scholar]
- 31.Ferrannini E: The theoretical bases of indirect calorimetry: a review. Metabolism 1988; 37: 287–301 [DOI] [PubMed] [Google Scholar]
- 32.Tappy L, Owen OE, Boden G: Effect of hyperinsulinemia on urea pool size and substrate oxidation rates. Diabetes 1988; 37: 1212–1216 [DOI] [PubMed] [Google Scholar]
- 33.Randle PJ, Garland PB, Hales CN, Newsholme EA: The glucose-fatty acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963; 1: 785–789 [DOI] [PubMed] [Google Scholar]
- 34.Bonadonna RC, Zych K, Boni C, Ferrannini E, DeFronzo RA: Time dependence of the interaction between lipid and glucose in humans. Am J Physiol 1989; 257( Pt. 1): E49–E56 [DOI] [PubMed] [Google Scholar]
- 35.Clore JN, Brennan JR, Gebhart SP, Newsome HH, Nestler JE, Blackard WG: Prolonged insulin resistance following insulin-induced hypoglycaemia. Diabetologia 1987; 30: 851–858 [DOI] [PubMed] [Google Scholar]
- 36.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI: Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 1999; 103: 253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peino R, Cordido F, Peñalva A, Alvarez CV, Dieguez C, Casanueva FF: Acipimox-mediated plasma free fatty acid depression per se stimulates growth hormone (GH) secretion in normal subjects and potentiates the response to other GH-releasing stimuli. J Clin Endocrinol Metab 1996; 81: 909–913 [DOI] [PubMed] [Google Scholar]
- 38.Paramore DS, Fanelli CG, Shah SD, Cryer PE: Forearm norepinephrine spillover during standing, hyperinsulinemia, and hypoglycemia. Am J Physiol 1998; 275: E872–E881 [DOI] [PubMed] [Google Scholar]
- 39.Newrick PG, Braatvedt G, Stansbie D, Corrall RJ: Suppression of lipolysis in normal man does not inhibit recovery from insulin-induced hypoglycaemia. Eur J Clin Invest 1993; 23: 53–56 [DOI] [PubMed] [Google Scholar]
- 40.Davis SN, Tate D: Effects of morning hypoglycemia on neuroendocrine and metabolic responses to subsequent afternoon hypoglycemia in normal man. J Clin Endocrinol Metab 2001; 86: 2043–2050 [DOI] [PubMed] [Google Scholar]
- 41.Cryer PE: Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 2005; 54: 3592–3601 [DOI] [PubMed] [Google Scholar]
- 42.U.K. prospective diabetes study 16: overview of 6 years' therapy of type II diabetes: a progressive disease: U.K. Prospective Diabetes Study Group. Diabetes 1995; 44: 1249–1258 [PubMed] [Google Scholar]
- 43.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christie AW, McCormick DK, Emmison N, Kraemer FB, Alberti KG, Yeaman SJ: Mechanism of anti-lipolytic action of acipimox in isolated rat adipocytes. Diabetologia 1996; 39: 45–53 [DOI] [PubMed] [Google Scholar]
- 45.Aktories K, Schultz G, Jakobs KH: Inhibition of adenylate cyclase and stimulation of a high affinity GTPase by the antilipolytic agents, nicotinic acid, acipimox and various related compounds. Arzneimittelforschung 1983; 33: 1525–1527 [PubMed] [Google Scholar]
- 46.Fuccella LM, Goldaniga G, Lovisolo P, Maggi E, Musatti L, Mandelli V, Sirtori CR: Inhibition of lipolysis by nicotinic acid and by acipimox. Clin Pharmacol Ther 1980; 28: 790–795 [DOI] [PubMed] [Google Scholar]
- 47.Fulcher GR, Walker M, Catalano C, Farrer M, Alberti KG: Acute metabolic and hormonal responses to the inhibition of lipolysis in non-obese patients with non-insulin-dependent (type 2) diabetes mellitus: effects of acipimox. Clin Sci (Lond) 1992; 82: 565–571 [DOI] [PubMed] [Google Scholar]
- 48.Fulcher GR, Walker M, Catalano C, Agius L, Alberti KG: Metabolic effects of suppression of nonesterified fatty acid levels with acipimox in obese NIDDM subjects. Diabetes 1992; 41: 1400–1408 [DOI] [PubMed] [Google Scholar]
- 49.Vaag A, Skött P, Damsbo P, Gall MA, Richter EA, Beck-Nielsen H: Effect of the antilipolytic nicotinic acid analogue acipimox on whole-body and skeletal muscle glucose metabolism in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 1991; 88: 1282–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker M, Agius L, Orskov H, Alberti KG: Peripheral and hepatic insulin sensitivity in non-insulin-dependent diabetes mellitus: effect of nonesterified fatty acids. Metabolism 1993; 42: 601–608 [DOI] [PubMed] [Google Scholar]