Abstract
OBJECTIVE
Regulatory T-cells (Tregs) recognizing islet autoantigens are proposed as a key mechanism in the maintenance of self-tolerance and protection from type 1 diabetes. To date, however, detailed information on such cells in humans, and insight into their mechanisms of action, has been lacking. We previously reported that a subset of CD4 T-cells secreting high levels of the immunosuppressive cytokine interleukin-10 (IL-10) is significantly associated with late onset of type 1 diabetes and is constitutively present in a majority of nondiabetic individuals. Here, we test the hypothesis that these T-cells represent a naturally generated population of Tregs capable of suppressing proinflammatory T-cell responses.
RESEARCH DESIGN AND METHODS
We isolated and cloned islet-specific IL-10–secreting CD4+ T-cells from nondiabetic individuals after brief ex vivo exposure to islet autoantigens using cytokine capture technology and examined their phenotype and regulatory potential.
RESULTS
Islet-specific IL-10+ CD4 T-cells are potent suppressors of Th1 effector cells, operating through a linked suppression mechanism in which there is an absolute requirement for the cognate antigen of both the regulatory and effector T-cells to be presented by the same antigen-presenting cell (APC). The regulatory T-cells secrete perforin and granzymes, and suppression is associated with the specific killing of APCs presenting antigen to effector T-cells.
CONCLUSIONS
This hitherto undescribed population of islet autoantigen–specific Tregs displays unique characteristics that offer exquisite specificity and control over the potential for pathological autoreactivity and may provide a suitable target with which to strengthen β-cell–specific tolerance.
There is now overwhelming evidence from both mice and humans that regulatory T-cells (Tregs) play a key role in the process of controlling the expansion of pathogenic autoreactive T-cells and that defects in this key cell population can lead to the development of autoimmune disease including type 1 diabetes (1–4). A view has therefore emerged that in the majority of individuals for the majority of life, destructive autoimmunity is held in check by populations of Tregs, recognizing autoantigens and responding through a variety of regulatory pathways (5–8).
Given the apparent importance of this cell type in promoting self-tolerance, there has been much interest in investigating whether strategies to increase the number or functional potency of Tregs are capable of influencing the progression of autoimmune diseases such as type 1 diabetes. Indeed, numerous studies in animal models have demonstrated that such strategies are able not only to prevent the onset of type 1 diabetes but also to reverse established diabetes, making them a logical target for intervention strategies in humans (9–13). Critically, these studies highlighted the importance of antigen specificity: islet-specific Tregs have far greater potency than those derived from polyclonal populations. This presents a substantial challenge to translating advances in our understanding of Treg biology into immunotherapy for human type 1 diabetes, because the literature on the natural repertoire of human islet antigen–specific Tregs that may contribute to the tolerant state in vivo is scant.
In recent years, we have developed approaches that allow the functional interrogation of human autoreactive T-cells directly ex vivo for their ability to respond to islet autoantigens (14,15). In a previous study, we reported the existence of a population of islet autoreactive CD4 T-cells that produce the signature immunosuppressive cytokine interleukin-10 (IL-10) after brief coculture with naturally processed and presented epitopes of proinsulin and IA-2 (16). Of note, such cells were frequently found in nondiabetic individuals, and when present in patients, they primarily segregated with a significantly older age of onset. We reasoned that such cells could have regulatory properties, because they were associated with both the absence and delayed onset of disease.
The present study tests this hypothesis. We isolated and cloned CD4+ T-cells secreting IL-10 after brief ex vivo exposure to islet autoantigens. As subjects, we elected to use islet autoantibody–negative nondiabetic individuals, reasoning that in such individuals immune regulation would be most operative. We find that the islet-specific IL-10–secreting CD4+ T-cells are potent suppressors of proinflammatory effector Th1 cells, of the type believed to mediate islet β-cell destruction. Surprisingly, despite the cells' IL-10 production, we find that their primary modus operandi in vitro is the specific destruction of antigen-presenting cells presenting peptides from islet autoantigens. Our study represents an unequivocal demonstration of autoantigen-specific Tregs in human type 1 diabetes and provides a novel platform for the development of strategies to promote their development.
RESEARCH DESIGN AND METHODS
Subjects.
Fresh heparinized blood samples were obtained from 11 healthy nondiabetic control subjects with no family history of type 1 diabetes. Ethical approval for this study was granted by the local Ethics Committee and informed consent obtained.
Antigens and synthetic peptides and cytokine ELISpot.
Previously identified peptides, representing naturally processed and presented epitopes of IA-2 and proinsulin (16,17), and a set of overlapping peptides encompassing the sequence of human insulin were synthesized by Keck Biotechnology Resource Laboratory, Yale University. Tetanus toxoid was obtained from Pasteur Merieux (Maidenhead, U.K.); hemagglutinin, from Solvay Pharmaceuticals, Inc. (Marietta, GA); and human insulin, from Sigma Chemical Corporation (Poole, U.K.). Enzyme-linked immunosorbent spot (ELISpot) assays for the detection of IL-10–producing T-cells were performed and analyzed as previously described (16).
Isolation and cloning of IL-10–secreting CD4+ T-cells.
IL-10–secreting CD4+ T-cells were isolated using the Miltenyi IL-10 secretion, detection, and enrichment kit following the manufacturer's protocol (Miltenyi Biotec, Surrey, U.K.). Cells were labeled with monoclonal anti-CD3, -CD4, and -CD14 antibodies and 7-aminoactinomycin D (7-AAD; Merck Chemical Ltd., Nottingham, U.K.) and analyzed on a MoFlo flow cytometer (Cytomation Bioinstruments, Freiburg, Germany), and single, viable, IL-10–secreting T-cells were deposited into the wells of a 96-well tissue culture plate. Single cells were expanded by the addition of mixed-donor irradiated peripheral blood mononuclear cells (PBMCs; 105/well), phytohemagglutinin-L (4 μg/ml; Biostat Diagnostic Systems, Stockport, U.K.), and human recombinant IL-15 (10 ng/ml; Peprotech, London, U.K.) in 200 μl/well complete media (X-Vivo15 + 5% pooled human AB serum; BioWhittaker) and tested for antigen specificity. Antigen-specific clones were restimulated every 21–28 days, and human recombinant IL-15 (10 ng/ml; Peprotech) was added at day 3 and as required during expansion. T-cell clones were subjected to a maximum of three cycles of stimulation. T-cell clones were used for the experiments described below when fully rested, a minimum of 21 days after stimulation.
Characterization of IL-10–secreting T-cell clones.
IL-10 and tumor necrosis factor-α (TNF-α) secretion by T-cell clones was measured by ELISA (ImmunoTools, Friesoythe, Germany); all other cytokines were measured by multiplex bead array (Millipore Ltd., Watford, U.K.) on the Luminex 100 LabMAP System (Luminex Corporation, Oosterhout, The Netherlands). Analysis of cytokine production by intracellular flow cytometry was performed as previously described (18).
T-cell clones was studied by flow cytometry, using anti-CD4, -CD3, CD69, –HLA-DR, -CD39, –glucocorticoid-induced TNF receptor (GITR), -CD62L -α4 integrin, and -β7 integrin antibodies (BD Biosciences, Oxford, U.K.); anti–inducible costimulator (ICOS) antibody (Biolegend, San Diego, CA); and anti-CD25 (AbD Serotec, Oxford, U.K.). FoxP3 staining was performed using the eBioscience anti-human Foxp3 Staining Set (Insight Biotechnology, Wembley, U.K.), and granzyme and perforin expression were detected using BD Biosciences staining sets, following the manufacturer's instructions. Flow cytometry was performed on a FACSCalibur or FACSAria (BD Biosciences) and data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Suppression assays.
Suppression assays were performed through coculture of putative regulatory T-cell clones with effector populations and antigen-presenting cells (APCs) that had been differentially labeled with intracellular fluorescent dyes carboxyfluorescein diacetate, succinimidyl ester (CFSE) and CellTrace Far Red DDAO-SE (DDAO) (Invitrogen, Paisley, U.K.). Autologous suppression assays were established in 96-well plates with 1 × 105 CFSE-labeled PBMCs/well ± 3 × 104 DDAO-labeled Treg clone. To facilitate further analysis of the mechanisms of suppression, a panel of CD4+ Th1 clones specific for recall antigens was generated as detailed in supplementary Table 1, available in an online appendix at http://diabetes.diabetesjournals.org/content/early/2010/03/10/db09-0503/suppl/DC1. Clonal suppression assays were established in 96-well plates with 3 × 104 CFSE-labeled clone cells, 1 × 105 DDAO-labeled irradiated (3,000 rad) PBMCs as a source of antigen presenting cells ± 3 × 104 DDAO-labeled Treg clone. To study the role of cytokines in suppression, anti–IL-10Rα (clone 37607.11), anti–transforming growth factor-β1,2,3 (TGF-β1,2,3; clone 1Δ11) (both R&D Systems, Abingdon, U.K.), and mouse IgG1 isotype control (clone MOPC31C; Sigma Chemical Corporation) antibodies were added to the cultures to a final concentration of 10 μg/ml. Transwell assays were established in 24-well plates (Costar, Corning, NY) using 3 × 105 CFSE-labeled responder clone, 1 × 105 DDAO-labeled irradiated PBMCs, and 3 × 105 DDAO-labeled Treg clones per well as indicated. Percentage suppression was calculated as previously described (19).
Cytotoxicity assay.
Autologous PBMCs were labeled with either 0.1 or 1 μmol/l CFSE and incubated in complete media at 1 × 106/ml for 3 h at 37°C with 25 μg/ml peptide (0.1 μmol/l labeled cells) or peptide diluent (DMSO; 1 μmol/l labeled cells). Unbound peptide was removed by extensive washing, target populations were combined at a ratio of 1:1, and 105 PBMCs were incubated in the presence or absence of 105 resting Treg clones. After incubation for 16 h at 37°C, cells were stained with anti-CD4, anti-CD14, and anti-CD19 fluorochrome-labeled antibodies (BD Biosciences) and 7-AAD and analyzed by flow cytometry, and percentage specific killing was calculated as follows: 100 − [(% peptide-labeled targets in absence of Tregs − % peptide-labeled targets in absence of Tregs)/% peptide-labeled targets in absence of Tregs] × 100. In experiments using Epstein-Barr virus B-cell lines, target populations were pulsed with peptide as described above, and killing was assessed by calculating the percentage of 7-AAD+ cells. To assess the role of the granzyme/perforin and Fas/Fas ligand (FasL) pathways in killing, cells were treated with EGTA (4 mmol/l; Merck Chemical Ltd.), concanamycin A (ConA; 100 nmol/l; Sigma Chemical), and anti-CD95L antibody (1 μg/ml ZB4; Immunotech, Marseilles, France).
Single-cell multiplex RT-PCR.
Multiplex RT-PCR was performed on single IL-10–secreting CD4 T-cells isolated from fresh PBMC preparations after coculture with islet autoantigen as described above for cloning, based on the technique described by Kelso et al. (20), using the primer pairs detailed in supplementary Table 2. The frequency of expression of cytotoxic molecules in different groups was compared by χ2 analysis.
RESULTS
Detection and cloning of islet peptide–specific IL-10–secreting T-cells from nondiabetic individuals.
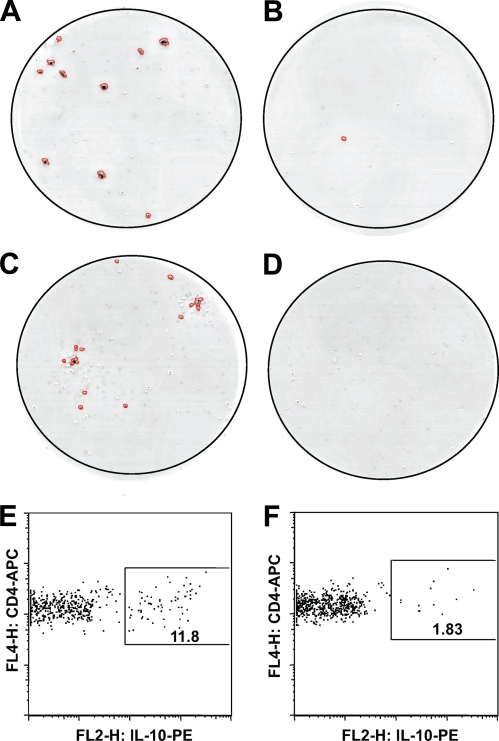
We first examined IL-10 responses to a panel of naturally processed IA-2 and proinsulin peptides and a set of overlapping peptides encompassing the insulin molecule in a group of healthy nondiabetic individuals (n = 11). Using a highly sensitive and specific cytokine ELISpot, we found that the majority of individuals (8/11, 72%) mounted a significant IL-10 response (insulin sensitivity index ≥3) to at least one peptide (data not shown), consistent with our previous work (16). Individuals with positive responses by ELISpot were recalled a minimum of 1 month later, and IL-10–secreting CD4 T-cells were detected, isolated by sensitive cytokine secretion assay, and immediately single-cell sorted by flow cytometry. The cytokine secretion assay confirmed the reproducible detection of IL-10–secreting, islet peptide–specific CD3+CD4+ T-cells in nondiabetic individuals (Fig. 1). Cloning was initiated after brief (48 h) culture with antigen and was carried out in the absence of any IL-10 biasing culture conditions. T-cell clones specific for insulin B11–30 (clone MHB10.3) and IA-2 709–736 (clone RAR5.3) were isolated from nondiabetic individuals, M.H. (age 27 years; HLA-DRB1*0301-DRB1*0404) and R.A. (age 27 years; HLA-DRB1*0101-DRB1*0407), respectively. Both clones produced large amounts of IL-10 (>1 ng/ml), in response to submicrogram doses of the peptide used in their isolation (Fig. 2A).
FIG. 1.
Representative IL-10 responses to islet antigen peptides by PBMCs from nondiabetic individuals stimulated with (A) insulin B11–30 peptide compared with (B) peptide diluent alone in nondiabetic individual M.H.; (C) IA-2 709–736 peptide compared with (D) peptide diluent alone in nondiabetic individual R.A. E and F: Isolation of IL-10–secreting cells by cytokine secretion assay. Plots represent flow cytometric analysis of magnetically enriched IL-10–secreting cells from M.H. stimulated with insulin B11–30 peptide (E) compared with background response to (F) diluent alone.
FIG. 2.
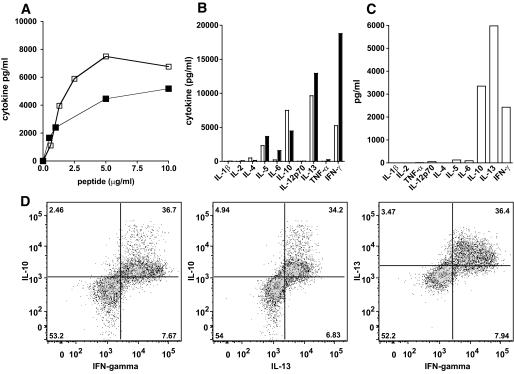
Cytokine production profiles of islet-specific T-cell clones. A: MHB10.3 (open symbols) and RAR5.3 (closed symbols) were stimulated with insulin B11–30 and IA-2 709–736, respectively, in the presence of autologous irradiated PBMCs, and IL-10 production was measured by enzyme-linked immunosorbent assay after 3 days. B: Cytokine production by MHB10.3 (open bars) and RAR5.3 (closed bars) clones cultured as above with suboptimal doses of peptide (1 μg/ml) and by (C) MHB10.3 stimulated with whole recombinant insulin (100 μg/ml) in the presence of autologous irradiated dendritic cells. Cytokines were measured in 3-day supernatants by multiplex bead technology. D: Cytokine production by MHB10.3 was assessed by flow cytometry for intracellular synthesis of IL-10, IL-13, and IFN-γ after stimulation with plate-bound anti-CD3 and soluble anti-CD28 antibodies for 2 days.
Phenotypic analysis of IL-10–secreting islet-specific T-cells.
Analysis of cultures stimulated with suboptimal doses of peptide demonstrated that both clones have a similar cytokine secretion profile, producing large amounts of IL-10, IL-13, γ-interferon (IFN-γ), and IL-5 but little or no IL-2, IL-4, TNF-α, or IL-1β (Fig. 2B). A similar cytokine secretion profile was seen when MHB10.3 was incubated with whole recombinant insulin (Fig. 2C). Analysis of cytokine production at a single-cell level shows that when activated and producing cytokines, the same cells produce IL-10, IFN-γ, and IL-13 (Fig. 2D). The HLA class II restriction of the clones was investigated using monoclonal antibodies that block HLA-DR or HLA-DQ and partially HLA-matched irradiated PBMCs. These experiments demonstrated that clone MHB10.3 was restricted by HLA-DR3 (B1*0301) and clone RAR5.3, by HLA-DR4 (DRB1*0407) (data not shown).
Expression of activation and regulatory T-cell markers on islet-specific T-cells was assessed by flow cytometry. A small number of resting IL-10–secreting islet-specific clone cells (21 days after peptide stimulation) expressed a low level of CD69 (14.4 and 7.5% for RAR5.3 and MHB10.3, respectively), whereas much higher proportions expressed HLA-DR (94.6 and 60.4%), CD25 (99.8 and 92.8%), FoxP3 (97.5 and 61.9%), CD39 (100 and 99.8%), and ICOS (92 and 97.2%); lower levels of GITR (42.2 and 23.4%) and CD62L (10.5 and 18.7%); and variable amounts of CD127 (32.7 and 64.1%) (supplementary Fig. 1). In addition, both MHB10.3 and RAR5.3 expressed both α4 and β7 integrins (supplementary Fig. 2), which were absent on the TT-specific RATT6 and HA-specific RAHA5 Th1-type T-cell clones.
Regulatory potential of IL-10–secreting islet-specific T-cells.
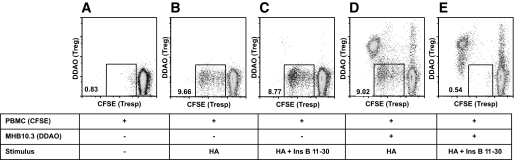
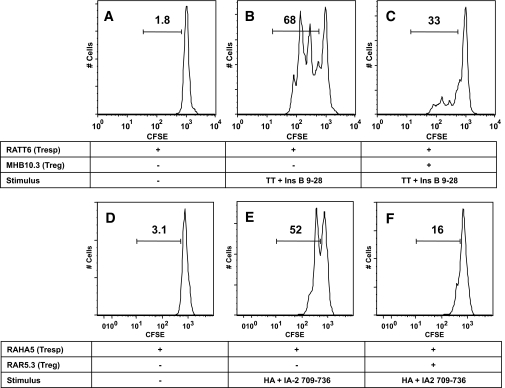
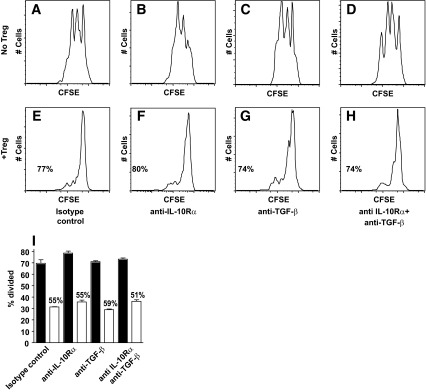
To investigate the regulatory potential of IL-10–secreting islet-specific T-cells, a dual fluorescence–based suppression assay was used. Regulation was first assessed in an autologous assay system, measuring the response of PBMCs to recall antigen HA. Individual M.H. exhibited a robust proliferative response to HA (9.6% of CD3+ PBMCs; Fig. 3B). Addition of either T-cell clone MHB10.3 or its epitope insulin B11–30 had little effect on proliferation (Fig. 3C and D), whereas addition of MHB10.3 and its epitope insulin B11–30 together resulted in complete suppression of the HA-specific proliferative response (Fig. 3E). These data demonstrate that IL-10–secreting islet-specific T-cells are potent regulators, and that regulation is dependent on activation of Tregs by APC presentation of cognate peptide. To pursue further analysis of the mechanisms of suppression, we used a panel of CD4+ Th1 clones specific for recall antigens TT and HA (RATT6 and RAHA5, respectively). Both clones proliferate rapidly in response to cognate antigen with 68 and 52% of cells divided after 3 days, respectively (Fig. 4B and E). However, when cultured with MHB10.3 (Fig. 4C) or RAR5.3 (Fig. 4F) in the presence of the cognate islet peptide, proliferation was dramatically reduced (33 and 16%), resulting in suppression rates of 51 and 69%, respectively. Blocking IL-10 and TGF-β either individually or in combination had no effect on the ability of MHB10.3 or RAR5.3 to suppress proliferation of RATT6 (74–80% suppression, Fig. 5A–H) or RAHA5 (51–59% suppression, Fig. 5I). This absence of IL-10 dependency of regulation was also present in studies in which Treg number and peptide concentration were titrated to extinction of activity (supplementary Fig. 3). Furthermore, blockade of IL-10 during the activation and expansion of IL-10–secreting islet-specific T-cell clones also did not affect the ability of the cells to regulate (supplementary Fig. 4).
FIG. 3.
Regulation of autologous T-cell responses by MHB10.3. A–E: PBMCs from M.H. were labeled with CFSE and stimulated with combinations of recombinant influenza hemagglutinin (HA; 45 ng/ml) and insulin B11–30 (10 μg/ml) and in the presence or absence of DDAO-labeled MHB10.3 IL-10–secreting islet-specific T-cell clone as indicated. Proliferation of CD3+ T-cells was assessed after 6 days by flow cytometry. Numbers represent the percentage of CD3+ PBMCs in the gated area. Data are representative of more than three independent experiments. When activated by cognate antigen, clone MHB10.3 completely abrogates the proliferative response of recall memory T-cells specific for HA. Ins, insulin; Tresp, T-cell responses.
FIG. 4.
Regulation of clonal T-cell responses by MHB10.3 and RAR5.3. A–C: The tetanus toxoid–specific Th1 clone RATT6 was labeled with CFSE and stimulated with combinations of tetanus toxoid (100 ng/ml) and insulin B11–30 (10 μg/ml) in the presence or absence of DDAO-labeled MHB10.3 as indicated. D–F: The hemagglutinin-specific Th1 clone RAHA5 was labeled with CFSE and stimulated with combinations of recombinant hemagglutinin (45 ng/ml) and IA-2 709–736 (25 μg/ml) in the presence or absence of DDAO-labeled RAR5.3 as indicated. HLA-matched DDAO-labeled irradiated PBMCs were used as a source of antigen-presenting cells. Proliferation of Th1 clones (DDAO− cells) was assessed after 3 days by flow cytometry, and the gated regions represent the percentage of live clone cells that have undergone division. Proliferation of the Th1 clones RATT6 and RAHA5 is suppressed by activated MHB10.3 and RAR5.3 Treg clones. Data are representative of a minimum of three independent experiments.
FIG. 5.
Regulation of clonal T-cell responses by IL-10–secreting islet-specific Tregs is not dependent on IL-10 or TGF-β. A–H: CFSE-labeled RATT6 and HLA-matched DDAO-labeled irradiated PBMCs were stimulated with tetanus toxoid (100 ng/ml) and insulin B11–30 (10 μg/ml) in the absence (A–D) or presence (E–H) of DDAO-labeled MHB10.3 and monoclonal antibodies as indicated. Proliferation of RATT6 (DDAO− cells) was assessed after 3 days by flow cytometry. Numbers indicate the percentage suppression in the presence of MHB10.3. I: A similar experiment in which CFSE-labeled RAHA5, DDAO-labeled RAR5.3, and HLA-matched DDAO-labeled irradiated PBMCs were stimulated with either hemagglutinin (45 ng/ml) alone (black bars) or hemagglutinin and IA-2 709–736 (25 μg/ml) (white bars) in the presence of monoclonal antibodies as indicated. Proliferation of RAHA5 (DDAO− cells) was assessed after 3 days by flow cytometry. Numbers indicate the percentage suppression in the presence of IA-2 709–736, and error bars indicate SE from the mean for triplicate cultures. Data are representative of at least three independent experiments.
However, IL-10 secreted by these Tregs was found to upregulate monocyte expression of the molecule B7-H4 (a B7 family member that negatively regulates T-cell immunity through inhibition of T-cell proliferation, cytokine production, and cell cycle progression [21]), which is inhibited by anti–IL-10R antibody (supplementary Fig. 5).
IL-10–secreting islet-specific Tregs require cell-cell contact to mediate suppression.
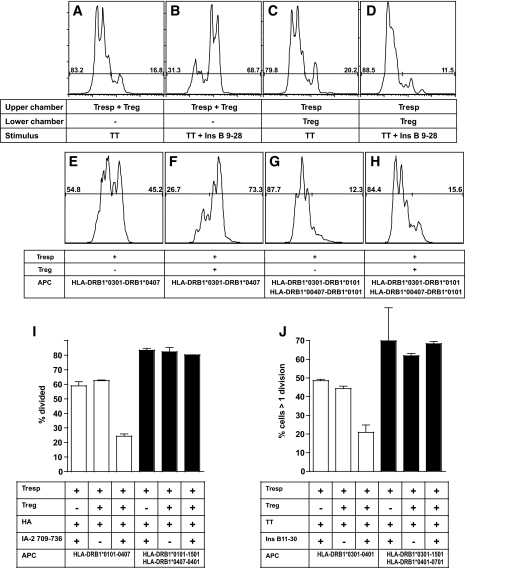
We used a transwell system to investigate whether suppression by IL-10–secreting islet-specific Tregs is mediated by soluble factors or dependent on cell-cell contact. When activated by peptide, MHB10.3 is able to suppress proliferation of RATT6 in coculture, but this is completely ablated if responder and regulatory T-cells are physically separated (Fig. 6A–D). The role of the APC in suppression was then more closely investigated. First, a standard suppression assay was established using irradiated PBMCs from a donor with both HLA-DRB1*0301 and HLA-DRB1*0407 genotypes, which can therefore present antigens to both regulatory (MHB10.3) and responder (RATT6) T-cell clones (Fig. 6E and F). Second, assays were established using irradiated PBMCs from two different donors (one with the HLA-DRB1*0301 genotype but not HLA-DRB1*0407 and another with the HLA-DRB1*0407 genotype but not HLA-DRB1*0301) in which presentation to regulatory and responder clones is therefore separated on two different APC populations (Fig. 6G and H). Whereas suppression occurs when both responder and regulatory T-cell clones are stimulated by the same APC (Fig. 6E and F), no suppression is seen when presentation to regulatory and responder clones takes place on two different APC populations (Fig. 6G and H). The lack of suppression in these latter cultures was observed at both high and low levels of effector clone stimulation strength (supplementary Fig. 6). Similar assays were established using IL-10–secreting islet-specific Treg RAR5.3 using two independent responder T-cell clones and sources of APCs with identical results (Fig. 6I and J).
FIG. 6.
Regulation of clonal T-cell responses by IL-10–secreting islet-specific Tregs is dependent on cell-cell contact and operates by a linked suppression mechanism. A–D: CFSE-labeled RATT6 and DDAO-labeled MHB10.3 were stimulated with tetanus toxoid (100 ng/ml) or tetanus toxoid and insulin B11–30 (10 μg/ml) in the upper or lower chamber of a transwell plate as indicated. HLA-matched DDAO-labeled irradiated PBMCs as a source of antigen-presenting cells were present in both upper and lower chambers. Proliferation of RATT6 (DDAO− cells) was assessed after 4 days by flow cytometry. E–H: CFSE-labeled RATT6 (HLA-DRB1*0407 restricted) Th1 clone cells were stimulated with tetanus toxoid (100 ng/ml) and insulin B11–30 (10 μg/ml) in the presence or absence of DDAO-labeled MHB10.3 (HLA-DRB1*0301 restricted) Treg clone as indicated. Partially or fully HLA-matched DDAO-labeled irradiated PBMCs were used as a source of antigen-presenting cells as indicated. Proliferation of RATT6 (DDAO− cells) was assessed after 4 days by flow cytometry. I and J: The HLA-DRB1*0101 restricted Th1 clone RAHA5 (I) and the HLA-DRB1*0401 restricted Th1 clone TTTT6 (J) were labeled with CFSE and stimulated with hemagglutinin (45 ng/ml) or tetanus toxoid (100 ng/ml), respectively, in the presence or absence of DDAO-labeled RAR5.3 (HLA-DRB1*0407 restricted) Treg clone and IA-2 709–736 (25 μg/ml) as indicated. Partially or fully HLA-matched DDAO-labeled irradiated PBMCs were used as a source of antigen-presenting cells as indicated. Proliferation of Th1 clones (DDAO− cells) was assessed after 4 days by flow cytometry. Numbers indicate percentage of suppression, and error bars indicate SE from the mean for triplicate cultures.
IL-10–secreting islet-specific Tregs express cytotoxic molecules and kill islet antigen-presenting cells.
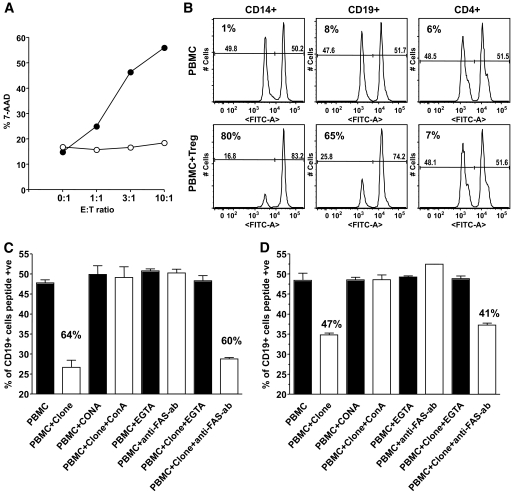
Because IL-10–secreting islet-specific Treg-mediated suppression requires cell-cell contact and is not mediated by cytokines conventionally associated with regulation, we sought alternative mechanisms to account for these effects. We noted that resting IL-10–secreting islet-specific Treg clones express granzymes A and B and perforin and that expression of perforin and granzyme B was upregulated upon activation (supplementary Fig. 7). To investigate whether expression of these cytotoxic molecules translated into an ability to kill APCs, a flow cytometry–based cytotoxicity assay was used. High levels of cytotoxicity were seen against APCs loaded with islet autoantigen (56% at an effector/target ratios of 10:1), whereas APCs lacking islet peptide were not killed, even at high effector/target ratios (Fig. 7A). To investigate the specificity of killing, a dual fluorescence-intensity killing assay was used. In this assay, peptide-pulsed and unpulsed PBMCs were incubated in the same culture along with IL-10–secreting islet-specific Tregs. In these experiments, only peptide-loaded APCs (i.e., CD19+ B cells and CD14+ monocytes) were killed (specific killing of 80 and 65%, respectively), whereas APCs lacking islet peptide and CD4+ T-cells were not killed despite juxtaposition with highly activated Tregs in the cultures (Fig. 7B). To investigate the mechanism of killing, cells were preincubated with inhibitors of the perforin pathway (ConA or EGTA) or Fas/FasL ligand pathway (neutralizing anti-FasL antibody) (Fig. 7C and D). Whereas killing was unaffected in the presence of anti-FasL antibodies, it was completely abrogated in the presence of ConA or EGTA, demonstrating that IL-10–specific islet-specific Tregs exhibit perforin-granzyme–dependent cytotoxicity.
FIG. 7.
Islet peptide–pulsed antigen-presenting cells are specifically killed by islet-specific Tregs. A: The HLA-DRB1*0401 Epstein-Barr virus B-cell line Preiss was labeled with CFSE and pulsed with either IA-2 709–736 (filled symbols) or peptide diluent alone (open symbols) and then incubated with various effector/target (E:T) ratios with resting RAR5.3 T-cells. Death of CFSE+ antigen-presenting cells was assessed by uptake of 7-AAD. Similar results were obtained with MHB10.3. B: Autologous PBMCs from individual R.A. were labeled with either 0.1 μmol/l CFSE and pulsed with IA-2 709–736 or 1 μmol/l CFSE and pulsed with DMSO diluent alone. After 3 h, excess peptide was removed by rigorous washing and the peptide-pulsed, and unpulsed PBMCs were combined at a 1:1 ratio and incubated either alone or with resting RAR5.3 T-cells as indicated. After incubation for 16 h at 37°C, cultures were harvested and death in different cell populations was assessed by multicolor flow cytometry. Numbers indicate percentage of specific death of peptide-pulsed cells in the gated population. C and D: Autologous PBMCs from individuals R.A. (C) and M.H. (D) were labeled with CFSE and pulsed with IA-2 709–736 and insulin B11–30, respectively, or DMSO as described for (B). Peptide-pulsed and unpulsed PBMCs were combined at a 1:1 ratio and incubated either alone (black bars) or with resting IL-10–secreting islet-specific Tregs (white bars) in the presence of inhibitors of cytotoxicity as indicated. After incubation for 16 h at 37°C, cultures were harvested and death in different cell populations was assessed by multicolor flow cytometry. Plots show percentage of viable CD19+ lymphocytes that were peptide pulsed. Numbers indicate percentage of specific death of peptide-pulsed cells in the gated population, and error bars indicate SE from the mean for triplicate cultures. Results indicate inhibition of APC killing by Tregs in the presence of inhibitors of cytotoxic granule release. Data are representative of at least three independent experiments. ab, antibody.
Using cell viability dyes, we demonstrate that during assays in which Tregs are activated by peptide-loaded APCs, the responder T-cells undergoing regulation are not themselves killed (supplementary Fig. 8). The kinetics of suppression in relation to responder T-cell proliferation are shown in supplementary Fig. 9 and demonstrate that suppression is apparent and most potent when measured at day 2 (the earliest time point at which proliferation of responder T-cells can be detected; supplementary Fig. 9A). Furthermore, if Tregs are placed in the APC/peptide culture for 24 h prior to addition of the T responders suppression is greatly enhanced, presumably due to killing of most APCs before they have the chance to stimulate responder T-cells (supplementary Fig. 9B–D).
IL-10–secreting islet autoantigen–specific Tregs express cytotoxic molecules directly ex vivo.
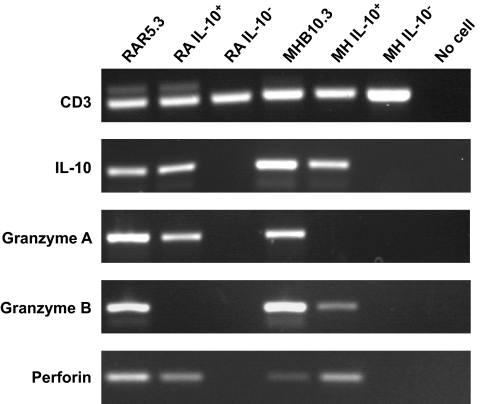
To establish whether IL-10–secreting islet-specific Tregs express cytotoxic molecules directly ex vivo, or gain this expression as a consequence of the cloning process in vitro, we performed a multiplex RT-PCR on single IL-10+ CD4+ T-cells freshly isolated from islet peptide–stimulated PBMC cultures (Fig. 8). These experiments were performed with the original donors used to clone MHB10.3 and RAR5.3 Tregs. All sorted T-cells were examined for CD3 expression as a template quality control and also for IL-10 expression. Only IL-10+ sorted T-cells that were also IL-10 transcript–positive were included in the analysis. These studies demonstrated that 67% (6/9) of the IL-10 transcript–positive T-cells expressed at least one cytotoxic molecule, compared with 5.6% (1/18) of the IL-10 transcript–negative T-cells (P = 0.0006).
FIG. 8.
IL-10–producing islet-specific CD4+ T-cells express cytotoxic molecules directly ex vivo. PBMCs from donors M.H. and R.A. were stimulated with islet peptides, and after 48 h single CD4+ IL-10+ or CD4+ IL-10− T-cells isolated by flow cytometry were deposited into 96-well plates containing 8 μl lysis buffer. RT and first-round PCR (30 cycles) were performed using gene-specific primers and the One Step RT-PCR kit following the manufacturer's instructions (Qiagen, Crawley, U.K.). After multiplex RT-PCR, 1 μl of a 1:100 dilution of the reaction was used in individual gene, second round, nested PCRs (30 cycles) using internal primers and HotStar Taq Polymerase kit (Qiagen). Products were analyzed by agarose gel electrophoresis. Data were considered for analysis only from cells that yielded a CD3ε product. Each lane represents products amplified from a single cell. As a positive control, pools of five cells of the clones RAR5.3 and MHB10.3 were used after peptide stimulation.
DISCUSSION
The present study builds upon our previous observation that in type 1 diabetes, a distinction can be made between tolerant and disease states in terms of the polarization of islet-specific CD4 T-cell responses. Notably, we previously showed that islet autoreactive T-cells identified by IL-10 secretion are associated with both the nondiabetic state and late onset disease. On this basis, we proposed that IL-10 production by CD4 T-cells in response to islet autoantigens represents a signature of immune regulation. As proof of this concept, in the present study we report the first cloning of such human, IL-10–secreting islet-specific T-cells (which we term ISIS-Tregs) and demonstrate that their marked capacity for linked suppression of proinflammatory T-cells is associated with APC killing.
We first screened a cohort of healthy individuals to identify those with robust IL-10 polarization of islet autoreactive CD4 T-cell responses. In agreement with our earlier studies, we found that the majority of nondiabetic individuals mounted a significant IL-10 response to at least one islet autoantigen. We next used a sensitive, direct, ex vivo cloning strategy to isolate and expand the IL-10–producing cells, to derive sufficient numbers with which to examine our original hypothesis that these cells have regulatory properties. We demonstrate that not only are IL-10–secreting islet-specific T-cells capable of suppressing responses of resting CD4 memory T-cells present in PBMC preparations, they are also potent suppressors of both the proliferation and cytokine production of highly activated Th1 T-cell clones similar to those believed to play a role in the pathogenesis of type 1 diabetes. In contrast, in our experience neither freshly isolated nor expanded populations of the prototypic polyclonal CD4+CD127loCD25+ naturally occurring Tregs (nTregs) are able to suppress activated Th1 clones to such a degree (J.L., personal communication). Further examination of their function revealed that ISIS-Tregs have a highly distinctive mode of action. Their ability to suppress effector T-cell function is mediated entirely independently of production of cytokines or any other paracrine soluble factors, requiring instead direct-cell contact. Using ISIS-Treg and effector T-cell clones with differing HLA restriction elements, we limited antigen presentation to occur on the same APC or on two different APC populations. Suppression was dependent on presentation of the cognate antigen for both ISIS-Treg and effector T-cells on the same APCs. In investigating the molecular and cellular interactions required for this effect, we noted that ISIS-Tregs express perforin and granzymes A and B and therefore examined the APC killing potential of these cells. Only APCs bearing cognate antigen were killed by ISIS-Tregs in a perforin-dependent manner, and as a result APC-mediated activation of effector clones was effectively negated. Critically, we confirmed expression of perforin and granzymes in freshly isolated ISIS-Tregs directly ex vivo, excluding the possibility that their expression is an artifact of clonal expansion in vitro.
Based on our studies in vitro, we hypothesize that in vivo, ISIS-Tregs achieve regulation by a “kill the messenger approach” in which APCs displaying high levels of self-antigens are rapidly deleted, thus preventing the priming and/or expansion of potentially pathogenic autoreactive CD4 T-helper and CD8 cytotoxic T-cells. We propose a three-cell model of linked suppression in which professional APCs will display peptide-HLA complexes representing a range of autoantigens including insulin, IA-2, and GAD-65 released by β-cells (perhaps damaged by an environmental insult such as a virus [22]). ISIS-Tregs will kill these APCs, while sparing bystander APCs not displaying islet antigens. Although not providing “classical” antigen-specific regulation (i.e., only regulating T-cells responding to the same antigen), this mechanism provides “tissue-specific” regulation, including suppression of T-cells recognizing other islet autoantigens. Such a mechanism of suppression has several important attributes, most notably the highly specific nature and location of regulation as a means of maintaining tissue-specific tolerance. In sum, our data appear to support a model in which the most potent regulatory function of these Tregs in vitro requires cell-cell contact and is independent of IL-10. However, as also pointed out by others in this field (23,24) and supported by our finding in relation to B7-H4, we cannot exclude the possibility that there are additional pathways via IL-10 secretion through which ISIS-Tregs are able to regulate in vivo and these remain to be elucidated.
Expression of cytotoxic molecules has been reported in other natural and adaptive human polyclonal Treg populations (25–28). However, comparison with the highly specific killing mediated by ISIS-Tregs is difficult, as these other Tregs are of unknown antigen specificity and require polyclonal stimuli to mediate their suppressor function. The importance of the cytotoxic function of Tregs has recently been demonstrated in a model of transplantation in which Tregs from granzyme B–deficient mice were unable to mediate transplant tolerance (29). Furthermore, Benoist and colleagues have also highlighted the importance of a population of CD25+ Tregs characterized by the expression of IL-10 and granzyme B in preventing disease in a mouse model of type 1 diabetes (30), supporting the suggestion that cytotoxic ISIS-Tregs may play an important role in maintaining islet-specific tolerance in vivo.
At present, it is not possible to be certain where ISIS-Tregs stand in the current, complex taxonomy of regulatory T-cells. ISIS-Tregs share some features in common with other Treg populations (e.g., constitutive expression of CD25, FoxP3, CD39, and ICOS; requirement for cell-cell contact to mediate suppression; and expression of cytotoxic effector molecules) but not others (e.g., expression of GITR and absence of CD127), and the relevance of these findings to their function and ontogeny will require further investigation.
We speculate that ISIS-Tregs are generated in the periphery as a consequence of low-level exposure to autoantigens occurring early during development or chronically, in the absence of inflammatory signals, as has been suggested for the generation of peripheral tolerance (31). Intriguingly, ISIS-Tregs do bear striking similarities (IL-10 secretion and dependence on cell-cell contact) to Tregs generated from naive cord blood cells by repeated stimulation with immature allogeneic dendritic cells (32), suggesting the possibility that ISIS-Tregs may be generated early in development.
In summary, we have isolated and characterized a novel population of human islet autoantigen–specific regulatory T-cells associated with late development of type 1 diabetes. These ISIS-Tregs are potent suppressors of Th1 effector cells and mediate suppression via direct contact with autoantigen-presenting APCs, providing exquisite specificity of suppression. This hitherto undescribed population of antigen-specific Tregs, which appears to be constitutively present in the majority of nondiabetic individuals, may provide a suitable target to strengthen tolerance to islets by clinical immunointervention.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Diabetes UK and the Sir Jules Thorn Charitable Trust. T.I.M.T. is a Diabetes UK RD Lawrence Research Fellow and J.L. is the recipient of a Diabetes UK PhD studentship. T.I.M.T., A.S., and M.P. acknowledge financial support from the Department of Health via the NIHR Comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London.
No potential conflicts of interest relevant to this article were reported.
We are grateful to subjects for blood donation, especially M.H., R.A., and T.F.B.H.; to Adrian Hayday and Maria Grazia Roncarolo for helpful discussions; and to Wayne Turnbull for performing flow cytometry and cell sorting.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Sakaguchi S: Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004; 22: 531–562 [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T: Organ-specific autoimmune diseases induced in mice by elimination of T cell subset: I, evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med 1985; 161: 72–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M: Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995; 155: 1151–1164 [PubMed] [Google Scholar]
- 4.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME: X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 2001; 27: 18–20 [DOI] [PubMed] [Google Scholar]
- 5.Baecher-Allan C, Hafler DA: Human regulatory T cells and their role in autoimmune disease. Immunol Rev 2006; 212: 203–216 [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T: Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 2006; 212: 8–27 [DOI] [PubMed] [Google Scholar]
- 7.Roncarolo MG, Levings MK: The role of different subsets of T regulatory cells in controlling autoimmunity. Curr Opin Immunol 2000; 12: 676–683 [DOI] [PubMed] [Google Scholar]
- 8.Bach JF: Regulatory T cells under scrutiny. Nat Rev Immunol 2003; 3: 189–198 [DOI] [PubMed] [Google Scholar]
- 9.Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA: Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J Immunol 2005; 175: 3053–3059 [DOI] [PubMed] [Google Scholar]
- 10.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA: In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 2004; 199: 1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM: Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med 2007; 204: 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM: CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 2004; 199: 1467–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staeva-Vieira T, Peakman M, von Herrath M: Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol 2007; 148: 17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peakman M, Stevens EJ, Lohmann T, Narendran P, Dromey J, Alexander A, Tomlinson AJ, Trucco M, Gorga JC, Chicz RM: Naturally processed and presented epitopes of the islet cell autoantigen IA-2 eluted from HLA-DR4. J Clin Invest 1999; 104: 1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schloot NC, Meierhoff G, Karlsson Faresjö M, Ott P, Putnam A, Lehmann P, Gottlieb P, Roep BO, Peakman M, Tree T: Comparison of cytokine ELISpot assay formats for the detection of islet antigen autoreactive T cells: report of the third immunology of diabetes society T-cell workshop. J Autoimmun 2003; 21: 365–376 [DOI] [PubMed] [Google Scholar]
- 16.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M: Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 2004; 113: 451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Lorenzo TP, Peakman M, Roep BO: Translational mini-review series on type 1 diabetes: systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol 2007; 148: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skowera A, Cleare A, Blair D, Bevis L, Wessely SC, Peakman M: High levels of type 2 cytokine-producing cells in chronic fatigue syndrome. Clin Exp Immunol 2004; 135: 294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI: Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes 2005; 54: 92–99 [DOI] [PubMed] [Google Scholar]
- 20.Kelso A, Groves P, Ramm L, Doyle AG: Single-cell analysis by RT-PCR reveals differential expression of multiple type 1 and 2 cytokine genes among cells within polarized CD4+ T cell populations. Int Immunol 1999; 11: 617–621 [DOI] [PubMed] [Google Scholar]
- 21.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L: B7–H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 2003; 18: 849–861 [DOI] [PubMed] [Google Scholar]
- 22.Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P: Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 2007; 104: 5115–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wraith DC: Role of interleukin-10 in the induction and function of natural and antigen-induced regulatory T cells. J Autoimmun 2003; 20: 273–275 [DOI] [PubMed] [Google Scholar]
- 24.O'Garra A, Vieira PL, Vieira P, Goldfeld AE: IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest 2004; 114: 1372–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ: Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol 2005; 174: 1783–1786 [DOI] [PubMed] [Google Scholar]
- 26.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ: Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 2004; 21: 589–601 [DOI] [PubMed] [Google Scholar]
- 27.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ: Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 2004; 104: 2840–2848 [DOI] [PubMed] [Google Scholar]
- 28.Kawamura K, Kadowaki N, Kitawaki T, Uchiyama T: Virus-stimulated plasmacytoid dendritic cells induce CD4+ cytotoxic regulatory T cells. Blood 2006; 107: 1031–1038 [DOI] [PubMed] [Google Scholar]
- 29.Gondek DC, Devries V, Nowak EC, Lu LF, Bennett KA, Scott ZA, Noelle RJ: Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells. J Immunol 2008; 181: 4752–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman AE, Freeman GJ, Mathis D, Benoist C: CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med 2004; 199: 1479–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carbone FR, Belz GT, Heath WR: Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol 2004; 25: 655–658 [DOI] [PubMed] [Google Scholar]
- 32.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH: Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med 2000; 192: 1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.