Abstract
OBJECTIVE
The nonobese diabetic (NOD) mouse is a well-established mouse model of spontaneous type 1 diabetes, which is characterized by an autoimmune destruction of the insulin-secreting pancreatic β-cells. In this study, we address the role of tertiary lymphoid organs (TLOs) that form in the pancreas of NOD mice during disease progression.
METHODS
We developed a model designed to “lock” lymphocytes in the pancreatic lymph node (PLN) and pancreas by the use of FTY720, which blocks the exit of lymphocytes from lymph nodes. A combination of flow cytometry, immunofluorescence, and analysis of clinical scores was used to study the effects of long-term FTY720 treatment on TLO development and development of diabetes.
RESULTS
Continuous treatment of NOD mice with FTY720 prevented diabetes development even at a time of significant insulitis. Treatment withdrawal led to accelerated disease independent of the PLN. Interestingly, naive T-cells trafficked to and proliferated in the TLOs. In addition, morphological changes were observed that occurred during the development of the disease. Remarkably, although the infiltrates are not organized into T/B-cell compartments in 8-week-old mice, by 20 weeks of age, and in age-matched mice undergoing FTY720 treatment, the infiltrates showed a high degree of organization. However, in naturally and FTY720-induced diabetic mice, T/B-cell compartmentalization was lost.
CONCLUSION
Our data show that TLOs are established during diabetes development and suggest that islet destruction is due to a loss of TLO integrity, which may be prevented by FTY720 treatment.
The nonobese diabetic (NOD) mouse is a well-established mouse model of spontaneous type 1 diabetes, which is characterized by an autoimmune attack against the insulin-secreting pancreatic β-cells in the islets (1). Multiple factors play a role in the development of diabetes in the NOD mouse model starting with the activation of T-cells by antigen-presenting cells, leading to T-cell differentiation and, ultimately, destruction of the target tissue. However, the location of these key events remains unclear.
Lymphocytic infiltrates can be detected surrounding the islets in NOD mice as early as 3–4 weeks of age (2). The infiltrate continues to amass and by 15–18 weeks of age completely invades the islet and destroys it. Although virtually all NOD mice show some level of insulitis, only 60–80% of females and 20–30% of males, depending on the colony, develop fulminant disease (1). Therefore, the autoimmune destruction of pancreatic islets in NOD mice has been suggested to include two phases: insulitis, which involves infiltration of lymphocytes into the pancreas, and overt diabetes, which involves killing of the β-cells in the islets and resultant hyperglycemia (3). Interestingly, it has been shown that removal of the draining pancreatic lymph node at 3 weeks of age protects from disease development but removal at 10 weeks of age has no effect on disease development, suggesting that disease can become lymph node independent (4).
Organized lymphocytic infiltrates forming ectopically in nonlymphoid tissues have been termed tertiary lymphoid organs (TLOs) because of their morphological similarities to secondary lymphoid organs (5). These include the presence and compartmentalization of T- and B-cells controlled by chemokine gradients, high endothelial venules (HEVs), lymphatic vessels, and antigen-presenting cells. TLOs have been described in a variety of autoimmune diseases including gastritis, thyroiditis, and systemic lupus erythematosus (5). However, a detailed analysis of TLOs in the pancreas of NOD mice has not been performed and could provide insights into the development of the disease, including whether antigen presentation occurs in the pancreas leading to epitope spreading and expansion of the immune response; whether continuous recruitment of lymphocytes to the site is necessary to cause disease; and whether priming of T-cells in these structures sufficiently activates them to become pathogenic. In this study, we used the S1P1 agonist FTY720, which blocks T-cell egress from lymphoid tissues (6), to “lock” cells in the draining pancreatic lymph node and the pancreas to study the role of TLOs in the pathogenesis of diabetes in NOD mice.
RESEARCH DESIGN AND METHODS
Mice.
NOD, NOD.BDC25 (7), and NOD.Rag2 knockout mice were bred at the University of California, San Francisco. NOD mice were also purchased from Taconic. All mice were housed under specific pathogen–free conditions at the University of California, San Francisco Animal Barrier Facility. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco. For diabetes incidence, urine glucose was followed twice weekly.
Human pancreas samples.
Samples from human diabetic patients were obtained through the Network for Pancreatic Organ Donors with Diabetes (nPOD) project of the Juvenile Diabetes Research Foundation (JDRF). The age of the patients ranged from 12 to 22 years old and they were diagnosed with type 1 diabetes 1–8 years prior to death. Details regarding their collection and methods of preparation can be found at http://www.jdrfnpod.org.
FTY720 administration.
FTY720 was obtained from Novartis Pharmaceuticals and was dissolved in normal saline. A dose of 20 μg/day was administered by intraperitoneal injection three times per week.
Pancreatic lymphadenectomy.
Pancreatic lymph nodes were removed as previously described (4).
Reagents.
For flow cytometry, labeled antibodies specific for CD4 clone RM4–5, CD8 clone 53–6.7, CD25 clone PC61, FoxP3 clone FJK-16s, CD45 clone I3/2.3, CD11c clone N418, CD86 cone GL1, CD40 clone 3/23, CD80 clone 16–10A1, and I-Ag7clone 10.2.16 were used. Cells were fixed and permeabilized using Foxp3 Staining Kit per manufacturer's protocol (ebiosciences, San Diego, CA). For immunofluorescence, primary antibodies specific for mCD4 clone L3T4, hCD4 clone RPA-T4, mCD45R/B220-biotin clone RA3.6B2, hCD19 clone H1B19, mCD31 clone 390, murine mucosal addressin cell adhesion molecule (mMAdCAM) clone MECA367, murine lymphatic vessel endothelial hyaluronan receptor-1 (mLyve-1) clone 223322, and murine fibroblast specific clone ERTR7 were used. MECA79 antibody specific for peripheral node addressin (PNAd) was obtained from Dr. Steve Rosen (University of California, San Francisco).
Immunofluorescence.
Pancreata were isolated and frozen in optimum cutting temperature embedding compound. Consecutive sections (7 μm) were cut with a Leica CM 3050S cryomicrotome and fixed for 15 min in acetone at −20°C. Sections were blocked with 5% BSA and 3% normal serum corresponding to the secondary antibody and/or Avidin/Biotin Blocking Kit per manufacturer's protocol (Vector, Burlingame, CA). Slides were incubated with primary and secondary antibodies for 1–2 h at room temperature and counterstained with Gill hematoxylin. Digital images were captured with an Optiphot microscope equipped with an Axiocam digital camera and analyzed using Adobe Illustrator CS2.
Isolation of lymphocytes from pancreas.
Lymphocytes were isolated from the pancreas as previously described (8).
Isolation of dendritic cells.
Dendritic cells were isolated from lymph nodes and islets as previously described (9).
Adoptive transfer and CFSE dilution analysis.
CD4+CD25−CD62L+ cells were sorted from NOD.BDC2.5Tg+Thy1.1 mice. Cells were then labeled with carboxyfluorescein succinimidyl ester (CFSE) and 1 × 106 transferred to NOD.Thy1.2 mice. Immediately after transfer and daily until time of harvest, mice were injected with FTY720. On day 5, single-cell suspensions from pancreas, spleen, pancreatic lymph node (PLN), and inguinal lymph nodes (ILN) were stained for flow cytometric analysis. To calculate the percentage of T-cells that entered cell cycle, the original number of cells that entered cell cycle was determined by dividing the number of events at each division (i.e., CFSE peak) by the number of divisions that occurred. This number was divided by the total number of cells (original number of cells that entered cell cycle + number of cells that never entered cell cycle).
Statistical analysis.
The statistical significance between Kaplan-Meier survival curves in Figs. 1 and 4 was determined by a log-rank test using Prism software. The statistical significance of differences between groups in Table 1 and Fig. 3 was determined by two-tailed Student t test using Excel. The statistical significance of differences between groups in supplementary Fig. 2 (available in an online appendix at http://diabetes.diabetesjournals.org/content/early/2010/03/10/db09-1129/suppl/DC1) was determined by the Mann-Whitney test using Prism software.
FIG. 1.
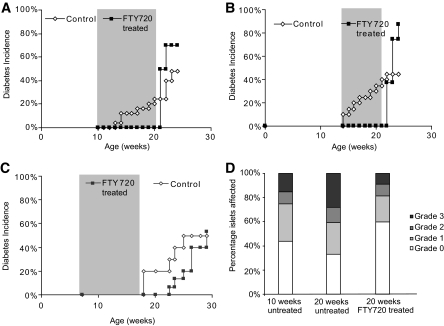
FTY720 treatment of NOD mice. A: Female NOD mice treated three times per week with 20 μg/day FTY720 from 12 to 22 weeks of age (shaded). Control n = 25; FTY720 treated n = 10. P = 0.0091 after FTY720 withdrawal. B: Female NOD mice treated as in (A) starting at 14 weeks of age for 7 weeks. Control n = 20; FTY720 treated n = 8. P = 0.0004 after FTY720 withdrawal. C: Female NOD mice treated as in (A) starting at 7 weeks of age for 9 weeks (shaded). Control n = 10; FTY720 treated n = 15 pooled from two independent experiments. P = 0.7723 after FTY720 withdrawal. D: Histological analysis of pancreas sections at the end of FTY720 treatment in panel (A). Ten weeks untreated n = 6 mice, 1,001 individual islets; 20 weeks untreated n = 6 mice, 576 individual islets; 20 weeks FTY720 treated n = 7 mice from two independent treatments, 816 individual islets. Grade 0 = no infiltrate. Grade 1 = <25% of islet infiltrated. Grade 2 = 25–75% of islet infiltrated. Grade 3 = >75% of islet infiltrated.
FIG. 4.
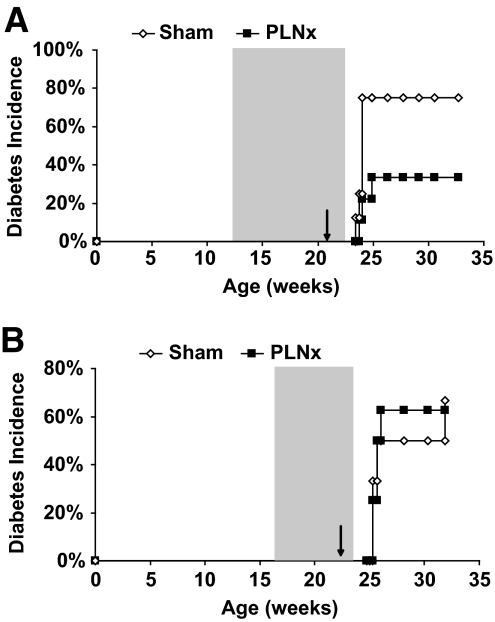
Role of draining PLN on FTY720-induced diabetes. A: NOD female mice were treated for 9–10 weeks (shaded) with FTY720 beginning at weeks 11–13. During the last week of treatment, the PLNs were surgically removed. Disease incidence was monitored after FTY720 withdrawal. Data are pooled from two independent experiments. Sham n = 8, PLNx n = 9. P = 0.0817. B: NOD female mice were treated for 8 weeks (shaded) with FTY720 beginning at week 16. During the last week of treatment, the pancreatic lymph nodes were surgically removed. Disease incidence was monitored after FTY720 withdrawal. Sham n = 6, PLNx n = 8. P = 0.8456.
TABLE 1.
Analysis of T-cells during FTY720 treatment
| Pancreas | PLN | ILN | Spleen | |
|---|---|---|---|---|
| Total cell number (×106) | ||||
| Control | ND | 2.73 ± 1.40* | 9.97 ± 3.50* | 79.6 ± 25.9* |
| FTY720 treated | ND | 3.16 ± 0.80 | 3.65 ± 1.03 | 31.7 ± 15.2 |
| % CD4+ of CD45+ cells | ||||
| Control | 26.2 ± 9.6* | 48.5 ± 1.5* | 51.4 ± 7.5* | 28.3 ± 4.7* |
| FTY720 treated | 9.3 ± 1.9 | 32.4 ± 1.8 | 27.8 ± 3.3 | 13.1 ± 2.6 |
| Total CD4+ cell number (×106) | ||||
| Control | ND | 1.32 ± 0.67 | 4.99 ± 1.47* | 21.6 ± 4.48* |
| FTY720 treated | ND | 1.02 ± 0.26 | 1.00 ± 0.25 | 4.07 ± 1.95 |
| % Foxp3+ of CD4+ cells | ||||
| Control | 17.3 ± 6.8 | 21.6 ± 5.7 | 11.8 ± 2.6* | 17.9 ± 4.2 |
| FTY720 treated | 26.5 ± 9.8 | 24.3 ± 3.3 | 27.2 ± 2.3 | 20.0 ± 3.1 |
| Total CD4+Foxp3+ cell number (×106) | ||||
| Control | ND | 0.28 ± 0.15 | 0.60 ± 0.23* | 3.91 ± 1.33* |
| FTY720 treated | ND | 0.25 ± 0.07 | 0.27 ± 0.07 | 0.84 ± 0.45 |
| % CD8+ of CD45+ cells | ||||
| Control | 7.2 ± 5.3 | 19.8 ± 2.6* | 20.5 ± 2.4* | 12.5 ± 2.0* |
| FTY720 treated | 4.0 ± 4.1 | 31.9 ± 3.3 | 31.5 ± 3.4 | 4.0 ± 1.0 |
| Total CD8+ cell number (×106) | ||||
| Control | ND | 0.54 ± 0.29* | 2.02 ± 0.73* | 9.86 ± 3.31* |
| FTY720 treated | ND | 1.01 ± 0.28 | 1.13 ± 0.26 | 1.25 ± 0.59 |
NOD female mice were treated for 10 weeks with FTY720 beginning at 10–12 weeks of age. Mice were analyzed on the last day of treatment. Total cell number and percentages of CD4+ and CD8+ lymphocytes among CD45+ cells and CD4+Foxp3+ cells among CD4+ cells were determined by flow cytometry. Mean values of 3 independent treatment experiments are shown. Control n = 8. FTY720 treated n = 11. ND, not determined.
*P < 0.01 between control and FTY720 treated in the same tissue.
FIG. 3.
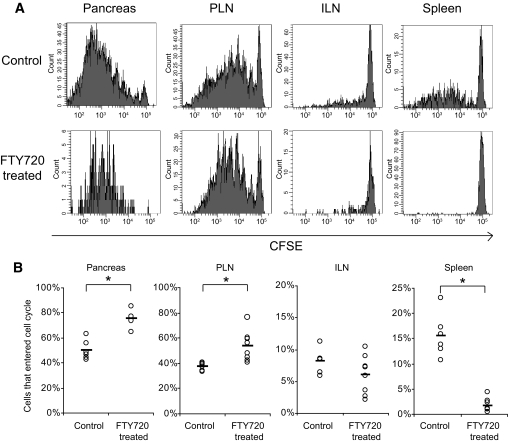
Proliferation of BDC2.5 Tg T-cells in NOD mice. CD25−CD62L+ naive BDC2.5 TCR transgenic cells (1 × 106) were CFSE labeled and transferred to 8- to 10-week-old NOD females. After transfer, mice were treated with 20 μg FTY720 daily. On day 5, BDC2.5 proliferation was analyzed by CFSE dilution in the pancreas, draining PLN, nondraining ILN, and spleen. A: Representative histograms of control (top) and FTY720-treated (bottom) mice. B: Percentage of cells that entered the cell cycle was calculated as described in the “Research Design and Methods” section. Data were pooled from two independent experiments. *P < 0.01.
RESULTS
Continuous FTY720 treatment prevents diabetes but leads to rapid disease development upon withdrawal.
FTY720 blocks T-cell egress from lymph nodes and has been shown to be effective clinically in the treatment of autoimmunity and transplant rejection (10). We set up a model using FTY720 that was designed to examine the effects of “locking” lymphocytes in the PLN and pancreas. Treatment of female NOD mice with 20 μg/day 3 times per week starting at 10 or 14 weeks of age, when insulitis had already begun or was established, completely protected mice from diabetes development (Fig. 1A and B). Withdrawal of drug treatment after 10 or 7 weeks, respectively, led to rapid development of diabetes. Of mice, 70–88% were diabetic 18 days after treatment withdrawal in multiple independent experiments (Fig. 1A and B). This compares with 48% incidence among untreated control mice. Similarly, continuous treatment until 25 weeks led to protection during treatment and acceleration of disease upon withdrawal (data now shown). To assess whether insulitis at the beginning of treatment was necessary for the rapid disease onset seen after withdrawal, we began treatment at 7 weeks of age, when insulitis was present but minimal (Fig. 1C). Unlike the previous regimens, withdrawal 10 weeks later did not lead to rapid diabetes development. By 29 weeks of age, 12 weeks after FTY720 withdrawal, only 50% of FTY720-treated mice had become diabetic with similar kinetics as observed in control mice. As expected, continuous FTY720 treatment led to a marked decreased of circulating lymphocytes, which returned to normal levels 7 days after treatment withdrawal (data not shown). These results suggest that the disease acceleration was not due to direct effects of the drug on the pancreas.
Continuous FTY720 treatment does not clear pancreatic infiltrate.
A possible explanation for disease protection during FTY720 treatment is clearance of pancreatic infiltrate. Histological analysis and scoring of islet infiltrates of 20-week-old mice that had been treated with FTY720 from 10–12 to 20–22 weeks of age and age-matched controls showed that treatment had limited effects on islet infiltration (Fig. 1D). Of the islets, 40% had some degree of peri-islet infiltration similar to that observed in untreated 10- to 12-week-old NOD mice, suggesting that FTY720 treatment did not clear tissue infiltrate but rather blocked trafficking of new cells from the periphery. Interestingly, the overall fraction of pancreatic islets exhibiting grades 1, 2, or 3 insulitis remained consistent between 10- and 20-week-old FTY720-treated mice, suggesting that FTY720 blocked further islet invasion.
FTY720 treatment leads to minor changes in infiltrating CD4+ T-cells.
Next, we compared the infiltrate in 20- to 22-week-old mice undergoing continuous FTY720 treatment from 10–12 weeks of age (as in Fig. 1A) to aged-matched untreated controls. The total number of cells in the nondraining ILN and the spleen was threefold less in FTY720-treated mice (Table 1) likely due to sequestration of cells in the thymus (data not shown) and systemic lymphopenia, respectively. Interestingly, the total cell number in the PLN remained unchanged (Table 1), a result that could reflect continued expansion of cells specific for pancreatic antigens. Similar results were observed when examining the CD4+ and CD8+ T-cells (Table 1). In the pancreas, the percentage of CD4+ T-cells decreased twofold to threefold, but there was no change in infiltrating CD8+ T-cells (Table 1). These results suggest that disease protection during treatment, but not the disease acceleration seen after withdrawal, may be due to a decrease in infiltrating CD4+ T-cells. Importantly, these data suggest that potentially pathogenic T-cells were able to persist at the site of inflammation without continuous trafficking from peripheral lymphoid tissues.
Because FTY720 has been suggested to increase the proportion of regulatory T cells (Tregs;11,12), we assessed whether a proportional increase in Tregs could account for the disease protection during treatment. Analysis of absolute numbers of Tregs showed that the total number of CD4+Foxp3+ cells decreased in the ILN and spleen but remained the same in the PLN (Table 1). The percentage of CD4+Foxp3+ cells in the pancreas remained the same; however, the absolute number of Tregs likely decreased in treated mice, suggesting that changes in Tregs were unlikely to be responsible for disease prevention.
In addition to inhibiting the migration of lymphocytes, FTY720 has also been suggested to have effects on dendritic cells (DCs;13). To test this hypothesis, we treated 16- to 18-week-old NOD mice with FTY720 for 10 days and analyzed the percentage of CD11c+ cells in the islets, PLNs, and ILNs as well as their expression of CD86, CD40, CD80, and I-Ag7 (major histocompatibility complex class II). The treatment did not affect the percentage of DCs or their maturation state in any tissue analyzed (supplementary Fig. 1), suggesting that disease protection observed during FTY720 treatment or disease acceleration observed after treatment withdrawal was not due to changes in DCs.
Analysis of tertiary lymphoid organs in the pancreas.
Because the total cell number in the PLN was unaffected by FTY720 treatment and a significant number of cells remained in the pancreas, it was unlikely that disease protection during FTY720 treatment could be attributed solely to a reduction in infiltrating cells. Therefore, we analyzed the effect of long-term FTY720 treatment on the structure and characteristics of TLOs. Because FTY720 blocks lymphocyte egress from the lymph nodes, we hypothesized that disease was prevented by inhibiting lymphocyte egress from the TLOs in the pancreas to the site of tissue destruction—the β-cells. First, we characterized the TLOs in pre-diabetic 8-week-old, nondiabetic 20-week-old and naturally diabetic mice by immunofluorescent staining of individual markers on consecutive pancreas sections using markers described in the “Research Design and Methods” section.
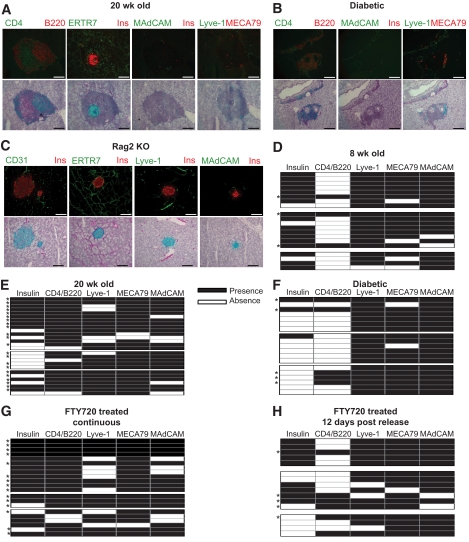
ERTR7 reactivity and CD31+ vessels were observed surrounding all islets and throughout the acinar tissue at all time points analyzed (Fig. 2A and data not shown). In addition, ERTR7 reactivity was found within the infiltrate, suggesting that these structures have a supporting stromal network, as is seen in T-cell zones in the spleen and lymph nodes (14). However, although the results trend toward an increase in the association of the presence of MAdCAM+ or Lyve+ vessels and disease progression, these differences were not statistically significant (supplementary Fig. 2B). In addition, most infiltrates in both pre-diabetic and naturally diabetic mice contained PNAd+ HEVs (Fig. 2D–G), suggesting that, like secondary lymphoid organs, these structures are capable of recruiting naive T-cells. Analysis of at least three consecutive sections showed that infiltrates of pre-diabetic 20-week-old mice were usually, but not always, associated with insulin-producing islets (Fig. 2A and E). Although T- and B-cells were present in the pancreas of 8-week-old mice, only 15% (3/20) of infiltrates showed clear compartmentalization, whereas 89% (24/27) of infiltrates in 20-week-old mice showed distinct T- and B-cell zones. In the majority of these, the T-cell zone was closer to the islet, whereas the B-cell zone was on the edge of the infiltrate, reminiscent of lymph nodes (Fig. 2A). Only 15% of infiltrates (3/19) in naturally diabetic mice showed T/B-cell compartmentalization, which correlated with an absence of insulin-positive islets that we attribute to destroyed β-cells (Fig. 2B and G). However, β-cells may have been present but not producing insulin because of inflammation (15). True TLOs, defined as infiltrates with T/B compartmentalization and association with at least two of the vascular markers analyzed, were present in 15% (3/20) of infiltrates in 8-week-old mice, increasing to 81% (22/27) of infiltrates in 20-week-old mice but decreasing to only 26% (5/19) of infiltrates in naturally diabetic mice (Fig. 2D–F). Therefore, TLOs peaked as a proportion of the infiltrate at the “peak” of the inflammatory response before diabetes onset.
FIG. 2.
Analysis of TLOs in the pancreas of NOD mice. Pancreas sections from (A) 20-week-old, (B) naturally diabetic, and (C) Rag2 knockout NOD mice were costained with anti-insulin and anti-CD31, anti-ERTR7 and anti-insulin, anti–Lyve-1 and anti-insulin, anti-MAdCAM and anti-insulin, anti-CD4 and anti-B220, or anti–Lyve-1 and MECA79 antibodies. Representative immunofluorescence images of consecutive sections (except for [C]) stained with the indicated antibodies are shown on the top and are superimposed on hematoxylin counterstain bright-field images on the bottom (green fluorophore appears as pink, red fluorophore appears as blue). (D–H) Infiltrates were scored based on their association with insulin-producing islets (column 1), whether there was T/B-cell zone compartmentalization (column 2), and association with Lyve-1+ (column 3), MECA79/PNAd+ (column 4), or MAdCAM+ (column 5) vessels. Each row indicates a separate infiltrate/islet analyzed. Each block indicates a separate mouse. Bar represents 100 μm. *Infiltrates considered to be true TLOs based on T/B compartmentalization and association with at least two of the vascular markers analyzed. (A high-quality digital representation of this figure is available in the online issue).
We analyzed NOD.Rag2 knockout mice to assess whether the appearance of TLO markers was due to inflammation or a normal characteristic of the NOD pancreas (Fig. 2C). As in NOD mice, there was ERTR7 reactivity and CD31+ vessels around islets and throughout the acinar tissue. MAdCAM+ and Lyve-1+ vessels were associated with 30 and 59% of islets, respectively, which is significantly lower than in mice with an intact immune system (supplementary Fig. 2B). There was no PNAd staining, suggesting an adaptive immune response was necessary for the development of HEVs in the pancreas (data not shown).
Analysis of FTY720-treated mice after 8–10 weeks of continuous treatment (20 weeks old) showed that the majority of the infiltration was associated with insulin-producing islets (Fig. 2G). Moreover, the infiltrates in FTY720-treated mice showed a similar degree of organization, based on T/B compartmentalization (19/21), as age-matched untreated controls (Fig. 2G). Similar to pre-diabetic and naturally diabetic mice, the TLOs in these mice had PNAd+ HEVs as well as Lyve-1+ and MAdCAM+ vessels (supplementary Fig. 2A). Analysis of mice on day 12 after FTY720 release, when 30–60% mice have become diabetic, showed that the infiltrates lose T/B compartmentalization and are similar to those of naturally diabetic mice; only 31% (5/16) of infiltrates were true TLOs based on our criteria (Fig. 2H).
In an attempt to define TLOs in humans with type 1 diabetes, we analyzed pancreatic sections from four such patients obtained through JDRF's nPOD program (http://www.jdrfnpod.org). Although CD4+ T-cells and CD19+ B-cells were found surrounding insulin+ islets (supplementary Fig. 3), the cells were scattered and did not form large clusters as was observed in pre-diabetic and early-onset diabetic NOD mice. However, at present, we cannot distinguish whether the absence of TLOs in the human samples is due to temporal differences in sampling (i.e., the human tissues represented those obtained from individuals with longer disease duration than those we analyzed in NOD mice) or actual differences between the disease in the two species.
Accelerated disease upon FTY720 withdrawal is independent of the draining pancreatic lymph node.
A possible explanation for the accelerated disease after cessation of FTY720 is that the relevant antigen-specific cells continue to expand to sufficient numbers and activation status to cause rapid disease upon treatment withdrawal and restoration of trafficking. This would suggest that during long-term FTY720 treatment there are reservoirs of islet-reactive cells that accumulate either within or outside of the pancreas. To determine in which lymphoid tissues islet antigens are presented that can lead to antigen-specific T-cell expansion, we transferred 1 × 106 CFSE-labeled naive CD4+CD25−CD62L+ islet antigen–specific BDC2.5 TCR-Tg cells to 8-week-old NOD mice and treated them with FTY720 daily to prevent recirculation. After 5 days, cells were harvested and proliferation was evaluated by CFSE dilution (Fig. 3). In control treated mice, 37 and 50% of transferred cells had entered the cell cycle in the PLN and the pancreas, respectively. The proliferation was antigen dependent, as only 8 and 14% of the TCR-Tg T-cells went into cycle in the ILN and spleen, respectively. In FTY720-treated mice, a higher percentage of cells that entered the cell cycle were observed in the PLN (54%) and pancreas (75%). These results suggested that naive cells were able to home directly to and proliferate in the pancreas and that FTY720 treatment did not prevent antigen presentation or antigen-driven proliferation. Similar to untreated mice, 6 and 2% of the cells in the ILN and spleen, respectively, proliferated in FTY720-treated mice, suggesting that the PLN is the only secondary lymphoid tissue where islet antigens are presented and that the proliferating cells seen in the spleens of untreated mice are a result of recirculation.
We reasoned that the cells causing rapid onset of diabetes upon FTY720 withdrawal originated from either the PLN or the pancreas. Thus, we tested the effect of surgical removal of the PLN in mice undergoing FTY720 treatment. FTY720 treatment was begun at 12 weeks of age, the PLN was surgically removed (PLNx) 9 weeks later, and treatment was withdrawn 1 week after the surgery. Of mice that underwent PLNx, 40% developed diabetes within 15 days of treatment withdrawal compared with 80% of sham controls (Fig. 4A). As our previous experiments had shown, age at beginning of treatment influences whether there is accelerated disease upon treatment withdrawal, suggesting that although the PLN may be important early in disease progression, the requirement of this lymph node diminishes as disease progresses. We hypothesized that if we began treatment late in life, a greater percentage of mice would develop disease independently of the PLN because of the increased level of infiltration at the time of treatment. Therefore, for the second series of experiments, we began treatment at 16 weeks of age, removed the PLN at 23 weeks of age, and withdrew FTY720 1 week later. Under these conditions, 50% of sham control mice and 65% of mice that underwent PLNx became diabetic within 15 days of FTY720 withdrawal (Fig. 4B). These results suggest that the diabetes acceleration observed after FTY720 can be attributed to antigen-reactive T-cells present in both pools. However, the PLN is not required for the accelerated disease in mice with the most progressed disease. In this setting, the cells present in the TLOs of the pancreas at the beginning of the treatment are sufficient to cause diabetes upon FTY270 withdrawal.
DISCUSSION
In this study, we used FTY720 treatment of NOD mice to prevent trafficking of lymphocytes into the pancreas and studied the role of TLOs in diabetes development. Our results show that continuous FTY720 treatment prevents diabetes development and, when withdrawn, leads to an accelerated disease that is independent of the PLN if treatment is begun when insulitis is established. Analysis of NOD mice at various stages of disease and during FTY720 treatment showed that lymphocyte infiltration into the pancreas leads to the formation of organized TLOs characterized by T/B compartmentalization and specialized vasculature, including lymphatic vessels, HEVs, and MAdCAM-expressing vessels. Furthermore, our data showed that these structures do not exhibit T/B compartmentalization in naturally diabetic mice even though the specialized vasculature remains unchanged. We propose a model where cells that infiltrate the pancreas organize into TLOs that, like secondary lymphoid organs, have T/B compartmentalization and specialized vasculature. Once these structures are established, the immune response is amplified and eventually is sufficient to destroy the neighboring islet. In doing so, T/B compartmentalization is lost and TLO integrity is lost.
Although there is agreement that the main mode of action of FTY720 is by blocking lymphocyte egress from the thymus and lymph nodes, some controversy remains concerning other effects on T-cells. Indeed, protection from disease development even in the presence of significant infiltration in the pancreas suggested the possibility of direct effects of FTY720 on T-cells. Previous studies have suggested that FTY720 induces Tregs or enhances their function (11,12). In our study, increased Tregs were observed only in the ILN, not in the PLN or in the pancreas, the locations where an increase in Tregs would be likely to have an effect on disease outcome. Interestingly, it is unclear whether this increase was due to preferential homing, proliferation, or conversion from effectors to Tregs. FTY720 has also been suggested to induce apoptosis of T-cells (10). Similar to our results, Maki et al. reported that continuous treatment of NOD mice with FTY720 prevented diabetes development during treatment (16). They observed diabetes protection even after withdrawal in mice that started treatment at 4 weeks of age, which led to the suggestion that therapy eliminated autoreactive effector T-cells by FTY720-mediated apoptosis (16). Rather than previous interpretations, we propose that the lack of accelerated disease after treatment of young pre-diabetic NOD mice is a result of a low number of antigen-specific T-cells in TLOs at the time of treatment initiation, which was not sufficient to develop a pathogenic T-cell population capable of rapid disease induction once drug treatment was discontinued. This interpretation fits with the analysis of 8-week-old mice, where TLOs were not yet organized into distinct T- and B-cells zones and therefore not fully established by this point. Moreover, in a model of allograft rejection, Habicht et al. showed that FTY720 treatment had no effect either alloreactive T-cell apoptosis or T-cell activation, proliferation, and cytokine production (17). Our data are consistent with the documented mechanism of action of FTY720, namely, that FTY720 controls exit from primary and secondary lymphoid organs. In addition, our data suggest that FTY720 also controls exit out of TLOs and entry into the neighboring pancreatic tissue, thereby stabilizing these structures and preventing islet destruction and diabetes development.
Tertiary lymphoid organs have been previously described in the pancreas of NOD mice; however, a characterization at different stages of disease including association with specialized vessels had not been described. Our data show that Lyve-1+ and MAdCAM+ vessels are present even in noninflammatory conditions, such as the pancreas of NOD.Rag2 knockout mice. Inflammation in NOD mice results in a higher percentage of islets associated with specialized vasculature. Whether this increase reflects neovascularization or expression of these markers on vessels that were previously present is not known.
The role of TLOs in the amplification of the immune response is unclear (18). Our results expand on the role of TLOs in T-cell responses. The adoptive transfer of naive BDC2.5 Tg T-cells during FTY720 treatment shows that naive cells can in fact be recruited directly to the pancreas rather than having to be primed first in the PLN. In addition, proliferation of these cells in the pancreas suggests that there is local antigen presentation (19). However, it is not known how the antigen reaches the TLOs. It is possible that islet-resident dendritic cells traffic to the TLO bringing antigen with them; that antigen is picked up by TLO resident antigen-presenting cells, such as macrophages and DCs, at the TLO/islet boundary; or that antigen travels through the lymph from the islet to the TLO.
The requirement of the lymphotoxin axis on TLO development has been explored because of its importance in lymph node development. Disruption of TLOs by overexpression of LIGHT, a LTβR ligand, in the pancreas of NOD mice (NOD-RIP-LIGHT) results in accelerated diabetes compared with nontransgenic controls (20). This suggests that during the natural course of disease and TLO breakdown, additional signals are present that allow pathogenic cells to destroy the islets rather than instructing them to leave the pancreas. Therefore, preventing islet destruction by altering TLO maintenance will require a deeper understanding of the signals that control islet destruction. In addition, targeting of TLOs may be difficult because the same signals that induce, and presumably maintain, TLOs are also necessary for lymph node integrity (18).
Analysis of TLOs in type 1 diabetic patients has been challenging. In accordance with our findings, Willcox et al. analyzed pancreas samples from patients who died within 18 months of diagnosis (21) and found scattered infiltrating cells surrounding glucagon- or insulin-positive islets. We hypothesize that the lack of true TLOs in the nPOD samples is because of timing: analyzing long-term clinically diabetic patients likely misses the peak of the immune response in the pancreas. In fact, the NOD mouse data support this interpretation because we found 81% of infiltrates were localized to TLOs in 20-week-old pre-diabetic mice but only 26% of infiltrates were localized to TLOs in diabetic mice. This highlights the need for screening and analysis of pre-diabetic, autoantibody-positive individuals to elucidate the role of TLOs in type 1 diabetes.
Promising clinical trials using FTY720 as a treatment for multiple sclerosis are under way (22), suggesting its possible use in treating other autoimmune diseases such as type 1 diabetes. However, our data suggest that autoreactive cells continue to expand during treatment and become highly pathogenic, such that if treatment were advertently or inadvertently discontinued the stealth T-cells rapidly destroy the target tissue. This has not been documented thus far in the clinical trials; whether the rebound effect is restricted to diabetes or this mouse model or whether it requires longer treatment remains to be seen.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants R37 AI-46643 and P30 DK-63720. nPOD tissues were obtained with support from the Juvenile Diabetes Research Foundation. C.P. was supported by National Institute of General Medical Sciences (NIGMS) Grant 1 R25 GM56847.
No potential conflicts of interest relevant to this article were reported.
We thank Navdeep Grewal, Shuwei Jiang, Mark Singer, Patrick Rowe, and Josh Beilke for technical assistance; Nicolas Martinier and Dorothy Fuentes for animal husbandry; Dr. Steve Rosen for the MECA79 antibody; and Novartis Pharmaceuticals Corporation for FTY720. We thank Drs. Abul Abbas, Mark Anderson, Steve Rosen, and Hans Dooms and members of the Bluestone laboratory for helpful discussions and reading the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson MS, Bluestone JA: The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 2005; 23: 447–485 [DOI] [PubMed] [Google Scholar]
- 2.Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA: Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and beta-cell destruction in NOD mice. Diabetes 1994; 43: 667–675 [DOI] [PubMed] [Google Scholar]
- 3.André I, Gonzalez A, Wang B, Katz J, Benoist C, Mathis D: Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc Natl Acad Sci U S A 1996; 93: 2260–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagnerault MC, Luan JJ, Lotton C, Lepault F: Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J Exp Med 2002; 196: 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carragher DM, Rangel-Moreno J, Randall TD: Ectopic lymphoid tissues and local immunity. Semin Immunol 2008; 20: 26–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H: Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002; 296: 346–349 [DOI] [PubMed] [Google Scholar]
- 7.Katz JD, Wang B, Haskins K, Benoist C, Mathis D: Following a diabetogenic T cell from genesis through pathogenesis. Cell 1993; 74: 1089–1100 [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA: Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 2009; 10: 1000–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melli K, Friedman RS, Martin AE, Finger EB, Miao G, Szot GL, Krummel MF, Tang Q: Amplification of autoimmune response through induction of dendritic cell maturation in inflamed tissues. J Immunol 2009; 182: 2590–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brinkmann V, Lynch KR: FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr Opin Immunol 2002; 14: 569–575 [DOI] [PubMed] [Google Scholar]
- 11.Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM: FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol 2007; 178: 2458–2468 [DOI] [PubMed] [Google Scholar]
- 12.Sawicka E, Dubois G, Jarai G, Edwards M, Thomas M, Nicholls A, Albert R, Newson C, Brinkmann V, Walker C: The sphingosine 1-phosphate receptor agonist FTY720 differentially affects the sequestration of CD4+/CD25+ T-regulatory cells and enhances their functional activity. J Immunol 2005; 175: 7973–7980 [DOI] [PubMed] [Google Scholar]
- 13.Lan YY, De Creus A, Colvin BL, Abe M, Brinkmann V, Coates PT, Thomson AW: The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am J Transplant 2005; 5: 2649–2659 [DOI] [PubMed] [Google Scholar]
- 14.Van Vliet E, Melis M, Foidart JM, Van Ewijk W: Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J Histochem Cytochem 1986; 34: 883–890 [DOI] [PubMed] [Google Scholar]
- 15.Sherry NA, Tsai EB, Herold KC: Natural history of beta-cell function in type 1 diabetes. Diabetes 2005; 54( Suppl. 2): S32–S39 [DOI] [PubMed] [Google Scholar]
- 16.Maki T, Gottschalk R, Monaco AP: Prevention of autoimmune diabetes by FTY720 in nonobese diabetic mice. Transplantation 2002; 74: 1684–1686 [DOI] [PubMed] [Google Scholar]
- 17.Habicht A, Clarkson MR, Yang J, Henderson J, Brinkmann V, Fernandes S, Jurewicz M, Yuan X, Sayegh MH: Novel insights into the mechanism of action of FTY720 in a transgenic model of allograft rejection: implications for therapy of chronic rejection. J Immunol 2006; 176: 36–42 [DOI] [PubMed] [Google Scholar]
- 18.Motallebzadeh R, Bolton EM, Pettigrew GJ: Lymphoid tissue formation in allografts: innocent until proven guilty. Transplantation 2008; 85: 309–311 [DOI] [PubMed] [Google Scholar]
- 19.Pabst R: Plasticity and heterogeneity of lymphoid organs: what are the criteria to call a lymphoid organ primary, secondary or tertiary? Immunol Lett 2007; 112: 1–8 [DOI] [PubMed] [Google Scholar]
- 20.Lee Y, Chin RK, Christiansen P, Sun Y, Tumanov AV, Wang J, Chervonsky AV, Fu YX: Recruitment and activation of naive T cells in the islets by lymphotoxin beta receptor-dependent tertiary lymphoid structure. Immunity 2006; 25: 499–509 [DOI] [PubMed] [Google Scholar]
- 21.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG: Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009; 155: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, Pohlmann H, Kappos L: FTY720 D2201 Study Group Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology 2009; 72: 73–79 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.