Abstract
OBJECTIVE
Costimulation blockade has emerged as a selective nontoxic maintenance therapy in transplantation. However, these drugs must be combined with other immunomodulatory agents to ensure long-term graft survival.
RESEARCH DESIGN AND METHODS
Recent work has demonstrated that caspase inhibitor therapy (EP1013) prevents engraftment phase islet loss and markedly reduces the islet mass required to reverse diabetes. The “danger” hypothesis suggests that reduction in graft apoptosis should reduce the threshold for immunosuppression and increase the possibility for tolerance induction. Thus, the impact of combination of EP1013 treatment with costimulation blockade (CTLA4-Ig) was investigated in this study.
RESULTS
Islet allografts were completed in fully major histocompatibility complex (MHC)-mismatched mice (Balb/C to B6). When animals received vehicle or EP1013, there was no difference in graft survival. CTLA4-Ig resulted in prolonged graft survival in 40% of the animals, whereas EP1013+CLTA4-Ig resulted in a significant increase in graft survival (91% >180 days; P = 0.01). Ex vivo analysis revealed that animals receiving EP1013 or EP1013+CTLA4-Ig had a reduced frequency of alloreactive interferon (IFN)-γ–secreting T-cells and an increased frequency of intragraft Foxp3+ Treg cells. Alloantibody assays indicated that treatment with EP1013 or CTLA4-Ig prevented allosensitization.
CONCLUSIONS
This study suggests that addition of caspase inhibitor therapy to costimulation blockade will improve clinical transplantation by minimizing immune stimulation and thus reduce the requirement for long-term immunosuppressive therapy. The approach also prevents allosensitization, which may be an important component of chronic graft loss in clinical transplantation.
Strategies aimed at minimizing donor organ injury and the induction of immunological tolerance have been a major area of research in transplantation over the past decade. One of the most promising new immunosuppressive agents involves costimulatory blockade, which prevents signal 2 during T-cell activation, resulting in T-cell anergy. Belatacept, a high-affinity version of CTLA4-Ig, the most widely studied costimulatory blockade agent, is currently in phase III studies in renal transplantation. Whereas CTLA4-Ig has been shown to be an effective immunomodulatory agent in preclinical animal models, long-term graft survival has only been achieved when this agent is combined with other immunomodulatory agents, such as anti-CD154 or sirolimus (1–3). In clinical renal transplantation, belatacept has proven to be as effective as cyclosporine for maintenance immunosuppression, with a reduced rate of chronic allograft nephropathy (4). Taken together, these data suggest that costimulation blockade maintenance therapy will minimize end organ damage, but further development of combination strategies must be undertaken to further minimize post-transplant immunosuppression regimens or induce tolerance.
Over the past several years, our group has explored caspase inhibitor therapies as a means to prevent early graft loss in islet transplantation. During the procurement, preservation, implantation, and reperfusion of an allograft, considerable damage occurs, resulting in intragraft inflammation and shedding of donor antigen. In the setting of islet transplantation, this effect is profound, since an estimated 60% or more of the implanted tissue fails to engraft after portal infusion (5). As a result, islets derived from at least two cadaveric organ donors are generally required to achieve insulin independence (>10,000 islet equivalents/kg recipient body weight) (6). The majority of these transplanted islets never become functional and are lost via apoptosis (7). In an effort to prevent postimplantation graft loss, we used synthetic peptidyl pan caspase inhibitors (zVAD-FMK and EP1013 [zVD-FMK]) as a transient systemic therapy in marginal islet mass models using both syngeneic rodent islet grafts and human islets transplanted into immunodeficient chemically diabetic mice (8,9). These small molecule therapies bind to the active site of both initiation and effector caspases, thereby preventing apoptosis resulting from extrinsic signals (i.e., cytokines, Fas pathway) and intrinsic signals (i.e., hypoxia, nutrient deprivation). In these studies, our data demonstrated that the caspase inhibitors are required as a post-transplant therapy for up to 5 days to maximize islet graft survival during the engraftment period. Using both mouse and human islet grafts in mice, caspase inhibitor therapy for only 5 days post-transplant resulted in a majority of animals achieving insulin independence, with a 70–80% reduction in islet mass. In addition, our data demonstrated that a brief period of caspase inhibitor therapy can stabilize a marginal mass islet graft, preventing metabolic burnout over time post-transplant (9).
In these studies, the effect of caspase inhibitor therapy during islet engraftment was examined in animal models that do not generate an immune response to the graft. However, our data have shown that caspase inhibitor therapy prevents islet cell death post-transplant, which may translate to less allosensitization in transplant recipients that receive this therapy. In the context of allograft rejection, the overall health of the donor tissue has been implicated in directing the immunological behavior of the graft recipient. Data investigating the “danger” hypothesis suggests that death of donor tissue and its associated inflammatory response sends out warning signals to the immune system, resulting in activation of alloreactive T-cells (10–12). Conversely, when a graft is allowed to “heal in” before immune reconstitution, there are less “danger signals” being transmitted, and as such, the immune system is less activated and more likely to become tolerant to the allograft (10–12). Although this hypothesis has never been challenged in the face of an apoptosis-resistant graft (versus a healed-in graft), the model suggests that marked reduction in graft apoptosis should result in lower requirements for immunosuppression and a greater possibility for tolerance induction. For these reasons, the effect of combination therapy using CTLA4-Ig and EP1013 in murine islet allograft transplantation was explored in the present study.
RESEARCH DESIGNS AND METHODS
Animals.
Mice were obtained from Jackson Labs (Bar Harbor, ME) and housed under specific pathogen-free conditions. Ethical approval was obtained from the animal welfare committee at the University of Alberta, and all animal care was in accordance with the guidelines of the Canadian Council on Animal Care.
Caspase inhibitor therapy.
EP1013 was obtained from Epicept Corporation (San Diego, CA), and CTLA4-Ig was obtained from Bioexpress (West Lebanon, NH). Stock preparations of EP1013 were prepared in DMSO and diluted into sterile saline.
Islet transplantation studies.
Mouse islets were isolated from Balb/C (H-2d) and C3H (H-2k) donors using established methods (13). Streptozotocin (Sigma-Aldrich, Canada, Mississauga, ON) was used to induce diabetes in C57BL/6 (“B6”, H-2b) recipients (250 mg/kg i.p.). Before transplantation, mouse islet preparations were incubated in medium containing caspase inhibitor (EP1013 at 100 μmol/l in Dulbecco's modified Eagle's medium with 10% FCS, Invitrogen Canada) or vehicle for 2 h. Transplant recipients were treated with either vehicle solution (DMSO in sterile saline) or caspase inhibitor (3 mg/kg s.c., days 0–10). CTLA4-Ig was administered on days 0, 2, 4, and 6 post-transplant (0.25 mg i.p.) (14). Animals were monitored three times per week, and two consecutive blood glucose values >18 mmol/l was considered to be evidence of rejection. Euglycemic animals from each cohort were selected randomly, and the graft-bearing kidney was removed to establish that the islet graft was functional, as determined by a return to hyperglycemia postnephrectomy (>18 mmol/l).
Immunofluorescence.
Cryostat sections (10 μm) of islet grafts were stained as previously described (15). Rat anti-mouse CD4 (clone GK1.5), rat anti-mouse CD8 (clone 53-6.7), rat-anti-mouse Foxp3 (clone FJK-16s), and polyclonal guinea pig anti-insulin were used. All slides were photographed at 100–200× magnification, and the total number of fluorescein isothiocyanate (FITC)+ cells per section were quantified from N = 4–5 animals per treatment group (minimum three sections per animal analyzed).
Alloantibody assays.
Serum was harvested from treated animals and stored until the completion of the study for bulk analysis. Alloantibody levels were determined using the indirect cellular enzyme-linked immunosorbent assay (ELISA) method described by Fan et al. (16).
Mixed lymphocyte reactions and ELISPOT assays.
Animals were killed at 11 days post-transplant, and splenocytes were harvested and purified using Lympholyte-M (Cedarlane). Donor-type Balb/C splenocytes were harvested, purified, and irradiated (1,500 Rad). The 5 × 105 recipient splenocytes were incubated with 5 × 105 irradiated Balb/C splenocytes in RPMI medium supplemented with 10% FCS (Invitrogen) for 48 h (ELISPOT) or 96 h (mixed lymphocyte reaction [MLR]).
ELISPOT.
After the incubation period, IFN-γ ELISPOT plates were washed and processed according to the manufacturer's protocols (Ebioscience).
MLR.
After the culture period, cells were pulsed for an additional 18 h with 1 μCi 3H thymidine/well and harvested, and thymidine incorporation was determined using a scintillation counter. For both experiments, splenocytes from individual transplanted animals in each experimental group were analyzed in triplicate. Also, for both MLR and ELISPOT assays, wells containing splenocytes from each treatment group, as well as irradiated target cells, were analyzed as negative control samples to rule out any nonspecific proliferation or IFN production.
Statistical analysis.
All statistical analyses in this study were carried out using SigmaPlot 10 and SigmaStat 3.5 (Systat, Inc.), and results are expressed as mean ± SEM. Mann-Whitney rank-sum tests and ANOVA with Bonferroni post hoc analysis were used to analyze multiple groups. Kaplan-Meier survival analyses were compared using the log-rank test.
RESULTS
Combination of EP1013 and CTLA4-Ig results in long-term islet allograft acceptance and operational tolerance.
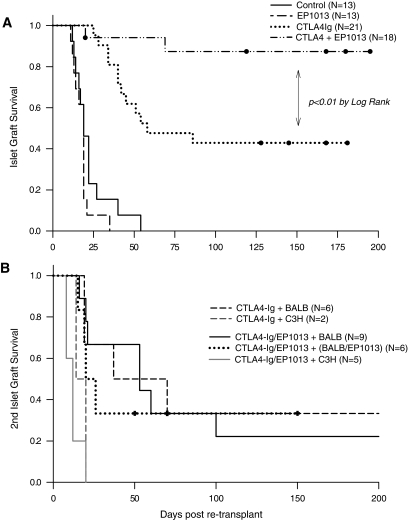
Initially, fully MHC-mismatched islet allografts were completed using chemically diabetic recipients, and treatment with either EP1013 (days 0–10), CTLA4-Ig (days 0, 2, 4, and 6), combination of EP1013 and CTLA4-Ig, or vehicle (days 0–10) was commenced. As shown in Fig. 1A, treatment with EP1013 resulted in no prolongation of islet allograft survival (mean survival time 18.2 days) compared with vehicle-treated animals (mean survival time 22.8 days, P = NS). CTLA4-Ig monotherapy resulted in prolonged graft survival in 40% of the animals (mean survival time 103.0 days), whereas the addition of EP1013 to CTLA4-Ig resulted in a significant increase in graft survival (n = 16/18 with long-term survival resulting in mean survival time >180 days post-transplant; P < 0.01 vs. CTLA4-Ig by log-rank).
FIG. 1.
EP1013 therapy, when combined with costimulation blockade using CTLA4-Ig, results in long-term islet graft survival and operational tolerance. A: Islet allografts were completed across a complete MHC mismatch using Balb/C (H-2d) donors and chemically diabetic B6 (H-2b) recipients. Caspase inhibitor therapy (EP1013) did not prolong graft survival compared with vehicle-treated animals. Monotherapy with CTLA4-Ig prevented graft rejection in 40% of recipients, whereas combination of EP1013 and CTLA4-Ig resulted in graft survival of >200 days in 89% of recipients (P < 0.01 vs. CTLA4-Ig). B: To test for donor-specific tolerance, graft-bearing kidneys were removed, and animals were re-transplanted with either donor-type (Balb/C) or third-party (C3H, H-2k) islets without further treatment or with a second 10-day course of EP1013. When using Balb/C islets, donor-specific tolerance was demonstrated in ∼30% of the transplant recipients (either CTL4-Ig or CTLA4-Ig+EP1013 cohorts). Re-transplantation with third-party islets resulted in robust rejection in all recipients.
To further understand the enhanced outcomes using EP1013+CTLA4Ig versus CTLA4-Ig alone, a series of experiments was carried out in animals with long-term graft function in each group. To test for tolerance, recovery nephrectomies were performed in recipients with graft survival >180 days. After 2 days of hyperglycemia, either same-donor (Balb/C, H2-d) or third-party (C3H; H2-k) islets were implanted with no additional therapeutic intervention (Fig. 1B). Re-transplantation with Balb/C islets led to prolonged graft survival in 20–30% of the recipients, regardless of the initial treatment group. This demonstrates that true donor-specific tolerance was only achieved in a subgroup of the transplant recipients, despite the presence of indefinite graft survival after the initial transplant (i.e., “operational tolerance”). Implantation of C3H islets resulted in rejection in all recipients in both treatment groups, confirming that operational tolerance to the initial graft was achieved without compromising the recipient's immunological function (Fig. 1B). One possible explanation for the lack of donor-specific tolerance in this model is the effect of the tissue injury, local inflammation, and graft cell death at the time of and after the second transplant, which could alter the recipient's ability to maintain donor-specific tolerance. To minimize these effects, an additional cohort of animals with long-term islet graft survival were re-transplanted with Balb/C islets and also treated with a 10-day course of EP1013, using the same dosing as the initial treatment period. As shown in Fig. 1B, this intervention did not improve the rate of donor-specific tolerance in animals previously treated with EP1013+CTLA4-Ig.
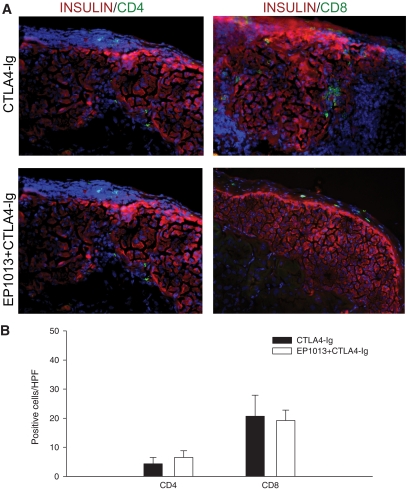
To explore the immune response to these grafts at this late time point post-transplant, immunofluorescence staining for insulin, CD4, or CD8 was completed. As shown in Fig. 2, histological analysis of grafts harvested from tolerant animals previously treated with either CTLA4-Ig alone or EP1013+CTLA4-Ig demonstrated intact islets with very few intragraft CD4+ or CD8+ cells. Quantification of these cells per high-power field (HPF) revealed that there was no difference in immune infiltrate between treatment groups. Thus, while combination of EP1013 and CTLA4-Ig resulted in a dramatic increase in islet allograft survival over time, analysis at this late time point could not explain the difference in outcomes observed between animals treated with EP1013+CTLA4-Ig versus CTLA4-Ig alone.
FIG. 2.
Allogeneic islet grafts harvested from tolerant animals >150 days post-transplant exhibit minimal immune infiltrate and intact islets. Renal subcapsular islet grafts were harvested from animals surviving >150 days after receiving CTLA4-Ig monotherapy or CTLA4-Ig/EP1013 combination therapy. A: Cryosections of islet grafts were co-stained for insulin (TRITC; red) and either CD4 (FITC, green; left panels) or CD8 (FITC; right panels). DAPI nuclear stain (blue) was used to identify all cells present on the section. Representative slides from both treatment groups are shown at 200× magnification and demonstrate that the transplanted islets were largely intact with no invasive immune infiltrate and rare CD4+ or CD8+ lymphocytes. B: Quantification of CD4 and CD8 staining confirmed that CD4+ and CD8+ lymphocytes were infrequently observed within the sections, regardless of the initial treatment intervention. For these data, n = 10 sections from >5 islet grafts per group were blinded and subsequently analyzed. (A high-quality digital color representation of this fiugre is available in the online issue.)
Caspase inhibitor therapy (EP1013) alters early allogeneic immune responses.
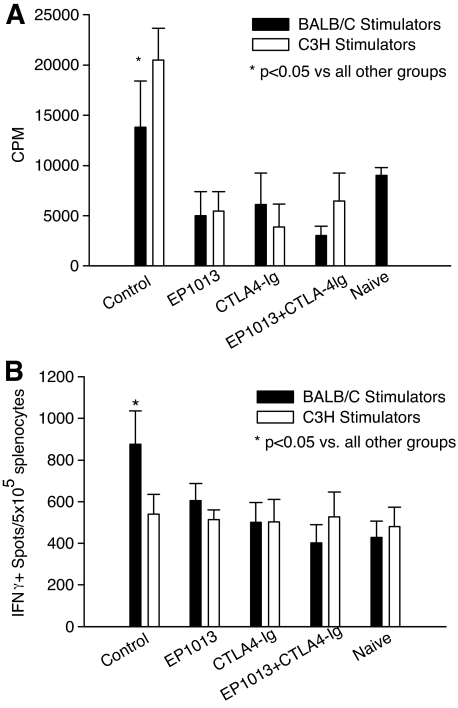
To characterize the impact of EP1013 on the allogeneic immune response in this model, ex vivo analysis was conducted at an early time point post-transplant, 1 day after the final treatment (day 11 post-transplant). As shown in Fig. 3A, co-incubation with either donor type or third-party stimulators resulted in a robust proliferative response in splenocytes harvested from vehicle-treated animals, which was significantly higher than all other treatment groups (P < 0.05 by ANOVA). Splenocytes harvested from recipients treated with CTLA4-Ig or EP1013+CTLA4-Ig exhibited a markedly reduced proliferation, which was comparable to that observed in naïve B6 animals. Interestingly, splenocytes harvested from animals treated with EP1013 monotherapy also displayed a markedly reduced proliferation compared with vehicle-treated animals (P < 0.05 by ANOVA).
FIG. 3.
EP1013 therapy inhibits early allogeneic T-cell responses, both as a monotherapy and in combination with CTLA4-Ig. Transplanted animals were sacrificed on day 11 post-transplant for ex vivo immune analysis using MLR and IFN-γ ELISPOT assays to measure the allogeneic immune response. A: MLR assays revealed that splenocytes harvested from vehicle-treated (“control”) recipients reacted with a nearly twofold increase in proliferation to both donor and third-party stimulators as compared with naïve animals (P < 0.05). In contrast, splenocytes harvested with animals treated with EP1013, CTLA4-Ig, or EP1013+CTLA4-Ig proliferated at rates that were comparable to naïve animals, irrespective of the stimulator source (P < 0.05 vs. control for all treatment groups). B: IFN-γ ELISPOT assays demonstrated that splenocytes from vehicle-treated control animals mounted a robust donor-specific allogeneic IFN-γ response, with a significant increase in number of spots compared with naïve animals (P < 0.05 by ANOVA). Prior treatment with EP1013, CTLA4-Ig, or EP1013+CTLA4-Ig markedly reduced the number of IFN-γ–secreting alloreactive splenocytes, to levels comparable to those observed in naïve animals (P < 0.05 for each treatment group vs. control by ANOVA). For these assays, n = 3–4 animals per treatment group were analyzed in triplicate in two separate experiments.
Similar results were obtained using IFN-γ ELISPOT to analyze allo-specific immune responses (Fig. 3B). In these assays, splenocytes harvested from vehicle-treated animals generated a large number of IFNγ+ spots when exposed to donor-type stimulators (P < 0.05 vs. all other groups by ANOVA), while co-incubation with third-party stimulators resulted in a response that was similar to naïve B6 splenocytes. Splenocytes harvested from animals treated with CTLA4-Ig or EP1013+CTLA4-Ig generated few IFNγ+ spots when exposed to either donor-type or third-party stimulators, and no difference was observed when compared with the response generated using naïve B6 splenocytes. Of note, splenocytes harvested from animals treated with EP1013 monotherapy also displayed a marked reduction in IFNγ+ spots after exposure to donor-type stimulators, as compared with splenocytes harvested from vehicle-treated animals (P < 0.001 by ANOVA).
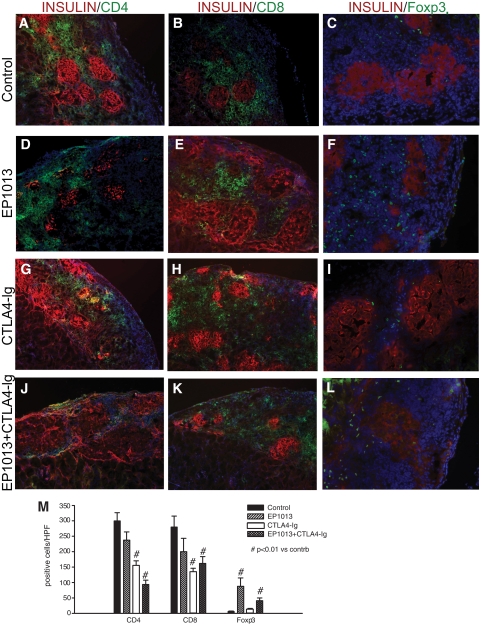
Immunofluorescence staining of sections cut from the islet grafts harvested at this early time point was completed to characterize the relative contribution of different T-cell subsets to the allogeneic immune response in each treatment group (Fig. 4). Sections were co-stained for insulin and CD4, CD8, or Foxp3, and the immunological markers were quantified per HPF. Representative tissue sections demonstrate that treatment with either CTLA4-Ig (155.0 ± 15.5 cells/HPF, Fig. 4G) or EP1013+CTLA4-Ig (93.5 ± 14.2 cells/HPF, Fig. 4J) resulted in a 50–70% decrease in CD4+ T-cell infiltration compared with vehicle-treated controls (299.8 ± 26.8 CD4+ cells/HPF, Fig. 4A; P < 0.01 by ANOVA). Similar results were observed when CD8+ staining was quantified, with both CTLA4-Ig (134.5 ± 11.9 cells/HPF, Fig. 4H) and EP1013+CTLA4-Ig (162.0 ± 21.8 cells/HPF, Fig. 4K) treatment groups exhibiting a nearly 50% decrease in CD8+ T-cell infiltration compared with vehicle-treated animals (279.8 ± 39.7, Fig. 4B; P < 0.01 by ANOVA). No significant difference in either CD4+ or CD8+ infiltration was observed between animals treated with EP1013 (237.3 ± 37.9 CD4+ cells/HPF, Fig. 4D, and 200.0 ± 43.5 CD8+ cells/HPF, Fig. 4E) when compared with vehicle-treated animals.
FIG. 4.
Histological analysis in the early post-transplant period demonstrates that EP1013 therapy promotes intragraft accumulation of Foxp3+ lymphocytes. Animals were sacrificed on day 11 post-transplant, and graft-bearing kidneys were harvested for immunfluorescent quantification of intragraft lymphocytic infiltrates. Insulin staining was used to identify transplanted islets (TRITC; red), and the immune markers CD4 (left column), CD8 (middle column), and Foxp3 (right column) were labeled with FITC (green). DAPI counterstaining (blue) was used to identify all cells present within the sections, which are shown at 100× (CD4, CD8) or 200× (Foxp3). Representative sections from each treatment group are shown. M: Quantification of CD4, CD8, and Foxp3 staining was conducted in sections from each treatment group. A significant reduction in the number of CD4+ cells/HPF was observed after treatment with CTLA4-Ig or EP1013+CTLA4-Ig compared with vehicle (P < 0.01 vs. control for both groups). Similar results were observed for CD8+ cells/HPF, with CTLA4-Ig or EP1013+CTLA4-Ig treatment cohorts exhibiting a significant decrease compared with vehicle-treated animals (P < 0.01 vs. control for both groups). Quantification of Foxp3+ cells/HPF revealed that prior treatment with EP1013, either alone or in combination with CTLA4-Ig, resulted in a significant increase in the frequency of intragraft regulatory T-cells (P < 0.01 vs. control and vs. CTLA4-Ig alone by ANOVA). Serial tissue sections from n = 3–4 animals per group were stained, blinded, and analyzed for this experiment. (A high-quality digital color representation of this figure is available in the online issue.)
Quantification of regulatory T-cell (Treg) infiltration was carried out using the marker Foxp3 (17). Grafts harvested from vehicle-treated animals exhibited almost no intragraft Foxp3+ Treg cells (5.7 ± 1.7 cells/HPF, Fig. 4C), which was similar to that observed in animals treated with CTLA4-Ig (12.3 ± 3.9 cells/HPF, Fig. 4I). In contrast, treatment with EP1013, either alone (87.8 ± 27.8 cells/HPF, Fig. 4F) or in combination with CTLA4-Ig (41.0 ± 8.9 cells/HPF, Fig. 4L), resulted in a 7- to 15-fold increase in the frequency of intragraft Foxp3+ Treg cells (P < 0.01 for each vs. control by ANOVA).
EP1013 therapy prevents allosensitization after islet transplantation.
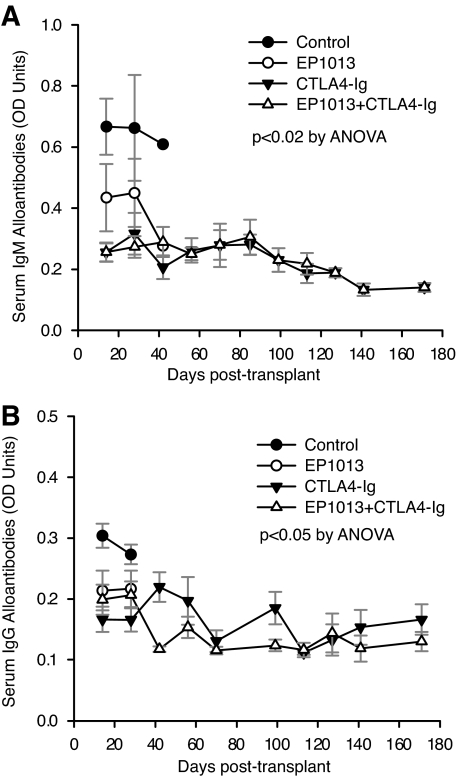
Development of alloantibodies and donor-specific sensitization is increasingly being recognized as an important prognostic factor in long-term allograft survival (18). To understand the effect of caspase inhibitor therapy on the B-cell compartment during the allogeneic immune response, both IgM and IgG alloantibodies were measured prospectively post-transplant using a cellular ELISA (16). Vehicle-treated animals generate a robust IgM (Fig. 5A) and IgG (Fig. 5B) donor-specific alloantibody response. Treatment with EP1013, CTLA4-Ig, or EP1013+CTLA4-Ig prevented the formation of either IgM or IgG alloantibodies over time, even after the discontinuation of treatment at day 10 (P < 0.02 for all treatment groups versus control for IgM, P < 0.05 vs. control for all treatment groups for IgG, ANOVA). Of note, naïve B6 serum demonstrated a consistent mean optical density of 0.30 ± 0.09 for IgM and a mean optical density of 0.19 ± 0.07 for IgG, which is statistically significant only when compared with control transplant mice at 2 and 4 weeks post-transplant. Thus, treatment with either EP1013 or CTLA4-Ig prevents allosensitization post-transplant in this model.
FIG. 5.
EP1013 therapy prevents both IgM and IgG alloantibody formation, both as a single agent and in combination with CTLA4-Ig. Sera were collected via tail vein bleeds prospectively and stored for bulk analysis at the completion of the study using a donor-specific cellular ELISA to measure alloantibody production. A: IgM alloantibody formation was detected in vehicle-treated control animals (●), while treatment with EP1013 (○), CTLA4-Ig (▲), or EP1013+CTLA4-Ig (△) resulted in a significant reduction in IgM alloantibody formation (P < 0.02 for all treatment groups vs. control). B: Similar results were obtained when IgG alloantibody levels were assessed, with vehicle-treated control animals becoming highly sensitized within 2 weeks post-transplantation (●). IgG allosensitization was prevented after treatment with EP1013 (○), CTLA4-Ig (▲), or EP1013+CTLA4-Ig (△) (P < 0.05 for each treatment group vs. control). A minimum of n = 5 serum samples from each treatment group were analyzed in duplicate for these assays. OD, optical density.
DISCUSSION
The present study demonstrates for the first time that combination of EP1013, a selective pan-caspase inhibitor therapy with CTLA4-Ig, a selective immunomodulatory therapy, results in long-term islet allograft acceptance. This approach had a global effect on the allogeneic immune response, as shown by experiments evaluating both T- and B-cell compartments. EP1013+CTLA4-Ig reduced the frequency of intragraft CD4+ and CD8+ T-cells both in the short term (Fig. 4) and over time (Fig. 2), and it reduced the functional alloreactive T-cell response, both in production of IFN-γ–secreting allo-specific lymphocytes and overall allo-stimulated T-cell proliferation (Fig. 3). Moreover, this combined approach reduced B-cell allosensitization (Fig. 5). Of note, the immunomodulatory effects of combination of EP1013+CTL4-Ig far exceeded the short treatment period post-transplant, without further requirements for immunosuppression. Taken together, these data indicate that prevention of donor tissue apoptosis using caspase inhibitor therapy during the engraftment period can dramatically lower the threshold for anti-rejection therapy, such that brief treatment with the costimulation blockade agent CTLA4-Ig results in robust indefinite islet allograft survival.
In the setting of islet transplantation, a significant fraction of the transplanted islets fail to engraft, resulting in a large requirement for donor tissue and ultimately a flood of alloantigen during the post-transplant period. Our group has proven that caspase inhibitor therapy protects islets during the engraftment period and thus minimizes the total number of islets required to reverse diabetes (8,9). From these previous experiments, it is clear that intragraft apoptosis is markedly reduced, demonstrating the anticipated cytoprotective effect of caspase inhibitor therapy on the graft. However, data in the present study illustrate another potential beneficial effect of caspase inhibitor therapy—modulation of the allogeneic immune response. Previous data in other experimental models have suggested that caspase inhibitors can alter the function of lymphocytes. In vitro studies have shown that caspase inhibitors can have a negative regulatory effect on the immune system, given that caspases play a critical role in cell cycle regulation of lymphocyte populations (19–21). Studies using a pan caspase inhibitor (zVAD) on human lymphocytes demonstrated that this agent potently suppressed lymphocyte proliferation; recall antigen response; the upregulation of MHC-II, CD25, and CD69; and IL-2 production after stimulation (19). However, confirming these effects in vivo has been more elusive (22,23). Data in the present study clearly indicate that caspase inhibitor therapy strikingly increases the frequency of intragraft Treg cells, while at the same time reducing the frequency of alloreactive IFN-γ–secreting lymphocytes (Figs. 3 and 4). Whether this effect is the result of enhanced proliferation or a lower threshold for activation selectively within these key populations is not clear and is on an ongoing area of research in our lab. Despite clear differences in intragraft T-cell populations and alloreactive T-cell responses ex vivo (Figs. 3 and 4), no functional difference in graft survival was observed using EP1013 monotherapy in the traditional allograft model (Fig. 1). It is likely that the multiple noxious stimuli surrounding the transplant procedure (anesthesia, surgical manipulation, stress-induced cytokines, etc.) provide too much nonspecific immune stimulation and thus limit the effectiveness of EP1013 monotherapy as an immunomodulatory agent. Thus, while the mechanism of caspase inhibitor–mediated immunomodulation is complex, it is evident from data in this study that a brief post-transplant therapeutic period is of marked benefit in transplantation.
With regard to tolerance, our data suggest that the combination of a potent cytoprotective agent (EP1013) with a selective immunomodulator (CTLA4-Ig) promotes a state of immunological “ignorance,” where the immune system is in a state of passive “tolerance” to the graft, versus mounting an ongoing and reproducible state of active donor-specific tolerance. It may be that early upregulation of regulatory T-cells promotes this type of “operational tolerance,” which could explain the observations in this study, especially given the almost complete absence of lymphocytes within the graft at late time points (Fig. 2) versus findings at an early time point (Fig. 4). Similarly, the lack of allospecific IFN-γ production in animals treated with EP1013 (Fig. 3B) supports this theory, since IFN-γ production has been implicated as an important component of donor-specific tolerance induction (24).
The present study was conducted using islet allograft transplantation, and our results indicate that this approach could be of particular benefit in the clinical setting. Brief treatment with caspase inhibitors can minimize the amount of donor tissue required to restore euglycemia by as much as 80%, thereby increasing the “transplantability” of high-quality clinical islet preparations of low yield by current standards (<300,000 islet equivalents) (8,25). Whereas true donor-specific tolerance was not universally achieved in our study, our data strongly suggest that this approach will reduce the intensity and/or duration of immunosuppression required, which would still represent a major advance in the field. Another important consideration is the lack of donor sensitization in this model, which has recently been identified as a potential cause of chronic graft failure in clinical islet transplantation (26,27). Previous studies have shown that costimulation blockade can prevent allo-sensitization post-transplant (28). Our data support these findings and demonstrate that combination therapy with EP1013 does not reduce the efficacy of CTLA4-Ig in this regard.
While this study was limited to islet allograft transplantation, it is likely that the combined use of caspase inhibitors and costimulation blockade will be of benefit in all areas of solid organ transplantation. As the demand for organs continues to outpace donation rates worldwide, it has increasingly become the trend to use organs procured from extended criteria donors or even after donation after cardiac death. Use of extended criteria donor and donation after cardiac death organs increases the rate of delayed graft function and reduces long-term graft survival rates (29). The inclusion of a caspase inhibitor in the preservation solution in a murine model of acute kidney injury dramatically reduced renal tubular apoptosis and brush border injury, two processes that have been linked to delayed graft function (30). Similar results have been obtained in a cardiac allograft model, where early treatment with a caspase inhibitor reduced intimal proliferation and vessel occlusion, which are both important markers of chronic allograft vasculopathy (31). Thus, it is likely that the cytoprotective benefits of caspase inhibitor therapy combined with selective nontoxic immunosuppression using costimulation blockade will broadly improve both graft function and long-term survival in many areas of organ transplantation.
In summary, data in this study indicate that caspase inhibitors represent the first single agent therapy that has the potential to benefit the graft directly, while also regulating the allogeneic immune response. Caspase inhibitors have been used clinically in liver transplantation and so far have proven to be safe and effective in improving graft function post-transplant (32–34). It is likely that inclusion of caspase inhibitors in clinical transplantation protocols will be of greatest benefit in situations where considerable stress to the donor organ is generated, particularly after the process of brain death, prolonged ischemic time, or use of extended criteria donors. It is also likely that this approach will reduce the amount and intensity of anti-rejection therapy, accelerating accommodation and drug minimization. If this could be achieved, or if the stable immunological tolerance enhanced by caspase inhibitor co-treatment could be translated to the clinic, transplantation would be potentially safer and therefore more available to a broader spectrum of patients.
ACKNOWLEDGMENTS
J.E. was supported by fellowships from the American Society for Transplantation, Juvenile Diabetes Research Foundation, and Alberta Heritage Foundation for Medical Research (AHFMR) and by the Rhind Autoimmunology Award. S.M. was supported by studentship awards from the AHFMR and the Canadian Institutes for Health Research. C.T. was the recipient of a Swiss National Science Foundation fellowship, the FS Chia Scholarship, and an AHFMR fellowship. A.M.J.S. is a Senior Clinical Scholar with the AHFMR. This study was funded by Juvenile Diabetes Research Foundation Clinical Center Grant 4-2001-920.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Jr, Knechtle SJ: CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A 1997; 94: 8789–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams AB, Shirasugi N, Durham MM, Strobert E, Anderson D, Rees P, Cowan S, Xu H, Blinder Y, Cheung M, Hollenbaugh D, Kenyon NS, Pearson TC, Larsen CP: Calcineurin inhibitor-free CD28 blockade-based protocol protects allogeneic islets in nonhuman primates. Diabetes 2002; 51: 265–270 [DOI] [PubMed] [Google Scholar]
- 3.Adams AB, Shirasugi N, Jones TR, Durham MM, Strobert EA, Cowan S, Rees P, Hendrix R, Price K, Kenyon NS, Hagerty D, Townsend R, Hollenbaugh D, Pearson TC, Larsen CP: Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol 2005; 174: 542–550 [DOI] [PubMed] [Google Scholar]
- 4.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B: Belatacept Study Group Costimulation blockade with belatacept in renal transplantation. N Engl J Med 2005; 353: 770–781 [DOI] [PubMed] [Google Scholar]
- 5.Biarnés M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E: Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 2002; 51: 66–72 [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV: Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343: 230–238 [DOI] [PubMed] [Google Scholar]
- 7.Emamaullee JA, Shapiro AM: Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant 2007; 16: 1–8 [PubMed] [Google Scholar]
- 8.Emamaullee JA, Davis J, Pawlick R, Toso C, Merani S, Cai SX, Tseng B, Shapiro AM: The caspase selective inhibitor EP1013 augments human islet graft function and longevity in marginal mass islet transplantation in mice. Diabetes 2008; 57: 1556–1566 [DOI] [PubMed] [Google Scholar]
- 9.Emamaullee JA, Stanton L, Schur C, Shapiro AM: Caspase inhibitor therapy enhances marginal mass islet graft survival and preserves long-term function in islet transplantation. Diabetes 2007; 56: 1289–1298 [DOI] [PubMed] [Google Scholar]
- 10.Anderson CC, Carroll JM, Gallucci S, Ridge JP, Cheever AW, Matzinger P: Testing time-, ignorance-, and danger-based models of tolerance. J Immunol 2001; 166: 3663–3671 [DOI] [PubMed] [Google Scholar]
- 11.Matzinger P: Tolerance, danger, and the extended family. Annu Rev Immunol 1994; 12: 991–1045 [DOI] [PubMed] [Google Scholar]
- 12.Matzinger P: An innate sense of danger. Semin Immunol 1998; 10: 399–415 [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Singh B, Warnock GL, Rajotte RV: Prevention of recurrence of IDDM in islet-transplanted diabetic NOD mice by adjuvant immunotherapy. Diabetes 1992; 41: 114–117 [DOI] [PubMed] [Google Scholar]
- 14.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC: Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 1996; 381: 434–438 [DOI] [PubMed] [Google Scholar]
- 15.Merani S, Pawlick RL, Edgar RL, Toso C, Emamaullee J, Anderson CC, Shapiro AM: Protein kinase C inhibitor, AEB-071, acts complementarily with cyclosporine to prevent islet rejection in rats. Transplantation 2009; 87: 59–65 [DOI] [PubMed] [Google Scholar]
- 16.Fan X, Tyerman K, Ang A, Koo K, Parameswaran K, Tao K, Mai L, Lang H, West LJ: A novel tool for B-cell tolerance research: characterization of mouse alloantibody development using a simple and reliable cellular ELISA technique. Transplant Proc 2005; 37: 29–31 [DOI] [PubMed] [Google Scholar]
- 17.Hori S, Nomura T, Sakaguchi S: Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057–1061 [PubMed] [Google Scholar]
- 18.Zeevi A, Girnita A, Duquesnoy R: HLA antibody analysis: sensitivity, specificity, and clinical significance in solid organ transplantation. Immunol Res 2006; 36: 255–264 [DOI] [PubMed] [Google Scholar]
- 19.Falk M, Ussat S, Reiling N, Wesch D, Kabelitz D, Adam-Klages S: Caspase inhibition blocks human T cell proliferation by suppressing appropriate regulation of IL-2, CD25, and cell cycle-associated proteins. J Immunol 2004; 173: 5077–5085 [DOI] [PubMed] [Google Scholar]
- 20.Kennedy NJ, Kataoka T, Tschopp J, Budd RC: Caspase activation is required for T cell proliferation. J Exp Med 1999; 190: 1891–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pozzetto U, Aguzzi MS, Maggiano N, Scala E, Capelli A, Castagneto M, Capogrossi MC, Citterio F, Serino F, Facchiano A: RGDS peptide inhibits activation of lymphocytes and adhesion of activated lymphocytes to human umbilical vein endothelial cells in vitro. Immunol Cell Biol 2005; 83: 25–32 [DOI] [PubMed] [Google Scholar]
- 22.Nussbaum AK, Whitton JL: The contraction phase of virus-specific CD8+ T cells is unaffected by a pan-caspase inhibitor. J Immunol 2004; 173: 6611–6618 [DOI] [PubMed] [Google Scholar]
- 23.Weber P, Wang P, Maddens S, Wang P, Wu R, Miksa M, Dong W, Mortimore M, Golec JM, Charlton P: VX-166: a novel potent small molecule caspase inhibitor as a potential therapy for sepsis. Crit Care 2009; 13: R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishimoto K, Sandner S, Imitola J, Sho M, Li Y, Langmuir PB, Rothstein DM, Strom TB, Turka LA, Sayegh MH: Th1 cytokines, programmed cell death, and alloreactive T cell clone size in transplant tolerance. J Clin Invest 2002; 109: 1471–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kin T, Zhai X, Murdoch TB, Salam A, Shapiro AM, Lakey JR: Enhancing the success of human islet isolation through optimization and characterization of pancreas dissociation enzyme. Am J Transplant 2007; 7: 1233–1241 [DOI] [PubMed] [Google Scholar]
- 26.Campbell PM, Salam A, Ryan EA, Senior P, Paty BW, Bigam D, McCready T, Halpin A, Imes S, Al Saif F, Lakey JR, Shapiro AM: Pretransplant HLA antibodies are associated with reduced graft survival after clinical islet transplantation. Am J Transplant 2007; 7: 1242–1248 [DOI] [PubMed] [Google Scholar]
- 27.Campbell PM, Senior PA, Salam A, Labranche K, Bigam DL, Kneteman NM, Imes S, Halpin A, Ryan EA, Shapiro AM: High risk of sensitization after failed islet transplantation. Am J Transplant 2007; 7: 2311–2317 [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim S, Jakobs F, Kittur D, Hess A, Linsley PS, Sanfilippo F, Baldwin WM, 3rd:: CTLA4Ig inhibits alloantibody responses to repeated blood transfusions. Blood 1996; 88: 4594–4600 [PubMed] [Google Scholar]
- 29.Saidi RF, Elias N, Kawai T, Hertl M, Farrell ML, Goes N, Wong W, Hartono C, Fishman JA, Kotton CN, Tolkoff-Rubin N, Delmonico FL, Cosimi AB, Ko DS: Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: realities and costs. Am J Transplant 2007; 7: 2769–2774 [DOI] [PubMed] [Google Scholar]
- 30.Jani A, Ljubanovic D, Faubel S, Kim J, Mischak R, Edelstein CL: Caspase inhibition prevents the increase in caspase-3, -2, -8 and -9 activity and apoptosis in the cold ischemic mouse kidney. Am J Transplant 2004; 4: 1246–1254 [DOI] [PubMed] [Google Scholar]
- 31.Balsam LB, Mokhtari GK, Jones S, Peterson S, Hoyt EG, Kofidis T, Tanaka M, Cooke DT, Robbins RC: Early inhibition of caspase-3 activity lessens the development of graft coronary artery disease. J Heart Lung Transplant 2005; 24: 827–832 [DOI] [PubMed] [Google Scholar]
- 32.Pockros PJ, Schiff ER, Shiffman ML, McHutchison JG, Gish RG, Afdhal NH, Makhviladze M, Huyghe M, Hecht D, Oltersdorf T, Shapiro DA: Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology 2007; 46: 324–329 [DOI] [PubMed] [Google Scholar]
- 33.Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, Burgart L, Garrity-Park M, van Vilsteren FG, Oliver LK, Rosen CB, Gores GJ: Clinical trial of the pan-caspase inhibitor, IDN-6556, in human liver preservation injury. Am J Transplant 2007; 7: 218–225 [DOI] [PubMed] [Google Scholar]
- 34.Valentino KL, Gutierrez M, Sanchez R, Winship MJ, Shapiro DA: First clinical trial of a novel caspase inhibitor: anti-apoptotic caspase inhibitor, IDN-6556, improves liver enzymes. Int J Clin Pharmacol Ther 2003; 41: 441–449 [DOI] [PubMed] [Google Scholar]