Abstract
OBJECTIVE
Oxidative stress is a key pathogenic factor in diabetic retinopathy. We previously showed that lovastatin mitigates blood-retinal barrier (BRB) breakdown in db/db mice. The purpose of this study is to determine the mechanisms underlying the salutary effects of lovastatin in diabetic retinopathy.
RESEARCH DESIGN AND METHODS
Expression of NADPH oxidase (Nox) 4, vascular endothelial growth factor (VEGF), and hypoxia-inducible factor (HIF)-1α; production of reactive oxygen species (ROS); and retinal vascular permeability were measured in cultured retinal capillary endothelial cells (RCECs) and in db/db mice treated with lovastatin.
RESULTS
Expressions of Nox4 and VEGF were significantly increased in retinas of db/db mice and reduced by lovastatin treatment. In cultured RCECs, hypoxia and high glucose upregulated mRNA and protein expression of Nox4, ROS generation, and VEGF level. These changes were abrogated by pretreatment with lovastatin or NADPH oxidase inhibitor diphenyleneiodonium chloride. Overexpression of Nox4 increased basal level of ROS generation, HIF-1α, and VEGF expression in RCECs. In contrast, blockade of Nox4 activity using adenovirus-expressing dominant-negative Nox4 abolished hypoxia- and high-glucose–induced ROS production and VEGF expression. Moreover, inhibition of Nox4 attenuated hypoxia-induced upregulation of HIF-1α and high-glucose–elicited phosphorylation of STAT3. Finally, depletion of Nox4 by adenovirus-delivered Nox4 small interfering RNA significantly decreased retinal NADPH oxidase activity and VEGF expression and reduced retinal vascular premeability in db/db mice.
CONCLUSIONS
Activation of Nox4 plays an important role in high-glucose– and hypoxia-mediated VEGF expression and diabetes-induced BRB breakdown. Inhibition of Nox4, at least in part, contributes to the protective effects of lovastatin in diabetic retinopathy.
Diabetic retinopathy is a common complication of diabetes and one of the most frequent causes of blindness in the U.S. (1–3). Hallmark sequential pathological changes in diabetic retinopathy include increased vascular permeability, pericyte and endothelial cell death, capillary occlusion and aberrant retinal new vessel growth, or neovascularization (4). Increased vascular permeability caused by the breakdown of the blood-retinal barrier (BRB) results in diabetic macular edema, a major cause of vision loss in diabetic patients (2,5,6). Vascular endothelial growth factor (VEGF) is a potent angiogenic factor playing a crucial role in angiogenesis (7,8). VEGF is also referred as vascular permeability factor (VPF) based on its ability to induce vascular hyperpermeability (9). Significantly elevated VEGF levels in the eye have been reported in diabetic patients with diabetic macular edema and correlated with the severity of vascular leakage (10). Overexpression of VEGF is also responsible for retinal hyperpermeability in streptozotocin (STZ)-induced diabetic rats (11). These findings suggest that VEGF is a key mediator of retinal vascular leakage in diabetic retinopathy.
Oxidative stress plays an important role in vascular endothelial dysfunction in diabetes (12–15). Increased level of hydrogen peroxide, a reactive oxygen species (ROS), was colocalized with VEGF expression at the inner BRB and associated with vascular leakage in the retina in diabetic BBZ/Wor rats, suggesting a role of ROS in regulation of VEGF in diabetic retinopathy (16). In addition, suppression of ROS generation by NADPH oxidase inhibitor or antioxidants significantly attenuated retinal vascular leakage in diabetic animals, suggesting that activation of NADPH oxidase contributes to retinal ROS generation and vascular damage in diabetic retinopathy (17). NADPH oxidase (Nox) 4, which is originally identified in the kidney and termed renox (renal oxidase), is a novel isoform of NADPH oxidase expressed in nonphagocytes, such as vascular endothelial cells and smooth muscle cells (18,19). In aorta isolated from the STZ-induced diabetic apolipoprotein E–deficient mice or the db/db mice, Nox4 expression is significantly upregulated, associated with increased ROS production and inflammation, indicating a potential role of Nox4 in diabetic macrovascular disease (20). Moreover, inhibition of Nox4 expression using antisense oligonucleotides attenuates ROS generation and ameliorates glomerular hypertrophy in STZ-induced diabetic mice, suggesting that Nox4 is the major source of ROS in the diabetic kidney, contributing to renal hypertrophy in diabetic nephropathy (21). However, the role of Nox4 in diabetic retinopathy has not been investigated.
3-Hydroxy-3-methylglutaryl CoA reductase inhibitors (statins) are potent inhibitors of cholesterol biosynthesis commonly used in dyslipidemia and type 2 diabetes (22). Moreover, statins have demonstrated impressive beneficial effects, such as improvement of endothelial function, neuroprotection, and anti-inflammation, which are independent of their lipid-lowering effects (23). In a previous study, we have shown that lovastatin protects retinal tight junction and ameliorates BRB breakdown in db/db mice, a type 2 diabetes model (24). However, the mechanisms remain elusive. In the present study, we have tested the hypothesis that Nox4 is a key mediator of oxidative stress and BRB breakdown in diabetic retinopathy and that inhibition of Nox4 is, at least in part, responsible for the salutary effect of lovastatin on retinal endothelial function.
RESEARCH DESIGN AND METHODS
Lovastatin, NG-nitro-l-arginine methyl ester hydrochloride (l-NAME), rotenone, and allopurinol were obtained from Sigma-Aldrich (St .Louis, MO). Diphenyleneiodonium chloride (DPI) and NADPH were purchased from Calbiochem (San Diego, CA). 2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA), dihydroethidium (DHE), and N-acetyl-3,7-dihydroxyphenoxazine (Amplex red) were obtained from Molecular Probe (Invitrogen, Carlsbad, CA). Protease inhibitor cocktail, phenylmethylsulfonyl fluoride, sodium orthovanadate, rabbit anti-Nox4, and anti-VEGF antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti–hypoxia-inducible factor (HIF)-1α antibody was obtained from BD Bioscience (San Jose, CA). Anti-phophorylated and total STAT3 antibodies were obtained from Cell Signaling Technology (Boston, MA). Anti–β-actin antibody was obtained from Abcam (Cambridge, MA). Fluorescein isothiocyanate–conjugated or horseradish peroxidase–conjugated secondary antibodies, Hoechst dye, and DAPI were purchased from Jackson Immunoresearch Laboratories (West Grove, PA) and Vector Laboratories (Bulingame, CA), respectively.
Male db/db (BKS.Cg-m+/+ Leprdb) were purchased from the Jackson Laboratory (Bar Harbor, ME). All the experiments were performed in accordance with the statement for the use of animals in ophthalmic and vision research from the Association for Research in Vision and Ophthalmology and the guidelines in the care and use of laboratory animals set forth by the University of Oklahoma. db/db mice at age 13 weeks were randomly selected to receive daily gastric gavage of lovastatin dissolved in vegetable oil (5 mg/ml) at 10 mg/kg or same amount of vehicle for 6 weeks. Nondiabetic littermates received the same vehicle treatment. Blood glucose and body weight were measured every other week.
Cell culture.
Primary bovine retinal capillary endothelial cells (RCECs) were isolated and cultured as described previously (25). Primary human retinal microvascular endothelial cells were purchased from Cell Systems (Kirkland, WA) and maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS, 30 μg/ml endothelial cell growth supplement, 90 μg/ml heparin, 1% Insulin-Transferrin-Selenium-A supplement (ITS), and 1% antibiotic/antimycotic solution. Confluent monolayer cells were quiescent in DMEM with 1% FBS for 8 h followed by treatment with desired conditions. To evaluate the effect of hypoxia, cells were exposed to hypoxia (2% O2) or normoxia (21% O2), with oxygen concentration being correctly regulated using the ProOxC system (BioSpherix, Lacona, NY). All experiments were conducted with cells of passages three to eight.
Adenovirus infection of RCECs.
Adenoviral vectors expressing human Nox4 (Ad-Nox4) (26), or a dominant-negative mutant of Nox4 lacking the FAD-NADPH binding site (Ad-Nox4ΔFAD) (26), or RNA interference targeting human Nox4 (nucleotides 418–436 from the start codon) (Ad-Nox4i), or control small interfering RNA (siRNA) (Ad-Ctrli) (27) were amplified in 293 AD cells and purified using an Adeno-X Maxi Purification kit (Clontech Laboratories, Mountain View, CA) according to manufacturer's protocol. Adenovirus expressing β-galactose (Ad-LacZ) was used as a control. Subconfluent human RCECs were grown in DMEM supplemented with 10% FBS and infected by adenovirus at a multiplicity of infection (MOI) of 50. Forty-eight hours after infection, cells were quiescent with DMEM containing 1% FBS for 8 h before experiments.
Intravitreal injection of adenovirus.
Intravitreal injection of adenovirus was performed in deeply anesthetized mice using a UltraMicroPump (World Precision Instruments, Sarasota, FL) following a documented protocol (28). Briefly, under a dissection microscope, an incision was made 1 mm behind limbus with the tip of a shape-edge 31-gauge needle. The 34-gauge blunt needle mounted on a 10-μl microsyringe was inserted into vitreous cavity. Microsyringe was calibrated to deliver a 1-μl vehicle containing 109 viral particles with one depression of the foot switch. After 3 weeks, mice were subjected to retinal vascular permeability assay or humanly killed. The retinas were dissected for Western blot analysis or sectioned for measurement of in situ NADPH-dependent ROS generation.
Measurement of retinal vascular permeability.
Retinal vascular permeability was quantified by measurement albumin leakage from blood vessels into the retina using the Evans blue-albumin method. Details please see the online supplement, available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1057/DC1.
Detection of intracellular ROS generation.
Intracellular ROS generation was assessed by CM-H2DCFDA and DHE (29). Briefly, RCECs were seeded into 96-well plates and grown to confluence. After quiescence, cells were incubated with or without 1 μmol/l lovastatin for 24 h and 0.1 μmol/l DPI for 4 h and then exposed to hypoxia for 16 h. In separate experiments, RCECs were infected with adenoviruses Ad-LacZ, Ad-Nox4, or Ad-Nox4ΔFAD for 48 h. Cells were washed with warm Hank's balanced salt solution and then incubated at 37°C with 10 μmol/l CM-H2DCFDA for 45 min or 5 μmol/l DHE for 30 min in phenol-red free DMEM. Cells were photographed under a fluorescent microscope (Olympus, Hamburg, Germany), and fluorescence intensity was quantified using a fluorescence microplate reader (Perkin Elmer, Waltham, MA) with excitation at 485 nm and emission at 535 nm for dichlorofluorescein (DCF) and with excitation of 485 nm and emission of 645 nm for DHE.
Real-time RT-PCR.
Total RNA was extracted from human RCECs and human neutrophil by using an RNeasy mini kit (Qiagen, Valencia, CA), according to manufacturer's protocol. cDNA were synthesized from 1 μg of RNA, using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) following manufacturer's instructions. Real-time RT-PCR was performed using a SYBR green PCR master mix (Bio-Rad Laboratories) as described in our previous study (25). Specific primers used for real-time PCR are as follows: Nox1: forward: 5′-CTTCCTCACTGGCTGGGATA-3′, reverse: 5′-TGACAGCATTTGCGCAGGCT-3′ (30); gp91phox/Nox2: forward: 5′-CCAGTGAAGATGTGTTCAGCT-3′, reverse: 5′-GCACAGCCAGTAGAAGTAGAT-3′ (30); and Nox4: forward: 5′-ACTTTTCATTGGGCGTCCTC-3′, reverse: 5′-AGAACTGGGTCCACAGCAGA-3′ (31). The mRNA levels of target genes were normalized by 18S rRNA.
Immunocytochemistry.
Cells were fixed with 10% formaldehyde for 10 min and permeabilizated with 0.5% Triton X-100 for 5 min. After blocking with 10% normal donkey serum in PBS for 1 h, cells were incubated with rabbit anti-Nox4 antibody (1:250) overnight at 4°C followed by secondary fluorescein isothiocyanate–conjugated affinity-purified donkey anti-rabbit IgG (1:200) at room temperature for 1 h. Nuclei were stained by VECTASHIELD mounting medium with DAPI. The slides were visualized and photographed under a fluorescent microscope (Olympus). Images represented three independent experiments.
Western blot analysis.
Retinas and cells were lysed in radioimmunoprecipitation assay lysis buffer with protease inhibitor cocktail, phenylmethylsulfonyl fluoride, and sodium orthovanadate. The lysates were sonicated and centrifuged at 14,000 rpm for 10 min. Protein concentration was measured by a bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL). Twenty-five micrograms of protein were dissolved by 12% SDS-PAGE and transferred to nitrocellular membranes. After blocking, the membranes were blotted overnight at 4°C with following primary antibodies: anti-Nox4 (1:1,000), anti-VEGF (1:500), anti–HIF-1α (1:1,000), anti-phosphorylated STAT3 (1:1,000), and anti–β-actin (1:5,000) antibodies. After incubation with horseradish peroxidase–conjugated secondary antibodies (1:2000) at room temperature for 1 h, the membranes were developed with enhanced chemiluminescence substrate using Bio Imaging System (Syngene, Frederick, MD). The bands were semiquantified using densitometry.
Statistical analysis.
Data are expressed as means ± SE. Statistical analysis was performed using Student t test when comparing two groups or ANOVA with Bonferroni's post hoc test when comparing three or more groups. Statistical significance was accepted as P value <0.05.
RESULTS
Lovastatin downregulates retinal Nox4 and VEGF expression in db/db mice.
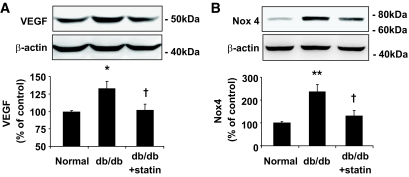
Overexpression of VEGF in the retina induced by diabetes plays a critical role in BRB breakdown and vascular leakage in diabetic retinopathy (32). To address if the protection of BRB by lovastatin is associated with the inhibition of VEGF signaling, we determined the effects of lovastatin on retinal VEGF expression in db/db mice. As shown in Fig. 1A, VEGF expression was significantly increased in retinas of db/db mice, which was normalized by lovastatin treatment to nearly the normal level. These results suggest that inhibition of VEGF by lovastatin is associated with its salutary effect on diabetes-induced BRB breakdown. To determine whether Nox4 is implicated in diabetic retinopathy, we measured Nox4 level in the retina of db/db mice. We found that Nox4 expression in the retina was significantly increased by twofold in db/db mice when compared with age- and sex-matched nondiabetic littermates (P < 0.01) (Fig. 1B). Lovastatin treatment almost completely reversed the increase in Nox4 expression in the db/db retina (P < 0.05) (Fig. 1B). These results suggest a potential role of Nox4 in VEGF upregulation and BRB breakdown in diabetic retinopathy.
FIG. 1.
Effect of lovastatin on VEGF and Nox4 expression in retinas of db/db mice. Db/db mice and nondiabetic littermate controls were gastric gavaged with or without lovastatin (10 mg/kg/day) for 6 weeks. VEGF (A) and Nox4 (B) expression in retinas were determined by Western blotting analysis and semiquantified by densitometry (means ± SE, n = 6). *P < 0.05 or **P < 0.01 vs. nondiabetic control; †P < 0.05 vs. db/db mice.
Nox4 is the major isoform of NADPH oxidase in RCECs.
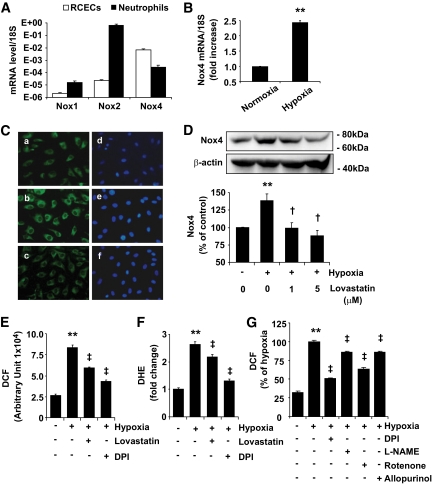
NADPH oxidase is a foremost source of ROS in endothelial cells; however, the exact role and mechanisms of NADPH oxidase in RCECs remains unknown. We first determined expression of major NADPH oxidase isoforms, including Nox1, Nox2, and Nox4 in human RCECs and compared them with human neutrophils. As shown in Fig. 2A, mRNA expression of Nox4 was at least 100-fold higher than Nox1 and Nox2 in RCECs. In contrast, expression of Nox2 is significantly higher than Nox1 and Nox4 in neutrophils (Fig. 2A). These results indicate that although all of the isoforms are expressed in both cell types, Nox4 is the major isoform of NADPH oxidase in RCECs, while Nox2 is the major one in neutrophils.
FIG. 2.
Expression of Nox isoforms and regulation of Nox4 by hypoxia and lovastatin in RCECs. A: mRNA expression of Nox1, Nox2, and Nox4 in human RCECs and neutrophil was determined by real-time RT-PCR and expressed relative to 18S rRNA level (means ± SE, n = 3). B: Human RCECs were exposed to hypoxia for 4 h, and Nox4 mRNA was quantified by real-time RT-PCR (means ± SE, n = 3). C: Cellular distribution of Nox4 was determined by immunocytochemistry in human RCECs. A–C: Nox4 staining. D–F: Nuclear staining. Nox4 displayed perinuclear distribution in RCECs under normoxia (A) and hypoxia (B). Exposure of cells to hypoxia for 16 h resulted in increased intensity of Nox4 (B), which was reversed by lovastatin (1 μmol/l) (C). D: Expression of Nox4 in bovine RCECs were determined by Western blot analysis and semiquantified by densitometry (means ± SE, n = 3). E and F: Bovine RCECs were preincubated with 1 μmol/l lovastatin for 24 h or 100 nmol/l DPI for 4 h, followed by exposure to hypoxia for 16 h. Intracellular ROS generation was measured by dichlorofluorescein (DCF) (E) and DHE (F). Results were obtained from 30 replicates in each condition and represent three independent experiments (means ± SE). G: Bovine RCECs were preincubated with 100 nmol/l DPI, 100 μmol/l l-NAME, 1 μmol/l rotenone, or 10 μmol/l allopurinol for 4 h, followed by exposure to hypoxia for 16 h. Intracellular ROS generation was measured by DCF (means ± SE, n = 3). **P < 0.01 vs. normoxia; †P < 0.05; ‡P < 0.01 vs. hypoxia. (A high-quality digital representation of this figure is available in the online issue.)
Lovastatin attenuated hypoxia-induced Nox4 expression and ROS generation in RCECs.
Hypoxia is a potent inducer of VEGF expression in endothelial cells (33). We next investigated Nox4 expression and ROS generation in RCECs exposed to hypoxia. The results show that hypoxia treatment for 4 h induced a significant increase in Nox4 mRNA expression by 3.2-fold (Fig. 2B). Consistently, Nox4 protein level was also significantly increased after hypoxia treatment for 16 h (Fig. 2C and D). Moreover, intracellular ROS generation was significantly increased in hypoxia-treated cells (Fig. 2E and F). Interestingly, an immunocytochemical study showed that Nox4 was localized in the perinuclear compartment (Fig. 2C). Hypoxia treatment resulted in an increase in the intensity of Nox4 signals but did not cause any translocation to other subcellular compartments.
To determine the effects of lovastatin on Nox4 expression in RCECs, cells were pretreated with lovastatin or DPI, a well-known inhibitor of NADPH oxidase, for 4 h followed by exposure to hypoxia for 16 h. The results showed that lovastatin almost completely abolished hypoxia-induced Nox4 upregulation in a dose-dependent manner (Fig. 2C and D). Furthermore, lovastatin and DPI significantly ameliorated hypoxia-induced ROS generation in RCECs (Fig. 2E and F). As DPI also acts as an inhibitor of other flavoproteins (34), to delineate the source of ROS induced by hypoxia, RCECs were pretreated with different inhibitors that suppress several ROS-producing systems, including rotenone (an inhibitor of mitochondrial electron transport chain), allopurinol (an inhibitor of xanthine oxidase), and l-NAME (an inhibitor of nitric oxide synthase inhibitor). Results show that each of these inhibitors attenuated hypoxia-induced ROS levels to some extent, suggesting that hypoxia may activate multiple pathways that contribute to ROS generation (Fig. 2G). However, DPI at a very low concentration (100 nmol/l) showed the most significant effect (Fig. 2G).
Downregulation of Nox4 and VEGF expression by lovastatin in RCECs exposed to high glucose.
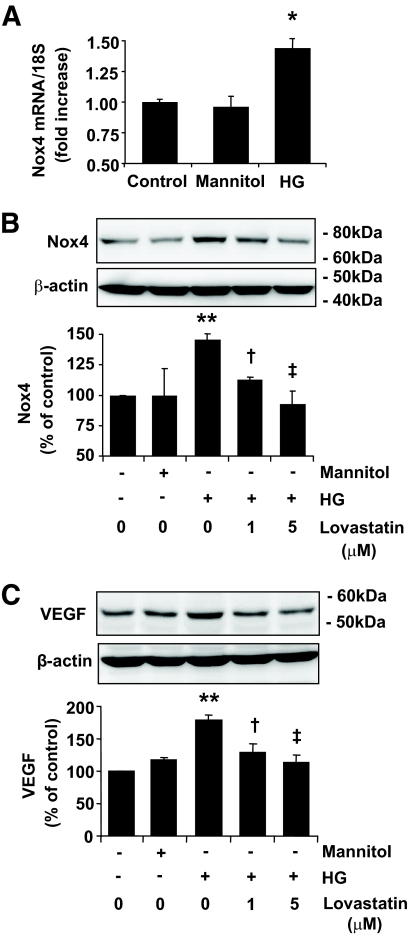
Hyperglycemia is one of the major pathogenic causes of vascular complications in diabetes. To evaluate the effects of high glucose on Nox4 expression, RCECs were exposed to 25 mmol/l d-glucose or same concentration of mannitol as osmotic control for 6–24 h, and the mRNA and protein levels of Nox4 expression were measured by real-time RT-PCR and Western blot analysis, respectively. The results showed that Nox4 mRNA was significantly increased in the cells exposed to high glucose for 6 h, when compared with osmotic control (Fig. 3A). The protein level of Nox4 was also increased by high glucose at 24 h, which was significantly decreased by lovastain in a dose-dependent manner (Fig. 3B). In parallel, VEGF expression was significantly increased in high-glucose–treated cells and attenuated by lovastatin treatment (Fig. 3C).
FIG. 3.
Lovastatin downregulates Nox4 and VEGF expression in RCECs exposed to high glucose. A: mRNA expression of Nox4 was quantified by real-time RT-PCR in human RCECs treated with 25 mmol/l glucose (HG, high glucose) or mannitol for 6 h. B and C: Bovine RCECs were treated by 25 mmol/l glucose (HG) or mannitol for 24 h with or without lovastatin (0–5 μmol/l). Expression of Nox4 (B) and VEGF (C) was determined by Western blot analysis and quantified by densitometry (means ± SE, n = 3). *P < 0.05; **P < 0.05 vs. control; †P < 0.05; ‡P < 0.01 vs. high glucose.
Inhibition of NADPH oxidase activity decreased HIF-1α and VEGF expression in RCECs.
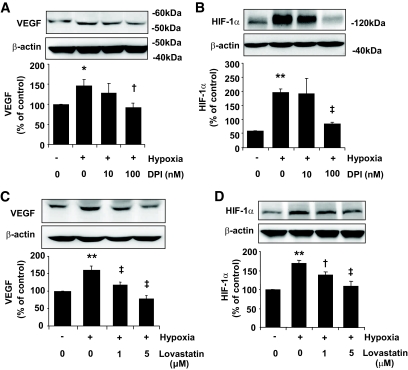
To determine whether increased Nox4 activity is a potential mediator of VEGF expression in RCECs in a hypoxic condition, cells were pretreated with DPI for 4 h, followed by exposure to hypoxia for 16 h. The results showed that a low dose (100 nmol/l) of DPI was sufficient to decrease VEGF expression induced by hypoxia (Fig. 4A). Moreover, this effect is associated with the downregulation of HIF-1α, a master transcription factor responsible for hypoxia-induced VEGF expression (Fig. 4B). In addition, pretreatment of cells with lovastatin significantly attenuated cellular VEGF expression and decreased HIF-1α level in a dose-dependent manner (Fig. 4C and D).
FIG. 4.
Downregulation of HIF-1α and VEGF expression by NADPH oxidase inhibitor or lovastatin in RCECs. Bovine RCECs were pretreated with DPI (A and B) for 4 h or lovastatin (C and D) for 24 h followed by exposure to hypoxia for 16 h. Expression of VEGF (A and C) and HIF-1α (B and D) was determined by Western blot analysis and quantified by densitometry. Results were expressed as means ± SE. (n = 3). *P < 0.05; **P < 0.01 vs. normoxia; †P < 0.05; ‡P < 0.01 vs. hypoxia.
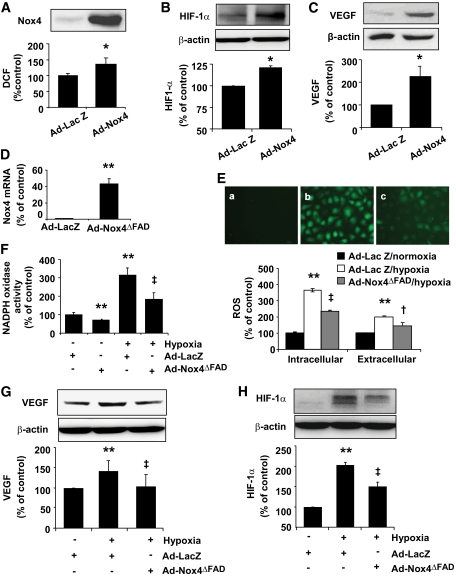
Overexpression of Nox4 increased ROS generation and upregulated VEGF expression in RCECs.
To further determine effect of Nox4 on ROS generation in retinal endothelial cells, we infected human RCECs with adenovirus-expressing Nox4 (Ad-Nox4) or adenovirus-expressing Lac Z (Ad-Lac Z) as control. Overexpression of Nox4 resulted in a moderate but significant increase in the basal level of intracellular ROS generation (Fig. 5A). The reasons for the discrepancy between Nox4 expression and ROS production are not clear. A possible explanation is that Nox4, while generating ROS, may also regulate other redox enzymes that enhance ROS scavenging and/or suppress ROS production to maintain a low basal level of ROS. While this speculation warrants further investigation, we found that knockdown of Nox4 with siRNA increases Nox2 mRNA expression (not shown) in RCECs. Similarly, an upregulation of Nox4 mRNA was observed in Nox2−/− knockout mice (35). These data suggest a reciprocal regulation of Nox enzymes. In addition, Ad-Nox4 treatment significantly increased HIF-1α (Fig. 5B) and VEGF expression (Fig. 5C). These results support a critical role of NADPH oxidase in the regulation of HIF-1α and VEGF in RCECs.
FIG. 5.
Nox4 regulates ROS generation, HIF-1α, and VEGF expression in RCECs. A–C: Human RCECs were infected with Ad-LacZ or Ad-Nox4 for 48 h. Expression of Nox4 and ROS generation (A), expression of HIF-1α (B), and VEGF (C) were determined (means ± SE, n = 3). *P < 0.05 vs. Ad-LacZ. D–H: Human RCECs were infected with Ad-LacZ or Ad-Nox4ΔFAD for 48 h followed by exposure to hypoxia for 16 h. D: mRNA expression of dominant-negative Nox4 were detected by real-time PCR and normalized by 18S rRNA (means ± SD, n = 3). E, upper panel: Representative images of DCF that indicate ROS generation in RCECs. E, a: Ad-LacZ + normoxia. E, b: Ad-LacZ + hypoxia. E, c: Ad-Nox4ΔFAD + hypoxia. E, lower panel: Intracellular ROS generation was measured by DCF. Extracellular ROS level was determined by Amplex red assay. Results were obtained from 30 replicates in each condition and represent three independent experiments (means ± SE). F: NADPH oxidase activity was measured as described in research design and methods (means ± SD, n = 3). G and H: Expression of VEGF (G) and HIF-1α (H) were determined by Western blot analysis and quantified by densitometry (means ± SE, n = 3). **P < 0.01 vs. Ad-LacZ + normoxia; †P < 0.05; ‡P < 0.01 vs. Ad-LacZ + hypoxia.(A high-quality digital representation of this figure is available in the online issue).
Inhibition of Nox4 reduced ROS generation and VEGF expression in RCECs exposed to hypoxia.
We next asked whether Nox4 activity is essential for hypoxia-induced ROS generation and VEGF expression in human RCECs. Inhibition of Nox4 activity was achieved by adenovirus overexpressing a dominant-negative mutant of Nox4 (Ad-Nox4ΔFAD). mRNA expression of Nox4ΔFAD was confirmed by real-time RT-PCR (Fig. 5D). Intracellular ROS generation was measured by fluorescence probe CM-H2DCFDA and H2O2 released into the medium was measured by Amplex red assay. In addition, NADPH oxidase activity was measured in cell homogenates. Results show that Ad-Nox4ΔFAD significantly attenuated intracellular ROS level and extracellular ROS (H2O2) released from RCECs exposed to hypoxia (Fig. 5E). In keeping, inhibition of Nox4 significantly decreased NADPH-dependent ROS generation and reduced hypoxia-mediated increase in NADPH oxidase activity (Fig. 5F).
To further elucidate the role of Nox4 in hypoxia-induced HIF-1α and VEGF expression, we determined HIF-1α and VEGF levels in human RCECs infected with Ad-Nox4ΔFAD after exposure to hypoxia for 16 h. As expected, hypoxia induced a significant increase in HIF-1α and VEGF expression. Ad-Nox4ΔFAD treatment almost completely abolished hypoxia-induced VEGF upregulation (Fig. 5G). Moreover, HIF-1α level was significantly attenuated in Ad-Nox4ΔFAD-treated cells when compared with cells infected with control virus (Fig. 5H).
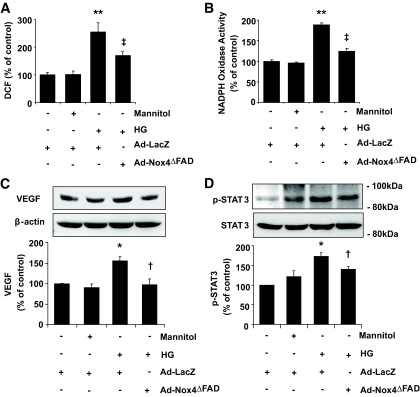
Nox4 is required for high-glucose–mediated ROS generation, STAT3 activation, and VEGF expression in RCECs.
To determine whether inhibition of Nox4 affects high-glucose–induced ROS and VEGF expression, bovine RCECs were infected with Ad-LacZ and Ad-Nox4ΔFAD for 48 h, followed by exposure to high glucose for 24 h. Ad-Nox4ΔFAD significantly attenuated high-glucose–induced ROS generation (Fig. 6A) and largely blocked the increase of NADPH oxidase activity in cells exposed to high glucose (Fig. 6B). In addition, inhibition of Nox4 abrogated high-glucose–induced upregulation of VEGF expression (Fig. 6C). Interesting, high glucose did not alter the expression of HIF-1α (not shown), suggesting that HIF-1α is not involved in high-glucose–induced VEGF expression in RCECs. Conversely, high glucose significantly increased phosphorylation of STAT3, another key transcription factor that activates VEGF expression (36) (Fig. 6D). Treatment with Ad-Nox4ΔFAD attenuated high-glucose–elicited STAT3 phosphorylation, suggesting Nox4 is involved in high-glucose–mediated ROS generation and VEGF expression through activation of STAT3.
FIG. 6.
Inhibition of Nox4 attenuates high-glucose–induced ROS generation, STAT3 activation, and VEGF expression in RCECs. Bovine RCECs were infected with Ad-LacZ or Ad-Nox4ΔFAD for 48 h followed by treatment with 25 mmol/l glucose. Mannitol was used as osmotic control. A: Intracellular ROS generation was detected by DCF. Ad-Nox4ΔFAD significantly alleviated high-glucose–induced ROS generation. Results were obtained from 30 replicates in each condition and represent three independent experiments (means ± SE). B: NADPH oxidase activity was measured as described in research design and methods (means ± SE, n = 3). C and D: Expression of VEGF (C) and phosphorylation of STAT3 (D) were determined by Western blot analysis and quantified by densitometry (means ± SE, n = 3). *P < 0.05; **P < 0.01 vs. control; †P < 0.05; ‡P < 0.01 vs. high glucose.
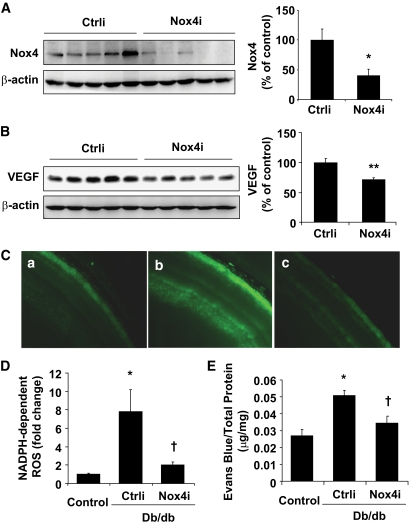
Genetic inhibition of Nox4 reduced NADPH-dependent ROS generation and ameliorated vascular leakage in retinas of db/db mice.
To establish the causative role of Nox4 in retinal VEGF expression and vascular leakage in db/db mice, we downregulated Nox4 expression in the retina using an adenovirus-delivered siRNA against mouse Nox4. Three weeks after an intravitreal injection of Ad-Nox4i or Ad-Ctrli, mice were humanely killed, and retinal expression of Nox4 and VEGF was determined by Western blot analysis. As shown in Fig. 7A, intravitreal injection of Ad-Nox4i led to a significant decrease of Nox4 expression in the retina by approximately 60%. Moreover, Ad-Nox4i significantly decreased retinal VEGF expression in db/db mice, when compared with Ad-Ctrli treatment (Fig. 7B). In parallel, NADPH-dependent ROS generation was markedly increased by eightfold in retinas of db/db mice, indicating enhanced NADPH oxidase activity in the diabetic retina, which was significantly blunted in Ad-Nox4i–treated eyes (Fig. 7C and D). In addition, intravitreal injection of Ad-Nox4i almost completely abolished the increase in retinal vascular permeability in db/db mice (Fig. 7E). These results strongly suggest a causal role of Nox4 in vascular hyperpermeability in diabetic retinopathy, further supporting the notion that lovastatin inhibits retinal vascular leakage through suppression of Nox4 expression.
FIG. 7.
Genetic depletion of Nox4 downregulate retinal VEGF expression and ameliorated vascular leakage in db/db mice. Db/db mice were randomly selected to receive an intravitreal injection of Ad-Nox4i or Ad-Ctrli. Nondiabetic littermate mice received same treatment as control. Three weeks after injection, retinal levels of Nox4 and VEGF, NADPH-dependent ROS generation, and vascular permeability were evaluated. A and B: Expression of Nox4 (A) and VEGF (B) was determined by Western blot analysis and quantified by densitometry (means ± SE, n = 5). *P < 0.05 vs. Ad-Ctrli. C and D: In situ NADPH-dependent ROS generation was measured in freshly prepared retinal sections. When compared with nondiabetic littermate controls (C, a), NADPH-dependent ROS generation was markedly increased in retinas of db/db mice (C, b), and dramatically decreased after Ad-Nox4i treatment (C, c). The fluorescent intensity of DCF was semiquantified (D) (means ± SE, n = 4). *P < 0.05 vs. nondiabetic control; †P < 0.05 vs. db/db + Ad-Ctrli. E: Retinal vascular permeability was measured by the Evans blue method. Results were expressed as micrograms per milligram total retinal protein (means ± SE, n = 6). Db/db mice showed retinal vascular hyperpermeability compared with nondiabetic littermate controls. Intravitreal injection of Ad-Nox4i significantly decreased retinal vascular permeability in db/db mice. *P < 0.05 vs. nondiabetic control; †P < 0.05 vs. db/db + Ad-Ctrli. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
Several studies, including ours, have demonstrated the protective effects of statins on retinal vascular function by maintaining the endothelial tight junction integrity and vasculature homeostasis in diabetic retinopathy (37–39); however, the mechanisms are poorly understood. The present study provided the first evidence that Nox4, a nonphagocyte NADPH oxidase isoform, is upregulated in diabetic retinas of db/db mice and in RCECs exposed to diabetic stressors (hypoxia and high glucose). Inhibition of Nox4 activity by lovastatin downregulates HIF-1α– and STAT3-mediated VEGF expression and ameliorates retinal vascular leakage in diabetic retinopathy. These findings suggest that Nox4 is a key player in diabetes-induced oxidative stress and VEGF expression in retinal endothelial cells and that activation of Nox4 is a novel mechanism of retinal vascular leakage in diabetic retinopathy. Inhibition of Nox4 is at least in part responsible for the salutary effects of lovastatin on retinal endothelial and BRB function in diabetes.
The NADPH oxidase (Nox) family consists of the phagocyte NADPH oxidase (Nox2/gp91phox) and six homolog members identified in nonphagocytes: Nox1, Nox3, Nox4, Nox5, Duox1, and Duox2 (18,40). In endothelial cells, Nox1, Nox2, and Nox4 are mainly expressed (41). We found that Nox4 expression is significantly higher than Nox2 and Nox1 in RCECs. In contrast, Nox2 appears to be the major form expressed in human neutrophils (Fig. 2). Similar findings have been reported in human umbilical vein endothelial cells (42) and human aorta endothelial cells (43), indicating a potential role of Nox4 in endothelial cells. In addition, Nox4 has several unique features that make Nox4 particularly important in controlling the redox status and intracellular signaling in endothelial cells. In contrast to other Nox members, Nox4 is constitutively active even in the absence of cytosolic regulators (44–47). We have shown that overexpression of Nox4 increases while inhibition of Nox4 activity reduces the basal level of ROS generation, suggesting that Nox4 is a major enzyme responsible for ROS generation in unstimulated endothelial cells. Moreover, Nox4 upregulates HIF-1α and VEGF expression in RCECs. In contrast, suppression of Nox4 attenuates cell proliferation in human aorta endothelial cells (27). These findings imply an important role of Nox4 in endothelial function through regulation of VEGF expression.
Hypoxia is a potent inducer of VEGF expression through stabilizing HIF-1α in vascular endothelial cells. Intriguingly, recent studies (48) have provided evidence that increased ROS production contributes to HIF-1α stabilization and VEGF overexpression induced by hypoxia. In the present study, we show that hypoxia stimulates multiple sources of ROS production in RCECs and that activation of NADPH oxidase plays an important role in these processes. Exposure of RCECs to hypoxia upregulates Nox4 mRNA and protein expression, which is attenuated by pretreatment with lovastatin. Interestingly, hypoxia also increases Nox2 protein expression, although at a much lower level when compared with Nox4, and lovastatin decreases Nox2 level in RCECs exposed to hypoxia (not shown). These results suggest that Nox4 and Nox2 may both contribute to ROS generation in hypoxia in RCECs. Indeed, downregulation of Nox4 expression or activity significantly decreases NADPH oxidase–derived ROS generation and alleviates H2O2 production in RCECs under hypoxic or high glycemic conditions. In addition, inhibition of Nox4 significantly mitigates hypoxia-induced upregulation of HIF-1α and VEGF in RCECs, indicating that Nox4 is essential for HIF-1α–dependent VEGF expression. Moreover, inhibition of Nox4 attenuates high-glucose–mediated STAT3 activation and consequent VEGF upregulation, suggesting that Nox4-derived ROS may also regulate VEGF through HIF-1α–independent pathways, such as STAT3.
The subcellular localization of Nox4 has been controversial. In previous studies, Nox4 was detected in various compartments, such as mitochondria, cell membrane, nucleus, focal adhesion, and endoplasmic reticulum (27,31,49). Our results indicate that Nox4 localizes in the perinuclear compartment in RCECs. This observation corroborates several recent studies in various cell types, such as vascular smooth muscle cells and endothelial cells (27,31). Further, we found that Nox4 expression is colocalized with the endoplasmic reticulum marker KDEL (Lys-Asp-Glu-Leu) in RCECs (not shown), suggesting that Nox4 may be an endoplasmic reticulum resident protein. However, the exact role of Nox4 in the endoplasmic reticulum remains unknown. It is also unclear how Nox4 is regulated by high glucose and hypoxia and how statin inhibits Nox4 expression in retinal endothelial cells. Our previous study (25) showed that hypoxia induces endoplasmic reticulum stress in cultured RCECs. Pedruzzi et al. (46) showed that knockdown of IRE-1 and Jun NH2-terminal kinase inhibition downregulated Nox4 expression in human aorta smooth muscle cells. Moreover, a recent study showed that deletion of C/EBP-homologous protein reduces oxidative stress and improves β-cell function (50). These findings suggest that endoplasmic reticulum stress–induced cellular response is involved in the regulation of redox status. Future studies are warranted to investigate if Nox4 is a potential target linking endoplasmic reticulum stress and intracellular redox signaling in retinal endothelial cells and how this crosstalk contributes to endothelial dysfunction and vascular abnormality in diabetic retinopathy.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health Grant P30 EY12190, P20RR024215, Juvenile Diabetes Federation Grants 5-2009-475, American Health Assistance Foundation (AHAF) grant M2010088 and a research award from Oklahoma Center for the Advancement of Science and Technology (OCAST).
No potential conflicts of interest relevant to this article were reported.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Parts of this study were presented at The Association for Research in Vision and Ophthalmology (ARVO).
The authors thank Drs. Lily Wong and Olga Nikolgeva for excellent technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Klein R, Klein BE, Moss SE: Epidemiology of proliferative diabetic retinopathy. Diabetes Care 1992; 15: 1875–1891 [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, Cruickshanks KJ: The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XV. the long-term incidence of macular edema. Ophthalmology 1995; 102: 7–16 [DOI] [PubMed] [Google Scholar]
- 3.Miller JW, Adamis AP, Aiello LP: Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev 1997; 13: 37–50 [DOI] [PubMed] [Google Scholar]
- 4.De La Cruz JP, Gonzalez-Correa JA, Guerrero A, De La Cuesta FS: Pharmacological approach to diabetic retinopathy. Diabetes Metab Res Rev 2004; 20: 91–113 [DOI] [PubMed] [Google Scholar]
- 5.Moss SE, Klein R, Klein BE: The 14-year incidence of visual loss in a diabetic population. Ophthalmology 1998; 105: 998–1003 [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL: The Wisconsin Epidemiologic Study of diabetic retinopathy: IV. diabetic macular edema. Ophthalmology 1984; 91: 1464–1474 [DOI] [PubMed] [Google Scholar]
- 7.Dvorak HF, Brown LF, Detmar M, Dvorak AM: Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995; 146: 1029–1039 [PMC free article] [PubMed] [Google Scholar]
- 8.Aiello LP, Wong JS: Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int Suppl 2000; 77: S113–S119 [DOI] [PubMed] [Google Scholar]
- 9.Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M: Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol 1996; 149: 293–305 [PMC free article] [PubMed] [Google Scholar]
- 10.Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S: Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol 2002; 133: 70–77 [DOI] [PubMed] [Google Scholar]
- 11.Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP: VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci 2001; 42: 2408–2413 [PubMed] [Google Scholar]
- 12.Baynes JW: Role of oxidative stress in development of complications in diabetes. Diabetes 1991; 40: 405–412 [DOI] [PubMed] [Google Scholar]
- 13.Du Y, Miller CM, Kern TS: Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med 2003; 35: 1491–1499 [DOI] [PubMed] [Google Scholar]
- 14.Abu El-Asrar AM, Meersschaert A, Dralands L, Missotten L, Geboes K: Inducible nitric oxide synthase and vascular endothelial growth factor are colocalized in the retinas of human subjects with diabetes. Eye 2004; 18: 306–313 [DOI] [PubMed] [Google Scholar]
- 15.Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP: Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol 2002; 160: 501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis EA, Guberski DL, Somogyi-Mann M, Grant MB: Increased H2O2, vascular endothelial growth factor and receptors in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med 2000; 28: 91–101 [DOI] [PubMed] [Google Scholar]
- 17.Chen P, Guo AM, Edwards PA, Trick G, Scicli AG: Role of NADPH oxidase and ANG II in diabetes-induced retinal leukostasis. Am J Physiol Regul Integr Comp Physiol 2007; 293: R1619–R1629 [DOI] [PubMed] [Google Scholar]
- 18.Geiszt M, Kopp JB, Varnai P, Leto TL: Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 2000; 97: 8010–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orient A, Donkó A, Szabó A, Leto TL, Geiszt M: Novel sources of reactive oxygen species in the human body. Nephrol Dial Transplant 2007; 22: 1281–1288 [DOI] [PubMed] [Google Scholar]
- 20.San Martín A, Du P, Dikalova A, Lassègue B, Aleman M, Góngora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK: Reactive oxygen species-selective regulation of aortic inflammatory gene expression in type 2 diabetes. Am J Physiol Heart Circ Physiol 2007; 292: H2073–H2082 [DOI] [PubMed] [Google Scholar]
- 21.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE: Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 2005; 280: 39616–39626 [DOI] [PubMed] [Google Scholar]
- 22.Danesh FR, Kanwar YS: Modulatory effects of HMG-CoA reductase inhibitors in diabetic microangiopathy. FASEB J 2004; 18: 805–815 [DOI] [PubMed] [Google Scholar]
- 23.Ludwig S, Shen GX: Statins for diabetic cardiovascular complications. Curr Vasc Pharmacol 2006; 4: 245–251 [DOI] [PubMed] [Google Scholar]
- 24.Li J, Wang JJ, Chen D, Mott R, Yu Q, Ma JX, Zhang SX: Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res 2009; 89: 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Wang JJ, Yu Q, Wang M, Zhang SX: Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett 2009; 583: 1521–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ: The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol 2004; 24: 1844–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JFJ: Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 2008; 181: 1129–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori K, Gehlbach P, Ando A, Wahlin K, Gunther V, McVey D, Wei L, Campochiaro PA: Intraocular adenoviral vector-mediated gene transfer in proliferative retinopathies. Invest Ophthalmol Vis Sci 2002; 43: 1610–1605 [PubMed] [Google Scholar]
- 29.Zhang SX, Wang JJ, Dashti A, Wilson K, Zou MH, Szweda L, Ma JX, Lyons TJ: Pigment epithelium-derived factor mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized low-density lipoprotein. J Mol Endocrinol 2008; 41: 135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M: Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 2004; 109: 227–233 [DOI] [PubMed] [Google Scholar]
- 31.Mittal M, Roth M, König P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hänze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N: Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 2007; 101: 258–267 [DOI] [PubMed] [Google Scholar]
- 32.Zhang SX, Ma J-X, Sima J, Chen Y, Hu MS, Ottlecz A, Lambrou GN: Genetic difference in susceptibility to the blood-retina barrier breakdown in diabetes and oxygen-induced retinopathy. Am J Pathol 2005; 166: 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang SX, Wang JJ, Gao G, Parke K, Ma JX: Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol 2006; 37: 1–12 [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Trush MA: Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochim Biophys Res Commun 1998; 253: 295–299 [DOI] [PubMed] [Google Scholar]
- 35.Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V: Antioxid redox signal: role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells 2008; 11: 747–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB:: Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci 2008; 49: 3239–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sen K, Misra A, Kumar A, Pandey RM: Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract 2002; 56: 1–11 [DOI] [PubMed] [Google Scholar]
- 38.Medina RJ, O'Neill CL, Devine AB, Gardiner TA, Stitt AW: The pleiotropic effects of simvastatin on retinal microvascular endothelium has important implications for ischaemic retinopathies. PLoS ONE 2008; 3: e2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyahara S, Kiryu J, Yamashiro K, Miyamoto K, Hirose F, Tamura H, Katsuta H, Nishijima K, Tsujikawa A, Honda Y: Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am J Pathol 2004; 164: 1697–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD: Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001; 269: 131–140 [DOI] [PubMed] [Google Scholar]
- 41.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Görlach A: NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 2006; 8: 1473–1484 [DOI] [PubMed] [Google Scholar]
- 42.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL: Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 2005; 7: 308–317 [DOI] [PubMed] [Google Scholar]
- 43.Park HS, Chun JN, Jung HY, Choi C, Bae YS: Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res 2006; 72: 447–455 [DOI] [PubMed] [Google Scholar]
- 44.Tyagi N, Moshal KS, Sen U, Lominadze D, Ovechkin AV, Tyagi SC: Ciglitazone ameliorates homocysteine-mediated mitochondrial translocation and matrix metalloproteinase-9 activation in endothelial cells by inducing peroxisome proliferator activated receptor-gamma activity. Cell Mol Biol (Noisy-le-grand) 2006; 52: 21–27 [PubMed] [Google Scholar]
- 45.Hwang J, Kleinhenz DJ, Rupnow HL, Campbell AG, Thulé PM, Sutliff RL, Hart CM: The PPARgamma ligand, rosiglitazone, reduces vascular oxidative stress and NADPH oxidase expression in diabetic mice. Vascul Pharmacol 2007; 46: 456–462 [DOI] [PubMed] [Google Scholar]
- 46.Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O'dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E: NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 2004; 24: 10703–10717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI: Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol 2008; 28: 25–33 [DOI] [PubMed] [Google Scholar]
- 48.Irwin DC, McCord JM, Nozik-Grayck E, Beckly G, Foreman B, Sullivan T, White MT, Crossno JJ, Bailey D, Flores SC, Majka S, Klemm D, van Patot MC: A potential role for reactive oxygen species and the HIF-1alpha-VEGF pathway in hypoxia-induced pulmonary vascular leak. Free Radic Biol Med 2009; 47: 55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Block K, Gorin Y, Abboud HE: Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 2009; 106: 14385–14390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ: Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 2008; 118: 3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.