Abstract
Objective
Mouse aorta smooth muscle cells (SMC) express tumor necrosis factor receptor superfamily member 1A (TNFR-1) and lymphotoxin β-receptor (LTβR). Circumstantial evidence has linked the SMC LTβR to tertiary lymphoid organogenesis in hyperlipidemic mice. Here, we explored TNFR-1 and LTβR signaling in cultured SMC.
Methods and Results
TNFR-1 signaling activated the classical RelA NF-κB pathway, whereas LTβR signaling activated the classical RelA and alternative RelB NF-κB pathways, and both signaling pathways synergized to enhance p100 inhibitor processing to the p52 subunit of NF-κB. Microarrays showed that simultaneous TNFR-1/LTβR activation resulted in elevated mRNA encoding leukocyte homeostatic chemokines CCL2, CCL5, CXCL1, and CX3CL1. Importantly, SMC acquired features of lymphoid tissue organizers, which control tertiary lymphoid organogenesis in autoimmune diseases through hyperinduction of CCL7, CCL9, CXCL13, CCL19, CXCL16, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1. TNFR-1/LTβR cross-talk resulted in augmented secretion of lymphorganogenic chemokine proteins. Supernatants of TNFR-1/LTβR–activated SMC markedly supported migration of splenic T cells, B cells, and macrophages/dendritic cells. Experiments with ltbr−/− SMC indicated that LTβR-RelB activation was obligatory to generate the lymphoid tissue organizer phenotype.
Conclusion
SMC may participate in the formation of tertiary lymphoid tissue in atherosclerosis by upregulation of lymphorganogenic chemokines involved in T-lymphocyte, B-lymphocyte, and macrophage/dendritic cell attraction.
Keywords: smooth muscle cells, immune response, chemokines, signaling pathways, tumor necrosis factor receptor, lymphotoxin-β receptor
Most studies of atherosclerosis have focused on intima lesions, which are composed of lipid-laden macro-phage/foam cells, T cells, and smooth muscle cells (SMC). Plaque leukocytes1,2 interacting with SMC3–6 are thought to initiate adaptive immune responses toward arterial wall-derived antigens. However, evidence that macrophage/foam cells/dendritic-like cells (DC)7 and T cells may mediate antigen-dependent T-cell responses within plaques remains circumstantial. The anatomic location for the initiation of antigen-dependent B-cell responses is similarly puzzling,8,9 and impacts of antigen-specific T and B cells on disease progression remain to be delineated.8 T-cell and B-cell responses typically require antigen-presenting DC in T-cell areas, follicular DC in activated germinal centers of B-cell follicles, and high rates of T-cell recirculation, none of which has been shown to occur in atherosclerotic plaques.1,2,8,10 Although immune responses toward atherosclerosis may occur in lymph nodes or spleen, evidence to support this possibility is limited.8 Thus, how and where (auto)immune reactions generate self-reactive T cells and B cells to trigger plaque instability and myocardial infarction are all important issues that remain to be defined.
We and others11–13 reported that T-cell and B-cell aggregates emerge in adventitia of aorta segments adjacent to atherosclerotic lesions of apolipoprotein E-deficient (apoE−/−) mice. These aggregates were precursors of well-structured aorta tertiary lymphoid organs that showed a high degree of organization akin to lymph nodes.14 These data provided evidence that aorta tertiary lymphoid organs may organize antigen-dependent T-cell and B-cell (auto)immune responses toward atherosclerosis.14 In addition, medial SMC underlying intimal plaques became activated and expressed the lymphorganogenic chemokines CXCL13 (B-lymphocyte chemoattractant) and CCL21 (secondary lymphoid tissue chemokine).14,15 Moreover, aorta tertiary lymphoid organ integrity depended on the lymphotoxin β-receptor (LTβR).15–17 Together, these data indicate that media SMC were activated by plaques to express features of lymphoid tissue organizers (LTO) through an LTβR-dependent NF-κB signaling pathway.14,18 –21
Mesenchymal cell-derived LTO have been studied during embryonic lymphoid organ neogenesis, when they interact with CD3−/CD4+ hematopoietic cells referred to as lymphoid tissue inducer cells to give rise to secondary lymphoid organs.10 It has been proposed that LTO also may be functional in adult animals in chronic inflammatory diseases and infection. Activated synoviocytes in rheumatoid arthritis and intestinal fibroblasts in inflammatory bowel disease recapitulate features of embryonic LTO by controlling tertiary lymphoid organ neogenesis.10,13–21 Importantly, action of LTO in tertiary lymphoid organs is often associated with tissue destruction and significant disease pathology.14 Here, we examined the effects of tumor necrosis factor (TNF) and of an agonistic anti-LTβR mAb (α-LTβR) in cultured mouse aorta SMC. Our data show that SMC stimulated by TNF and α-LTβR, but not with either TNF or α-LTβR alone, adopt a phenotype that strikingly resembles LTO.
Materials and Methods
Mice
Mice on the C57BL/6J background were from The Jackson Laboratories and housed and fed as reported.14 Ltbr−/− mice21 were a kind gift from Klaus Pfeffer, Institute for Medical Microbiology, Heinrich-Heine University of Düsseldorf, Germany. Animal procedures were approved by the Animal Care and Use Committee of Thuringia.
Cell Culture
SMC were obtained from aortae of 8- to 12-week-old C57BL/6 mice by sequential dissection and enzyme digestion.14 Aortic endothelial cells were harvested by scraping using a Teflon policeman before digestion. SMC were used at passages 1 to 3 and purity was ≥99% as shown by α-smooth muscle actin positivity of cytospins. Cells were stimulated with 10 μg/mL agonistic rat anti-mouse-LTβR mAb (α-LTβR)22 or 1 ng/mL mouse recombinant TNF (R&D Systems) as indicated.
Assays
Quantitative reverse-transcription polymerase chain reaction was performed as described using primers as reported in the Data Supplement, available online at http://atvb.ahajournals.org.14 Enzyme-linked immunosorbent assays were performed as recommended by the supplier (R&D Systems). Splenocyte migration was analyzed according to Guo et al23 and detailed in the supplementary materials. FACS and microarray analyses were performed as described previously and are detailed in the supplementary materials.14,24
Results
SMC TNFR-1 and LTβR Signaling Differentially Activates Classical and Alternative NF-κB Pathways
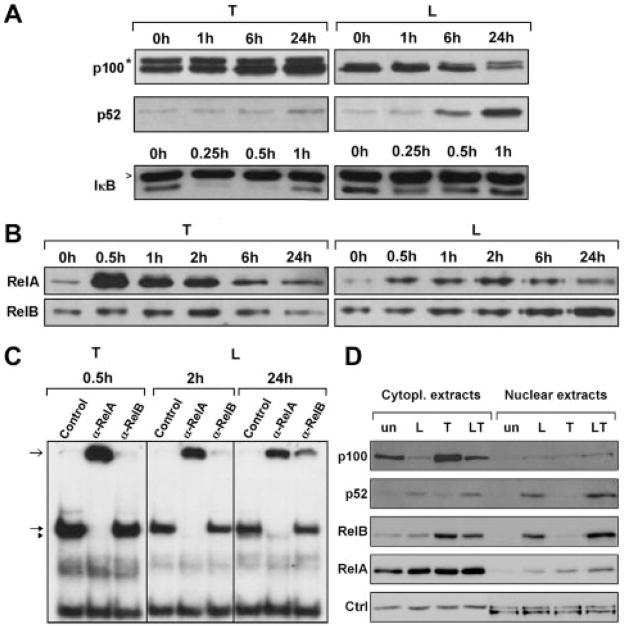
As mouse aorta SMC express TNFR-1 and LTβR in vivo and in vitro,14 we examined their signaling. TNF increased p100 protein levels and triggered rapid and complete IκBα degradation, indicating that TNF activated the classical NF-κB pathway (Figure 1A, left). In contrast, α-LTβR induced processing of the p100 inhibitor to the p52 subunit of NF-κB and triggered a moderate decrease in IκBα levels, indicating that α-LTβR predominantly activated the alternative NF-κB signaling pathway (Figure 1A, right). Furthermore, TNF strongly induced nuclear translocation of RelA within 30 minutes, as shown by Western blotting of nuclear extracts (Figure 1B, left). In contrast, α-LTβR also induced RelA translocation to the nucleus, albeit to a lesser extent, but predominantly induced nuclear translocation of RelB at late time points (Figure 1B, right). Early TNFR-1–induced and delayed LTβR-induced nuclear translocation of RelA and RelB, respectively, was confirmed by immunofluorescence staining of SMC (Figure I). Electrophoretic mobility shift assay supershift analysis confirmed TNF-induced RelA but not RelB NF-κB binding within 30 minutes (Figure 1C, left). In contrast, α-LTβR induced both RelA and RelB complexes, although with slower kinetics (Figure 1C, right). These data show that in SMC TNFR-1 signaling activated the classical NF-κB pathway, whereas LTβR signaling resulted in the activation of classical RelA and alternative RelB NF-κB pathways. Cross-talk of TNFR-1 and LTβR signaling was reported in embryonic fibroblasts to occur through upregulation of RelB and p100 levels by TNF in combination with enhanced p100-to-p52 processing by α-LTβR.25–28 To examine TNFR-1/LTβR signaling cross-talk, we analyzed cytoplasmic and nuclear p100, p52, RelB, and RelA levels after treatment with TNF, α-LTβR, or both. Whereas TNF treatment (24 hours) markedly increased cytoplasmic p100 and RelB levels without inducing nuclear translocation, combined TNFR-1/LTβR stimulation resulted in p100 degradation and significantly increased nuclear accumulation of p52 and RelB compared to LTβR signaling alone. In contrast, nuclear RelA levels were only marginally increased on combined TNFR-1/LTβR activation (Figure 1D). Thus, TNFR-1 and LTβR in SMC differentially engage the classical and alternative NF-κB pathways, respectively, and they synergize to enhance nuclear translocation of p52 and RelB.
Figure 1.
SMC TNFR-1 and LTβR signaling differentially activates classical and alternative NF-κB pathways. SMC were stimulated with 1 ng/mL TNF or 10 μg/mL α-LTβR or buffer for the indicated time points. A, Immunoblot analysis of TNF-induced p100 accumulation and IκBα degradation (left) and α-LTβR–induced p100 to p52 processing (right) in total cell homogenates. *,>Unspecific bands. B, Immunoblot analysis of TNF-induced and α-LTβR–induced nuclear translocation of RelA and RelB in SMC. C, Electrophoretic mobility shift assay/super-shift analysis of TNF-induced RelA complexes and α-LTβR–induced RelA and RelB complexes. Open arrow, shifted complexes; closed arrow, RelA complexes; arrowhead, RelB complexes; control, pre-immune serum. D, Combined stimulation of TNFR-1 and LTβR in SMC hyperinduces p52 and RelB nuclear translocation. Immunoblot analysis of cytoplasmic and nuclear p100, p52, RelB, and RelA levels in untreated (un) and in 24-hour α-LTβR–stimulated (L), TNF-stimulated (T), or α-LTβR+TNF–stimulated (LT) SMC. Actin and polymerase II Western blotting for cytoplasmic and nuclear extracts, respectively (Ctrl in D), or by Coomassie staining of immunoblots (A–C; not shown) served as loading controls.
TNFR-1 and LTβR Signaling Induces Differential Transcription Responses
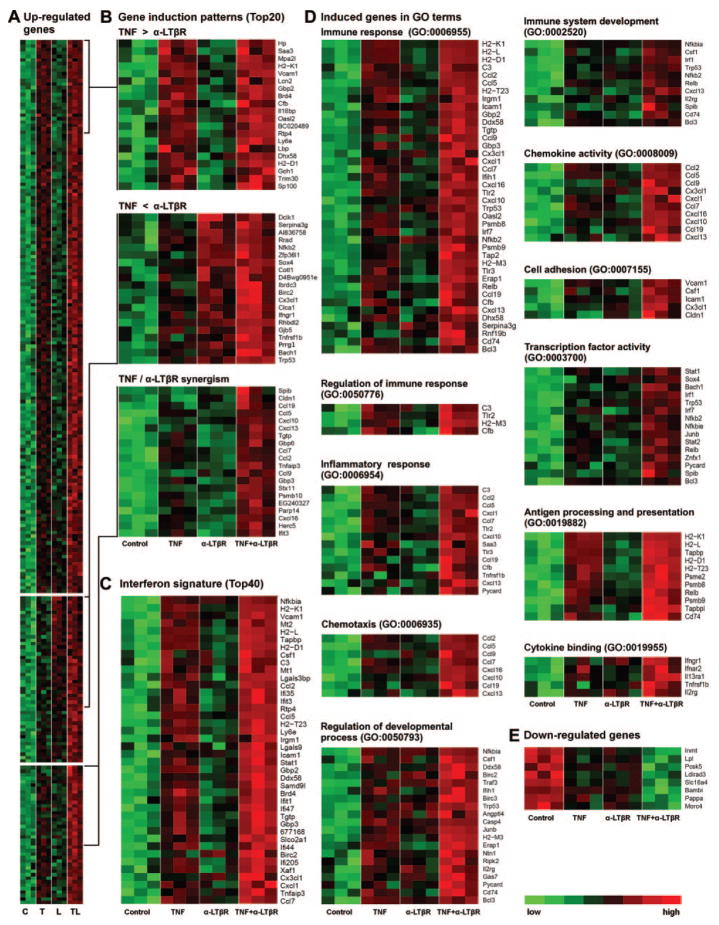
To assess kinetics of TNFR-1– dependent vs LTβR-dependent transcription responses, preliminary microarray screening experiments were performed in single-array experiments. SMC were stimulated with TNF or α-LTβR, and microarrays were prepared at 2, 6, and 24 hours following MIAME guidelines (www.ncbi.nlm.nih.gov/geo/info/MIAME.html; data were deposited in National Center for Biotechnology Information’s gene expression omnibus, GEO accession number GSE19139). TNF induced a stronger response at 2 and 6 hours when compared to 24 hours. In contrast, α-LTβR induced a muted response at 2 and 6 hours and a robust response at 24 hours (Figure IIA–F; Table I). Thus, TNF-induced and LTβR-induced transcription followed kinetics that paralleled their NF-κB signaling kinetics (compare Figure 1A–D and Figure IIA–F). Accordingly, we chose the 24-hour time point to explore transcription after TNFR-1 and LTβR activation in detail. SMC were stimulated with each agonist alone or with a combination of TNF/α-LTβR, and microarrays were prepared. TNF induced 86, α-LTβR induced 23, and both agonists induced 177 genes (Figure III; Table II). These data show that the combination of TNF/α-LTβR elicited transcription of 85 previously untranscribed genes at 24 hours. Moreover, for 40 genes both agonists hyperinduced mRNA expression (Figure 2A; Table III). Three groups of TNF/α-LTβR–induced genes were apparent: TNF-dominated group (Figure 2A; no hyperinduction; TNF>α-LTβR); α-LTβR–dominated group (Figure 2A; no hyperinduction; α-LTβR>TNF); and hyperinduction group (TNF/α-LTβ; R≥1.5-fold the sum of gene expression after single stimulation; Figure 2A; Table III). Thus, concomitant activation of TNFR-1/LTβR hyperinduced transcription of 40 genes and induced transcription of 85 mRNA that were not significantly expressed in quiescent SMC or SMC exposed to each agonist alone.
Figure 2.
TNFR-1 and LTβR activation induces mRNAs encoding lymphorganogenic molecules. A, SMC were stimulated with TNF (T) or α-LTβR (L) or both (LT) as described in Figure 1, and microarrays were prepared after 24 hours. Heat maps (A) represent upregulated genes (left heat map) and (B) the top 20 induced genes of 1 of 3 induction patterns. C, Top 40 of interferon signature derived from www.interferome.com data bank are displayed for ease of reading. D, Induced genes in gene ontology terms. E, Downregulated genes.
Differentiation to LTO-Like SMC
We inspected those genes that showed a statistically significant hyperinduced response29–31 (Figure 2A; Table III). TNF or α-LTβR, when added alone, induced small or no increases in cxcl13 and ccl19 mRNA levels (Figure 2); however, when incubated with TNF and α-LTβR, there was a marked supra-additive increase in cxcl13 and ccl19 mRNA (Figure 2A; lower heat map at right). Similar responses were observed for myeloid homeostatic chemokines ccl2 (MCP-1), ccl5 (RANTES), ccl7 (MCP-3), ccl9 (MIP-1γ), cxcl1 (Groα), cxcl10 (IP10), cxcl16 (SR-PSOX; scavenger receptor for oxidized low-density lipoprotein), and for the interferon-γ–inducible genes gbp3, gbp6, and mpa2l (Figure 2). Hyperinduced mRNA included adhesion molecules vcam1 and icam1 (Figure 2A; Table III). In addition, matching the upregulated genes with public data banks, we observed a large interferon signature (www.interferome.com; for ease of reading, only the top 40 genes are displayed as heat map in Figure 2C; see Table III). This indicates that multiple agonists may affect the LTO phenotype of SMC in addition to TNF/α-LTβR. We next analyzed the induced transcriptomes in a more global way. When gene ontology terms related to immune responses were analyzed, significant numbers of genes were upregulated (Figure 2D). Moreover, when gene ontology terms chemokine activity and cytokine activity were inspected, 10 chemokine genes were hyperinduced (Figure 2D). Notably, and not represented in Figure 2, SMC constitutively express other lymphorganogenic genes at high levels, including tnfr1, ltbr, and cxcl12 (stromal-derived factor 1), whose signal intensities are available at the GEO data bank (GEO accession number GSE19139). Interestingly, chemokine receptor CXCR7 known to bind CXCL12 was found to be the only chemokine receptor to be constitutively expressed by SMC at high levels. Finally, genes in gene ontology terms related to inflammation were markedly induced (Figure 2D). These data show that cross-talk of TNFR-1 and LTβR signaling hyper-induces genes that are known to control a wide range of immune responses, lymphorganogenesis, lymphocyte homeostasis, stromal–lymphocyte interaction, and autoimmunity. To examine the specificity of the α-LTβR reagent (Figures 1, 2) we used SMC prepared from ltbr−/− mice.32,33 The ltbr−/− SMC responded toward TNF but not toward α-LTβR (Figure IV). Applying rather strict filter criteria, a small group of genes was downregulated by TNF, α-LTβR, and, to a larger extent, by a combination of TNF/α-LTβR including lipoprotein lipase (lpl), BMP, and activin membrane-bound inhibitor involved in second heart field mesoderm signaling (bambi), and pregnancy-associated plasma protein A (pappa) associated with atherosclerosis and vascular injury (Figure 2E). These data demonstrate that genes controlling lymphorganogenesis and a variety of known arterial wall biology-related genes in vivo either are constitutively expressed in SMC or are hyperinduced by a combination of TNF/α-LTβR. These transcription responses appear to be SMC-specific, because similar experiments using aortic endothelial cells did not show comparable transcription responses or an LTO phenotype, although several chemokines were induced in both cell types (Figure V; Table IV). Although we found in preliminary experiments that both human aorta and saphenous vein SMC and human umbilical vein endothelial cells expressed both TNFR-1 and LTβR at levels that were similar to those expressed by their mouse aorta counterparts, available reagents to activate the human LTβR yielded weak responses. Unfortunately, this observation precludes a comprehensive examination of a synergistic activation response of TNFR members in primary human vascular cells at this time.
Prolonged Hyperinduction of mRNA by TNF/α-LTβR Signaling
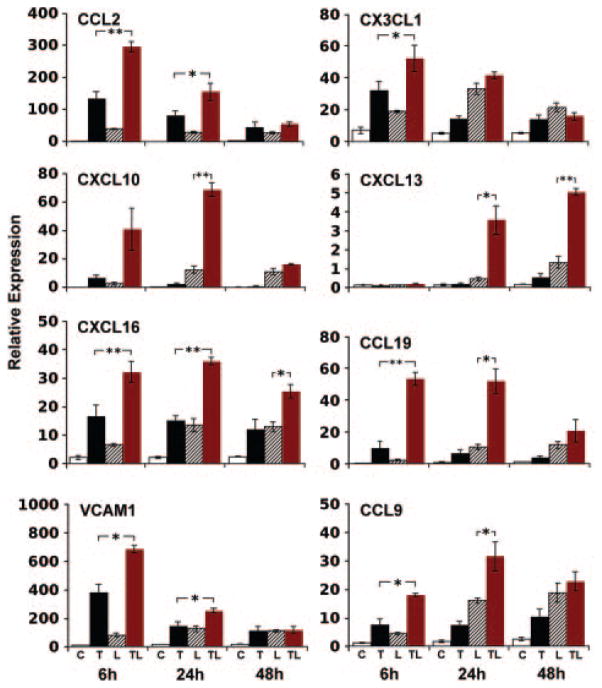
To verify major microarray data, we analyzed mRNA levels of selected genes by quantitative reverse-transcription polymerase chain reaction. For each mRNA, there were small effects when each agonist was added alone but pronounced actions of both agonists (Figure 3). Distinct mRNA showed different kinetics and quantitative responses (Figure 3). Vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 FACS analyses revealed a marked TNF effect for the surface expression of these adhesion molecules (Figure VI). Although ccl21 was found to be expressed by medial SMC in hyperlipidemic mouse aortae in vivo,14 it was not detectable in SMC. This observation is consistent with data from mouse fibroblasts,31,34 suggesting that ccl21 expression cannot be recapitulated under these culture conditions.
Figure 3.
Induction kinetics of inflammatory and lymphorganogenic chemokine and vascular cell adhesion molecule-1 mRNA. SMC were stimulated with agonists as described in Figure 1. At the indicated time points, quantitative reverse-transcription polymerase chain reaction analyses were performed using β-actin as internal control. Data represent means of 3 independent experiments±SEM. Unpaired Student t test. *P<0.05. **P<0.01.
Hyperinduction of De Novo Synthesis and Prolonged Secretion of CCL5, CX3CL1, CXCL13, and CCL19
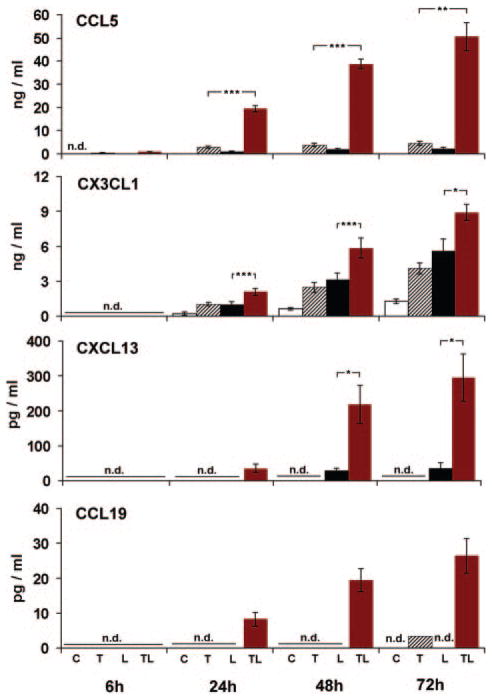
We chose selected lymphorganogenic genes to examine protein hyperinduction. CCL5 determined at 6 hours was undetectable in control or in α-LTβR–stimulated SMC but was secreted in TNF-stimulated SMC at low levels and progressively increased in TNF/α-LTβR–stimulated SMC (Figure 4). For CXCL13 and CCL19, the kinetics of chemokine accumulation were comparable to CCL5 with a pronounced hyperinduction. Similarly, CX3CL1 was absent at 6 hours but it became detectable after 24 hours of TNF/α-LTβR stimulation, further increasing up to 72 hours (Figure 4).
Figure 4.
Hyperinduction of de novo synthesis and prolonged secretion of CCL5, CX3CL1, CXCL13, and CCL19 by TNF/α-LTβR activation. SMC were stimulated with TNF, α-LTβR, or both, as described in Figure 1. At the indicated time points, chemokine levels in supernatants were determined by enzyme-linked immunosorbent assays. Data represent means of 3 or 5 (6 hours, n=3; 24 hours, n=5; 48 hours, n=5; 72 hours, n=3) independent SMC cultures±SEM. Student unpaired t test. *P<0.05. **P<0.01. ***P<0.001. Only probability values for chemokine levels with the highest values are shown for reasons of clarity. At 72 hours, in 2 experiments, SMC that received TNF CCL19 levels were below the detection limit and the CCL19 level within the detection limit is displayed for the remaining data point.
Activated SMC Promote Migration of Naive Splenic T Cells, B Cells, and Macrophages/DC Through Soluble Chemotactic Molecules
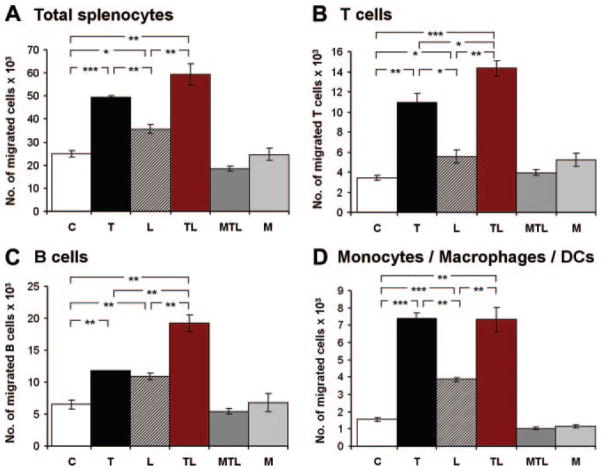
To examine whether the LTO SMC phenotype (Figures 1–4) resulted in biological activity toward leukocytes, supernatants of SMC were examined in a migration assay using naive splenocytes from young C57BL/6J mice as targets. There was no difference in chemotactic activity between cell-free medium and unstimulated SMC, indicating that quiescent SMC did not elaborate significant chemotactic activity toward total splenocytes (Figure 5, upper left). However, TNF, α-LTβR, and both TNF/α-LTβR caused elaboration by SMC of marked migration activity toward total splenocytes (Figure 5, upper left). In view of our hypothesis that SMC may acquire an LTO-like phenotype, we next examined the leukocyte lineage composition of migrated cells by FACS using CD3 for total T cells, CD19 for B cells, and CD11b for macrophages/DC. For splenic T and B lymphocytes, there was a significant effect for TNF and α-LTβR, and a supra-additive effect of the combination of TNF/α-LTβR (Figure 5, upper right and lower left). Moreover, splenic CD11b+ macrophages/DC strongly responded to supernatants of SMC stimulated with TNF, α-LTβR, and both agonists (Figure 5, lower right). These data demonstrate that activated—but not quiescent—SMC elaborate soluble chemotactic molecules toward T cells, B cells, and macrophages/DC.
Figure 5.
Activated—but not quiescent—SMC promote chemotaxis of splenic T cells, B cells, and monocytes/macrophages/DC. SMC were cultured and stimulated with TNF, α-LTβR, or both, as described in Figure 1. After 48 hours, supernatants were collected and examined for migration activity as described in Materials and Methods. C, control SMC; T, TNF-stimulated SMC; L, α-LTβR-stimulated SMC; TL, TNF/α-LTβR-stimulated SMC; MTL, medium+ TNF/α-LTβR; M, medium. Numbers represent means of triplicate migration chambers±SEM of a representative experiment (3 independent experiments of 2 independent SMC preparations). Student t test. *P ≤0.05. **P≤0.01. ***P≤0.001.
Discussion
Here, we show that mouse aorta SMC stimulated with TNF/α-LTβR differentiate into a phenotype that strikingly resembles LTO.10, 18–20, 35 The LTO phenotype resulted from cross-talk of the classical and alternative NF-κB signaling pathways, most likely through RelA and RelB dimer complex-mediated transcription at target gene promoters. The majority of genes with additive, synergistic, and newly recruited mRNA induction patterns mediate inflammation, leukocyte adhesion, autoimmunity, lymphocyte homeostasis, or lymphoid organ neogenesis in vivo6,31,36–41 (Table V). These data strongly support our hypothesis that activated SMC may participate in the immunologic characteristics of the diseased arterial wall by defining a signaling pathway, its resulting transcription responses, and the elaboration of chemotactic activity toward 3 leukocyte lineages involved in inflammation and tertiary lymphoid organogenesis (Figure 6).
Figure 6.

Cross-talk of NF-κB pathways in mouse aorta SMC stimulated with TNF and α-LTβR generates a phenotype resembling LTO. Cross-talk between TNFR-1–induced and LTβR-induced NF-κB target gene activation in SMC is likely to be attributable to p100 and RelB accumulation in response to TNF. Subsequent LTβR-mediated processing of the p100 inhibitor-to-p52 and nuclear translocation of p52-RelB complexes activates expression of molecules involved in adaptive immune responses, lymphorganogenic chemokines, and adhesion molecules in SMC.
During development10 and maintenance of secondary lymphoid organs, hematopoietic cells identified as lymphoid tissue-inducer cells interact with mesenchymal LTO to coordinate lymphoid organ growth and organization. LTO in these organs include myofibroblastic reticular cells in T-cell areas.10 Yet, the identity of the lymphoid tissue-inducer cells in the artery wall that interact with medial SMC-differentiated LTO remains to be uncovered.14 Our data provide the first comprehensive delineation of transcriptional cross-talk after combined TNFR-1/LTβR activation in any cell type and confirm and extend previous in vivo data of activated SMC by defining a selected set of genes that might mediate tertiary lymphoid organogenesis in atherosclerosis.
Conclusion
The hyperinduction of SMC genes coding for lymphorganogenic chemokines provides strong support for the cross-talk via the NF-κB signaling network model proposed by Basak and Hoffmann.26–28 The conclusion that the alternative LTβR NF-κB pathway was critical in determining the LTO-like phenotype was based on pharmacological (α-LTβR) and genetic (ltbr−/− SMC) evidence together with the finding that TNFR-1 activation triggered the classical but not the alternative NF-κB pathway, whereas LTβR activation triggered both.
Given the complexities of NF-κB signaling cross-talk in SMC and the known activity of NF-κB not only in SMC but also in endothelial cells and macrophage/foam cells, it is not surprising that the role of NF-κB in atherogenesis has produced controversial results in hyperlipidemic mouse models.42–46 The remarkable ability of SMC to modify their phenotype in vivo depending on environmental conditions has been widely recognized.3–5 Our data provide evidence for a new role of media SMC, ie, to function as LTO that translate inflammatory cues from plaques and convey them as lymphorganogenic signals to the adventitia.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the German Research Organization (HA 1083/15-1; WE2224/5; SP 713/4-1) to A.J.R.H., R.G., F.W., and R.S.
Footnotes
Supplement information is available on the ATVB web site (http://atvb.ahajournals.org). Microarray data have been deposited in NCBI GEO (www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession number GSE19139. GEO makes supplement files available for FTP download by series accession at the following FTP site: ftp://ftp.ncbi.nih.gov/pub/geo/DATA/supplement/series.
Disclosure
None.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 4.Raines EW, Ferri N. Thematic review series: The immune system and atherogenesis. Cytokines affecting endothelial and smooth muscle cells in vascular disease. J Lipid Res. 2005;46:1081–1092. doi: 10.1194/jlr.R500004-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 6.Zeiffer U, Schober A, Lietz M, Liehn EA, Erl W, Emans N, Yan ZQ, Weber C. Neointimal smooth muscle cells display a proinflammatory phenotype resulting in increased leukocyte recruitment mediated by P-selectin and chemokines. Circ Res. 2004;94:776–784. doi: 10.1161/01.RES.0000121105.72718.5C. [DOI] [PubMed] [Google Scholar]
- 7.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 9.Randolph GJ, Jakubzick C, Qu C. Antigen presentation by monocytes and monocyte-derived cells. Curr Opin Immunol. 2008;20:52–60. doi: 10.1016/j.coi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Moos MP, Grabner R, Pedrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lotzer K, Huang L, Cui J, Rader DJ, Evans JF, Habenicht AJ, Funk CD. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 12.Moos MP, John N, Grabner R, Nossmann S, Gunther B, Vollandt R, Funk CD, Kaiser B, Habenicht AJ. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 13.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, Fu YX, Hehlgans T, Mebius RE, van der Wall M, Kruspe D, Englert C, Lovas A, Hu D, Randolph GJ, Weih F, Habenicht AJ. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206:233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 16.Ettinger R, Munson SH, Chao CC, Vadeboncoeur M, Toma J, McDevitt HO. A critical role for lymphotoxin-beta receptor in the development of diabetes in nonobese diabetic mice. J Exp Med. 2001;193:1333–1340. doi: 10.1084/jem.193.11.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weyand CM, Kurtin PJ, Goronzy JJ. Ectopic lymphoid organogenesis: a fast track for autoimmunity. Am J Pathol. 2001;159:787–793. doi: 10.1016/S0002-9440(10)61751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181:6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- 19.Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol. 2005;174:21–25. doi: 10.4049/jimmunol.174.1.21. [DOI] [PubMed] [Google Scholar]
- 20.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 21.Braun A, Takemura S, Vallejo AN, Goronzy JJ, Weyand CM. Lymphotoxin beta-mediated stimulation of synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2004;50:2140–2150. doi: 10.1002/art.20356. [DOI] [PubMed] [Google Scholar]
- 22.Stopfer P, Mannel DN, Hehlgans T. Lymphotoxin-beta receptor activation by activated T cells induces cytokine release from mouse bone marrow-derived mast cells. J Immunol. 2004;172:7459–7465. doi: 10.4049/jimmunol.172.12.7459. [DOI] [PubMed] [Google Scholar]
- 23.Guo F, Weih D, Meier E, Weih F. Constitutive alternative NF-kappaB signaling promotes marginal zone B-cell development but disrupts the marginal sinus and induces HEV-like structures in the spleen. Blood. 2007;110:2381–2389. doi: 10.1182/blood-2007-02-075143. [DOI] [PubMed] [Google Scholar]
- 24.Uzonyi B, Lotzer K, Jahn S, Kramer C, Hildner M, Bretschneider E, Radke D, Beer M, Vollandt R, Evans JF, Funk CD, Habenicht AJ. Cysteinyl leukotriene 2 receptor and protease-activated receptor 1 activate strongly correlated early genes in human endothelial cells. Proc Natl Acad Sci U S A. 2006;103:6326–6331. doi: 10.1073/pnas.0601223103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yilmaz ZB, Weih DS, Sivakumar V, Weih F. RelB is required for Peyer’s patch development: differential regulation of p52-RelB by lymphotoxin and TNF. Embo J. 2003;22:121–130. doi: 10.1093/emboj/cdg004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basak S, Kim H, Kearns JD, Tergaonkar V, O’Dea E, Werner SL, Benedict CA, Ware CF, Ghosh G, Verma IM, Hoffmann A. A fourth IkappaB protein within the NF-kappaB signaling module. Cell. 2007;128:369–381. doi: 10.1016/j.cell.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basak S, Shih VF, Hoffmann A. Generation and activation of multiple dimeric transcription factors within the NF-kappaB signaling system. Mol Cell Biol. 2008;28:3139–3150. doi: 10.1128/MCB.01469-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basak S, Hoffmann A. Crosstalk via the NF-kappaB signaling system. Cytokine Growth Factor Rev. 2008;19:187–197. doi: 10.1016/j.cytogfr.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suto H, Katakai T, Sugai M, Kinashi T, Shimizu A. CXCL13 production by an established lymph node stromal cell line via lymphotoxin-beta receptor engagement involves the cooperation of multiple signaling pathways. Int Immunol. 2009;21:467–476. doi: 10.1093/intimm/dxp014. [DOI] [PubMed] [Google Scholar]
- 30.Kutsch S, Degrandi D, Pfeffer K. Immediate lymphotoxin beta receptor-mediated transcriptional response in host defense against L. monocytogenes. Immunobiology. 2008;213:353–366. doi: 10.1016/j.imbio.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Hara T, Katakai T, Lee JH, Nambu Y, Nakajima-Nagata N, Gonda H, Sugai M, Shimizu A. A transmembrane chemokine, CXC chemokine ligand 16, expressed by lymph node fibroblastic reticular cells has the potential to regulate T cell migration and adhesion. Int Immunol. 2006;18:301–311. doi: 10.1093/intimm/dxh369. [DOI] [PubMed] [Google Scholar]
- 32.Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, Rajewsky K, Nedospasov SA, Pfeffer K. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci U S A. 1997;94:9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 34.Lovas A, Radke D, Albrecht D, Yilmaz ZB, Moller U, Habenicht AJ, Weih F. Differential RelA- and RelB-dependent gene transcription in LTbetaR-stimulated mouse embryonic fibroblasts. BMC Genomics. 2008;9:606. doi: 10.1186/1471-2164-9-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 36.Heller EA, Liu E, Tager AM, Yuan Q, Lin AY, Ahluwalia N, Jones K, Koehn SL, Lok VM, Aikawa E, Moore KJ, Luster AD, Gerszten RE. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 37.Sheikine Y, Sirsjo A. CXCL16/SR-PSOX–a friend or a foe in atherosclerosis? Atherosclerosis. 2008;197:487–495. doi: 10.1016/j.atherosclerosis.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig A, Weber C. Transmembrane chemokines: versatile ‘special agents’ in vascular inflammation. Thromb Haemost. 2007;97:694–703. [PubMed] [Google Scholar]
- 39.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martinez AC, Mackay CR, Mackay F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol. 2008;28:1897–1908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- 41.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 42.Blessing E, Bea F, Kuo CC, Campbell LA, Chesebro B, Rosenfeld ME. Lesion progression and plaque composition are not altered in older apoE−/− mice lacking tumor necrosis factor-alpha receptor p55. Atherosclerosis. 2004;176:227–232. doi: 10.1016/j.atherosclerosis.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 43.Schreyer SA, Vick CM, LeBoeuf RC. Loss of lymphotoxin-alpha but not tumor necrosis factor-alpha reduces atherosclerosis in mice. J Biol Chem. 2002;277:12364–12368. doi: 10.1074/jbc.M111727200. Epub 12002 Jan 12323. [DOI] [PubMed] [Google Scholar]
- 44.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Peppel K, Sivashanmugam P, Orman ES, Brian L, Exum ST, Freedman NJ. Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1087–1094. doi: 10.1161/ATVBAHA.0000261548.49790.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de Winther MP, Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.