Abstract
The role of vasculogenesis as opposed to angiogenesis in tumor formation has been little explored genetically. Endothelial cells that lack the MEK kinase MEKK3 cannot form vessels. In this study, we employed mice with hematopoietic deletions of the Mekk3 gene to evaluate the importance of vasculogenesis in the formation of Ewing’s sarcoma tumors. Bone marrow cells (BM) from LacZ+ Mekk3-deficient conditional knockout mice (Mekk3Δflox/− mice) were transplanted into irradiated nude mice before injection of Ewing’s sarcoma cells. Because the grafted Mekk3Δflox/− BM cells cannot contribute to vessel development in the same way as the host Mekk3+/+ endothelial cells, angiogenesis is normal in the model whereas vasculogenesis is impaired. Four weeks after BM transplant, Ewing’s sarcoma TC71 or A4573 cells were injected, and tumor growth and vessel density were compared. Strikingly, chimeric mice transplanted with Mekk3Δflox/− BM exhibited a reduction in tumor growth and vessel density compared with mice transplanted with Mekk3Δflox/+ BM cells. Mekk3Δflox/− cells that were LacZ positive were visualized within the tumor; however, few of the LacZ+ cells colocalized with either CD31+ endothelial cells or desmin+ pericytes. Quantification of double-positive LacZ+ and CD31+ endothelial cells or LacZ+ and desmin+ pericytes confirmed that chimeric mice transplanted with Mekk3Δflox/− BM were impaired for tumor vessel formation. In contrast, siRNA-mediated knockdown of Mekk3 in TC71 Ewing’s sarcoma cells had no effect on tumor growth or vessel density. Our findings indicate that vasculogenesis is critical in the expansion of the tumor vascular network.
Introduction
New blood vessel formation is critical for tumor growth and metastasis (1). Endothelial cells play an essential role in this process (2–5). We previously showed that in addition to angiogenesis, the migration of bone marrow (BM) cells into the Ewing’s sarcoma tumor and their subsequent differentiation into endothelial cells and pericytes (vasculogenesis) contributes to the growth of Ewing’s sarcoma (6, 7). It is well established that inhibiting angiogenesis suppresses tumor growth (8, 9). However, the consequences of blocking vasculogenesis are unclear. Evaluating the importance of BM cells and vasculogenesis to tumor growth requires an in vivo model in which vasculogenesis is selectively blocked but local angiogenesis can occur. Mitogen-activated protein kinases are common mediators in the signal transduction pathways from membrane to nucleus. These kinases sequentially activate the downstream kinases and relay signals from extracellular agonists to the designated targets. Mekk3 is a Ser/Thr protein kinase of the mitogen-activated protein kinase kinase kinase gene family (10, 11). Mekk3 is involved in early embryonic cardiovascular and blood vessel development. Deletion of Mekk3 is embryonically lethal at embryonic day (E) 9.5 (12). The Mekk3-deficient yolk sacs have few or no vessels, indicating that this gene plays an essential role in embryonic blood vessel formation. Moreover, cells that lack Mekk3 do not form normal vessels.
To evaluate the importance of vasculogenesis in the growth and development of Ewing’s sarcoma, we used Mekk3-deleted BM cells from Mekk3Δflox/− conditional knockout mice as the donor cells to reconstitute irradiated Mekk3+/+ nude mice before tumor injection. Our rationale was that Mekk3-deleted (Mekk3Δflox/−) BM cells cannot participate in vasculogenesis, yielding a model in which vasculogenesis is blocked in the experimental group but angiogenesis in both the experiment and control groups is equal. If vasculogenesis is critical, tumor growth in the Mekk3Δflox/− BM–transplanted mice will be smaller compared with tumors from Mekk3Δflox/+ BM–transplanted mice. Here, we show that Mekk3Δflox/− BM cells do not participate in tumor vessel formation and that tumor growth is significantly impaired when vasculogenesis is inhibited.
Materials and Methods
Mice
Tie2-GFP-Mekk3+/− mice
Tie2-GFP mice express green fluorescent protein (GFP) under the direction of the endothelial-specific receptor tyrosine kinase (Tek, formerly, Tie2) promoter (13). Tie2-GFP mice were crossed with Mekk3+/− mice (12) to create Tie2-GFP-Mekk3+/− mice. Male and female Tie2-GFP-Mekk3+/− mice were set up for time mating to get Tie2-GFP-Mekk3+/− or Tie2-GFP-Mekk3−/− day 9.5 embryos.
Mekk3 flox/+-Cre-ER™-R26R mice
Rosa26R mice (14) were purchased from The Jackson Laboratory. Mekk3flox/− mice (15) were crossed with Rosa26R mice to generate Mekk3flox/−/Rosa26R mice. Mekk3+/− mice were crossed with Cre-ER mice to generate Cre-ER/Mekk3+/− mice. These mice were then crossed with Mekk3flox/− Rosa26R mice to yield Mekk3flox/+-Cre-ER™-R26R and Mekk3flox/− Cre-ER™-R26R mice Before BM transplantation, these mice were injected i.p. with tamoxifen (2 mg) in 100 μL corn oil every 48 h for 14 d to delete the floxed-Mekk3 gene, generating Mekk3Δflox/+ and Mekk3Δflox/− mice. The Cre recombinase activation after tamoxifen was monitored by lacZ expression resulting from the deletion of a loxp-flanked DNA STOP sequence upstream of the lacZ gene at the Rosa26 locus (14, 16).
Isolation of Mekk3+/− Tie2-GFP and Mekk3−/− Tie2-GFP Endothelial Cells from E9.5–10.5 Embryos and Yolk Sacs
To obtain Tie2-GFP-Mekk3+/− and Tie2-GFP-Mekk3−/− embryos and yolk sacs, Tie2-GFP-Mekk3+/− female mice were set up for time mating with Tie2-GFP-Mekk3+/− male mice (12). Vaginal-plugged female mice were sacrificed on day 9.5–10.5. Embryos and yolk sacs were dissected to remove decidu and Reichert’s membrane. A small piece of yolk sac was removed from each embryo for immediate PCR genotyping. After PCR genotyping, Tie2-GFP-Mekk3+/− and Tie2-GFP-Mekk3−/− yolk sacs were minced into small pieces by repetitive pipetting before incubating with 1 mL of digestion cocktail containing 2 μg/mL collagenase A and 25 μg/mL DNase A (Sigma Chemical) in DMEM (Invitrogen/Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 1 mmol/L sodium pyruvate, 2× MEM vitamins, 1× nonessential amino acids, and 2 mmol/L glutamine (Invitrogen/Life Technologies). The cells were cultured for 1 wk. Endothelial cells and endothelial precursor cells were isolated by sorting for GFP+ cells (17).
Vessel Formation Assay In vivo
GFP+ endothelial cells (5 × 105) isolated from Tie2-GFP-Mekk3+/− or Tie2-GFP-Mekk3−/− embryos and yolk sacs were placed in 0.5 mL of Matrigel (BD Biosciences-Discovery Lab-ware). This Matrigel was injected s.c. into nude mice. Mice were sacrificed 4 wk later. Matrigel plugs were removed, frozen, and analyzed by immunohistochemistry using rat anti-mouse CD31 (BD Bioscience Pharmingen).
Western Blot Analysis
BM cells, heart, lung, kidney, and liver from the Mekk3Δflox/+ and Mekk3Δflox/− mice were homogenized in Triton X-100 lysis buffer with protease and phosphatase inhibitors, and supernatants were harvested. The protein content of each lysate was determined using the Bradford reagent (Bio-Rad Lab). Tissue lysate (30 μg) was resolved by SDS-PAGE. The gels were then electroblotted onto Hybond-ECL nitrocellulose membranes (Amersham). Specific protein bands were detected with monoclonal mouse anti-Mekk3 antibody (BD Transduction Laboratories). Protein loading was assessed with the monoclonal β-actin antibody (Sigma Chemical) using chemiluminescence and Western blotting analysis (Amersham).
PCR Analysis for Mekk3 Genotypes
Genomic DNA from 7-day-old mice tails or BM from donor and recipient mice were isolated and amplified by PCR. The PCR primers used were as follows: P1 (5′-TCGCAGCGCATCGCCTTC-3′), P2 (5′-ATGTGAAGCTTGGGGATTTTG-3′), P3 (5′-TGGTTAGACTCACTGGTCAGAGAC-3′), and P4 (5′-TTGTGCATCGGGACATCA-3′). Primer combination P1, P2, and P3 yields amplified fragments of 1,000 bp (Mekk3 floxed), 900 bp (Mekk3 wild-type), and 830 bp (Mekk3 knockout). Primer combination P3 and P4 yields amplified fragments of 1,500 bp (Mekk3 floxed), 1,300 bp (Mekk3 wild-type), and 650 bp (Mekk3 Δfloxed), which represents the deleted allele following tamoxifen treatment. Two separate PCR assays are necessary because one assay cannot detect all four alleles.
Matrigel Plug Assay
Mekk3Δflox/+ and Mekk3Δflox/− mice were injected s.c. at the abdominal midline with 0.6 mL of Matrigel (BD Biosciences-Discovery Labware). Tamoxifen (2 mg) was administered daily for 14 d. Mice were sacrificed, and the Matrigel plugs were removed, frozen, fixed with acetone and chloroform, incubated in 4.5% fish gelatin (Aurion) for 20 min to block nonspecific protein, and then analyzed by immunohistochemistry. Blood vessels were visualized by immunohistochemistry using rat anti-mouse CD31 as the primary antibody and goat anti-rat Texas red (Jackson Immunoresearch Lab) as the secondary antibody. Hoechst 33342 (1:10,000 in PBS; Molecular Probes) was used for nuclear staining. The mean vessel density (MVD) was determined by averaging the number of CD31+ vessels in five random high-power microscopic fields from different Matrigel sections.
Cell Lines and Lewis Lung Carcinoma 3LL Mouse Tumor Model
TC71 and A4573 human Ewing’s sarcoma cells and Lewis lung carcinoma 3LL cells were cultured as previously described (6, 7, 18). 3LL cells (105 in 0.1 mL) were injected into the right and left flanks in Mekk3Δflox/+ Cre-ER-R26R and Mekk3Δflox/− Cre-ER-R26R mice from the same litter.
Ewing’s Sarcoma Mouse Model and BM Transplantation
BM cells from the Mekk3flox/+ Cre-ER-R26R and Mekk3flox/− Cre-ER-R26R mice are LacZ+. These mice were treated with 2 mg of tamoxifen every 48 h for 14 d to achieve deletion of the floxed Mekk3. BM from Mekk3Δflox/+ and Mekk3Δflox/− mice were obtained by flushing the femur and tibia with PBS containing 5% fetal calf serum. Freshly isolated BM cells (5 × 106 in 100 μL PBS) were injected into the lateral tail vein of 6-wk-old female nude mice that had been irradiated with 8.0 Gy using an external cesium source (137Cs Mark 1 irradiator, J.L. Shepherd & Associates).
Four weeks after BM transplantation, mice were injected s.c. with 2 × 106 TC71 or A4573 cells in 100 μL of PBS. Mekk3Δflox/+ and Mekk3Δflox/− BM–transplanted mice were injected with 2 mg of tamoxifen every 48 h to maintain deletion of floxed Mekk3, thereby maintaining the Mekk3Δflox/+ genotypes and Mekk3Δflox/− genotypes. Tumor diameter was measured every other day with calipers. Tumor volumes were calculated as v = 1/2ab2, in which a is the longer diameter and b is the shorter diameter. The chimeric mice were euthanized 3 wk after tumor cell injection. Tumor tissue was analyzed by immunohistochemistry.
Immunohistochemistry and LacZ Staining
Tumor specimens from Mekk3Δflox/+ and Mekk3Δflox/− BM–transplanted mice were fixed and cryoprotected in sucrose (35%) overnight, embedded in Tissue-Tek optimum cutting temperature compound, and frozen. LacZ staining was done (19–21). Immunohistochemistry using anti–PECAM-1/CD31 antibody (clone MEC13.3; Pharmingen) or anti-desmin (Ab-cam) on LacZ-stained tissues was performed followed by counterstaining with nuclear fast red (17, 22). The MVD and mural cell density was determined by averaging the number of CD31 or desmin and LacZ double-positive cells in five random high-power microscopic fields from different tumors.
Expression Plasmids and Transfection
siRNA expression vector pSilencer2.1-U6 hygro was purchased from Ambion. siRNA-expressing plasmids targeting human Mekk3 mRNA GTGCAGAAAATTCCTTGTC (23) were annealed at 90°C for 3 min, cooled to 37°C, and incubated for 1 h. The annealed dsDNA oligonucleotides were ligated between BamHI and HindIII sites on the pSilencer2.1-U6 hygro vector. The control vector (si-) was constructed by inserting a sequence that expresses an siRNA with limited homology to sequences in the human and mouse genomes. Transfection of TC71 cells was performed with Lipofectamine 2000 (Invitrogen/Life Technologies). Transfected clones were selected in 400 μg/mL hygromycin B (Invitrogen/Life Technologies). Human Mekk3 expression in the stable clones was quantified by Western blot.
In vivo Experiments Using TC71/Mekk3 si-transfected Clones
TC71, TC71/si-control, TC71/Mekk3 si-clone 8, or TC71/Mekk3 si-clone 10 cells (2 × 106) were injected s.c. into four groups of nude mice. Tumor growth was quantified every other day. All the tumors were harvested on day 27 and frozen sections were stained using rat anti-mouse CD31 (primary antibody) and goat anti-rat Texas red (secondary antibody). Hoechst 33342 (1:10,000 in PBS) was used for nuclear staining. The MVD was determined by averaging the number of CD31+ cells in five random high-power microscopic fields. Apoptotic cells were quantified using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling.
Results
Mekk3 deficiency inhibits the formation of normal vessel structures
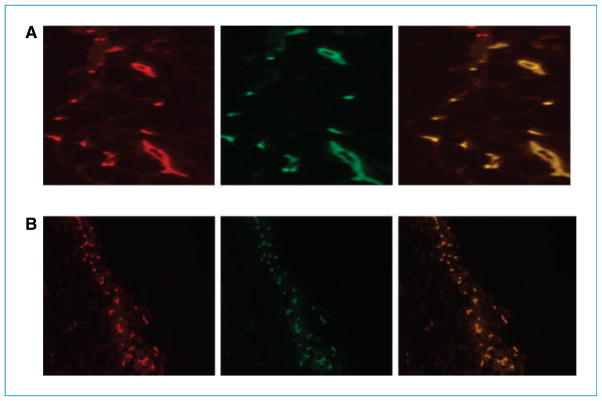
Endothelial cells from Tie2-GFP-Mekk3+/− and Tie2-GFP-Mekk3−/− E9.5 yolk sacs were isolated and injected with Matrigel s.c. into nude mice. Vessel formation was assessed after 2 weeks. Mekk3+/− endothelial cells formed typical round vessels (Fig. 1A), whereas Mekk3−/− endothelial cells failed to form vessel structures (Fig. 1B).
Figure 1.
Mekk3 plays a critical role in the formation of normal vessel structures. E9.5 yolk sacs from (A) Tie2-GFP-Mekk3+/− or (B) Tie2-GFP-Mekk3−/− endothelial cells in Matrigel were injected into nude mice. Vessel formation was assessed 2 wk later by immunohistochemistry. Left, CD31 (red); middle, GFP (green); right, merged image showing colocalization (orange). Immunofluorescent microscopy was performed using a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Inc.) equipped with a 100-W Hg lamp and narrow bandpass excitation filters. Representative images were obtained using a cooled charge-coupled device Hamamatsu C5810 camera (Hamamatsu Photonics) and Optimas software (Media Cybernetics). Composite images were constructed with Photoshop software (Adobe Systems, Inc.). Original magnification, ×200.
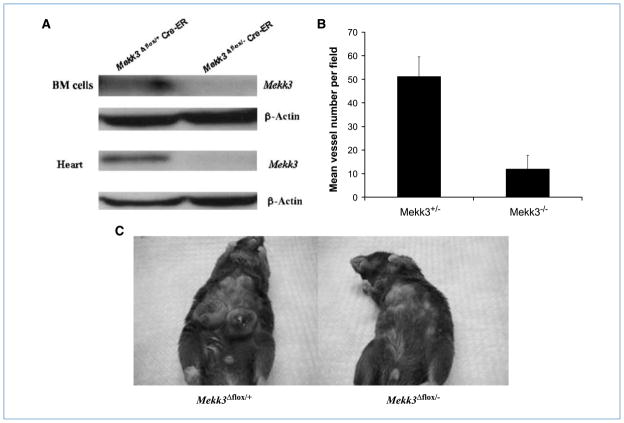
Because deletion of Mekk3 is embryonically lethal (12), we elected to use tamoxifen-dependent, Cre-mediated Mekk3 deletion in Mekk3flox/− Cre-ER-R26R mice to obtain Mekk3-deleted (Mekk3Δflox/−) BM cells. Mekk3Δflox/+ BM cells from tamoxifen-treated Mekk3flox/+ Cre-ER-R26R mice were used as the control. To confirm Mekk3 deletion, mice were sacrificed 2 weeks after tamoxifen treatment. Western blotting showed no Mekk3 in BM cells, heart (Fig. 2A), lung, kidney, and liver (data not shown) from the Mekk3Δflox/− mice. By contrast, MEKK3 was present in the cells and organs taken from the Mekk3Δflox/+ mice.
Figure 2.
Tamoxifen-induced deletion of Mekk3 in Mekk3flox/− Cre-ER mice results in decreased vessel formation and tumor growth in vivo. BM cells and heart (A) were obtained from Mekk3flox/+ Cre-ER and Mekk3flox/− Cre-ER mice 2 wk after tamoxifen administration. The resulting Mekk3Δflox/+ and Mekk3Δflox/− cells were assessed for Mekk3 expression by Western blot. B, following tamoxifen treatment, mice were injected with Matrigel. The Matrigel plugs were excised 2 wk later and assessed by immunohistochemistry using CD31. The average number of CD31+ vessels was calculated by counting five random high-power microscopic fields from different Matrigel plug sections. C, 3LL cells were injected into the Mekk3Δflox/+ and Mekk3Δflox/− mice 2 wk after tamoxifen administration. Tumor growth was assessed 3 wk after tumor cell injection. Error bars represent SD.
Having confirmed that we can successfully create Mekk3Δflox/− mice with no cellular expression of MEKK3, we next evaluated whether new blood vessel formation could be induced. Anti-CD31 staining was assessed 2 weeks after Matrigel implantation. Matrigel plugs excised from Mekk3Δflox/− mice showed a significant decrease in vessel density compared with those from Mekk3Δflox/+ control mice (Fig. 2B). These data confirm that the ability of endothelial cells to form vessels is impaired following deletion of Mekk3. To confirm that tumor vessel formation was also impaired in mice in which Mekk3 was deleted, we injected 3LL lung cancer cells into Mekk3Δflox/− and Mekk3Δflox/+ control mice. Rapid tumor growth was seen in the Mekk3Δflox/+ control mice. By contrast, no tumor growth was seen in the Mekk3Δflox/− mice, confirming that this gene is also critical for tumor vessel formation (Fig. 2C). This Mekk3Δflox/− mouse model, however, could not be used to assess the importance of vasculogenesis in tumor vessel development because both angiogenesis and vasculogenesis are inhibited. Furthermore, no assessment could be performed with regard to Ewing’s sarcoma, as human tumor cells will not grow in immunocompetent mice.
Deletion of Mekk3 in BM cells inhibits vasculogenesis and tumor growth
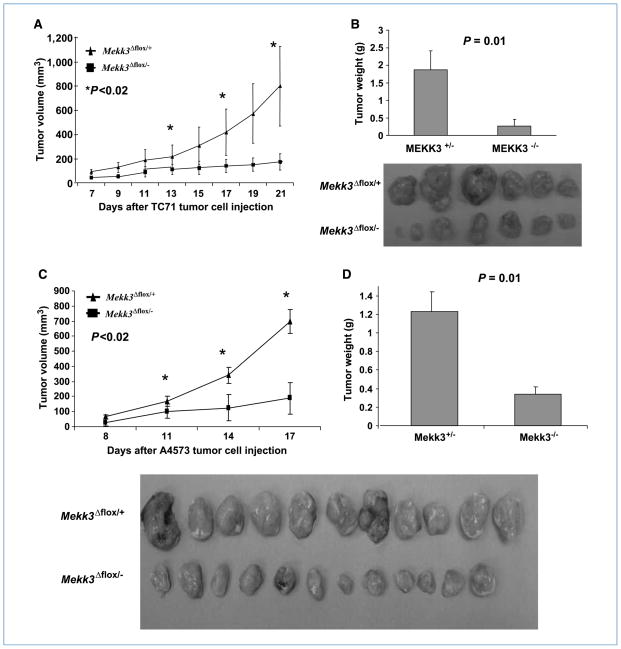
We showed that Mekk3-deficient cells do not form blood vessels and that Mekk3-deficient mice do not support tumor growth. We next used our previously described Ewing’s sarcoma transplant model (6) to selectively inhibit vasculogenesis to determine the importance of BM cells in the formation of tumor blood vessels. Recipient nude mice were lethally irradiated and then transplanted with BM cells from either the Mekk3Δflox/+ or Mekk3Δflox/− mice. The absence of Mekk3 in the Mekk3Δflox/− BM cells was confirmed as described in Fig. 2A before transplant. Mekk3Δflox/+ BM cells were positive for Mekk3 and served as the control transplant cells. Engraftment was confirmed 4 weeks after transplant by demonstrating the absence of Mekk3 in the BM cells of the Mekk3Δflox/− BM–transplanted mice (Fig. 3). Mice were then injected with TC71 or A4573 cells, and tumor growth was monitored. Because nude mice are Mekk3+/+, the local endothelial cells can participate in vascular expansion. However, because the Mekk3Δflox/− BM cells lack Mekk3, their ability to contribute to tumor vessel formation (vasculogenesis) is impaired. This experimental design results in selective inhibition of vasculogenesis. Both TC71 and A4573 tumor growth were impaired in the Mekk3Δflox/− BM–transplanted mice compared with the mice transplanted with Mekk3Δflox/+ BM cells (Fig. 4A and C). The tumors from the Mekk3Δflox/− BM–transplanted mice remained small and stable over 17 to 21 days, and seemed to be less vascular in addition to being smaller (Fig. 4B and D).
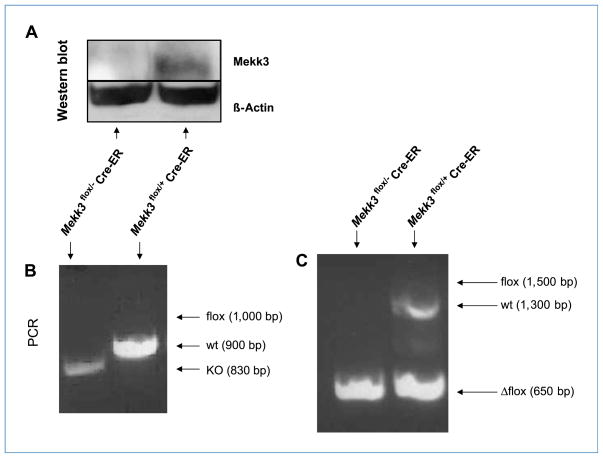
Figure 3.
Confirmation of engraftment 4 wk after transplant. LacZ+ Mekk3Δflox/+ or LacZ+ Mekk3Δflox/− BM cells were injected into lethally irradiated nude mice. To confirm engraftment, BM cells from the Mekk3Δflox/+ and Mekk3Δflox/− BM–transplanted mice were obtained 4 wk following transplantation and analyzed by Western blot (A) and genotyping (B and C). B, PCR primers P1, P2, and P3 were used to detect the floxed Mekk3 allele (flox, 1,000 bp), wild-type Mekk3 allele (wt, 900 bp), and Mekk3 germline knockout allele (KO, 830 bp). C, primers P3 and P4 were used to detect wild-type (1,300 pb), floxed (1,500 bp), and the loxp-deleted Mekk3 allele (Δflox, 650 bp). Primer design necessitates using two separate PCR reactions to confirm the presence of the Mekk3 Δflox allele, which represents the deleted Mekk3 allele following tamoxifen treatment. Floxed allele is equivalent to the wild-type allele and the Δflox allele is equivalent to the knockout allele.
Figure 4.
Deletion of Mekk3 in BM cells inhibited tumor growth. Mekk3Δflox/+ or Mekk3Δflox/− BM cells were injected into lethally irradiated nude mice. Following engraftment, mice were injected with TC71 or A4573 cells. TC71 (A) and A4573 (C) tumor growth was monitored for 3 wk. Error bars represent SD. Representative TC71 (B) and A4573 (D) tumors from Mekk3Δflox/+ BM–transplanted mice (top) and Mekk3Δflox/− BM–transplanted mice (bottom). Average weight of TC71 (B) and A4573 (D) tumors on day 21 and 17, respectively. Error bars represent SD.
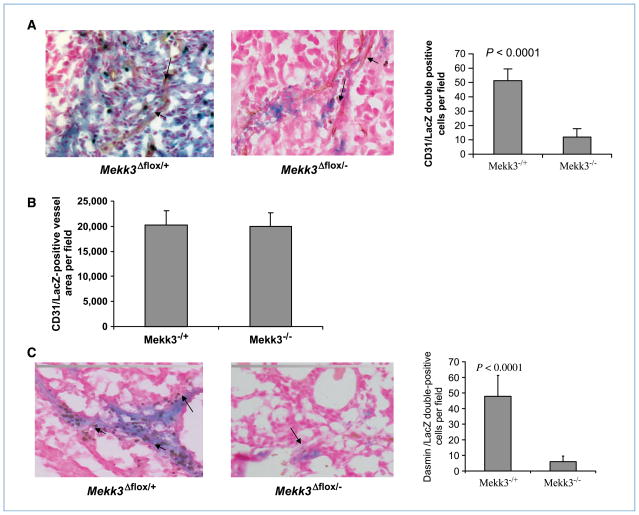
Because the BM cells from the donor mice were LacZ+, β-gal staining was used to distinguish cells of donor origin from cells of the host. Colocalization of LacZ and CD31 on tumor vessels was seen in tumors excised from the Mekk3Δflox/+ BM–transplanted mice (Fig. 5A). Whereas Lac Z+ cells were visualized within the tumor in the Mekk3Δflox/− BM–transplanted mice, very few of these cells colocalized with the CD31+ cells (Fig. 5A). Quantification of double-positive LacZ and CD31 cells confirmed that BM cell contribution to tumor vessel formation was impaired following deletion of Mekk3 (Fig. 5A). These data indicate that whereas the Mekk3-deficient BM cells were able to migrate to the tumor area, their contribution to tumor vessel formation was minimal.
Figure 5.
Deletion of Mekk3 inhibits the participation of BM cells in tumor vessel formation. LacZ+ Mekk3Δflox/+ or LacZ+ Mekk3Δflox/− BM cells were injected into lethally irradiated nude mice. Following engraftment, mice were injected with TC71 cells. Tumors were removed 3 wk later and analyzed by immunohistochemistry. A, left, colocalization of LacZ+ BM-derived cells (blue staining) and brown-stained CD31+ vessels (arrow) was visualized in a tumor from Mekk3flox/+ Cre-ER–transplanted mice. Middle, no colocalization of LacZ+ BM-derived cells (blue staining) with brown-stained CD31+ vessels (arrow) in tumors from Mekk3flox/− Cre-ER BM–transplanted mice. Right, the average number of vessels showing colocalization of LacZ+ and CD31+ was calculated by counting five random high-power microscopic fields from different tumor sections. B, CD31+/LacZ− staining was used to identify vessels formed by local endothelial cells. The average number of CD31+/LacZ− vessels was quantified by counting five random high-power microscopic fields from three different tumor sections. C, left, colocalization of LacZ+ BM-derived cells (blue staining) and brown-stained desmin+ pericytes (arrow) in a tumor from Mekk3flox/+ Cre-ER–transplanted mice. Middle, no colocalization of LacZ+ BM-derived cells (blue staining) with brown-stained desmin+ pericytes (arrow) in tumors from Mekk3flox/− Cre-ER BM–transplanted mice. Right, colocalization of LacZ+ BM–derived cells (blue staining) and brown-stained desmin+ vessels was used to quantify BM-derived pericytes. The average number of double-positive cells was determined by counting five random high-power microscopic fields from different tumor sections. Error bars represent SD.
To confirm that angiogenesis was at a similar level in Mekk3Δflox/+ and Mekk3Δflox/− BM–transplanted mice, we quantified the number CD31+/LacZ− vessels in the tumors. CD31+/LacZ− represent tumor vessels with no BM-derived cells and thus can be used as a way to quantify local angiogenesis. There was no difference in the number of CD31+/LacZ− vessels (Fig. 5B). These data indicate that the angiogenesis process was similar in the two groups, confirming that the inhibition in tumor growth was secondary to the defect in vasculogenesis.
Deletion of Mekk3 inhibits the contribution of BM-derived mural cells/pericytes to Ewing’s sarcoma vasculature
Interaction between endothelial cells and mural cells/pericytes plays an important role in vascular formation (24). We showed that BM-derived mural cells/pericytes contribute to Ewing’s sarcoma vessel development (7, 25). Tumors were therefore analyzed by immunohistochemistry for the presence of BM-derived mural cells/pericytes. Colocalization of LacZ+ (donor BM cells) and desmin+ cells (mural cell/pericytes marker) allowed us to quantify BM-derived mural cells/pericytes in tumor vessels and assess whether deleting Mekk3 affected the ability of BM-derived mural cells/pericytes to contribute to the tumor vascular structure (Fig. 5C). The quantification of double-positive cells in the tumors from control Mekk3Δflox/+ BM–transplanted mice (Fig. 5C) confirmed our previous findings that BM-derived mural cells/pericytes contribute to tumor vessel formation (7, 25). Deletion of Mekk3 inhibited this process. Very few BM-derived mural cells/pericytes were detected in the tumor vessels from the Mekk3Δflox/− BM–transplanted mice (Fig. 5C). By contrast, macrophage marker F4-80 staining showed no difference between the tumors from mice transplanted with Mekk3Δflox/− versus Mekk3Δflox/+ BM cells (data not shown), indicating that the migration and differentiation of myeloid cells was not impaired following deletion of Mekk3.
Effect of downregulating Mekk3 in TC71 cells on tumor growth and tumor vessel formation
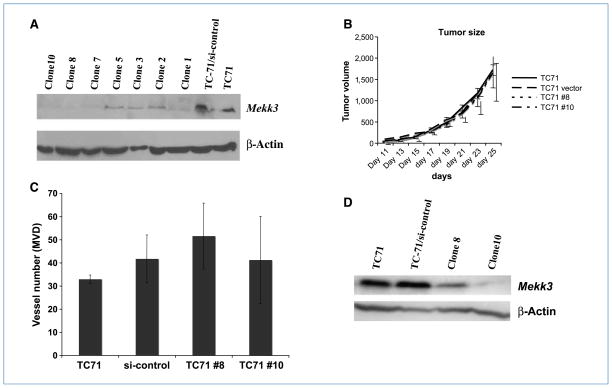
We have shown that deletion of Mekk3 in BM cells impairs their ability to participate in tumor vessel expansion, resulting in decreased tumor vascular density and mural cells/pericytes coverage. To determine whether tumor cell expression of Mekk3 is required for tumor-induced vessel expansion, Mekk3 in TC71 cells was knocked down using siRNA. Two stable clones were isolated (TC71 clone 8 and TC71 clone 10; Fig. 6A). The downregulation of Mekk3 in the TC71 cells had no effect on the ability of these cells to form tumors or stimulate neovascularization. Tumor growth and MVD were similar to the control-transfected cells (Fig. 6B and C). Tumors were harvested on day 25 to confirm that the Mekk3 downregulation had been maintained (Fig. 6D).
Figure 6.
Downregulation of Mekk3 in TC71 cells did not affect tumor growth or tumor vessel formation. TC71 cells were transfected with Mekk3 siRNA. Several clones were isolated following transfection. A, downregulation of Mekk3 was confirmed by Western blot in clone 8 and clone 10. B, mice were injected with TC71, TC71 control siRNA transfected (si-control), clone 8, or clone 10 cells. Tumor growth was monitored for 4 wk. C, tumors were excised on day 25 and analyzed by immumohistochemistry using CD31 staining. The average number of vessels was quantified by counting five random high-power microscopic fields from different tumor sections. Error bars represent SD. D, excised tumors were also analyzed by Western blot for Mekk3 expression.
Discussion
Tumor vessels are formed through at least two different processes: angiogenesis (2, 4, 26), defined as endothelial cell proliferation from the preexisting vasculature, and vasculogenesis (2, 3, 27), defined as the recruitment of BM-derived precursors into the tumor area with subsequent differentiation into endothelial cells. The contribution of vasculogenesis varies considerably depending on the tumor type. We have shown that vasculogenesis, in addition to angiogenesis, contributes to the expansion of the Ewing’s tumor vascular network with 10% of the vessels containing BM-derived as opposed to locally derived cells (6, 7, 28). Using a transplant mouse model with GFP+ BM cells, we showed that both locally derived and BM-derived cells make up the tumor vessel wall and the surrounding pericyte population (7, 28). Our previous studies (6, 7, 28) support the concept that BM cells contribute to the endothelial and peri-endothelial components that form the Ewing’s tumor vasculature. Inhibiting BM cell chemotaxis into the tumor area resulted in the formation of significantly smaller and less vascular tumors (28). Furthermore, stimulating the migration of BM cells into vascular endothelial growth factor (VEGF)–inhibited Ewing’s tumors in vivo enhanced the neovasculature development and rescued tumor growth (29). Taken together, these data indicated that the vasculogenesis process is vital in the formation of tumor vessels.
Recent studies have questioned the importance of vasculogenesis to tumor development and growth (20). Proving that vasculogenesis is a critical process in tumor vessel expansion requires a model in which angiogenesis is intact but vasculogenesis is impaired. In the study presented here, we elected to take advantage of the recent finding that the Mekk3 gene is required for blood vessel development (12). We first confirmed that Mekk3−/− cells failed to form vessels. Next, we transplanted Mekk3Δflox/− BM cells into lethally irradiated nude mice, thereby creating a mouse that had Mekk3+/+ cells in the peripheral organs (cells that could participate in angiogenesis) and Mekk3Δflox/− cells in the BM (cells that could not participate in vasculogenesis). The absence of Mekk3 did not affect engraftment. Mice survived the irradiation procedure and engraftment was confirmed 4 weeks after transplant. BM cells from the Mekk3Δflox/− BM–transplanted mice were negative for Mekk3, as assessed by both genotyping and Western blot (Fig. 3). Mice transplanted with Mekk3Δflox/+ BM cells were positive for Mekk3 and served as the controls. Tumor growth was significantly impaired in the Mekk3Δflox/− BM–transplanted mice (Fig. 4). Although tamoxifen was used to generate and maintain the Mekk3Δflox/− status in the BM cells, tamoxifen treatment played no role in this tumor suppression as both the Mekk3Δflox/− and Mekk3Δflox/+ BM–transplanted mice received identical tamoxifen treatment. The tumors from the Mekk3Δflox/− BM–transplanted mice remained small for over 2 weeks (Fig. 4A and C). These tumors were less vascular with a significant decrease in MVD and pericyte coverage (Fig. 5). Chemotaxis of the Mekk3Δflox/− BM cells into the tumor area was not impaired. The Mekk3Δflox/− and Mekk3Δflox/+ cells were LacZ+, allowing us to identify BM-derived cells in the tumor tissue using β-gal staining. Tumor sections from the BM-transplanted mice showed LacZ+ cells with significant numbers of LacZ+/CD31+ double-positive cells, indicating that BM cells were migrating into the tumor area, differentiating into endothelial cells, and participating in tumor vessel formation. Whereas LacZ+ cells were also identified in tumor sections from the Mekk3Δflox/− BM–transplanted mice, the number of LacZ+/CD31+ double-positive cells was significantly diminished (Fig. 5). These data suggest that the Mekk3Δflox/− BM cells were able to migrate to the tumor but unable to participate in vessel formation. This resulted in few BM-derived tumor vessels, confirming the selective inhibition of vasculogenesis. The vessels that were present were derived from the host cells in the surrounding tumor area as they were LacZ−. Our data show for the first time that selectively inhibiting vasculogenesis severely compromised tumor growth.
The transplant model used to selectively inhibit BM cell participation in tumor vessel formation required whole-body irradiation. Radiation has been shown to inhibit local angiogenesis to some degree (30). Although we cannot exclude the fact that the level of angiogenesis may have been decreased in the mice after radiation, the level of angiogenesis was the same in the Mekk3Δflox/+ and Mekk3Δflox/− BM–transplanted mice. Therefore, radiation effect cannot explain the decreased tumor growth rate seen. We confirmed this by quantifying the number of tumor vessels that contained no BM-derived cells. This was done by identifying the CD31+/LacZ− tumor vessels. Both the Mekk3Δflox/+ and Mekk3Δflox/− BM cells were LacZ+. Therefore, a vessel that contained no LacZ+ cells was derived by the local endothelial cells or angiogenesis. Vasculogenesis contributed to the LacZ+ vessels. The number of CD31+/LacZ− vessels was not significantly different in the Mekk3Δflox/+ and Mekk3Δflox/− BM–transplanted mice (P > 0.05). These data confirmed that the decreased tumor growth rate seen in the Mekk3Δflox/− –transplanted mice using both the TC71 and A4573 models was a direct effect of defective vasculogenesis and the inability of BM cells to participate in tumor vessel formation. Although our model may favor vasculogenesis, this does not lessen the importance of our finding. Exponential tumor growth was seen in the irradiated mice transplanted with Mekk3Δflox/+ BM cells that can participate in vasculogenesis, whereas the tumors in the Mekk3Δflox/− –transplanted mice remained unchanged in size (Fig. 4). If anything, this shows that vasculogenesis may provide a mechanism for tumor cells to circumvent the negative effect of radiation on angiogenesis, thereby rescuing tumor growth.
Our studies indicate that tumor vessel expansion and tumor growth are compromised when BM cells are unable to participate in tumor vascular formation. Altering Mekk3 expression in the TC71 tumor cells had no effect on tumor growth. This is not surprising because altering the status of Mekk3 in tumor cells does not affect VEGF production or the ability of cells in the microenvironment to form vessel (31). Both the local endothelial cells and the BM cells in this model are Mekk3+/+ and can therefore participate in tumor vessel formation. These data indicate that the status of Mekk3 in the tumor cell itself is of no consequence to the development of the tumor vasculature.
Others have shown that the growth of Mekk3 deficient tumor-like embryonic stem cells was similar to Mekk3+/+ embryonic stem cells (31). MVD was also not affected. This supports our findings that the status of Mekk3 in tumor cells does not affect tumor-induced vessel formation. However, this study did not examine whether Mekk3 deletion in the microenvironment affected tumor growth or tumor vessel formation. Our current study examined both.
In summary, we have shown that MEKK3 plays a key role in the ability of BM stem cells to participate in the formation of tumor vessels, which subsequently significantly affect tumor growth. We also showed that MEKK3 may control critical signaling pathways that are involved in pericyte differentiation and interaction with the vascular endothelium. Finally, we showed for the first time that vasculogenesis and BM cells not only contribute to, but play a critical role in, the development and the expansion of the tumor vascular network that supports the growth of Ewing’s tumors. The importance of BM-derived vasculogenesis has also been shown in other tumor models (32, 33). Inhibiting this process suppresses the ability of Ewing’s sarcoma cells to grow. These are important new findings particularly for Ewing’s sarcoma, as cure rates have remained stagnant for >20 years. In addition, the long-term effects associated with intensive chemotherapy and radiation underscores the need for more targeted approaches. Targeting the vasculogenesis process may therefore offer novel therapeutic opportunities.
Acknowledgments
We thank Dr. Thomas Ludwig (Columbia University, New York, NY) for providing the Cre-ER mice.
L. Yu performed research, designed experiments, analyzed data, and co-wrote the paper; B. Su helped to design the experiments and co-wrote the paper; M. Hollomon, Y. Deng, and V. Facchinetti performed research; E.S. Kleinerman conceived and designed the research and co-wrote the paper.
Grant Support
National Cancer Institute grants ROI 103986 (E.S. Kleinerman), core grant CA 16672, and NIH grant HL070225 (B. Su).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Walsh JE, Lathers DM, Chi AC, Gillespie MB, Day TA, Young MR. Mechanisms of tumor growth and metastasis in head and neck squamous cell carcinoma. Curr Treat Options Oncol. 2007;8:227–38. doi: 10.1007/s11864-007-0032-2. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J, D’Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–5. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 5.Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–66. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- 6.Bolontrade MF, Zhou RR, Kleinerman ES. Vasculogenesis plays a role in the growth of Ewing’s sarcoma in vivo. Clin Cancer Res. 2002;8:3622–7. [PubMed] [Google Scholar]
- 7.Reddy K, Zhou Z, Schadler K, Jia SF, Kleinerman ES. Bone marrow subsets differentiate into endothelial cells and pericytes contributing to Ewing’s tumor vessels. Mol Cancer Res. 2008;6:929–36. doi: 10.1158/1541-7786.MCR-07-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 10.Blank JL, Gerwins P, Elliott EM, Sather S, Johnson GL. Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. Regulation of sequential phosphorylation pathways involving mitogen-activated protein kinase and c-Jun kinase. J Biol Chem. 1996;271:5361–8. doi: 10.1074/jbc.271.10.5361. [DOI] [PubMed] [Google Scholar]
- 11.Ellinger-Ziegelbauer H, Brown K, Kelly K, Siebenlist U. Direct activation of the stress-activated protein kinase (SAPK) and extracellular signal-regulated protein kinase (ERK) pathways by an inducible mitogen-activated protein kinase/ERK kinase kinase 3 (MEKK) derivative. J Biol Chem. 1997;272:2668–74. doi: 10.1074/jbc.272.5.2668. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Boerm M, McCarty M, et al. Mekk3 is essential for early embryonic cardiovascular development. Nat Genet. 2000;24:309–13. doi: 10.1038/73550. [DOI] [PubMed] [Google Scholar]
- 13.Motoike T, Loughna S, Perens E, et al. Universal GFP reporter for the study of vascular development. Genesis. 2000;28:75–81. doi: 10.1002/1526-968x(200010)28:2<75::aid-gene50>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Chang X, Facchinetti V, Zhuang Y, Su B. MEKK3 is essential for lymphopenia-induced T cell proliferation and survival. J Immunol. 2009;182:3597–608. doi: 10.4049/jimmunol.0803738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–18. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 17.Balconi G, Spagnuolo R, Dejana E. Development of endothelial cell lines from embryonic stem cells: a tool for studying genetically manipulated endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2000;20:1443–51. doi: 10.1161/01.atv.20.6.1443. [DOI] [PubMed] [Google Scholar]
- 18.Giermasz A, Makowski M, Kozlowska E, et al. Potentiating antitumor effects of a combination therapy with lovastatin and butyrate in the Lewis lung carcinoma model in mice. Int J Cancer. 2002;97:746–50. doi: 10.1002/ijc.10119. [DOI] [PubMed] [Google Scholar]
- 19.Elefanty AG, Begley CG, Hartley L, Papaevangeliou B, Robb L. SCL expression in the mouse embryo detected with a targeted lacZ reporter gene demonstrates its localization to hematopoietic, vascular, and neural tissues. Blood. 1999;94:3754–63. [PubMed] [Google Scholar]
- 20.Gothert JR, Gustin SE, van Eekelen JA, et al. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–77. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 21.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi H, Fukuda Y, Yoshida R, et al. Suppressing effects of dietary supplementation of soybean trypsin inhibitor on spontaneous, experimental and peritoneal disseminated metastasis in mouse model. Int J Cancer. 2004;112:519–24. doi: 10.1002/ijc.20430. [DOI] [PubMed] [Google Scholar]
- 23.Kim K, Duramad O, Qin XF, Su B. MEKK3 is essential for lipopolysaccharide-induced interleukin-6 and granulocyte-macrophage colony-stimulating factor production in macrophages. Immunology. 2007;120:242–50. doi: 10.1111/j.1365-2567.2006.02495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neurooncol. 2005;7:452–64. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy K, Cao Y, Zhou Z, Yu L, Jia SF, Kleinerman ES. VEGF165 expression in the tumor microenvironment influences the differentiation of bone marrow-derived pericytes that contribute to the Ewing’s sarcoma vasculature. Angiogenesis. 2008;11:257–67. doi: 10.1007/s10456-008-9109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 27.Aghi M, Chiocca EA. Contribution of bone marrow-derived cells to blood vessels in ischemic tissues and tumors. Mol Ther. 2005;12:994–1005. doi: 10.1016/j.ymthe.2005.07.693. [DOI] [PubMed] [Google Scholar]
- 28.Lee TH, Bolontrade MF, Worth LL, Guan H, Ellis LM, Kleinerman ES. Production of VEGF165 by Ewing’s sarcoma cells induces vasculogenesis and the incorporation of CD34+ stem cells into the expanding tumor vasculature. Int J Cancer. 2006;119:839–46. doi: 10.1002/ijc.21916. [DOI] [PubMed] [Google Scholar]
- 29.Reddy K, Zhou Z, Jia SF, et al. Stromal cell-derived factor-1 stimulates vasculogenesis and enhances Ewing’s sarcoma tumor growth in the absence of vascular endothelial growth factor. Int J Cancer. 2008;123:831–7. doi: 10.1002/ijc.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udagawa T, Birsner AE, Wood M, D’Amato RJ. Chronic suppression of angiogenesis following radiation exposure is independent of hematopoietic reconstitution. Cancer Res. 2007;67:2040–5. doi: 10.1158/0008-5472.CAN-06-2877. [DOI] [PubMed] [Google Scholar]
- 31.Deng Y, Yang J, McCarty M, Su B. MEKK3 is required for endothelium function but is not essential for tumor growth and angiogenesis. Am J Physiol Cell Physiol. 2007;293:C1404–11. doi: 10.1152/ajpcell.00058.2007. [DOI] [PubMed] [Google Scholar]
- 32.Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 33.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–95. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]