Abstract
OBJECTIVES
The goal of this study was to determine whether subclinical thyroid dysfunction was associated with incident heart failure (HF) and echocardiogram abnormalities.
BACKGROUND
Subclinical hypothyroidism and hyperthyroidism have been associated with cardiac dysfunction. However, long-term data on the risk of HF are limited.
METHODS
We studied 3044 adults ≥65 years initially free of HF in the Cardiovascular Health Study (CHS). We compared adjudicated HF events over a mean 12-year follow-up and changes in cardiac function over 5 years among euthyroid participants, those with subclinical hypothyroidism (subdivided by TSH levels: 4.5–9.9, ≥10.0 mU/L), and those with subclinical hyperthyroidism.
RESULTS
Over 12 years, 736 participants developed HF events. Participants with TSH≥10.0 mU/L had a greater incidence of HF compared to euthyroid participants (41.7 vs. 22.9/1000 person years, p=0.01, adjusted hazard ratio=1.88, 95% confidence interval 1.05–3.34). Baseline peak E velocity, an echocardiographic measurement of diastolic function associated with incident HF in the CHS cohort, was higher in those with TSH≥10.0 compared to euthyroid participants (0.80 vs. 0.72 m/sec, p=0.002). Over 5 years, left ventricular mass increased among those with TSH≥10.0, but other echocardiographic measurements were unchanged. Those with TSH 4.5–9.9 or with subclinical hyperthyroidism had no increase in risk of HF.
CONCLUSIONS
Compared to euthyroid older adults, those with a TSH≥10.0 mU/L have a moderately elevated risk of HF and alterations in cardiac function, but not older adults with TSH<10. Clinical trials should assess whether the risk of HF might be ameliorated by thyroxine replacement in individuals with TSH≥10.0 mU/L.
Keywords: Subclinical Thyroid Dysfunction, Heart Failure, Echocardiography, Cohort Study
Subclinical thyroid dysfunction is present in patients who have an abnormal thyrotropin (thyroid-stimulating hormone, TSH) level and a normal free thyroxine (FT4) level(1). Subclinical thyroid dysfunction is common, particularly in older individuals, with a prevalence of subclinical hypothyroidism of 10% and subclinical hyperthyroidism of 1.5%(2,3). Whereas it is generally accepted to treat the abnormal free thyroxine levels of overt thyroid dysfunction, the indications and threshold TSH for treatment of subclinical hypothyroidism and subclinical hyperthyroidism are areas of clinical controversy(1,3,4), as current evidence about the risks is limited(1,3).
Based on the known effects of thyroid hormone on the heart, it is reasonable to expect adverse cardiac effects in subclinical thyroid dysfunction(5). Subclinical thyroid disease has been associated with systolic and diastolic cardiac dysfunction, and small studies have shown that thyroxine replacement improved measurements of cardiac function in subjects with subclinical hypothyroidism(6,7). However, the clinical importance of these effects is unclear(3,8). Data on cardiovascular risks are conflicting(9–11), and no randomized clinical trials (RCT) have assessed the impact of thyroxine replacement on clinical cardiac endpoints(3). Only one study has examined the relationship between subclinical thyroid dysfunction and HF events. In a population-based study of adults aged 70–79, participants with TSH≥7.0 mU/L had a more than 2-fold higher risk of HF events, compared to euthyroid subjects(10), but echocardiography was not performed. No study has directly addressed the relationship between subclinical hyperthyroidism and HF events.
As the number of hospitalizations for HF has greatly increased(12), examining a common and easily treatable potential risk factor for HF is warranted. To determine whether subclinical thyroid dysfunction was associated with HF and cardiac dysfunction, we examined a large cohort study of community-dwelling older adults.
Methods
Study Population
Participants were part of the Cardiovascular Health Study (CHS), a population-based, longitudinal study of risk factors for the development of cardiovascular disease (CVD) in 5888 adults ≥65 years(13). Enrolment of an original cohort of 5201 adults occurred in 1989–1990, and an additional cohort of 687, predominantly African Americans, was enrolled in 1992–1993. Eligible individuals were identified from an age- and sex-stratified random sample of the Medicare-eligible adults in 4 US communities. Details of the eligibility criteria have been previously described(9). All participants gave written informed consent; the Institutional Review Boards at study sites approved the protocol.
Fasting TSH was measured at baseline in a subsample (n=3678) of CHS participants, selected according to availability of stored serum for analysis. Because our primary study question pertained to unrecognized thyroid function abnormalities, participants taking thyroid hormone preparations at baseline (n=339) or other medications that could affect thyroid testing, including antithyroid drugs (n=1), corticosteroids (n=77) and amiodarone (n=2), were excluded. We also excluded participants with known HF at baseline (n=144) to examine incident HF.
Measurements
Thyroid Hormones
Serum TSH and FT4 concentrations were assayed, as previously described(9), with FT4 (normal range: 0.7–1.7 ng/dL [9–22 pmol/L]) measured in individuals with TSH <0.10 or >4.50 mU/L for the 95% of samples with sufficient serum for this additional test. Compared to those without thyroid function testing (n=1523), participants with thyroid function testing were less likely to be men (38 vs. 55%, p<0.001) and to have prevalent CVD (22 vs. 26%, p=0.002), but mean ages, race proportions, thyroxine use, and prevalent HF were similar.
Study participants were classified into 3 groups based on their thyroid function tests(9):
Subclinical hyperthyroidism: TSH≥0.10 and <0.45 mU/L(3) (n=38), or <0.10 with a normal FT4 (n=6).
Euthyroidism: normal TSH (0.45–4.50 mU/L) (n=2526).
Subclinical hypothyroidism: TSH>4.50 and <20 mU/L with a normal FT4 (n=474). Based upon the definitions used by the United States Preventive Services Task Force (USPSTF)(1) and on expert consensus(3), subclinical hypothyroidism was subclassified according to TSH levels: 4.5–9.9 (n=428), and ≥10 (marked elevation, n=46), because of possible higher risks above this cut-off. In additional analyses, mild subclinical hypothyroidism was further subclassified between those with TSH 4.5–6.9 (mild elevation, n=337) and 7.0–9.9 (moderate elevation, n=91), based upon USPSTF definitions(1).
Participants whose testing suggested nonthyroidal illness (low TSH and FT4, n=2), ≥TSH 4.50 and a missing FT4 (n=21), and overt thyrotoxicosis (TSH<0.10 with an elevated FT4, n=1) or overt hypothyroidism (TSH≥20 mU/L or >4.50 with a FT4<0.70 ng/dL, n=47) were excluded from analyses. The sample for our analyses was 3044.
HF events
During the 15-year follow-up, we assessed incident HF events among participants free of HF at baseline. Clinical outcomes were ascertained every six months. Diagnoses have been adjudicated until 6/30/2004 based on interview, review of medical records, and other support documents without knowledge of thyroid status. HF events were defined on the basis of diagnosis from a physician and consideration of symptoms, signs, chest radiographs, and treatment of heart failure (current prescription for a diuretic agent and digitalis or a vasodilator)(14,15).
Echocardiography
As previously described(16), echocardiographic images were obtained in 1989–1990 and 1994–1995, using a standardized protocol with core reading centers. The following measurements were examined without knowledge of thyroid status; left ventricular (LV) mass and fractional shortening at the midwall, a quantitative measurement of global chamber function, were calculated, as reported(17). Qualitative assessment of LV ejection fraction (LVEF) was classified as normal, borderline, or abnormal, approximately corresponding to values ≥55%, ≥45% and <45%, respectively(17). Agreement between baseline and 5-year follow-up echocardiography core centers has been previously reported(17). For diastolic function, left atrial size, Doppler peak E and A velocities were measured(18,19). Doppler filling velocities and their ratios, consistent with impaired relaxation and restrictive filling, were previously found in CHS to be predictive of incident HF(14). 896 participants had missing 1994–1995 echocardiograms, because of death (36%), missed visit (17%) or follow-up visit outside the clinics with no echocardiogram (47%). The 576 alive participants without follow-up echocardiograms were older, more likely to be nonwhite, smoker, have diabetes, atrial fibrillation and subclinical hyperthyroidism (2.9 vs. 1.4%); prevalence of hypertension, CVD, subclinical hypothyroidism and gender proportions did not differ significantly. LVEF at the time of incident HF was based on data abstracted from reports of echocardiograms performed during hospitalization for HF, and was classified as described above.
Covariates
We collected self-reported race and smoking status, classified as never, current, or former. Thyroid medication use was assessed annually via medication bottle examination. Physical examination included blood pressure, heart rate, and body mass index (BMI). Hypertension was defined as self-report and use of anti-hypertensive medications, or blood pressure ≥140/90 mmHg. Diabetes was defined as a fasting glucose ≥126 mg/dL (7.0 mmol/L) or use of hypoglycemic medication. Atrial fibrillation at baseline was self-reported or determined on ECG or Holter monitor(9). For prevalent HF at baseline, self-reports were confirmed by physical examination or, if necessary, by a validation protocol that included surveys of treating physicians or review of medical records(20). Prevalent CVD (defined as coronary heart disease, stroke, or transient ischemic attack) was based on self-report, medical record verification and use of selected drugs.
Statistical analysis
We calculated HF incidence per 1000 person-years of follow-up, and used log-rank tests to compare Kaplan-Meier estimates of HF incidence across the four thyroid groups. We used Cox proportional hazard models to examine associations between the four groups and HF. The multivariate models adjusted for known clinical risk factors of HF in older adults(12,15,21,22) that might be potential confounders in the relationship between subclinical thyroid disease and HF. To check the sensitivity of our results to the selection of covariates, we assessed models that excluded potential confounders with p-values >0.2 after adjustment, and obtained similar results. We hypothesized some interactions a priori: the relationship between subclinical thyroid disease and HF might differ by gender, prevalent CVD or thyroxine use during follow-up. As previously(9), we first examined models in which follow-up was censored at the time of first thyroxine use, as the main analysis. Because of interaction with thyroxine use, we also used models in which baseline hazard was stratified by thyroxine use during follow-up as a time-dependent covariate, and participants changed stratum when thyroxine use was initiated.
We graphically examined smooth estimates of hazard function for HF against TSH to examine cut-points at which HF risk might be increased, as well as tabulation of event rates within smaller TSH intervals, and used Schoenfeld residuals to check proportional hazards assumption for thyroid status and for covariates included in the multivariate models. We used models with baseline hazard jointly stratified by four variables (prevalent atrial fibrillation, CVD, diabetes and hypertension) that did not meet this assumption(23). We also categorized age, the only continuous covariate that did not meet the assumption of log-linearity. Results were reported as hazard ratios (HR), with 95% confidence intervals (CI).
Among participants with echocardiographic data, multiple linear regression was used to assess baseline differences and changes over time in echocardiographic measurements across thyroid groups, after adjustment for age and gender, similar to previous analyses of echocardiograms in CHS(14). We conducted analyses using Stata 9.2 (Stata Corporation, Texas).
Results
Baseline characteristics
The mean age was 72.6 years; 60% were women (Table 1). Mild subclinical hypothyroidism (TSH 4.5–9.9 mU/L) was present in 428 (14.5%) participants, severe subclinical hypothryoidism (TSH 10.0–19.9 mU/L) in 46 (1.5%), and subclinical hyperthyroidism in 44 (1.4%). Subclinical hypothyroidism was more common in women, and associated with lower systolic blood pressure. Subclinical hyperthyroidism was associated with higher BMI and lower LDL-cholesterol.
Table 1.
Baseline characteristics of study population according to thyroid status in the Cardiovascular Health Study (n = 3044)*
| Total | Subclinical Hyperthyroid | Euthyroid | Subclinical Hypothyroid | ||
|---|---|---|---|---|---|
| TSH categories, mU/L | (N=3044) | < 0.45 (n=44) | 0.45–4.5 (n=2526) | 4.5–9.9 (n=428) | 10.0–19.9 (n=46) |
| TSH, mU/L | 2.88 (2.14) | 0.24 (0.13) | 2.21 (0.99) | 6.02 (1.36) | 12.9 (2.70) |
| Free thyroxine, ng/dL | 1.27 (0.27) | 1.01 (0.16) | 0.89 (0.15) | ||
| Age, y | 72.6 (5.5) | 73.8 (6.9) | 72.5 (5.5) | 73.0 (5.5) | 74.0 (6.2) |
| Female, No. (%) | 1826 (60.0) | 29 (65.9) | 1488 (58.9) | 281 (65.7)†† | 28 (60.9) |
| White race, No. (%) | 2867 (94.2) | 42 (95.5) | 2373 (93.9) | 409 (95.6) | 43 (93.5) |
| Smoking status, No. (%) | |||||
| Current | 326 (10.7) | 8 (18.2) | 277 (11.0) | 36 (8.4) | 5 (10.9) |
| Former | 1236 (40.6) | 16 (36.4) | 1039 (41.2) | 164 (38.4) | 17 (37.0) |
| Never | 1479 (48.7) | 20 (45.5) | 1208 (47.9) | 227 (53.2) | 24 (52.2) |
| Alcohol, drinks/wk | |||||
| <1, No. (%) | 2084 (68.6) | 34 (77.3) | 1704 (67.6) | 311 (72.8) | 35 (76.1) |
| 1–6, No. (%) | 510 (16.8) | 5 (11.4) | 434 (17.2) | 67 (15.7) | 4 (8.7) |
| ≥7, No. (%) | 443 (14.6) | 5 (11.4) | 382 (15.2) | 49 (11.5) | 7 (15.2) |
| Diabetes mellitus, No. (%) | 405 (13.3) | 9 (20.5) | 329 (13.0) | 61 (14.3) | 6 (13.0) |
| Hypertension, No. (%) | 1219 (40.2) | 18 (40.9) | 1029 (40.9) | 150 (35.1)† | 22 (47.8) |
| Prevalent CVD, No. (%) | 595 (19.6) | 9 (20.5) | 489 (19.4) | 87 (20.3) | 10 (21.7) |
| Prevalent atrial fibrillation, No. (%) | 59 (1.9) | 1 (2.3) | 49 (1.9) | 9 (2.1) | 0 (0) |
| Body mass index, kg/m2 | 26.2 (4.4) | 27.6 (5.4)† | 26.2 (4.3) | 26.4 (4.7) | 25.7 (4.7) |
| Systolic blood pressure, mmHg | 135.6 (20.9) | 140.4 (18.5) | 135.9 (21.1) | 133.3 (19.1)† | 137.1 (25.7) |
| Diastolic blood pressure, mmHg | 69.9 (11.0) | 70.6 (14.0) | 70.1 (11.2) | 69.1 (9.7) | 69.0 (12.4) |
| Lipid values | |||||
| Total cholesterol, mg/dL | 213 (39) | 203 (40) | 213 (38) | 211 (41) | 213 (42) |
| LDL-cholesterol, mg/dL | 131 (35) | 120 (28)† | 131 (35) | 129 (35) | 130 (39) |
| HDL-cholesterol, mg/dL | 55 (16) | 53 (16) | 55 (16) | 54 (16) | 56 (19) |
| Triglycerides, mg/dL | 141 (74) | 140 (83) | 140 (73) | 146 (78) | 131 (60) |
| Fasting glucose, mg/dL | 108 (32) | 110 (38) | 109 (32) | 108 (31) | 104 (22) |
| Creatinine, mg/dL | 1.04 (0.32) | 0.97 (0.31) | 1.04 (0.33) | 1.06 (0.26) | 1.08 (0.29) |
| Lipid-lowering medication, No. (%) | 154 (5.1) | 2 (4.6) | 137 (5.4) | 14 (3.3) | 1 (2.2) |
Abbreviations: TSH: thyroid-stimulating hormone; CVD: prevalent clinical cardiovascular disease defined as prevalent coronary heart disease or cerebrovascular disease.
SI conversions: To convert free thyroxine to pmol/L, multiply by 12.87; LDL, HDL, and total cholesterol to mmol/L, multiply by 0.0259; and glucose to mmol/L, multiply by 0.0555.
Values are mean (SD) or No. (%). Percentages may not sum to 100 due to rounding and some numbers may not add to the total due to missing information.
P<0.05,
p<0.01 for pairwise comparison with euthyroid category. P values based on chi-square tests and t-tests, as appropriate.
Baseline Echocardiographic Data
Most echocardiographic measurements at baseline did not differ significantly by thyroid status (Table 2). Participants with TSH≥10.0 had a higher peak E velocity (0.80 vs 0.72 m/sec, p=0.002), and this difference persisted after adjustment for age, gender, heart rate and systolic blood pressure. Peak E velocity was associated with incident HF in the overall study sample (HR 1.14 for each 0.1 m/sec increment, 95%CI:1.09–1.18, p<0.001) and in those with TSH≥10.0 (HR 1.45, 95%CI:1.20–1.76, p<0.001) after adjustment for age, gender and systolic blood pressure. Compared to euthyroidism, subclinical hyperthyroidism was associated with larger left atrial size, higher proportions with E/A ratio <0.7, and increased heart rate (Table 2), differences that persisted after excluding those with atrial fibrillation.
Table 2.
Echocardiographic characteristics at baseline according to thyroid status *
| Measure (n) | Subclinical Hyperthyroid | Euthyroid | Subclinical Hypothyroid | |
|---|---|---|---|---|
| TSH categories, mU/L | TSH < 0.45 (n=44) | TSH 0.45–4.5 (n=2526) | TSH 4.5–9.9 (n=428) | TSH 10.0–19.9 (n=46) |
| Systolic function | ||||
| LV ejection fraction, qualitative assessment (3020) | ||||
| Normal (≥55%), % | 88.1 | 93.4 | 91.0 | 93.5 |
| Borderline (<55%), % | 9.5 | 4.5 | 5.2 | 6.5 |
| Abnormal (<45%), % | 2.4 | 2.2 | 3.8† | 0 |
| LV mass, g (2031) | 149 ± 58 | 149 ± 48 | 144 ± 45 | 146 ± 42 |
| Fractional shortening at the midwall, % (2006) | 43.3 ± 10.1 | 42.2 ± 8.1 | 42.5 ± 7.9 | 43.1 ± 7.8 |
| Diastolic function | ||||
| Left atrial dimension, mm (2947) | 40.7 ± 6.4† | 38.6 ± 6.6 | 38.5 ± 6.7 | 39.5 ± 6.5 |
| Peak E, m/sec (2951) | 0.72 ± 0.18 | 0.72 ± 0.18 | 0.72 ± 0.20 | 0.80 ± 0.25††‡ |
| Peak A, m/sec (2951) | 0.85 ± 0.29 | 0.79 ± 0.22 | 0.79 ± 0.23 | 0.83 ± 0.26 |
| E/A ratio (2951)§ | 0.81 [0.68–0.98] | 0.89 [0.74–1.07] | 0.90 [0.75–1.08] | 0.92 [0.82–1.13] |
| <0.7: impaired relaxation, % | 35.7†† | 17.4 | 16.1 | 13.0 |
| 0.7–1.5, % | 59.5 | 77.0 | 78.1 | 82.6 |
| >1.5: restrictive filling pattern, % | 4.8 | 5.6 | 5.8 | 4.4 |
| Other measurements | ||||
| Heart rate, sec (3034) | 69 ± 11† | 64 ± 10 | 65 ± 10 | 64 ± 7 |
Abbreviations: TSH: thyroid-stimulating hormone, LV = left ventricular, Peak E = doppler early diastolic peak filling velocity, Peak A = doppler late diastolic peak filling velocity, E/A ratio = early/late transmitral peak flow velocity ratio. E/A ratio was used to categorize impaired relaxation (low E/A) and restrictive patterns (high E/A).
Values are mean ± SD or percentages. For each outcome measure, those for whom the outcome measure was not available were not included in the analysis.
P <0.05,
p < 0.01 compared to euthyroid category, after adjustment for age and gender. No significant differences for other comparisons. For mitral filling velocities, differences were similar after further adjustment for heart rate and systolic blood pressure.
P = 0.008 according to Kruskal-Wallis test.
Expressed as median [25%–75%], and reciprocal transformation of the variable (1/x) to normalize the distribution for adjusted comparisons.
Risk of Heart Failure Events
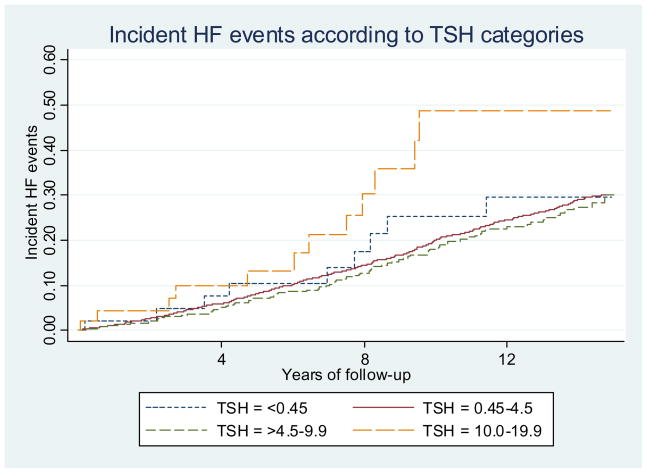
Over a median (interquartile range) follow-up of 12 years (7.0–14.4), 736 (24%) participants developed HF events. Among the 474 participants with sublinical hypothyroidism, 109 received thyroxine replacement during follow-up, with 28 reporting intermittent use. Among the 46 participants with TSH≥10.0, 22 received thyroxine during follow-up (7 intermittent use). In the analysis censoring follow-up at first thyroxine use, participants with TSH≥10.0 had a greater incidence of HF compared to euthyroid participants (41.7 vs. 22.9/1000 person-years, p=0.01), but rates were similar for those with subclinical hyperthyroidism or TSH 4.5–9.9 (Figure). In multivariate analysis censoring at first thyroxine use, the risk of HF was increased among those with TSH≥10.0 (HR: 1.88, 95%CI: 1.05–3.34; Table 3, model 1). Multivariate model omitting lipids yielded similar results.
Figure. Incident Heart Failure Events According to TSH Levels.
Abbreviations: TSH: thyroid-stimulating hormone.
Participants with TSH≥10.0–19.9 mU/L who were untreated by thyroxine replacement (participants censored at the time of first thyroxine use) had a greater incidence of HF events compared to euthyroid participants (41.7 vs. 22.9/1000 person-years, p=0.01), but rates were similar for those with subclinical hyperthyroidism or those with TSH between 4.5 and 9.9 mU/L.
Table 3.
Subclinical thyroid dysfunction and the risk of incident heart failure events (n = 3044)
| Thyroid status | Subclinical Hyperthyroid | Euthyroid | Subclinical Hypothyroid | |
|---|---|---|---|---|
| TSH, mU/L | < 0.45 | 0.45–4.5 | 4.5–9.9 | 10.0–19.9 |
| No. at risk | 44 | 2526 | 428 | 46 |
| No. of events | 10 | 621 | 89 | 16 |
| Incidence rate (95% CI) per 1000 person-years* | 23.9 (12.4–45.9) | 22.9 (21.1–24.8) | 20.5 (16.5–25.5) | 41.7 (23.7–73.5)† |
| Adjusted HR (95% CI) | ||||
| Model 1‡ | 0.94 (0.48–1.83) | 1.0 | 0.92 (0.73–1.17) | 1.88 (1.05–3.34) |
| Model 2§ | ||||
| No thyroxine use during follow-up | 1.0 | 0.92 (0.73–1.16) | 1.83 (1.05–3.20) | |
| With thyroxine use during follow-up | 1.0 | 0.28 (0.11–0.74) | 0.50 (0.14–1.74) | |
Abbreviation: TSH: thyroid-stimulating hormone.
Participants were censored at the time of first use of thyroxine replacement. Those treated by thyroxine replacement at baseline were excluded from the study sample.
P = 0.01 for pairwise comparison with euthyroid category. No significant differences in HF rates for participants with TSH between 4.5 and 9.9 mU/L and those with TSH <0.45 mU/L compared to euthyroid participants.
Adjusted for age, gender, race, clinical cardiovascular disease at baseline, atrial fibrillation at baseline, alcohol use, smoking status, diabetes mellitus, hypertension, body mass index, LDL-cholesterol, HDL-cholesterol and creatinine. Hypertension was defined as blood pressure ≥140/90 mmHg, or self-report of hypertension and use of blood pressure medications (diuretics, β-blockers, angiotensin-converting enzyme inhibitors, or calcium-channel blockers). Participants were censored at the time of first use of thyroxine replacement. Those treated by thyroxine replacement at baseline were excluded from the study sample.
Stratified model by periods of use or no use of thyroxine replacement during follow-up, because of interaction by thyroxine replacement use in those with subclinical hypothyroidism (p=0.02), and adjusted for the same covariates than in model 1 plus thyroxine use as a time-dependent covariate during all follow-up. Participants changed stratum when thyroxine use was initiated. No significant interactions in those with subclinical hyperthyroidism.
The relationship between subclinical hypothyroidsim and HF differed by thyroxine use during follow-up (p=0.02 for interaction). In a model (model 2) including all follow-up and thyroxine use as a time-dependent covariate, participants with TSH≥10.0 had an increased risk of HF during periods where thyroxine use was not reported, but no increased risk during periods of thyroxine use. Almost identical results were obtained using a model that incorporated history of thyroxine use as a time-dependent covariate (participants classified as user from first use) and allowing for interaction of this measure with thyroid status (data not shown).
Stratifying sublinical hypothyroidism into those with TSH 4.5–6.9 and 7.0–9.9 mU/L, we similarly found no significantly increased risk of HF; graphical examination of hazard function showed no increased risk up to around 10 mU/L. A cubic spline analysis also showed the non-linearity of this relationship. None of the 6 participants with TSH<0.10 had HF events. The relationship between subclinical thyroid disease and HF did not differ by prevalent CVD or gender (each interaction p value >0.20). Excluding 595 participants with prevalent CVD yielded similar results, including in those with TSH≥10.0 (HR: 2.17, 95%CI: 1.15–4.07) in multivariate analysis censoring at first thyroxine use. Based on baseline echocardiographic data, peak E velocity might be a potential mediator of this relationship; further adjusting Model 1 for peak E velocity yielded an HR of 1.69 (95%CI: 0.94–3.02) for those with TSH≥10, showing that this relationship was not fully explained by increased peak E velocity and that other factors, including unmeasured echocardiographic parameters, might play a role in this relationship.
Echocardiographic Changes over Time
Repeat echocardiograms were obtained on 2148 participants after 5 years; those with TSH≥10.0 had a larger increase in LV mass (+21 vs. +4 g, p=0.04, Table 4) and a greater proportion of HF events associated with low EF than in euthyroid participants (80 vs. 45%, p=0.08). Peak E velocity decreased more in subjects with TSH≥10 than in euthyroid participants (−0.10 vs. −0.01m/sec, p=0.005), which might be related to increase in LV mass over time, increasing impairment of LV relaxation, a plateau effect and/or regression to the mean due to higher baseline values.
Table 4.
Change in echocardiographic characteristics between baseline (1988–1989) and 5-year follow-up (1994–1995) according to thyroid status *
| Measure (n) | Subclinical Hyperthyroid | Euthyroid | Subclinical Hypothyroid | ||
|---|---|---|---|---|---|
| TSH categories, mU/L | TSH < 0.45 (n=44) | TSH 0.45–4.5 (n=2526) | TSH 4.5–9.9 (n=428) | TSH 10.0–19.9 (n=46) | |
| Systolic function | |||||
| LV mass, g (1191) | −5 ± 71 | +4 ± 39 | +3 ± 29 | +21 ± 31† | |
| Fractional shortening measured at the midwall, % (1121) | + 2.3 ± 10.2 | − 0.3 ± 9.6 | − 1.3 ± 8.6 | - 1.4 ± 10.7 | |
| Echocardiograms at 5-year | |||||
| LV ejection fraction, qualitative assessment‡ (2032) | |||||
| Normal (≥55%), % | 90.9 | 89.8 | 91.4 | 90.0 | |
| Borderline (<55%), % | 9.1 | 7.2 | 6.5 | 6.7 | |
| Abnormal (<45%), % | 0 | 3.0 | 2.2 | 3.3 | |
| Diastolic function | |||||
| Left atrial dimension, mm (2046) | −0.9 ± 6.2† | +1.7 ± 6.0 | +1.2 ± 6.2 | +1.1 ± 5.4 | |
| Peak E, m/sec (2017) | −0.02 ± 0.20 | −0.01 ± 0.17 | −0.01 ± 0.15 | −0.10 ± 0.14†† | |
| Peak A, m/sec (1965) | −0.08 ± 0.23†† | +0.03 ± 0.17 | +0.04 ± 0.17 | −0.01 ± 0.16 | |
| Echocardiograms at the time of incident HF | |||||
| LV ejection fraction, qualitative assessment (185) | |||||
| Normal, % | 33.3 | 36.3 | 51.6 | 0 | |
| Borderline, % | 33.3 | 19.2 | 9.7 | 20.0 | |
| Abnormal, % | 33.3 | 44.5 | 38.7 | 80.0§ | |
Abbreviations: TSH: thyroid-stimulating hormone, LV = left ventricular, Peak E = doppler early diastolic peak filling velocity, Peak A = doppler late diastolic peak filling velocity.
Values are mean ± SD or percentages. + = increase in the measurement from baseline to 5-year follow-up; − = decrease in the measurement from baseline to 5-year follow-up.
P<0.05,
p<0.01 compared to euthyroid category, after adjustment for age and gender. No significant differences for other comparisons. For mitral filling velocities, differences were similar after further adjustment for heart rate and systolic blood pressure.
Cross-sectional measures at 5-year follow-up.
P=0.08 compared to euthyroid category.
Compared to euthyroidism, subclinical hyperthyroidism was associated with a smaller increase in left atrial size and peak A velocity over time, but cross-sectional year-5 left atrial sizes and peak A velocities did not differ (40.5 vs. 40.0 mm, p=0.65; 0.81 vs. 0.81 m/sec, p=0.90). Excluding participants with atrial fibrillation at baseline or those who developed HF before year 5 examination (n=237) yielded similar results. For the 499 participants who developed HF after year 5 examination, the time interval±SD between the 5-year echocardiograms and HF was 4.4±2.6 years (3.5±1.9 for those with TSH 10.0).
Discussion
In this large, population-based study of older adults, subclinical hypothyroidism was associated with a moderately elevated risk of HF among older adults with TSH≥10.0 mU/L, consistent with another large cohort study of older individuals(10). The risk of CHF was not increased among the high proportions of older adults with TSH levels between 4.5 and 9.9 mU/L or among those with TSH<0.45. Adverse alterations in two echocardiographic measurements, peak E velocity at baseline - an echocardiographic measurement of diastolic function associated with HF in this cohort sample and previous CHS analysis(14) - and increase in left ventricular mass over 5 years, were also found exclusively in the subgroup of subclinical hypothyroidism with TSH≥10.0 mU/L.
To our knowledge, only one previous population-based prospective study directly examined the relationship between subclinical hypothyroidism and HF events, and found that adults aged 70–79 with TSH≥7.0 mU/L had a higher risk of HF (HR: 2.88, 95%CI 1.39–5.99) in multivariate analysis compared to euthyroid participants, with a more pronounced risk in those with TSH≥10 (HR 3.10, 95%CI: 1.30–7.39), and no increased risk in those with TSH 4.5–6.9(10). Possible explanations for the weaker point estimate found in CHS as compared to the Health, Aging, and Body Composition (Health ABC) Study include differences in the study population that was mainly white in CHS (40% were black in Health ABC), younger mean age (72.6 vs. 74.7), different length of follow-up (12 vs. 4 years), no upper TSH cut-off in Health ABC analysis (1% with TSH≥20) and less precision due to lower power in the Health ABC. In the present study, the fact that HF risk was limited to participants with TSH≥10 who were not taking thyroxine, with a HF risk similar to the euthyroid group under thyroxine replacement, strengthens the case for a potential causal relationship, which could only be definitively proven by RCTs. Potential mechanisms for the impact of thyroid dysfunction on HF may be related to the effects of thyroid hormones on the heart(5,7), notably by the regulation of genes coding for cardiac proteins(5). Overt hypothyroidism also alters cardiovascular function(24).
For most echocardiographic measurements, we found no significant differences for subclinical hypothyroidism or hyperthyroidism compared to euthyroidism, in contrast to several non population-based studies(6,25–27), but consistent with other population-based studies(28,29). Several explanations exist for these discrepant findings, including the age of the underlying study population and the degree of subclinical thyroid dysfunction(8). All of these previous studies had insufficient sample sizes with subclinical hypothyroidism (n=8–66 as compared to 474 in the present study) to investigate different TSH cutoffs(7,28), and none linked abnormalities in the surrogate echocardiographic markers with HF events(8). Indeed, our participants with subclinical hyperthyroidism demonstrated some differences in baseline echocardiographic measurements, but no increase in HF events, suggesting that these echocardiographic abnormalities, while statistically significant, do not result in HF. In our cohort, those with TSH≥10.0 had a higher peak E velocity at baseline, an echocardiographic measurement of diastolic function that was associated with incident HF in this cohort sample and previous CHS analysis(14). The higher early diastolic filling velocity we found in participants with TSH≥10 may reflect increased left atrial pressure and diastolic dysfunction from more marked subclinical hypothyroidism. Some small RCTs have been performed that have shown improvement in echocardiographic measures of cardiac function(30–32), supporting our findings of reduction of risk of HF after thyroxine initiation.
Limitations and Strengths
Among limitations, our data may not be generalizable to younger age groups and it has been suggested that there may be age differences in the associations between subclinical thyroid dysfunction and adverse outcomes(33). Thyroid function testing was performed at a single point in time, which is a limitation of all published observational cohorts(10,11,34). Our power was limited in those with TSH≥10, as this group was small. Our echocardiographic findings could be affected by multiple comparisons and missing data for 5-year and incident HF echocardiograms; we could not definitively conclude on the type of cardiac dysfunction (systolic or diastolic) involved in those with TSH≥10, which needs to be explored in future studies.
These data have a number of strengths: the large, population-based cohort of older adults, designed to examine cardiovascular risk factors; the mean 12-year follow-up; the formal adjudication of HF events; the exclusion of individuals taking thyroxine or other medications that could affect thyroid function testing; and the incorporation of thyroxine use over time analytically(9). In addition, few large prospective studies have the availability of baseline and follow-up echocardiography data.
Clinical Implications
Our data suggest that subclinical hypothyroidism with a TSH≥10.0 mU/L represents a potentially modifiable risk factor for HF in older adults, but not subclinical hypothyroidism with moderate TSH levels (TSH 4.5–9.9 mU/L) and subclinical hyperthyroidism. Our study builds on the prior study demonstrating increased HF risk in marked subclinical hypothyroidism(10), with additional mechanistic support in our study from echocardiographic data and information on reversibility of HF risk with thyroxine replacement. Our findings of a lack of HF risk in the high proportions of older adults with less severe subclinical hypothyroidism are also important, as many patients with TSH levels of 4.5–9.9 mU/L are treated in clinical practice(35), without consistent evidence to support increased risk without thyroxine replacement and improved risk with replacement(1,3). This and previous studies have mostly found no increased cardiovascular risk in subjects with TSH<10(9,10,36), with some conflicting data(11). Moreover, about 20 % of patients are currently overtreated by thyroxine replacement with an increased risk of subclinical hyperthyroidism that has been associated with atrial fibrillation and increased fracture risk(3,37). In aggregate, our findings might help refine a treatment threshold at which clinical benefit would be expected(8) and demonstrate a subpopulation at risk for a life-threatening condition. Clinical trials should examine the efficacy of screening for and treating subclinical thyroid dysfunction, and assess whether the risk of HF might be ameliorated by thyroxine replacement in individuals with TSH levels above 10.0 mU/L.
Acknowledgments
Funding sources
The research reported in this article was supported by contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org. The TSH measurement and this study were supported by an American Heart Association Grant-in-Aid (to Dr Fried) with funding from July 1991 to June 1993.
Role of the Sponsor
This study was funded through contracts with the NHLBI and included substantial NHLBI involvement in study design and oversight. A member of the NHLBI serves on the executive committee of the study, and NHLBI reviewed the manuscript, and approved its publication.
Abbreviations list
- HF
heart failure
- TSH
thyroid-stimulating hormone
- FT4
free thyroxine
- CHS
Cardiovascular Health Study
- CVD
cardiovascular disease
- RCT
randomized controlled trial
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- BMI
body mass index
Footnotes
Conflict of interest
No author has a financial interest in the subject matter or materials discussed in the manuscript.
Statistical Evaluation
Dr. Vittinghoff, Professor of Biostatistics in the Department of Epidemiology and Biostatistics University of California, San Francisco, reviewed the statistical analyses of the paper and is included in the authors of this paper.
References
- 1.Helfand M. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140:128–41. doi: 10.7326/0003-4819-140-2-200401200-00015. [DOI] [PubMed] [Google Scholar]
- 2.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 3.Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. Jama. 2004;291:228–38. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 4.Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT. Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. J Clin Endocrinol Metab. 2005;90:581–5. doi: 10.1210/jc.2004-1231. discussion 586–7. [DOI] [PubMed] [Google Scholar]
- 5.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–9. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 6.Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med. 2002;137:904–14. doi: 10.7326/0003-4819-137-11-200212030-00011. [DOI] [PubMed] [Google Scholar]
- 7.Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid. 2007;17:625–30. doi: 10.1089/thy.2007.0158. [DOI] [PubMed] [Google Scholar]
- 8.Cappola AR. Subclinical thyroid dysfunction and the heart. J Clin Endocrinol Metab. 2007;92:3404–5. doi: 10.1210/jc.2007-1575. [DOI] [PubMed] [Google Scholar]
- 9.Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. Jama. 2006;295:1033–41. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165:2460–6. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 11.Walsh JP, Bremner AP, Bulsara MK, et al. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med. 2005;165:2467–72. doi: 10.1001/archinte.165.21.2467. [DOI] [PubMed] [Google Scholar]
- 12.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 14.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–8. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 15.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–37. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 16.Gardin JM, Siscovick D, Anton-Culver H, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–48. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 17.Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–15. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 18.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 19.Stoddard MF, Pearson AC, Kern MJ, Ratcliff J, Mrosek DG, Labovitz AJ. Left ventricular diastolic function: comparison of pulsed Doppler echocardiographic and hemodynamic indexes in subjects with and without coronary artery disease. J Am Coll Cardiol. 1989;13:327–36. doi: 10.1016/0735-1097(89)90507-x. [DOI] [PubMed] [Google Scholar]
- 20.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 21.Bibbins-Domingo K, Chertow GM, Fried LF, et al. Renal function and heart failure risk in older black and white individuals: the Health, Aging, and Body Composition Study. Arch Intern Med. 2006;166:1396–402. doi: 10.1001/archinte.166.13.1396. [DOI] [PubMed] [Google Scholar]
- 22.Ingelsson E, Bjorklund-Bodegard K, Lind L, Arnlov J, Sundstrom J. Diurnal blood pressure pattern and risk of congestive heart failure. Jama. 2006;295:2859–66. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 23.Vittinghoff E, Glidden D, Shiboski S, McCulloch C. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. New York: Springer-Verlag; 2005. [Google Scholar]
- 24.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–35. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 25.Tseng KH, Walfish PG, Persaud JA, Gilbert BW. Concurrent aortic and mitral valve echocardiography permits measurement of systolic time intervals as an index of peripheral tissue thyroid functional status. J Clin Endocrinol Metab. 1989;69:633–8. doi: 10.1210/jcem-69-3-633. [DOI] [PubMed] [Google Scholar]
- 26.Forfar JC, Wathen CG, Todd WT, et al. Left ventricular performance in subclinical hypothyroidism. Q J Med. 1985;57:857–65. [PubMed] [Google Scholar]
- 27.Biondi B, Fazio S, Palmieri EA, et al. Left ventricular diastolic dysfunction in patients with subclinical hypothyroidism. J Clin Endocrinol Metab. 1999;84:2064–7. doi: 10.1210/jcem.84.6.5733. [DOI] [PubMed] [Google Scholar]
- 28.Iqbal A, Schirmer H, Lunde P, Figenschau Y, Rasmussen K, Jorde R. Thyroid stimulating hormone and left ventricular function. J Clin Endocrinol Metab. 2007;92:3504–10. doi: 10.1210/jc.2007-0727. [DOI] [PubMed] [Google Scholar]
- 29.Dorr M, Wolff B, Robinson DM, et al. The association of thyroid function with cardiac mass and left ventricular hypertrophy. J Clin Endocrinol Metab. 2005;90:673–7. doi: 10.1210/jc.2004-1554. [DOI] [PubMed] [Google Scholar]
- 30.Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab. 2001;86:1110–5. doi: 10.1210/jcem.86.3.7291. [DOI] [PubMed] [Google Scholar]
- 31.Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-Thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med. 1984;101:18–24. doi: 10.7326/0003-4819-101-1-18. [DOI] [PubMed] [Google Scholar]
- 32.Franzoni F, Galetta F, Fallahi P, et al. Effect of L-thyroxine treatment on left ventricular function in subclinical hypothyroidism. Biomed Pharmacother. 2006;60:431–6. doi: 10.1016/j.biopha.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Cooper DS. Thyroid disease in the oldest old: the exception to the rule. Jama. 2004;292:2651–4. doi: 10.1001/jama.292.21.2651. [DOI] [PubMed] [Google Scholar]
- 34.Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132:270–8. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 35.Fatourechi V, Lankarani M, Schryver PG, Vanness DJ, Long KH, Klee GG. Factors influencing clinical decisions to initiate thyroxine therapy for patients with mildly increased serum thyrotropin (5.1–10.0 mIU/L) Mayo Clin Proc. 2003;78:554–60. doi: 10.4065/78.5.554. [DOI] [PubMed] [Google Scholar]
- 36.Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet. 2001;358:861–5. doi: 10.1016/S0140-6736(01)06067-6. [DOI] [PubMed] [Google Scholar]
- 37.Bauer DC, Ettinger B, Nevitt MC, Stone KL. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001;134:561–8. doi: 10.7326/0003-4819-134-7-200104030-00009. [DOI] [PubMed] [Google Scholar]