Abstract
Purpose
Thiamine is an essential component of cellular metabolism, and lack of this vitamin results in a potentially life-threatening biochemical lesion. The stress of surgery and critical disease depletes electrolytes, minerals, and essential biochemical substrates. We hypothesized that critical illness (represented by major surgery) will result in decreased thiamine levels over time.
Methods
We performed a prospective, observational study of serial thiamine levels of 15 patients who underwent non-emergent coronary artery bypass graft (CABG) surgery. The primary endpoint was change in thiamine levels from pre- to immediately post-surgery. Secondary endpoints included change in thiamine levels between pre- surgery and 6 and 24 hour time-points.
Results
Of the 15 study patients, one did not have a plasma thiamine measurement at time zero because of lab error and could not be accounted for in paired comparisons over time. Plasma thiamine levels decreased significantly from the pre to post-CABG period (p = 0.0004). In addition, there was a statistically significant decrease in thiamine levels from pre-surgery to 24 hours (P = 0.003).
Conclusion
Our data suggest that major surgery (as a surrogate for the stress of critical illness) depletes thiamine levels; further study is needed to determine whether routine replacement of thiamine in the critically ill is warranted.
Keywords: Thiamine deficiency, critical illness, coronary artery bypass graft surgery
Introduction
Thiamine is an essential component of cellular metabolism with a key role in mitochondrial machinery. Specifically, thiamine is a co-factor for pyruvate dehydrogenase, the enzyme responsible for the conversion of pyruvate into acetyl-CoA. A deficiency of this vitamin results in a potentially life-threatening biochemical lesion, as pyruvate cannot enter the Citric Acid Cycle and is instead converted to lactic acid.[1-3] Thiamine deficiency may result in neurologic dysfunction (Wernicke's Encephalopathy), cardiac dysfunction (wet beriberi), peripheral polyneuropathy (dry beriberi), lactic acidosis, gastrointestinal beriberi, and death.[1, 3-10] Moreover, lower levels of thiamine have been associated with increased mortality in the critically ill[11] and thiamine deficiency syndromes have been described in the intensive care setting.[5, 12-14]
While electrolyte replacement therapy is routine for patients in a critical disease state or those undergoing the stress of surgery, little research or clinical consideration has been given to the potential loss of essential biochemical substrates or vitamins, specifically thiamine. As previously noted, the clinical consequences of thiamine deficiency may be severe and result in permanent neurologic dysfunction or death. We hypothesized that the stress of critical disease would result in decreased thiamine levels from increased metabolic demand. In order to test our hypothesis, we performed a prospective, observational study of serial thiamine levels of patients who underwent non-emergent coronary artery bypass surgery (major surgical stress).
Methods
Design
Prospective, observational study conducted at an urban tertiary care center with 50,000 emergency department visits/year and intensive care units numbering a total of approximately 50 beds. This study was approved by the Beth Israel Deaconess Medical Center Institutional Review Board and all patients provided informed written consent in order to participate.
Inclusion criteria
All outpatients over the age of 18 who were undergoing elective Coronary Artery Bypass Graft Surgery (CABG) were eligible for inclusion. Data from all patients were included in the analysis. All patients were screened, consented, and enrolled prior to surgery.
Exclusion Criteria
Exclusion criteria consisted of use of multivitamins, age < 18, or unwilling to give consent.
Procedures
Plasma thiamine levels were measured at pre-operation, immediately post-operation, six hours post operation and 24 hours post operation. The pre-operation blood draw was performed in the pre-op area prior to the initiation of anesthesia. Blood was collected from patients by venipuncture, or via a pre-existing arterial or venous catheter, into two 5 mL EDTA tubes. Blood was centrifuged at 2000 XG for 10 minutes after which 2 milliliters of this plasma was aliquoted into crytotubes and frozen. Blood was protected from light during the collection and freezing process. Frozen samples were sent to Quest Diagnostics. At Quest Diagnostics, plasma was deproteinized and then incubated with acid phosphatase to convert thiamine phosphate esters to free thiamine. The free thiamine was then oxidized to thiochrome by the addition of alkaline potassium ferricyanide. Depending on the age of the column and the temperature of the room, thiochrome retention time varied from 2.5 minutes to 3.0 minutes. The mixture was injected to a Supelco (Bellefonte, PA 16823) HPLC column (7.5 cm × 4.6 mm, particle size: 3 μm) connected to an HPLC system using Hitachi (Pleasant, CA 94588) pump, autosampler and fluorescent detector (excitation wavelength: 365 nm, emission wavelength: 440 nm). Seventy-five mM potassium phosphate at pH 7.5 with 25% methanol was used as the mobile phase for the HPLC system. The flow rate was set at 1.0 mL per minute. Through this process, the thiochrome was then separated from other interfering substances and then measured fluorometrically. The amount of total thiamine in an unknown sample is proportional to the amount of thiochrome formed. Absolute thiamine deficiency was determined using previously established standard lab reference range from Quest Diagnostics; specifically, absolute thiamine deficiency was defined as a level less than or equal to 9 nmol/Liter.
Statistical Methods
Baseline characteristics were reported with simple descriptive statistics. We used a paired t-test to compare pre- and post-CABG thiamine levels over time. The primary endpoint was the difference in thiamine levels pre- to immediately post-operative. A p-value of 0.05 was used to determine statistical significance. Secondary endpoints included 6 and 24 hour thiamine levels. In order to account for multiple measurements, we used a Bonferroni correction factor to adjust the p value for significance such that a p value of 0.025 was considered statistically significant for time point differences between pre- and 6 and 24 hour thiamine levels.
Results
25 patients were screened yielding a total of 15 study patients. The baseline characteristics of the study patients are shown in Table 1. Of the 15 study patients, one did not have a plasma thiamine measurement at time zero because of lab error and could not be accounted for in paired comparisons over time. Two patient samples may have had partial, premature thawing during transport, however independent laboratory experts at Quest Diagnostics felt results were unaffected. Moreover, all samples for each of these patients in question were equally affected (time zero through time twenty-four) and therefore all would have the same “relative” effect, if any did indeed occur. Thus, both of these patients were counted in the analysis.
Table 1.
Baseline Characteristics
| Baseline Characteristics | |
|---|---|
| No. | 15 |
| Age, ys. | 68.5 ± 6.7 |
| Male/Female, | 60:40 |
| Ethnicity, % | |
| White | 100 |
| African American | |
| Hispanic | |
| Asian | |
| Co-morbidity, % | |
| Hypertension | 73.3 |
| Coronary Artery Disease | 40 |
| Myocardial Infarction | 20 |
| Chronic Obstructive Lung Disease | 13.3 |
| Diabetes Mellitus | 40 |
| Vital Signs (baseline) | |
| Temperature, F | 98.0 ± 0.9 |
| Heart rate, beats/min | 77 ± 19.9 |
| MAP, mmHg | 78.2 ± 25 |
| Respiratory Rate, breaths/min | 17.8 ± 2.6 |
| Laboratory Values | |
| WBC, per mm3 | 11.4 ± 3.2 |
| Hematocrit, g/dl | 36 ± 7.5 |
| Sodium, mmol/l | 138 ± 3.1 |
| Potassium, mmol/l | 4.3 ± 0.4 |
| Bicarbonate, mmol/l | 26 ± 3.4 |
| Creatinine, mg/dl | 2.1 ± 1.3 |
| Total Bilrubin, mg/dl | 0.5 ± 0.28 |
| Alanine Aminotransferase (ALT) | 24 ± 7 |
| Aspartate Aminotransferase (AST) | 23 ± 1.4 |
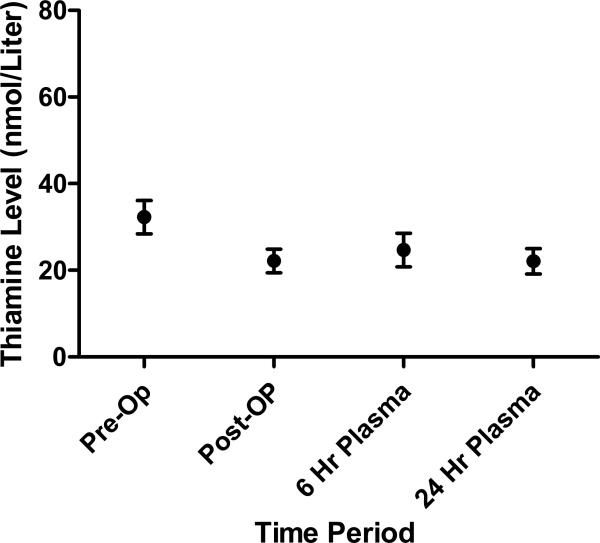
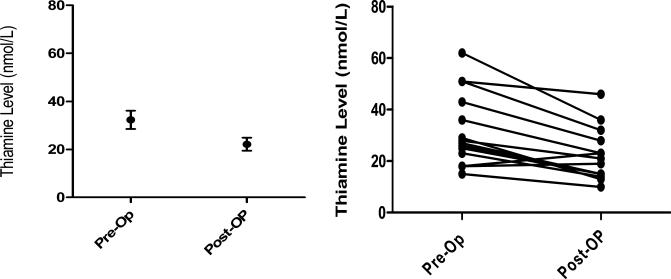
Plasma thiamine levels over time are shown in Figure 1. For the primary endpoint, there was a statistically significant decrease in thiamine levels between the baseline (pre-CABG) and post-CABG serum thiamine levels (mean of differences = 10.14 nmol/Liters; [95% CI 5.6 – 14.7], p = 0.0004) (Figure 2). The secondary endpoint of pre- to 24 hour thiamine levels were also statistically significantly lower (mean of differences= 8.5 nmol/Liters; [95% CI 3.6-13.3], p = 0.003), however pre- to 6 hour levels were not significantly lower (mean of differences = 5.8 nmol/Liters [−.06 – 11.7], p = 0.052).
Figure 1.
Thiamine levels (mean and standard error of the mean) over time.
Figure 2.
(Left) Group mean +/− SE pre- and post- thiamine levels. (Right) Paired comparison of pre- and post-operative CABG thiamine levels were statistically significantly lower than pre-op thiamine levels (p = 0.0004).
Discussion
In patients undergoing CABG, thiamine levels decreased over time. Specifically, we found a statistically significant drop in thiamine levels from pre- to immediately post-CABG surgery. In addition, there was a statistically significant decrease in thiamine levels from pre-surgery to 24 hours post-surgery. Thiamine is an essential component for multiple biochemical processes in the body, and depleted states may result in serious neurologic morbidity and even death.[1] The current investigation raises the possibility that the stress of critical illness (or, in this case, surgery) may deplete or decrease thiamine. The implications of this study are important in that routine replacement of thiamine for critically ill patients is not widely performed particularly when patients are not known alcoholics (a population which traditionally receives thiamine supplementation).
Several small clinical investigations have evaluated thiamine levels in the critically ill but none within the CABG population using the methodology employed in the current investigation.[11, 15] Thiamine deficiency in the critically ill has been described in two previous investigations using indirect measures. Cruickshank et al. examined thiamine deficiency in critically ill adults requiring parenteral nutrition.[11] Using an indirect and functional measure of thiamine deficiency (erythrocyte transketolase), they performed a retrospective analysis of 158 patients. Patients with higher body thiamine status as determined by erythrocyte transketolase had a lower mortality than those with lower levels of thiamine (72% vs. 50%; p < 0.05).[11] However, this study was retrospective, utilized an indirect measure of thiamine, and focused only on those who required parenteral nutritional support. In the pediatric intensive care unit, Seear et al. found that 10 out of 80 children (12.5%) were thiamine deficient, again based upon erythrocyte transketolase levels.[15] Both of these investigations raised considerable concern for the prevalence of thiamine deficiency in the critically ill. In contrast to previous investigations in the critically ill, we measured both baseline and “stressed” levels rather than levels only after entry into the critical care setting. Thus, we are able to judge the change in baseline levels after introduction of a significant stressor to the body. Second, we utilized direct rather than indirect measurements therefore mitigating the potential for confounding from other biochemical processes. A previous investigation evaluated thiamine levels pre- and post- orthopedic surgery again using the indirect thiamine assay and found that levels decreased over time.[16] Their findings are consistent with the current investigations. Moreover, the decrease of thiamine with increased cellular metabolism is also consistent with laboratory models of thiamine activity. Specifically, McCourt found that despite the catalytic role of thiamine, destruction occurs when thiamine is utilized in a reaction with pyruvate decarboxylase in a model derived from E. Coli.[17] Thus, increased metabolic demand would logically increase usage and therefore lead to increased depletion consistent with the findings in this report.
As stated, thiamine is an essential component of cellular metabolism without which neurologic dysfunction, cardiovascular dysfunction, or death may occur.[1] Thus, the implications of the current investigation are potentially of high importance for the routine management of critically ill patients. Thiamine is essential to a well-functioning mitochondrium, however despite much attention to mitochondrial dysfunction in sepsis, few have considered the importance of this vitamin.[18] Like other electrolytes and biochemical products in the body, thiamine may need to be replaced in those with critical illness. Moreover, critical illness may actually require higher than normal levels similar to that of corticosteroids however this possibility remains unexplored. That stated, the current study was not designed to determine the clinical significance of a decrease in thiamine over time but instead was designed to test the hypothesis of whether levels would change over time in the face of a significant stressor. We chose to evaluate non-emergent CABG patients since this population is relatively healthy prior to surgery and then undergoes a tremendous stressor followed by a typically rapid recovery. Thus, the current study does not necessarily support widespread thiamine administration to the critically ill and post-surgical patient but instead illustrates that thiamine levels may decrease in the setting of physical stress and the consideration for replacement may need to be explored through future investigation.
Limitations
The sample size of the current study is small; however, there was adequate statistical power to conclude that thiamine levels decreased over time. This investigation was performed only on CABG patients rather than other surgical conditions. While we would expect similar findings in cohorts undergoing other surgical procedures or the stress of critical illness, this extrapolation is not definitive and may need to be tested. We chose to evaluate patients utilizing a direct assay for thiamine; whether the results of the study would differ if a functional assay of thiamine deficiency (i.e., erythrocyte transketolase) was utilized remains unknown.
Conclusion
Thiamine levels are depleted over time in patients undergoing CABG surgery. The depletion of thiamine raises concern that patients with critical illness or in the post-operative period may need thiamine replacement.
Funding
This project was funded from NIH grant P30 DK040561
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33(11):1892–9. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 2.Donnino MW, Miller J, Goyal N, Loomba M, Sankey SS, Dolcourt B, Sherwin R, Otero R, Wira C. Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation. 2007;75(2):229–34. doi: 10.1016/j.resuscitation.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Campbell CH. Lacticacidosis and thiamine deficiency. Lancet. 1984;2(8414):1282. doi: 10.1016/s0140-6736(84)92835-6. [DOI] [PubMed] [Google Scholar]
- 4.Donnino M. Gastrointestinal beriberi: a previously unrecognized syndrome. Ann Intern Med. 2004;141(11):898–9. doi: 10.7326/0003-4819-141-11-200412070-00035. [DOI] [PubMed] [Google Scholar]
- 5.Fried RT, Levy M, Leibowitz AB, Bronster DJ, Iberti TJ. Wernicke's encephalopathy in the intensive care patient. Crit Care Med. 1990;18(7):779–80. doi: 10.1097/00003246-199007000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Oriot D, Wood C, Gottesman R, Huault G. Severe lactic acidosis related to acute thiamine deficiency. JPEN J Parenter Enteral Nutr. 1991;15(1):105–9. doi: 10.1177/0148607191015001105. [DOI] [PubMed] [Google Scholar]
- 7.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. 1989.
- 8.Francini-Pesenti F, Brocadello F, Manara R, Santelli L, Laroni A, Caregaro L. Wernicke's syndrome during parenteral feeding: not an unusual complication. Nutrition. 2009;25(2):142–6. doi: 10.1016/j.nut.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Koike H, Ito S, Morozumi S, Kawagashira Y, Iijima M, Hattori N, Tanaka F, Sobue G. Rapidly developing weakness mimicking Guillain-Barre syndrome in beriberi neuropathy: two case reports. Nutrition. 2008;24(7-8):776–80. doi: 10.1016/j.nut.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Nakasaki H, Ohta M, Soeda J, Makuuchi H, Tsuda M, Tajima T, Mitomi T, Fujii K. Clinical and biochemical aspects of thiamine treatment for metabolic acidosis during total parenteral nutrition. Nutrition. 1997;13(2):110–7. doi: 10.1016/s0899-9007(96)00384-x. [DOI] [PubMed] [Google Scholar]
- 11.Cruickshank AM, Telfer AB, Shenkin A. Thiamine deficiency in the critically ill. Intensive Care Med. 1988;14(4):384–7. doi: 10.1007/BF00262893. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura K, Yamaguchi T, Tanaka H, Hashimoto S, Yang M, Takahashi T. TPN-induced fulminant beriberi: a report on our experience and a review of the literature. Surg Today. 1996;26(10):769–76. doi: 10.1007/BF00311635. [DOI] [PubMed] [Google Scholar]
- 13.Fattal-Valevski A, Kesler A, Sela BA, Nitzan-Kaluski D, Rotstein M, Mesterman R, Toledano-Alhadef H, Stolovitch C, Hoffmann C, Globus O, Eshel G. Outbreak of life-threatening thiamine deficiency in infants in Israel caused by a defective soy-based formula. Pediatrics. 2005;115(2):e233–8. doi: 10.1542/peds.2004-1255. [DOI] [PubMed] [Google Scholar]
- 14.Kountchev J, Bijuklic K, Bellmann R, Joannidis M. A patient with severe lactic acidosis and rapidly evolving multiple organ failure: a case of shoshin beri-beri. Intensive Care Med. 2005;31(7):1004. doi: 10.1007/s00134-005-2648-7. [DOI] [PubMed] [Google Scholar]
- 15.Seear M, Lockitch G, Jacobson B, Quigley G, MacNab A. Thiamine, riboflavin, and pyridoxine deficiencies in a population of critically ill children. J Pediatr. 1992;121(4):533–8. doi: 10.1016/s0022-3476(05)81140-0. [DOI] [PubMed] [Google Scholar]
- 16.Older MW, Dickerson JW. Thiamine and the elderly orthopaedic patient. Age Ageing. 1982;11(2):101–7. doi: 10.1093/ageing/11.2.101. [DOI] [PubMed] [Google Scholar]
- 17.McCourt JA, Nixon PF, Duggleby RG. Thiamin nutrition and catalysis-induced instability of thiamin diphosphate. Br J Nutr. 2006;96(4):636–8. [PubMed] [Google Scholar]
- 18.Singer M. Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit Care Med. 2007;35(9 Suppl):S441–8. doi: 10.1097/01.CCM.0000278049.48333.78. [DOI] [PubMed] [Google Scholar]