Abstract
This study examined the effects of the Dietary Approaches to Stop Hypertension (DASH) diet on insulin sensitivity and lipids. In a randomized control trial, 144 overweight (body mass index 25–40) men (N= 47) and women (N= 97) with high blood pressure (130–159/85–99 mm Hg) were randomly assigned to either: (1) DASH diet alone (DASH-A); (2) DASH diet with aerobic exercise and caloric restriction (DASH-WM); or usual diet controls (UC). Body composition, fitness, insulin sensitivity, and fasting lipids were measured before and following 4 months of treatment. Insulin sensitivity was estimated based on glucose and insulin levels in the fasting state and after an oral glucose load. Participants in the DASH-WM condition lost weight (−8.7 [95% CI = −2.0, −9.7] kg,), and exhibited a significant increase in aerobic capacity, while the DASH-A and UC participants maintained their weight (−0.3 [95% CI = −1.2, 0.5] kg and +0.9 [95% CI = 0.0, 1.7] kg, respectively) and had no improvement in exercise capacity. DASH-WM demonstrated lower glucose levels following the oral glucose load, improved insulin sensitivity, and lower total cholesterol and triglycerides compared to both DASH-A and UC, and lower fasting glucose and low-density lipoprotein cholesterol compared to UC; DASH-A participants generally did not differ from UC in these measures. Combining the DASH diet with exercise and weight loss resulted in significant improvements in insulin sensitivity and lipids. Despite clinically significant reductions in blood pressure, the DASH diet alone, without caloric restriction or exercise, resulted in minimal improvements in insulin sensitivity or lipids.
Keywords: Diet, Hypertension, Lipids, Insulin Resistance, Exercise
Introduction
High blood pressure (HBP) affects more than 70 million Americans and is among the most common reasons for outpatient visits to physicians’ offices1. Although HBP can be lowered pharmacologically2, 3 anti-hypertensive medications may be costly, oftentimes must be used in combination to achieve adequate blood pressure (BP) control, and can be associated with side effects that impair quality of life and reduce adherence2, 4. Moreover, metabolic abnormalities associated with HBP, such as insulin resistance and hyperlipidemia, may persist or may be exacerbated by some medications5. Consequently, there is great deal of interest in the use of non-pharmacologic interventions in the prevention and management of HBP.
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7)6 recommends that lifestyle modifications such as weight loss and regular aerobic exercise be the initial treatment strategy for lowering HBP, and specifically recommends the Dietary Approaches to Stop Hypertension (DASH) diet-- a diet rich in fiber, fruits, vegetables and low-fat dairy products, and low in fat. This diet was established as efficacious in reducing BP in a series of 4 to 8 week “feeding” trials, in which HBP patients were provided DASH meals in a controlled environment7, 8. A subsequent randomized trial to examine the efficacy of the DASH diet in an outpatient setting, the PREMIER study9 demonstrated that the DASH diet could be successfully implemented in free-living persons. Both “established” JNC 610 recommendations and the JNC 6 recommendations plus the DASH diet (i.e., JNC 7 recommendations6) were associated with significant BP reductions compared to advice only controls. In an ancillary study, Ard et al.11 reported results from a subsample of 52 PREMIER participants who received an oral glucose tolerance test (OGTT) at baseline and following 6-months of treatment. Those who received the “established” intervention with or without the DASH diet showed greater improvements in fasting insulin and glucose compared to controls, but only the “established plus DASH” intervention achieved greater improvements in insulin sensitivity. However, because participants in the “established plus DASH” treatment tended to lose more weight and reduce their waist circumference compared to participants in both the advice only control condition and the JNC 6 “established” intervention condition, the incremental benefit of the DASH diet to lifestyle modifications of weight loss, exercise, and sodium restriction could not be determined.
In an effort to examine the independent and combined effects of the DASH diet and weight loss plus exercise on BP and biomarkers of risk, the ENCORE study examined 4 months of treatment with the DASH diet alone, without exercise or weight loss (DASH-A), or the DASH diet combined with a behavioral weight management program including caloric restriction and aerobic exercise (DASH-WM), in 144 men and women with HBP12. Results showed that both DASH-A and DASH-WM were associated with larger BP reductions compared to a usual diet control (UC) group, although the DASH-WM condition achieved larger BP reductions and greater improvements in such cardiovascular biomarkers as pulse wave velocity, baroreflex sensitivity, and left ventricular mass. The present study reports the findings from the ENCORE study on the secondary outcomes of insulin sensitivity and lipids.
Methods
Participants
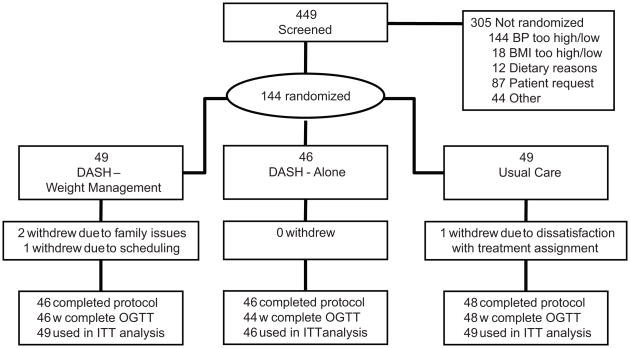
As described in our primary paper12, the ENCORE trial enrolled 144 healthy, but overweight adults with HBP (Figure 1). Persons were eligible if they were not taking anti-hypertensive medication and had a mean systolic blood pressure (SBP) 130–159 or diastolic blood pressure (DBP) 85–99 mm Hg averaged over four separate BP screening visits. Potential participants were asked to refrain from smoking or ingesting caffeine for at least 30-min prior to their appointment time. BP measurements were standardized for cuff size, position, environment, and time of day. Other inclusion criteria included age 35 years or older, body mass index (BMI) of 25–40 kg/m2, sedentary (i.e., not engaged in regular exercise), and no other medical comorbidities that would preclude safe participation in the trial, including diabetes requiring insulin or oral hypoglycemic agents. Clinic BPs were determined according to JNC 7 guidelines using a standard mercury sphygmomanometer and stethoscope.
Figure 1.
Participant flow in the ENCORE clinical trial. OGTT indicates oral glucose tolerance test; ITT refers to intent-to-treat.
Trial Overview
The ENCORE study was approved by the Institutional Review Board at Duke University Medical Center and written informed consent was obtained from all participants. Following completion of a series of baseline assessments (see below), participants were randomized to the DASH diet alone (DASH-A) or the DASH diet combined with a behavioral weight management program (DASH-WM), or to usual diet controls (UC). At the conclusion of the 4-month treatment period, assessments were repeated.
Assessments of Body Composition, Dietary Content, and Aerobic Fitness
Body weight was measured by a standard balance scale with participants dressed in light clothing without shoes. Body composition and fat distribution were assessed by dual energy absorptiometry (DEXA). This procedure provides measurements of fat mass, lean body mass, and percent body fat for both the whole body and designated anatomical subregions13. An independent assessment of dietary and nutritional content was obtained by two separate self-report measures of diet: a retrospective food frequency questionnaire (FFQ)14, requiring participants to recall typical consumption over a 4-week period, and a 4-day food diary. The FFQ was analyzed by NutritionQuest (Berkeley, CA), while the diary data were analyzed using Food Processor SQL Edition software (version 10.3, ESHA Research, Salem, OR)15. Fitness was measured with a maximal graded exercise treadmill test in which workloads were increased at a rate of one metabolic equivalent per minute16. Expired air was collected by mouthpiece for quantification of minute ventilation, oxygen consumption, and carbon dioxide production with a Parvo Medics True One measurement system (Model 2400; Parvo Medics, Sandy, Utah).
Assessments of Insulin Sensitivity and Lipids
Measures of glucose tolerance and insulin sensitivity were based on results of an oral glucose tolerance test (OGTT) using an oral glucose load of 75g, with measurement of plasma glucose (by Beckman auto-analyzer) at 0, 30, 60, 90, and 120 minutes and insulin (by double-antibody radioimmunoassay) at 0 and 120 minutes. Insulin sensitivity was assessed using the quantitative insulin sensitivity check index (QUICKI), as described by Katz, et al.17 and using a method based on dynamic glucose and insulin levels -- the Insulin Sensitivity Index (ISI0, 120), as described by Gutt, et al18. Both of these surrogate measures of insulin sensitivity provide estimates of insulin sensitivity that correlate closely with glucose clamp measurements and are predictive of the onset of type 2 diabetes19, 20.
Lipid profiles, including total cholesterol, high-density lipoprotein (HDL)- and low-density lipoprotein (LDL)-cholesterol, very-low-density lipoprotein (VLDL)-cholesterol, and triglycerides were obtained from fasting blood samples drawn between 0800 and 0900 hrs; assays were measured enzymatically (Labcorp Inc, Burlington, NC).
Randomization
Upon completion of the baseline assessments, patients were randomized in blocks of 2–5 participants. Participants were provided their group assignments in sealed envelopes; staff performing assessments was unaware of participants’ treatment group assignments. Assignments were stratified by baseline clinic BP, BMI, and age.
Interventions
Immediately following randomization, participants received 2-week controlled feeding on the Duke Clinical Research Unit, in which they ate the assigned dietary patterns (controlled usual diet, DASH diet or a reduced calorie DASH diet). Participants ate their evening meal on the unit, and took home their breakfast, lunch and snack for the following day. The controlled feeding period was modeled after the original DASH feeding studies7, 8. Participants in the DASH-A and UC conditions consumed study meals isocalorically for weight maintenance, whereas the caloric level in the DASH-WM arm was set at a 500 calories per day deficit to allow weight loss of about 0.5–1.0 pound a week.
After the first 2 weeks of controlled feeding, participants were instructed to maintain the DASH diet either with (DASH-WM) or without weight loss (DASH-A). Participants in the DASH-A condition met weekly with a nutritionist and modified the content of their diet to meet DASH guidelines but did not exercise or attempt to lose weight.
Participants in the DASH-WM condition received the same instruction in the DASH diet from the same nutritionist as in the DASH-A group, but also met with a clinical health psychologist who provided a structured, cognitive behavioral weight loss intervention that employed cognitive behavioral strategies21 and appetite awareness training22; the DASH recommendations provided participants with guidance regarding what to eat, while weight management (WM) was designed to help individuals learn when, how, and how much to eat. Participants also engaged in supervised exercise 3-times per week for 30 minutes at a level of 70–85% of their initial heart rate reserve determined during their baseline treadmill test.
Participants in the UC condition were asked to maintain their usual dietary and exercise habits for 4 months until they were re-evaluated. Weight and BP were monitored biweekly.
Statistical Analysis
Treatment effects were evaluated using the general linear model in the SAS 9.1 software (SAS Institute, Cary, NC), with separate models for each outcome. Each model included treatment condition as a three-level factor, and the corresponding pre-treatment value of the outcome, age, gender, and ethnicity (Caucasian vs. non Caucasian) as adjustment covariables. We compared post-treatment group means using pairwise treatment group comparisons that were adjusted using Tukey’s Honestly Significant Difference procedure. Data for all outcomes were analyzed following the intent-to-treat principle, with missing data managed using the multiple imputation method available in SAS PROC MI. For a given outcome, we estimated that we would have about 80% power to detect a 0.5 standard deviation difference between the active treatments and UC, and a 0.6 standard deviation difference between DASH-A and DASH-WM.
Results
Participant flow
As described previously12, 3129 participants initially inquired about the study, 447 met our initial inclusion criteria, and 144 participants were randomized to the DASH-WM (N=49); DASH-A (N=46); or UC (N=49). Post-treatment glucose and lipid data were available for 46 participants in DASH-WM, 44 in DASH-A, and 48 in UC. For body composition variables, post-treatment data were available for 46 participants in DASH-WM, 46 in DASH-A, and 47 in UC.
Participant characteristics
Table 1 displays the demographic and medical characteristics of the sample across the three treatment groups at baseline. On average, participants were 52 years old; 39% were African American and 67% were women. The mean clinic BP was 138/86 mm Hg. The majority of participants were college-educated and relatively affluent. The groups were generally comparable across the background variables.
Table 1.
Background characteristics of the sample
| Demographics | DASH-WM | DASH-A | UC | All |
|---|---|---|---|---|
| N=49 | N = 46 | N = 49 | N = 144 | |
| Age (years) | 52.3 (10) | 51.8 (10) | 51.8 (9) | 52.0 (10) |
| Gender: Female | 69% (34) | 63% (29) | 69% (34) | 67% (97) |
| Ethnicity | ||||
| Caucasian | 69% (34) | 50% (23) | 59% (29) | 60% (86) |
| African American | 31% (15) | 48% (22) | 39% (19) | 39% (56) |
| Asian | 0% (0) | 2% (1) | 2% (1) | 1% (2) |
| Level of Education | ||||
| High School | 31% (15) | 30% (14) | 42% (20) | 34% (49) |
| Some College | 8% (4) | 9% (4) | 14% (7) | 11% (15) |
| Completed College | 29% (14) | 30% (14) | 18% (9) | 22% (32) |
| Post-Graduate School | 20% (10) | 28% (13) | 20% (10) | 24% (34) |
| Other | 12% (6) | 13% (6) | 2% (1) | 9% (13) |
| Weight (kg) | 93.9 (14) | 93.0 (14) | 92.6 (15) | 93.1 (14.1) |
| BMI (kg/m2) | 33.5 (4.4) | 32.8 (3.4) | 33.0 (3.9) | 33.1 (3.9) |
Values are mean (standard deviation) for continuous variables and group percent (n) for categories.
Adherence to protocol
Attendance to the exercise and diet classes was excellent. DASH-WM participants attended 90% (median = 38) of scheduled exercise sessions and spent most time (median = 94%) at or above their target heart rate training range. DASH dietary class attendance also was excellent, with the median number of sessions attended 12 (92%) in both intervention groups. As reported previously12, compared to DASH-A and UC, participants in DASH-WM on average consumed significantly fewer total calories (1648 [95% CI = 1521–1774] kcal, 1962 [95% CI = 1833–2090] kcal, 2095 [95% CI = 1961–2228] kcal for DASH-WM, DASH-A, and UC respectively), and both DASH conditions consumed more calories from protein (19.5%, 19.4%, 16.7% for DASH-WM, DASH-A, and UC respectively), less saturated fat (26.3%, 27.8%, 36.8% for DASH-WM, DASH-A, and UC respectively), and more fiber (25g, 26g, 16 g for DASH-WM, DASH-A, and UC respectively) compared to those in UC (P’s < .001).
Changes in body weight and body composition
Adjusting for baseline weight, age, gender, and ethnicity, the mean post-treatment weight for the DASH-WM group was significantly lower (84.5 kg) compared to DASH-A (92.9 kg; p < .001) and to UC (94.1 kg; p < .001). The weight change was −8.7 kg in DASH-WM, −0.3 kg in DASH-A, and +0.9 kg in UC.
Following treatment, the DASH-WM group showed lower percent body fat and trunk fat compared to DASH-A and UC (Table 2). DASH-WM also had lower lean body mass compared to the other groups. DASH-A did not differ significantly from UC on any body composition measure.
Table 2.
Body Composition (DEXA) measures before and after treatment
| Treatment Group | P-value from pairwise comparison after treatment | ||||||
|---|---|---|---|---|---|---|---|
| Variable | DASH-WM | DASH-A | UC | DASH-WM vs. DASH-A | DASH-WM vs. UC | DASH-A vs UC | |
| Total % Body Fat | Before | 37.6 (35.5, 39.7) | 35.4 (33.2, 37.5) | 36.4 (34.3, 38.5) | |||
| After | 33.1 (32.4, 33.8) | 36.2 (35.5, 36.8) | 36.9 (36.2, 37.6) | < .001 | < .001 | .293 | |
| Total Lean Body Mass (Kg) | Before | 56.0 (53.0, 59.1) | 57.5 (54.2, 60.7) | 56.7 (53.7, 59.8) | |||
| After | 54.3 (53.8, 54.9) | 56.8 (56.2, 57.4) | 56.5 (55.9, 57.0) | < .001 | < .001 | .694 | |
| Total Trunk Fat (Kg) | Before | 17.7 (16.4, 19.0) | 16.2 (14.9, 17.5) | 16.9 (15.6, 18.2) | |||
| After | 13.6 (13.1, 14.1) | 16.6 (16.1, 17.1) | 17.1 (16.7, 17.6) | < .001 | < .001 | .217 | |
Values are mean and 95% confidence interval. Values after treatment are adjusted for pretreatment levels of outcome variable, age, gender, and ethnicity. P-values are adjusted using Tukey’s Honestly Significant Difference procedure.
Changes in aerobic fitness
Adjusting for pretreatment levels, age, gender, and ethnicity, the mean post-treatment peak maximal oxygen consumption (VO2) was higher in DASH-WM (29 ml/kg/min) compared to DASH-A (23 ml/kg/min, p < .001) and UC (22 ml/kg/min) (p < .001). Participants in the DASH-WM group showed a 19% increase in peak VO2, compared to small, and non-significant, decreases in the DASH-A (−1.2%) and UC (−3.2%).
Glucose Tolerance and Insulin Sensitivity
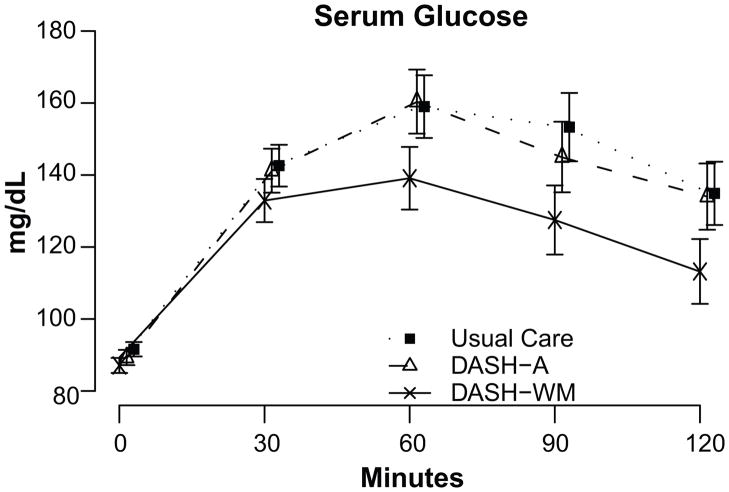
Results of the OGTT revealed that participants in the DASH-WM condition achieved greater improvements in glucose response compared to DASH-A and UC (Figure 2). Compared to UC, participants in the DASH-WM group showed lower fasting glucose levels (Table 3). DASH-WM also exhibited lower glucose AUC and greater insulin sensitivity, as measured by both QUICKI and ISI0, 120 compared to DASH-A or UC. DASH-A did not differ from UC on any measure of glucose metabolism or insulin sensitivity.
Figure 2.
Post-treatment glucose response during oral glucose tolerance test. Values are adjusted for pretreatment glucose levels, gender, age, and ethnicity. Error bars represent 95% confidence limits.
Table 3.
Glucose and insulin values before and after treatment
| Treatment Group | P-value from pairwise comparison after treatment | ||||||
|---|---|---|---|---|---|---|---|
| Variable | DASH-WM | DASH-A | UC | DASH-WM vs. DASH-A | DASH-WM vs. UC | DASH-A vs UC | |
| Fasting glucose (mg/dl) | Before | 89.4 (86.4, 92.3) | 90.4 (87.3, 93.5) | 91.3 (88.4, 94.3) | |||
| After | 87.2 (85.1, 89.3) | 89.4 (87.3, 91.5) | 91.9 (89.9, 93.9) | .324 | .006 | .214 | |
| Fasting insulin (μU/ml) | Before | 18.1 (15.7, 20.4) | 16.6 (14.2, 19.0) | 18.0 (15.7, 20.3) | |||
| After | 12.5 (10.8, 14.3) | 17.6 (15.9, 19.4) | 18.6 (16.9, 20.2) | < .001 | < .001 | .711 | |
| Glucose AUC (mg/dl· minutes) | Before | 6057 (5221, 6893) | 6087 (5224, 6951) | 6345 (5508, 7181) | |||
| After | 4947 (4340, 5554) | 6238 (5637, 6838) | 6334 (5756, 6912) | .011 | .005 | .958 | |
| ISI0,120 (mg·L2/mmol·μU·min) | Before | 74.4 (67.1, 81.8) | 70.9 (63.5, 78.3) | 66.0 (58.8, 73.2) | |||
| After | 75.3 (71.8, 78.8) | 68.7 (65.1, 72.3) | 68.8 (65.4, 72.2) | .031 | .026 | .981 | |
| QUICKI | Before | 0.319 (0.313, 0.325) | 0.319 (0.313, 0.325) | 0.315 (0.309, 0.321) | |||
| After | 0.334 (0.329, 0.339) | 0.318 (0.313, 0.323) | 0.316 (0.312, 0.321) | < .001 | < .001 | .850 | |
Values are mean and 95% confidence interval. Values after treatment are adjusted for pretreatment levels of outcome variable, age, gender, and ethnicity. P-values are adjusted using Tukey’s Honestly Significant Difference procedure.
We also noted that 24% (N = 34) of participants were considered overweight (BMI = 25–29.9), while 76% (N = 110) were considered obese (BMI >30) at baseline. The treatment group by BMI interaction was not significant, however, for glucose AUC (p = .385), QUICKI (p = .528), or ISI0, 120 (p = .142), suggesting that pre-treatment body weight did not moderate the effects of treatment on glucose metabolism or insulin sensitivity.
In a post hoc analysis, participants were classified as diabetic (>199 mg/dl), pre-diabetic (141–199 mg/dl), or normal (<140 mg/dl) based upon their glucose levels at 2 hrs during the OGTT. Overall, 72% (N=13) of the 18 participants in DASH-WM who were either prediabetic or diabetic at study entry improved by at least one category over the course of the trial, compared to 54% (7/13) in DASH-A and 42% (8/19) in UC. Among participants who were either not diabetic or pre-diabetic upon study entry, diabetic classification worsened in only 2% (1/44) of participants in DASH-WM, compared to 16% (7/43) in DASH-A and 11% (5/46) in UC.
Serum Lipids
Participants in the DASH-WM group obtained significantly lower total cholesterol and triglyceride levels compared to DASH-A and UC participants and lower LDL-cholesterol levels compared to UC, but not DASH-A (Table 4). Participants in DASH-A had marginally lower HDL-cholesterol levels than UC, but otherwise participants in DASH-A were not different from UC participants on any other lipid measure.
Table 4.
Serum lipids before and after treatment
| Treatment Group | P-value from pairwise comparison after treatment | ||||||
|---|---|---|---|---|---|---|---|
| Variable | DASH-WM | DASH-A | UC | DASH-WM vs. DASH-A | DASH-WM vs. UC | DASH-A vs UC | |
| Total Cholesterol (mg/dl) | Before | 209 (198, 220) | 199 (188, 211) | 206 (195, 217) | |||
| After | 184 (177, 199) | 199 (192, 205) | 205 (199, 211) | .008 | < .001 | .364 | |
| Low Density Lipoprotein-Cholesterol (mg/dl) | Before | 128 (118, 138) | 122 (112, 132) | 126 (116, 136) | |||
| After | 112 (106, 117) | 122 (116, 127) | 125 (119, 130) | .054 | .005 | .715 | |
| High Density Lipoprotein-Cholesterol (mg/dl) | Before | 55 (50, 59) | 53 (49, 57) | 55 (51, 59) | |||
| After | 54 (52, 55) | 51 (50, 53) | 54 (53, 56) | .115 | .911 | .047 | |
| Triglycerides (mg/dl) | Before | 133 (116, 149) | 122 (106, 139) | 122 (106, 139) | |||
| After | 93 (81, 106) | 129 (117, 142) | 130 (118, 142) | < .001 | < .001 | .900 | |
Values are mean and 95% confidence interval. Values after treatment are adjusted for pretreatment levels of outcome variable, age, gender, and ethnicity. P-values are adjusted using Tukey’s Honestly Significant Difference procedure.
Discussion
Our findings demonstrate that adherence to the DASH diet alone, although sufficient to modify BP values12, resulted in significant improvements in metabolic indices of cardiovascular risk only when accompanied by aerobic exercise and weight loss. In the DASH-WM group, participants lost an average of 19 pounds over 4 months and increased their aerobic capacity by 19%. While both the DASH-A and DASH-WM groups achieved clinically meaningful reductions in BP and improvements in other cardiovascular biomarkers of risk, as described in our earlier publication12, only DASH-WM participants demonstrated significant improvements in glucose tolerance and insulin sensitivity.
Although the DASH diet has been shown to reduce BP in controlled “feeding” studies7, 8 and in studies of free living individuals9, 12 the present study found that ENCORE participants who adhered to the DASH diet but did not exercise or lose weight achieved minimal improvements in glucose metabolism or insulin sensitivity, and also in lipids, relative to controls. Our findings contrast with results from the PREMIER substudy11, in which addition of the DASH diet to an established intervention of weight loss, reduced sodium intake, increased physical activity, and moderation of alcohol intake resulted in a significant improvement in insulin sensitivity relative to controls. However, because there was no difference in insulin sensitivity between groups randomized to the established intervention with or without the DASH diet, and there was a trend toward greater weight loss in the DASH group, the added value of the DASH diet is uncertain. The present ENCORE study findings indicate that despite DASH-related reductions in BP12, the DASH diet by itself produced minimal improvements in insulin sensitivity.
Our study was designed to evaluate only the DASH diet, and it is possible, even likely, that other diets, either alone or combined with exercise, could be beneficial. Many studies have examined the impact of various diets on weight loss23–26. Sacks et al.23, for example, randomized overweight adults to one of four diets in which the targeted percentages of energy derived from fat, protein and carbohydrates varied. After 2 years, groups achieved similar benefits in weight loss and lipid-related risk factors and fasting insulin levels. It was concluded that reduced calorie diets result in significant weight loss regardless of the macronutrient content. Foster and colleagues25 reported that a low carbohydrate, high protein and high fat (Atkins) diet was associated with greater weight loss after 6 months compared to a conventional low fat, low calorie, high carbohydrate diet, but that the differences were not significant after 12 months. With respect to body composition, the present findings confirm the results of previous findings suggesting that a low fat, weight loss diet (50% carbohydrate, 30% fat, 20% protein) results in reduced lean body mass. However, very low carbohydrate diets have been found to result in even greater reductions in weight and lean body mass compared to low fat diets27–29. Lipid changes were generally similar over time, and both diets were associated with lower DBP and insulin response to an oral glucose load.
While weight loss is associated with improved lipids, particularly LDL-cholesterol30, and increased insulin sensitivity31–33, diet composition also may affect lipids and glucose metabolism independent of weight loss. For example, with a 4-week, isocaloric weight maintenance diet, both the Ornish diet and South Beach diet have been shown to favorably reduce lipids, while high fat diets may be associated with increased LDL and total cholesterol levels34. However, the number of calories consumed appears to be more important relative to the content of the calories with regard to the development of diabetes35.
Exercise also was a key component of the DASH-WM intervention, but its effects on insulin sensitivity could not be determined independent from weight loss. Although exercise is widely considered to be important for successful weight loss, studies of the effects of exercise in the absence of weight loss on glucose, insulin sensitivity and lipids have produced mixed results. Exercise has been shown to improve insulin sensitivity, either due to chronic effects of exercise training or to the residual effects of acute exercise. Studies of both healthy adults and patients with type 2 diabetes have demonstrated that improved insulin sensitivity is maintained up to 16 hr after a single bout of exercise36, 37 but may be diminished 60 hours after the final exercise training session38, 39. Some studies have demonstrated that exercise training is associated with reduced glucose levels and improved glycemic control40–44, while others have not45–50. Because studies that have shown improvements in glucose control after exercise training have not established that these effects are due to exercise independent of weight loss51, the extent to which the exercise component of the DASH-WM condition contributed to the metabolic improvements observed in the ENCORE study is not known. The effects of exercise training on lipids also have provided mixed results52 although recent evidence suggests that high levels of exercise without weight loss may be required to achieve improvements in lipid and lipoprotein variables53.
Finally, it should be noted that some studies also have suggested that obesity may moderate the effects of exercise training on insulin sensitivity. Poirier et al.48, for example, reported no improvement in insulin sensitivity in obese type 2 diabetic patients after 12 weeks of aerobic training, although insulin sensitivity was improved in nonobese type 2 diabetic subgroups. Our data, in overweight but non-diabetic patients revealed no evidence that obesity moderated the effects of treatment. Therefore, our findings suggest that the improvements in insulin sensitivity observed in the DASH-WM intervention are generalizable to both obese and non-obese populations.
Perspectives
In summary, the results of the ENCORE study indicate that while the DASH diet alone can reduce BP in overweight, sedentary adults with HBP, there was little evidence that the DASH diet improved insulin sensitivity or lipids without the addition of exercise and weight reduction. It would appear that caloric consumption rather than nutrient composition is most salient for improved metabolic function.
Acknowledgments
Sources of Funding
Supported by grants from the National Heart, Lung, and Blood Institute (HL074103) and the General Clinical Research Center, National Institutes of Health (M01-RR-30). This publication was made possible by Grant Number 5UL1RR024128-03 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. There are no conflicts of interest to report. The principal investigator (JAB) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical Trial Registration: clinicaltrials.gov Identifier: NCT00571844
Disclosures
None reported.
References
- 1.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 2.Neal B, MacMahon S, Chapman N Blood Pressure Lowering Treatment Trialists C. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet. 2000;356:1955–1964. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 3.Adverse reactions to bendrofluazide and propranolol for the treatment of mild hypertension. Report of Medical Research Council Working Party on Mild to Moderate Hypertension. Lancet. 1981;2:539–543. [PubMed] [Google Scholar]
- 4.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–207. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention DE, Treatment of High Blood Pressure. National Heart L, Blood I, National High Blood Pressure Education Program Coordinating C. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH Group DA-SCR. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 9.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, Stedman SW, Young DR Writing Group of the PCRG. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 10.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 11.Ard JD, Grambow SC, Liu D, Slentz CA, Kraus WE, Svetkey LP. The effect of the PREMIER interventions on insulin sensitivity. Diabetes Care. 2004;27:340–347. doi: 10.2337/diacare.27.2.340. [DOI] [PubMed] [Google Scholar]
- 12.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin P-H, Caccia C, Johnson J, Waugh RA, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: The ENCORE Study. Arch Intern Med. 2010;170:126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treuth MS, Hunter GR, Kekes-Szabo T. Estimating intraabdominal adipose tissue in women by dual-energy X-ray absorptiometry. Am J Clin Nutr. 1995;62:527–532. doi: 10.1093/ajcn/62.3.527. [DOI] [PubMed] [Google Scholar]
- 14.Eck LH, Klesges RC, Hanson CL, Slawson D, Portis L, Lavasque ME. Measuring short-term dietary intake: development and testing of a 1-week food frequency questionnaire. J Am Diet Assoc. 1991;91:940–945. [PubMed] [Google Scholar]
- 15.Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, Whelton PK. Agreement on nutrient intake between the databases of the First National Health and Nutrition Examination Survey and the ESHA Food Processor. Am J Epidemiol. 2002;156:78–85. doi: 10.1093/aje/kwf003. [DOI] [PubMed] [Google Scholar]
- 16.Blumenthal JA, Emery CF, Rejeski WJ. The effects of exercise training on psychosocial functioning after myocardial infarction. J Cardpulm Rehabil. 1988;8:183–193. [Google Scholar]
- 17.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 18.Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, Schneiderman N, Skyler JS, Marks JB. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000;47:177–184. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 19.Hanley AJ, Williams K, Gonzalez C, D’Agostino RB, Jr, Wagenknecht LE, Stern MP, Haffner SM. Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes. 2003;52:463–469. doi: 10.2337/diabetes.52.2.463. [DOI] [PubMed] [Google Scholar]
- 20.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 21.Fairburn CG. Cognitive-behavioral treatment of obesity: a clinican’s guide. New York: Guilford Press; 2003. [Google Scholar]
- 22.Craighead L. The Appetite Awareness Workbook: How to listen to your body and overcome binge eating, overeating, and obsession with food. Oakland, CA: New Harbinger; 2006. [Google Scholar]
- 23.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Bluher M, Stumvoll M, Stampfer MJ. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 25.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 26.Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–293. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 27.Noakes M, Foster P, Keogh J, James A, Mamo J, Clifton P. Comparison of isocaloric very low carbohydrate/high saturated fat and high carbohydrate/low saturated fat diets on body composition and cardiovascular risk. Nutr Metab. 2006;3:7. doi: 10.1186/1743-7075-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meckling KA, O’Sullivan C, Saari D. Comparison of a low-fat diet to a low-carbohydrate diet on weight loss, body composition, and risk factors for diabetes and cardiovascular disease in free-living, overweight men and women. J Clin Endocrinol Metab. 2004;89:2717–2723. doi: 10.1210/jc.2003-031606. [DOI] [PubMed] [Google Scholar]
- 29.Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–1623. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 30.Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 31.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–847. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 32.Rice B, Janssen I, Hudson R, Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–691. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- 33.Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol. 1996;81:318–325. doi: 10.1152/jappl.1996.81.1.318. [DOI] [PubMed] [Google Scholar]
- 34.Miller M, Beach V, Sorkin JD, Mangano C, Dobmeier C, Novacic D, Rhyne J, Vogel RA. Comparative effects of three popular diets on lipids, endothelial function, and C-reactive protein during weight maintenance. J Am Diet Assoc. 2009;109:713–717. doi: 10.1016/j.jada.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feinglos MN, Totten SE. Are you what you eat, or how much you eat?: The case of type 2 diabetes mellitus. Arch Intern Med. 2008;168:1485–1486. doi: 10.1001/archinte.168.14.1485. [DOI] [PubMed] [Google Scholar]
- 36.Mikines KJ, Sonne B, Tronier B, Galbo H. Effects of training and detraining on dose-response relationship between glucose and insulin secretion. Am J Physiol. 1989;256:E588–596. doi: 10.1152/ajpendo.1989.256.5.E588. [DOI] [PubMed] [Google Scholar]
- 37.Devlin JT, Hirshman M, Horton ED, Horton ES. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes. 1987;36:434–439. doi: 10.2337/diab.36.4.434. [DOI] [PubMed] [Google Scholar]
- 38.Burstein R, Polychronakos C, Toews CJ, MacDougall JD, Guyda HJ, Posner BI. Acute reversal of the enhanced insulin action in trained athletes. Association with insulin receptor changes. Diabetes. 1985;34:756–760. doi: 10.2337/diab.34.8.756. [DOI] [PubMed] [Google Scholar]
- 39.Giacca A, Groenewoud Y, Tsui E, McClean P, Zinman B. Glucose production, utilization, and cycling in response to moderate exercise in obese subjects with type 2 diabetes and mild hyperglycemia. Diabetes. 1998;47:1763–1770. doi: 10.2337/diabetes.47.11.1763. [DOI] [PubMed] [Google Scholar]
- 40.Barnard RJ, Jung T, Inkeles SB. Diet and exercise in the treatment of NIDDM. The need for early emphasis. Diabetes Care. 1994;17:1469–1472. doi: 10.2337/diacare.17.12.1469. [DOI] [PubMed] [Google Scholar]
- 41.Yamanouchi K, Shinozaki T, Chikada K, Nishikawa T, Ito K, Shimizu S, Ozawa N, Suzuki Y, Maeno H, Kato K. Daily walking combined with diet therapy is a useful means for obese NIDDM patients not only to reduce body weight but also to improve insulin sensitivity. Diabetes Care. 1995;18:775–778. doi: 10.2337/diacare.18.6.775. [DOI] [PubMed] [Google Scholar]
- 42.Schneider SH, Khachadurian AK, Amorosa LF, Clemow L, Ruderman NB. Ten-year experience with an exercise-based outpatient life-style modification program in the treatment of diabetes mellitus. Diabetes Care. 1992;15:1800–1810. doi: 10.2337/diacare.15.11.1800. [DOI] [PubMed] [Google Scholar]
- 43.Vanninen E, Uusitupa M, Siitonen O, Laitinen J, Lansimies E. Habitual physical activity, aerobic capacity and metabolic control in patients with newly-diagnosed type 2 (non-insulin-dependent) diabetes mellitus: effect of 1-year diet and exercise intervention. Diabetologia. 1992;35:340–346. doi: 10.1007/BF00401201. [DOI] [PubMed] [Google Scholar]
- 44.Trovati M, Carta Q, Cavalot F, Vitali S, Banaudi C, Lucchina PG, Fiocchi F, Emanuelli G, Lenti G. Influence of physical training on blood glucose control, glucose tolerance, insulin secretion, and insulin action in non-insulin-dependent diabetic patients. Diabetes Care. 1984;7:416–420. doi: 10.2337/diacare.7.5.416. [DOI] [PubMed] [Google Scholar]
- 45.Skarfors ET, Wegener TA, Lithell H, Selinus I. Physical training as treatment for type 2 (non-insulin-dependent) diabetes in elderly men. A feasibility study over 2 years. Diabetologia. 1987;30:930–933. doi: 10.1007/BF00295876. [DOI] [PubMed] [Google Scholar]
- 46.Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984;74:1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, Wagner O, Georg P, Prager R, Kostner K, Dunky A, Haber P. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil. 2005;86:1527–1533. doi: 10.1016/j.apmr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Poirier P, Tremblay A, Broderick T, Catellier C, Tancrede G, Nadeau A. Impact of moderate aerobic exercise training on insulin sensitivity in type 2 diabetic men treated with oral hypoglycemic agents: is insulin sensitivity enhanced only in nonobese subjects? Med Sci Monit. 2002;8:CR59–65. [PubMed] [Google Scholar]
- 49.Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–2982. doi: 10.2337/diacare.26.11.2977. [DOI] [PubMed] [Google Scholar]
- 50.Blumenthal JA, Sherwood A, Gullette ECD, Babyak M, Waugh RH, Georgiades A, Craighead LW, Tweedy D, Feinglos M, Appelbaum M, Hayano J, Hinderliter A. Exercise and weight loss reduce blood pressure in men and women with mild hypertension. Arch Intern Med. 2000;160:1942–1958. doi: 10.1001/archinte.160.13.1947. [DOI] [PubMed] [Google Scholar]
- 51.Feuerstein BL, Weinstock RS. Diet and exercise in type 2 diabetes mellitus. Nutrition. 1997;13:95–99. doi: 10.1016/s0899-9007(96)00398-x. [DOI] [PubMed] [Google Scholar]
- 52.Leon AS, Sanchez OA. Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc. 2001;33:S502–515. doi: 10.1097/00005768-200106001-00021. [DOI] [PubMed] [Google Scholar]
- 53.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]