SUMMARY
Gaucher disease is caused by mutations in the gene that encodes the lysosomal enzyme acid β-glucosidase (GCase). We have shown previously that the small molecule pharmacological chaperone isofagomine (IFG) binds and stabilizes N370S GCase, resulting in increased lysosomal trafficking and cellular activity. In this study, we investigated the effect of IFG on L444P GCase. Incubation of Gaucher patient-derived lymphoblastoid cell lines (LCLs) or fibroblasts with IFG led to approximately 3.5- and 1.3-fold increases in L444P GCase activity, respectively, as measured in cell lysates. The effect in fibroblasts was increased approximately 2-fold using glycoprotein-enrichment, GCase-immunocapture, or by incubating cells overnight in IFG-free media prior to assay, methods designed to maximize GCase activity by reducing IFG carryover and inhibition in the enzymatic assay. IFG incubation also increased the lysosomal trafficking and in situ activity of L444P GCase in intact cells, as measured by reduction in endogenous glucosylceramide levels. Importantly, this reduction was seen only following three-day incubation in IFG-free media, underscoring the importance of IFG removal to restore lysosomal GCase activity. In mice expressing murine L444P GCase, oral administration of IFG resulted in significant increases (2- to 5-fold) in GCase activity in disease-relevant tissues, including brain. Additionally, eight-week IFG administration significantly lowered plasma chitin III and IgG levels, and 24-week administration significantly reduced spleen and liver weights. Taken together, these data suggest that IFG can increase the lysosomal activity of L444P GCase in cells and tissues. Moreover, IFG is orally available and distributes into multiple tissues, including brain, and may thus merit therapeutic evaluation for patients with neuronopathic and non-neuronopathic Gaucher disease.
Keywords: Lysosomal storage disorder, Gaucher disease, pharmacological chaperone, isofagomine, L444P, β-glucocerebrosidase
INTRODUCTION
Gaucher disease is caused by inherited mutations in the gene (GBA) that encodes acid β-glucosidase (EC 3.2.1.45; GCase), the lysosomal enzyme responsible for the metabolism of glucosylceramide (GC) into ceramide and glucose [1]. Mutations in GCase result in reduced cellular enzyme activity and progressive accumulation of GC mainly within macrophages (Gaucher cells) leading to clinical manifestations that include anemia, thrombocytopenia, hepatosplenomegaly, bone lesions, and in some cases, central nervous system (CNS) impairment [2, 3]. Gaucher patients without CNS involvement are classified as type I, while those with CNS involvement are type II or type III [4, 5]. The two most prevalent missense mutant forms of GCase reported in Gaucher patients are N370S and L444P [5]. Patients homozygous or heterozygous for N370S GCase typically present with a non-neuronopathic form of Gaucher disease, whereas those homozygous for L444P GCase usually display a more severe neuronopathic form. More than 70% of Gaucher patients within the Ashkenazi Jewish population carry at least one N370S allele, while 38% of non-Jewish Gaucher patients carry the L444P allele [5–7].
Currently, enzyme replacement therapy (ERT) and small-molecule substrate reduction therapy (SRT) are the only approved treatment options for patients with the non-neuronopathic form of Gaucher disease [8–12]. ERT, based on the intravenous administration of recombinant GCase, is the most effective treatment for type I and the visceral manifestations of types II and III disease. ERT generally leads to reduced spleen and liver weights, as well as increased platelet counts and hemoglobin levels [13–16]. However, the CNS manifestations of type II and III Gaucher disease do not respond well to ERT due to the inability of exogenous enzyme to cross the blood-brain barrier [17]. SRT drugs have the potential for better CNS penetration and some neurological benefit as the therapeutic agent is a small molecule, such as N-butyl-1-deoxynojirimycin (NB-DNJ, miglustat, Zavesca®), which acts as a weak inhibitor of glucosyl-transferase, thus reducing the synthesis of GC. Miglustat has been approved for use in patients with mild-to-moderate type I Gaucher disease [9, 11, 12], and is currently being evaluated in neuronopathic Gaucher patients, though a recent report showed no significant benefit for the neurological manifestations of type III patients [18]. Furthermore, many patients treated with miglustat have experienced side effects including diarrhea, weight loss, tremor, and peripheral neuropathy [19].
More recently, pharmacological chaperone therapy has been proposed as a potential treatment for Gaucher disease [20–23]. Small molecule pharmacological chaperones are designed to selectively bind and stabilize mutant GCase, thereby facilitating proper folding and trafficking to lysosomes, and increasing total cellular GCase activity [24, 25]. In addition, pharmacological chaperones have the potential to cross the blood-brain barrier and to be orally available. A number of iminosugar-based pharmacological chaperones have been shown to increase cellular activity of various mutant forms of GCase in Gaucher patient-derived cell lines [26–34]. The iminosugar isofagomine (IFG) has been shown to stabilize and promote lysosomal trafficking of N370S GCase [24, 25]. To date however, the effects of pharmacological chaperones on L444P GCase in vitro are varied, with some reports showing small increases in enzyme activity [33] and others showing no response at all [28, 31, 34]. Importantly, IFG has not been extensively evaluated in vitro against L444P GCase, and in vivo testing of IFG has been hampered by the lack of a suitable Gaucher mouse model. Initial attempts to create mice with an L444P GCase point mutation resulted in a perinatal lethal phenotype [35]. However, rescue of lethality was achieved using a genetically-modified background (GC synthase heterozygosity), optimized breeding schemes, and improved husbandry [36]. Phenotypically, the L444P GCase mice do not exhibit the severe features generally associated with the L444P mutation in humans, such as GC accumulation, Gaucher cells, gross hepatosplenomegaly, or neurological symptoms. However, they do manifest an attenuated, Gaucher-related phenotype characterized by reduced GCase activity in disease-relevant tissues such as liver, spleen, lung, and brain, moderate increases in spleen and liver weights, and elevated plasma chitin III and IgG levels [36]. Given that other mouse models generated for Gaucher disease do not carry the L444P mutation [37, 38] and that the L444P GCase mice were readily available, viable, and easy to breed, we chose this mouse model to test the effects of the pharmacological chaperone IFG on L444P GCase in vivo.
In this study we report the effects of IFG on human L444P GCase activity and GC levels in Gaucher patient-derived cell lines and on murine L444P GCase activity in mice. Five-day incubation of Gaucher patient-derived lymphoblastoid cell lines with IFG led to approximately 3.5-fold increases in L444P GCase activity as measured in cell lysates; the magnitude was much smaller in patient-derived fibroblasts (up to 1.3-fold). The measured effect of IFG on L444P GCase activity in fibroblasts could be increased approximately 2-fold after glycoprotein- or GCase-enrichment using concanavalin A- and immunocapture, respectively, prior to assay, or by incubating the cultured cells in IFG-free media for 24 hours prior to direct assay of cell lysates. IFG incubation also increased lysosmal trafficking of L444P GCase in fibroblasts and reduced GC levels in situ in L444P GCase fibroblasts and LCLs. Oral administration of IFG to L444P mice for four weeks resulted in selective and significant increases in GCase activity (2- to 5-fold) in liver, spleen, lung, and brain, and after 24 weeks resulted in significant increases in GCase activity (up to 2-fold) in mineralized bone and bone marrow. Furthermore, oral administration of IFG for eight weeks significantly lowered plasma chitin III and IgG levels, and after 24 weeks significantly reduced spleen and liver weights. Collectively, these data indicate that IFG increases L444P GCase activity both in vitro and in vivo, and may warrant clinical evaluation for patients with both neuronopathic and non-neuronopathic Gaucher disease.
RESULTS
IFG increases L444P GCase activity in Gaucher patient-derived cells
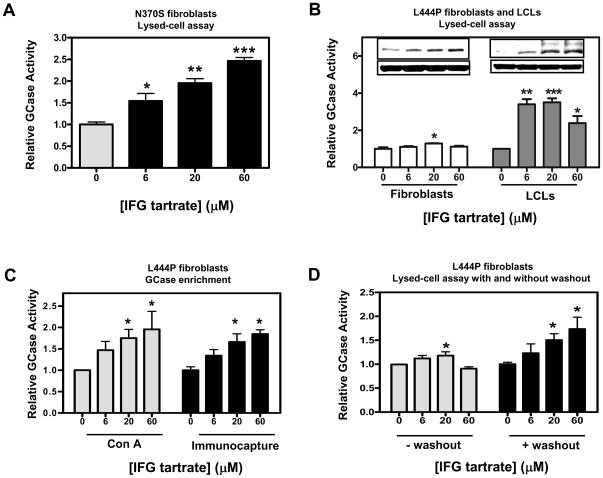
Primary skin fibroblasts and lymphoblastoid cell lines (LCLs) derived from Gaucher patients homozygous for either N370S or L444P GCase were used to investigate the effects of the pharmacological chaperone isofagomine (IFG). As previously reported, incubation of N370S GCase fibroblasts for five days with IFG tartrate resulted in a statistically significant and concentration-dependent increase in GCase activity, as measured directly in cell lysates using the fluorogenic substrate 4-MUG (±CBE) [24, 25, 31–33, 39] (Fig. 1A). In contrast, incubation of L444P GCase fibroblasts under the same conditions resulted in small, but reproducible, increases in GCase activity (Fig. 1B, left panel), again as previously reported [33]. This effect was seen in L444P fibroblast cell lines derived from four different Gaucher patients, with maximal increases from 1.2- to 1.3-fold above baseline (Table 1). Importantly, incubation of L444P GCase LCLs for five days with IFG tartrate resulted in robust increases in GCase activity, as measured in lysed cells (Fig. 1B, right panel). Again, similar responses were seen in L444P LCL cell lines derived from five different Gaucher patients, with maximal increases from 2.5- to 3.5-fold above baseline (Table 1). The effects of IFG were also seen directly on GCase protein levels as assessed by Western blotting. Here, IFG incubation increased the mature, lysosomal 69 kD form of GCase in fibroblasts (Fig. 1B, left panel inset), and both the immature, Golgi 59 kD and mature 69 kD forms of GCase in LCLs (Fig. 1B, right panel inset). These data indicate that IFG increases the total quantity of L444P GCase that is capable to traffic through the Golgi and to lysosomes [25].
Figure 1. IFG increases N370S and L444P GCase activity in Gaucher patient-derived cells.
Panel A. N370S fibroblasts (DMN89.45) were incubated with the indicated concentrations of IFG tartrate for five days and GCase activity was directly measured in lysed cells as described in ‘Materials and Methods’. In the experiment shown, a concentration-dependent increase of approximately 2.5-fold was seen in GCase activity. The increase in GCase activity was found significant for a linear trend (one-way ANOVA), indicating a concentration-dependent effect. Panel B. L444P fibroblasts (GM07968) and LCLs (GS0501) were incubated with the indicated concentrations of IFG tartrate for five days and GCase activity was directly measured in lysed cells. In the experiments shown, a small but reproducible 1.3-fold increase in GCase activity was seen in fibroblast lysates (left panel), and a 3.5-fold increase was seen in LCL lysates (right panel). The increase in GCase activity measured in LCLs was found significant for a linear trend (one-way ANOVA). Summary data from the fibroblast and LCL cell lines shown here, as well as others, are presented in Table 1. Insets, GCase protein levels were increased in Gaucher fibroblasts and LCLs after five-day incubation with IFG, as directly measured by Western blotting (50 μg total protein per lane). Blots were probed with an anti-human GCase antibody and a β-actin antibody (loading control). The data shown are representative of three independent experiments. Panel C. Gaucher fibroblasts homozygous for L444P GCase (GM07968) were incubated for five days with the indicated concentrations of IFG tartrate. Cell lysates were then subjected to either glycoprotein- or GCase-enrichment using Con A- and immunocapture, respectively, as described in ‘Materials and Methods’. GCase activity was measured on the precipitated beads. In the experiments shown, concentration-dependent increases (approximately 2-fold) were seen in GCase activity. The increases were found significant for a linear trend (one-way ANOVA). Panel D. Gaucher fibroblasts homozygous for L444P GCase (GM07968) were incubated for five days with the indicated concentrations of IFG tartrate, followed by 24-hour washout (media only). GCase activity was measured directly in lysed cells. In the experiments shown, an approximately 1.7-fold increase was seen in L444P GCase activity after 24-hour washout. This increase was found significant for a linear trend (one-way ANOVA). In all panels, the data have been normalized to baseline (untreated) values and are representative of three or six independent experiments as indicated in Tables 1 and 2, with each point the mean±SEM of triplicate determinations. Statistically significant differences from untreated were determined using a two-tailed, unpaired student’s t-test with *p<0.05, **p<0.01, and ***p<0.001.
Table 1.
Effect of IFG tartrate on N370S and L444P GCase activity in lysates from Gaucher patient-derived fibroblasts and LCLs.
| Cell ID | Mutation | Cell Type | GCase Activity | ||||
|---|---|---|---|---|---|---|---|
| Baseline nmol/mg/hour | IFG tartrate -μM (% increase) | n | |||||
| 6 | 20 | 60 | |||||
| DMN89.45 | N370S | F | 4.0±0.3 | 35±2 | 95±1 | 115±3 | 3 |
| GM07968 | L444P | F | 0.2±0.03 | 15±10 | 30±10 | 17±7 | 3 |
| GM00877 | L444P | F | 1.0±0.1 | 25±5 | 30±4 | 17±7 | 3 |
| GM10915 | L444P | F | 3.0±0.04 | 25±5 | 30±4 | 16±7 | 3 |
| GM08760 | L444P | F | 2.3±0.1 | 20±10 | 20±10 | 15±5 | 3 |
| GS0501 | L444P | L | 3.1±0.2 | 227±23 | 251±15 | 146±28 | 3 |
| GS0505 | L444P | L | 0.4±0.1 | 220±18 | 232±27 | 124±13 | 3 |
| GS0502 | L444P | L | 0.8±0.2 | 120±14 | 150±15 | 134±19 | 6 |
| GS0503 | L444P | L | 0.7±0.1 | 141±14 | 146±24 | 124±13 | 6 |
| GS0504 | L444P | L | 0.7±0.2 | 129±28 | 152±34 | 129±32 | 6 |
GCase activity in cell lysates was determined after five-day incubation of Gaucher fibroblasts or LCLs with the indicated concentrations of IFG tartrate. The GCase activity in fibroblasts derived from three different healthy volunteers (CRL1509, CRL2076, and CRL2097) was 25±2, 30±0.9, and 16±1.0 nmol/mg protein/hour, respectively. The average GCase activity in LCLs derived from two different healthy volunteers (GM02184 and GM03201) was 15±4 nmol/mg protein/hour. All cell lines were homozygous for the specified GCase mutations. The data for each cell line have been normalized to the GCase activity in untreated cells and are presented as the mean±SEM. ‘F’, fibroblast; ‘L’, lymphoblastoid cell line.
As patient-derived L444P GCase cell lines have very low GCase levels (Table 1), we developed methods to enrich GCase and simultaneously remove IFG, thereby increasing the sensitivity of the GCase measurements. To this end, the lysed-cell assay protocol was modified to include either glycoprotein-enrichment using concanavalin A (Con A) precipitation or GCase-immunocapture, followed by extensive washing of the pellets to remove bound IFG from immobilized GCase. GCase activity was then measured in the absence or presence of CBE using 4-MUG [24, 25, 31–33, 39]. Under these conditions, five-day incubation of patient-derived fibroblasts with IFG tartrate significantly increased L444P GCase activity approximately 2.0-fold (Fig. 1C). This effect was seen in fibroblast cell lines derived from four different Gaucher patients, with maximal increases from 1.8- to 2.2-fold (Table 2). Lastly, three different L444P GCase fibroblast cell lines incubated with IFG for five days followed by a one-day incubation in growth media only (washout) showed maximal increases in GCase activity from 1.6- to 1.7-fold, as measured directly in cell lysates (Fig. 1D; Table 2). Collectively, these data indicate that IFG can increase L444P GCase activity and protein levels in vitro, though the effect is more pronounced in Gaucher patient-derived LCLs compared to fibroblasts. In addition, the measured response in fibroblast lysates is larger after removal of IFG, which can otherwise inhibit the enzyme activity if carried into the assay.
Table 2.
Effect of IFG tartrate on L444P GCase activity in lysates from Gaucher patient-derived fibroblasts after IFG removal.
| Cell ID | GCase Activity (% increase) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IFG tartrate (μM) | |||||||||
| 6 | 20 | 60 | 6 | 20 | 60 | 6 | 20 | 60 | |
| Glycoprotein-enrichment | Immunocapture | IFG Washout | |||||||
| GM07968 | 64±13 | 88±19 | 115±33 | 33±16 | 55±17 | 115±46 | 40±12 | 55±13 | 74±5 |
| GM00877 | 99±21 | 113±39 | 100±20 | 27±6 | 41±10 | 39±8 | 33±3 | 51±7 | 61±12 |
| GM10915 | 25±5 | 55±1 | 100±7 | 55±12 | 84±19 | 73±19 | 25±3 | 45±2 | 55±2 |
| GM08760 | ND | ND | ND | 38±7 | 62±6 | 72±7 | ND | ND | ND |
GCase activity was measured in Gaucher patient-derived fibroblasts homozygous for L444P GCase after five-day incubation with the indicated concentrations of IFG tartrate. Prior to assay, glycoproteins or GCase were enriched from cell lysates using either Con A- or immunocapture, respectively, or cells were incubated for 24 hours in media only (IFG washout), as described in the ‘Materials and Methods’. The data presented are the mean±SEM from three independent experiments. ND, not determined.
IFG increases the lysosomal pool of L444P GCase
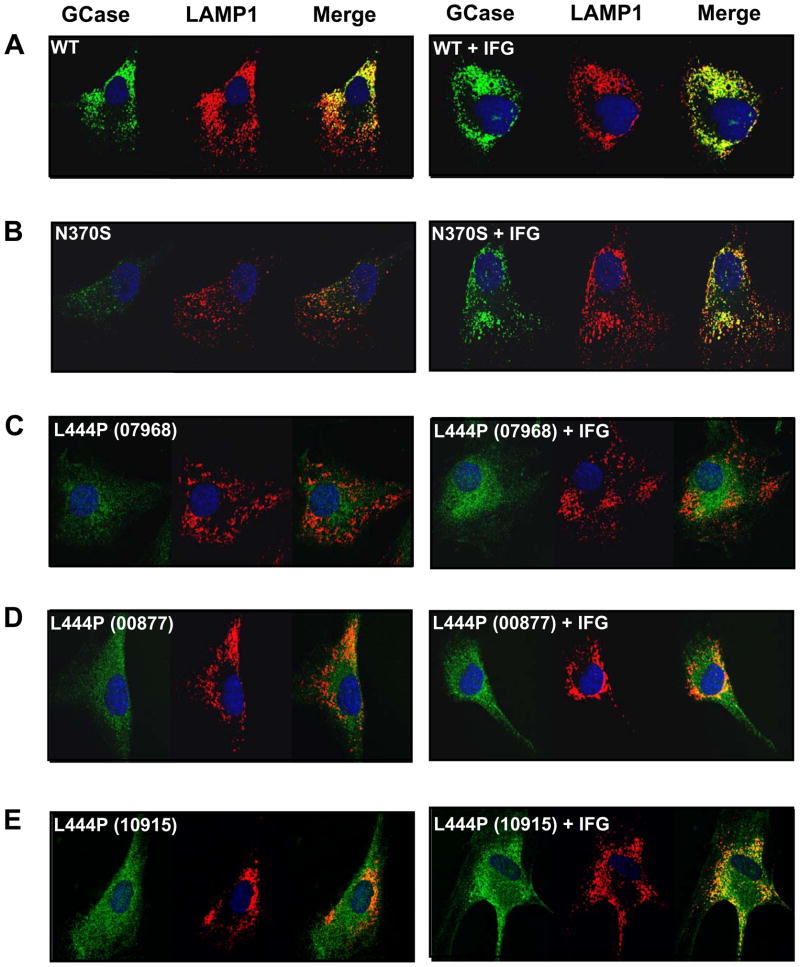
Indirect immunofluorescence staining and confocal microscopy imaging were used to determine if IFG increases the trafficking of L444P GCase to lysosomes. Fibroblasts derived from healthy volunteers (wild-type) or Gaucher patients homozygous for the N370S or L444P mutant forms of GCase were incubated for 14 days without or with 100 μM IFG tartrate. Fixed cells were incubated with primary antibodies against GCase and the lysosomal marker LAMP-1, followed by labeling with secondary antibodies conjugated with different fluorophores. Strong, punctate signals for GCase and LAMP-1 were recorded in wild-type fibroblasts in the absence or presence of IFG; the degree of colocalization of the GCase and LAMP-1 signal was increased after IFG incubation (Fig. 2A). By comparison, the overall GCase signal in untreated N370S fibroblasts was weaker. However, N370S GCase levels were significantly increased and showed increased colocalization with LAMP-1 after incubation with IFG (Fig. 2B), as previously reported [39]. The GCase signal was even weaker with a more diffuse pattern in the three L444P GCase fibroblast lines investigated (Fig. 2C–E), with lines 00877 and 10915 showing some low-level co-localization with LAMP-1 prior to IFG incubation. Importantly, IFG increased the overall GCase signal (more intense, punctate signals) in all three L444P GCase cell lines, resulting in clear colocalization with LAMP-1. Collectively, these data indicate that IFG can increase the lysosomal content of L444P GCase in Gaucher patient-derived cells.
Figure 2. IFG increases the lysosomal pool of L444P GCase in Gaucher patient-derived fibroblasts.
Fibroblasts derived from healthy volunteers (WT; CRL2097) and Gaucher patients homozygous for the N370S (DMN89.45) or L444P (GM07968, GM00877, GM10915) mutant forms of GCase were incubated in the absence or presence of 100 μM IFG tartrate for 14 days. GCase (green) and the lysosomal marker LAMP-1 (red) were visualized by confocal microscopy after indirect immunofluorescence staining as described in ‘Materials and Methods’. In the merged images, yellow denotes co-localization of the two proteins, indicative of their lysosomal localization. Nuclei are stained with DAPI (blue). IFG treatment increased the lysosomal pool of GCase in wild-type as well as N370S and L444P GCase fibroblasts (as shown by the increased amount of yellow in the merged images). Representative cells are shown to demonstrate the degree of co-localized GCase and LAMP-1. Magnification: 63x.
IFG reduces GC levels in L444P GCase fibroblasts and LCLs
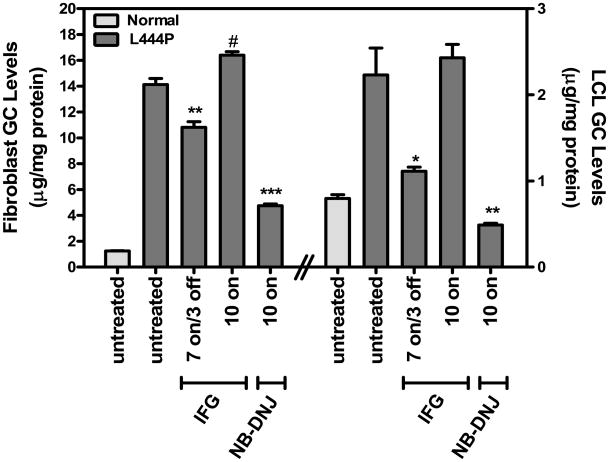
We next determined whether increased L444P GCase levels and lysosomal trafficking result in increased substrate turnover in situ (Fig. 3 and Table 3). GC levels in Gaucher fibroblasts and LCLs were measured after 7-day incubation in the absence or presence of 30 μM IFG tartrate, followed by a 3-day washout period to minimize potential GCase inhibition by IFG in situ (‘7 on/3 off’). For comparison, the effects of continuous 10-day incubation (‘10 on’) with either 30 μM IFG or the glucosylceramide synthase inhibitor N-butyl-DNJ (500 μM) [40] were also assessed. Baseline GC levels in the Gaucher fibroblast cell lines 07968 and 10915 were elevated 7.1±2.2- and 1.9±0.6-fold, respectively, compared to the normal fibroblast cell line CRL2076. Similarly, baseline GC levels in the Gaucher LCL cell lines GS0501 and GS0505 were elevated 3.4±0.8- and 3.4±0.5-fold, respectively, compared to the normal LCL cell line WT0003. Importantly, all tested L444P GCase fibroblasts and LCLs incubated with IFG in the ‘7 on/3 off’ regimen showed significant decreases in GC levels. In contrast, IFG incubation in the ‘10 on’ regimen did not reduce GC levels in any cell line tested. As expected, 10-day incubation with NB-DNJ significantly decreased GC levels in these three cell lines. These data indicate that the IFG-mediated increases in cellular and lysosomal GCase can lead to reduction of GC levels in L444P GCase fibroblasts and LCLs, provided that IFG is sufficiently washed from the cells for several days.
Figure 3. IFG reduces GC levels in Gaucher fibroblasts and LCLs.
Fibroblasts (GM07968, left side of panel) and LCLs (GS0501, right side of panel) homozygous for L444P GCase were incubated in the absence or presence of 30 μM IFG for 7 days followed by a 3-day washout (‘7 on/3 off’). Parallel cultures of these cell lines were incubated for 10 days with 30 μM IFG or 500μM NB-DNJ (‘10 on’). GC levels were then measured as described in ‘Materials and Methods’ as well as in normal control fibroblasts (CRL2076) or LCLs (WT0003). The data are expressed as the mean±SEM from 3 flasks for each condition tested. Statistically significant differences from untreated in GC levels were determined using a two-tailed, unpaired student’s t-test with *p<0.05, **p<0.01, and ***p<0.001, or #p<0.05 for untreated versus ‘10 on’. Similar results were seen in two other L444P GCase cell lines (GM10915 fibroblasts and GS0505 lymphoblasts; see Table 3).
Table 3.
Effect of IFG and NB-DNJ on GC levels in Gaucher patient-derived cells homozygous for the L444P mutation.
| Cell ID | Cell Type | GC Levels | |||
|---|---|---|---|---|---|
| Baseline | Compound/Regimen (% decrease) | ||||
| μg/mg protein | IFG ‘7 on/3 off’ | IFG ‘10 on’ | NB-DNJ ‘10 on’ | ||
| GM07968 | F | 14±0.5 | 23±3** | - | 66±1*** |
| GM10915 | F | 2.5±0.1 | 32±4** | - | 76±1*** |
| GS0501 | L | 2.2±0.3 | 50±2* | - | 77±1** |
| GS0505 | L | 2.7±0.2 | 26±1* | - | 75±1*** |
GC levels in Gaucher fibroblasts and LCLs homozygous for L444P GCase were determined after 7-day incubation in the absence or presence of 30 μM IFG followed by a 3-day washout (‘7 on/3 off’). For comparison, cells were also incubated for 10 days (‘10 on’) with 30 μM IFG or 500μM NB-DNJ. The data for each cell line have been normalized to the GC levels in untreated cells and are expressed as the mean±SEM from three flasks for each condition tested. Differences in GC levels between treated and untreated cells were determined using a two-tailed, unpaired student’s t-test (*p<0.05, **p<0.01, ***p<0.001). While incubation with 30 μM IFG for 10 days did not significantly reduce GC levels in any cell line tested, significant increases were seen in fibroblast cell lines 07968 and 10915 (16% and 35%, respectively; p<0.05 compared to untreated). GC levels in fibroblasts and LCLs derived from healthy volunteers (CRL2076 and WT0003, respectively) were 1.2±0.02 and 0.85±0.2 μg/mg protein, respectively (see Fig. 3). ‘F’, fibroblast; ‘L’, lymphoblastoid cell line; ‘-‘, no decrease.
IFG is orally available and has broad tissue distribution
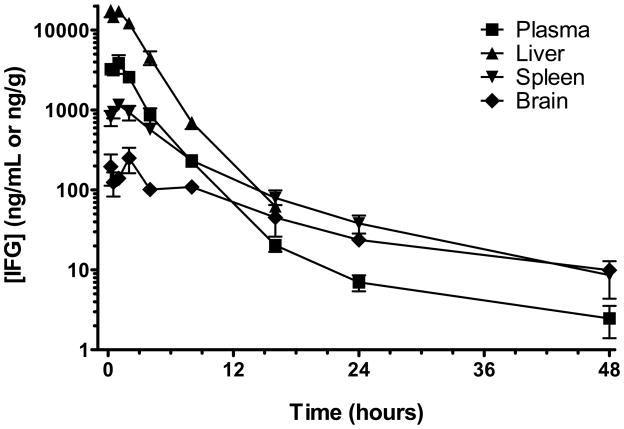
Two different salt forms of isofagomine, IFG hydrochloride (HCl) and IFG tartrate, were used for the in vivo studies. We first determined the tissue distribution and rate of clearance of IFG in plasma, liver, spleen, and brain of male Sprague-Dawley rats. Animals were administered a single oral dose (by gavage) of IFG tartrate (600 mg/kg, equivalent to 300 mg/kg free base), and plasma and tissue concentrations of IFG were measured by LC-MS/MS as a function of time after administration (Fig. 4). Maximal IFG levels were attained within 1 hour after administration. IFG was detected in all peripheral tissues tested, with peak levels of 3.9±1.0 μg/mL, 17.7±3.3 μg/g, and 1.2±0.1μg/g in plasma, liver, and spleen respectively (approximately 26 μM, 120 μM, and 8 μM, assuming 1 gram of tissue is equivalent to 1 mL of volume). Twenty-four hours post-administration, concentrations fell to 0.007±0.002 μg/mL and 0.04±0.01 μg/g in plasma and spleen, respectively, with levels in liver below the limit of quantitation (0.025 μg/g). By 48 hours, IFG concentrations in plasma and spleen were less than 0.003 μg/mL and 0.01 μg/g, respectively. IFG penetration into the brain was slower and reached lower levels than in other tissues, with a maximal concentration of 0.25±0.09 μg/g (approximately 1.7 μM) at 2 hours; 48 hours post-administration, brain levels were less than 0.01 μg/g. The total brain exposure was approximately 20% of the plasma exposure. The terminal half-life of IFG was estimated to be 4.4, 2.6, 4.6, and 9 hours in plasma, liver, spleen, and brain, respectively. Collectively, these results indicate that IFG is orally available and has a wide tissue distribution profile, including the CNS.
Figure 4. Tissue distribution pharmacokinetics of IFG.
Eight-week old male Sprague-Dawley rats were fasted overnight prior to administration of IFG tartrate (600 mg/kg, equivalent to 300 mg/kg free base) by oral gavage. Tissue and blood samples were drawn as a function of time. IFG levels were assessed by LC-MS/MS in plasma and tissue homogenates as described in ‘Materials and Methods’. Each point represents the mean±SEM from 3 rats.
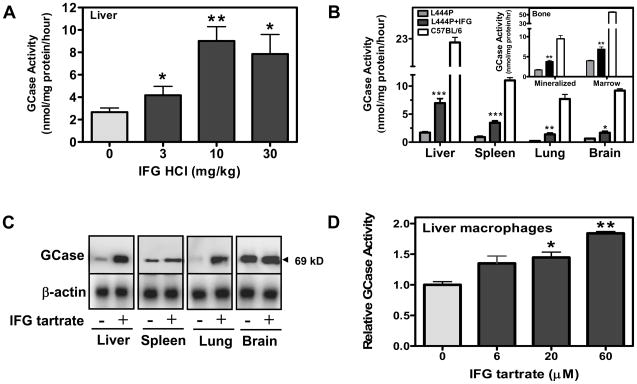
IFG selectively increases L444P GCase activity in mouse tissues
To investigate the effect of IFG on L444P GCase in vivo, two-month old male L444P GCase mice were administered IFG HCl (3, 10, or 30 mg/kg per day, equivalent to 2.5, 8.2, and 25 mg/kg free base, respectively) ad libitum in drinking water for two weeks. Mice were then euthanized and GCase activity measured in liver homogenates. A statistically significant and dose-dependent increase in L444P GCase activity (approximately 4-fold) was seen (Fig. 5A), with a maximal increase at a daily dose of 10 mg/kg per day. In a follow-up study, two-month old male L444P GCase mice were administered IFG tartrate (20 mg/kg per day, equivalent to 10 mg/kg free base) ad libitum in drinking water for four weeks (Fig. 5B). Again, a statistically significant increase (approximately 4-fold) in L444P GCase activity was seen in liver. In addition, L444P GCase activity was also elevated in spleen, lung, and brain, with increases of approximately 4-, 5-, and 2-fold, respectively (Fig. 5B). In separate studies, oral administration (ad libitum) of IFG tartrate (20 mg/kg per day) to six-month old L444P GCase mice for 24 weeks resulted in a significant increase in L444P GCase activity (up to 2-fold) in mineralized bone and bone marrow (Fig. 5B, inset). Oral administration of IFG tartrate increased tissue L444P GCase activity to 15% to 40% of that measured in the respective tissues of age-matched, untreated wild-type C57BL/6 mice (Fig. 5B). IFG administration did not affect tissue activity of four other lysosomal hydrolases, including α-galactosidase A, acid α-glucosidase, β-glucuronidase, and β-galactosidase in L444P GCase mice (data not shown), indicating that the increase of L444P GCase activity in vivo is selective. Furthermore, the 69 kDa form of L444P GCase was increased 3-, 2-, 4-, and 1.2-fold in liver, spleen, lung, and brain homogenates, respectively, of L444P GCase mice administered 20 mg/kg per day IFG tartrate for four weeks as measured by Western blotting (Fig. 5C). Lastly, the increased activity of murine L444P GCase in liver tissue correlated with increased quantities of GCase protein in lysosomal fractions isolated from liver homogenates of mice administered IFG tartrate (20 mg/kg per day) for 24 weeks (Supplementary Figure).
Figure 5. IFG increases tissue L444P GCase activity in vivo.
Panel A. Two-month old male L444P GCase mice were administered IFG HCl (3, 10, or 30 mg/kg per day, equivalent to 2.5, 8.2, and 25 mg/kg free base, respectively) ad libitum in drinking water for two weeks. GCase activity in liver lysates was measured as described in ‘Materials and Methods’. Significant increases in GCase activity were seen at all three doses. Each bar represents the mean±SEM of GCase activity from 4 mice/group analyzed in triplicate. The treatment was also found significant for a linear trend (one-way ANOVA), indicating a dose-dependent effect. Panel B. Two-month old male L444P GCase mice were administered IFG tartrate (20 mg/kg per day, equivalent to 10 mg/kg free base) ad libitum for four weeks. GCase activity was measured in tissue lysates as described in ‘Materials and Methods’. Significant increases in GCase activity were seen in liver (4-fold), spleen (4-fold), lung (5-fold), and brain (2-fold). Tissue GCase activity from untreated wild-type C57BL/6 mice are also shown. Each bar represents the mean±SEM of GCase activity from 4 mice/group analyzed in triplicate. Inset. Six-month old male L444P GCase mice were administered IFG tartrate (20 mg/kg per day, equivalent to 10 mg/kg free base) ad libitum for 24 weeks and GCase activity was measured in mineralized bone and bone marrow lysates as described in ‘Materials and Methods’. Significant increases in L444P GCase activity (up to 2-fold) were seen with IFG administration. Each bar represents the mean±SEM of GCase activity from 7–8 mice/group analyzed in triplicate. Panel C. GCase protein levels in the tissue samples (50 μg) used in panel B were directly measured by Western blotting using anti-mouse GCase and β-actin (loading control) antibodies as described in ‘Materials and Methods’. IFG tartrate administration increased GCase activity in liver (3-fold), spleen (2-fold), lung (4-fold), and brain (1.2-fold). Each lane represents one mouse from each group and is representative of two experiments with two different mice from each group. Panel D. Primary cultures of mouse liver macrophages were derived from two-month old untreated male L444P GCase mice and incubated with IFG tartrate for five days at the concentrations indicated as described in ‘Materials and Methods’. In the experiment shown, a significant and concentration-dependent increase (approximately 2-fold) in L444P GCase activity was seen in macrophage lysates. The increase was also found significant for a linear trend (one-way ANOVA). The data shown have been normalized to untreated values and are representative of three independent experiments, with each point the mean±SEM of triplicate determinations. In panels A, B, and D, statistically significant differences from untreated were determined using a two-tailed, unpaired student’s t-test with *p<0.05, **p<0.01, and ***p<0.001.
To determine whether the effect of IFG tartrate on L444P GCase could be reproduced in cells derived from the L444P GCase mice, primary macrophage cultures were established from liver. Five-day ex vivo incubation with increasing concentrations of IFG tartrate resulted in a 2±0.3-fold increase in L444P GCase activity as measured in lysates from the cultured macrophages (Fig. 5D). Collectively, these data indicate that IFG can selectively increase murine L444P GCase activity and lysosomal levels both in vitro and in vivo.
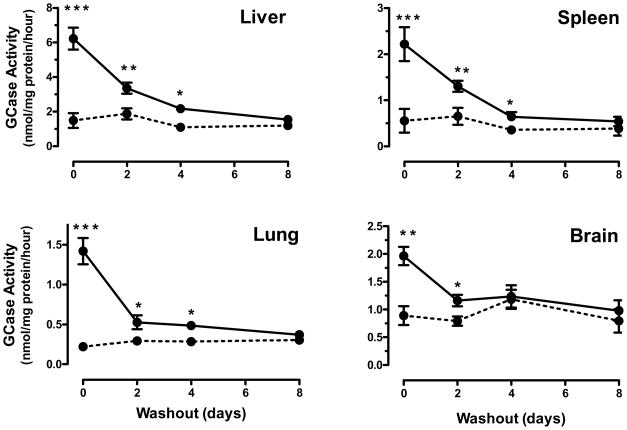
Tissue L444P GCase activity is elevated for days after withdrawal of IFG
To determine the duration of elevated L444P GCase after IFG withdrawal in vivo, four-month old, male L444P GCase mice were administered IFG tartrate (20 mg/kg per day, equivalent to 10 mg/kg free base) ad libitum in drinking water. After four-week administration, IFG tartrate was removed and mice were provided access to drinking water only. Groups of mice were then euthanized and tissue GCase activity was measured 0, 2, 4, and 8 days after IFG tartrate withdrawal (Fig. 6). On the last day of IFG administration (Day 0), L444P GCase activity was significantly increased in liver, spleen, lung, and brain. The elevated tissue GCase activity was sustained for a minimum of two days in all tissues before returning to pre-dose levels. The half-life of elevated L444P GCase was estimated to be 1.7, 1.6, 1.2, and 2.0 days in liver, spleen, lung, and brain, respectively.
Figure 6. Time course for decay of increased L444P GCase activity after IFG withdrawal.
Four-month old male L444P GCase mice were administered drinking water (dotted lines) or IFG tartrate (20 mg/kg per day, equivalent to 10 mg/kg free base (solid line)) ad libitum in drinking water for four weeks followed by a washout period (drinking water only) for up to eight days. Groups of mice were then euthanized on Days 0, 2, 4, or 8 after IFG tartrate withdrawal and GCase activity was measured in tissue lysates. Statistically significant increases above baseline were maintained in liver, spleen, and lung GCase activity for up to four days, and in brain for up to two days. Each data point represents the mean±SEM of tissue GCase activity from 6 mice/group analyzed in triplicate. Statistically significant differences from untreated were determined using a two-tailed, unpaired student’s t-test with *p<0.05, **p<0.01, and ***p<0.001.
Tissue IFG concentrations in L444P GCase mice administered IFG tartrate were measured using liquid chromatography tandem mass spectrometry (LC-MS/MS). With the exception of brain, IFG was clearly detectable in all tissues on the last day (Day 0) of administration (Table 4). Estimated molar concentrations of IFG in plasma, liver, spleen, and lung were 0.48±0.04 μM, 1.20±0.11 μM, 0.47±0.02 μM, and 0.47±0.06 μM, respectively. In contrast, IFG was not detected in any tissue two days after withdrawal, indicating that the small molecule is cleared relatively rapidly from the body (Table 4). These data demonstrate that tissue GCase activity remains elevated even after IFG is cleared (Fig. 6), and support a long half-life of the enzyme. The inability to detect IFG in brain of the L444P GCase mice is most likely due to the dose of IFG tartrate administered (20 mg/kg per day) and the relatively low sensitivity for measurement of IFG in brain tissue (limit of quantitation 50 ng/g). Importantly however, the ability of IFG to cross the blood-brain barrier was confirmed previously in the rat tissue distribution studies described above (Fig. 4), as well as in primate studies. In cynomolgus monkeys, a single oral dose (by gavage) of IFG tartrate (1000 mg/kg, equivalent to 500 mg/kg free base) resulted in measurable levels of IFG in cerebrospinal fluid two hours post-administration (Table 4).
Table 4.
Tissue levels of IFG.
| Species | IFG tartrate (mg/kg) | Tissue | [IFG] (ng/g or ng/mL) | LOQ (ng/g or ng/mL) | |
|---|---|---|---|---|---|
| Day 0 | Day 2 | ||||
| L444P GCase mice | 20 | Plasma | 71±7 | <LOQ | 5 |
| Liver | 177±17 | <LOQ | 20 | ||
| Spleen | 70±4 | <LOQ | 20 | ||
| Lung | 69±10 | <LOQ | 20 | ||
| Brain | <LOQ | <LOQ | 50 | ||
| Monkey | 1000 | CSF | 673±128 | ND | 100 |
Four-month old L444P GCase mice were administered IFG tartrate (20 mg/kg per day, equivalent to 10 mg/kg free base) ad libitum in drinking water for four weeks. Mice were euthanized on the last day of dosing (Day 0) or two days after IFG tartrate withdrawal (Day 2). For monkeys (Cynomolgus), a single dose of IFG tartrate (1000 mg/kg, equivalent to 500 mg/kg free base) was administered by oral gavage with cerebrospinal fluid collected two hours post-administration. IFG levels were quantitated by LC-MS/MS and expressed as ng/mL (plasma and CSF) or ng/g (liver, spleen, lung, and brain). Values represent the mean±SEM for groups of 6 (L444P GCase mice) or 10 (monkeys). LOQ, limit of quantitation.
IFG reduces Gaucher disease markers in L444P GCase mice
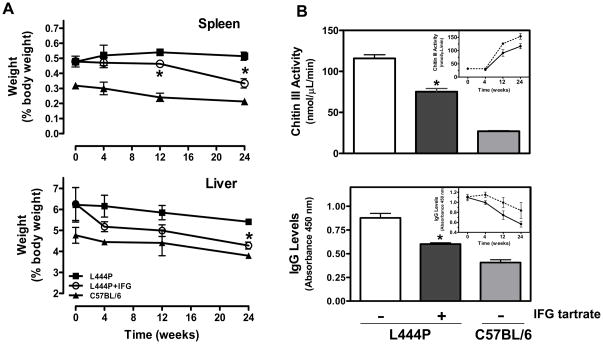
Two-month old L444P GCase mice have moderately elevated liver (20%) and spleen (30%) weights compared to age-matched, isogenic wild-type littermates [36]. To assess the effects of IFG on organ size, one-month old male L444P GCase mice were administered IFG HCl (10 mg/kg per day, equivalent to 8.2 mg/kg free base) ad libitum in drinking water for up to 24 weeks. Groups of 4–6 mice were euthanized after 4, 12, or 24 weeks, together with age-matched untreated L444P GCase and wild-type C57BL/6 mice. Total body, liver, and spleen weights were determined (Table 5). Compared to untreated L444P GCase mice, a statistically significant increase in absolute body weight was seen at 24 weeks in mice that received IFG HCl. Significant reductions in absolute spleen weights at 12 and 24 weeks (13% and 20%, respectively) and liver weights at 24 weeks (6%) were also seen. When expressed as a percentage of total body weight, reductions in organ weights were more pronounced, with 14% and 35% decreases in spleen at 12 and 24 weeks, respectively, and a 21% decrease in liver at 24 weeks (Fig. 7A).
Table 5.
Effect of IFG HCl on absolute spleen and liver weights in L444P GCase mice.
| Mice | Age (weeks) | Treatment (weeks) | Weight (g) | ||
|---|---|---|---|---|---|
| Whole Body | Spleen | Liver | |||
| L444P | 4 | 0 | 20.6±1.2 | 0.1±0.02 | 1.3±0.1 |
| 8 | 0 | 22.0±2.0 | 0.11±0.01 | 1.4±0.08 | |
| 8 | 4 | 23.8±1.8 | 0.11±0.01 | 1.2±0.05 | |
| 16 | 0 | 27.9±2.2 | 0.15±0.01 | 1.6±0.07 | |
| 16 | 12 | 27.7±0.8 | 0.13±0.01* | 1.4±0.04 | |
| 28 | 0 | 29.5±0.6 | 0.15±0.01 | 1.6±0.02 | |
| 28 | 24 | 35.1±0.9* | 0.12±0.01* | 1.5±0.06* | |
| C57BL/6 | 4 | 0 | 22.4±0.03 | 0.08±0.01 | 1.06±0.05 |
| 8 | 0 | 26.7±0.4 | 0.08±0.01 | 1.18±0.08 | |
| 16 | 0 | 32.2±4.0 | 0.08±0.01 | 1.5±0.04 | |
| 28 | 0 | 41.1±1.3 | 0.09±0.003 | 1.56±0.02 | |
Four-week old male L444P GCase mice (n=4–6/group; age at start of treatment) were administered IFG HCl (10 mg/kg per day, equivalent to 8.2 mg/kg free base) ad libitum in drinking water for the times indicated and were compared to untreated L444P GCase (n=4–6/group) and C57BL/6 mice (n=4/group). Mice were euthanized at the indicated ages and the total body, liver, and spleen weights were measured. Statistically significant differences (*) from age-matched, untreated L444P GCase mouse were determined by t test, with p<0.05.
Figure 7. IFG reduces organ weights and the levels of plasma markers for Gaucher disease in L444P GCase mice.
Panel A. One-month old male L444P GCase mice were administered IFG HCl (10 mg/kg per day, equivalent to 8.2 mg/kg free base) ad libitum for up to 24 weeks. Mice (4–6/time point) were euthanized after 4, 12, or 24 weeks of administration and total body, liver, and spleen weights were recorded and compared to those of age-matched untreated L444P GCase and wild-type C57BL/6 mice (4 mice/time point). Statistically significant reductions in organ weights compared to untreated L444P GCase mice were seen in spleen (upper panel) and liver (lower panel). Organ weights are expressed as a percentage of body weight. Panel B. Four-month old male L444P GCase mice were administered IFG tartrate (20 mg/kg per day, equivalent to 10 mg/kg free base) ad libitum for eight weeks (11 mice/time point). Chitin III (upper panel) and IgG (lower panel) levels were measured in plasma samples and compared to those of age-matched untreated L444P GCase (11 mice/time point) and wild-type C57BL/6 mice (6 mice/time point). Statistically significant reductions in both markers compared to untreated L444P GCase mice were seen. Insets. One-month old male L444P GCase mice were administered drinking water (dotted lines) or IFG HCl (10 mg/kg per day, equivalent to 8.2 mg/kg free base (solid lines)) ad libitum for up to 24 weeks. Mice were euthanized after 4, 12, or 24 weeks of treatment (4–6 mice/time point). A trend of reduction in both chitin III and IgG levels was seen with time compared to age-matched untreated L444P GCase mice. Statistically significant differences from untreated were determined using a two-tailed, unpaired student’s t-test with *p<0.05.
The effects of IFG on elevated plasma chitin III and IgG levels [36] in the L444P GCase mice were also assessed. Administration of IFG HCl (10 mg/kg per day, equivalent to 8.2 mg/kg free base) ad libitum for up to 24 weeks resulted in a time-dependent reduction in both markers (Fig. 7B, insets), although statistical significance relative to untreated L444P GCase mice was not attained due to the small number of animals per group (4–6 mice/time point). In a follow-up study, four-month old L444P GCase mice (11 mice/group) were administered IFG tartrate (20 mg/kg per day, equivalent to 10 mg/kg free base) ad libitum for eight weeks and compared to age-matched untreated L444P GCase (n=11) and wild-type C57BL/6 mice (n=6). Compared to untreated L444P GCase mice, statistically significant reductions in both chitin III and IgG levels were seen (Fig. 7B). These data indicate that administration of IFG can reduce tissue weights as well as circulating chitin III and IgG levels in L444P GCase mice.
DISCUSSION
We have shown previously that the pharmacological chaperone IFG selectively binds and stabilizes N370S GCase, promoting trafficking of the enzyme to lysosomes and increasing total cellular enzyme activity [24, 25]. Other pharmacological chaperones have been identified that also bind and stabilize N370S GCase [22, 26–29, 31–34, 39]. To date, however, only small increases in L444P GCase have been reported in Gaucher patient-derived fibroblasts after incubation with the pharmacological chaperone c-octyldeoxynojorimycin [33], whereas other pharmacological chaperones have shown no effect at all [28, 31, 34, 39]. As a result, it has been generally concluded that L444P GCase is not responsive to pharmacological chaperones. In this study, we show for the first time a reproducible, statistically significant, and concentration-dependent increase in L444P GCase activity in cell lysates prepared from LCLs derived from Gaucher patients homozygous for L444P GCase that have been incubated with IFG for five days. The methodologies employed are well known and have been previously described [24, 25, 31–33, 39]. Using the same assay conditions however, fibroblasts derived from Gaucher patients homozygous for L444P GCase showed only small, but reproducible, increases in GCase activity after incubation with IFG, consistent with the previous report using c-octyldeoxynojorimycin [33]. Due to the very low level of GCase activity in the L444P fibroblasts, alternative methods were developed to reduce the amount of IFG in the assay, thereby increasing the overall sensitivity. This was accomplished two ways. First, Con A- or immunocapture (for glycoprotein and GCase enrichment, respectively) was used in combination with multiple wash steps to remove residually-bound IFG prior to assay. Second, cultured cells were subjected to a one-day IFG washout (following five-day incubation with IFG), thereby reducing the quantity of drug in lysates prior to assay. Overall, the maximal increase in L444P GCase activity after five-day incubation with IFG ranged from mild (20% to 30% in fibroblasts using the lysed-cell assay; Fig. 1B, left panel and Table 1), to moderate (100% to 115% in fibroblasts after glycoprotein- or GCase-enrichment, or following a one-day IFG washout prior to assay; Figs. 1C and D; Table 2), to robust (150% to 250% in LCLs using the lysed-cell assay; Fig. 1B, right panel and Table 1). The response mediated by IFG on L444P GCase was selective, as two other small molecule pharmacological chaperones designed to bind lysosomal enzymes other than GCase, 1-deoxygalactonojiromycin (DGJ) [21] and deoxynojiromycin [41], tested in the same cells and under the same conditions did not increase L444P GCase activity (data not shown).
The lack of a robust response for L444P GCase measured directly in fibroblast lysates shown here as well as in previous studies with different small molecule chaperones may be due to a number of factors. First, a clear difference is seen between the magnitude of the response in fibroblasts and LCLs using the lysed-cell assay (Fig. 1 and Table 1), suggesting that cell type may influence the response to a pharmacological chaperone. Importantly, the effects of pharmacological chaperones on L444P GCase had been reported previously only in fibroblasts derived from Gaucher patients [28, 31, 34, 39]. Second, carryover of chaperone into the cell lysates could result in GCase inhibition in the subsequent activity measurements. This possibility is supported by the lower activity measured in L444P fibroblast and LCL lysates after incubation with 60 μM IFG compared to the activity measured after incubation with 6 or 20 μM IFG (Table 1). Western blotting data also support this hypothesis, as maximal increases in GCase protein levels were seen after incubation of fibroblasts with 60 μM IFG (Fig. 1B). Importantly, the GCase activity measured after 60 μM IFG incubation was increased by washing the cells for 24 hours prior to lysis, or by using the glycoprotein-enrichment or GCase immunocapture assays (Figs. 1C and D; Tables 1 and 2). The potential for GCase inhibition may also be lower in LCLs compared to fibroblasts due to the ability of IFG to more freely diffuse into and out of the LCLs grown in suspension compared to the monolayers of adherent fibroblast cells, thus resulting in less IFG carryover. Also, it should be noted that L444P GCase has been reported to bind a number of small molecule pharmacological chaperones with high affinity, similar to wild-type enzyme [41]. This may necessitate a more thorough washout of IFG to ensure accurate measurement of enzyme activity. In contrast, N370S GCase has lower affinity for these molecules, which may make inhibition from chaperone carryover less significant than for L444P or wild-type GCase. Lastly, it has been postulated that active-site-binding pharmacological chaperones may not efficiently stabilize proteins with perturbations caused by spatially distant amino acid substitutions like L444P, which is located in the immunoglobulin-like domain of GCase, especially if the domains are not thermodynamically coupled [24, 42]. However, IFG does clearly increase cellular and lysosomal quantities and activity of L444P GCase (Fig. 2 and Supp. Fig.), suggesting that the binding of some active-site-specific pharmacological chaperones can impart stability to proteins destabilized by mutations in spatially distant protein domains. Taken together, these data indicate that IFG significantly increases cellular L444P GCase activity in multiple cell types in vitro.
In rats, IFG has a broad tissue distribution (Fig. 4) and attains tissue concentrations in excess of its Ki value for GCase inhibition (40 nM), confirming the potential for interaction between chaperone and enzyme in vivo. Oral administration of IFG to L444P GCase mice resulted in a selective, statistically significant, and dose-dependent increase in tissue GCase activity. In liver, spleen, lung, and brain tissue, GCase activity was increased 2- to 5-fold as measured by enzyme activity and 1.2- to 4-fold as measured by semi-quantitative Western blotting (Fig. 5A–C), indicating that IFG can increase both the absolute level and the relative specific activity of the mutant enzyme in vivo. This observation is in agreement with the previous finding that IFG can increase the relative specific activity of N370S GCase in Gaucher patient-derived fibroblasts [25]. The effect of IFG on GCase activity in vivo was selective, as IFG did not alter the activity of four other lysosomal hydrolases, specifically, α-galactosidase A (α-Gal A), acid α-glucosidase, β-glucuronidase, and β-galactosidase. Lastly, L444P GCase activity was elevated approximately 2-fold in macrophage cultures derived from livers of L444P GCase mice after five-day ex vivo incubation with IFG (Fig. 5D), indicating that IFG can increase L444P GCase activity in tissues as well as in cells derived from tissues of L444P GCase mice.
The increase in GCase activity seen in the brains of L444P GCase mice after oral dosing for four weeks suggests that IFG can cross the blood-brain barrier, although brain levels are lower than in plasma and other tissues (Table 4). Access to the brain was confirmed in parallel studies in which IFG was detected in monkey cerebrospinal fluid and rat brain tissue after oral administration at higher doses (Table 4 and Fig. 4, respectively). Furthermore, in separate studies, orally administered IFG tartrate (100 mg/kg per day) increased wild-type GCase activity in rat brains after two-week administration (data not shown). This result extends previous observations indicating that IFG incubation can increase wild-type human GCase activity in vitro [25]. The ability of IFG to cross the blood-brain barrier and to increase the activity of L444P GCase encourages further evaluation of this molecule for the treatment of neuronopathic forms of Gaucher disease.
Administration of IFG to L444P GCase mice for up to 24 weeks reduced liver and spleen weights (Fig. 7A) and lowered plasma chitin III and IgG levels (Fig. 7B), all of which are elevated in these animals [36]. The L444P GCase mice do not show significant GC accumulation and do not have observable Gaucher cells, suggesting that elevations in organ weights and plasma markers may result from systemic inflammation that is characteristic of these animals [36]. In addition, accumulation of misfolded GCase protein in the ER may also contribute. Importantly, systemic inflammation has been reported in a majority of Gaucher patients [43–45]. Furthermore, accumulation of misfolded GCase in the ER has been associated with cellular dysfunction and may be a contributing factor to Gaucher disease [46, 47]. In our studies, IFG was able to increase lysosomal levels of L444P GCase in cultured cells and in tissues of the mutant mice, indicating improved trafficking of this mutant form in vitro and in vivo (Fig. 2 and Supp. Fig.). Given that these mice do not accumulate GC and do not have Gaucher cells, it is possible that a reduction in the amount of misfolded L444P GCase in pre-lysosomal compartments could lead to reduced cellular stress, which in turn, could affect organ weights and plasma marker levels of these animals. Whether the effects of IFG on organ weights and plasma markers of inflammation in L444P GCase mice are mediated by reduced systemic inflammation, reduced ER stress, or both is currently unknown, but will be the focus of future mechanistic studies.
Our data show that exposure to IFG followed by a washout period leads to a sustained increase in L444P GCase levels in cells (Fig. 1C and D) as well as in animals (Fig. 6). In addition, the tissue half-life of IFG is significantly shorter (hours) than that of elevated L444P GCase (days) in vivo (Figs. 4, 6 and Table 4), consistent with the long lysosomal half-life of the mutant enzyme [47]. Taken together, these data indicate that the increased GCase activity can be sustained following IFG clearance, and that the difference in half-lives between IFG and elevated L444P GCase may allow for improved efficacy by using a less-frequent dosing regimen. In this case, a period of IFG administration to provide protein stabilization and trafficking to lysosomes could be followed by a period of IFG withdrawal to allow for dissociation and cellular/tissue clearance of the small molecule, thus minimizing enzyme inhibition in situ and maximizing the net gain in lysosomal enzyme activity. We tested this hypothesis in Gaucher-patient derived cells homozygous for L444P GCase via direct measurement of GC levels. Incubation with 30 μM IFG for seven days, which led to maximal increases in GCase activity in the present study, followed by a 3-day washout (‘7 on/3 off’), significantly reduced GC levels in every cell line tested (Fig. 3 and Table 3). In contrast, no GC reduction was seen in any L444P cell line after 10-day continuous incubation with IFG, demonstrating that the washout period is required for GC reduction. This washout requirement is consistent with previous studies using DGJ, the pharmacological chaperone for mutant α-Gal A, the enzyme deficient in Fabry disease. DGJ washout from Fabry patient-derived cell lines in vitro and less-frequent oral administration to mice that express a mutant form of α-Gal A in vivo maximized substrate reduction [48, 49]. It should also be noted that IFG incubation did not reduce GC levels to those seen in normal control cells or in L444P-expressing cells incubated with NB-DNJ. It is possible that IFG-mediated GC reduction could be maximized further by varying the IFG concentration, incubation duration, washout time, and/or by providing single or multiple optimized incubation/washout cycles. However, testing of multiple incubation/washout cycles requires significantly longer times that are not feasible with fibroblasts or LCLs due to the inability to control for such factors as cell division, contact inhibition, and cell senescence. Such studies would best be conducted using an appropriate Gaucher mouse model that accumulates GC in relevant tissues and cell types. Importantly, the present in vitro results provide the first demonstration of a pharmacological chaperone-mediated increase in cellular L444P GCase activity as directly measured by reduction of endogenous substrate in different Gaucher patient cell lines and cell types. Furthermore, future studies with the L444P GCase mice will determine whether ‘on/off’ dosing regimens can also lead to greater reductions in organ weights and plasma markers of inflammation.
In conclusion, our data indicate that IFG is orally available and has a broad tissue distribution that includes access to the CNS. Furthermore, incubation of Gaucher patient-derived cells with IFG increases the lysosomal trafficking and activity of human L444P GCase, leading to reduced cellular GC levels in intact cells. In addition, oral administration of IFG to L444P GCase knock-in mice increased the activity and lysosomal content of GCase in cells and tissues, resulting in the reduction of several markers of Gaucher disease. Recently, IFG was evaluated in a Phase 2 randomized, open-label clinical study to assess safety, tolerability, and preliminary efficacy in adult type 1 Gaucher patients. Two less-frequent dosing regimens (225 mg administered three days on/four days off or seven days on/seven days off) were studied during this six-month trial. Preliminary results indicate that the treatment was generally well-tolerated, as no serious adverse events were reported. Importantly, all patients that were enrolled showed increased GCase activity as measured in white blood cells, including those homozygous for L444P GCase. However, clinically meaningful improvements in key measures of disease (e.g., reduced spleen weight, glucosylceramide, and plasma markers) were observed in only one of the eighteen patients who completed the study. The results of this trial provide preliminary proof-of-concept that IFG can increase GCase activity and improve the pathophysiological manifestations of Gaucher disease in some patients, although the optimal dose, regimen, and treatment duration are still to be determined. These clinical results, combined with our preclinical observations, suggest that a pharmacological chaperone approach merits a more thorough evaluation as a treatment for patients with neuronopathic and non-neuronopathic forms of Gaucher disease.
MATERIALS AND METHODS
Materials
The HCl salt form of isofagomine (IFG HCl) was purchased from Toronto Research Chemicals; the tartrate salt form (IFG tartrate) was synthesized at Amicus Therapeutics. For tartrate salt conversion, IFG HCl was dissolved in a minimum volume of aqueous NH4OH and filtered through a short silica gel column with 9:1 absolute alcohol (EtOH):aq NH4OH. Evaporation at temperatures below 40 °C yielded IFG free base which was re-dissolved in EtOH and filtered to remove particulates. Separately, 1.3 molar equivalents of L-tartaric acid was dissolved in EtOH, filtered, and added to the free base solution at room temperature. The resulting suspension was stirred at room temperature for 45 minutes, filtered, washed with EtOH, and dried in a vacuum oven to yield IFG tartrate.
Fibroblasts derived from healthy subjects (CRL1509, CRL2076, and CRL2097) were purchased from the American type culture collection (ATCC, Manasses, VA); fibroblasts derived from Gaucher patients homozygous for L444P GCase (GM07968, GM10915, GM08760, and GM00877) or N370S GCase (DMN89.45) were purchased from Coriell (Camden, NJ). Lymphoblastoid cell lines (LCLs) (GS0501, GS0502, GS0503, GS0504, and GS0505) were derived from Gaucher patients’ blood collected in the laboratory of Dr. Raphael Schiffmann (National Institute of Neurological Disorders and Stroke, Bethesda, MD) [50]. LCLs from healthy volunteers (GM02184 and GM03201, WT0003) were obtained from Coriell. For Western blotting, rabbit anti-human GCase and rabbit anti-mouse GCase polyclonal antibodies were gifts from Dr. Gregory Grabowski (University of Ohio, Cincinnati, OH); rabbit anti-human GCase antibody was purchased from Sigma Aldrich (St. Louis, MO). For immunofluorescence analyses, rabbit polyclonal IgG anti-human GCase (raised against recombinant GCase in the laboratory of Don Mahuran) and mouse monoclonal IgG1 anti-human LAMP-1 (from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA)) were used as primary antibodies; Alexa Fluor 488 chicken anti-rabbit IgG and Alexa Fluor 594 goat anti-mouse IgG used as secondary antibodies were purchased from Molecular Probes, Inc. (Eugene, OR). Mice homozygous for L444P GCase were obtained from Dr. Richard Proia (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD). Wild-type C57BL/6 mice and Sprague-Dawley rats were purchased from Taconic Farms (Germantown, NY). Animal husbandry and all in vivo experiments in mice, rats, and monkeys were conducted under Institutional Animal Care and Use Committee (IACUC) approved protocols. All other reagents were purchased from Sigma Aldrich unless noted otherwise.
Measurement of L444P GCase activity in Gaucher patient-derived cell lines
Fibroblasts were seeded at 3×105 cells in T25 flasks in 6 mL Dulbecco’s Modified Eagle’s Media (DMEM) supplemented with 15% fetal bovine serum (FBS), 1% penicillin/streptomycin (P/S), and 1% L-glutamine (GIBCO, Grand Island, NY), and incubated at 37 °C, 8% CO2 for 1 hour for cell attachment. Subsequently, cells were incubated in the absence or presence of IFG tartrate in supplemented DMEM at 37 °C, 8% CO2 for 5 days. When noted, media containing IFG were removed and replaced overnight with media alone to further remove IFG in the cells (IFG washout). After incubation, cells were washed 3 × 10 minutes at 37 °C with DMEM, 2 × 5 minutes with phosphate-buffered saline (PBS) and detached using 1 mL TrypLE Express (Corning, NY) to prepare cell pellets. The pellets were frozen overnight and subsequently lysed in McIlvaine (MI) buffer (100 mM sodium citrate, 200 mM sodium phosphate dibasic, 0.25% sodium taurocholate, and 0.1% Triton X-100, pH 5.2). Lysed cell pellets were refrigerated at 4 °C overnight and GCase activity was then measured in cell lysates using a, previously-described assay [24, 25]. Briefly, lysates (10 μL) were incubated at room temperature without and with 1.25 mM conduritol-B-epoxide (CBE) in MI buffer for 30 minutes. After, 6.0 mM 4-methylumbeliferryl-β-glucoside (4-MUG) substrate in MI buffer (50 μL) was added and incubated at 37 °C for 60 minutes. Reactions were stopped by addition of 0.4 M glycine, pH 10.6 (70 μL). Fluorescence was measured on a Victor2 plate reader (Perkin Elmer, Waltham, MA) for one second per well using 355 nm excitation and 460 nm emission. Total protein was determined in lysates using the MicroBCA kit according to the manufacturer’s instructions (Pierce, Rockford, IL). A 4-methylumbelliferone (4-MU) standard curve ranging from 1 nM to 30 μM was run in parallel for conversion of raw fluorescence intensity to absolute GCase activity expressed as nanomoles of 4-MU released per milligram of protein per hour (nmol/mg protein/hour).
To derive LCLs from Gaucher patients homozygous for the L444P mutation, blood was collected in Vacutainer Blood Collection Tubes (Becton Dickinson, Franklin Lakes, NJ) and spun at 1800g for 10 minutes at room temperature. The buffy-coat was transferred to a 15 mL centrifuge tube. The volume was adjusted with RPMI media (Mediatech, Manassas, VA) to 6 mL and the blood/RPMI solution was gently layered on top of a fresh tube containing 5 mL pre-dispensed Ficoll (Amersham Biosciences, Piscataway, NJ). The solution was centrifuged for 30 minutes at 1000g at room temperature. After centrifugation, the white blood cells at the Ficoll/plasma gradient layer interface were carefully removed with more than half of the overlaying supernatant and dispensed into a fresh 15 mL centrifuge tube. The volume was adjusted to 10 mL with RPMI and centrifuged at 700g for 10 minutes at room temperature. The RPMI wash was repeated twice. White cells were then transformed with Epstein-Barr Virus as described previously to derive LCLs [51]. For the GCase assay, 1.0×105 LCLs were grown in T25 flasks in 5 mL supplemented RPMI and incubated at 37 °C, 5% CO2 overnight. Cells were incubated in the absence or presence of IFG tartrate for 5 days, washed 10 minutes with RPMI at 37 °C, washed 3 × 5 minutes with PBS at room temperature, and subsequently lysed in MI buffer. GCase activity was then measured in the absence and presence of CBE as described above.
For immunocaptured and Con A-enriched GCase assays, 1–1.25×106 fibroblasts were seeded in T25 flasks in 6 mL supplemented DMEM and incubated at 37 °C, 8% CO2 for 3–6 hours. Cells were incubated in the absence or presence of IFG tartrate in supplemented DMEM at 37 °C, 8% CO2 for 5 days. After incubation, cells were washed 2 × 5 minutes at 37 °C with DMEM, and detached using 1 mL TrypLE Express. Cells were then transferred to 15 mL tubes (Fisher Scientific, Pittsburgh, PA), centrifuged for 5 minutes at 200g, washed twice in PBS at room temperature, and suspended in 400 μL of either PBS + 1% NP40 (immunocapture) or Bis-Tris/NaCl (25 mM Bis-Tris, 150 mM NaCl, pH 6.5) + 1% Tween 20 (Con A-capture). Cells were triturated with a pipette, incubated on ice for 30 minutes, and centrifuged for 5 minutes at 16000g. Supernatants (50 to 100 μg protein) were transferred to fresh microcentrifuge tubes and incubated at 4 °C for 12–24 hours with either 50 μL Con A Sepharose beads (GE Healthcare, Piscataway, NJ) for enrichment of glycoproteins or 0.25 μL rabbit anti-human GCase polyclonal antibody for immunocapture. For the immunocapture assays, 10 μL protein G beads (Pierce) were then added to each tube and incubated for an additional 1 hour at 4 °C with rocking. The beads for both the immuno- and Con A-capture assays were collected by centrifugation at 4 °C for 1 minute at 16000g and washed 3 × 10 minutes with 500 μL of either PBS + 0.1% NP40 or Bis-Tris/NaCl + 0.1% Tween 20, respectively.
Immunofluorescence staining and confocal microscopy imaging
For immunofluorescence, 1×105 fibroblasts were seeded in T25 flasks in 6 mL supplemented DMEM and incubated at 37 °C, 8% CO2 for 3–6 hours. Cells were then incubated in the absence or presence of 100 μM IFG tartrate in supplemented DMEM for 14 days. Cells were split after 7 days and fresh media containing IFG were added. After 13 days, cells were washed 2 × 5 minutes with DMEM at 37 °C, and detached using 1 mL TrypLE Express. Cells were then transferred to 15 mL tubes (Fisher Scientific), centrifuged for 5 minutes at 200g, and washed twice in PBS at room temperature. 5×105 cells were then seeded overnight on 18 mm glass coverslips (Fisher Scientific) in 12-well plates (BD Biosciences) in fresh media in the absence or presence of 100 μM IFG. After overnight incubation, cells were washed 2 × 5 minutes with PBS and fixed in 2.5% paraformaldehyde at room temperature for 30 minutes followed by incubation at 37 °C for 10 minutes. The cells were then rinsed three times with PBS followed by indirect immunofluorescence and DAPI (1:50,000 dilution in PBS for 10 minutes at room temperature; Molecular Probe Inc, Eugene, OR) staining and confocal microscopy imaging as previously reported [32, 39]. To provide a qualitative estimate of GCase levels from the fluorescent signals, the same confocal microscope settings were maintained throughout all confocal sessions for wild-type and N370S GCase fibroblasts; however, the detector gain was increased for image recording of L444P GCase fibroblasts to produce a detectable signal. The confocal microscope settings were not changed when collecting LAMP-1 images between the wild-type, N370S, or L444P GCase fibroblasts. Confocal images were imported and contrast/brightness adjusted using Volocity 5 software (Improvision, Inc., Waltham, MA).
GC quantitation in Gaucher fibroblasts and LCLs
LCLs were seeded at 3×105 cells per ml in 9 ml of growth media (RPMI + 10% FBS) per T25 flask. Fibroblasts were seeded at 5.6×104 cells per ml in 19 ml of growth media (DMEM + 15% FBS) per T75 flask and were incubated at 37°C, 8% CO2 for 1 hour. IFG or NB-DNJ were prepared in growth media and incubated with cells for 7 or 10 days under the culture conditions described above. Following the 7-day incubation, cells receiving a 3-day washout period were rinsed 3 × 30 minutes with fresh media at 37 °C followed by incubation with media only for 3 additional days. Fibroblasts were trypsinized and pelleted by centrifugation at 2000 rpm for 5 minutes; lymphoblasts were rinsed twice with PBS and pelleted by centrifugation at 2000 rpm for 5 minutes. Pellets were stored at −80 °C until GC analysis.
Cell pellets were resuspended in 200 μL Lysis Buffer (2.7 mM citrate, 4.6 mM phosphate buffer, pH 5.5), and vortexed. Samples were incubated at room temperature for 15 minutes, then extracted in acetone:methanol (50:50). Samples were vortexed, sonicated, and centrifuged at 10600g for 10 minutes at room temperature. Supernatants (200 μL of the upper organic portion) were mixed with 100 μL N-palmitoyl-d3-glucopsychosine internal standard (625 ng/mL) (Matreya, LLC, Pleasant Gap, PA) and 800 μL of water:methanol (13:87). Solid phase extraction was conducted on a pre-conditioned Bond Elut 40 μm, 100 mg C-18 column (Varian Inc, Palo Alto, CA) by washing with methanol:acetone:water (7:2:1) and elution with 1 mL acetone:methanol (9:1) into silanized glass tubes. Samples were evaporated to dryness at 40 °C, and reconstituted with 200 μL acetone:methanol:water (45:45:10) containing 4 mM lithium acetate. Total GC levels were determined from 30 μL of each sample extract by liquid chromatographic tandem mass spectrometry (LC-MS/MS) (LC: Shimadzu SIL-HT system, Columbia, MD; MS/MS: Sciex API 4000 MS/MS, AME BioSciences, Toten, Norway). LC was conducted using an acetone:methanol mobile phase system containing lithium acetate (mobile phase A: 100% water and 2 mM lithium acetate; mobile phase B: acetone:methanol (60:40) and 2 mM lithium acetate) with a flow rate of 0.4 mL/minute on a C6 phenyl column (Gemini C6 phenyl 3 μm 150 × 3.0 mm, Phenomenex, Torrance, CA). The final GC elution condition was 95% mobile phase B. MS/MS analysis was conducted under positive ion mode (ESI+) and seven different isoforms of GC [C14:0, C16:0, C18:0, C18:1, C20:0, C21:0, C22:0, C23:0, C24:0, C24:1] as well as internal standard [C16:0 D3] were identified in each sample. The following transitions were monitored: m/z 706.7→m/z 496.5 for C16:0; m/z 734.4→m/z 524.6 for C18:0; m/z 762.7→m/z 552.6 for C20:0; m/z 790.9→m/z 580.6 for C22:0; m/z 804.9→m/z 594.7 for C23:0; m/z 818.9→m/z 608.6 for C24:0; m/z 816.9→m/z 606.6 for C24:1; and m/z 709.7→m/z 499.6 for the C16:0 D3 internal standard. For quantitation, the area counts for each isoform were determined and summed to obtain the total GC area counts. The ratio of the total GC area counts to that of the internal standard was used to calculate the final concentration of GC in each sample based on a linear least squares fit equation applied to an 11-point calibration curve (GC reference standard purchased from Matreya LLC) prepared in 20% Lysis Buffer and methanol:acetone (50:50). Total GC measurements were normalized to the total protein in each sample, determined from 50 μL cell lysate using a BCA protein assay (Pierce). No significant differences in GC levels from three normal LCL lines (WT0003, WT0007, and WT0009) or three normal fibroblast lines (CRL1509, CRL2076, and CRL2097) were seen.
Isolation of primary macrophages from L444P GCase mouse liver
Macrophages were isolated from L444P GCase mouse liver as described previously [52]. For the GCase activity assay, 2.5×106 macrophages were grown in 6-well plates in 2 mL RPMI (containing 15% FBS, 1% P/S, and 1% L-glutamine) in the absence or presence of IFG tartrate at 37 °C, 5% CO2 for 5 days; GCase activity in cell lysates was measured as described above.
Administration of IFG to L444P GCase mice and measurement of tissue enzyme activity
Mice were administered either IFG HCl or IFG tartrate in drinking water (ad libitum). The dosing solutions were prepared based on the daily water consumption of L444P GCase mice (approximately 10 mL/day per mouse) and were made fresh each week. After administration, mice were euthanized with CO2 and body weights were recorded. Whole blood was drawn into lithium heparin tubes from the inferior vena cava after CO2 euthanization. Plasma was collected by spinning blood at 2700g for 10 minutes at 4 °C. Liver, spleen, lung, and brain tissues were removed, washed in cold PBS, blotted dry, and weighed before storing on dry ice. Femurs were collected from the hind legs and were cleaned of all muscle tissue. The ends of each bone were then removed and a 23 gauge needle containing 200 μL PBS was carefully inserted into the medullary cavity to flush out the bone marrow.
Tissue GCase activity was measured as described above except that lysates of liver, spleen, lung, and brain were prepared by homogenizing 50 mg of tissue in MI buffer at pH 5.2 for 3–5 seconds on ice with a microhomogenizer (Pro Scientific, Thorofare, NJ). Mineralized bone lysates were prepared by crushing tissue in MI buffer in a liquid nitrogen cooled mortar (Fisher Scientific). Bone marrow lysates were prepared by gently triturating cells with a pipette. For selectivity measurements, substrates specific for the lysosomal hydrolases α-galactosidase A, acid α-glucosidase, β-glucuronidase, and β-galactosidase (4-methylumbeliferryl α-D-galactopyranoside A, 4-methylumbeliferryl α-D-glucopyranoside, 4-methylumbeliferryl β-glucuronide, and 4-methylumbeliferryl β-galactopyranoside, respectively) were used according to methodologies previously described [22].
Tissue distribution of IFG
Eight-week old male Sprague-Dawley rats were administered a single dose (600 mg/kg) of IFG tartrate by oral gavage. Blood and tissues were collected at various time points over a 48-hour period and IFG levels were measured by LC-MS/MS (described below). Whole blood was drawn into lithium heparin tubes from the inferior vena cava after CO2 euthanization. Plasma was collected by spinning blood at 2700g for 10 minutes at 4 °C. Liver, spleen, and brain tissues were removed, washed in cold PBS, blotted dry, and weighed before storing on dry ice.
Tissue levels of IFG in monkey CSF
The in-life portion of the study was performed at MDS Pharma Services (Saint Germain sur I’ Arbresle, France) under Animal Care and Committee (ACC) approval. Five young male and female cynomolgus monkeys (2–4 kilogram) were administered a single dose of IFG tartrate (1000 mg/kg, equivalent to 500 mg/kg free base) via oral gavage, and cerebrospinal fluid (CSF) was collected two hours post-administration. For CSF collection, animals were subjected to general anesthesia using either isofluorane or tiletamine HCl/zolazepam HCl (Telazol®). The hair over the lumbosacral region was clipped and skin was wiped with a povidone-iodine surgical scrub alternating with an alcohol wipe at least three times. Aseptic techniques were followed throughout the procedure. The needle with the syringe was carefully inserted into the intervertebral space until fluid entered the hub. Care was taken to not advance the needle too far, upon which blood is obtained. No more than 2 mL of CSF were collected. IFG levels were quantitated by LC-MS/MS (described below) and expressed as ng/mL.
Tissue IFG quantitation
Mouse or rat tissue (50 mg), plasma or CSF (50 μL) were homogenized in water:acetone:0.5% formaldehyde (1:1:8) containing IFG internal standard (0.5μg/mL). The samples were vortexed, sonicated for 10 minutes, and centrifuged at 10000g for 10 minutes at 4 °C. IFG levels in supernatants were determined by LC-MS/MS using 10 mM NH4HCO3:acetonitrile mobile phase (22:78) at 50 °C with a flow rate of 0.3 mL/min on a 4.6×150 mm 5 μm amine column (Tosoh Bioscience, Cincinnati, OH). Tissue and plasma concentrations of IFG were reported as ng/g or ng/mL respectively, and approximate molar concentrations within tissues were derived based on the molecular weight of IFG (147.17) and the assumption that one gram of tissue is equivalent to 1 mL of volume.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of L444P GCase transcripts
To confirm the genotype of L444P GCase mice, a codon-specific RT-PCR approach was used on mRNA isolated from livers of L444P GCase and wild-type C57BL/6 mice. Codon specificity was attained using forward primers designed to terminate at the proline codon of the L444P transcript or the leucine codon of the wild-type transcript. For this purpose, total RNA from liver tissue of two-month old L444P GCase and wild-type C57BL/6 mice was isolated using the RNAeasy kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). Using oligo dT primers, cDNA was synthesized using the Superscript III first strand kit (Invitrogen, Carlsband, CA) followed by amplification of a 115 base pair (bp) fragment from exon 10 of the murine GCase gene (Gba). The two codon-specific primers, 5′-AGTGAGAGCACTGACCC-3′ (murine L444P GCasec DNA) or 5′-AGTGAGAGCACTGACTT-3′ (murine wild-type GCase cDNA) were used with reverse primer, 5′-CAGGTCAGGATCACTGAG-3′. The PCR amplification consisted of 30 reaction cycles (30 seconds denaturation at 94 °C; 30 seconds annealing at 65 °C; 30 seconds elongation at 72 °C). Using the L444P codon-specific primer, a 115 bp fragment was amplified from L444P cDNA but not from wild-type cDNA. Similarly, the wild-type codon-specific primer yielded a 115 bp fragment from wild-type cDNA but not from L444P cDNA (data not shown). The amplified products were sequenced (Genewiz Technology, South Plainfield, NJ) to confirm the presence of L444P GCase cDNA in the L444P GCase mice.
Western blot analyses
Cell lysates from fibroblasts or LCLs (50 μg total protein per lane) were subjected to SDS-PAGE on 12% gels (Bio-Rad, Hercules, CA), transferred to PVDF membranes (Bio-Rad), and immunoblotted with a rabbit anti-human GCase polyclonal antibody (1:500 dilution); a goat anti-mouse β-actin monoclonal antibody was used as a loading control (1:2500 dilution). Protein bands were detected using peroxidase-conjugated goat anti-rabbit (GCase), or donkey anti-mouse (β-actin) secondary antibodies (Jackson Immunosearch Labs, West Grove, PA) in combination with enhanced chemiluminescence (Pierce). The blots were scanned on an Image Station 4000R (Kodak, Rochester, NY) and the amount of tissue GCase protein relative to β-actin was quantified using Molecular Imaging Software, version 4.0 (Kodak).
Plasma chitin III and IgG assays
Plasma chitin III activity was measured in a 96-well plate assay as described previously [53]. Briefly, 5 μL of plasma were incubated with 95 μL of 0.1 M citric acid/0.2 M sodium phosphate buffer (pH 5.2) containing 3 mM 4-methyl umbelliferyl-β-D-N,N′N″-triacetylchitotriose at 37 °C for 15 minutes. The reaction was stopped with 150 μL of 1 M glycine (pH 10.6). Enzymatic activity was expressed as nanomoles of 4-MU released per μL of plasma per minute (nmol/μL/min). The IgG levels in mouse plasma were measured using a mouse IgG ELISA kit following the manufacturer’s instructions (Bethyl Laboratories, Inc., Montgomery, TX).
Data analysis
All curve fitting was conducted using non-linear regression analyses in GraphPad Prism, version 4.02 (La Jolla, CA). The time required for elevated tissue GCase activity to decay to half the maximum value (half-life) after IFG withdrawal in mouse-based experiments was calculated using a one-phase exponential decay curve fitting function. Statistical significance was determined by calculating p values using a two-tailed unpaired t test in Microsoft Office Excel 2003 (Redmond, WA) or GraphPad Prism. Linear trends of significance (p<0.05) to estimate a dose-dependent increase were calculated using a one-way ANOVA analysis in GraphPad Prism. Tissue exposure to IFG was calculated using WinNonlin software (Pharsight Corporation, Mountain View, CA). GCase activity and protein levels were calculated using Microsoft Excel and GraphPad Prism.
Supplementary Material
Figure S1. IFG increases the lysosomal content of L444P GCase in mouse liver.
Acknowledgments
The authors wish to thank Drs. Benjamin Mugrage and Kamlesh Sheth for synthesizing IFG tartrate, and Dr. Philip Rybczynski for providing the methodology for the synthesis. Sincere thanks are also due to Corey W. Pine for initial observations on the effects of IFG in Gaucher LCLs, Dr. Jim Fan for obtaining the L444P GCase mice, Dr. Shihong Li for the initial observations on L444P mice, Dr. Sheela A. Sitaraman for GC method development, Dr. Mei Hu for IFG method development, and PPD, Inc. (Middleton, WI) for measurement of GC by LC-MS/MS. Lastly, the authors also wish to thank Drs. Matthew J. Toth, Hung V. Do, and Pedro Huertas for helpful discussions.
Abbreviations
- GCase
acid β-glucosidase
- IFG
isofagomine
- GC
glucosylceramide
- CNS
central nervous system
- NB-DNJ
N-butyl-1-deoxynojirimycin
- LCLs
lymphoblastoid cell lines
- IACUC
Institutional Animal Care and Use Committee
- MI
McIlvaine
- DMEM
Dulbecco’s Modified Eagle’s Media
- FBS
fetal bovine serum
- CBE
conduritol-B-epoxide
- 4-MUG
4-methylumbeliferryl-β-glucoside
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- Con A
concanavalin A
- RT-PCR
reverse transcriptase-polymerase chain reaction
- ERT
enzyme replacement therapy
- SRT
substrate reduction therapy
- kD
kilodalton
- SEM
standard error of mean
- FTCD
formiminotransferase cyclodeaminase
- PBS
phosphate-buffered saline
- P/S
penicillin/streptomycin
- RPMI
Roswell Park Memorial Institute
- α-Gal A
α-Galactosidase A
References
- 1.Brady RO, Kanfer JN, Bradley RM, Shapiro D. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher’s disease. J Clin Invest. 1966;45:1112–1115. doi: 10.1172/JCI105417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler E, Grabowski G. In: The Metabolic and Molecular Bases of Inherited Disease 2006. Scriver C, Beaudet A, Sly W, Valle D, editors. McGraw-Hill; New York: 2001. [Google Scholar]
- 3.Butters TD. Gaucher disease. Curr Opin Chem Biol. 2007;11:412–418. doi: 10.1016/j.cbpa.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Beutler E, Gelbart T, West C. Identification of six new Gaucher disease mutations. Genomics. 1993;15:203–205. doi: 10.1006/geno.1993.1035. [DOI] [PubMed] [Google Scholar]
- 5.Grabowski GA. Gaucher disease: Gene frequencies and genotype/phenotype correlations. Genet Test. 1997;1:5–12. doi: 10.1089/gte.1997.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz M, Pasmanik-Chor M, Borochowitz Z, Falik-Zaccai T, Heldmann K, Carmi R, Parvari R, Beit-Or H, Goldman B, Peleg L, Levy-Lahad E, Renbaum P, Legum S, Shomrat R, Yeger H, Benbenisti D, Navon R, Dror V, Shohat M, Magal N, Navot N, Eyal N. Prevalence of glucocerebrosidase mutations in the Israeli Ashkenazi Jewish population. Hum Mutat. 1998;12:240–244. doi: 10.1002/(SICI)1098-1004(1998)12:4<240::AID-HUMU4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NST, Abeysinghe S, Krawczak M, Cooper DN. Human Gene Mutation Database (HGMDR): 2003 update. Hum Mutat. 2003;21:577–581. doi: 10.1002/humu.10212. [DOI] [PubMed] [Google Scholar]
- 8.Barton NW, Brady RO, Dambrosia JM, Di Bisceglie AM, Doppelt SH, Hill SC, Mankin HJ, Murray GJ, Parker RI, Argoff CE, et al. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher’s disease. N Engl J Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- 9.Cox T, Lachmann R, Hollak C, Aerts J, van Weely S, Hrebicek M, Platt F, Butters T, Dwek R, Moyses C, Gow I, Elstein D, Zimran A. Novel oral treatment of Gaucher’s disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. The Lancet. 2000;355:1481–1485. doi: 10.1016/S0140-6736(00)02161-9. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa ML, Rosenbloom BE, Kay AC, Garver P, Thurston DW, Koziol JA, Gelbart T, Beutler E. A less costly regimen of alglucerase to treat Gaucher’s disease. N Engl J Med. 1992;327:1632–1636. doi: 10.1056/NEJM199212033272304. [DOI] [PubMed] [Google Scholar]
- 11.Platt FM, Jeyakumar M, Andersson U, Priestman DA, Dwek RA, Butters TD, Cox TM, Lachmann RH, Hollak C, Aerts JM, Van Weely S, Hrebicek M, Moyses C, Gow I, Elstein D, Zimran A. Inhibition of substrate synthesis as a strategy for glycolipid lysosomal storage disease therapy. J Inherit Metab Dis. 2001;24:275–290. doi: 10.1023/a:1010335505357. [DOI] [PubMed] [Google Scholar]
- 12.Tifft CJ, Proia RL. Stemming the tide: glycosphingolipid synthesis inhibitors as therapy for storage diseases. Glycobiology. 2000;10:1249–1258. doi: 10.1093/glycob/10.12.1249. [DOI] [PubMed] [Google Scholar]
- 13.Barton NW, Furbish FS, Murray GJ, Garfield M, Brady RO. Therapeutic response to intravenous infusions of glucocerebrosidase in a patient with Gaucher disease. Proc Natl Acad Sci U S A. 1990;87:1913–1916. doi: 10.1073/pnas.87.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabowski GA, Leslie N, Wenstrup R. Enzyme therapy for Gaucher disease: the first 5 years. Blood Rev. 1998;12:115–133. doi: 10.1016/s0268-960x(98)90023-6. [DOI] [PubMed] [Google Scholar]
- 15.Lebel E, Dweck A, Foldes AJ, Golowa Y, Itzchaki M, Zimran A, Elstein D. Bone density changes with enzyme therapy for Gaucher disease. J Bone Miner Metab. 2004;22:597–601. doi: 10.1007/s00774-004-0529-8. [DOI] [PubMed] [Google Scholar]
- 16.Schiffmann R, Mankin H, Dambrosia JM, Xavier RJ, Kreps C, Hill SC, Barton NW, Rosenthal DI. Decreased bone density in splenectomized Gaucher patients receiving enzyme replacement therapy. Blood Cells Mol Dis. 2002;28:288–296. doi: 10.1006/bcmd.2002.0517. [DOI] [PubMed] [Google Scholar]
- 17.Futerman AH, Sussman JL, Horowitz M, Silman I, Zimran A. New directions in the treatment of Gaucher disease. Trends Pharmacol Sci. 2004;25:147–151. doi: 10.1016/j.tips.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Schiffmann R, FitzGibbon EJ, Harris C, DeVile C, Davies EH, Abel L, Schaik INV, Benko WS, Timmons M, Ries M, Vellodi A. Randomized, controlled trial of miglustat in Gaucher’s disease type 3. Ann Neurol. 2008;64:514–522. doi: 10.1002/ana.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimran A, Elstein D. Gaucher disease and the clinical experience with substrate reduction therapy. Philos Trans R Soc Lond B Biol Sci. 2003;358:961–966. doi: 10.1098/rstb.2003.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck M. New therapeutic options for lysosomal storage disorders: enzyme replacement, small molecules and gene therapy. Hum Genet. 2007;121:1–22. doi: 10.1007/s00439-006-0280-4. [DOI] [PubMed] [Google Scholar]
- 21.Fan JQ. A contradictory treatment for lysosomal storage disorders: inhibitors enhance mutant enzyme activity. Trends Pharmacol Sci. 2003;24:355–360. doi: 10.1016/S0165-6147(03)00158-5. [DOI] [PubMed] [Google Scholar]
- 22.Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Chemical chaperones increase the cellular activity of N370S beta - glucosidase: A therapeutic strategy for Gaucher disease. Proc Natl Acad Sci U S A. 2002;99:15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawkar AR, D’Haeze W, Kelly JW. Therapeutic strategies to ameliorate lysosomal storage disorders--a focus on Gaucher disease. Cell Mol Life Sci. 2006;63:1179–1192. doi: 10.1007/s00018-005-5437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieberman RL, Wustman BA, Huertas P, Powe AC, Jr, Pine CW, Khanna R, Schlossmacher MG, Ringe D, Petsko GA. Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol. 2007;3:101–107. doi: 10.1038/nchembio850. [DOI] [PubMed] [Google Scholar]
- 25.Steet RA, Chung S, Wustman B, Powe A, Do H, Kornfeld SA. The iminosugar isofagomine increases the activity of N370S mutant acid {beta}-glucosidase in Gaucher fibroblasts by several mechanisms. Proc Natl Acad Sci U S A. 2006;109:13813–13818. doi: 10.1073/pnas.0605928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfonso P, Pampin S, Estrada J, Rodriguez-Rey JC, Giraldo P, Sancho J, Pocovi M. Miglustat (NB-DNJ) works as a chaperone for mutated acid beta-glucosidase in cells transfected with several Gaucher disease mutations. Blood Cells Mol Dis. 2005;35:268–276. doi: 10.1016/j.bcmd.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Chang HH, Asano N, Ishii S, Ichikawa Y, Fan JQ. Hydrophilic iminosugar active-site-specific chaperones increase residual glucocerebrosidase activity in fibroblasts from Gaucher patients. Febs J. 2006;273:4082–4092. doi: 10.1111/j.1742-4658.2006.05410.x. [DOI] [PubMed] [Google Scholar]
- 28.Lei K, Ninomiya H, Suzuki M, Inoue T, Sawa M, Iida M, Ida H, Eto Y, Ogawa S, Ohno K, Suzuki Y. Enzyme enhancement activity of N-octyl-[beta]-valienamine on [beta]-glucosidase mutants associated with Gaucher disease. Biochimica et Biophysica Acta. 2007;1772:587–596. doi: 10.1016/j.bbadis.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Lin H, Sugimoto Y, Ohsaki Y, Ninomiya H, Oka A, Taniguchi M, Ida H, Eto Y, Ogawa S, Matsuzaki Y, Sawa M, Inoue T, Higaki K, Nanba E, Ohno K, Suzuki Y. N-octyl-beta-valienamine up-regulates activity of F213I mutant beta-glucosidase in cultured cells: a potential chemical chaperone therapy for Gaucher disease. Biochim Biophys Acta. 2004;1689:219–228. doi: 10.1016/j.bbadis.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Mu T-W, Ong DST, Wang Y-J, Balch WE, Yates Iii JR, Segatori L, Kelly JW. Chemical and Biological Approaches Synergize to Ameliorate Protein-Folding Diseases. Cell. 2008;134:769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawkar AR, Adamski-Werner SL, Cheng WC, Wong CH, Beutler E, Zimmer KP, Kelly JW. Gaucher disease-associated glucocerebrosidases show mutation-dependent chemical chaperoning profiles. Chem Biol. 2005;12:1235–1244. doi: 10.1016/j.chembiol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Tropak MB, Kornhaber GJ, Rigat BA, Maegawa GH, Buttner JD, Blanchard JE, Murphy C, Tuske SJ, Coales SJ, Hamuro Y, Brown ED, Mahuran DJ. Identification of Pharmacological Chaperones for Gaucher Disease and Characterization of Their Effects on beta-Glucocerebrosidase by Hydrogen/Deuterium Exchange Mass Spectrometry. Chem Bio Chem. 2008;9:2650–2662. doi: 10.1002/cbic.200800304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu L, Ikeda K, Kato A, Adachi I, Godin G, Compain P, Martin O, Asano N. alpha-1-C-Octyl-1-deoxynojirimycin as a pharmacological chaperone for Gaucher disease. Bioorg Med Chem. 2006;14:7736–7744. doi: 10.1016/j.bmc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Yu Z, Sawkar AR, Whalen LJ, Wong CH, Kelly JW. Isofagomine- and 2,5-Anhydro-2,5-imino-D-glucitol-Based Glucocerebrosidase Pharmacological Chaperones for Gaucher Disease Intervention. J Med Chem. 2007;50:94–100. doi: 10.1021/jm060677i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Suzuki K, Reed JD, Grinberg A, Westphal H, Hoffmann A, Doring T, Sandhoff K, Proia RL. Mice with type 2 and 3 Gaucher disease point mutations generated by a single insertion mutagenesis procedure. Proc Natl Acad Sci U S A. 1998;95:2503–2508. doi: 10.1073/pnas.95.5.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizukami H, Mi Y, Wada R, Kono M, Yamashita T, Liu Y, Werth N, Sandhoff R, Sandhoff K, Proia RL. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J Clin Invest. 2002;109:1215–1221. doi: 10.1172/JCI14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y, Quinn B, Witte DP, Grabowski GA. Gaucher disease mouse models: point mutations at the acid beta-glucosidase locus combined with low-level prosaposin expression lead to disease variants. J Lipid Res. 2005;46:2102–2113. doi: 10.1194/jlr.M500202-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Xu YH, Quinn B, Witte D, Grabowski GA. Viable mouse models of acid beta-glucosidase deficiency: the defect in Gaucher disease. Am J Pathol. 2003;163:2093–2101. doi: 10.1016/s0002-9440(10)63566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigat B, Mahuran D. Diltiazem, a L-type Ca2+ channel blocker, also acts as a pharmacological chaperone in Gaucher patient cells. Mol Genet Metab. 2009;96:225–232. doi: 10.1016/j.ymgme.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butters TD, Dwek RA, Platt FM. Inhibition of glycosphingolipid biosynthesis: application to lysosomal storage disorders. Chem Rev. 2000;100:4683–96. doi: 10.1021/cr990292q. [DOI] [PubMed] [Google Scholar]
- 41.Liou B, Kazimierczuk A, Zhang M, Scott CR, Hegde RS, Grabowski GA. Analyses of variant acid beta-glucosidases: effects of Gaucher disease mutations. J Biol Chem. 2006;281:4242–4253. doi: 10.1074/jbc.M511110200. [DOI] [PubMed] [Google Scholar]
- 42.Dvir H, Harel M, McCarthy AA, Toker L, Silman I, Futerman AH, Sussman JL. X-ray structure of human acid-beta-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep. 2003;4:1–6. doi: 10.1038/sj.embor.embor873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen MJ, Myer BJ, Khokher AM, Rushton N, Cox TM. Pro-inflammatory cytokines and the pathogenesis of Gaucher’s disease: increased release of interleukin-6 and interleukin-10. Qjm. 1997;90:19–25. doi: 10.1093/qjmed/90.1.19. [DOI] [PubMed] [Google Scholar]
- 44.Michelakakis H, Spanou C, Kondyli A, Dimitriou E, Van Weely S, Hollak CEM, Van Oers MHJ, Aerts JMFG. Plasma tumor necrosis factor-a (TNF-a) levels in Gaucher disease. Biochimica et Biophysica Acta. 1996;1317:219–222. doi: 10.1016/s0925-4439(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 45.Zimran A, Bashkin A, Elstein D, Rudensky B, Rotstein R, Rozenblat M, Mardi T, Zeltser D, Deutsch V, Shapira I, Berliner S. Rheological determinants in patients with Gaucher disease and internal inflammation. Am J Hematol. 2004;75:190–194. doi: 10.1002/ajh.20011. [DOI] [PubMed] [Google Scholar]
- 46.Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet. 2005;14:2387–2398. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 47.Ron I, Horowitz M. Intracellular cholesterol modifies the ERAD of glucocerebrosidase in Gaucher disease patients. Mol Genet Metab. 2008;93:426–436. doi: 10.1016/j.ymgme.2007.10.132. [DOI] [PubMed] [Google Scholar]
- 48.Benjamin E, Flanagan J, Schilling A, Chang H, Agarwal L, Katz E, Wu X, Pine C, Wustman B, Desnick R, Lockhart D, Valenzano K. The pharmacological chaperone 1-deoxygalactonojirimycin increases α-galactosidase A levels in Fabry patient cell lines. Journal of Inherited Metabolic Disease. 2009;32:424–440. doi: 10.1007/s10545-009-1077-0. [DOI] [PubMed] [Google Scholar]
- 49.Khanna R, Soska R, Lun Y, Feng J, Frascella M, Young B, Brignol N, Pellegrino L, Sitaraman SA, Desnick RJ, Benjamin ER, Lockhart DJ, Valenzano KJ. The Pharmacological Chaperone 1-Deoxygalactonojirimycin Reduces Tissue Globotriaosylceramide Levels in a Mouse Model of Fabry Disease. Mol Ther. 2009 doi: 10.1038/mt.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wustman B, Pine C, Ranes B, Flanagan J, Palling D, Do H, Insinga F, Grabowski G, Weinreb N, Pastores G, Fernhoff P, Kaplan P, Lockhart D. 114. Pharmacological chaperone therapy for Gaucher disease: Mechanism of action, a survey of responsive mutations and phase I clinical trial results. Mol Genet Metab. 2008;93:44. [Google Scholar]
- 51.Neitzel H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet. 1986;73:320–326. doi: 10.1007/BF00279094. [DOI] [PubMed] [Google Scholar]
- 52.Chan KW, Waire J, Simons B, Karey K, Fung J, Copeland D, Andrews L. Measurement of lysosomal glucocerebrosidase activity in mouse liver using a fluorescence-activated cell sorter assay. Anal Biochem. 2004;334:227–233. doi: 10.1016/j.ab.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 53.Hollak CE, van Weely S, van Oers MH, Aerts JM. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93:1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. IFG increases the lysosomal content of L444P GCase in mouse liver.