Abstract
Angiogenesis inhibitors targeting the vascular endothelial growth factor (VEGF) signalling pathways are affording demonstrable therapeutic efficacy in mouse models of cancer and in an increasing number of human cancers. However, in both preclinical and clinical settings, the benefits are at best transitory and are followed by a restoration of tumour growth and progression. Emerging data support a proposition that two modes of unconventional resistance underlie such results: evasive resistance, an adaptation to circumvent the specific angiogenic blockade; and intrinsic or pre-existing indifference. Multiple mechanisms can be invoked in different tumour contexts to manifest both evasive and intrinsic resistance, motivating assessment of their prevalence and importance and in turn the design of pharmacological strategies that confer enduring anti-angiogenic therapies.
The long-standing proposition that induction of chronic angiogenesis is a hallmark of cancer is now solidly grounded in a substantial body of research involving genetic and pharmacological perturbation of elements in the vascular regulatory circuitry. The ‘angiogenic switch’1 is increasingly recognized as a rate-limiting secondary event in multistage carcinogenesis2, as documented in animal models of cancer and correlated in advanced pre malignant stages, as well as their malignant derivatives, in a growing list of human cancer types. That this acquired capability is functionally important for manifestation of the disease has been further validated by the approval of angiogenesis inhibitors as cancer therapeutics, most notably ones targeting the vascular endothelial growth factor (VEGF) pro-angiogenic signalling pathways3. The pioneers of the clinical proof-of-concept for angiogenesis inhibitors are bevacizumab (Avastin, Genentech/Roche), a ligand-trapping monoclonal antibody, and two kinase inhibitors (sorafenib (Nexavar, Bayer) and sunitinib (Sutent, Pfizer)) targeting the VEGF receptor (VEGFR) tyrosine kinases, principally VEGFR2 (also known as KDR). Since March 2008, bevacizumab has been approved for treating patients with late-stage colon cancer, non-small-cell lung cancer and breast cancer, all in combination with chemotherapy. Sorafenib and sunitinib have both been approved for treating renal carcinoma, a highly vascularized (and angiogenic) tumour type. In addition, sunitinib has been approved for treating gastrointestinal stromal tumours, and sorafenib for hepatocel-lular carcinomas3–6. Numerous ongoing clinical trials seek to expand the applications of each of these VEGF pathway inhibitors, and dozens of other angiogenesis inhibitors (many also targeting VEGF signalling) are being clinically evaluated (see Angiogenesis Inhibitors Therapy URL and clinical trials URLs in Further information). Moreover, two VEGF pathway inhibitors (the RNA aptamer pegaptanib and a Fab derivative of bevacizumab) have been approved for treating the angiogenic (wet) form of macular degeneration7–9.
Many of the demonstrable clinical benefits and side effects (such as hypertension) of the kinase inhibitors targeting the VEGF signalling pathway (sorafenib, sunitinib and dozens more in various stages of preclinical and clinical testing) can be attributed to inhibition of the activity of the VEGFRs, but it must be emphasized that all are intrinsically (owing to their chemistry) selective but not specific. Thus, virtually every VEGFR kinase inhibitor has an attendant variety of additional moderate-to-high affinity kinase targets in the ‘kinome’10, of which some may convey added therapeutic benefit (such as inhibition of platelet-derived growth factor receptor (PDGFR), in the case of sunitinib), and others may evoke new toxicities. The components of the VEGF signalling pathway, the constellation of drugs developed to inhibit VEGF signalling, the specific mechanistic effects of VEGF signalling and VEGF inhibition, and the clinical trials evaluating their effects are described in depth and discussed in a Review by L. Ellis and D. J. Hicklin also in this Focus issue11. Additional reviews, in this Focus on targeting angiogenesis12–14 and elsewhere15–21, describe and discuss other targets and strategies for inhibiting tumour angiogenesis.
The clinical achievements with bevacizumab, sunitinib and sorafenib constitute a milestone event for the field of angiogenesis research, eliciting survival benefits in many aggressive tumours, but there is a sobering addendum: these VEGF pathway inhibitors are failing to produce enduring clinical responses in most patients5,22–24. Rather, the introduction of anti-angiogenic therapy results in transitory improvements, in the form of tumour stasis or shrinkage and in some cases increased survival. Inevitably, however, the tumours begin to grow again and the disease progresses, after a fleeting period of clinical benefit that is typically measured in months25. This seemingly preordained return to growth and progression in the face of ostensibly potent angiogenesis inhibitors conflicts with the widely accepted proposition that angiogenesis is an essential capability for the manifestation of lethal cancer2,26. There is, therefore, a clear need to understand the mechanistic basis of this apparent conundrum for the therapeutic targeting of tumour angiogenesis.
In this Review, we elaborate a hypothesis for the transitory efficacy of the current generation of pathway-specific angiogenesis inhibitors, one we predict will prove general to potent angiogenesis inhibitors. our proposition is based both on emerging data from clinical and preclinical investigations, and on mechanistic insights from studying the biological regulatory mechanisms operative in the tumour microenvironment that govern tumour phenotypes, most notably angiogenesis and invasion. We envision two general modes of resistance to angiogenesis inhibitors, in particular those targeting the VEGF pathways: first, adaptive (evasive) resistance; and second, intrinsic (pre-existing) non-responsiveness (FIG. 1). Multiple mechanisms are suspected to underlie both modes of resistance, as outlined below.
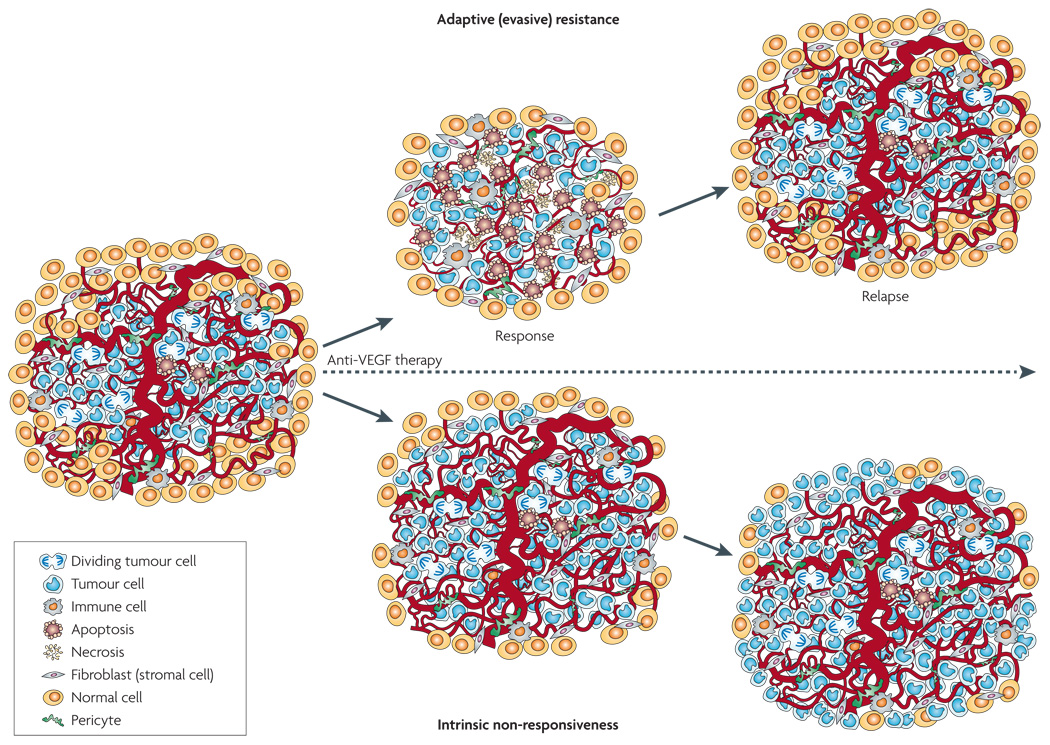
Figure 1. Two modes of resistance in response to anti-angiogenic therapy imply adaptive evasion and intrinsic non-responsiveness of tumours.
Adaptive or evasive resistance refers to the ability of a tumour, after an initial response phase, to adapt so as to evade the therapeutic blockade by inducing or accentuating mechanisms that enable neovascularization despite the therapeutic blockade, or reduce dependence on such growth of new blood vessels by other means, leading to renewed tumour growth and progression. By contrast, intrinsic non-responsiveness is a pre-existing condition defined by the absence of any (even transitory) beneficial effect of an anti-angiogenic therapy, ranging from the inability to shrink or stabilize tumours to the lack of improvement in quality of life. Consequently tumours grow and progress unabated during the course of anti-angiogenic therapy. VEGF, vascular endothelial growth factor.
Mode I: evasion of anti-angiogenic therapy
Over the past few years a provocative ‘contrarian’ concept has emerged from the hopeful but simplistic view that angiogenesis inhibition would prove insurmountable27,28; the new postulates arose from considering the results of both preclinical and clinical research into angiogenic mechanisms and the largely modest effects that angiogenesis inhibitors have had on tumours and cancer patients. The evolving hypothesis is that angiogenic tumours can adapt to the presence of angiogenesis inhibitors, variously acquiring the means to functionally evade the therapeutic blockade of angiogenesis25,29–36. In contradistinction to traditional concepts of drug resistance being acquired by mutational alteration of the gene encoding a drug target or by alterations in drug uptake and efflux37,38, evasive resistance is largely indirect: alternative ways to sustain tumour growth are activated but the specific therapeutic target of the anti-angiogenic drug remains inhibited30,31,33,36,39–41. The current experimental evidence, which is not yet definitive, suggests that there are at least four distinct adaptive mechanisms that manifest evasive resistance to anti-angiogenic therapies: first, activation and/or upregulation of alternative pro-angiogenic signalling pathways within the tumour (FIG. 2); second, recruitment of bone marrow-derived pro-angiogenic cells, both of which can obviate the necessity of VEGF signalling, thereby effecting reinitiation and continuance of tumour angiogenesis (FIG 2,FIG 3); third, increased pericyte coverage of the tumour vascu-lature, serving to support its integrity and attenuate the necessity for VEGF-mediated survival signalling (FIG. 4); and fourth, activation and enhancement of invasion and metastasis to provide access to normal tissue vasculature without obligate neovascularization (FIG. 5).
Figure 2. Induced pro-angiogenic factor substitution re-establishes tumour neovascularization.
Activation and/or upregulation of other pro-angiogenic signalling pathways, including those involving members of the fibroblast growth factor (FGF), ephrin and angiopoietin families, can circumvent the anti-angiogenic therapy and, in due course, provoke neovascularization and subsequent tumour relapse.
Figure 3. Recruitment of bone marrow-derived cells can endorse restored neovascularization.
Low oxygen conditions in tumours (hypoxia), acting in part through induced increases in hypoxia-inducible factor 1α and its targets stromal cell-derived factor 1α and vascular endothelial growth factor, can attract a heterogeneous population of bone marrow-derived cells consisting of vascular progenitors and pro-angiogenic monocytic cells. Endothelial and pericyte progenitors are incorporated as components of new vessels to directly build new blood vessels, and pro-angiogenic monocytes fuel the tumours with pro-angiogenic cytokines, growth factors and proteases, all of which facilitate neovascularization.
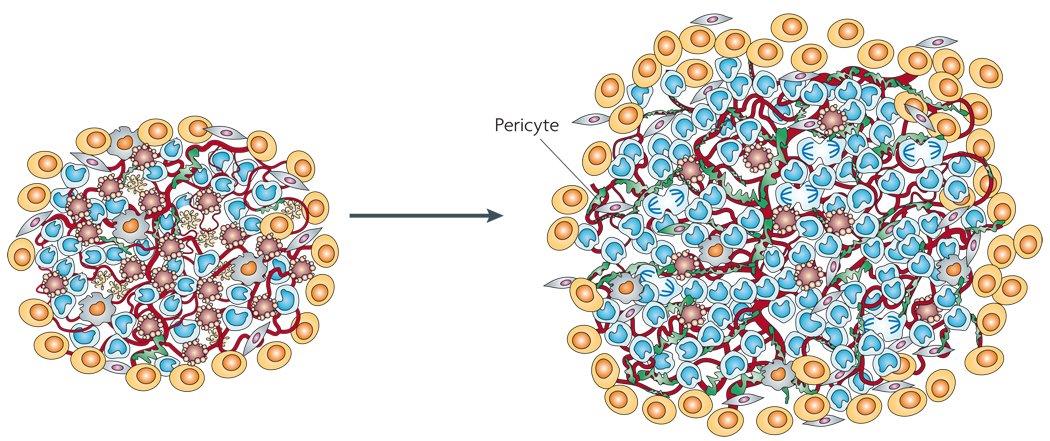
Figure 4. Increased pericyte coverage protects tumour blood vessels.
Although inhibition of vascular endothelial growth factor signalling pathways can lead to vessel regression, a few ‘normal-appearing’ slim and functional vessels remain; these vessels are densely and tightly covered with pericytes, and are markedly distinct from the vessels that are seen in tumours of untreated animals, which are typically dilated, tortuous and irregularly shaped, and variably covered with less closely associated pericytes. Such coating by pericytes arguably helps the tumour endothelium to survive and function, and thereby enables tumours to grow (perhaps more slowly) during the course of an anti-angiogenic therapeutic regimen.
Figure 5. Increased tumour cell invasiveness to escape oxygen and nutrient deprivation.
When tumours are not able to satisfactorily reinitiate angiogenesis, tumour cells may invade adjacent normal tissue to achieve vascular sufficiency in a dispersed fashion. Tumour cells can migrate along the outside of blood vessels (perivascu lar invasion), using them as conduits into normal tissue, or infiltrate through the extracellular matrix. In either case they depend on normal vasculature for sustenance.
The logical bases for these four adaptive mechanisms and the emerging experimental evidence that supports their existence are presented in the subsections below.
At a glance
Angiogenesis inhibitors targeting the vascular endothelial growth factor (VEGF)-mediated pro-angiogenic signalling pathways are producing demonstrable clinical benefit for an increasing number of cancer types. However, in some cases (both in humans and in mouse models of cancer) anti-angiogenic therapies produce initial responses followed almost inevitably by progression, thereby affording appreciable but limited survival advantage. In other cases there is no objective benefit. Increasing evidence supports the proposition that progression and mortality following a period of benefit reflects an adaptive response by tumours, manifesting ‘evasive resistance’ to angiogenesis inhibitors. By contrast, patients for whom there is no tangible benefit indicate that an intrinsic resistance to angiogenesis inhibitors exists.
Evasive resistance to VEGF pathway inhibitors (and arguably other angiogenesis inhibitors) involves a number of distinct and interrelated mechanisms that may be variably important. The emergent mechanisms of evasive resistance include revascularization consequent to upregulation of alternative pro-angiogenic signals; protection of the tumour vasculature either by recruiting pro-angiogenic inflammatory cells or by increasing protective pericyte coverage; accentuated invasiveness of tumour cells into local tissue to co-opt normal vasculature; and increased metastatic seeding and tumour cell growth in lymph nodes and distant organs.
Intrinsic resistance is likely to involve similar molecular and cellular mechanisms to those that mediate evasive resistance. Whereas rapid adaptive responses (fast evasion) may underlie some cases of apparent intrinsic resistance, there is evidence to suggest that certain tumours, owing to their stage of progression, treatment history, genomic constitution and/or host genotype, may have a pre-existing tumour microenvironment that conveys such indifference.
If the postulate of evasive and intrinsic resistance to angiogenesis inhibitors is further validated in preclinical and clinical investigations, we foresee a future of cancer therapeutics in which combinatorial strategies involving angiogenesis inhibition are integrated with drugs targeting resistance mechanisms to afford enduring efficacy.
Upregulation of alternative pro-angiogenic signalling circuits
Evidence for the existence of evasive resistance manifested by alternative pro-angiogenic signalling was revealed during preclinical trials in a genetically engineered mouse model of pancreatic neuroendocrine (islet cell) cancer, Rip1–Tag2 (REF. 30). When Rip1–Tag2 mice were treated with a monoclonal antibody (DC101) that specifically blocked VEGFR signalling (in particular VEGFR2), there was an initial response denoted by tumour stasis and reductions in tumour vascularity. However, the response phase was transitory (10–14 days) and was followed by tumour regrowth, during which the typically dense tumour vasculature was restored, indicative of re-initiation and persistence of tumour angiogen esis. The relapsing tumours were found to express higher levels of the mRNAs for the pro-angiogenic factors fibroblast growth factor 1 (Fgf1) and Fgf2, ephrin A1 (Efna1) and Efna2, and angiopoietin 1 (Angpt1) than did unperturbed tumours. Notably, the tumours had regions of acute hypoxia at the peak of the response phase, and tumour-derived cells subjected to hypoxic conditions similarly upregulated most of these genes. In order to begin assessing the functional significance of these upregulated genes in the observed revasculariza tion, mice were first treated with the VEGFR inhibitor alone, and then at the peak of the response phase they were also treated with an FGF-trap (FGFR–Fc fusion protein) to suppress signalling through the FGF ligands. The combination attenuated the revascularization and slowed tumour growth, indicating that FGF signalling was involved in regulating the restored angiogenesis. A concurrent study showed that induction of the pro-angiogenic cytokine IL8 served to maintain an angiogenic capability in tumours that were otherwise impaired owing to absence of the transcription factor hypoxia-inducible factor 1α (HIF1 α), an inducer of VEGF expression36. Further corroboration comes from a recent study that implicated evasive resistance (through upregulation of pro-angiogenic factors such as VEGF, PDGFA and FGF2) in tumours whose growth is impaired by ectopic expression of the endogenous angiogenesis inhibitors thrombospondin 1, tumstatin and endostatin (also known as CoL4A3 and CoL18A1 fragments, respectively)40.
A suggestion of analogous evasive resistance by FGF-dependent revascularization has come from a clinical investigation involving a study of glioblastoma patients being treated with the VEGFR inhibitor cediranib (Recentin, Astra Zeneca)42. There was a demonstrable response phase, and then a relapse or progression phase. Provocatively, levels of FGF2, one of the factors associated with evasive resistance in the preclinical models, were found to be higher in the blood of relapsing patients than in that of the same patients during the response phase, indicative of an analogous adaptive mechanism involving upregulation of FGF2. The fact that receptor tyrosine kinase inhibitors can transiently increase plasma levels of pro-angiogenic factors such as VEGF and placental growth factor (PlGF, also known as PGF) has been previously recognized in the clinic, and even proposed as a predictive biomarker for tumour response43–45. Such propositions, however, may require refinement or reconsideration in light of recent experiments that revealed increased levels of pro-angiogenic factors in the circulation of sunitinib-treated control (non-tumour-bearing) mice, implicating a systemic endocrine response to inhibition of VEGF and PDGF signalling in normal tissues46. Increased VEGF expression was detected in a number of tissues in the sunitinib-treated mice, as were dose-dependent increases in plasma levels of granulocyte colony-stimulating factor (GCSF, also known as CSF3), stromal cell-derived factor 1α (SDF1α, also known as CXCL12), stem cell factor (SCF, also known as KIT ligand) and osteopontin. Thus, the presence of pro-angiogenic factors in the blood may not be strictly indicative of tumour revascularization nor of their involvement in it. on the other hand, vascular imaging of the glioma patients treated with cediranib indicated that relapse was accompanied by re-initiation of tumour angiogen esis and loss of vascular normalization42, implicating a mechanism to evade the VEGF blockade. This could well involve upregulated FGF2, consistent with its increased level in the bloodstream.
The resulting uncertainties about the instructive value of increased levels of pro-angiogenic ligands in the circulation underscore the importance of analysing treated human tumour tissue to assess the angiogenic and histopathological phenotypes in responding and relapsing tumours in the face of anti-VEGF therapy. In mouse models, the capability to do this has been instrumental in revealing the existence and importance of alternative pro-angiogenic signalling for evasive resistance. Critical evaluation of the existence (and prevalence) of this mechanism in human tumours would be markedly facilitated by increased use of trial designs involving surgical resection following a regimen of anti-angiogenic therapy, more frequent imaging (such as dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) and possibly Doppler ultrasound) of tumours to assess vascular status during the course of treatment, and development of refined pre- and post-treatment biopsy strategies amenable to more routine use.
Recruitment of vascular progenitor cells and pro-angiogenic monocytes from the bone marrow
Low oxygen conditions (hypoxia) caused by vessel regression during the course of anti-angiogenic therapy can not only lead to upregulation of pro-angiogenic factors within the tumours but also to the recruitment of various bone marrow-derived cells (BMDCs) that have the capacity to fuel tumours by eliciting new blood vessels (FIG. 3). Pro-angiogenic BMDCs consist of vascular progenitors and vascular modulatory cells. Endothelial and pericyte progenitors differentiate into endothelial cells, which form the inner lining of blood vessel walls, or pericytes, the cells that envelop blood vessels, respectively47,72 (BOX 1). By contrast, pro-angiogenic monocytes, such as tumour-associated macrophages48, and immature mono cytic cells — including TIE2+ (also known as TEK+) monocytes49, VEGFR1+ hemangiocytes50,51 and CD11b+ (also known as ITGAM+) myeloid cells52,53 — exert their functions as vascular modulators by expressing a variety of cytokines, growth factors and proteases without being physically part of the vasculature54,55. Evidence that low oxygen tension triggers the recruitment of BMDCs stems from observations in experimentally induced ischaemic tissue, wherein endothelial progenitors and other CXCR4+ BMDCs are recruited, in part through increases in HIF1α and its downstream effectors SDF1α (a CXCR4 ligand) and VEGF itself18,56,57. In a study of neovascularization in glioblastoma multiforme (GBM), a tumour type characterized by extensive hypoxia and necrosis, HIF1α was found to promote angiogenesis and tumour growth by inducing recruitment of various pro-angiogenic bone marrow-derived CD45+ (also known as PTPRC+) myeloid cells, including subpopulations defined by expression of TIE2, VEGFR1 and/or CD11b, as well as mature F4/80+ tumour-associated macrophages; endothelial and pericyte progenitor cells were also prevalent55,58. GBM tumours lacking HIF1α had few of these abundant BMDCs, and were severely impaired in their angiogenic and tumour growth phenotypes55.
Box 1|Pericytes
Pericytes (mural or vascular smooth muscle cells) are perivascular cells with a prominent nucleus, a small content of cytoplasm and long processes that wrap around blood capillaries (peri- meaning around; -cyte meaning cell). They communicate with endothelial cells by direct physical contact through a jointly synthesized basement membrane and reciprocal paracrine signalling. Pericytes and endothelial cells are thereby interdependent and, as such, defects in either endothelial cells or pericytes can affect the vascular system. Pericytes have various demonstrable functions in different physiological contexts, including stabilization and homeostatic regulation of mature blood vessels; facilitation of vessel maturation in the context of neovascularization; provision by their intimate association of endothelial cell survival signals; and limitation of cell transit across the vascular wall. The functional significance of pericytes in development is underscored by genetic depletion or disruption of pericyte association with the developing vasculature in mice, which results in blood vessel dilation, widespread microvascular leakage and microaneurysms, and subsequent lethality during late gestation. In tumours, pericytes are typically less abundant and more loosely attached to blood vessels than in normal tissues, but their association is still important, as shown in a growing body of experimental evidence which indicates that pericytes help to maintain the integrity and functionality of the tumour vasculature.
These results collectively suggest that anti-angiogen-esis-induced hypoxia can in some contexts elicit BMDC recruitment and thereby foster an adaptive mechanism that enables tumours to overcome hypoxia. Given that many tumours contain hypoxic areas, influx of BMDCs may depend on a threshold of or correlate with the degree of low oxygen tension59. Evidence supporting the link between therapy-induced hypoxia and BMDC recruitment has come from a study demonstrating that vascula-ture-disrupting agents, which ablate blood vessels within a tumour and thereby cause acute hypoxia and necrosis, can trigger a transient accumulation of endothelial progenitor cells at the tumour margins in sufficient numbers to facilitate revascularization60. Notably, the untreated transplant tumours did not contain appreciable levels of BMDCs, consistent with the interpretation that this was an adaptive response enabling evasive resistance to potent anti-angiogenesis therapy.
There is intriguing evidence from clinical investigations that this evasion mechanism operates in patients with GBM who are undergoing anti-VEGF therapy. one recent study suggests that hypoxia determines survival outcome in bevacizumab-treated GBM patients61, and a second reports that SDF1α, a hypoxia-regulated retention factor for CXCR4+ BMDCs, was detectable in the blood of patients with GBM at the time of tumour progression and relapse (discussed above and in ReF.42). Therefore, SDF1α could be an effector and biomarker of response for relapsing tumours, raising the possibility that both forms of evasive resistance (upregulation of pro-angiogenic factors such as FGF2 and recruitment of BMDCs) could be operative and collaborative in GBM. However, there remains the challenge to develop means to distinguish systemic paracrine reactions of drug-treated normal tissue from those of tumour-specific adaptive responses. We predict that such mechanisms will prove to be involved in other tumour types, and anticipate that both these clinical investigations and the aforementioned preclinical studies will motivate further investigation of the possibility.
Increased and tight pericyte coverage protects tumour blood vessels
A growing body of evidence indicates that pericytes, the periendothelial support cells of the microvasculature62–64, are also important cell constituents of the aberrant tumour vasculature. When therapies impair neovascularization and/or elicit vascular regression, some tumours evidently rely on pericytes to help keep a core of pre-existing blood vessels alive and functional (FIG. 3). This concept has evolved from the observation by several groups that, although inhibition of VEGF signalling can lead to substantial reduction in tumour vascularity, distinctive functional vessels remain that are slim and tightly covered with pericytes19,65–68. Notably, the morphology of these surviving tumour vessels is readily distinguishable from the typically dilated tumour vessels of untreated animals, which are, by contrast, variably covered with less closely associated pericytes19,66,67,69. These observations suggest that endothelial cells can induce pericyte recruitment to protect themselves from death consequent to the lack of the crucial tumour-derived survival signals conveyed by VEGF. This hypothesis is supported by the findings that tumour vessels lacking adequate pericyte coverage are more vulnerable to VEGF inhibition19,70 and that tumour pericytes, which are juxtacrine to endothelial cells, express appreciable levels of VEGF and potentially other factors that support endothelial cell survival71,72. In addition, pericytes are capable of attenuating the proliferation rate of endothelial cells73,74, a necessity for proper maturation and stabilization of newly formed vascular structures. one can therefore speculate that in treated tumours, blood vessels heavily covered with peri cytes survive because pericytes mediate endothelial cell quiescence and survival (this is also the case for normal tissue), therefore rendering tumour endothelial cells less responsive to anti-angiogenic agents.
Based on the stabilizing effects of pericytes and their functional capability to ameliorate the effects of VEGFA depletion on angiogenic endothelium, there is a rationale for targeting both cell types. Indeed, improved efficacy from dual targeting of endothelial cells and pericytes has been observed in a variety of mouse tumour models, beginning with the Rip1–Tag2 transgenic mouse model of pancreatic neuroendocrine cancer in studies combining inhibitors of VEGF and PDGF signalling to target endothelial cells and pericytes, respectively19,20,75,76. Clinical trials are currently ongoing or in development that aim to target endothelial cells and pericytes simultaneously and assess the potential benefits for anti-tumoural efficacy. A related agenda will be to clarify the clinical situations in which singular VEGF pathway inhibition (with its attendant transitory normalization) is preferable, and those in which combining VEGF inhibitors with agents that disturb vascular ‘normalization’ by disrupting pericyte support (such as PDGFR inhibitors) has greater benefit.
The strategy of dissociating pericytes from the tumour vasculature (with inhibitors of PDGFR signalling, for example) to render the tumour endothelium more sensitive to VEGF inhibitors is attractive, but emerging clues suggest an additional and undesirable effect: severe reduction or lack of pericyte coverage may disrupt the integrity of the vasculature, enabling tumour cells to transit into the circulatory system, thereby facilitating metastasis. In one study, genetic disruptions of pericyte coverage elicited increased metastasis in the Rip1–Tag2 pancreatic islet tumour model77. In several experimental therapeutic studies, both anti-VEGF strategies, as well as treatment modalities involving dual targeting of endothelial cells and pericytes, increased the incidence of metastasis concomitant with severe impairment of the vasculature and growth of the primary tumour (o. Casanovas, unpublished observations; R. Kerbel, unpublished observations). If substantiated by further studies in mouse models and in human patients, the result would solidify another clinically significant mechanism of evasive resistance: when angiogenesis is severely inhibited and the integrity of the remaining tumour vas culature disrupted by anti-angiogenic therapeutic strategies, increased intravasation might provide an alternative means for tumour cells to survive and grow, through blood-borne metastasis. However, resistance mechanisms involving increased intravasation and metastatic seeding will probably remain dependent either on evading angiogenesis inhibition at the new sites, or on co-opting normal tissue vasculature to support tumour expansion, as discussed below.
Increased capabilities for invasion without angiogenesis
There are increasing clues for another insidious form of adaptation: change to a condition of increased inva siveness. It is increasingly evident that in some tumours in which angiogenesis is thwarted genetically or pharmacologically, cancer cells adapt by migrating more aggressively into normal tissue. The evasive phenotype of increased invasiveness was first described in mouse models of orthotopic GBM in which neovascularization was blocked by genetically deleting angiogenic factors such as VEGF, HIF1α and matrix metalloproteinase 9 (MMP9), or inhibited pharmacologically with the VEGF inhibitor SU5416 (semaxanib). In this setting the tumours became more invasive and continued to grow, albeit more slowly31,39,55. The glioblastoma cells were seen to co-opt normal blood vessels, evidently using them as highways or conduits to journey deep into the brain, thereby achieving vascular sufficiency in a dispersed fashion, a phenotype referred to as perivascular tumour invasion. Congruent with the results obtained in ortho-topic mouse models of GBM, three recent studies implicate pro-invasive adaptation in humans, as observed by MRI in a subset of GBM patients who had developed multifocal recurrence of tumours during the course of anti-VEGF therapy with bevacizumab78,79 (M. Prados and N. Butowski, unpublished observations).
A pro-invasive adaptation mechanism has also been observed in the Rip1–Tag2 pancreatic islet tumour model in the course of assessing the effects of inhibitors targeting VEGFR30 or multiple receptor tyrosine kinases implicated in pro-angiogenic signalling (D.H., unpublished observations, and o. Casanovas, unpublished observations). The reduction in tumour vascularity evoked by the angiogenesis inhibitors was accompanied by clear signs of increased tumour cell invasiveness. How might such tumours become more invasive? We envision multiple adaptive mechanisms. First, tumours may increase the activity of a pre-existing invasion programme that was not previously the driving force of expansive tumour growth, given the capability for angiogenesis. Alternatively, some tumours may switch on a distinctive invasive growth programme to that arising spontaneously during progression. For example, untreated GBMs often invade normal brain tissue by infiltrating as single cells into the brain parenchyma, migrating along basement membranes of ventricles, leptomeninges and blood vessels80. By contrast, when GBMs were genetically or pharmacologically impaired in their angiogenic capability, tumour cells were observed to predominantly migrate as multicellular layers along normal blood vessels31,39,55,81. Additionally, VEGF signalling may in some contexts serve to directly restrict perivascular tumour cell invasion: addition of VEGF to cultured GBM cells, or the generation of GBM cells that ectopically express high levels of VEGF, reduced the propensity of those cells to invade both in vitro and in vivo. Notably, these tumour cells express VEGFR1 and VEGFR2 (REF. 55). Adaptive mechanisms in the face of angiogenesis inhibition involving increased invasiveness are also predicted to culminate in increased metastasis in some tumour types, and indeed current studies with VEGF pathway inhibitors are uncovering both increased invasiveness and metastasis, as discussed above. Importantly, the existence and the prevalence of such adaptive mechanisms across the spectrum of human cancers remain to be established, but there are sufficient clues and rationale to raise this possibility here in order to provoke future investigations.
We have presented a set of non-traditional (evasive) therapeutic resistance mechanisms that stand in contrast to the well-established mechanisms operative in cancer cells, where genes are mutationally altered, deleted and amplified so as to afford genetic resistance to drugs targeting the genetically unstable tumour cells. We cannot exclude the possibility that such genetic resistance mechanisms will be discovered in tumour endothelial cells in response to chronic therapy with anti-angiogenic drugs, perhaps in particular metronomic chemotherapy. Indeed, some tumour endothelial cells have been reported to be aneuploid82, suggesting the capability to develop mutations that convey drug resistance. Additionally, it is increasingly evident that angiogenic signalling pathways are not unilaterally specific, and indeed are found to be functioning in certain tumour types, as reviewed by L. Ellis and D. J. Hicklin in this Focus issue11 and in REF. 83, which raises the possibility of classical resistance circumventing the direct impairment of tumour cells treated with ‘angiogenesis inhibitors’. We suspect, however, that the aforementioned evasive resistance mechanisms will prove to be the primary basis for the transitory efficacy of anti-angiogenic drugs, in particular those targeting VEGF signalling.
Mode 2: indifference to anti-angiogenic therapy
We envision a second mode of resistance to anti-angio genic therapy, one for which the experimental clues are more diffuse but the rationale is nevertheless persuasive: intrinsic, pre-existing non-responsiveness of a tumour (FIG. 1). one such clue involves the clinical trials of the currently approved VEGF pathway inhibitors. A substantial minority of the individuals tested in clinical trials for bevacizumab, sorafenib and sunitinib failed to show even transitory clinical benefit24,42. Although the trial designs did not typically involve frequent serial monitoring that could document intrinsic resistance, it seems likely that some of these non-responding patients fall into this category of no discernible response, with their tumours evidently ‘growing through’ the therapy. Although this resistance could reflect rapid adaptation and the onset of evasive resistance, we expect that pre-existing resistance will prove to be in force in some cases. Its basis could be in tumours that, by virtue of their particular developmental ontogeny, have already activated one or more of the aforementioned evasive resistance mechanisms, not in response to therapy but rather in response to the selective pressures of their microenvironment during pre-malignant development and malignant progression.
The operative definition of this mode of pre-existing resistance is the absence of a discernable (even transitory) beneficial effect of an angiogenesis inhibitor, even when the subject’s tumour(s) is serially analysed. There is no tumour shrinkage (that is, no partial or complete responses in the clinical parlance), no cessation of tumour growth (stasis), nor even retardation in growth rate; neither is there quality of life benefit, nor increased survival. In short, the patient (or the tumour-bearing animal) is intrinsically refractory to the anti-angiogenic therapy, such that disease progression continues unabated. Rigorous characterization and discrimination of this mode of non-responsiveness as distinctive to the potentially rapid adaptive mechanisms discussed above is experimentally challenging because the major difference may be in the timing of activating the two modes, as variations of the same basic mechanistic themes. Nonetheless, there is good reason to suspect that intrinsic resistance exists in some individuals afflicted with cancer, appreciation of which may prove important for clinical management of anti-angiogenic therapies. The clues and rationales for these prospective mechanisms of pre-existing resistance are discussed briefly below.
Pre-existing multiplicity of redundant pro-angiogenic signals
There are intriguing clues of pre-existing resistance to angiogenesis inhibition consequent to treatment with bevacizumab, which captures the VEGF ligands. Bevacizumab is currently approved by the US Food and Drugs Administration (FDA) for treatment of late-stage metastatic colon, breast and lung cancer, but only in combination with conventional chemotherapy for all three indications (see FDA approval of bevacizumab URL in Further information). In the case of breast cancer, an analysis of human breast cancer biopsies covering a spectrum from low- to high-grade malignancies revealed that late-stage breast cancers expressed a plethora of pro-angiogenic factors, including FGF2, in contrast to earlier stage lesions, which preferentially expressed VEGF84. Thus one can imagine that the pre-existence of FGF2 and the other pro-angiogenic factors in late-stage metastatic tumours could enable continuing angiogenesis in the face of bevacizumab therapy, such that inhibition of VEGF signalling does not affect angiogenesis. Notably, bevacizumab trials in late-stage metastatic breast cancer have been equivocal, as reviewed by L. Ellis and D. J. Hicklin in this Focus issue11 and in REF. 85. The exception involved a small subgroup of patients with metastatic ERBB2– (also known as HER2–) breast cancer who had not received prior chemotherapy. Under these particular conditions, a regimen of bevacizumab in combination with the cytotoxic drug paclitaxel extended progression-free survival (but not overall survival)86. By contrast, trials in patients pretreated with chemotherapy showed no benefit from bevacizumab as second-line therapy, alone or in combination with additional chemotherapy (see New approval for bevacizumab (Avastin) URL in Further information). The latter result suggests that the pretreated tumours may have already activated mechanisms that coincidentally convey intrinsic resistance to subsequent anti-angiogenic therapy. What, then, could be the basis for the modest albeit demonstrable value of bevacizumab when combined in a first-line setting with chemotherapy, which led to its approval by the FDA? Certainly bevacizu mab might be transiently blocking angiogenesis in these tumours. Another possible explanation is that VEGF blockade in the context of these late-stage breast cancers is serving primarily to transiently normalize the permeability of tumour vasculature, reducing VEGF-induced haemorrhaging and oedema and therefore relieving the physiological effects of tumour burden. In addition, one cannot exclude the possibility that the induction of vascular normalization by VEGF pathway inhibitors serves to improve blood flow, consequently facilitating intratumoural bioavailability of efficacious levels of the attendant cytotoxic chemotherapy66. This latter effect could also explain the selective clinical benefits of anti-VEGF therapy only in combination with chemotherapy in metastatic colon and lung cancer. This view of VEGF inhibitors as ‘chemosensitizers’ does not imply that bona fide anti-angiogenic therapy has no potential benefit in treating such late-stage cancers, but rather implies that we must find ways to finesse pre-existing resistance by targeting the alternative pro-angiogenic signals in addition to the vascular normalization (and contributions to angiogenesis inhibition) afforded by VEGF inhibition. Here again, there is clear need to histologically analyse treated human tumours in response, relapse and progression phases (in this case with bevacizumab plus chemotherapy), to ascertain the temporal ontogeny of effects on the tumour vasculature (for example, vascular dropout, revascularization and normalization).
Pre-existing inflammatory cell-mediated vascular protection
A recent preclinical study found that a subset of murine transplant tumours growing in mice showed no responsiveness to an anti-mouse VEGF monoclonal antibody that mimicked bevacizumab34. The non-responsive tumours, which had not previously been treated with chemotherapy, were characterized by a pre-existing infiltration of inflammatory cells, principally CD11b+Gr1+ myeloid cells, which were shown to express a number of pro-angiogenic factors. By contrast, the responsive tumour types had comparatively low levels of such inflammatory cells. Pharmacological impairment of myeloid cell recruitment rendered the otherwise resistant tumours responsive to the VEGF blockade. Although the experimental design and data cannot discriminate between rapid adaptation and pre-existing resistance, there is reason to suspect that these pro-angiogenic myeloid cells, when sufficiently abundant as a consequence of chemoattract ants produced by such tumours, will convey pre-existing resistance. Certainly this result is not definitive of the possible general principle, but it motivates both preclini cal and clinical studies to further assess this prospective mechanism.
Characteristic hypovascularity and indifference toward angiogenesis inhibitors
Pancreatic ductal adenocarci noma (PDAC) may display another class of pre-existing resistance, manifest as a tumour type that is characteristically hypovascularized with a massive (largely avascular) desmoplastic stroma87. The fact that treatment-naive PDAC tumours are intrinsically poorly vascularized and nevertheless not widely necrotic suggests that PDAC tumori genesis involves adaptation to survive and prosper in the harsh, presumably hypoxic microenvironment that results from the sparse vascularity. It is unclear why these tumours fail to induce a dense neovasculature and instead evolve to flourish in the absence of prominent angiogenesis. Irrespectively, the PDAC tumour physiology may render it intrinsically indifferent to angiogenesis inhibitors. For example, ∼75% of PDACs carry inactivating mutations in the TP53 tumour suppressor gene88,89, loss of which in another tumour type has been shown to improve survival in hypoxic conditions, including ones induced by angio genesis inhibition90,91. Consistent with this line of reasoning, it is notable that bevacizumab showed no efficacy in a clinical trial of patients with late-stage PDAC, alone or in combination with the standard-of-care chemotherapeutic agent gemcitabine24. one can infer, therefore, that normalization is either not induced or lacks the ability to augment the limited utility of gemcitabine. Preliminary studies in a mouse model of PDAC with another VEGF pathway inhibitor also showed no efficacy alone or combined with gemcitabine and no alterations in vascularity, consistent with this clinical observation (P. olson and D.H., unpublished observations). Although this class of intrinsic indifference to VEGF and other angiogenesis inhibitors may be applicable to some or all cases of PDAC and perhaps to rare subtypes of other organ-specific cancers, appreciation of its potential existence may provoke further investigation and, if validated, will probably prove influential for evaluating potential therapeutic trials with angiogenesis inhibitors in PDAC and other similar tumour types.
Invasive (and metastatic) co-option of normal vessels without requisite angiogenesis
Extrapolation from the observations of adaptive resistance by increased invasion and metastasis predicts the intrinsic resistance of tumours that have already switched on highly invasive and/or meta static capabilities early in their ontogeny, serving to effect local or distant co-option of quiescent normal tissue vessels so as to sustain growth without requisite angiogenic switching and development of neovasculature. Grade II and III astrocytoma may be an example. These diffusely growing tumours do not form lesions with a prominent neovasculature containing proliferative endothelial cells80,92. We suspect that such tumours will prove refractory to angiogenesis inhibitors and even to inducers of normalization such as bevacizumab, given that they have not acquired the aberrant, angiogenic vasculature that is typical of the more aggressive grade IV lesions (GBM).
Although the rationales posed above for the existence of different forms of pre-existing (intrinsic) resistance to anti-angiogenic therapy may seem speculative based on the current data, we are confident in predicting that intrinsic resistance will prove to be operative in some tumours, with consequent implications for anti-angiogenic therapies. Certainly, the observation that patients bearing the same tumour type respond differently to the same therapy is well recognized. Such a heterogeneous response to anti-VEGF therapy is exemplified in the aforementioned report of a clinical trial of the VEGFR inhibitor cediranib, in which a subset of patients with GBM responded transiently, whereas another group of patients had essentially no response42. It remains to be elucidated whether the intrinsic indifference to anti-angiogenic therapy in the latter group is due to one or more of the described intrinsic resistance mechanisms. The results underscore the importance of biomarkers that would predict whether a patient will respond to anti-VEGF therapy. We raise the hypothesis herein to set seeds of future inquiry that should, in due course, establish (or not) its accuracy and prevalence, and clarify the alternative mechanisms that most commonly manifest this proposed mode of intrinsic resistance or indifference to angiogenesis inhibitors.
Conclusions
These considerations, about modes and underlying mechanisms of resistance to anti-angiogenic therapy, present both an agenda for future research and the outlines of a strategy for improving the treatment of human cancer with angiogenesis inhibitors. All of the mechanisms, both of adaptive and of intrinsic resistance, deserve further investigation and rigorous evaluation of their prevalence and significance, both in animal models of cancer and in humans. We also expect that other evasive mechanisms will be uncovered. Additionally, it will be important to clarify the circumstances that elicit particular mechanistic pathways of resistance, alone and in the context of standard-of-care chemotherapy and radiotherapy for different cancer types, recognizing that the likely future of most anti-angiogenic therapies will be in such combinations. The constraints on clinical investigation pose challenges indeed, but we envision that advancing technologies, in non-invasive imaging for example, and in collecting and analysing tumour biopsies to assess histological parameters of response and evasive resistance, will be instrumental. Another challenge is to identify robust biomarkers for tumour angiogenesis and for vascular normalization93, as well as for the modes of intrinsic and evasive resistance.
As for cancer therapeutics, we foresee several applications of these principles. First and foremost is the emerging role of invasion and metastasis, both in intrinsic and adaptive resistance. The combination of anti-invasive and anti-metastatic drugs with anti-angiogenic drugs would seem to have particular promise. For example, multiple drugs targeting the pro-invasive hepatocyte growth factor (HGF)–MET and insulin-like growth factor 1 (IGF1) receptor pathways are in clinical trials94,95 and should be tested in combination with VEGF pathway inhibitors. The HIF regulatory network also holds promise as a target, given its global effects on angiogenesis, invasion and stress-adaptive cell physiology96–99. Another promising avenue involves multi-targeting of parallel pro-angiogenic signalling circuits, aimed to circumvent pre-existing redundancy or evasive mechanisms involving upregula tion of alternative angiogenic signalling circuits. Drugs targeting such signals (such as FGFs) are entering clinical trials, now aimed in part to assess their impact on evasive resistance to VEGF blockade100–102.
Finally, although the transitory efficacy of the groundbreaking VEGF pathway inhibitors might be construed as disappointing, the results must be considered in the context of the current standards of care for most of the major human cancers, which typically have transitory efficacy, inevitable progression and/or resistance, toxicity and poor quality of life effects. Angiogenesis inhibitors, despite their evident limitations, are an important milestone in cancer therapeutics, where they are becoming components of standard-of-care therapy, for example for colorectal and renal cancers. Moreover, the growing knowledge about their effects and efficacy, and about the existence and mechanistic basis for adaptive evasive resistance and abject failures (intrinsic indifference), presents an exciting future of opportunity for improving and sustaining the benefits of anti-angiogenic therapy.
DATABASES.
Entrez gene:
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene Angpt1 | COL18A1 | COL4A3 | CSF3 | CXCL12 | CXCR4 | Efna1 | Efna2 | ERBB2 | Fgf1 | Fgf2 | HGF | HIF1 α | IGF1 | IL8 | ITGAM | KDR | KIT ligand | MET | MMP9 | osteopontin | PDGFA | PGF | PTPRC | TEK | TP53
National cancer Institute: http://www.cancer.gov/ breast cancer | colon cancer | glioblastoma | lung cancer | non-small-cell lung cancer | renal cancer
National cancer Institute Drug Dictionary: http://www.cancer.gov/drugdictionary/ bevacizumab | gemcitabine | paclitaxel | semaxanib | sorafenib | sunitinib
FURTHER INFORMATION
G. Bergers’ homepages: http://www.ucsf.edu/bms/faculty/bergers.html; http://neurosurgery.medschool.ucsf.edu/neurosurgery research/btrc/bergers lab.html
Angiogenesis Inhibitors Therapy: http://www.cancer.gov/cancertopics/factsheet/Therapy/angiogenesis-inhibitors
Clinical trials in patients with brain cancer: http://www.cancer.gov/search/ResultsClinicalTrialsAdvanced. aspx?protocolsearchid=4816487
Clinical trials in patients with breast cancer: http://www.cancer.gov/search/ResultsClinicalTrials.aspx?pr otocolsearchid=4586362
Clinical trials in patients with gastrointestinal stromal tumours: http://www.cancer.gov/search/ResultsClinicalTrials.aspx?protocolsearchid=4586407
Clinical trials in patients with kidney cancer: http://www.cancer.gov/search/ResultsClinicalTrials.aspx?pr otocolsearchid=4586416
Clinical trials in patients with liver cancer: http://www.cancer.gov/search/ResultsClinicalTrials.aspx?protocolsearchid=4586421
Clinical trials in patients with lymphoma: http://www.cancer.gov/search/ResultsClinicalTrials.aspx?protocolsearchid=4586451
Clinical trials in patients with lung cancer: http://www.cancer.gov/search/ResultsClinicalTrials.aspx?protocolsearchid=4586453
Clinical trials in patients with pancreatic cancer: http://www.cancer.gov/search/ResultsClinicalTrials.aspx?protocolsearchid=4586434
FDA approval of bevacizumab: http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab
New approval for bevacizumab (Avastin): http://www.fda.gov/cder/Offices/OODP/whatsnew/bevacizumab200802.htm
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Acknowledgements
We thank R. Kerbel, M. Aghi, H. Rugo and O. Casanovas for insightful discussions. Research in the authors’ laboratories was supported by grants from the US National Cancer Institute (to G.B. and D.H.), and by an award to D.H. from the William F. Bowes Charitable Foundation. D.H. is an American Cancer Society Research Professor and G.B. holds the Neill H. and Linda S. Brownstein Chair in Brain Tumour Research.
Glossary
- Hypertension
A medical condition in which the blood pressure is chronically increased.
- Doppler ultrasound
A test that uses reflected sound waves to evaluate blood flow.
- Hemangiocytes
CXCR4+ VEGFR1+ haematopoietic progenitors.
- Intravasation
Part of the metastasis process in which cancer cells invade through the basal membrane into blood vessels.
- Co-option
Blood vessel co-option: tumour cells grow around existing blood vessels in the tissue.
- Leptomeninges
The two innermost layers, comprised of the arachnoid mater and pia mater, that envelop the brain and spinal cord.
- Metronomic chemotherapy
Administration of chemotherapeutic drugs at comparatively low doses on a frequent or continuous schedule, with no extended interruptions.
- Oedema
An observable swelling due to an increase of interstitial fluid in any organ.
- Desmoplastic stroma
Abnormal and excessive growth of stromal cells that is often associated with invasive cancers.
Contributor Information
Gabriele Bergers, Email: gabriele.bergers@ucsf.edu.
Douglas Hanahan, Email: dh@biochem.ucsf.edu.
References
- 1.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature Rev. Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 5.Jain RK. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology (Williston Park) 2005;19:7–16. [PubMed] [Google Scholar]
- 6.Smith JK, Mamoon NM, Duhe RJ. Emerging roles of targeted small molecule protein-tyrosine kinase inhibitors in cancer therapy. Oncol. Res. 2004;14:175–225. doi: 10.3727/000000003772462298. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an antivascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 8.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 9.Apte RS. Pegaptanib sodium for the treatment of age-related macular degeneration. Expert Opin. Pharmacother. 2008;9:499–508. doi: 10.1517/14656566.9.3.499. [DOI] [PubMed] [Google Scholar]
- 10.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nature Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 11.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature Rev. Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 12.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nature Rev. Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nature Rev. Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 14.Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nature Rev. Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 15.Yan M, Plowman GD. Delta-like 4/Notch signaling and its therapeutic implications. Clin. Cancer Res. 2007;13:7243–7246. doi: 10.1158/1078-0432.CCR-07-1393. [DOI] [PubMed] [Google Scholar]
- 16.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nature Rev. Cancer. 2007;7:327–331. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 17.Shojaei F, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 18.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erber R, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 21.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saltz LB, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J. Clin. Oncol. 2007;25:4557–4561. doi: 10.1200/JCO.2007.12.0949. [DOI] [PubMed] [Google Scholar]
- 23.Shojaei F, Ferrara N. Antiangiogenic therapy for cancer: an update. Cancer J. 2007;13:345–348. doi: 10.1097/PPO.0b013e31815a7b69. [DOI] [PubMed] [Google Scholar]
- 24.Kindler HL, et al. A double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC) J. Clin. Oncol. 2007;25:4508. [Google Scholar]
- 25.Miller KD, Sweeney CJ, Sledge GW., Jr Can tumor angiogenesis be inhibited without resistance? EXS. 2005;2005:95–112. doi: 10.1007/3-7643-7311-3_7. [DOI] [PubMed] [Google Scholar]
- 26.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nature Rev. Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 27.Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anticancer therapeutic agents. Bioessays. 1991;13:31–36. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- 28.Kerbel RS. A cancer therapy resistant to resistance. Nature. 1997;390:335–336. doi: 10.1038/36978. [DOI] [PubMed] [Google Scholar]
- 29.Kerbel RS, et al. Possible mechanisms of acquired resistance to anti-angiogenic drugs: implications for the use of combination therapy approaches. Cancer Metastasis Rev. 2001;20:79–86. doi: 10.1023/a:1013172910858. [DOI] [PubMed] [Google Scholar]
- 30. Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. This study demonstrates the existence of evasive resistance by alternative pro-angiogenic signalling.
- 31.Blouw B, et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell. 2003;4:133–146. doi: 10.1016/s1535-6108(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 33.Kerbel RS. Therapeutic implications of intrinsic or induced angiogenic growth factor redundancy in tumors revealed. Cancer Cell. 2005;8:269–271. doi: 10.1016/j.ccr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 34. Shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD 11 b+ Gr 1+ myeloid cells. Nature Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. This study reveals CD1 1b+Gr1+ monocytes to be mediators of intrinsic resistance to anti-VEGF treatment in murine transplant tumours.
- 35.Glade Bender J, Cooney EM, Kandel JJ, Yamashiro DJ. Vascular remodeling and clinical resistance to antiangiogenic cancer therapy. Drug Resist. Updat. 2004;7:289–300. doi: 10.1016/j.drup.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Mizukami Y, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1 α-deficient colon cancer cells. Nature Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 37.Gorre ME, Sawyers CL. Molecular mechanisms of resistance to STI571 in chronic myeloid leukemia. Curr. Opin. Hematol. 2002;9:303–307. doi: 10.1097/00062752-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 38.O’Connor R, Clynes M, Dowling P, O’Donovan N, O’Driscoll L. Drug resistance in cancer — searching for mechanisms, markers and therapeutic agents. Expert Opin. Drug Metab. Toxicol. 2007;3:805–817. doi: 10.1517/17425255.3.6.805. [DOI] [PubMed] [Google Scholar]
- 39. Rubenstein JL, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. First study demonstrating vessel co-option of tumour cells in response to anti-VEGF therapy.
- 40.Fernando NT, et al. Tumor escape from endogenous, extracellular matrix-associated angiogenesis inhibitors by up-regulation of multiple proangiogenic factors. Clin. Cancer Res. 2008;14:1529–1539. doi: 10.1158/1078-0432.CCR-07-4126. [DOI] [PubMed] [Google Scholar]
- 41.Kadenhe-Chiweshe A, et al. Sustained VEGF blockade results in microenvironmental sequestration of VEGF by tumors and persistent VEGF receptor-2 activation. Mol. Cancer Res. 2008;6:1–9. doi: 10.1158/1541-7786.MCR-07-0101. [DOI] [PubMed] [Google Scholar]
- 42. Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. This clinical study describes the ability of the pan-VEGFR inhibitor AZD1271 to normalize tumour vessels in recurrent patients with glioblastoma using MRI technology. FGF2 and SDF 1 α blood levels increased when tumours escaped treatment.
- 43.Bertolini F, Mancuso P, Shaked Y, Kerbel RS. Molecular and cellular biomarkers for angiogenesis in clinical oncology. Drug Discov. Today. 2007;12:806–812. doi: 10.1016/j.drudis.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nature Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 45.Bocci G, et al. Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res. 2004;64:6616–6625. doi: 10.1158/0008-5472.CAN-04-0401. [DOI] [PubMed] [Google Scholar]
- 46.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc. Natl Acad. Sci. USA. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 48.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 49.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Hattori K, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1+ stem cells from bone-marrow microenvironment. Nature Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J. Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 53.Yang L, et al. Expansion of myeloid immune suppressor Gr+CD 1 1b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 54.Grunewald M, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 55. Du R, et al. HIF 1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. This study reveals that monocytic cells from the bone marrow are sufficient to drive neovascularization in GBM. Impairment of VEGF signalling leads to an adaptive pro-invasive tumour phenotype, which can be directly blocked by VEGF.
- 56.Ceradini DJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 57.De Falco E, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 58.Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. doi: 10.1158/0008-5472.CAN-05-3759. [DOI] [PubMed] [Google Scholar]
- 59.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nature Rev. Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shaked Y, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. This study reveals that influx of bone marrow-derived endothelial progenitors is an essential step in re-neovascularization of tumours that have undergone treatments with vascular disrupting agents.
- 61.Sathornsumetee S, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J. Clin. Oncol. 2008;26:271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 63.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 64.Bergers G, Song S. The role of pericytes in bloodvessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jain RK, Booth MF. What brings pericytes to tumor vessels? J. Clin. Invest. 2003;112:1134–1136. doi: 10.1172/JCI20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 67.Mancuso MR, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005;15:102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Benjamin L, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 71.Darland DC, et al. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev. Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 72.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRβ+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nature Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirschi KK, D’Amore PA. Control of angiogenesis by the pericyte: molecular mechanisms and significance. EXS. 1997;79:419. doi: 10.1007/978-3-0348-9006-9_18. [DOI] [PubMed] [Google Scholar]
- 74.Orlidge A, D’Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J. Cell. Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pietras K, Hanahan D. A multitarge ted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J. Clin. Oncol. 2005;23:939–952. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 76.Sun J, et al. Inhibiting angiogenesis and tumorigenesis by a synthetic molecule that blocks binding of both VEGF and PDGF to their receptors. Oncogene. 2005;24:4701–4709. doi: 10.1038/sj.onc.1208391. [DOI] [PubMed] [Google Scholar]
- 77. Xian X, et al. Pericytes limit tumor cell metastasis. J. Clin. Invest. 2006;116:642–651. doi: 10.1172/JCI25705. This study demonstrates that disruption of pericyte coverage from tumour vessels can elicit increased metastasis in a transgenic model of pancreatic islet carcinogenesis.
- 78.Norden AD, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 79.Narayana A, et al. J. Neurosurg. Anti-angiogenic therapy using bevacizumab in recurrent high grade glioma: impact on local control and survival. (in the press) [DOI] [PubMed] [Google Scholar]
- 80.Berger MS, Wilson CB. The Gliomas. Philadelphia: W.B. Saunders; 1999. [Google Scholar]
- 81.Kunkel P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61:6624–6628. [PubMed] [Google Scholar]
- 82.Hida K, et al. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–8255. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 83.Lesslie DP, et al. Vascular endothelial growth factor receptor-1 mediates migration of human colorectal carcinoma cells by activation of Src family kinases. Br. J. Cancer. 2006;94:1710–1717. doi: 10.1038/sj.bjc.6603143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Relf M, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor β-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 85.Marty M, Pivot X. The potential of anti-vascular endothelial growth factor therapy in metastatic breast cancer: clinical experience with anti-angiogenic agents, focusing on bevacizumab. Eur. J. Cancer. 2008;44:912–920. doi: 10.1016/j.ejca.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Miller K, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 87.Sofuni A, et al. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J. Gastroenterol. 2005;40:518–525. doi: 10.1007/s00535-005-1578-z. [DOI] [PubMed] [Google Scholar]
- 88.Schneider G, Schmid RM. Genetic alterations in pancreatic carcinoma. Mol. Cancer. 2003;2:15. doi: 10.1186/1476-4598-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cowgill SM, Muscarella P. The genetics of pancreatic cancer. Am. J. Surg. 2003;186:279–286. doi: 10.1016/s0002-9610(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 90.Yu JL, et al. Heterogeneous vascular dependence of tumor cell populations. Am. J. Pathol. 2001;158:1325–1334. doi: 10.1016/S0002-9440(10)64083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science. 2002;295:1526–1528. doi: 10.1126/science.1068327. [DOI] [PubMed] [Google Scholar]
- 92.Kaur B, Tan C, Brat DJ, Post DE, Van Meir EG. Genetic and hypoxic regulation of angiogenesis in gliomas. J. Neurooncol. 2004;70:229–243. doi: 10.1007/s11060-004-2752-5. [DOI] [PubMed] [Google Scholar]
- 93.Sessa C, Guibal A, Del Conte G, Ruegg C. Biomarkers of angiogenesis in the development of antiangiogenic therapies in oncology: tools or decorations? Nature Clin. Pract. Oncol. 2008;5:378–391. doi: 10.1038/ncponc1150. [DOI] [PubMed] [Google Scholar]
- 94.Wang X, et al. Potent and selective inhibitors of the Met [hepatocyte growth factor/scatter factor (HGF/SF) receptor] tyrosine kinase block HGF/SF-induced tumor cell growth and invasion. Mol. Cancer Ther. 2003;2:1085–1092. [PubMed] [Google Scholar]
- 95.Feng Y, Dimitrov DS. Monoclonal antibodies against components of the IGF system for cancer treatment. Curr. Opin. Drug Discov. Devel. 2008;11:178–185. [PubMed] [Google Scholar]
- 96.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nature Rev. Drug Discov. 2003;2:803–811. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- 97.Semenza GL. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 98.Maxwell PH. The HIF pathway in cancer. Semin. Cell Dev. Biol. 2005;16:523–530. doi: 10.1016/j.semcdb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 99.Tan C, et al. Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Res. 2005;65:605–612. [PubMed] [Google Scholar]
- 100.Sarker D, et al. A phase I pharmacokinetic and pharmacodynamic study of TKI258, an oral, multitargeted receptor tyrosine kinase inhibitor in patients with advanced solid tumors. Clin. Cancer Res. 2008;14:2075–2081. doi: 10.1158/1078-0432.CCR-07-1466. [DOI] [PubMed] [Google Scholar]
- 101.Garrett CR, et al. A phase I study of BMS-582664 (brivanib alaninate), an oral dual inhibitor of VEGFR and FGFR tyrosine kinases, in combination with full-dose cetuximab in patients (pts) with advanced gastrointestinal malignancies (AGM) who failed prior therapy. J. Clin. Oncol. 2007 ASCO Annu. Meeting Proc. Pt I. 2007;25:14018. [Google Scholar]
- 102.Von Pawel J, et al. A double blind phase II study of BIBF 1,120 in patients suffering from relapsed advanced non-small cell lung cancer (NSCLC) J. Clin. Oncol. 2007 ASCO Annu. Meeting Proc. Pt I. 2007;25:7635. [Google Scholar]