Abstract
Immunotherapy of canine visceral leishmaniasis (CVL) may provide an alternative to both marginally effective chemotherapy and undesired euthanasia of infected dogs and could have a great impact not only on animal welfare, but also on control of human disease. Therefore, we examined the potential immunotherapeutic efficacy of the subunit vaccine Leish-111f + MPL-SE, which has undergone rigorous preclinical testing and been demonstrated safe in human clinical trials. Two separate trials were performed in Salvador, Brazil, to evaluate the vaccine for therapeutic efficacy against CVL caused by natural infection: an open trial and a blinded trial. In the open trial 59 dogs with clinically active CVL were sequentially allocated to four groups: group 1 received Leish-111f + MPL-SE; group 2 was treated with Glucantime; group 3 received a combination of the vaccine and Glucantime; and group 4 was given no treatment. At the 6-month assessment, the 13 non-treated dogs had either died or showed no clinical improvement. In contrast, most dogs in groups 1-3 showed initial improvement (100%, 80%, and 92% respectively). Upon evaluation for a mean of 36 months after therapy, the following cure rates were observed: 75% for group 1 dogs (exact 95% confidence interval [CI] 43-95%), 64% for group 2 dogs (exact 95% CI 31-89%), and 50% for group 3 dogs (exact 95% CI 19-81%). Therapeutic efficacy of the Leish-111f + MPL-SE vaccine was reconfirmed in a subsequent blinded trial. The vaccine was effective for mild cases of CVL and was compromised in dogs with severe disease. Although further studies are required to understand mechanisms of action, the Leish-111f + MPL-SE vaccine is a promising tool to control VL in both dogs and humans.
Keywords: visceral leishmaniasis, Leishmania infantum, dog, immunotherapy, vaccine, adjuvant
1. Introduction
Zoonotic visceral leishmaniasis (VL), caused by the protozoan parasite Leishmania infantum (chagasi), is a vector-borne disease found in South America and areas surrounding the Mediterranean Sea [1, 2]. Dogs are the major reservoirs for L. infantum in these regions [3, 4], and control of the disease in dogs could have a significant impact on human disease [5-8]. Beginning in the 1960's, Brazilian health authorities began culling infected dogs in the largest endemic areas of northeast Brazil as a major strategy for reducing transmission to humans [9]. However, judging from the prevalence of VL in humans and its recent spread into several metropolitan areas [10, 11], this strategy has been inadequate.
There are a limited number of chemotherapeutic agents available to treat canine VL, and they are often toxic and ineffective [12]. Moreover, the WHO recommends against their use in dogs out of concern for selecting drug-resistant parasites that might then be untreatable in subsequent human infections [13]. Also, primary resistance to these drugs is considerable [14, 15], and treated dogs may still be infectious even if asymptomatic [16]. Other means of control, such as insecticides and deltamethrin-impregnated collars, have been tried, but have had limited efficacy [7, 17, 18].
Immunotherapy is one of the most attractive alternatives for treatment of canine visceral leishmaniasis at this time. Indeed, some vaccine protein candidates have given encouraging results in controlled trial settings [19, 20]. The recombinant polyprotein vaccine antigen Leish-111f, formulated with monophosphoryl lipid A in stable emulsion (MPL-SE), is the first subunit vaccine to be evaluated in humans. The vaccine is protective against both cutaneous and visceral leishmaniasis in mice [21, 22], and has been demonstrated to be safe and well-tolerated in humans [23]. MPL-SE serves as an efficacious adjuvant to induce protective Th1 responses and is more affordable than rIL-12 [24].
Two studies have previously reported on the therapeutic efficacy of a canine vaccine composed of Leish-111f + MPL-SE against CVL. In a study conducted in southern Italy, Gradoni et al. [25] concluded that the vaccine was not effective at preventing either the on-set or progression of leishmaniasis in dogs. Although the vaccine improved the survival rates of dogs with VL in a separate Brazil study, the curative effect was limited [26]. A common feature in those two studies is that the vaccine was given three times at 3 or 4-week intervals. We performed two separate clinical trials with this vaccine in the endemic area of Monte Gordo, Bahia, Brazil. Because our trials used several weekly vaccinations, these trials effectively evaluated whether more frequent injections of the vaccine leads to improvement of existing CVL. The first trial was an open randomized study focused on evaluating efficacy in terms of clinical improvement using vaccine either by itself or in conjunction with chemotherapy. The second trial was single-blinded and randomized with the purpose of evaluating immunotherapeutic efficacy along with immunological evaluations. Here we show that weekly injections of the Leish-111f+MPL-SE vaccine can provide a clinical cure for many dogs with VL.
2. Materials and methods
The treatment clinic for this study is located in Monte Gordo (State of Bahia, Brazil), an area endemic for leishmaniasis [10]. To evaluate therapeutic efficacy of the Leish-111f + MPL-SE vaccine on dogs with CVL, two separate clinical studies were performed: an Open trial followed by a single Blinded trial. At the time of these studies, the Universidade Federal da Bahia did not have an Institutional Review Board for animals; however, the investigators performed the studies under generally recognized standards of care and medical practice. Dogs from the area surrounding the clinic were used in these studies. Enrollment of all the dogs in these studies was performed with the owner's consent.
2.1. Study #1: Open trial
2.1.1. Enrollment of dogs with CVL
The study was conducted between July, 2001 and June, 2005. The dogs were suspected of CVL based on clinical symptoms including cachexia, alopecia, splenomegaly, lymphadenopathy, onychogryphosis, and skin lesions. CVL was confirmed by the presence of parasites in bone marrow, lymph node, or spleen upon examination of Giemsa-stained smears, or after culture of bone marrow or spleen aspirates in 57 of the 59 dogs; CVL was serologically confirmed in the remaining two dogs using two ELISAs, one with recombinant K39 antigen [27] and one with soluble antigens from a lysate of L. infantum promastigotes [28]. Information on the breed and sex of dogs enrolled in the study are shown in Table S1 (Supplementary Data).
2.1.2 Treatment arms
Fifty-nine pre-screened dogs were enrolled in the study. The dogs were sequentially allocated to one of the following groups in an open fashion, and treatment was started. There were four cohorts in this study: Group 1 (Vaccine) dogs (n=18) were given four weekly subcutaneous vaccinations with 20 μg of Leish-111f plus 20 μg of MPL in SE; Group 2 (Glucantime) dogs (n=15) were given intravenous injections of 20 mg/kg/day of meglumine antimoniate (Glucantime®: Sanofi Aventis, Paris, France) daily for 30 days; Group 3 (Vaccine + Glucantime) dogs (n=13) were given both vaccine and Glucantime injections following the same schedule/dose as for groups 1 and 2, respectively; and Group 4 (Control) dogs (n=13) were given no treatment. Leish-111f protein was produced at the Infectious Disease Research Institute (Seattle, WA) as previously described [22], MPL-SE was obtained from GlaxoSmithKline Biologicals (Rixensart, Belgium), and Glucantime was provided by the Bahia State health department.
2.1.3 Follow-up clinical evaluations
The dogs were followed for a mean interval of 36 months. Dogs in groups 1, 2 and 3 were kept in the clinic during the entire treatment period, and then returned to their owners. The dogs received no additional protection or treatment in the clinic or in the care of their owners other than normal clinical care and standard immunizations. To reduce the chance of spreading disease in Monte Gordo, the group 4 Control dogs were donated to the clinic by their owners and kept in kennels outside the sand fly transmission area. Although seven dogs out of 13 in this control group were still alive after 6 months, all of them showed unimproved symptoms of leishmaniasis. Those dogs were withdrawn from the study at that time and started on a course of chemotherapy.
Six months after beginning treatment, dogs were classified as either “initial clinical improvement” or “no improvement” based on qualitative improvement of skin lesions and general health status (weight gain and regained strength). Survival of the dogs after being returned to their owners was monitored by contacting individual owners monthly during the first six months, and at the 12, 24 and 36 month anniversaries. Dogs were not clinically evaluated at other time points. At the end of the study period, the dogs were classified as either sick, dead, or cured. “Sick” dogs were those who were still clinically diseased with leishmaniasis, those still smear-positive for Leishmania parasites, or those who relapsed with disease during the follow-up and were sick at the evaluation. “Cured” dogs were those with no clinical disease for at least 6 months of follow-up. Immunological readouts were not included as part of the Open Trial protocol.
2.2. Study #2: Blinded trial
2.2.1. Enrollment of dogs with CVL
The study was conducted between May, 2006 and August, 2007. The same inclusion criteria were used for this trial as for Trial #1. Information on the breed and sex of dogs enrolled in the study are shown in Table S2 (Supplementary Data).
2.2.2 Treatment arms
Twenty pre-screened dogs were enrolled. They were sequentially allocated to one of three study cohorts without regard to their disease severity: Vaccine Group 1 (n=10) received the vaccine containing 20 μg of Leish-111f + 25 μg of MPL in SE; Adjuvant Group 2 (n=5) received the adjuvant formulation consisting of 25 μg of MPL in SE; and Saline Group 3 (n=5) received saline alone. Vaccine, adjuvant alone, and saline were administered weekly, either four or six times, via 0.5 mL subcutaneous injections. The Leish-111f and MPL-SE were obtained as described above. The first seven dogs enrolled (two Saline dogs; three Vaccine dogs; and two Adjuvant dogs) received four injections each before the immunization schedule was expanded to six weekly injections for the remaining nineteen dogs admitted into the trial. Rescue treatment (Glucantime or amphotericin B) was given to three Saline placebo dogs and seven dogs that failed to improve in the Vaccine or Adjuvant alone arms.
Two veterinarians were engaged in this trial: One veterinarian, who was not blinded, prepared and performed the injections. The second veterinarian (“the evaluating veterinarian”) was blinded from group assignment until the completion of the study and performed all the clinical evaluations. Disease severity was calculated at Day 0 and at subsequent clinical examinations using a clinical score (CS) rubric (Table 1 and as previously described [29]).
Table 1. Classification of disease severity (Sum of points = CS).
| Category | Number of points | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Hair | Good or excellent | Regular | Bad (alopecia, desquamation, etc) |
| Weight | Normal or obese | Skinny | Cachexia |
| Nails | Normal | Abnormal onychogryphosis | |
| Spleen | Normal | Enlarged (palpable) | |
| Lymph nodes | Not palpable/ Small* | Enlarged | Very Enlarged** |
| Oral mucosa | Normal | Hyperemia or Pale | |
| Oral lesions | Absent | Present | |
| Conjunctivitis | Absent | Mild (unilateral, serous or mucous) | Severe (bilateral, purulent) |
non pathologic,
Sizes of the lymph nodes are dependent on weight/breed of the dogs; 1∼2 cm lymph node is normal if the dog is large (over 40 kg).
2.2.3 Follow-up clinical evaluations
The dogs were kept in the clinic during the entire treatment period, and then returned to their owners. Following release to their owners, the dogs were monitored periodically until Day 180 with weekly clinical evaluations for the first six weeks and monthly evaluations thereafter. Hematological and biochemical analyses for hematocrit, blood hemoglobin, platelet, and serum alanine transaminase were performed at the time points indicated in Tables S3-6 in Supplementary Data. Parasitological evaluations of the dogs were carried out either by microscopic observations after Giemsa staining of a needle aspirate of the spleen / lymph node or a touch smear of an ear punch biopsy, or by culturing a spleen / lymph node aspirate in NNN-LIT medium. Samples were collected at the time points indicated in Table 4.
Table 4.
Parasitological evaluations of dogs in Trial #2
| Parasitologya | CS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | D0 | D30 | D60 | D90 | D120 | D150 | D180 | Day 0 | Endpointb |
| Saline | + | Out | Out | Out | Out | Out | Out | 9 | 8 |
| Saline | + | + | + | - | Out | Out | Out | 8 | 9 |
| Saline | + | + | Out | Out | Out | Out | Out | 6 | 11 |
| Saline | + | + | + | + | Out | Out | Out | 6 | 7 |
| Saline | + | - | - | + | + | - | - | 3 | 4 |
| Adjuvant | + | + | - | + | - | Out | Out | 8 | 6 |
| Adjuvant | + | - | - | Out | Out | Out | Out | 7 | 10 |
| Adjuvant | + | + | - | - | ND | + | ND | 7 | 3 |
| Adjuvant | + | + | + | - | - | + | - | 6 | 3 |
| Adjuvant | + | - | + | - | - | ND | - | 4 | 0 |
| Vaccine | + | + | + | + | Out | Out | Out | 12 | 11 |
| Vaccine | + | + | Out | Out | Out | Out | Out | 10 | 8 |
| Vaccine | + | Out | Out | Out | Out | Out | Out | 8 | 12 |
| Vaccine | + | - | + | Out | Out | Out | Out | 8 | 8 |
| Vaccine | + | + | - | - | - | - | - | 7 | 2 |
| Vaccine | + | ND | ND | - | - | - | - | 7 | 1 |
| Vaccine | + | + | - | Out | Out | Out | Out | 6 | 10 |
| Vaccine | + | - | ND | - | - | - | - | 6 | 6 |
| Vaccine | + | - | - | + | - | - | - | 6 | 0 |
| Vaccine | + | - | - | - | - | - | - | 5 | 1 |
Parasitology was performed by microscopic observation of a Giemsa-stained smear of spleen, lymph node or ear biopsy, or by culture of spleen aspirate: +, parasite detected in at least one of the samples; -, negative (no parasites detected) in the samples evaluated; ND, not done; Out, the dog was removed from the study either because of no improvement or death of the dog.
CS Endpoint, Clinical Score at the endpoint for each dog, either at the time of removal from the study or Day 180.
The dogs received no additional protection or treatment either in the clinic or in the care of their owners other than standard clinical care and immunizations. In the event the evaluating veterinarian determined a dog was getting sicker due to CVL, the dog was given rescue treatment with chemotherapy and continued in follow up. The last CS before death or rescue treatment was used for calculating a mean CS for the treatment group in the remaining time points through Day 180.
2.2.4 Antibody ELISA
Peripheral blood samples were collected from a radial vein at Day 0 and one week after the last vaccination (either Day 30 or Day 42) for plasma isolation. Those plasma samples were used for antibody ELISA to examine responses of dogs to Leish-111f, the vaccine antigen. For these analyses Leish-111f was diluted in sodium carbonate buffer, pH 9.6, and used to coat Nunc 96-well polysorp plates (Thermo Fisher Scientific Inc., Waltham, MA), as previously described [29]. HRP-conjugated protein G (1/5,000 dilution: Invitrogen corporation, Carlsbad, CA) was used as secondary antibody, washed plates were developed with 100 μl/well of tetramethylbenzidine peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD), and the enzyme-substrate reaction stopped after 4 min by adding 50 μl/well of 1N H2SO4. The plates were read by a microplate reader at 450 nm (570 nm reference). Reciprocal endpoint titers to individual antigens were calculated with GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA) using a cutoff value of 0.2 (all samples from eight healthy controls gave OD values below this cutoff at 1:100 dilution). Endpoint titers of samples were recorded as <100 if OD values of the samples were lower than the cutoff value at 1:100 or >312,500 if higher than that at 1:312,500 dilution. In these two cases, titers of 100 or 312,500 were used for graphing.
2.3. Statistical analyses
Statistical evaluations were performed using GraphPad Prism to perform a Mantel-Cox test for survival and a 2-tailed Fisher's exact test for study completion; and Stata v.9 (College Station, TX) for the exact 95% Confidence Interval (CI).
3. Results
3.1. Vaccine efficacy in Trial #1, the Open Trial
Dogs in the Open Trial were evaluated six months after the first vaccination (i.e., five months after completion of vaccinations). None of the 13 dogs in the Control group showed clinical improvement at this time point (Table 2). Five of the Control dogs died of CVL (and a sixth was lost to the study), and seven others remained clinically sick (Fig. 1). Since untreated dogs remain infectious, they had to be removed from the transmission area as culling is mandatory in Brazil (Vieira & Coelho, 1998), preventing further study of these dogs. Therefore, the sick dogs were withdrawn from the remainder of the study and given rescue treatment with Glucantime according to the study protocol.
Table 2. Outcomes at 6 and 36 months follow-up in Trial #1.
| Groups | Diagnosis at | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 months | 36 months | |||||||
| Treatment | N | Fraction showing initial improvement | Fraction surviving (%) [exact 95% CId] |
# Dead |
# Sick |
# Cured |
# Censorede |
% Cured [exact 95% CI] |
| Vaccine | 18 | 15/15a | 15/15(100%) [78.2 – 100] |
2 | 1 | 9 | 6 | 75.0 [42.8 – 94.5] |
| Glucantime | 15 | 12/15 | 12/15(80.0%) [51.9 – 95.7] |
4 | 0 | 7 | 4 | 63.6 [30.7 – 89.1] |
| Vaccine + Glucantime | 13 | 12/13 | 13/13(100%) [75.2 – 100] |
3 | 2 | 5 | 3 | 50.0 [18.7 – 81.3] |
| No treatment | 13 | 0/12b | 7/12c(58.3%) [27.7 – 84.8] |
|||||
Three dogs receiving vaccine died of unrelated causes during the 6 months after vaccination; their causes of death were cardiac infarction, canine distemper, and intoxication. These dogs are listed as censored.
One dog in the control group was lost to the study in the first month and was scored as censored.
Five dogs in the control group died and one was lost during the initial 6-month period. The remaining seven sick dogs were treated with Glucantime at the 6-month time point and not followed further.
CI, confidence interval.
Censored dogs include dogs lost to the study or dying of causes other than VL (e.g., osteosarcoma, ehrlichiosis, and those listed above).
Figure 1.
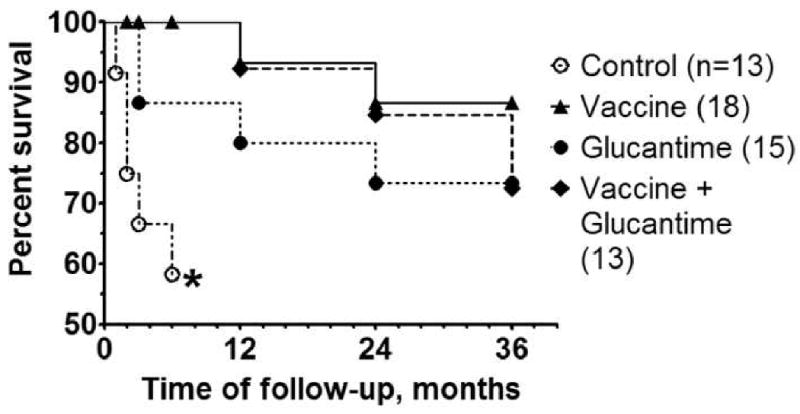
Survival curves for the Open Trial (Study #1) consisting of Control (untreated) dogs, or dogs treated with either the Leish-111f + MPL-SE Vaccine alone, Glucantime alone, or Vaccine + Glucantime. Dogs were monitored monthly for the first 6 months and then yearly. Percent survival of dogs in each group is shown. Dogs removed from the study because of non-compliance with the protocol (i.e., left the area or ran away from owner) are censored in this graph. *All the seven surviving dogs in the saline group remained clinically sick at six months. These dogs were withdrawn from the study and from survival monitoring according to the study protocol.
In general, dogs in the different treatment groups fared better than Control dogs although two out of 15 dogs in the Glucantime group died of CVL in the first six-month period. In contrast to the Control and Glucantime groups, none of the dogs in the two vaccine treatment groups died of CVL during the first 6 months (Fig. 1). All 15 dogs in the Vaccine group showed initial improvement at this same evaluation point (Table 2; three additional dogs that died of other causes -- cardiac infarction, canine distemper, and intoxication -- were censored). Similarly, 80% (12 out of 15) of Glucantime dogs and 92% (12 out of 13) of Vaccine + Glucantime dogs showed initial improvement (Table 2). During this initial six-month period, the survival curves of the immunotherapy and the immuno-chemotherapy groups (Fig. 1) were significantly different from the Control group (P = 0.003 and P = 0.010 for immunotherapy and immuno-chemotherapy, respectively, by the logrank test), while curves for the chemotherapy alone and Control groups were not significantly different (P = 0.081).
At the 36-month follow-up examination, 75% (9/12, exact 95% CI 43-95%) of dogs in the Vaccine group were considered cured. Similar, but slightly lower cure rates of 64% and 50% were observed for dogs in the Glucantime (7/11, exact 95% CI 31-89%) and Vaccine + Glucantime treatment groups (5/10, exact 95% CI 19-81%), respectively (Table 2). A response rate of the vaccine group was at least comparable, if not better than that observed in animals treated with Glucantime (64% cure) and contrasts with the poor outcome for dogs in the Control arm.
The survival curves for the Vaccine alone and Vaccine + Glucantime groups are nearly identical for the first 24 months (Fig. 1), only diverging at the 36-month evaluation mark (not statistically significant, P = 0.487 by the logrank test). While chemotherapy alone showed a relatively rapid decline during the first 6 months after initiation of treatment, its course thereafter mimicked the declines observed for the other two treatment groups. Over the life of the study, there were no significant differences in survival rates between the different treatment groups (P > 0.30 for all pair-wise comparisons by the logrank test).
3.2. Trial #2, a Blinded trial
3.2.1. Curative efficacy of the Leish-111f+MPL-SE vaccine
Because of the apparent therapeutic efficacy of the Vaccine when administered alone and because no immunological analyses were performed as part of the Open Trial, a second trial was performed. Trial #2 was performed as a blinded study. Dog allocation into a study group was based on the enrollment order and followed a chart prepared before the start of the study. Mean values ± SD of each study group's initial clinical scores were 6.4 ± 2.3 (range: 3 – 9, where a larger score means more severe clinical symptoms) for the Saline group, 6.4 ± 1.5 (range: 4 - 8) for the Adjuvant group, and 7.5 ± 2.1 (range: 5 - 12) for the Vaccine group. Thus, at study inclusion the Vaccine-group dogs had a higher mean CS (7.5 vs. 6.4) with a larger range (7 vs. 4 or 6) and the most severely diseased animal based on CS (CS=12) compared to the other two groups (CS=9 or 8); however, the clinical scores for the three groups were not statistically different (unpaired t-test). No adverse events were associated with injections of either adjuvant or vaccine, based on clinical observations and hematological / biochemical analyses (Tables S3-S7 in Supplemental Data).
In agreement with Trial #1, dogs in the Saline group did not spontaneously cure (Figure 2A). CS of five dogs in the Saline group increased by 1.4 (range: -1 to +5, where a positive difference equates with worsening disease symptoms and a negative difference indicates an improvement in clinical symptoms) between Day 0 and the endpoint (either Day 180 or at the time of death or rescue treatment) indicating increased disease severity in those dogs. Only one dog out of five (20%) in this group completed the 180-day study. In contrast to the Saline group, dogs in the Adjuvant and Vaccine groups showed clinical improvement (Fig. 2). Changes in CS for the Adjuvant group and the Vaccine group were -2 (range: -4 to +3) and -1.6 (range: -6 to +4), respectively. Three out of five dogs (60%) in the Adjuvant group and 5 out of 10 dogs (50%) in the Vaccine group completed the study alive and without drug treatment (Table 3). Of the three Adjuvant-group dogs completing the study, two dogs (Day 0 CS=6 and 7) received four injections; the third dog (Day 0 CS=4) received six injections of MPL-SE. The five dogs in the Vaccine group that finished the study alive and without rescue treatment all had a Day 0 CS <8; these dogs received six injections. In contrast, of the four dogs in the Vaccine group that were given rescue treatment (Glucantime and/or amphotericin B), three had a Day 0 CS ≥8 (and two of the three received only four vaccinations). Clinical improvement, including lower CS, brought by the vaccine or adjuvant was often associated with clearance of parasites. This was observed for many of the improved dogs in the vaccine and adjuvant groups that were parasitologically negative for most, if not all, of the post-enrollment time points examined (Table 4). In contrast, the saline placebo dogs and most of the other dogs that were eventually removed from the study, either because they showed no clinical improvement or because they died during the study period, remained parasitologically positive (Table 4).
Figure 2.
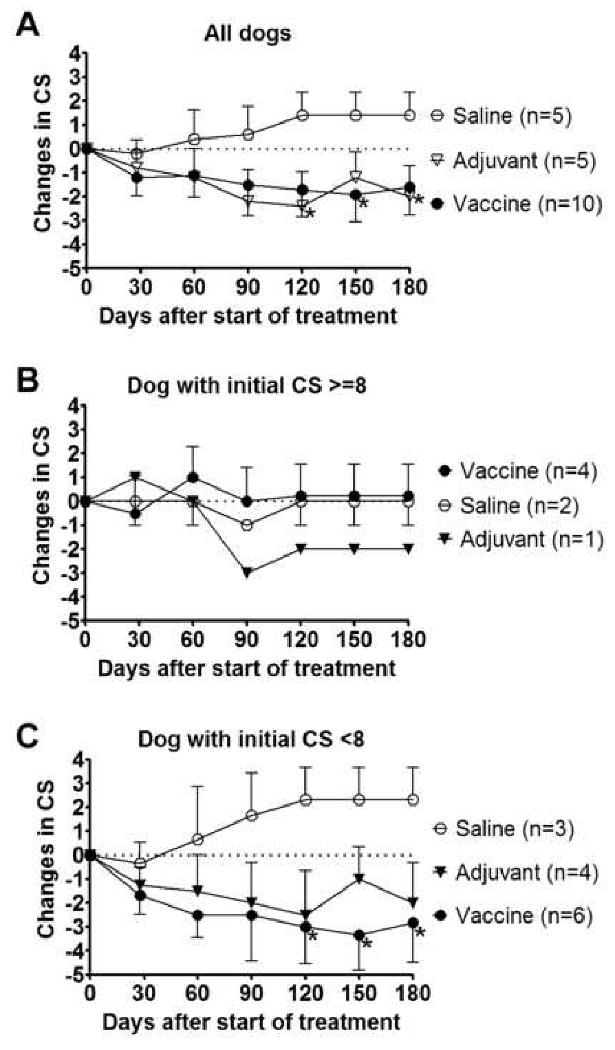
Efficacy of the Leish-111f/MPL-SE vaccine in the Blinded trial. (A) Clinical scores (CS) of all 20 dogs participating in this study were monitored for 180 days. Changes are shown for each group's CS (mean±SEM) relative to the Day 0 CS. In cases of death or chemotherapeutic intervention for a given dog, the CS value preceding the event was used for the remainder of the study period in calculating the mean. (B and C) Dogs were divided into two categories based on CS at Day 0, either CS ≥8 (B) or CS <8 (C), and changes in the CS are shown separately for the two categories. Two Vaccine-group dogs that were admitted with a CS ≥8 (CS=8,12) received only four injections of L110f + MPL-SE per the original protocol; all other dogs with a CS ≥8 on Day 0 received six injections. In the category of CS <8 on Day 0, two Saline dogs, two Adjuvant, and one Vaccine dog received only four injections before the vaccination protocol was modified to allow six weekly injections. *P<0.05 by one-tailed unpaired t-test compared with the Saline group.
Table 3. Completion of vaccine Trial #2, classified by Clinical Score.
| Clinical score | Vaccine | Adjuvant alone | Vaccine + Adjuvant alone | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial completed | *Trial not completed | Total | Trial completed | Trial not completed | Total | Trial completed | Trial not completed | Total | |
| ≥8 | 0 | 4 | 4 | 0 | 1 | 1 | 0 | 5 | 5 |
| <8 | 5 | 1 | 6 | 3 | 1 | 4 | 8 | 2 | 10 |
| Total | 5 | 5 | 10 | 3 | 2 | 5 | 8 | 7 | 15 |
| **P=0.0476 | **P=0.4 | **P=0.007 | |||||||
“Trial not completed” refers to dogs that failed to improve with the study injections and were treated with Glucantime or amphotericin B. Some of these dogs ultimately died from leishmaniasis.
2-tailed Fisher's exact test.
The observations recorded in Tables 3 and 4 and the graphs in Figure 2B and 2C suggest that the vaccine worked better in moderately sick dogs than in severely sick dogs. No clinical improvement was observed for dogs in the Vaccine group that were severely sick at the time of inclusion (CS ≥8 at Day 0, n=4). The kinetics of CS for dogs scoring ≥8 was very similar for the Saline group and Vaccine group (Figure 2B). In contrast, moderately sick dogs (CS <8 at Day 0, n=6) responded better to the vaccine; the CS for these dogs decreased by a mean 2.8 points, and 83% of them completed the 180-day study.
3.2.2. Diminished response to vaccine in severely sick dogs
Dogs were evaluated for anti-Leish-111f antibody titers at Day 0 (pre-vaccination) and Day 42 (post-vaccination). Some dogs in the Vaccine group showed an increase in titers over the vaccination period, whereas no such increase was found in the Saline and Adjuvant groups (Figure 3A). In contrast to the Leish-111f-specific antibody responses, no remarkable changes in pre- and post-vaccination antibody titers were found in any of the dogs when either parasite lysate antigens or the defined diagnostic antigen rK39 were used in ELISAs (data not shown). Thus, the elevated antibodies in the responding animals indicate a targeted immune response has occurred to the vaccine antigen, not a generalized response to pathogen antigens.
Figure 3.
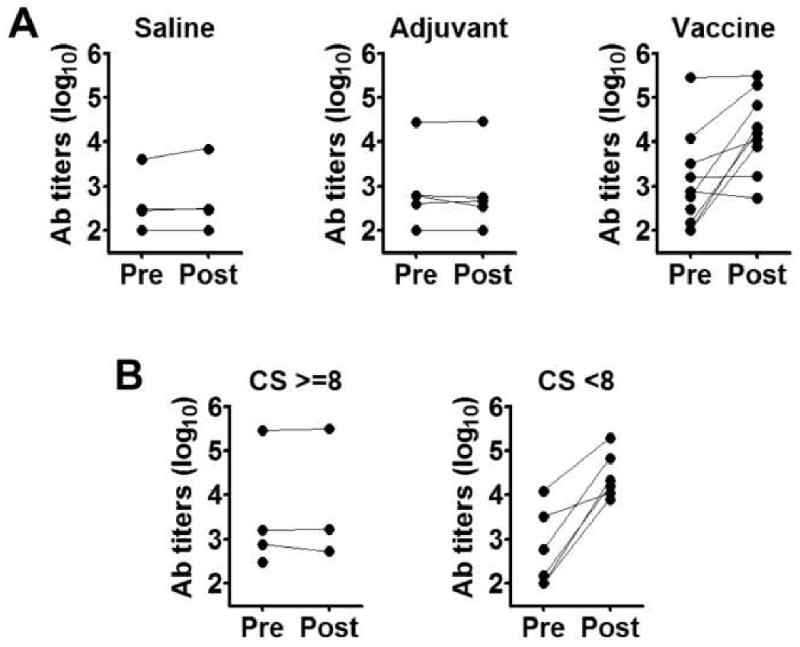
Leish-111f antibody titers in the three Blinded trial treatment groups and the relationship between disease severity and Leish-111f responsiveness within the Vaccine group. (A) Plasma samples were collected at pre- and post-vaccination time points from dogs in the Saline, Adjuvant, and Vaccine groups, and evaluated for antibody titers to Leish-111f by ELISA. Reciprocal endpoint titers for pre- and post-vaccination samples (filled circles) are shown for each dog; one dog in the Vaccine group died before a post-vaccination sample could be obtained. (B) ELISA titers of Vaccine-group dogs were re-plotted in the categories CS ≥8 and CS <8 based on CS at Day 0.
A striking difference in antibody responses was observed when dogs in the Vaccine group were divided into two categories based on their CS values: All the dogs with CS <8 at Day 0 showed increased antibody titers to Leish-111f after vaccination, regardless of whether they received four or six injections of vaccine. In contrast, no increase in anti-Leish-111f antibody titer was observed after vaccination in the three dogs who had an initial CS ≥8 (the fourth dog died before Day 42, Figure 3B). Thus, those dogs in the Vaccine group (dogs with a Day 0 CS ≥8) that did not improve clinically also failed to respond immunologically to the vaccine.
Discussion
The high mortality and morbidity that we observed in dogs with untreated CVL is consistent with earlier reports that L. infantum infection causes serious pathology in dogs and that spontaneous resolution of CVL is unusual [30]. Furthermore, we found that Glucantime treatment was not effective in many of the treated dogs, as reported [31]. In fact, failure rates of at least 45% have been reported using Glucantime alone [32] as a result of advanced disease, relapse, or drug resistance of the parasites [33]. This is why an alternative treatment, such as immunotherapy, is urgently needed. We designed Study #1 expecting an additive, if not a synergistic, effect of chemotherapy and immunotherapy since they have different modes of action. However, the combined effect was difficult to discern probably because of the good efficacy of immunotherapy itself, making any incremental increase in chemotherapeutic efficacy difficult to detect. Since chemotherapy has been the only available treatment option, our demonstrations that immunotherapy can treat CVL with an efficacy better than that observed for chemotherapy (and without the concern that drug-resistant parasites will be generated) will open a new window for CVL control.
In contrast to our present results, Gradoni et al. concluded that a Leish-111f + MPL-SE vaccine neither prevented infection nor prevented disease progression in a post-infection, pre-disease boost of immunity [25]. Because there are a number of differences between that study and our studies including vaccine dosages, schedules, the manufacturing processes used for producing the vaccine antigen and adjuvant formulations, geographical factors, and disease status of the different groups of dogs, we cannot be certain what accounts for the discrepant outcomes. Furthermore, the previous study did not evaluate the therapeutic effect of the vaccine on diseased dogs. Another study evaluating the therapeutic efficacy of the vaccine was performed by Miret et al. in Brazil [26] using vaccine components manufactured by the same organizations and processes as used for the present studies. Vaccinated dogs in the Miret et al. study responded immunologically to the vaccine antigen and had a better survival rate than either no treatment or Glucantime treatment, even though dogs in the Vaccine-alone group remained symptomatic and parasite-positive [26]. In contrast, improvements in both survival rate and clinical symptoms occurred with the weekly vaccination schedule (for a total of 4 or 6 injections) of the present studies. This vaccine schedule contrasts with the schedules used in the two previous studies in which three injections were given at either 3- or 4-week intervals [25, 26], and the schedule also differs from that typically used for a prophylactic vaccination. While prophylactic vaccination requires a good quality long-term memory T-cell response, a therapeutic vaccine may require large numbers of effector T-cells specialized at killing those Leishmania parasites already present in the infected host. Differences in vaccination schedules between pre- and post-exposure are well-known for rabies, and such an exhaustive schedule as weekly injections, which may prevent induction of memory responses, could still be beneficial for the purpose of a therapeutic treatment. In the future, it will be valuable to determine how the vaccination schedule affects immune responses (measurements that might include the ratio of antigen-specific effector vs. memory T cells) as well as the therapeutic efficacy of a vaccine. Also, it may be useful to evaluate the vaccine in other geographic areas that have a significant number of CVL cases, such as the European Mediterranean coastline.
As no plan was made to periodically check the treated dogs after the conclusion of the Open Trial (Study #1), it is not possible to determine whether there was differential long-term survival of the study groups. Although at least six dogs from the Vaccine group in this first study are known to still be alive and have remained leishmaniasis-free, it is not clear whether the vaccine provided longer term protection from reinfection in some dogs compared to a Glucantime cure. Moreover, in the absence of interim biopsies or serum evaluations and because no preventative measures (netting, insecticide-treated collars) were enforced on the owners, it cannot be ruled out that some dogs were re-infected over the course of the study. The possibility needs to be explored that periodic boosting with the therapeutic (or a different prophylactic) vaccine may be beneficial at, say, 12 or 24 month intervals after the initial course of treatment.
The second trial was intended to reproduce therapeutic efficacy of the vaccine in a blinded study as well as to evaluate the necessity of including the antigen component for vaccine efficacy. As seen in Trial #1, the vaccine improved the clinical symptoms of CVL dogs, whereas untreated dogs did not show improvement (Fig. 2). It is intriguing that the effectiveness of the vaccine depended on disease severity at the time of inclusion in the study. Severely sick dogs did not respond to the vaccine either clinically or immunologically (Fig. 2 and 3). The immunological hypo-responsiveness of the dogs may be due to an antigen-specific immunosuppressive status in severe CVL. It is accepted for dogs as well as for other mammalian hosts that a Th1 response is responsible for protection [34]. Production of Th1 cytokines such as IFN-γ, TNF, and IL-2 is associated with protection against CVL [35, 36]. For this reason we stimulated whole blood from the Study #2 dogs with antigen and attempted to measure IFN-γ production by ELISA. Unfortunately, the assay failed, and we were unable to detect IFN-γ production with even con A stimulation on many samples. This was likely a technical issue because in a previous study the vaccine induced cell-mediated immune responses in dogs [26]. The disease severity-related hypo-responsiveness of these dogs to the vaccine may be related to an IL-10 down-regulation of the Th1 response. Because IL-10 levels increase in the spleen as CVL progresses [37], some dogs with advanced disease may be rendered less responsive to such an extent that the immune system is refractory to the Leish-111f + MPL-SE vaccine. Other strategies, such as giving a vaccine along with anti-IL-10 antibody, should be considered for immunotherapy of dogs with advanced CVL.
The use of adjuvant alone also improved clinical outcomes in Study #2, and the efficacy was comparable to the vaccine (Fig. 2). Unlike with the Vaccine group, the single Adjuvant dog with a Day 0 CS ≥8 (whose CS changed by -2 vs. 0 for Vaccine) showed clinical improvement (Fig. 2) even though this dog exhibited no increased antibody titer to any of the antigens tested (Fig. 3A and data not shown). The clinical improvements observed in the Adjuvant group might be due to the immunostimulatory activity of MPL as a TLR4 ligand that directly activates cells within innate immune response pathways and, in conjunction with antigens present due to the existing parasite burden, may stimulate an effective anti-parasite, adaptive immune response. Such responses have previously been observed in immunotherapy settings; for example, in some cases the TLR ligands CpG oligonucleotides and imiquimod do not require exogenous antigens to improve clinical outcomes of leishmaniasis or to reduce parasite burdens [38-40]. Similar results have been obtained in our human clinical trials of the Leish-111f + MPL-SE vaccine: Injection of adjuvant without antigen accelerated the cure of CL by chemotherapy (Piazza F et al., unpublished data). MPL-SE alone may have worked in these situations because 1) it directly activated infected macrophages to kill parasites through TLR signaling, and/or 2) antigens derived from the killed parasites were presented to T-cells in the presence of Th1-inducing adjuvant. In these human vaccine trials, however, the vaccine clearly had better curative efficacy than adjuvant alone. We did not see any difference in curative efficacies between vaccine and adjuvant alone in this CVL therapy study, possibly due to the small size of the study. Therefore, it will be valuable to explore further the requirements of a therapeutic CVL vaccine with a larger number of dogs per group.
Supplementary Material
Acknowledgments
This research was funded in part by a grant from the Bill and Melinda Gates Foundation (No. 39129), the National Institutes of Health grant AI25038, and Fundação Bahiana de Infectologia. The authors gratefully acknowledge Drs. Karen Cowgill, Ajay Bhatia, Rhea Coler, and Sylvie Bertholet for their comments during the preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lukes J, Mauricio IL, Schonian G, Dujardin JC, Soteriadou K, Dedet JP, et al. Evolutionary and geographical history of the Leishmania donovani complex with a revision of current taxonomy. Proc Natl Acad Sci U S A. 2007;104(22):9375–80. doi: 10.1073/pnas.0703678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5(11):873–82. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 3.Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 2002;18(9):399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- 4.Dantas-Torres F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet Parasitol. 2007;149(3-4):139–46. doi: 10.1016/j.vetpar.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Dantas-Torres F. Leishmune vaccine: the newest tool for prevention and control of canine visceral leishmaniosis and its potential as a transmission-blocking vaccine. Vet Parasitol. 2006;141(1-2):1–8. doi: 10.1016/j.vetpar.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol. 2005;35(11-12):1169–80. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Gavgani AS, Hodjati MH, Mohite H, Davies CR. Effect of insecticide-impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children: a matched-cluster randomised trial. Lancet. 2002;360(9330):374–9. doi: 10.1016/s0140-6736(02)09609-5. [DOI] [PubMed] [Google Scholar]
- 8.Reithinger R, Davies CR. Canine leishmaniasis: novel strategies for control. Trends Parasitol. 2002;18(7):289–90. doi: 10.1016/s1471-4922(02)02296-1. [DOI] [PubMed] [Google Scholar]
- 9.Dietze R, Barros GB, Teixeira L, Harris J, Michelson K, Falqueto A, et al. Effect of eliminating seropositive canines on the transmission of visceral leishmaniasis in Brazil. Clin Infect Dis. 1997;25(5):1240–2. doi: 10.1086/516096. [DOI] [PubMed] [Google Scholar]
- 10.Cunha S, Freire M, Eulalio C, Critosvao J, Netto E, Johnson WD, Jr, et al. Visceral leishmaniasis in a new ecological niche near a major metropolitan area of Brazil. Trans R Soc Trop Med Hyg. 1995;89(2):155–8. doi: 10.1016/0035-9203(95)90474-3. [DOI] [PubMed] [Google Scholar]
- 11.Silva ES, Gontijo CM, Pacheco RS, Fiuza VO, Brazil RP. Visceral leishmaniasis in the Metropolitan Region of Belo Horizonte, State of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2001;96(3):285–91. doi: 10.1590/s0074-02762001000300002. [DOI] [PubMed] [Google Scholar]
- 12.Baneth G, Shaw SE. Chemotherapy of canine leishmaniosis. Vet Parasitol. 2002;106(4):315–24. doi: 10.1016/s0304-4017(02)00115-2. [DOI] [PubMed] [Google Scholar]
- 13.Tesh RB. Control of zoonotic visceral leishmaniasis: is it time to change strategies? Am J Trop Med Hyg. 1995;52(3):287–92. doi: 10.4269/ajtmh.1995.52.287. [DOI] [PubMed] [Google Scholar]
- 14.Alvar J, Molina R, San Andres M, Tesouro M, Nieto J, Vitutia M, et al. Canine leishmaniasis: clinical, parasitological and entomological follow-up after chemotherapy. Ann Trop Med Parasitol. 1994;88(4):371–8. doi: 10.1080/00034983.1994.11812879. [DOI] [PubMed] [Google Scholar]
- 15.Oliva G, Foglia Manzillo V, Pagano A. Canine leishmaniasis: evolution of the chemotherapeutic protocols. Parassitologia. 2004;46(1-2):231–4. [PubMed] [Google Scholar]
- 16.Riera C, Valladares JE, Gallego M, Aisa MJ, Castillejo S, Fisa R, et al. Serological and parasitological follow-up in dogs experimentally infected with Leishmania infantum and treated with meglumine antimoniate. Vet Parasitol. 1999;84(1-2):33–47. doi: 10.1016/s0304-4017(99)00084-9. [DOI] [PubMed] [Google Scholar]
- 17.Reithinger R, Coleman PG, Alexander B, Vieira EP, Assis G, Davies CR. Are insecticide-impregnated dog collars a feasible alternative to dog culling as a strategy for controlling canine visceral leishmaniasis in Brazil? Int J Parasitol. 2004;34(1):55–62. doi: 10.1016/j.ijpara.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Foglia Manzillo V, Oliva G, Pagano A, Manna L, Maroli M, Gradoni L. Deltamethrin-impregnated collars for the control of canine leishmaniasis: evaluation of the protective effect and influence on the clinical outcome of Leishmania infection in kennelled stray dogs. Vet Parasitol. 2006;142(1-2):142–5. doi: 10.1016/j.vetpar.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Saraiva EM, de Figueiredo Barbosa A, Santos FN, Borja-Cabrera GP, Nico D, Souza LO, et al. The FML-vaccine (Leishmune) against canine visceral leishmaniasis: a transmission blocking vaccine. Vaccine. 2006;24(13):2423–31. doi: 10.1016/j.vaccine.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 20.Lemesre JL, Holzmuller P, Goncalves RB, Bourdoiseau G, Hugnet C, Cavaleyra M, et al. Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: double-blind randomised efficacy field trial. Vaccine. 2007;25(21):4223–34. doi: 10.1016/j.vaccine.2007.02.083. [DOI] [PubMed] [Google Scholar]
- 21.Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD(4+) T cells. Infect Immun. 2007;75(9):4648–54. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coler RN, Skeiky YA, Bernards K, Greeson K, Carter D, Cornellison CD, et al. Immunization with a polyprotein vaccine consisting of the T-Cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infect Immun. 2002;70(8):4215–25. doi: 10.1128/IAI.70.8.4215-4225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velez ID, Gilchrist K, Martinez S, Ramirez-Pineda JR, Ashman JA, Alves FP, et al. Safety and immunogenicity of a defined vaccine for the prevention of cutaneous leishmaniasis. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 24.Reed SG, Coler RN, Campos-Neto A. Development of a leishmaniasis vaccine: the importance of MPL. Expert Rev Vaccines. 2003;2(2):239–52. doi: 10.1586/14760584.2.2.239. [DOI] [PubMed] [Google Scholar]
- 25.Gradoni L, Foglia Manzillo V, Pagano A, Piantedosi D, De Luna R, Gramiccia M, et al. Failure of a multi-subunit recombinant leishmanial vaccine (MML) to protect dogs from Leishmania infantum infection and to prevent disease progression in infected animals. Vaccine. 2005;23(45):5245–51. doi: 10.1016/j.vaccine.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Miret J, Nascimento E, Sampaio W, Franca JC, Fujiwara RT, Vale A, et al. Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime) and the recombinant Leish-110f + MPL-SE vaccine to treat canine visceral leishmaniasis. Vaccine. 2008;26(12):1585–94. doi: 10.1016/j.vaccine.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badaro R, Benson D, Eulalio MC, Freire M, Cunha S, Netto EM, et al. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J Infect Dis. 1996;173(3):758–61. doi: 10.1093/infdis/173.3.758. [DOI] [PubMed] [Google Scholar]
- 28.Porrozzi R, Santos da Costa MV, Teva A, Falqueto A, Ferreira AL, dos Santos CD, et al. Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic Leishmania infantum visceral infections in dogs. Clin Vaccine Immunol. 2007;14(5):544–8. doi: 10.1128/CVI.00420-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goto Y, Howard RF, Bhatia A, Trigo J, Nakatani M, Netto EM, et al. Distinct antigen recognition pattern during zoonotic visceral leishmaniasis in humans and dogs. Vet Parasitol. 2009;160(3-4):215–20. doi: 10.1016/j.vetpar.2008.10.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pozio E, Gradoni L, Bettini S, Gramiccia M. Leishmaniasis in Tuscany (Italy): VI. Canine leishmaniasis in the focus of Monte Argentario (Grosseto) Acta Trop. 1981;38(4):383–93. [PubMed] [Google Scholar]
- 31.Ikeda-Garcia FA, Lopes RS, Marques FJ, de Lima VM, Morinishi CK, Bonello FL, et al. Clinical and parasitological evaluation of dogs naturally infected by Leishmania (Leishmania) chagasi submitted to treatment with meglumine antimoniate. Vet Parasitol. 2007;143(3-4):254–9. doi: 10.1016/j.vetpar.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Denerolle P, Bourdoiseau G. Combination allopurinol and antimony treatment versus antimony alone and allopurinol alone in the treatment of canine leishmaniasis (96 cases) J Vet Intern Med. 1999;13(5):413–5. doi: 10.1892/0891-6640(1999)013<0413:caaatv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Rojas R, Valderrama L, Valderrama M, Varona MX, Ouellette M, Saravia NG. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J Infect Dis. 2006;193(10):1375–83. doi: 10.1086/503371. [DOI] [PubMed] [Google Scholar]
- 34.Barbieri CL. Immunology of canine leishmaniasis. Parasite Immunol. 2006;28(7):329–37. doi: 10.1111/j.1365-3024.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 35.Pinelli E, Gonzalo RM, Boog CJ, Rutten VP, Gebhard D, del Real G, et al. Leishmania infantum-specific T cell lines derived from asymptomatic dogs that lyse infected macrophages in a major histocompatibility complex-restricted manner. Eur J Immunol. 1995;25(6):1594–600. doi: 10.1002/eji.1830250619. [DOI] [PubMed] [Google Scholar]
- 36.Pinelli E, Killick-Kendrick R, Wagenaar J, Bernadina W, del Real G, Ruitenberg J. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect Immun. 1994;62(1):229–35. doi: 10.1128/iai.62.1.229-235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lage RS, Oliveira GC, Busek SU, Guerra LL, Giunchetti RC, Correa-Oliveira R, et al. Analysis of the cytokine profile in spleen cells from dogs naturally infected by Leishmania chagasi. Vet Immunol Immunopathol. 2007;115(1-2):135–45. doi: 10.1016/j.vetimm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Arevalo I, Ward B, Miller R, Meng TC, Najar E, Alvarez E, et al. Successful treatment of drug-resistant cutaneous leishmaniasis in humans by use of imiquimod, an immunomodulator. Clin Infect Dis. 2001;33(11):1847–51. doi: 10.1086/324161. [DOI] [PubMed] [Google Scholar]
- 39.Buates S, Matlashewski G. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. J Infect Dis. 1999;179(6):1485–94. doi: 10.1086/314782. [DOI] [PubMed] [Google Scholar]
- 40.Walker PS, Scharton-Kersten T, Krieg AM, Love-Homan L, Rowton ED, Udey MC, et al. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-gamma-dependent mechanisms. Proc Natl Acad Sci U S A. 1999;96(12):6970–5. doi: 10.1073/pnas.96.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


