Abstract
AMPA receptors consist of a family of hetero-oligomeric (tetrameric) receptors arising from four genes, each of which encodes a distinct receptor subunit (GluA1-4). Recombinant homo-tetrameric AMPA receptors, comprising four identical subunits, are functionally active and have been used in in vitro assays. However, the many different subunit permutations make possible the functional and anatomical diversity of AMPA receptors throughout the CNS. Furthermore, AMPA receptor subunit stoichiometry influences the biophysical and functional properties of the receptor. A number of chemically diverse positive modulators of AMPA receptor have been identified which potentiate AMPA receptor-mediated activity in vitro as well as improving cognitive performance in rodents and non-human primates with several being taken further in the clinic. This review article summarizes the current status in the research on positive allosteric modulation of AMPA receptors and outlines the challenges involved in identifying a chemically distinct series of AMPA receptor positive modulators, addressing the challenges created by the heterogeneity of the AMPA receptor populations and the development of structure-activity relationships driven by homomeric, recombinant systems on high-throughput platforms. We also review the role of X-ray crystallography in the selection and prioritization of targets for lead optimization for AMPA receptor positive modulators.
Keywords: AMPA, ion channel, electrophysiology, positive modulator, X-ray crystallography
AMPA receptors
AMPA (a-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors are the most highly expressed ionotropic glutamate receptors in the mammalian brain and are responsible for the majority of fast synaptic transmission (Ozawa et al., 1998). All AMPA receptors are a tetrameric combination of four subunits (GluA1-4, previously known as GluR1-4 or GluRA-D, see Alexander et al. 2009 and Collingridge et al., 2009), each encoded by a separate gene, allowing a wide variety of different subunit combinations and diverse function (Greger et al., 2003). Additional complexity arises from alternative splicing of RNA, which gives rise to flip and flop variants of each subunit (Seeburg et al., 1998). Each subunit contains approximately 900 amino acids and exhibits around 65–75% sequence homology to other subunits (Hollmann and Heinemann, 1994). Subunits are glycosylated and possess a long extracellular amino-terminus, a short intracellular carboxy-terminus and three membrane-spanning hydrophobic domains (M1, M3 and M4), as well as one intra-membrane re-entrant loop (M2) (Bennett and Dingledine, 1995). Furthermore, RNA editing results in a positively charged arginine (R) residue replacing the genomically encoded glutamine (Q) in the M2 re-entrant loop of the GluA2 subunit, which reduces both passage of divalent cations and block by intracellular polyamines (Sommer et al., 1991). GluA2 subunits in mature brain generally (>95%) contain the R residue, thus restricting Ca2+ flux through the channel and essentially rendering the receptor permeable to just Na+ and K+, which is deemed crucial for adult synaptic function and plasticity (Seeburg et al., 2001).
Recombinant homotetrameric AMPA receptors, comprising four identical subunits, are functionally active and can be used in various in vitro assays. However, the functional and anatomical diversity of AMPA receptors throughout the CNS is manifested by the different possible subunit permutations. AMPA receptor subunit stoichiometry influences the biophysical and functional properties of the receptor by modifying parameters such as receptor kinetics (Leever et al., 2003), channel open time (Klein and Howe, 2004), internalization and trafficking to and from the postsynaptic membrane (Collingridge et al., 2004; Greger et al., 2007), all of which are involved with fast excitatory synaptic transmission. In this respect, during periods of repetitive glutamatergic afferent input, AMPA receptor-mediated synaptic transmission activates the N-methyl d-aspartate (NMDA) subtype of glutamate receptor, relieving the voltage-dependent Mg2+ block of its channel and enabling Ca2+ influx into the postsynaptic neurone. This influx triggers Ca2+-dependent signal-transduction cascades, trafficking of extrasynaptic AMPA receptors and high conductance GluA1 homomers (Passafaro et al., 2001) to the postsynaptic density, leading to the induction of forms of synaptic plasticity, such as long-term potentiation (LTP), that are believed to be important in mnemonic processing. AMPA receptors further contribute to the maintenance of synaptic potentiation as more stable, GluA2-containing, receptors are recruited into the postsynaptic density of activated synapses (see Derkach et al., 2007).
A family of transmembrane AMPA receptor regulatory proteins (TARPs), which bind to the cytoplasmic carboxy-terminus domain (Barry and Ziff, 2002), has been identified. These AMPA receptor auxiliary subunits are intimately involved with not only the chaperoning of AMPA receptors to the synapse under both normal synaptic transmission and increased synaptic activity (Nicoll et al., 2006), but also with fundamental AMPA receptor properties such as open probability and channel kinetics (Milstein et al., 2008), single channel conductance (Tomita et al., 2005) and sensitivity to polyamine block (Soto et al., 2007), thus playing a crucial role in synaptic transmission of the CNS.
AMPA receptor positive modulators
Dysfunction of the glutamatergic system has been implicated in numerous psychiatric disorders, such as schizophrenia, Alzheimer's disease, depression, anxiety and attention-deficit hyperactivity disorder (ADHD) (see Javitt, 2004 for review). Re-instating the loss of glutamatergic function in such disease states via alterations in AMPA receptor function can be achieved by either direct agonism or positive modulation. Direct agonist-induced activation of AMPA receptors globally activates the CNS, thereby losing spatial and temporal aspects of AMPA receptor activation that are critical to generating a sufficient signal to noise ratio to enable appropriate CNS processing of sensory information. The alternative approach of positive modulation of AMPA receptors by, for example, slowing the rate at which the receptor (i) desensitizes in the continued presence of glutamate; or (ii) deactivates after removal of glutamate, enhances and/or prolongs glutamatergic synaptic currents, thereby promoting synaptic transmission and plasticity, without corrupting spatial and temporal information. This more attractive approach has been prosecuted extensively over the years, in order to identify a range of positive modulators of AMPA receptors. Such molecules potentiate AMPA receptor-mediated activity in vitro, exhibit selective potentiation at all AMPA receptor subunit types, compared to other ionotropic glutamate receptors, with little or no specificity for one particular subunit. Different chemical classes of modulator selectively and reversibly enhance AMPA receptor-mediated responses recorded from both recombinant AMPA receptor subunits and native AMPA receptors, slowing the rate at which responses desensitize and/or prolonging response deactivation (Arai et al., 1996; Quirk and Nisenbaum, 2002). In hippocampal slice recordings, polysynaptic circuit facilitation was increased to a greater degree than monosynaptic potentiation in the presence of AMPA receptor positive modulators, suggesting that such molecules amplify neurotransmission across a synaptic network (Sirvio et al., 1996). Additionally, AMPA receptor modulators lower the induction threshold, enhance the amplitude and increase the duration of LTP, widely viewed as the mechanism underlying many forms of learning and memory (Lynch, 2002; Arai et al., 2004). Brain derived neurotrophic factor (BDNF), which enhances synaptic plasticity and activates mechanisms that regulate induction, as well as early and late maintenance phases of LTP (Bramham and Messaoudi, 2005), is also up-regulated by AMPA receptor modulators in neuronal cultures (Legutko et al., 2001) and in vivo (Woolley et al., 2009). AMPA receptor positive modulators also improve cognitive performance in behavioural models in rodents, including improvement in olfactory discrimination, radial arm maze (Staubli et al., 1994), conditioned fear (Rogan et al., 1997), water maze performance (Zivkovic et al., 1995; Quirk and Nisenbaum, 2002), delayed non-match to sample (Hampson et al., 1998), novel object recognition (Lebrun et al., 2000), passive avoidance (Lebrun et al., 2000; Quirk and Nisenbaum, 2002) and attentional set shifting (Woolley et al., 2009). Similarly, in non-human primates, improved performance has been reported in the reversal of impaired multiple schedule task (Thompson et al., 1995), delayed match to sample in young (Buccafusco et al., 2004; Porrino et al., 2005) and aged rhesus monkeys (Buccafusco et al., 2004) and alleviation of sleep deprivation-impaired performance of delayed match to sample (Porrino et al., 2005). As well as cognition, preclinical data has suggested a role for AMPA receptor positive modulators in treatments for a range of diseases including depression (Quirk and Nisenbaum, 2002; O'Neill and Witkin, 2007), Huntington's disease (Simmons et al., 2009) stroke (Dicou et al., 2003) and Parkinson's disease (Bloss et al., 2008).
AMPA receptor positive modulators – clinical landscape
The first generation of AMPA positive modulators were derived from aniracetam, a nootropic compound shown to improve different phases of learning and memory impairment in rats and mice (Cumin et al., 1982). From that point, a number of AMPA receptor modulators have progressed into clinical evaluation (Figure 1). One of the earliest, CX516, was shown to improve cognitive performance in healthy volunteers (Ingvar et al., 1997), elderly subjects (Lynch et al., 1997) and schizophrenic patients (Goff et al., 2001), but failed to improve cognition or schizophrenic symptoms when added to antipsychotics (Goff et al., 2007). This molecule is weakly potent and has a very short half life in humans, and both could be major contributing factors to the study not achieving a statistically significant end point.
Figure 1.
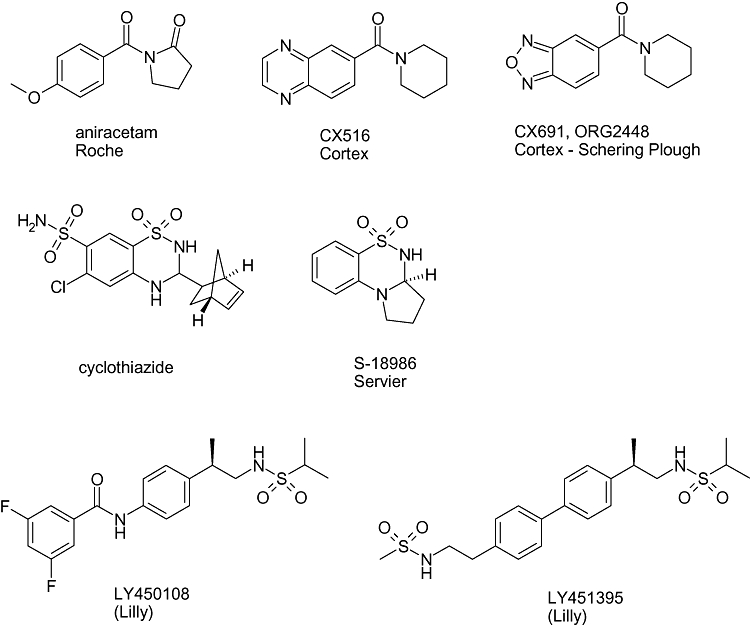
Structures of clinically evaluated AMPA receptor positive modulators.
Subsequently, however, more potent molecules have been identified with longer half lives, which have been or are currently in Phase II for the treatment of psychiatric and neurological disorders. Schering-Plough (formerly Organon), under license from Cortex, was developing farampator (Org24448; CX691; SCH900460), one of two lead, orally active, CX516 follow-on compounds, for the potential treatment of depression. However, by September 2008, the only apparent ongoing treatment trial, a phase II trial for the short-term treatment of depression sponsored by The National Institute of Mental Health, had been suspended, pending the results of an on-going follow-up study on cardiac safety, being carried out by Organon/Schering-Plough, in patients who had previously been treated with the compound. Farampator had previously also been in development for the potential treatment of schizophrenia, as a cognition enhancing agent in schizophrenia, and for the treatment of Alzheimer's disease. Schering-Plough is also developing ORG26576 for the potential treatment of depression and ADHD.
Cortex Pharmaceuticals has been the most active player in the field of AMPA positive modulators (see Table 1). CX717 has been registered for six Phase IIa clinical studies for Alzheimer's disease and respiratory depression and Cortex is also developing an intravenous formulation of the compound. A follow-on compound, CX1739, is also in a Phase II study for sleep apnoea, with trial completion expected in late 2009 and further plans to initiate another Phase II study in adult ADHD in the US within 2009. Other molecules such as CX701 have been reported to enter development, although they have not progressed to Phase II evaluation.
Table 1.
On-going Phase II trials with AMPA receptor positive modulators
| Drug | Current phase | Summary |
|---|---|---|
| CX717Cortex | Phase-II | Phase II Alzheimer's disease trial started (July 2005); however the FDA placed CX717 on clinical hold in April 2006 based on preclinical data. In July 2007, following an FDA data package review, the company announced that it was to resume its phase II Alzheimer's disease trial. In March 2006, Cortex completed a phase II trial of CX717 in adult ADHD; however in October 2007, the FDA rejected an IND for a phase IIb study and Cortex inactivated its application. On October 2008 Cortex reported positive top line results from the company's placebo-controlled, double-blind, randomized two-way crossover phase IIa CX717-RD-01 study of CX717 in opioid-induced respiratory depression. |
| LY451395Eli Lilly | Phase-II | LY451395 was negative in phase II trial for improving cognitive deficits in Alzheimer's disease (June 2003, published in Chappell et al., 2007). Trial is currently open in 180 patients with Alzheimer's disease, to assess LY451395 3 mg b.i.d orally for 12 weeks on the symptoms of aggression and agitation. Completion is expected in Nov 2010 (ClinicalTrials.gov, 23 Apr 2009, NCT00843518). |
| ORG26576Schering-Plough | Phase-II | In October 2007, a phase I/II trial in patients with major depressive disorder was initiated. Completion expected in December 2008. In January 2008, a phase II trial in adult ADHD subjects was initiated. Completion expected in March 2009. |
| CX1739Cortex | Phase-II | Currently under evaluation in a proof-of-concept Phase II trial in sleep apnoea. Top-line results are expected in mid-2009 (Press release, Cortex, 14 Apr 2009). A US Phase II trial in patients with ADHD to evaluate efficacy is expected to start in late 2009 (Press release, Cortex, 6 Sep 2008). |
LY451395 is an AMPA receptor potentiator under development by Lilly for the treatment of Alzheimer's disease (see Table 1). This compound has been in Phase II development since 2002 and is currently undergoing a Phase II trial in 180 patients with Alzheimer's disease; completion is expected in November 2010. Another molecule, LY450108, was evaluated in parallel with LY451395 in Phase I.
In addition to these compounds in clinical assessment, a number of other compounds exist in the pre-clinical phase of research, and published reviews give a comprehensive overview of the pre-clinical and clinical chemotypes in this area up to 2006 (Francotte et al., 2006; Morrow et al. 2006 and references within). Subsequently, further publications and patents have described newer analogues from within existing AMPA receptor chemical space as well as, more interestingly, some recent novel chemotypes from GlaxoSmithKline, Lilly, Pfizer and Cortex. A representative from each of these novel chemotypes across the most diverse of the new structures is represented in Figure 2.
Figure 2.
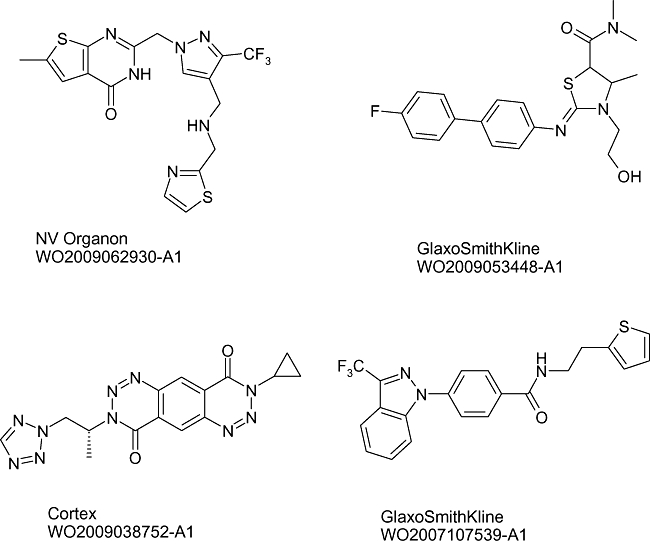
Recently disclosed new AMPA receptor positive modulator chemotypes.
Challenges of identifying new AMPA positive modulators
The approach of enhancing AMPA receptor function to ameliorate a number of psychiatric and neurological conditions is clearly well validated by extensive pre-clinical results. However, despite the extensive number of molecules that have been taken into various clinical studies by Cortex, Organon, Servier, GlaxoSmithKline and Lilly, there are disappointingly few positive findings, and no molecules have been identified which have progressed successfully into Phase III trials. A number of molecule-specific explanations have been provided for these failures, but nonetheless, they constitute, overall, a significant body of negative or equivocal data which frustrates assessment of the true clinical potential of AMPA receptor positive modulators. One of the major challenges to understanding these negative data relates to the lack of biomarker or translational approaches to assess the magnitude as well as spatial and temporal pattern of activation of AMPA receptor positive modulation that is observed for a given clinical dose, and thus to the ability to thoroughly interrogate and learn from the negative clinical outcome. Although a preliminary study with one class of AMPA modulator has shown changes in blood oxygenation levels using pharmacological magnetic resonance imaging in rat (Jones et al., 2005) and another chemotype increased regional cerebral glucose metabolism rates in non-human primates measured using positron emission tomography (Porrino et al., 2005). Furthermore, as has already been detailed, the very large number of reported AMPA positive modulators has, until recently, fallen into only three major chemotype groupings, and the majority of clinical trials reported have originated from CX516 and its close analogues.
Nevertheless, despite this potentially discouraging clinical landscape, there are still several active research programmes seeking to identify new AMPA receptor positive modulator chemotypes, as well as the active development programmes, detailed earlier. However, as would be assumed from the lack of chemical diversity in the clinical portfolio of AMPA receptor positive modulators, identification of new chemotypes to explore new chemical space is both desirable and challenging. As mentioned previously, the heterogeneity of the AMPA receptors, together with the various splice variants, sites of post-translational modification and well characterized accessory proteins in a native system combine to render replication in a recombinant cell-line extremely difficult. Indeed, the rationale of using homomeric recombinant cell lines for high throughput screening, whilst attractive in terms of the potential high volume of molecule screening, is questionable in terms of interpretation of data output and translation to the native system. Nonetheless, workers at Lilly have extensively described their chemical series which led to LY451395 and LY450108, and whose origin was identified by use of high-throughput screening technology against homomeric GluA4 (flip splice variant) using FLIPR technology (Figure 3) (Ornstein et al., 2000). This assay measured responses mediated through AMPA receptors by 100 µM l-glutamate in HEK293 cells by determining changes in concentration of calcium ions by means of a Ca2+-sensitive fluorescent dye relative to a positive control (100 µM cyclothiazide). From this, they were able to identify sets of molecules that were very potent potentiators of AMPA receptors in recombinant and native forms.
Figure 3.
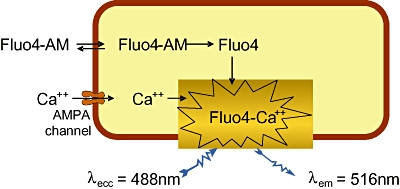
Schematic representation of FLIPR assay to detect new AMPA receptor positive modulators: •Intracellular calcium change can be detected by calcium-sensitive dyes using the fluorescent imaging plate reader (FLIPR). hGluA2(flip) Q/R edited to allow Ca2+ permeability •HEK293 cells expressing hGluA2 receptor are loaded with Fluo4-AM •AM-ester is cleaved by intracellular esterases •Fluo4 binds to intracellular Ca2+ and increases fluorescence.
Structural studies of AMPA receptors
Structural biology has a well-established role in lead optimization and discovery for soluble proteins (Congreve et al., 2005), and is used for nearly all soluble targets within GlaxoSmithKline. However, despite recent advances in membrane protein crystallography (Gouaux and MacKinnon, 2005; Rosenbaum et al., 2009), it is still unusual to be able to establish a robust crystallization system for routinely determining high resolution complexes of lead molecules to support lead discovery and optimization for an ion channel. However, the characterization of an isolated soluble ligand binding domain (LBD) for the GluA2 receptor has enabled many ligand-bound structures to be determined (Chen et al., 1998; Sun et al., 2002; Jin et al., 2005; Ptak et al., 2009).
The AMPA receptors have a modular structure (Mayer, 2006) with the extracellular region containing a LBD responsible for agonist binding and an amino-terminal domain (ATD) (Figure 4). Recent structures of the amino terminal domain of GluA2 (Clayton et al., 2009; Jin et al., 2009) show a dimer that is important in guiding subfamily specific receptor assembly. The three subfamilies of ionotropic glutamate receptors (AMPA, kainate and NMDA) all form tetramers, which appear to be composed of a dimer-of-dimers with twofold (not fourfold) symmetry (Tichelaar et al., 2004).
Figure 4.
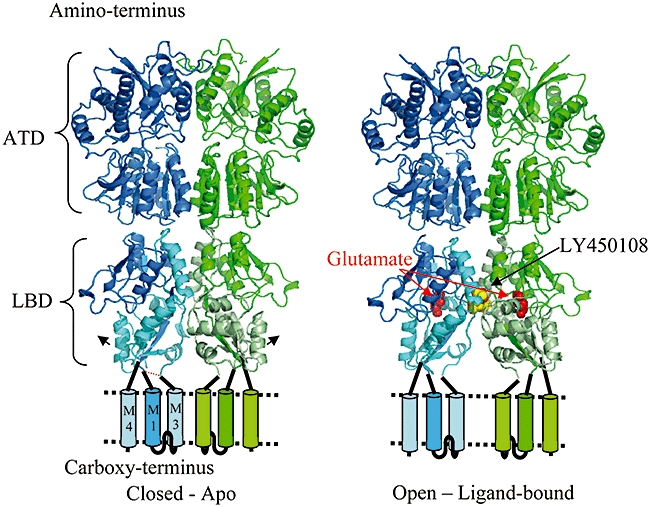
A model of the GluA2 receptor incorporating dimeric crystal structures of the amino-terminal domain (ATD – pdb code – 3h5v) and the ligand binding domain (LBD). Apo structure on left, ligand-bound structure on right, structures determined in GlaxoSmithKline. Two subunits are shown, one in blue and one in green.
In the full-length receptor, the LBD is tightly coupled to the ion channel domain. However, LBD constructs, in which membrane spanning helices M1 and M3 are deleted and replaced by a short loop (dotted red line Figure 4), and in which the carboxy-terminal membrane spanning region (M4) is also deleted, give soluble protein and well-diffracting crystals (Chen et al., 1998; Sun et al., 2002). We followed this literature precedent and solved in house over 40 high resolution structures of the GluA2 LBD bound with ligands from a wide variety of chemotypes (one such ligand is shown in Figure 5).
Figure 5.
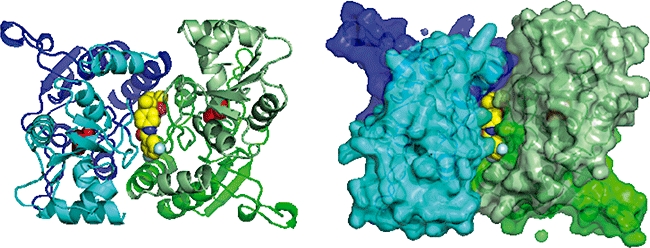
A view along the twofold axis showing a crystal structure of LY450108 binding to the GluA2 ligand binding domain. Glutamate molecules are shown in red.
Structural studies on the GluA2 LBD showed how the binding of glutamate causes the clam-shell-like domain to close (in Figure 4, the arrows on the apo structure show the direction of domain movement on binding glutamate). The LBD forms a dimer and the binding of glutamate increases the distance between the parts of the LBD that are covalently linked to the membrane-spanning helices in the full-length receptor (Figure 4) leading to the opening of the ion channel (Mayer, 2006). In the desensitized state, in which the glutamate is still bound but the ion channel is closed, the dimer interface observed between the two LBDs in crystal structures is broken. An L483Y mutation that stabilizes the crystallographic LBD dimer (Sun et al., 2002) greatly decreases AMPA receptor desensitization (Stern-bach et al., 1998). Many AMPA receptor positive modulators bind at the interface between the LBDs (Figure 5), increasing the stability of the glutamate-bound dimer and reducing the level of desensitization (Ptak et al., 2009). Aniracetam binds at this dimer interface and preferentially slows deactivation by stabilizing the clam-shell in its closed cleft, glutamate-bound, conformation (Jin et al., 2005). The compound binding site on the twofold axis of the GluA2 LBD dimer is formed by residues that act as ‘hinges’ between the two structural domains of each LBD. Modulators binding at this site can modulate deactivation as well as desensitization of the receptor (Jin et al., 2005).
GlaxoSmithKline approaches to identify new AMPA receptor positive modulators
The approaches at GlaxoSmithKline to identify new AMPA receptor positive modulators paralleled the approach of Lilly to run a high-throughput screen to identify new chemotypes. The screen was again established using a FLIPR/Ca2+ assay in 384 plate format with a dual addition protocol. This allowed for direct Ca2+ measurements with a good dynamic range for positive modulators, although there was a low signal for agonist addition. In contrast to Lilly's screen against the homomeric GluA4 homomer, we opted to screen against homomeric GluA2 (flip splice variant) given the relative distribution patterns of the two subunits (GluA2 is more highly expressed in cortical and subcortical brain areas than GluA4 which is predominant in the cerebellum (Petralia and Wenthold, 1992; Beneyto and Meador-Woodruff, 2004).
This assay allowed us to successfully establish a high-throughput screen and identify novel chemotypes and also to establish structure–activity relationships (SAR) within the chemical series. Nonetheless, we were cognizant of the limitations of this high throughput, recombinant cell-line assay to represent the situation in vivo with heterogeneous AMPA subunit receptors, the presence of splice variants and post-translational modifications and various accessory proteins. Furthermore, the molecules that had been reported as progressing to Phase II evaluation, cover a range of potencies in these FLIPR assays from low (e.g. CX516, CX691) to high (e.g. LY451395), but the efficacious doses in behavioural models of cognition do not necessarily correlate with these marked differences. With this in mind, we established a screening cascade as represented in Figure 6 in which molecules were progressed through a number of steps to profile the activity firstly in the recombinant FLIPR hGluA2 (flip) assay, then using more detailed conventional patch clamp electrophysiology evaluation to probe the AMPA receptor potentiation more fully in terms of effects on deactivation and desensitization. Importantly, this biophysical profile was conducted both on the recombinant cell line (to give confidence of eventual activity in man) and in rat neuronal cell cultures (to give confidence of efficacy in preclinical development).
Figure 6.
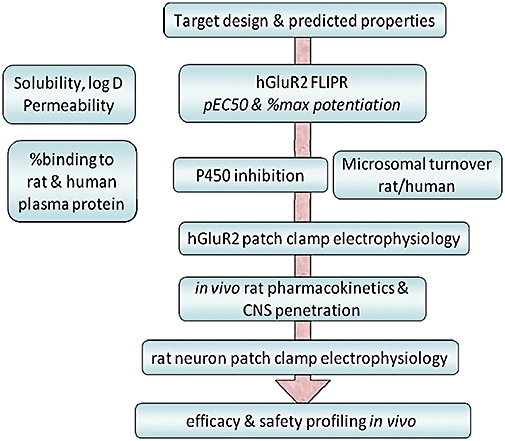
Screening cascade for identification of clinical candidates from new AMPA receptor positive modulators.
To both assist us in the rapid optimization of our early hit series and to be able to maximize the use of available information and technologies, we established a complementary crystallographic assay, as described earlier. This allowed us to generate regular ligand-bound crystal structures of our new AMPA receptor positive modulators in the GluA2-LBD constructs to facilitate our optimization strategies. By making a specific number of probing analogues to test the SAR, we were able to develop hypotheses of where modifications would be tolerated in the molecules and to identify the key protein-interacting residues to maintain. This composite of FLIPR data, electrophysiological characterization on recombinant and native systems coupled with structural evidence for the binding of the AMPA receptor modulators gave us a powerful set of lead optimization tools and allows informed navigation of the route through to molecules which can be taken on into downstream assays.
To illustrate this process, from our described high-throughput screen, we were able to identify a starting hit molecule, GSK-1 (Figure 7), which had modest potency in our hGluA2 FLIPR assay. However, there were limited analogues available around this molecule to establish an understanding of the SAR for the AMPA receptor. In order to focus our medicinal chemistry activities, we were able to generate a high resolution X-ray crystal structure of GSK-1 bound into the GluA2-LBD construct, and from there propose analogues to address potency and improve its potential for development. This sequence led to the preparation of new analogues, including GSK-2, for which we again generated a high resolution X-ray structure (Figure 8) from which we were able to make hypotheses regarding future analogue preparation and in particular to identify key conserved interactions. This work was then able to progress, through further iterations including GSK-3, towards a development candidate. This regular cycle of molecule design, biological profiling and X-ray crystallography with complete method description will be described fully in a later publication.
Figure 7.
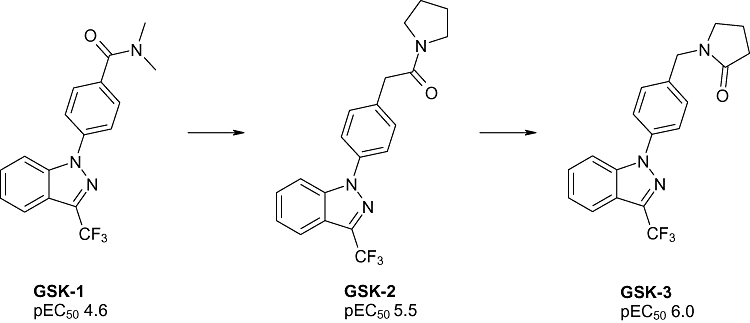
Examples of structures obtained by GlaxoSmithKline. Data reported are FLIPR generated pEC50 against hGluA2 flip isoform (full method reported in Bradley et al., 2008).
Figure 8.
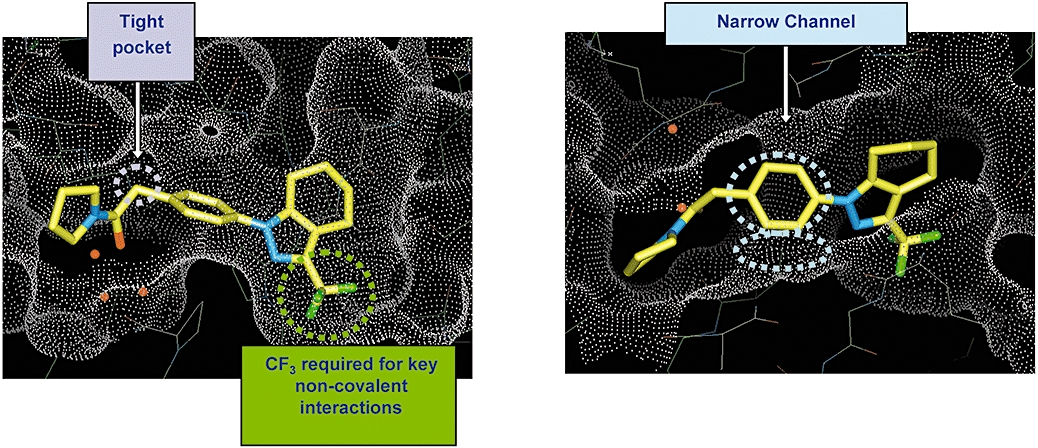
Two rotated views of crystal structures of GSK-2 bound into GluA2 LBD-construct.
Following assessment of the molecules in these assays, as well as assays designed to understand the physicochemical and pharmacokinetic properties and so assess the molecule's development potential, we were then able to proceed to evaluation in appropriate pharmacodynamic and behavioural models such as passive avoidance (Zivkovic et al., 1995) and novel object recognition (Woolley et al., 2009). For us, it was pivotal to the progression of both the molecules and the overall programme that we were able to use an in vivo electrophysiology readout, following previously reported protocols (Vandergriff et al., 2001), to establish a functionally relevant pharmacodynamic outcome to build confidence that the molecules were able to enhance neuronal transmission through AMPA receptors in vivo.
Conclusions
AMPA receptor positive modulators have the potential to be of value in treating a number of psychiatric and neurological conditions which is validated by an extensive body of preclinical data. The clinical scenario is more equivocal, with some early positive clinical data being superseded by later negative trial outcomes and thus preventing any molecules from progressing into large scale Phase III patient trials, which would better illustrate the true clinical potential of these molecules.
Nonetheless, a number of companies have expended considerable effort to identify new and differentiated AMPA receptor positive modulators and with the advent of high-throughput screening technologies, a broadening of the diversity of chemotypes has been reported.
Our in-house approaches at GlaxoSmithKline have been to combine biological characterization with routine high resolution X-ray crystal structure determinations, and this is the first report we are aware of where this has successfully been done prospectively to design new analogues for an ion channel programme.
Glossary
Abbreviations:
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- NMDA
N-methyl-D-aspartic acid
Conflict of interest
All authors are employees of GlaxoSmithKline and state no additional conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai A, Kessler M, Ambros-Ingerson J, Quan A, Yigiter E, Rogers G, et al. Effect of a centrally active benzoylpyrrolidine drug on AMPA receptor kinetics. Neuroscience. 1996;75:573–585. doi: 10.1016/0306-4522(96)00263-1. [DOI] [PubMed] [Google Scholar]
- Arai AC, Xia Y-F, Suzuki E. Modulation of AMPA receptor kinetics differentially influences synaptic plasticity in the hippocampus. Neuroscience. 2004;123:1011–1024. doi: 10.1016/j.neuroscience.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Expression of transcripts encoding AMPA receptor subunits and associated postsynaptic proteins in the macaque brain. J Comp Neurol. 2004;468:530–554. doi: 10.1002/cne.10981. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Dingledine R. Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining re-entrant membrane loop. Neuron. 1995;14:373–384. doi: 10.1016/0896-6273(95)90293-7. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Hunter RG, Waters EM, Munoz C, Bernard K, McEwen BS. Behavioural and biological effects of chronic S18986, a positive AMPA receptor modulator, during aging. Exp Neurol. 2008;210:109–117. doi: 10.1016/j.expneurol.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Bradley DM, Chan WN, Harrison SA, Thewlis KM, Ward SE. Compounds which potentiate AMPA receptor and uses thereof in medicine. 2008. International patent application WO 2008/110566.
- Bramham C, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Weiser T, Winter K, Klinder K, Terry AV. The effects of IDRA 21, a positive modulator of the AMPA receptor, on delayed matching performance by young and aged rhesus monkeys. Neuropharmacology. 2004;46:10–22. doi: 10.1016/j.neuropharm.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Chappell AS, Gonzales C, Williams J, Witte MM, Mohs RC, Sperling R. AMPA potentiator treatment of cognitive deficits in Alzheimer's disease. Neurology. 2007;68:1008–1012. doi: 10.1212/01.wnl.0000260240.46070.7c. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Sun Y, Rongsheng J, Gouaux E. Probing the ligand binding domain of the GluR2 receptor by proteolysis and deletion mutagenesis defines domain boundaries and yields a crystallisable construct. Protein Sci. 1998;7:2623–2630. doi: 10.1002/pro.5560071216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Siebold C, Gilbert RJC, Sutton GC, Harlos K, McIlhinney RAJ, et al. Crystal structure of the GluR2 amino-terminal domain provides insights into the architecture and assembly of ionotropic glutamate receptors. J Mol Biol. 2009;392:1125–1132. doi: 10.1016/j.jmb.2009.07.082. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nature Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacol. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congreve M, Murray CW, Blundell TL. Structural biology and drug discovery. Drug Disc Today. 2005;10:895–907. doi: 10.1016/S1359-6446(05)03484-7. [DOI] [PubMed] [Google Scholar]
- Cumin R, Bandle EF, Gamzu E, Haefely WE. Effects of the novel compound Aniracetam (Ro 13-5057) upon impaired learning and memory in rodents. Psychopharmacology. 1982;78:104–111. doi: 10.1007/BF00432244. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Dicou E, Rangon C-M, Guimiot F, Spedding M, Gressens P. Positive allosteric modulators of AMPA receptors are neuroprotective against lesions induced by an NMDA agonist in neonatal mouse brain. Br Res. 2003;970:221–225. doi: 10.1016/s0006-8993(03)02357-6. [DOI] [PubMed] [Google Scholar]
- Francotte P, de Tullio P, Fraikin P, Counerotte S, Goffin E, Pirotte B. In search of novel AMPA potentiators. Recent Pat CNS Drug Discov. 2006;1(3):239–246. doi: 10.2174/157488906778773661. [DOI] [PubMed] [Google Scholar]
- Goff DC, Leahy L, Berman I, Posever T, Herz L, Leon AC, et al. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J Clin Psychopharm. 2001;21:484–487. doi: 10.1097/00004714-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Goff DC, Lamberti JS, Leon AC, Green MF, Miller AL, Patel J, et al. A placebo-controlled add-on trial of the Ampakine, CX516, for cognitive deficits in schizophrenia. Neuropsychopharm. 2007;33:465–472. doi: 10.1038/sj.npp.1301444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaux E, MacKinnon R. Principles of selective ion transport in channels and pumps. Science. 2005;310:1461–1465. doi: 10.1126/science.1113666. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Greger IH, Ziff EB, Penn AC. Molecular determinants of AMPA receptor subunit assembly. TINS. 2007;30:407–416. doi: 10.1016/j.tins.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Rogers G, Lynch G, Deadwyler SA. Facilitative effects of the ampakine CX516 on short-term memory in rats: enhancement of delayed-nonmatch-to-sample performance. J Neurosci. 1998;18:2740–2747. doi: 10.1523/JNEUROSCI.18-07-02740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Ann Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, et al. Enhancement by an ampakine of memory encoding in humans. Exp Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatr. 2004;9:984–979. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Jin R, Clark S, Weeks AM, Dudman JT, Gouaux E, Partin KM. Mechanism of positive allosteric modulators acting on AMPA receptors. J Neurosci. 2005;25:9027–9036. doi: 10.1523/JNEUROSCI.2567-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Singh SK, Gu S, Furukawa H, Sobolevsky AI, Zhou J, et al. Crystal structure and association behaviour of the GluR2 amino-terminal domain. EMBO J. 2009;28:1812–1823. doi: 10.1038/emboj.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N, O'Neill MJ, Tricklebank M, Libri V, Williams SCR. Examining the neural targets of the AMPA receptor potentiator LY404187 in the rat brain using pharmacological magnetic resonance imaging. Psychopharmacology. 2005;180:743–751. doi: 10.1007/s00213-005-2254-y. [DOI] [PubMed] [Google Scholar]
- Klein RM, Howe JR. Effects of the lurcher mutation of GluR1 desensitization and activation kinetics. J Neurocsi. 2004;24:4941–4951. doi: 10.1523/JNEUROSCI.0660-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun C, Piliere E, Lestage P. Effects of S18986-1, a novel cognitive enhancer, on memory performance in an object recognition task in rats. Eur J Pharmacol. 2000;401:205–212. doi: 10.1016/s0014-2999(00)00429-5. [DOI] [PubMed] [Google Scholar]
- Leever JD, Clark S, Weeks AM, Partin KM. Identification of a site in GluR1 and GluR2 that is important for modulation of deactivation and desensitization. Mol Pharm. 2003;64:5–10. doi: 10.1124/mol.64.1.5. [DOI] [PubMed] [Google Scholar]
- Legutko B, Li X, Skolnick P. Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator. Neuropharmacology. 2001;40:1019–1027. doi: 10.1016/s0028-3908(01)00006-5. [DOI] [PubMed] [Google Scholar]
- Lynch G. Memory enhancement: the search for mechanism-based drugs. Nat Neurosci. 2002;5:1035–1038. doi: 10.1038/nn935. [DOI] [PubMed] [Google Scholar]
- Lynch G, Granger R, Ambros-Ingerson J, Davis M, Kessler M, Schehr R. Evidence that a positive modulator of AMPA-type glutamate receptors improves delayed recall in aged humans. Exp Neurol. 1997;145:89–92. doi: 10.1006/exnr.1997.6447. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440:456–462. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. Regulation of AMPA receptor gating and pharmacology by TARP auxiliary subunits. Trends Pharmacol Sci. 2008;29:333–339. doi: 10.1016/j.tips.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JA, Maclean JK, Jamieson C. Recent advances in positive allosteric modulators of the AMPA receptor. Curr Opin Drug Discov Devel. 2006;9(5):571–579. [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxilliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- O'Neill MJ, Witkin JM. AMPA receptor potentiators: application for depression and Parkinson's disease. Curr Drug Targets. 2007;8:603–620. doi: 10.2174/138945007780618517. [DOI] [PubMed] [Google Scholar]
- Ornstein PL, Zimmerman DM, Arnold BM, Bleisch TJ, Cantrell B, Simon R, et al. Biarylpropylsulfonamides as novel, potent potentiators of 2-amino-3-(5-methyl-3-hydroxyisoxazol-4-yl)-propanoic acid (AMPA) receptors. J Med Chem. 2000;43:4345–4358. doi: 10.1021/jm0002836. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron imunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak CP, Ahmed AH, Oswald RE. Probing the allosteric modulator binding site of GluR2 with thiazide derivatives. Biochemistry. 2009;48:8594–8602. doi: 10.1021/bi901127s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk JC, Nisenbaum ES. LY404187: a novel positive allosteric modulator of AMPA receptors. CNS Drug Rev. 2002;8:255–282. doi: 10.1111/j.1527-3458.2002.tb00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. AMPA receptor facilitation accelerates fear learning without altering the level of conditioned fear acquired. J Neurosci. 1997;17:5928–5935. doi: 10.1523/JNEUROSCI.17-15-05928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G-protein coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH, Higuchi M, Sprengel R. RNA editing of brain glutamate receptor channels: mechanism and physiology. Brain Res Brain Res Rev. 1998;26:217–229. doi: 10.1016/s0165-0173(97)00062-3. [DOI] [PubMed] [Google Scholar]
- Seeburg PH, Single F, Kuner T, Higuchi M, Sprengel R. Genetic manipulation of key determinants of ion flow in glutamate receptor channels in the mouse. Brain Res. 2001;907:233–243. doi: 10.1016/s0006-8993(01)02445-3. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Rex CS, Palmer L, Pandyarajan V, Fedulov V, Gall CM, et al. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proc Natl Acad Sci U S A. 2009;106:4906–4911. doi: 10.1073/pnas.0811228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirvio J, Larson J, Quach CN, Rogers GA, Lynch G. Effects of pharmacologically facilitating glutamatergic transmission in the trisynaptic intrahippocampal circuit. Neuroscience. 1996;74:1025–1035. doi: 10.1016/0306-4522(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeberg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;105:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Soto D, Coombs ID, Kelly L, Farrant M, Cull-Candy SG. Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nat Neurosci. 2007;10:1260–1267. doi: 10.1038/nn1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci U S A. 1994;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitisation of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Guidotti A, DiBella M, Costa E. 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA 21), a congener of aniracetam, potently abates pharmacologically induced cognitive impairments in patas monkeys. Proc Natl Acad Sci U S A. 1995;92:7667–7771. doi: 10.1073/pnas.92.17.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichelaar W, Safferling M, Keinanen K, Stark H, Madden DR. The three-dimensional structure of an ionotropic glutamate receptor reveals a dimer-of-dimers assembly. J Mol Biol. 2004;344:435–442. doi: 10.1016/j.jmb.2004.09.048. [DOI] [PubMed] [Google Scholar]
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Howe JR, Nicoll RA, et al. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- Vandergriff J, Huff K, Bond A, Lodge D. Potentiation of responses to AMPA on central neurones by LY392098 and LY404187 in vivo. Neuropharmacology. 2001;40:1003–1009. doi: 10.1016/s0028-3908(01)00031-4. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Waters KA, Gartlon JE, Lacroix LP, Jennings C, Shaugnessey F, et al. Evaluation of the pro-cognitive effects of the AMPA receptor positive modulator, 5-(1-piperidinylcarbonyl)-2,1,3-benzoxadiazole (CX691), in the rat. Psychopharmacology. 2009;202:343–354. doi: 10.1007/s00213-008-1325-2. [DOI] [PubMed] [Google Scholar]
- Zivkovic I, Thompson DM, Bertolino M, Uzunov D, DiBella M, Costa E, et al. 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA 21): a benzothidiazine derivative that enhances cognition by attenuating DL-alpha-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA) receptor desensitization. J Pharmacol Exp Ther. 1995;272:300–309. [PubMed] [Google Scholar]


