Abstract
Background and purpose:
Alkylphospholipid (APL) analogues constitute a new class of synthetic anti-tumour agents that act directly on cell membranes. We have previously demonstrated that hexadecylphosphocholine (HePC) alters intracellular cholesterol traffic and metabolism in HepG2 cells. We now extended our studies to analyse the effects of other clinically relevant APLs, such as edelfosine, erucylphosphocholine and perifosine on intracellular cholesterol homeostasis.
Experimental approach:
Using radiolabelled substrates we determined the effect of APLs on cholesterol metabolism and cholesterol traffic from the plasma membrane to the endoplasmic reticulum (ER). Protein levels and gene expression of the main proteins involved in cholesterol homeostasis were analysed by Western blot and RT-PCR respectively. Membrane raft and non-raft fractions were isolated from HepG2 cells by a detergent-free method.
Key results:
All APLs inhibited the transport of cholesterol from the plasma membrane to the ER, which induced a significant cholesterogenic response in HepG2 cells. This response involved an increased gene expression and higher levels of several proteins related to the biosynthesis and the receptor-mediated uptake of cholesterol. Cell exposure to the APL-representative HePC enhanced the content of cholesterol mainly in the membrane raft fractions, compared with the untreated cells.
Conclusions and implications:
Membrane-targeted APLs exhibited a novel and common mechanism of action, through disruption of cholesterol homeostasis, which in turn affected specific lipid microdomains of cellular membranes.
Keywords: alkylphospholipid analogues, cholesterol homeostasis, lipid rafts, HepG2 cells
Introduction
The synthetic lipid analogue, hexadecylphosphocholine (HePC) belongs to a new class of anti-tumour agents, which, in contrast to most of the currently used chemotherapeutic drugs, does not target DNA but acts at the cell membrane (van Blitterswijk and Verheij, 2008; Barratt et al., 2009). In addition to its anti-neoplastic activity, HePC is used as a drug for the oral treatment of human cutaneous and visceral leishmaniasis (Soto and Soto, 2006; Rakotomanga et al., 2007) and is also toxic in vitro to other protozoan parasites (Seifert et al., 2001; Saraiva et al., 2002; Walochnik et al., 2002).
Because of the hydrophobic nature of its long hydrocarbon chain, HePC may become incorporated into the plasma membrane of cells and resist catabolic degradation. The level of partitioning into membrane lipid bilayers is related to the degree of phospholipid alkyl chain unsaturation and the amount of cholesterol. The affinity of HePC for sterols suggests that it could interact with biological membranes thus interfering with lipid metabolism and lipid-dependent signal transduction (Barratt et al., 2009). Consistent with this suggestion, we found earlier that the alkylphosphocholine HePC interferes with phosphatidylcholine synthesis in human hepatoma HepG2 cells via both CDP-choline (Jiménez-López et al., 2002) and phosphatidylethanolamine methylation pathways (Jiménez-López et al., 2004).
HePC belongs to a second generation of the synthetic compounds called alkylphospholipid (APL) analogues, the prototype of the first generation being edelfosine, which structurally resembles lysophosphatidylcholine. In an attempt to improve anti-tumour activity with reduced side effects, erucylphosphocholine (ErPC) and perifosine were synthesized. Compared with HePC, ErPC contains a longer chain with a cis double bond and perifosine presents a piperidine moiety instead of the choline head group (van Blitterswijk and Verheij, 2008). All of them share structural features and act at cell membranes by interfering with the turnover and synthesis of phospholipids. Thus, they induce apoptosis through inhibition of CTP : phosphocholine cytidylyltransferase (CT), a key enzyme in phosphatidylcholine biosynthesis (van der Luit et al., 2007). These authors reported that, in mouse S49 lymphoma cells, edelfosine and other APL analogues accumulate in detergent-resistant, sphingolipid- and cholesterol-enriched lipid raft domains and are rapidly internalized by clathrin-independent, raft-mediated endocytosis to inhibit CT activity. A cell line made resistant to APLs was incapable of edelfosine internalization via this raft-dependent pathway. APL uptake in KB carcinoma cells, however, appears to be raft-independent and mediated by a yet undefined ATP-driven lipid transporter (Vink et al., 2007b). Particularly, it has been reported by Mollinedo's group that edelfosine treatment induces the formation of membrane raft aggregates containing Fas/CD95 death receptor and the adaptor molecule Fas-associated death domain-containing protein (FADD), which are critical in the triggering of apoptosis induced by edelfosine and other APLs in leukemic cells (Gajate et al., 2009).
Recently, we have established that HePC also alters intracellular cholesterol traffic and metabolism leading to an increased uptake, synthesis and accumulation of cholesterol in the cell (Jiménez-López et al., 2006; Carrasco et al., 2008; Marco et al., 2009). In the present work, we have extended our studies to analyse the effects of a variety of clinically relevant APLs on intracellular cholesterol homeostasis, thus affecting membrane lipid composition and their distribution in raft-non-raft domains. Alterations induced by APLs in the homeostasis of cholesterol provide a novel mechanism of action for membrane-targeted APLs that may well contribute to their anti-tumour activity.
Methods
Cell culture
The human hepatoma HepG2 cell line was from The European Collection of Animal Cell Cultures (Salisbury, UK). Cells were cultured in minimal essential medium (MEM) containing 10% heat-inactivated fetal bovine serum (FBS), supplemented with 2 mM l-glutamine, 1% non-essential amino acids, 100 U·mL−1 penicillin and 100 µg·mL−1 streptomycin. Cells were grown in a humidified atmosphere with 5% CO2 at 37°C and were subcultured at a 1:10 ratio once a week. Cells were plated on tissue-culture dishes (Nunc™, from LabClinics SA, Barcelona, Spain) at a density of 5 × 104 cells·cm−2 and maintained in culture medium for 3 days before being used in experimental radiolabelling assays at approximately 70% confluence.
Assays for cell viability and proliferation
Cells were seeded onto 24-well plates (25 000 cells·well−1) and maintained in MEM containing 10% FBS for 24 h. Then, the culture medium was replaced with fresh medium/10% FBS and the cells were incubated for 48 h in the absence or presence of APLs (up to 75 µM) before analyses. The anti-proliferative effect of the different APL analogues on HepG2 cells was assessed by the crystal violet staining assay using a cell number-based standard curve. The indexes of cytotoxicity used in this study were the measurements of lactate dehydrogenase (LDH) leakage and the change in cell morphology observed under the inverted microscope.
Metabolic labelling assays
The synthesis of cholesterol was determined by measuring the incorporation of radioactive exogenous acetate into cellular sterols. Log-phase HepG2 cells were incubated in medium containing 10% FBS either without (control) or with 25 µM HePC, edelfosine, ErPC or perifosine for 24 h. [1-14C]acetate (87 µM, 58 Ci·mol−1) was added during the last 6 h of incubation. After labelling, the medium was removed and the cells were washed twice with ice-cold PBS before being harvested by scraping into PBS. The lipids were extracted from the cells following the procedure of Bligh and Dyer (1959). Cholesterol was separated by TLC using a mixture of hexane/diethyl ether/acetic acid (80/20/2, v/v/v) as solvent.
To analyse the synthesis of cholesteryl esters, HepG2 cells were incubated in MEM/10% FBS without or with the APLs (25 µM) for 1 h at 37°C in the presence of [9,10 (n)-3H]oleate (100 µM, 34 Ci·mol−1). Cholesteryl [3H]oleate was isolated from the cells by TLC using hexane/diethyl ether/acetic acid (80/20/2, v/v/v) as solvent.
Radiometric measurements of scraped lipid spots, rendered visible by exposure to iodine vapour, were made by liquid scintillation counting.
Trafficking of cholesterol from the plasma membrane to the endoplasmic reticulum
An appropriate way to measure cholesterol transport from the plasma membrane to the endoplasmic reticulum (ER) is to determine the degree of esterification of radiolabelled cholesterol previously incorporated into the plasma membrane (Lange and Steck, 1997; Marco et al., 2009). Thus, HepG2 cells were incubated with 2 µCi of [7(n)3-3H]cholesterol (dissolved in 2-propanol) for 1 h at 37°C to label the plasma membrane cholesterol. To remove any unincorporated radioactivity, the cells were washed twice with PBS containing 0.5% BSA prewarmed to 37°C. The cells were then incubated at 37°C in MEM for 1 h in the absence or presence of 25 µM HePC, edelfosine, ErPC or perifosine. Then, the medium was removed and the lipids were extracted and analysed as described above.
Immunoblot analysis
HepG2 cells growing in log-phase were incubated with MEM containing 10% FBS in the absence (PBS as the vehicle) or presence of 25 µM HePC, edelfosine, ErPC or perifosine for three different periods (6, 24 or 48 h). The cells were washed twice and scraped into ice-cold PBS (pH 7.4), and centrifuged at 100×g for 10 min at 4°C. Cell pellets were suspended in 0.3 mL ice-cold lysis buffer consisting of 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100 and a protease inhibitor cocktail from Sigma (Madrid, Spain), and incubated on ice for 30 min with occasional shaking. Cell lysates were centrifuged at 10 000×g for 15 min at 4°C, and supernatants were stored at −80°C until use; an aliquot was taken to determine protein concentration. Equal amounts of lysate protein (30 µg) were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Prestained broad-range protein molecular-mass markers were used during electrophoresis. Membranes were blocked in PBS containing 5% non-fat dry milk and 0.05% Tween-20, and then probed with the indicated polyclonal anti-human primary Ig (1:1000) in blocking solution for 1 h. After several washes in PBS containing 0.05% Tween-20, the membranes were incubated with the corresponding horseradish peroxidase (HRP)-conjugated IgG (1:5000) as secondary antibody for 1 h. Immunoreactive proteins were detected by autoradiography using a chemiluminescent HRP substrate and exposure to Konica Minolta X-ray film (Tokyo, Japan). Following incubation with an antibody-stripping solution consisting of 60 mM Tris-HCl (pH 6.8), 100 mM β-mercaptoethanol and 2% SDS for 30 min at 60°C, blots were probed with rabbit polyclonal anti-human actin Ig (1:1000) to monitor the loading and transfer of the blotted samples. Densitometric analysis was carried out using the ImageJ gel-digitizing software from the National Institutes of Health (Bethesda, MD, USA).
Quantification of mRNA using real-time polymerase chain reaction array system
Exponentially growing HepG2 cells were incubated with MEM containing 10% FBS in the absence (PBS as the vehicle) or presence of 25 µM HePC or edelfosine for three different periods (6, 24 or 48 h). Total RNA was isolated using the RNeasy Mini kit (Qiagen, Iberia SL, Madrid, Spain) and reverse-transcribed into cDNA using the RT2 First Strand kit from SABiosciences (Frederick, MD, USA), which includes both oligo-dT and random hexamer primers. SYBR Green real-time polymerase chain reaction (PCR) was performed on a Chromo4 real-time PCR detector (Bio-Rad Laboratories SA, Madrid, Spain) using the human lipoprotein signalling and cholesterol metabolism RT2 profiler™ PCR array from SABiosciences, which profiles the expression of a focused panel of genes related to lipoprotein transport and cholesterol metabolism. The array also includes sets of replicate control wells that specifically detect non-transcribed genomic DNA contamination, the efficiency of reverse transcription or the efficiency of positive PCR reactions, and specific primer sets for the amplification of five housekeeping gene transcripts: β-2-microglobulin, hypoxanthine phosphoribosyltransferase 1, ribosomal protein L13a, glyceraldehyde-3-phosphate dehydrogenase and β-actin. Reactions were run in duplicate from three independent biological samples for each condition. As a quality control, a dissociation (melting) curve was acquired immediately after the PCR cycling programme. The relative expression ratio was determined from the averaged threshold cycle values, that is, ΔΔCT-based fold change calculations, using an integrated web-based software package from SABiosciences for the PCR array data analysis.
Detergent-free isolation of membrane fractions from HepG2 cells
Membrane raft and non-raft fractions were isolated from HepG2 cells by the detergent-free method of Macdonald and Pike (2005) with minor modifications. Briefly, the cells were washed and collected into a base buffer consisting of 20 mM Tris-HCl (pH 7.8), 250 mM sucrose, 1 mM CaCl2 and 1 mM MgCl2, supplemented with a protease inhibitor cocktail (Sigma). Cells were incubated on ice and lysed by successive passages through a 22 g × 3″ needle 10 times, then a 25 g × 3″ needle 20 times and, finally, a 30 g × 0.32″ needle 10 times, and lysates were centrifuged at 1000×g for 10 min at 4°C. The post-nuclear supernatant was collected, and the process was repeated on the pellet. Both resulting supernatants were combined and immediately subjected to centrifugation in a 12 mL step density gradient of 3–25% OptiPrep™[a 60% (w/v) iodixanol stock solution in water] for 90 min at 52 000×g using a SW-41 rotor in a Beckman ultracentrifuge. Gradients were fractionated into 0.67 mL aliquots collected from the top of the gradient; the fractions were stored at −80°C until use.
Distribution of the raft-marker GM1 ganglioside was examined by dot-blot immunodetection using the ability of HRP-conjugated cholera toxin B subunit to bind GM1 in the samples transferred individually onto a polyvinylidene difluoride membrane.
Other analyses
Lipids were isolated from both raft and non-raft fractions of controls and HePC-treated HepG2 cells. Total cholesterol content was measured by an enzymatic colorimetric kit from LabKit (Madrid, Spain). The levels of sphingomyelin (SM) were quantified by Bartlett's method (1959). Protein concentrations were determined by the Bradford's method (1976) using BSA as standard.
Statistics
The results were expressed as means ± SEM. One-way anova with subsequent post hoc comparisons by Scheffe's test was carried out (SPSS 13.0). P-values < 0.05 were considered to be statistically significant.
Materials
Radiolabelled compounds were supplied by American Radiolabeled Chemicals (St Louis, MO, USA). FBS was from The Cell Culture Company (Pasching, Austria). MEM and TLC plates were from Sigma-Aldrich (Madrid, Spain). HePC was supplied by Cayman Chemical (Ann Arbor, MI, USA), edelfosine by Calbiochem (Nottingham, UK), ErPC by Alexis Biochemicals (Exeter, UK) and perifosine by Selleck Chemical (London, ON, Canada). Polyclonal anti-human primary antibodies and HRP-conjugated secondary IgGs were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), except anti-human 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) primary Ig, which was acquired from Upstate (Lake Placid, NY, USA). HRP-conjugated cholera toxin B subunit was from Life Technologies Corp. (Carlsbad, CA, USA). The enhanced chemiluminescence detection system was from Millipore (Billerica, MA, USA). All other reagents were of analytical grade. Drug/molecular target nomenclature follows Alexander et al. (2009).
Results
Effects of alkylphospholipids on cell proliferation and viability in HepG2 cells
We first analysed the effect of various APLs – HePC, edelfosine, ErPC and perifosine – on cell proliferation. To do this, HepG2 cells were treated with increasing concentrations of APLs for up to 48 h, and cell number in control and APL-treated cells was assessed by crystal violet staining. All the tested APLs displayed growth inhibitory effects in a dose-dependent manner in the HepG2 cells, which became more pronounced as treatment time increased. However, it is important to note that the different APLs exhibited clear differences in their inhibitory potency. So, as shown in Figure 1 all the APLs similarly inhibited the growth rate up to 25 µM. However, at amounts higher than 25 µM, edelfosine and perifosine decreased the proliferation rate to a greater extent than either ErPC or HePC did after 48 h of cell exposure. After 48 h treatment, the values of IC50 derived from the growth inhibition curves were approximately 40 µM for edelfosine and perifosine, while the other two APLs needed double the concentration to produce the same inhibitory effect. The inhibitory potency of the four tested APLs shows the following pattern: edelfosine ≥ perifosine > ErPC > HePC.
Figure 1.
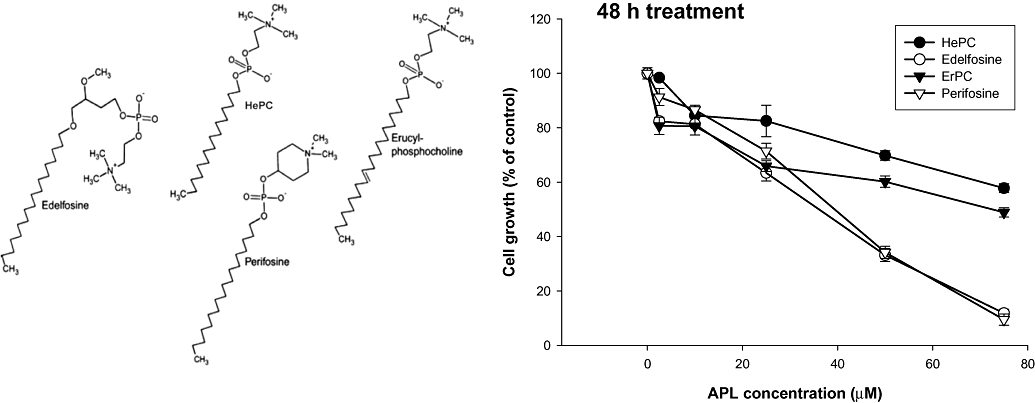
Inhibition curves in HepG2 cells exposed to different concentrations of alkylphospholipids. Cells growing in log-phase were incubated with MEM/10% FBS in the absence or presence of different concentrations of APLs for 48 h. Cell number was determined by the crystal violet staining assay and expressed as percentage of control (no addition) cells. Results represent the mean ± SEM of two independent experiments conducted in triplicate. Chemical structures of synthetic APLs used in this study are shown on the left of the Figure (adapted from Vink et al., 2007a). APL, alkylphospholipid; ErPC, erucylphosphocholine; FBS, fetal bovine serum; HePC, hexadecylphosphocholine; MEM, minimal essential medium.
It is important to note that the decrease in cell number observed after 48 h of APL treatment was not related to any acute cytotoxicity produced by plasma membrane leakage, as we did not detect LDH activity released into the culture medium after any of the treatments assayed up to 50 µM; slight toxicity was only apparent after prolonged treatment with 75 µM edelfosine or perifosine (data not shown).
Concomitantly, morphological changes were also found to be induced in cells treated with the different APLs such as condensation and rounding, traits of cell damage and initiation of apoptosis (Figure 2). Again, edelfosine and perifosine treatments affected HepG2 cells more profoundly than HePC and ErPC.
Figure 2.
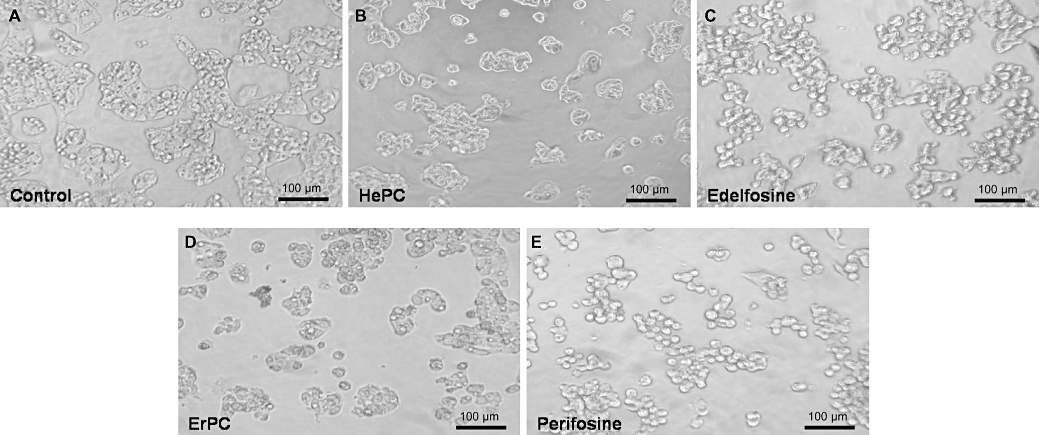
Alkylphospholipid-induced morphological changes in HepG2 cells. Cell morphology was examined with an inverted microscope (20× original magnification). The morphology of HepG2 cells incubated with MEM/10% FBS is shown in the absence of any addition (control, A), or in the presence of 25 µM of HePC (B), edelfosine (C), ErPC (D) or perifosine (E) for 24 h. ErPC, erucylphosphocholine; FBS, fetal bovine serum; HePC, hexadecylphosphocholine; MEM, minimal essential medium.
Effects of alkylphospholipids on cholesterol traffic and homeostasis
Previous studies in our laboratory have shown that treatment of HepG2 and Vero cells with HePC significantly modulates cholesterol metabolism and hence intracellular cholesterol levels are enhanced (Jiménez-López et al., 2006). The increase induced by HePC in cholesterogenic activity was found both in the presence or absence of exogenous LDL-cholesterol (Carrasco et al., 2008). To determine whether other APLs show the same effect, we determined cholesterol biosynthesis by the incorporation of radiolabelled acetate into cholesterol in HepG2 cells incubated with different APLs. Interestingly, similar to HePC, edelfosine, ErPC and perifosine all significantly stimulated de novo biosynthesis of cholesterol, leading to an increase in cholesterogenic activity. Edelfosine (90% activation) was the most potent followed by ErPC, HePC and perifosine, amounting to 80%, 60% and 40% activation respectively (Figure 3).
Figure 3.
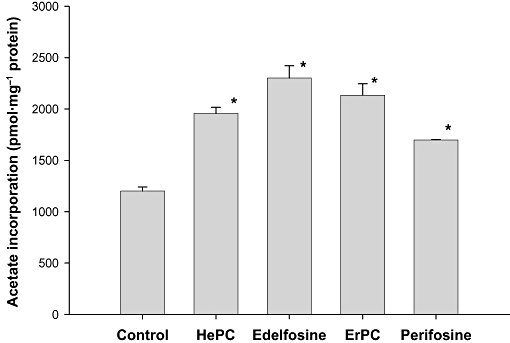
Effect of alkylphospholipids on the biosynthesis of cholesterol. HepG2 cells growing in log-phase were treated with 25 µM HePC, edelfosine, ErPC or perifosine for 24 h in MEM/10% FBS or without any additions (control). [1-14C]acetate (3.4 µM, 60 Ci·mol−1) was added during the last 6 h incubation period. The incorporation of acetate into cholesterol was determined. Results are expressed as pmol of acetate incorporated per mg of cell protein and represent the mean ± SEM of two independent experiments conducted in triplicate. *P < 0.001 when compared with control values. ErPC, erucylphosphocholine; FBS, fetal bovine serum; HePC, hexadecylphosphocholine; MEM, minimal essential medium.
Recently, we described that exposure of HepG2 cells to 50 µM HePC for 1 h causes a drastic reduction in the synthesis of [3H]cholesteryl oleate from radiolabelled oleate (Marco et al., 2009). To test whether it occurs also for other APLs, HepG2 cells were incubated with [9,10 (n)-3H]oleate in the absence or presence of the APLs and the synthesis of [3H]cholesteryl oleate was measured. The APLs were found to inhibit esterified cholesterol synthesis, HePC being the most potent, followed by edelfosine and ErPC (Figure 4).
Figure 4.
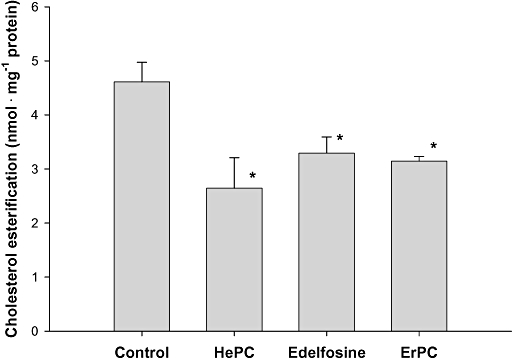
Effect of alkylphospholipids on the esterification of cholesterol. Log-phase HepG2 cells were incubated with [9,10 (n)-3H]oleate (100 µM, 34 Ci·mol−1) for 1 h in serum-free medium either with 25 µM HePC, edelfosine or ErPC or without any additions (control). The incorporation of oleate into cholesteryl oleate was determined. The results are expressed as nmol of oleate incorporated per mg of cell protein and represent the mean ± SEM of two independent experiments conducted in triplicate. *P < 0.02 when compared with control values. ErPC, erucylphosphocholine; HePC, hexadecylphosphocholine.
Previously, we demonstrated that 50 µM HePC blocked the esterification of plasma membrane cholesterol, confirming that the inhibition caused by HePC in the esterification of the intracellular cholesterol pool is produced by a disruption of its movement from the plasma membrane to the ER (Marco et al., 2009). We have used the esterification of plasma membrane cholesterol as a marker of cholesterol movement from the cell surface to the ER, the site of acyl-CoA : cholesterol acyltransferase activity (Marco et al., 2009). Our results indicated that edelfosine, ErPC and perifosine produce a remarkable decrease of 80% in cholesterol esterification. This effect was slightly higher than that obtained with the prototypical APL, HePC, around 70% (Figure 5).
Figure 5.
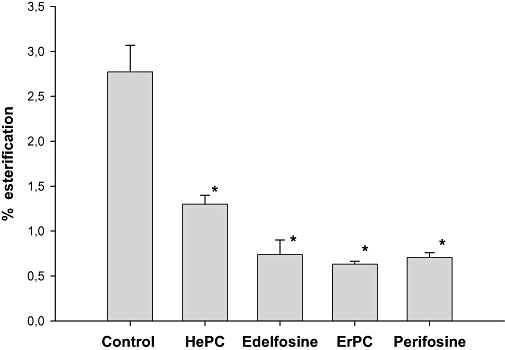
Effect of alkylphospholipids on trafficking of cholesterol from the plasma membrane to the endoplasmic reticulum. Plasma membrane cholesterol in the HepG2 cells was labelled during incubation with 2 µCi [7(n)3-3H]cholesterol for 1 h in serum-free medium. Then, cells were incubated for 1 h with 25 µM HePC, edelfosine, ErPC or perifosine or without any additions (control). The fraction of plasma membrane cholesterol that was esterified is expressed in terms of the percentage of esterification of the total labelled cholesterol and represents the mean ± SEM of two independent experiments conducted in triplicate. *P < 0.01 when compared with control values. ErPC, erucylphosphocholine; HePC, hexadecylphosphocholine.
Effect of alkylphospholipids on the expression of proteins involved in cholesterol homeostasis
Cholesterogenesis is known to be transiently induced by the translocation of the sterol regulatory element-binding protein 2 (SREBP2) transcription factor from the ER membrane (125 kDa precursor form) to the nucleus (70 kDa mature form) (Horton et al., 2003). To validate a role for SREBP2 in the actions of APLs, we analysed by immunoblotting the effect caused by APLs on the expression of SREBP2, and known key SREBP2 targets such as HMGCR and low-density lipoprotein receptor (LDLR) (Horton et al., 2003; Bennett et al., 2008) in the HepG2 cells. Incubation of cells with the different APLs produced a time-dependent increase of the mature form of SREBP2 in the assayed cell lysates, along with increased protein expression of its targets, HMGCR and LDLR (Figure 6). High expression levels of SREBP2 were observed after 24 h treatment with HePC, edelfosine, ErPC or perifosine when compared with basal levels in untreated cells, whereas the membrane-bound precursor form decreased in parallel with the appearance of the released active form (Figure 6 and data not shown).
Figure 6.

Effect of alkylphospholipids on the expression of proteins involved in the homeostasis of cholesterol. HepG2 cells were incubated with MEM/10% FBS without any additions (control) or containing 25 µM HePC, edelfosine, ErPC or perifosine for 6, 24 or 48 h. Cell lysate samples collected at selected times were analysed by immunoblotting to determine the content of mature SREBP2 form, HMGCR, LDLR and β-actin (loading control). The different bands were scanned and arbitrary units were assigned by densitometric analysis. SREBP2, HMGCR and LDL protein levels in the samples were normalized to their respective β-actin level and expressed as x-fold increase compared with the corresponding control ratio (1.0). The figure shows a representative experiment repeated twice. ErPC, erucylphosphocholine; FBS, fetal bovine serum; HePC, hexadecylphosphocholine; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; LDLR, low-density lipoprotein receptor; MEM, minimal essential medium; SREBP, sterol regulatory element-binding protein.
Exposure to HePC and edelfosine stimulates the expression of genes related to cholesterol homeostasis in HepG2 cells
Bearing in mind the results presented above, we proceeded to analyse how exposure to APL analogues modulated the pathway of cholesterol biosynthesis at the transcriptional level in HepG2 cells. For this purpose we selected HePC and edelfosine as representatives of this family of compounds.
Alterations in gene expression in response to treatment with HePC or edelfosine for 6, 24 or 48 h were analysed using the RT2 Profiler™ PCR Array System. Remarkably, Figure 7A shows that exposure of HepG2 cells to 25 µM HePC increased, in a time-dependent manner, the mRNA transcript levels of transcriptionally regulated, cholesterol-synthesizing enzymes such as HMG-CoA synthase (HMGCS1), HMGCR, farnesyl diphosphate synthase (FDPS) and farnesyl diphosphate farnesyltransferase-1 (FDFT1, also known as squalene synthase), which are rate-limiting enzymes of the cholesterol biosynthetic pathway (Sato and Takano, 1995). Similar to HePC, exposure of cells to 25 µM edelfosine also stimulated the main cholesterogenic genes (Figure 7B).
Figure 7.
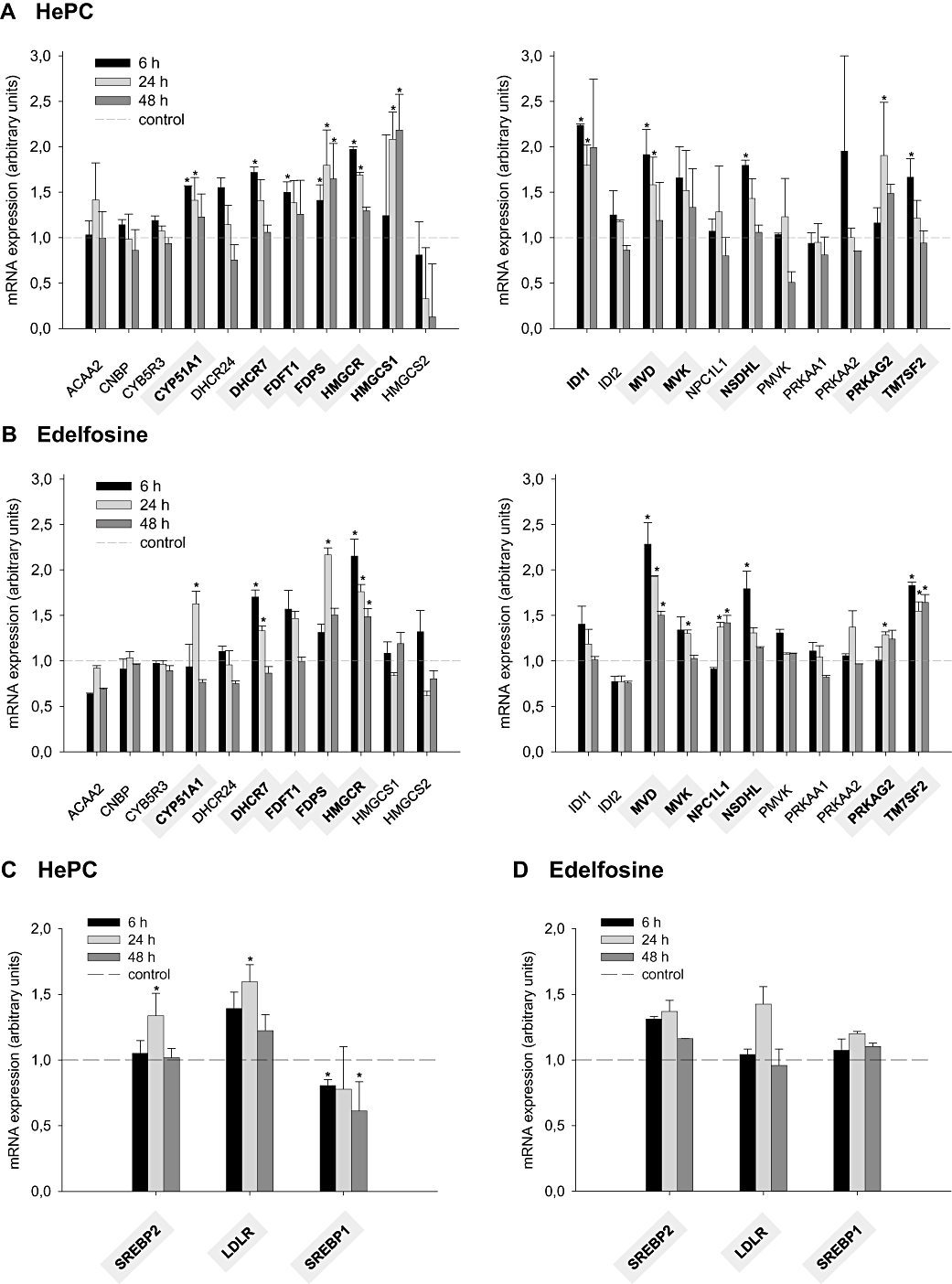
Effect of hexadecylphosphocholine and edelfosine on the expression of genes related to the homeostasis of cholesterol. HepG2 cells were incubated with MEM (minimal essential medium)/10% FBS (fetal bovine serum) in the absence (control) or presence of 25 µM HePC (hexadecylphosphocholine) or edelfosine for three different periods of time (6, 24 or 48 h). The levels of mRNA expression, relative to five housekeeping genes, are shown for a variety of transcriptionally regulated genes involved in cholesterol biosynthesis (A and B), and other genes involved in cholesterol homeostasis (C and D), using real-time PCR. Gene description: ACAA2 (acetyl-CoA acyltransferase 2); CNBP (CCHC-type zinc finger, nucleic acid binding protein); CYP5R3 (cytochrome b5 reductase 3); CYP51A1 (cytochrome P450, family 51, subfamily A, polypeptide 1); DHCR24 (24-dehydrocholesterol reductase); DHCR7 (7-dehydrocholesterol reductase); FDFT1 (farnesyl-diphosphate farnesyltransferase 1); FDPS (farnesyl diphosphate synthase); HMGCR (3-hydroxy-3-methylglutaryl-CoA reductase); HMGCS1 [3-hydroxy-3-methylglutaryl-CoA synthase 1 (soluble)]; HMGCS2 [3-hydroxy-3-methylglutaryl-CoA synthase 2 (mitochondrial)]; IDI1 (isopentenyl-diphosphate δ-isomerase 1); IDI2 (isopentenyl-diphosphate δ-isomerase 2); LDLR (low-density lipoprotein receptor); MVD [mevalonate (diphospho) decarboxylase]; MVK (mevalonate kinase); NPC1L1 [NPC1 (Niemann-Pick disease, type C1, gene)-like 1]; NSDHL [NAD(P) dependent steroid dehydrogenase-like]; PMVK (phosphomevalonate kinase); PRKAA1 (protein kinase, AMP-activated, α1 catalytic subunit); PRKAA2 (protein kinase, AMP-activated, α2 catalytic subunit); PRKAG2 (protein kinase, AMP-activated, γ2 non-catalytic subunit); SREBP1 (sterol regulatory element-binding protein 1, transcription factor); SREBP2 (sterol regulatory element-binding protein 2, transcription factor); TM7SF2 (transmembrane 7 superfamily member 2). *P < 0.05 when compared with control values.
The expression of another set of genes related to lipid homeostasis was analysed in the HepG2 cells exposed to HePC or edelfosine for several periods of time (Figure 7C and D). In mammalian cells, cholesterol-related genes tend to be more strongly activated by overexpression of nuclear SREBP2 form whereas genes that participate in the synthesis of fatty acids are more strongly affected by overexpression of the nuclear SREBP1 form (Horton et al., 2003). Incubation of cells with 25 µM HePC or edelfosine for 24 h stimulated transcription of SREBP2, that is, SREBP2 mRNA expression increased by 1.35-fold as compared with the untreated cells. Moreover, expression of the SREBP2-responsive LDLR gene was enhanced by both agents (Figure 7C and D), confirming earlier reported data obtained using TaqMan probes designed for amplification of LDLR (Carrasco et al., 2008). These results were closely correlated with the observed increases in the content of the mature SREBP2, HMGCR and LDLR proteins after APL treatment (Figure 6). Gene expression of the SREBP1 isoform, however, did not increase when the HepG2 cells were cultured in the presence of HePC or edelfosine (Figure 7C and D).
Effect of HePC on lipid composition of membrane fractions in HepG2 cells
In order to analyse possible interference by HePC with specific membrane domains, raft microdomains were isolated from HepG2 cells by a cell disruption procedure that avoids detergent or sonication, that is, the method of Macdonald and Pike (2005) with minor modifications. After cell disruption, whole cell lysates were passed through several syringes as described in the Methods section, and membrane fractions displaying distinct lipid–protein composition were obtained by density gradient centrifugation. The gradients were fractionated into 18 fractions and analysed for the distribution of several membrane markers (Figure 8). Fractions were characterized by Western blot analysis of protein markers for membrane raft (flotillin-1) or non-raft (clathrin heavy-chain) regions, dot-blot determination of raft-linked GM1 and measuring protein concentration and levels of cholesterol. As shown in Figure 8, lower-density regions of the gradient with relatively little protein (fractions 1–5) were highly enriched in cholesterol, as well as the raft markers GM1 and flotillin-1. Higher-density fractions (12–18) at the bottom of the gradient showed opposite, non-raft characteristics, that is, lower cholesterol with high protein levels, practically in the absence of raft markers but with a significant appearance of the non-raft marker clathrin heavy-chain. Fractions 6–11 constitute an intermediate fraction probably made up by a mixture of raft and non-raft domains.
Figure 8.
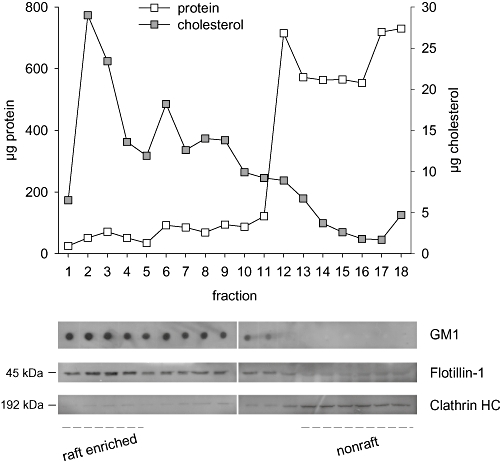
Application of a detergent-free raft isolation procedure to HepG2 cells: distribution of several markers across an Optiprep density gradient. Protein and cholesterol contents were determined in each gradient fraction. Raft-associated GM1 ganglioside was assayed by dot blotting (4 µL sample volumes) using the horseradish peroxidase-conjugated cholera toxin B subunit. A 25 µL aliquot of each gradient fraction was analysed by SDS-PAGE followed by Western blotting for the protein markers flotillin-1 (rafts) and clathrin heavy-chain (clathrin HC; non-rafts).
Although the cholesterol and sphingolipid enrichment of lipid rafts relative to whole plasma membrane is well established, relatively few studies have directly quantified lipid composition of membrane raft fractions isolated by detergent-free methods. Our results confirm the higher content of cholesterol and sphingomyelin in fractions 1–5 (rafts) versus the 6–11 (intermediate) and 12–18 (non-rafts) (Table 1). If only the lightest fractions of the Optiprep gradient are pooled, a relatively clean cholesterol- and sphingomyelin-enriched raft fraction can be obtained. Thus, further studies were carried out only with the raft and non-raft fractions.
Table 1.
Lipid composition of membrane raft, intermediate and non-raft fractions isolated from control HepG2 cells
| nmol of lipid/pooled fraction | ||
|---|---|---|
| Fractions | Cholesterol | Sphingomyelin |
| 1–5 (rafts) | 1530 | 2.17 |
| 6–11 (intermediate) | 570 | 1.87 |
| 12–18 (non-rafts) | 390 | 0.53 |
To investigate the effects of HePC treatment on plasma membrane lipid distribution, we analysed the lipid composition of both raft and non-raft membrane fractions after cell incubation with 25 µM HePC for 1, 12 and 24 h. We assessed GM1 localization after HePC treatment and did not detect any change in GM1 distribution along the different-density fractions (Figure 9).
Figure 9.
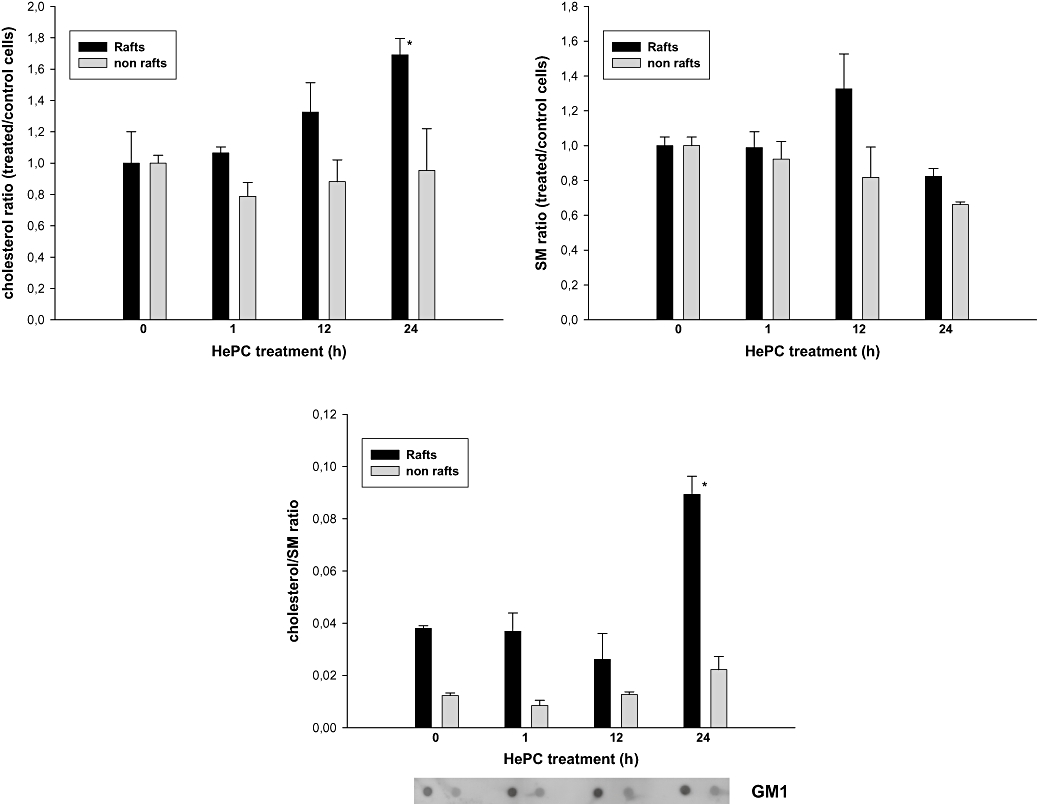
Effect of hexadecylphosphocholine on lipid composition of membrane fractions in HepG2 cells. Cholesterol and sphingomyelin (SM) levels were determined in both raft and non-raft membrane fractions after cell incubation with 25 µM hexadecylphosphocholine (HePC) for 1, 12 and 24 h. Results represent the mean ± SEM of two independent experiments conducted in triplicate. *P < 0.005 when compared with control values.
Results in Figure 9 show a marked increase in the content of cholesterol in rafts isolated from HepG2 cells treated with HePC for 24 h, when compared with the control, while only a little increase was found in the non-raft fraction. The content of sphingomyelin was not significantly altered after the same period of treatment. As a consequence of these changes, the cholesterol/sphingomyelin ratio was clearly increased, in a time-dependent manner, by HePC treatment, mainly in the raft-enriched fraction (Figure 9).
Discussion
It is widely accepted that synthetic APL derivatives have anti-tumour activity although their anti-proliferative action strongly depends on the cell type and the APL under study (van Blitterswijk and Verheij, 2008). Thus, IC50 values from 1 µM up to more than 150 µM have been reported in different cell lines for the different APLs. The IC50 values obtained in our study, related to the anti-proliferative activity exhibited by APLs in hepatoma HepG2 cells, agree well with those previously reported by other authors in several cell lines. For example, Jendrossek et al. (2002) have reported that in the U87 MG and C6 cell lines, ErPC concentrations of 80–90 µM decrease cell number up to 50% of controls after 72 h of treatment, while HePC needed concentrations higher than 150 µM to achieve the same anti-proliferative action. Similarly, IC50 values for perifosine after 24 h were higher than 40 µM in Ishikawa and HEC 1A human endothelial cancer cell lines (Engel et al., 2008). Few reports have explored the effects of several APLs on the same cell line to compare their inhibitory potency. Wieder et al. (1995) comparing edelfosine and HePC in MDCK cells concluded that HePC required twice the concentration, compared with edelfosine, to produce the same inhibitory effect on cell growth. Results in our study demonstrated that in the hepatoma HepG2 cell line, HePC had the lowest anti-proliferative activity while edelfosine presented higher potency in inhibiting cell growth. Due to their chemical structure, APLs easily insert into the membrane thereby causing detergent-like cell lysis at high concentrations. Nevertheless, in our study, the anti-proliferative action of the four APLs was not related to cytolysis, as prolonged incubation of cells with doses up to 50 µM did not cause a significant release of LDH from the cells into the culture medium.
Of particular interest in our previous studies were the effects caused by HePC upon cholesterol metabolism. We have previously reported that long-term HePC treatment of HepG2 cells causes a marked enhancement in cholesterol synthesis (Jiménez-López et al., 2006) that has been related to an impairment in the arrival of cholesterol at the ER (Carrasco et al., 2008; Marco et al., 2009). To our knowledge, there are no data concerning the effects of other APLs on the homeostasis of cholesterol and thus results presented in this paper demonstrate for the first time that, as observed for HePC, other APLs such as edelfosine, ErPC and perifosine increase the de novo synthesis and uptake of cholesterol, and inhibit cholesterol esterification, as a consequence of an impaired cholesterol transport from the plasma membrane to the ER in the HepG2 cells. In summary, our results demonstrate that all the APLs assayed affected cell growth and morphology similarly and also altered cholesterol traffic and homeostasis. It must be however stated that, among the four APLs tested, edelfosine exerted the greatest effects on the different parameters measured in HepG2 cells.
Presumably, in the presence of APL, the translocated SREBP2 remained active so as to stimulate gene expression of potential targets in the HepG2 cells. Thus, as a likely consequence of the APL-triggered translocation of SREBP2 transcription factor to the nucleus, gene expression of several SREBP2 targets appeared to be simultaneously induced by HePC or edelfosine treatment. Remarkably, some pivotal genes involved in the cholesterogenic pathway were up-regulated by HePC and edelfosine, for example HMGCR, FDPS and FDFT1. Likewise, transcript levels of the SREBP2-responsive LDLR were also high after HePC and edelfosine treatment in a time-dependent manner. Incubation with HePC was previously reported to increase the level of expression of HMGCR in a time-dependent manner in the HepG2 cell line (Carrasco et al., 2008). It is noticeable that exposure of these cells to any of the assayed APLs increased the expression of the SREBP2-responsive proteins HMGCR and LDLR after 24 h treatment, compared with the untreated cells (Figure 6). These results provide a mechanistic basis for the enhancement of cholesterol synthesis and LDL-cholesterol uptake after exposure of the cells to APLs.
Lipid biosynthesis may alter membrane lipid composition affecting lipid microdomain distribution or the amount of plasma membrane rafts. It has been previously reported that alteration in cholesterol biosynthesis affects lipid raft structure and function (Sánchez-Wandelmer et al., 2009). Therefore, we extended our studies to analyse the possible effect of HePC exposure on the lipid composition of membrane raft and non-raft domains. In the present work, we have obtained raft and non-raft fractions from HepG2 cells by using a free-detergent method; these non-detergent lipid rafts were shown to be enriched in both cholesterol and sphingomyelin, relative to bulk plasma membrane. As above mentioned, HePC exerted an important cholesterogenic effect and we measured the effect of this action on cholesterol distribution between the different membrane domains. Our results revealed higher cholesterol content in raft fractions isolated from HePC-treated cells, compared with control cells, while sphingomyelin levels practically did not change. These effects are of considerable interest because cholesterol is assumed to be a critical stabilizing component in several types of lipid microdomains (Bakht et al., 2007; Pike, 2009). For instance, depletion of cholesterol by methyl-β-cyclodextrin results in raft disruption and subsequent malfunction of numerous signal transduction pathways (Gajate et al., 2009; Park et al., 2009). Interestingly, enrichment of the membrane with cholesterol also destabilizes membrane rafts (Matkó and Szöllösi, 2005).
As cholesterol and sphingomyelin content are critical for the integrity and functionality of membrane lipid rafts, the disturbance of the cholesterol/ sphingomyelin ratio could alter signalling pathways associated to these membrane domains. Although further studies must be carried out, the changes induced by APLs on lipid composition of membrane rafts, in part due to alterations in the cholesterol content of cells, as indicated in this work, can destabilize such rafts and undoubtedly modulate their properties, thereby disturbing cell function in tumour cells.
Acknowledgments
We thank Xiomara Gálvez for her technical support. This work was aided by a Grant from the Carlos III Institute of Health of the Spanish Ministry of Health (PI061268). Pablo Ríos-Marco is the recipient of a Fellowship funded by the Spanish Ministry of Science and Innovation.
Glossary
Abbreviations:
- APL
alkylphospholipid
- CT
CTP : phosphocholine cytidylyltransferase
- ER
endoplasmic reticulum
- ErPC
erucylphosphocholine
- FBS
fetal bovine serum
- FDFT1
farnesyl diphosphate farnesyltransferase-1
- FDPS
farnesyl diphosphate synthase
- HePC
hexadecylphosphocholine
- HMGCR
3-hydroxy-3-methylglutaryl-CoA reductase
- HMGCS1
HMG-CoA synthase-1
- HRP
horseradish peroxidase
- LDH
lactate dehydrogenase
- LDLR
low-density lipoprotein receptor
- MEM
minimal essential medium
- PCR
polymerase chain reaction
- SREBP
sterol regulatory element-binding protein
Conflict of interest
None disclosed.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakht O, Pathak P, London E. Effect of the structure of lipids favoring disordered domain formation on the stability of cholesterol-containing ordered domains (lipid rafts): identification of multiple raft-stabilization mechanisms. Biophys J. 2007;93:4307–4318. doi: 10.1529/biophysj.107.114967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt G, Saint-Pierre-Chazalet M, Loiseau PM. Cellular transport and lipid interactions of miltefosine. Curr Drug Metab. 2009;10:247–255. doi: 10.2174/138920009787846332. [DOI] [PubMed] [Google Scholar]
- Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- Bennett MK, Seo YK, Datta S, Shin DJ, Osborne TF. Selective binding of sterol regulatory element-binding protein isoforms and co-regulatory proteins to promoters for lipid metabolic genes in liver. J Biol Chem. 2008;283:15628–15637. doi: 10.1074/jbc.M800391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des. 2008;14:2061–2074. doi: 10.2174/138161208785294636. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carrasco MP, Jiménez-López JM, Segovia JL, Marco C. Hexadecylphosphocholine interferes with the intracellular transport of cholesterol in HepG2 cells. FEBS J. 2008;275:1675–1686. doi: 10.1111/j.1742-4658.2008.06322.x. [DOI] [PubMed] [Google Scholar]
- Engel JB, Honig A, Schönhals T, Weidler C, Häusler S, Krockenberger M, et al. Perifosine inhibits growth of human experimental endometrial cancers by blockade of AKT phosphorylation. Eur J Obstet Gynecol Reprod Biol. 2008;141:64–69. doi: 10.1016/j.ejogrb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Gajate C, Gonzalez-Camacho F, Mollinedo F. Lipid raft connection between extrinsic and intrinsic apoptotic pathways. Biochem Biophys Res Commun. 2009;380:780–784. doi: 10.1016/j.bbrc.2009.01.147. [DOI] [PubMed] [Google Scholar]
- Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrossek V, Hammersen K, Erdlenbruch B, Kugler W, Krügener R, Eibl H, et al. Structure-activity relationships of alkylphosphocholine derivatives: antineoplastic action on brain tumor cell lines in vitro. Cancer Chemother Pharmacol. 2002;50:71–79. doi: 10.1007/s00280-002-0440-8. [DOI] [PubMed] [Google Scholar]
- Jiménez-López JM, Carrasco MP, Segovia JL, Marco C. Hexadecylphosphocholine inhibits phosphatidylcholine biosynthesis and the proliferation of HepG2 cells. Eur J Biochem. 2002;269:4649–4655. doi: 10.1046/j.1432-1033.2002.03169.x. [DOI] [PubMed] [Google Scholar]
- Jiménez-López JM, Carrasco MP, Segovia JL, Marco C. Hexadecylphosphocholine inhibits phosphatidylcholine synthesis via both the methylation of phosphatidylethanolamine and CDP-choline pathways in HepG2 cells. Int J Biochem Cell Biol. 2004;36:153–161. doi: 10.1016/s1357-2725(03)00193-6. [DOI] [PubMed] [Google Scholar]
- Jiménez-López JM, Carrasco MP, Marco C, Segovia JL. Hexadecylphosphocholine disrupts cholesterol homeostasis and induces the accumulation of free cholesterol in HepG2 tumour cells. Biochem Pharmacol. 2006;71:1114–1121. doi: 10.1016/j.bcp.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Lange Y, Steck TL. Quantitation of the pool of cholesterol associated with acyl-CoA:cholesterol acyltransferase in human fibroblasts. J Biol Chem. 1997;272:13103–13108. doi: 10.1074/jbc.272.20.13103. [DOI] [PubMed] [Google Scholar]
- van der Luit AH, Vink SR, Klarenbeek JB, Perrissoud D, Solary E, Verheij M, et al. A new class of anticancer alkylphospholipids uses lipid rafts as membrane gateways to induce apoptosis in lymphoma cells. Mol Cancer Ther. 2007;6:2337–2345. doi: 10.1158/1535-7163.MCT-07-0202. [DOI] [PubMed] [Google Scholar]
- Macdonald JL, Pike LJ. A simplified method for the preparation of detergent-free lipid rafts. J Lipid Res. 2005;46:1061–1067. doi: 10.1194/jlr.D400041-JLR200. [DOI] [PubMed] [Google Scholar]
- Marco C, Jiménez-López JM, Ríos-Marco P, Segovia JL, Carrasco MP. Hexadecylphosphocholine alters nonvesicular cholesterol traffic from the plasma membrane to the endoplasmic reticulum and inhibits the synthesis of sphingomyelin in HepG2 cells. Int J Biochem Cell Biol. 2009;41:1296–1303. doi: 10.1016/j.biocel.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Matkó J, Szöllösi J. Regulatory aspects of membrane microdomain (raft) dynamics in live cells. In: Mattson MP, editor. Membrane Microdomain Signalling: Lipid Rafts in Biology and Medicine. Totowa, NJ: Human Press Inc.; 2005. pp. 15–46. [Google Scholar]
- Park EK, Park MJ, Lee SH, Li YC, Kim J, Lee JS, et al. Cholesterol depletion induces anoikis-like apoptosis via FAK down-regulation and caveolae internalization. J Pathol. 2009;218:337–349. doi: 10.1002/path.2531. [DOI] [PubMed] [Google Scholar]
- Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50(Suppl):S323–S328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakotomanga M, Blanc S, Gaudin K, Chaminade P, Loiseau PM. Miltefosine affects lipid metabolism in Leishmania donovani promastigotes. Antimicrob Agents Chemother. 2007;51:1425–1430. doi: 10.1128/AAC.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Wandelmer J, Dávalos A, Herrera E, Giera M, Cano S, de la Peña G, et al. Inhibition of cholesterol biosynthesis disrupts lipid raft/caveolae and affects insulin receptor activation in 3T3-L1 preadipocytes. Biochim Biophys Acta. 2009;1788:1731–1739. doi: 10.1016/j.bbamem.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Saraiva VB, Gibaldi D, Previato JO, Mendonça-Previato L, Bozza MT, Freire-De-Lima CG, et al. Proinflammatory and cytotoxic effects of hexadecylphosphocholine (miltefosine) against drug-resistant strains of Trypanosoma cruzi. Antimicrob Agents Chemother. 2002;46:3472–3477. doi: 10.1128/AAC.46.11.3472-3477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R, Takano T. Regulation of intracellular cholesterol metabolism. Cell Struct Funct. 1995;20:421–427. doi: 10.1247/csf.20.421. [DOI] [PubMed] [Google Scholar]
- Seifert K, Duchêne M, Wernsdorfer WH, Kollaritsch H, Scheiner O, Wiedermann G, et al. Effects of miltefosine and other alkylphosphocholines on human intestinal parasite Entamoeba histolytica. Antimicrob Agents Chemother. 2001;45:1505–1510. doi: 10.1128/AAC.45.5.1505-1510.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto J, Soto P. Oral miltefosine to treat leishmaniasis. Biomedica. 2006;26(Suppl 1):207–217. [PubMed] [Google Scholar]
- Vink SR, van Blitterswijk WJ, Schellens JH, Verheij M. Rationale and clinical application of alkylphospholipid analogues in combination with radiotherapy. Cancer Treat Rev. 2007a;33:191–202. doi: 10.1016/j.ctrv.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Vink SR, van der Luit AH, Klarenbeek JB, Verheij M, van Blitterswijk WJ. Lipid rafts and metabolic energy differentially determine uptake of anti-cancer alkylphospholipids in lymphoma versus carcinoma cells. Biochem Pharmacol. 2007b;74:1456–1465. doi: 10.1016/j.bcp.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Walochnik J, Duchêne M, Seifert K, Obwaller A, Hottkowitz T, Wiedermann G, et al. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob Agents Chemother. 2002;46:695–701. doi: 10.1128/AAC.46.3.695-701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieder T, Haase A, Geilen CC, Orfanos CE. The effect of two synthetic phospholipids on cell proliferation and phosphatidylcholine biosynthesis in Madin-Darby canine kidney cells. Lipids. 1995;30:389–393. doi: 10.1007/BF02536296. [DOI] [PubMed] [Google Scholar]


