Abstract
Background and purpose:
Cysteinyl leukotrienes (CysLTs) have been implicated in the pathophysiology of inflammatory and cardiovascular disorders. Their actions are mediated by CysLT1 and CysLT2 receptors. Here we report the discovery of 3-({[(1S,3S)-3-carboxycyclohexyl]amino}carbonyl)-4-(3-{4-[4-(cyclo-hexyloxy)butoxy]phenyl}propoxy) benzoic acid (HAMI3379), the first potent and selective CysLT2 receptor antagonist.
Experimental approach:
Pharmacological characterization of HAMI3379 was performed using stably transfected CysLT1 and CysLT2 receptor cell lines, and isolated, Langendorff-perfused, guinea pig hearts.
Key results:
In a CysLT2 receptor reporter cell line, HAMI3379 antagonized leukotriene D4- (LTD4-) and leukotriene C4- (LTC4-) induced intracellular calcium mobilization with IC50 values of 3.8 nM and 4.4 nM respectively. In contrast, HAMI3379 exhibited very low potency on a recombinant CysLT1 receptor cell line (IC50 > 10 000 nM). In addition, HAMI3379 did not exhibit any agonistic activity on both CysLT receptor cell lines. In binding studies using membranes from the CysLT2 and CysLT1 receptor cell lines, HAMI3379 inhibited [3H]-LTD4 binding with IC50 values of 38 nM and >10 000 nM respectively. In isolated Langendorff-perfused guinea pig hearts HAMI3379 concentration-dependently inhibited and reversed the LTC4-induced perfusion pressure increase and contractility decrease. The selective CysLT1 receptor antagonist zafirlukast was found to be inactive in this experimental setting.
Conclusions and implications:
HAMI3379 was identified as a potent and selective CysLT2 receptor antagonist, which was devoid of CysLT receptor agonism. Using this compound, we showed that the cardiac effects of CysLTs are predominantly mediated by the CysLT2 receptor.
Keywords: CysLT2 receptor antagonist, cysteinyl leukotrienes, CysLT1 receptor, CysLT2 receptor
Introduction
Cysteinyl leukotrienes (CysLTs), namely leukotriene C4 (LTC4), leukotriene D4 (LTD4) and leukotriene E4 (LTE4), are the products of the 5-lipoxygenase pathway in arachidonic acid metabolism, and are predominantly synthesized by inflammatory cells, such as polymorphonuclear leukocytes, macrophages and mast cells (Funk, 2001). CysLTs have been implicated in a number of pathological inflammatory diseases, including asthma and allergic rhinitis (Drazen et al., 1999; Sharma and Mohammed, 2006; Capra et al., 2007; Riccioni et al., 2007). In addition, CysLTs are also involved in the pathophysiology of different cardiovascular disorders, including atherosclerosis, unstable angina pectoris and acute myocardial infarction. CysLTs reduce coronary blood flow, myocardial contractility and cardiac output without affecting the heart rate. They also increase vascular permeability, which ultimately leads to oedema formation (Burke et al., 1982; Letts, 1987; Fauler and Frölich, 1989; Funk, 2005; Bäck, 2009). In patients with ischemic heart disease and acute myocardial infarction, elevated LTC4 plasma levels and increased urinary LTE4 excretion have been observed (Carry et al., 1992; Takase et al., 1996). Human atherosclerotic coronary arteries have been shown to be hyperreactive in response to CysLTs, and all components of the 5-lipoxygenase pathway have been detected in the arterial walls of patients with various stages of atherosclerosis (Allen et al., 1993, 1998; Spanbroek et al., 2003). Variants of the gene encoding 5-lipoxygenase activating protein (FLAP), the key regulator of the CysLT synthetic pathway, have recently been linked to increased leukotriene production and to the pathogenesis of myocardial infarction (Helgadottir et al., 2004).
CysLT effects are mediated by at least two different G protein-coupled receptors (GPCRs), the CysLT1 and the CysLT2 receptor (nomenclature follows Alexander et al., 2009). These receptors were originally defined pharmacologically, based on their sensitivity to CysLT1 receptor-specific antagonists (Coleman et al., 1995). Both receptors have been isolated and functionally characterized. Activation of both receptors is coupled to intracellular calcium mobilization. Therefore, the Ca2+-sensitive photoprotein aequorin and fluorescent calcium indicator dyes have been used to study functional receptor activation in mammalian cell lines in vitro (Lynch et al., 1999; Sarau et al., 1999; Heise et al., 2000; Nothacker et al., 2000; Takasaki et al., 2000; Hui et al., 2001; Ogasawara et al., 2002). Recently, a third putative CysLT receptor subtype, formerly GPR17, has been identified (Ciana et al., 2006). In addition, a CysLT receptor subtype preferentially activated by LTE4 has also been described (Maekawa et al., 2008).
The most studied classes of CysLT receptor antagonists are CysLT1 receptor selective antagonists such as montelukast (Singulair™, Merck & Co, Whitehouse Station, NJ, USA), zafirlukast (Accolate™, AstraZeneca, London, UK) and pranlukast (Onon™, Ono Pharmaceutical, Osaka, Japan), which are used clinically for the treatment of bronchial asthma and allergic rhinitis (Drazen et al., 1999; Riccioni et al., 2007). The only CysLT2 receptor antagonist identified so far is the LTE4 analogue BAY u9773. However, BAY u9773 has been reported as a dual CysLT1 and CysLT2 receptor antagonist and as a partial agonist at the CysLT2 receptor (Labat et al., 1992; Tudhope et al., 1994).
CysLT2 receptors are strongly expressed in cardiovascular tissues. Cardiac expression of the CysLT2 receptor could be detected throughout the entire human heart, including ventricles, atrium, septum, apex and Purkinje fiber cells, as well as in vascular endothelial and smooth muscle cells (Heise et al., 2000; Nothacker et al., 2000; Takasaki et al., 2000; Hui et al., 2001; Kamohara et al., 2001; Moos et al., 2008). In contrast, expression of the CysLT1 receptor within the cardiovascular system is barely detectable (Lynch et al., 1999; Sarau et al., 1999; Kamohara et al., 2001). As CysLT2 receptors are highly expressed in heart and blood vessels, a role for this receptor in the pathogenesis of various cardiovascular diseases might be anticipated. In a study using CysLT2 receptor-deficient mice, a function for the CysLT2 receptor in inflammatory responses and pulmonary fibrosis could be demonstrated (Beller et al., 2004). However, the cardiovascular phenotype of these knockout mice was not further characterized. Using CysLT2 receptor transgenic, as well as knockout mice, a role for the CysLT2 receptor in vascular permeability and myocardial ischemia/reperfusion injury has recently been shown (Hui et al., 2004; Jiang et al., 2008; Moos et al., 2008).
Therefore, CysLT2 receptor antagonists are very desirable as both pharmacological tools to probe the CysLT pathway and as potential therapeutic agents. However, the characterization of the (patho)physiological role of the CysLT2 receptor is currently hampered by the fact that no selective CysLT2 receptor antagonists are available. We report here the identification of HAMI3379, a potent and selective CysLT2 receptor antagonist that does not exhibit agonistic activity at CysLT receptors. Using this compound, we showed that the effects of CysLTs on myocardial contractility and coronary blood flow were mediated by the CysLT2 receptor.
Methods
Characterization of recombinant CysLT1 and CysLT2 receptor cell lines
Full-length DNAs encoding human CysLT1 and CysLT2 receptors were amplified from human genomic DNA by PCR. A Kozak consensus sequence was artificially introduced, both PCR fragments were cloned into mammalian expression vectors (Invitrogen, Carlsbad, CA, USA), and receptor sequences were verified by sequencing. Both constructs were transfected by electroporation into recombinant apoaequorin-expressing Chinese hamster ovary (CHO) cells. After selection with geneticin (G418), positive clones were identified by stimulation with LTD4 and purified twice by the limited dilution technique.
Cells were cultured at 37°C and 5% CO2 in DMEM/F12 with Glutamax supplemented with 10% (v/v) inactivated fetal calf serum, 20 mM HEPES, 1.4 mM sodium pyruvate, 1.8 mM sodium bicarbonate, 50 U·mL−1 penicillin, 50 µg·mL−1 streptomycin and 1 mg·mL−1 geneticin. Confluent cultures were passaged using trypsin. All cell culture reagents were obtained from Invitrogen.
Measurement of agonist-induced calcium mobilization was performed according to Heise et al. (2000). Agonist testing was performed on opaque 384 well microtiter plates (MTP). Two thousand five hundred cells per well were cultured to confluence for 2 days. After removal of the cell culture medium, cells were loaded for 3 h at 37°C and 5% CO2 with 5 µg·mL−1 coelenterazine in Ca2+-free Tyrode (130 mM NaCl, 5 mM KCl, 20 mM HEPES, 1 mM MgCl2, 4.8 mM NaHCO3, pH 7.4). Test compounds were applied for 5 min in Ca2+-free Tyrode. Measurement of the aequorin luminescence was started for 1 min immediately before adding Ca2+ ions (3 mM final concentration).
Characterization of CysLT receptor antagonists by luminescence measurements
For the characterization of receptor antagonists, cells were loaded with coelenterazine in Tyrode containing 2 mM CaCl2 for 3 h. Cells were incubated with test compounds for 5 min at room temperature prior to the addition of 10 nM LTD4 (CysLT1 and CysLT2) or 10 nM LTC4 (CysLT2). Immediately before agonist addition, measurement of the aequorin luminescence was started for 1 min by using a charge-coupled device (CCD) camera (Hamamatsu Corporation, Shizuoka, Japan) in a light tight box.
Radioligand binding studies
CHO cells stably expressing the human CysLT1 or CysLT2 receptor were harvested after reaching 80% confluence. The cells were dispersed in buffer A containing 20 mM HEPES, pH 7.4, 5 mM MgCl2, 10 µg·mL−1 leupeptin, 10 µg·mL−1 pepstatin, 1 µg·mL−1 aprotinin, 1 mg·mL−1 pefabloc and 0.5 mg·mL−1 EDTA and subsequently homogenized by using an Ultra Turrax homogenizer (IKA, Staufen, Germany) for 10 min at 4°C. After centrifugation of the homogenate at 1000×g for 10 min at 4°C, the resulting supernatant was again centrifuged at 20 000×g for 30 min at 4°C. The supernatant was discarded, and the pellet was resuspended in binding buffer (20 mM HEPES, pH 7.4, 20 mM CaCl2, 1 mM cysteine, 0.1% BSA) at a protein concentration of 1 mg mL−1. For the competition studies, membrane preparations (300 µg mL−1) were incubated with 200 pM [3H]-LTD4 in binding buffer together with the test compounds at room temperature for 1 h. The specific activity of [3H]-LTD4 used for the binding assay was 180 Ci mmol−1. The [3H]-LTD4 concentration of 200 pM was chosen according to Heise et al. (2000) and Frey et al. (1993). Under these experimental conditions, approximately 5–10% of [3H]-LTD4 added to the incubation was bound. Non-specific binding of [3H]-LTD4 was determined in the presence of 1 µM unlabelled LTD4. Specific binding was linear with respect to radioligand and protein concentration, and represented 85–90% of the total [3H]-LTD4 binding to the membranes. After termination of the reaction by addition of washing buffer (10 mM HEPES, pH 7.4, 0.01% BSA), the mixture was centrifuged at 10 000×g for 10 min at 4°C. The resulting supernatant was removed and the remaining radioactivity in the pellet was measured by liquid scintillation counting.
Characterization of guinea pig CysLT1 and CysLT2 receptor expression by quantitative real-time PCR
Quantitative TaqMan analysis was performed using the ABI PRISM 7900HT sequence detection system (Applied Biosystems, Foster City, CA, USA). Guinea pig tissue mRNA probes were obtained from male guinea pigs (Crl:HA, Charles River, Sulzfeld, Germany) weighing 325–375 g. Due to the limited tissue size, coronary arteries from three animals were pooled. Genomic DNA was removed by DNAse digestion, and mRNAs were reverse transcribed using random hexamers. Comparable probe efficencies were assured by titration of genomic DNA. Normalization was performed using the house-keeping gene L32 as control, and relative expression was calculated using the formula: relative expression = 2(18-(Ct(probe)-Ct(L32))). The parameter Ct is defined as the threshold cycle number at which the amplification plot passed a fixed threshold above baseline. The resulting expression is given in arbitrary units.
The following primers and fluorescent probes were used: CysLT1: forward primer: 5′-TGGCTGATCTATTGTGTGTGTG-3′; probe: 5′-(FAM)ACACTGCCTCTCCGTGTGGCCTATT(TAMRA)-3′; reverse primer: 5′-AGCCAAATGCCTTTGTGAAC-3′; CysLT2: forward primer: 5′-TGCTGAGTGTGGTGCGTTTT-3′; probe: 5′-(FAM)TGGCTACTGTTCACCCCTTCCGGCT(TAMRA)-3′; reverse primer: 5′-CTGAAGCTGGTGACGTGGAG-3′; L32: forward primer: 5′-TGCTCACAATGTCTCCTCCA-3′; probe: 5′-(FAM)CAAGGCCATTGTGGAACGAGCAG(TAMRA)-3′; reverse primer: 5′-GTGACTCGGATGGCTAGCTG-3′
Effects of LTC4, HAMI3379 and zafirlukast on Langendorff-perfused guinea pig hearts
All animal care and experimental protocols complied with the German Law for the Protection of Laboratory animals and were approved by the local Laboratory Animals Science and Welfare Council. Hearts from male guinea pigs (Dunkin Hartley, 200–250 g, from Charles River) were Langendorff perfused according to Letts and Piper (1982) at 37°C with a non-recirculating system. The perfusion medium was a filtered Krebs–Henseleit solution containing 10 mM glucose and 1.8 mM CaCl2, equilibrated with O2+ CO2 (95% + 5%), to give a pH of 7.4 and a pO2 of 650–700 mmHg. Perfusion was performed at a constant flow rate of 10 mL·min−1. A latex balloon filled with saline and connected to a pressure transducer (TBD-1222, FMI GmbH, Seeheim, Germany) was inserted into the left ventricular cavity to measure the isovolumetric contractions of the left ventricle. Perfusion pressure was recorded by a second pressure transducer at the aortic cannula. Drug solutions were infused into the aortic cannula at a rate of 1% of the total flow rate. After an equilibration period, LTC4 was infused with increasing concentration steps for 20 min. For the characterization of receptor antagonists, 10 nM LTC4 was given continuously for the duration of the experiment. The antagonists were additionally infused with increasing concentration steps for 20 min.
Data analysis
The GraphPad Prism Software (version 4.02, GraphPad Software Inc., San Diego, CA, USA) was used for curve fitting and calculation of the half-maximal inhibitory concentration (IC50) and of the half-maximal effective concentration (EC50). The IC50 and EC50 values from the calcium mobilization assay were determined from four to six independent experiments performed in quadruplicate. The data from radioligand binding studies were derived from three to five independent experiments. IC50 and EC50 values are given as mean ± SEM.
Materials
HAMI3379 (3-({[(1S,3S)-3-carboxycyclohexyl]amino}carbonyl)-4-(3-{4-[4-(cyclo-hexyloxy)butoxy]phenyl}propoxy) benzoic acid) was synthesized by the chemistry department of Bayer Schering Pharma AG (Wuppertal, Germany) as described (Härter et al., 2004). BAY u9773 (4-({(1R,2E,4E,6Z,9Z)-1-[(1S)-4-carboxy-1-hydroxybutyl] pentadeca-2,4,6,9-tetraen-1-yl}thio) benzoic acid), LTC4 and LTD4 were purchased from Biomol (Hamburg, Germany). Zafirlukast (cyclopentyl{3-[2-methoxy-4-({[(2-methylphenyl)sulfonyl]amino}carbonyl)benzyl]-1-methyl-1H-indol-5-yl}carbamate) was eluted and purified from commercially available tablets.
Results
Characterization of recombinant CysLT1 and CysLT2 receptor cell lines
In the search for antagonists of the CysLT pathway, we identified 3-({[(1S,3S)-3-carboxycyclohexyl]amino}carbonyl)-4-(3-{4-[4-(cyclo-hexyloxy)butoxy]phenyl}propoxy) benzoic acid (HAMI3379) as a potent CysLT2 receptor antagonist (Figure 1A).
Figure 1.
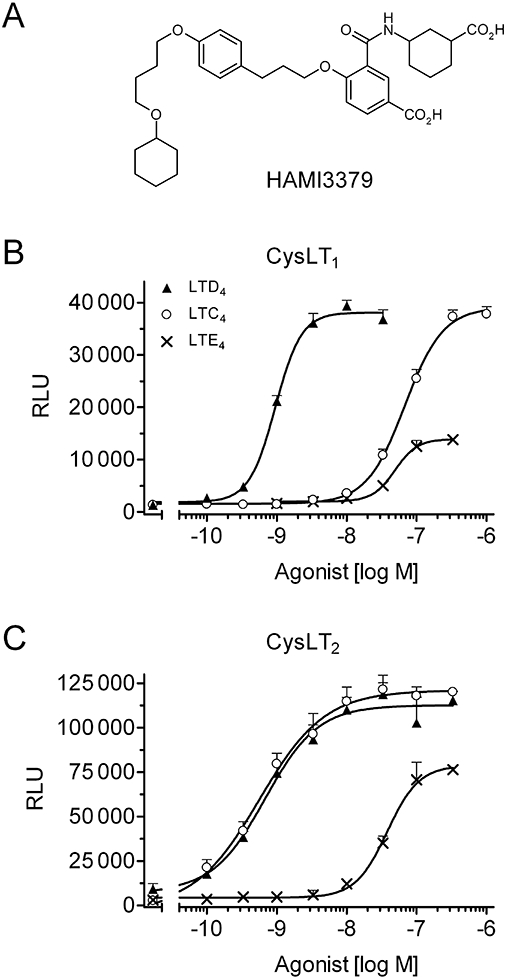
Characterization of recombinant CysLT1 and CysLT2 receptor cell lines. (A) Structure of HAMI3379. (B, C) Agonistic activities of LTC4, LTD4 and LTE4 on reporter cell lines stably transfected with (B) human CysLT1 or (C) human CysLT2 receptors. Results are expressed as relative light units (RLU), and data are presented as mean ± SEM (n= 4).
In order to assess the potency and selectivity of HAMI3379, CHO cells stably transfected with either the human CysLT1, or the human CysLT2 receptor were generated and intracellular calcium mobilization was monitored by aequorin luminescence measurements, according to Heise et al. (2000). For the characterization of our newly generated CysLT1 and CysLT2 receptor reporter cell lines, we used the natural receptor ligands LTC4, LTD4 and LTE4. Both recombinant CysLT receptor cell lines responded in a concentration-dependent manner to the agonists. The rank order for the activation of the CysLT1 receptor cell line was LTD4 > LTC4 > LTE4, with respective EC50 values of 1.12 ± 0.16 nM, 51.2 ± 9.0 nM and 55.9 ± 6.5 nM (Figure 1B). LTE4-mediated luminescence signals reached only ∼30–50% of the maximal stimulation obtained with the full agonists LTD4 and LTC4. The rank order for the activation of the CysLT2 receptor cell line was LTD4∼LTC4 > LTE4. LTD4 and LTC4 stimulated intracellular calcium mobilization in the recombinant CysLT2 receptor cell line with EC50 values of 0.99 ± 0.22 nM and 0.81 ± 0.13 nM respectively (Figure 1C). LTE4 stimulated the CysLT2 receptor cell line with lower potency (EC50= 59.0 ± 6.2 nM; Figure 1C). In addition, LTE4-mediated luminescence signals reached ∼40–70% of the maximal stimulation obtained with the full agonists LTD4 and LTC4.
Characterization of CysLT receptor antagonists by luminescence measurements
Preincubation with HAMI3379 only weakly inhibited 10 nM LTD4-induced (∼EC80–90) calcium release in the reporter cell line expressing the human CysLT1 receptor (IC50 > 10 000 nM; Figure 2A). In contrast, HAMI3379 was found to cause potent inhibition of 10 nM LTD4- and LTC4-induced (∼EC80–90) intracellular calcium release in the CysLT2 receptor cell line, with IC50 values of 3.8 ± 0.6 nM and 4.4 ± 0.7 nM respectively (Figure 2B,C). The structurally distinct CysLT1 receptor antagonist zafirlukast (Krell et al., 1990) potently inhibited LTD4-induced calcium mobilization in the CysLT1 receptor cell line (IC50= 20.6 ± 4.1 nM; Figure 2A). However, zafirlukast was only weakly active on the CysLT2 receptor cell line (IC50∼7000 nM; Figure 2B,C). The dual CysLT1/CysLT2 receptor antagonist and partial CysLT2 receptor agonist BAY u9773 (Labat et al., 1992; Tudhope et al., 1994) was also tested. In the CysLT1 receptor cell line, BAY u9773 inhibited 10 nM LTD4-induced luminescence signals with an IC50 value of ∼5000 nM (Figure 2A). In the CysLT2 receptor cell line, BAY u9773 inhibited 10 nM LTD4- and LTC4-induced calcium signals with IC50 values of 18.3 ± 1.1 nM and 19.5 ± 3.8 nM respectively (Figure 2B,C).
Figure 2.
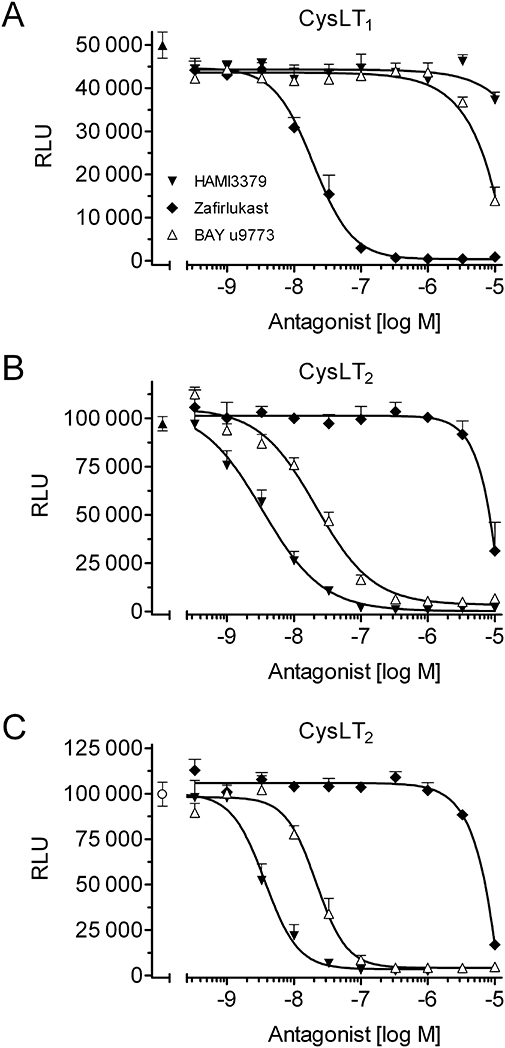
Characterization of CysLT receptor antagonists by luminescence measurements. (A) Inhibition curves of HAMI3379, zafirlukast and BAY u9773 using stably transfected CysLT1 receptor reporter cells challenged with 10 nM LTD4. (B, C) Corresponding inhibition curves using CysLT2 receptor reporter cells challenged with (B) 10 nM LTD4 or (C) 10 nM LTC4. Data are presented as mean ± SEM (n= 4).
Next, we tested if HAMI3379, zafirlukast and BAY u9773 were able to activate CysLT1 and CysLT2 receptors and stimulate intracellular calcium mobilization in the absence of LTC4 or LTD4. HAMI3379 was added to both the CysLT1 (Figure 3A) and the CysLT2 (Figure 3B) receptor cell lines and did not induce any intracellular calcium mobilization when tested up to 10 000 nM. The same results were obtained with the selective CysLT1 receptor antagonist zafirlukast (data not shown). BAY u9773 did not stimulate calcium mobilization when added to the CysLT1 receptor cell line (Figure 3A). In contrast, BAY u9773 stimulated luminescence signals in the CysLT2 receptor cell line with an EC50 value of 69.5 ± 8.3 nM (Figure 3B). The response to BAY u9773 was concentration dependent and reached ∼40–50% of the maximal luminescence signals obtained by stimulation with the full agonists LTC4 and LTD4. In addition, HAMI3379 (1 µM) effectively abolished the luminescence signals stimulated by BAY u9773 (Figure 3B).
Figure 3.
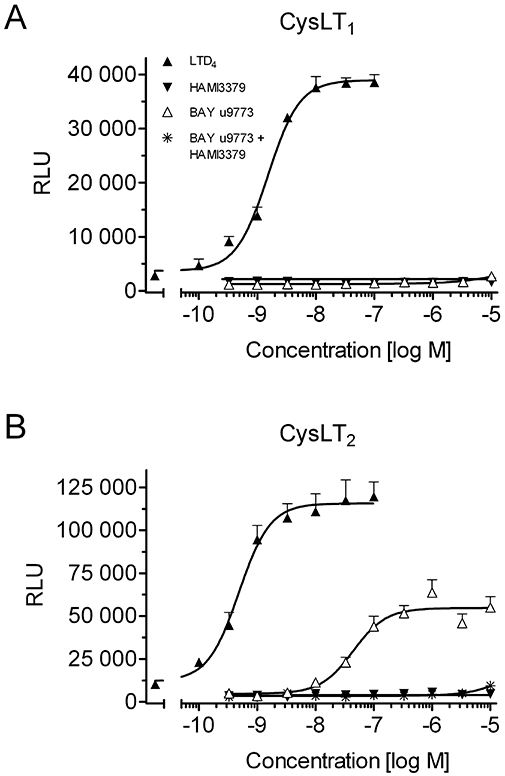
Characterization of CysLT receptor antagonists by luminescence measurements. Intracellular calcium mobilization induced by LTD4, HAMI3379, BAY u9773, and BAY u9773 in the presence of 1 µM HAMI3379 on reporter cell lines stably transfected with (A) human CysLT1 or (B) human CysLT2 receptors. Data are presented as mean ± SEM (n= 4).
Radioligand binding studies
We next performed saturation analysis of [3H]-LTD4 binding to membranes prepared from our recombinant CysLT1 and CysLT2 receptor cell lines. Membranes from CysLT receptor transfected CHO cells specifically bound [3H]-LTD4, whereas membranes prepared from non-transfected control cells did not exhibit specific [3H]-LTD4 binding (data not shown). This effect could be inhibited by increasing concentrations of unlabelled LTD4 (Figure 4A) with IC50 values of 2.9 ± 0.7 nM (CysLT1) and 1.3 ± 0.2 nM (CysLT2) respectively. HAMI3379 displaced [3H]-LTD4 bound to the CysLT2 receptor with an IC50 value of 37.9 ± 14.7 nM (Figure 4B). In contrast, HAMI3379 showed very low affinity to the CysLT1 receptor (IC50 > 30 000 nM).
Figure 4.
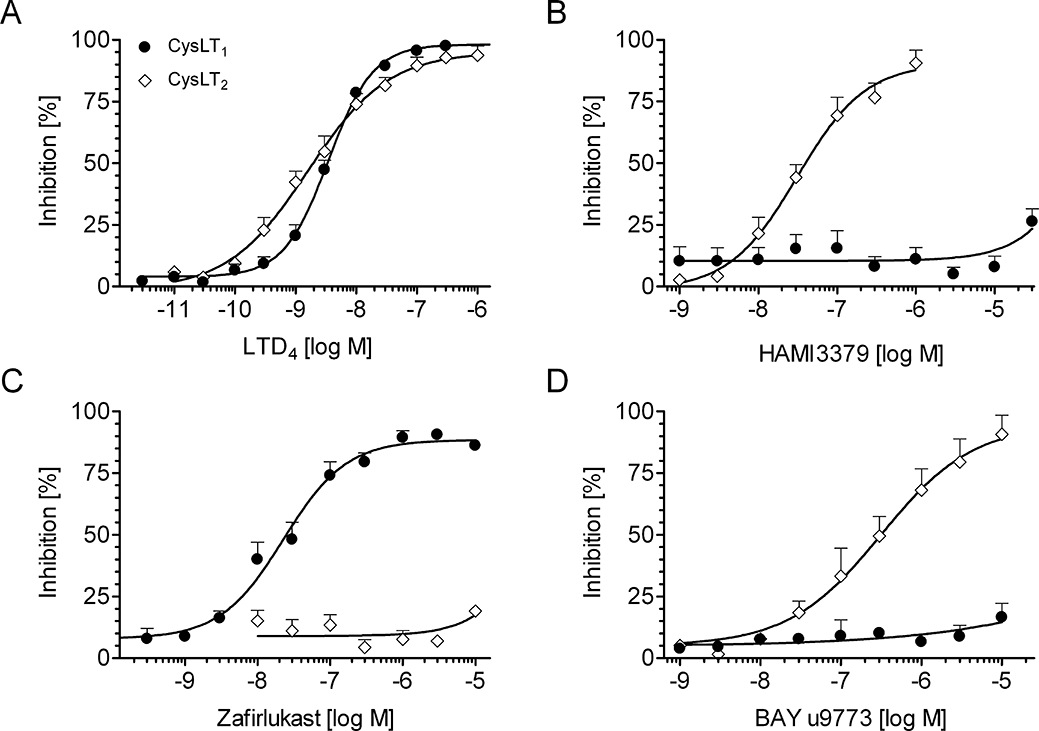
Radioligand binding studies. Effects of (A) LTD4, (B) HAMI3379, (C) zafirlukast and (D) BAY u9773 on competitive binding of [3H]-LTD4 to membranes prepared from the recombinant CysLT1 and CysLT2 receptor cell lines. Data are presented as mean ± SEM (n= 3–5).
The CysLT1 receptor selective antagonist zafirlukast displaced [3H]-LTD4 with an IC50 value of 45.4 ± 14.8 nM at the CysLT1 receptor (Figure 4C), but was found to be inactive at the CysLT2 receptor (IC50 > 10 000 nM). In addition, BAY u9773 inhibited radioligand binding at the CysLT2 receptor with an IC50 value of 496 ± 173 nM. However, BAY u9773 showed only very low activity (IC50 value > 10 000 nM) against membranes prepared from the human CysLT1 receptor cell line (Figure 4D).
Characterization of guinea pig CysLT1 and CysLT2 receptor expression by quantitative real-time RT-PCR
We next studied the expression of CysLT1 and CysLT2 receptors in guinea pig lung and heart tissue by quantitative real-time RT-PCR. As shown in Figure 5, we confirmed high expression levels of the CysLT1 receptor and medium expression levels of the CysLT2 receptor in guinea pig lung. In addition, we were able to detect high expression levels of the CysLT2 receptor in left and right heart ventricle and coronary artery. CysLT1 receptor expression was also detected in guinea pig heart ventricles and coronary artery, however, at significantly lower levels compared to the CysLT2 receptor.
Figure 5.
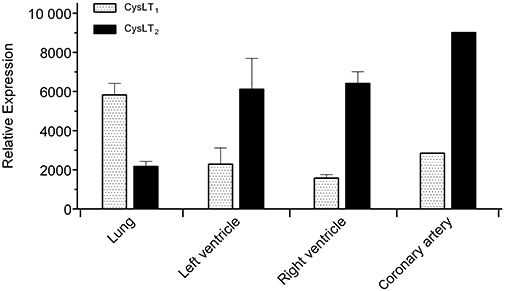
Characterization of CysLT1 and CysLT2 receptor expression in guinea pig tissues by quantitative real-time RT-PCR. Analysis was performed on guinea pig lung and heart tissue cDNAs using specific oligonucleotide probes. Expression levels were normalized to the house-keeping gene L32. Data are presented as mean ± SEM (n= 3).
Effects of LTC4, HAMI3379 and zafirlukast on Langendorff-perfused guinea pig hearts
The effects of LTC4, HAMI3379 and zafirlukast on isolated, Langendorff-perfused, guinea pig hearts were examined under constant flow conditions. Langendorff-perfused hearts were stimulated with LTC4 to preferentially stimulate the CysLT2 receptor and to monitor CysLT2 receptor-mediated cardiac effects. Continuous perfusion of the hearts with increasing concentrations (10−10–10−7 M) of LTC4 resulted in a significant increase in coronary perfusion pressure, a decrease of the left ventricular contractility (dp/dtmax) and of the left ventricular developed pressure (LVDP), whereas the heart rate remained unaffected (Figure 6). These effects persisted for the time of LTC4 perfusion if no antagonist was added. For the characterization of receptor antagonists, 10 nM LTC4 was given continuously for the duration of the experiment. Addition of HAMI3379 concentration-dependently antagonized the LTC4-induced perfusion pressure increase and the negative effects of LTC4 on contractility (Figure 7). Perfusion with HAMI3379 (10−8–10−6 M) in the absence of LTC4 had no influence on perfusion pressure, heart rate or contractility (data not shown). In contrast to HAMI3379, the CysLT1 receptor antagonist zafirlukast (10−8–10−6 M) was not able to antagonize the cardiac effects induced by 10−8 M LTC4 (Figure 8).
Figure 6.
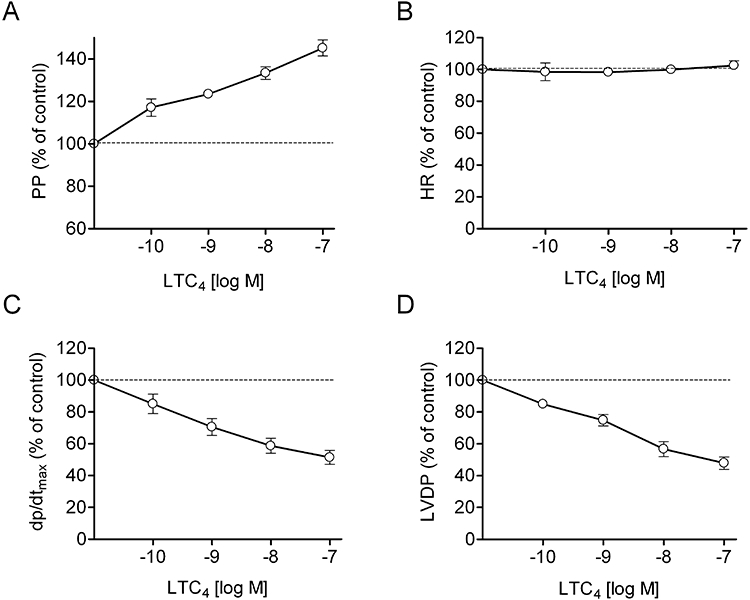
Effects of LTC4 in Langendorff-perfused guinea pig hearts. Effects of LTC4 on (A) perfusion pressure (PP), (B) heart rate (HR), (C) contractility (dp/dtmax) and (D) left ventricular developed pressure (LVDP). Hearts were perfused with increasing concentrations of LTC4. Data are presented as mean ± SEM (n= 16).
Figure 7.
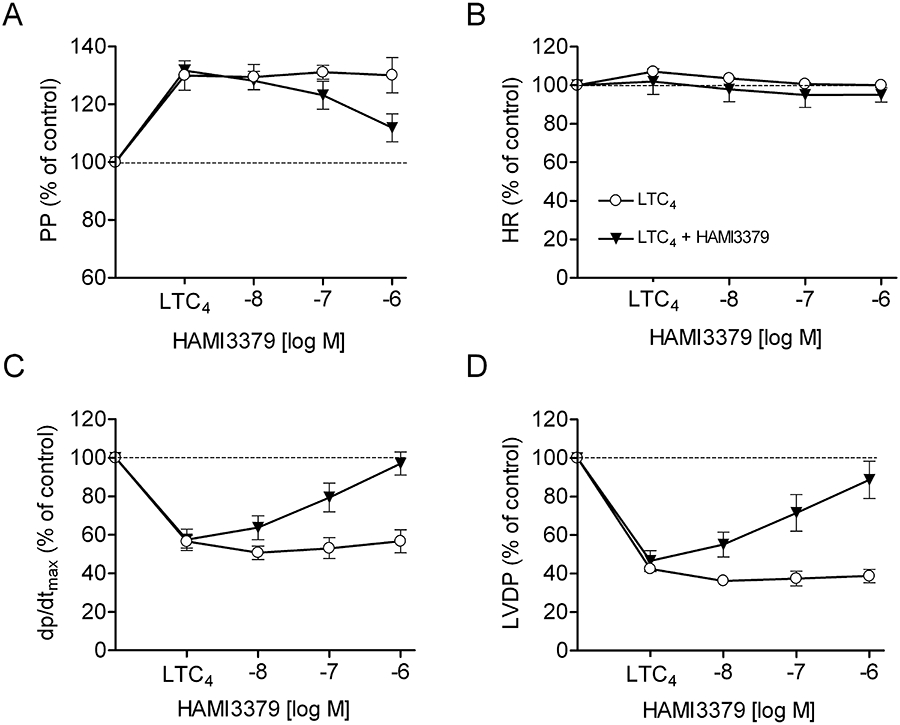
Effects of HAMI3379 in Langendorff-perfused guinea pig hearts. Effects of HAMI3379 on (A) perfusion pressure (PP), (B) heart rate (HR), (C) contractility (dp/dtmax) and (D) left ventricular developed pressure (LVDP) in the presence of LTC4. Hearts were constantly perfused either with 10 nM LTC4 alone or with LTC4 in the presence of increasing concentrations of HAMI3379. Data are presented as mean ± SEM (n= 10).
Figure 8.
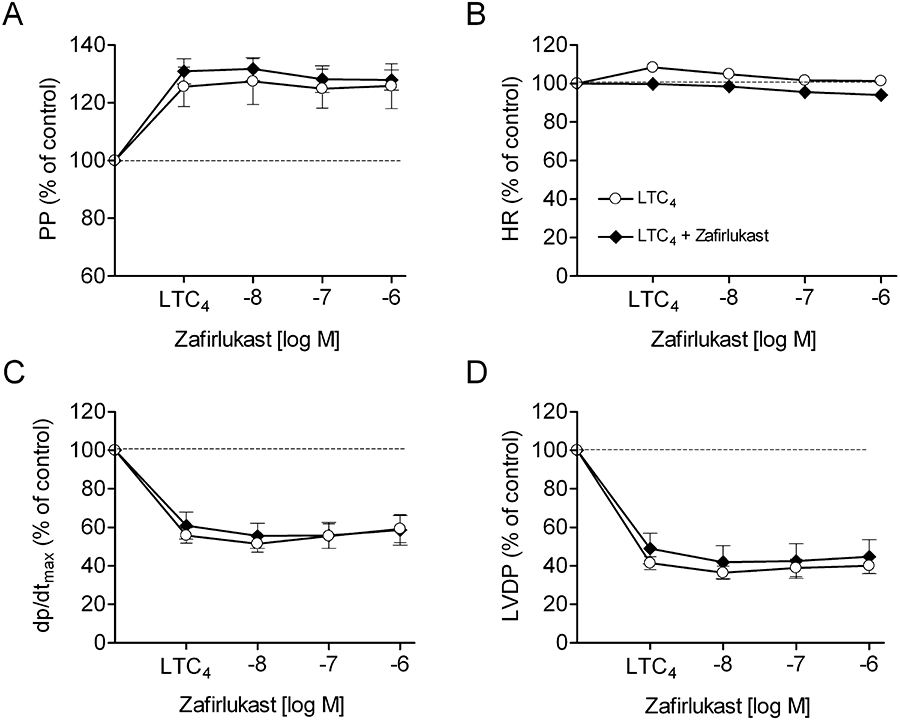
Effects of zafirlukast in Langendorff-perfused guinea pig hearts. Effects of zafirlukast on (A) perfusion pressure (PP), (B) heart rate (HR), (C) contractility (dp/dtmax) and (D) left ventricular developed pressure (LVDP) in the presence of LTC4. Hearts were constantly perfused either with 10 nM LTC4 alone or with LTC4 in the presence of increasing concentrations of zafirlukast. Data are presented as mean ± SEM (n= 5).
Discussion
CysLT effects are mediated by at least two different G protein-coupled receptors, the CysLT1 and the CysLT2 receptor, respectively. Both receptors have been cloned, and their tissue distribution has been characterized. Whereas the relevance of the CysLT1 receptor in inflammatory diseases such as asthma has been extensively investigated, an in-depth characterization of the (patho)physiological role of the CysLT2 receptor has been hindered by the lack of selective CysLT2 receptor antagonists. In this report, we describe the identification and initial pharmacological characterization of HAMI3379, the first potent and selective CysLT2 receptor antagonist, which is devoid of CysLT receptor agonism.
We have characterized the antagonistic and agonistic properties of HAMI3379 on human CysLT1 and CysLT2 receptors, respectively. The results show that HAMI3379 potently and selectively antagonizes LTD4- and LTC4-induced intracellular calcium mobilization and [3H]-LTD4 binding using a recombinant human CysLT2 receptor cell line. The potency of HAMI3379 on the human CysLT1 receptor cell line was found to be very low. In addition, the compound was tested for its ability to activate the two human CysLT receptors. In contrast to the partial CysLT2 receptor agonist BAY u9773 (Labat et al., 1992; Tudhope et al., 1994), HAMI3379 did not induce intracellular calcium mobilization. This is an important finding that clearly differentiates HAMI3379 from BAY u9773. A putative CysLT receptor agonism of HAMI3379 would preclude the use of this compound for the treatment of cardiovascular indications.
We also tried to characterize the activity of HAMI3379 on GPR17, recently identified as a CysLT receptor (Ciana et al., 2006). Unfortunately, we were not able to reproduce GPR17 activation by LTC4 or LTD4 in different expression systems (F. Wunder, unpubl. data). Similar observations have recently been made by other groups (Maekawa et al., 2009).
The LTE4 analogue BAY u9773 antagonized LTD4- and LTC4-mediated luminescence signals and acted as a partial agonist on CysLT2 receptor cells with comparable potencies. Interestingly, BAY u9773 was relatively weakly active on the human recombinant CysLT1 receptor cell line, in both the functional as well as the binding assay. The weak potency of BAY u9773 at the human CysLT1 receptor might be species related, as antagonistic activities against both CysLT receptors were observed in tissues from experimental animals (Tudhope et al., 1994). Similar differences in the antagonistic potency have been reported for pranlukast on human and murine CysLT2 receptors (Ogasawara et al., 2002). In addition, tissues with predominant CysLT1 receptor expression might also express certain (low) levels of the CysLT2 receptor. This would lead to mixed CysLT1/CysLT2 receptor populations and could interfere with the pharmacological characterization of CysLT receptor antagonists. Therefore, cell lines with recombinant receptors offer a valuable method for the pharmacological characterization of isolated CysLT1 and CysLT2 receptors and their respective ligands.
CysLTs have been implicated in the pathophysiology of atherosclerosis and coronary heart disease (Letts, 1987; Fauler and Frölich, 1989; Carry et al., 1992; Allen et al., 1993, 1998; Folco et al., 2000; Funk, 2005). However, the precise roles of CysLTs and their respective receptors in cardiovascular physiology remain largely to be explored. The tissue distribution of the CysLT2 receptor suggests an important function for this receptor in the cardiovascular system. High levels of CysLT2 receptor expression have been detected in the human myocardium and conduction system, as well as in human coronary artery smooth muscle cells and vascular endothelial cells. In contrast, expression of the CysLT1 receptor mRNA is barely detectable in human heart and coronary arteries (Heise et al., 2000; Nothacker et al., 2000; Takasaki et al., 2000; Hui et al., 2001; Kamohara et al., 2001; Sjöström et al., 2003). These published data are in good agreement to the results of our quantitative real-time RT-PCR experiments using guinea pig tissues. We could detect very high expression of the CysLT2 receptor in guinea pig heart ventricles and coronary artery. In contrast, expression of the CysLT1 receptor in these tissues was found to be significantly lower.
According to the expression data, the CysLT2 receptor may mediate cardiovascular CysLT effects, including coronary vasoconstriction, negative inotropy and increased vascular permeability. Therefore, we characterized the effects of the CysLT antagonists HAMI3379 and zafirlukast on LTC4-treated, Langendorff-perfused, guinea pig hearts. The results presented in this report show that HAMI3379 is an effective antagonist of the cardiac effects of LTC4. Since HAMI3379 blocked most (∼90–95%) of the cardiac LTC4 effects, and the CysLT1 receptor selective antagonist zafirlukast was found to be inactive in this experimental setting, the results imply that the cardiac CysLT effects are predominantly mediated by CysLT2 receptors and therefore are in good agreement to the high expression of the CysLT2 receptor in heart and blood vessels. However, according to the available data, an additional contribution of the CysLT1 receptor to cardiac CysLT effects cannot be completely ruled out. In previous studies, it has been shown that CysLT1 receptor antagonists were also able to antagonize cardiac CysLT effects (Burke et al., 1982; McLeod and Piper, 1991; Allen et al., 1993). Therefore, further studies are required to clarify whether cardiac CysLT effects are mediated by the CysLT2 receptor only.
It is tempting to speculate that HAMI3379 might have beneficial effects in various cardiovascular indications, for example during myocardial infarction, by antagonizing the negative inotropy and coronary constriction induced by endogenously synthesized CysLTs. HAMI3379 might also reduce the plasma leakage under these conditions, since the CysLT2 receptor is highly expressed in vascular endothelial cells and has been linked to increased vascular permeability (Sjöström et al., 2003; Beller et al., 2004; Jiang et al., 2008; Moos et al., 2008).
Besides being widely expressed in cardiovascular tissues, the CysLT2 receptor has also been detected in peripheral blood leukocytes, macrophages, mast cells, eosinophils, lymph nodes and spleen, which implies a role for the CysLT2 receptor in immune and inflammatory responses, as well (Heise et al., 2000; Nothacker et al., 2000; Takasaki et al., 2000; Kanaoka and Boyce, 2004).
In summary, we have identified the first potent and selective CysLT2 receptor antagonist, HAMI3379. Using this compound, we could clearly show that the CysLT2 receptor mediates cardiovascular CysLT effects in rodents. HAMI3379 will certainly help to elucidate further the (patho)physiological relevance of the CysLT2 receptor and may offer a novel therapeutic approach for the treatment of cardiovascular and inflammatory diseases.
Acknowledgments
The authors thank Nadia Mastroianni, Tanja Iselt, Birgit Krapp, Holger Schankin, Sandra Schopies, Karin Selbach and Annette Woermann for technical assistance. Special thanks to Dave Wood for critical comments on the manuscript.
Glossary
Abbreviations:
- CysLT
cysteinyl leukotriene
- FLAP
5-lipoxygenase activating protein
- LTC4
leukotriene C4
- LTD4
leukotriene D4
- LTE4
leukotriene E4
Conflicts of interest
None. All authors are employees of Bayer Schering Pharma AG.
References
- Alexander S, Mathie A, Peters J. Guide to receptors and channels (GRAC), 4th edition (2009 revision) Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SP, Dashwood MR, Chester AH, Tadjkarimi S, Collins M, Piper PJ, et al. Influence of atherosclerosis on the vascular reactivity of isolated human epicardial coronary arteries to leukotriene C4. Cardioscience. 1993;4:47–54. [PubMed] [Google Scholar]
- Allen S, Dashwood M, Morrison K, Yacoub M. Differential leukotriene constrictor responses in human atherosclerotic coronary arteries. Circulation. 1998;97:2406–2413. doi: 10.1161/01.cir.97.24.2406. [DOI] [PubMed] [Google Scholar]
- Bäck M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc Drugs Ther. 2009;23:41–48. doi: 10.1007/s10557-008-6140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller TC, Maekawa A, Friend DS, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of the cysteinyl leukotriene 2 receptor in increased vascular permeability and in bleomycin-induced pulmonary fibrosis in mice. J Biol Chem. 2004;279:46129–46134. doi: 10.1074/jbc.M407057200. [DOI] [PubMed] [Google Scholar]
- Burke JA, Levi R, Guo Z-G, Corey EJ. Leukotrienes C4, D4 and E4: effects on human and guinea pig cardiac preparations in vitro. J Pharmacol Exp Ther. 1982;221:235–241. [PubMed] [Google Scholar]
- Capra V, Thompson MD, Sala A, Cole DE, Folco G, Rovati GE. Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med Res Rev. 2007;27:469–527. doi: 10.1002/med.20071. [DOI] [PubMed] [Google Scholar]
- Carry M, Korley V, Willerson JT, Weigelt L, Ford-Hutchinson AW, Tagari P. Increased urinary leukotriene excretion in patients with cardiac ischemia. In vivo evidence for 5-lipoxygenase activation. Circulation. 1992;85:230–236. doi: 10.1161/01.cir.85.1.230. [DOI] [PubMed] [Google Scholar]
- Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25:4615–46127. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Eglen RM, Jones RL, Narumiya S, Shimizu T, Smith WL, et al. Prostanoid and leukotriene receptors: a progress report from the IUPHAR working parties on classification and nomenclature. Adv Prostaglandin Thromboxane Leukot Res. 1995;23:283–285. [PubMed] [Google Scholar]
- Drazen JM, Israel E, O'Byrne MB. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- Fauler J, Frölich JC. Cardiovascular effects of leukotrienes. Cardiovasc Drugs Ther. 1989;3:499–505. doi: 10.1007/BF01865508. [DOI] [PubMed] [Google Scholar]
- Folco G, Rossoni G, Buccellati C, Berti F, Maclouf J, Sala A. Leukotrienes in cardiovascular diseases. Am J Respir Crit Care Med. 2000;161:S112–S116. doi: 10.1164/ajrccm.161.supplement_1.ltta-22. [DOI] [PubMed] [Google Scholar]
- Frey EA, Nicholson DW, Metters KM. Characterization of the leukotriene D4 receptor in dimethylsulphoxide-differentiated U937 cells: comparison with the leukotriene D4 receptor in human lung and guinea-pig lung. Eur J Pharmacol. 1993;244:239–250. doi: 10.1016/0922-4106(93)90149-4. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Funk CD. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Discov. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- Härter M, Ergüden J, Wunder F, Tinel H, Köbberling J, Becker EM, et al. Isophthalic acid derivatives. 2004. Patent WO-2004052839-A 2004-06-24.
- Heise CE, O'Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- Helgadottir A, Manolescum A, Thorleifssonm G, Gretarsdottirm S, Jonsdottirm H, Thorsteinsdottir U, et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- Hui Y, Cheng Y, Smalera I, Jian W, Goldhahn L, Fitzgerald GA, et al. Directed vascular expression of human cysteinyl leukotriene 2 receptor modulates endothelial permeability and systemic blood pressure. Circulation. 2004;110:3360–3366. doi: 10.1161/01.CIR.0000147775.50954.AA. [DOI] [PubMed] [Google Scholar]
- Hui Y, Yang G, Galczenski H, Figueroa DJ, Austin CP, Copeland NG, et al. The murine cysteinyl leukotriene 2 (CysLT2) receptor. cDNA and genomic cloning, alternative splicing, and in vitro characterization. J Biol Chem. 2001;276:47489–47495. doi: 10.1074/jbc.M107556200. [DOI] [PubMed] [Google Scholar]
- Jiang W, Hall SR, Moos MP, Cao RY, Ishii S, Ogunyankin KO, et al. Endothelial cysteinyl leukotriene 2 receptor expression mediates myocardial ischemia-reperfusion injury. Am J Pathol. 2008;172:592–602. doi: 10.2353/ajpath.2008.070834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamohara M, Takasaki J, Matsumoto M, Matsumoto Si, Saito T, Soga T, et al. Functional characterization of cysteinyl leukotriene CysLT(2) receptor on human coronary artery smooth muscle cells. Biochem Biophys Res Commun. 2001;287:1088–1092. doi: 10.1006/bbrc.2001.5695. [DOI] [PubMed] [Google Scholar]
- Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–1510. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- Krell RD, Aharony D, Buckner CK, Keith RA, Kusner EJ, Snyder DW, et al. The preclinical pharmacology of ICI 204,219. A peptide leukotriene antagonist. Am Rev Respir Dis. 1990;141:978–987. doi: 10.1164/ajrccm/141.4_Pt_1.978. [DOI] [PubMed] [Google Scholar]
- Labat C, Ortiz JL, Norel X, Gorenne I, Verley J, Abram TS, et al. A second cysteinyl leukotriene receptor in human lung. J Pharmacol Exp Ther. 1992;263:800–805. [PubMed] [Google Scholar]
- Letts LG. Leukotrienes: role in cardiovascular physiology. Cardiovasc Clin. 1987;18:101–113. [PubMed] [Google Scholar]
- Letts LG, Piper PJ. The actions of leukotrienes C4 and D4 on guinea-pig isolated hearts. Br J Pharmacol. 1982;76:169–176. doi: 10.1111/j.1476-5381.1982.tb09203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KR, O'Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- Maekawa A, Balestrieri B, Austen KF, Kanaoka Y. GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc Natl Acad Sci U S A. 2009;106:11685–11690. doi: 10.1073/pnas.0905364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci U S A. 2008;105:16695–16700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod JD, Piper PJ. Effect of ICI 198,615, SK+F 104,353, MK-571 and CGP45715A on cysteinyl leukotriene-induced responses in guinea-pig heart. Prostaglandins. 1991;41:395–406. doi: 10.1016/0090-6980(91)90008-4. [DOI] [PubMed] [Google Scholar]
- Moos MP, Mewburn JD, Kan FW, Ishii S, Abe M, Sakimura K, et al. Cysteinyl leukotriene 2 receptor-mediated vascular permeability via transendothelial vesicle transport. FASEB J. 2008;22:4352–4362. doi: 10.1096/fj.08-113274. [DOI] [PubMed] [Google Scholar]
- Nothacker HP, Wang Z, Zhu Y, Reinscheid RK, Lin SH, Civelli O. Molecular cloning and characterization of a second human cysteinyl leukotriene receptor: discovery of a subtype selective agonist. Mol Pharmacol. 2000;58:1601–1608. doi: 10.1124/mol.58.6.1601. [DOI] [PubMed] [Google Scholar]
- Ogasawara H, Ishii S, Yokomizo T, Kakinuma T, Komine M, Tamaki K, et al. Characterization of mouse cysteinyl leukotriene receptors mCysLT1 and mCysLT2: differential pharmacological properties and tissue distribution. J Biol Chem. 2002;277:18763–18768. doi: 10.1074/jbc.M109447200. [DOI] [PubMed] [Google Scholar]
- Riccioni G, Bucciarelli T, Mancini B, Di Ilio C, D'Orazio N. Antileukotriene drugs: clinical application, effectiveness and safety. Curr Med Chem. 2007;14:1966–1977. doi: 10.2174/092986707781368522. [DOI] [PubMed] [Google Scholar]
- Sarau HM, Ames RS, Chambers J, Ellis C, Elshourbagy N, Foley JJ, et al. Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol Pharmacol. 1999;56:657–663. doi: 10.1124/mol.56.3.657. [DOI] [PubMed] [Google Scholar]
- Sharma JN, Mohammed LA. The role of leukotrienes in the pathophysiology of inflammatory disorders: is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology. 2006;14:10–16. doi: 10.1007/s10787-006-1496-6. [DOI] [PubMed] [Google Scholar]
- Sjöström M, Johansson AS, Schröder O, Qiu H, Palmblad J, Haeggström JZ. Dominant expression of the CysLT2 receptor accounts for calcium signaling by cysteinyl leukotrienes in human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:e37–e41. doi: 10.1161/01.ATV.0000082689.46538.DF. [DOI] [PubMed] [Google Scholar]
- Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K, et al. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci U S A. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki J, Kamohara M, Matsumoto M, Saito T, Sugimoto T, Ohishi T, et al. The molecular characterization and tissue distribution of the human cysteinyl leukotriene CysLT2 receptor. Biochem Biophys Res Commun. 2000;274:316–322. doi: 10.1006/bbrc.2000.3140. [DOI] [PubMed] [Google Scholar]
- Takase B, Kurita A, Maruyama T, Uehata A, Nishioka T, Mizuno K, et al. Change of plasma leukotriene C4 during myocardial ischemia in humans. Clin Cardiol. 1996;19:198–204. doi: 10.1002/clc.4960190312. [DOI] [PubMed] [Google Scholar]
- Tudhope SR, Cuthbert NJ, Abram TS, Jennings MA, Maxey RJ, Thompson AM, et al. BAY u9773, a novel antagonist of cysteinyl-leukotrienes with activity against two receptor subtypes. Eur J Pharmacol. 1994;264:317–323. doi: 10.1016/0014-2999(94)00485-4. [DOI] [PubMed] [Google Scholar]


