Abstract
Degradation of denuded collagen within adhesive resin-infiltrated dentin is a pertinent problem in dentin bonding. A biomimetic remineralization scheme that incorporates non-classic crystallization pathways of fluidic amorphous nanoprecursors and mesoscopic transformation has been successful in remineralizing resin-free, acid-etched dentin, with evidence of intrafibrillar and interfibrillar remineralization. This study tested the hypothesis that biomimetic remineralization provides a means for remineralizing incompletely infiltrated resin-dentin interfaces created by etch-and-rinse adhesives. The remineralization medium consists of a Portland cement/simulated body fluid that includes polyacrylic acid and polyvinylphosphonic acid biomimetic analogs for amorphous calcium phosphate dimension regulation and collagen targeting. Both interfibrillar and intrafibrillar apatites became readily discernible within the hybrid layers after 2-4 months. In addition, intra-resin apatite clusters were deposited within the porosities of the adhesive resin matrices. The biomimetic remineralization scheme provides a proof-of-concept for the adoption of nanotechnology as an alternative strategy to extend the longevity of resin-dentin bonds.
Keywords: biomineralization, biomimetics, dentin bonding agent, degradation, remineralization, intrafibrillar, nanocomposite
Introduction
Dentin bonding is a unique form of tissue engineering in which a demineralized collagen matrix continuous with the underlying mineralized dentin is created via acid-etching or acidic self-etching adhesives and used as the scaffold for resin infiltration (Tay and Pashley, 2002). The most compelling problem associated with resin-dentin bonds is their limited durability (De Munck et al., 2005), caused partially by water-sorption-induced hydrolysis of the hydrophilic resin components present in these adhesives (Ito et al., 2005), and partially by degeneration of collagen fibrils via endogenous matrix metalloproteinases (MMPs) derived from the demineralized dentin (Pashley et al., 2004).
A biomimetic remineralization scheme based on the interaction of calcium-hydroxide-releasing, set Portland cement with phosphate-containing fluids has recently been developed (Tay and Pashley, 2008). In this scheme, two biomimetic analogs of dentin non-collagenous proteins (Weiner, 2008) are used to create metastable amorphous calcium phosphate nanoprecursors (Eanes, 2001; Weiner et al., 2005) and to bind to dentin collagen (Dahl et al., 1998; He et al., 2005; Gajjeraman et al., 2007), so that the doped collagen can guide the dimension and hierarchy of remineralized apatite crystallites within the collagen matrix (Traub et al., 1989). This remineralization scheme represents an example of the recently recognized non-classic particle-mediated pathway of crystallization (Niederberger and Cölfen, 2006; Xu et al., 2007; Cai and Tang, 2008), wherein fluidic nanoprecursors stabilized by polymer molecules (Olszta et al., 2003; Cölfen, 2007) are transformed into mesocrystalline intermediates (Cölfen and Antonietti, 2005), which eventually fuse to create single microscopic crystals (Tao et al., 2007; Cölfen, 2008). The remineralized dentin created by this biomimetic remineralization scheme demonstrated both interfibrillar and intrafibrillar remineralization, the latter being crucial for maintenance of the mechanical properties of the mineralized dentin matrix (Kinney et al., 2003).
Although collagen degradation in imperfect hybrid layers may be postponed by the application of chlorhexidine as a MMP inhibitor (Carrilho et al., 2007), a zone of resin-sparse demineralized dentin inadvertently remains that is potentially susceptible to creep or cyclic fatigue rupture (Wang and Ker, 1995; Wang et al., 1995) during function. It is also doubtful whether the relatively short substantivity of chlorhexidine (Rosenthal et al., 2004) permits it to be retained permanently within the denuded collagen matrix. Biomimetic remineralization of denuded collagen fibrils appears to be an alternative strategy for the revival of resin-dentin bonds. However, unlike resin-free collagen matrices, amorphous fluidic nanoprecursors may not be able to diffuse through resin-infiltrated interfibrillar spaces to reach the underlying denuded collagen fibrils to establish intrafibrillar remineralization. Thus, the objective of this study was to test the hypothesis that intrafibrillar and interfibrillar remineralization of resin-dentin bonds created by etch-and-rinse adhesives is possible via a Portland cement-based biomimetic remineralization scheme.
Materials & Methods
Dentin Bonding
Twenty recently extracted human third molars were collected after donors’ informed consents were obtained under a protocol reviewed and approved by the Human Assurance Committee of the Medical College of Georgia. A flat dentin surface was prepared perpendicular to the longitudinal axis of each tooth by means of a slow-speed Isomet diamond saw (Buehler Ltd, Lake Bluff, IL, USA) under water-cooling. The bonding surface was further polished with a 400-grit silicon carbide paper attached to create a bonding surface in mid-coronal dentin. Two simplified etch-and-rinse adhesives were used: One-Step (Bisco Inc., Schaumburg, IL, USA), a water-free, acetone-based unfilled adhesive; and Single Bond Plus (3M ESPE, St. Paul, MN, USA), a water-containing, ethanol-based filled adhesive. Ten teeth were randomly assigned to each adhesive. Each dentin surface to be bonded was etched with a 32% phosphoric acid gel (Uni-Etch, Bisco, Inc.) for 15 sec to create a 5- to 8-µm-thick zone of completely demineralized dentin on top of a mineralized dentin base. The etched dentin surface was thoroughly rinsed with de-ionized water, and bonded with the respective adhesive by keeping the etched dentin visibly moist during bonding. After evaporation of the adhesive solvent, each adhesive was polymerized for 20 sec with a quartz-tungsten-halogen light-curing unit with an output intensity of 600 mW/cm2. This was followed by incremental placement of two 2-mm-thick layers of a resin composite that was light-cured separately for 40 sec. Each tooth was then sectioned occluso-gingivally into 1-mm-thick slabs, each containing the resin-dentin interface in the center of the slab.
Remineralization Medium
Type I white Portland cement (major components: 3CaO·SiO2, 2CaO·SiO2, 3CaO·Al2O3, and CaSO4·2H2O; Lehigh Cement Company, Allentown, PA, USA) was mixed with de-ionized water in a water-to-powder ratio of 0.35:1, placed in flexible silicone molds, and allowed to set and age at 100% relative humidity for 1 wk before use (Tay et al., 2007). When hydrated, tricalcium silicate [Eq. (1)] and dicalcium silicate [Eq. (2)] produce an amorphous calcium silicate hydrate (C-S-H) phase and calcium hydroxide, providing the calcium and hydroxyl ions for calcium phosphate precipitation.
| Eq. (1) |
| Eq. (2) |
We prepared a simulated body fluid (SBF) by dissolving 136.8 mM NaCl, 4.2 mM NaHCO3, 3.0 mM KCl, 1.0 mM K2HPO4·3H2O, 1.5 mM MgCl2·6H2O, 2.5 mM CaCl2, and 0.5 mM Na2SO4 in de-ionized water (Kokubo et al., 1990) and adding 3.08 mM sodium azide to prevent bacterial growth. The SBF was buffered to pH 7.4 with 0.1 M Tris Base and 0.1 M HCl and filtered. For biomimetic remineralization, 500 µg/mL of polyacrylic acid (MW 1800; Sigma-Aldrich, St. Louis, MO, USA) and 200 µg/mL of polyvinylphosphonic acid (MW 62,000; Sigma-Aldrich) (Munisamy et al., 2008; Tay and Pashley, 2008) were added to the SBF as biomimetic analogs, with the pH of the latter adjusted to 7.4.
Biomimetic Remineralization
Each experimental specimen slab was placed on top of a set Portland cement block (ca. 1 g) inside a glass scintillation vial. The latter was filled with 15 mL of SBF containing the two biomimetic analogs. The setup for the control specimens was the same, except that the liquid medium was replaced with SBF that did not contain biomimetic analogs. Each glass vial was capped to prevent evaporation of the solution and incubated at 37°C. Experimental specimens were retrieved after 1, 2, 3, and 4 mos (N = 5) for ultrastructural examination of the extent of remineralization. Control specimens were examined at the baseline period (i.e., before immersion) and after 4 mos of immersion in the Portland cement/SBF.
Transmission Electron Microscopy
Following retrieval from the respective solution, the specimen slices were fixed in Karnovsky’s fixative, rinsed in cacodylate buffer, post-fixed in 1% osmium tetroxide, and further processed as previously reported (Tay et al., 1999). Non-demineralized, epoxy-resin-embedded, 90-nm-thick sections were prepared and examined, without further staining, by means of a JEM-1230 transmission electron microscope (JEOL, Tokyo, Japan) operated at 80 kV.
For the control group, two additional specimens were immersed in 50 wt% ammoniacal silver nitrate for silver nanoleakage examination within the dentin hybrid layers, according to our previously reported protocol (Tay et al., 2002). After reduction of the diamine silver ions into metallic silver, the specimens were processed and examined in the manner described above.
Results
Control Specimens
Hybrid layers of One-Step or Single Bond Plus did not remineralize after 4 mos of immersion in Portland cement/SBF without biomimetic analogs. Nanoleakage could be identified from these hybrid layers after 4 mos of immersion in the control medium. In addition, the One-Step adhesive exhibited a porous region in which water eluting from the dentin surface was trapped by light-curing of the adhesive (Tay et al., 2005), with severe nanoleakage beneath the resin composite (Appendix 1).
Experimental Specimens
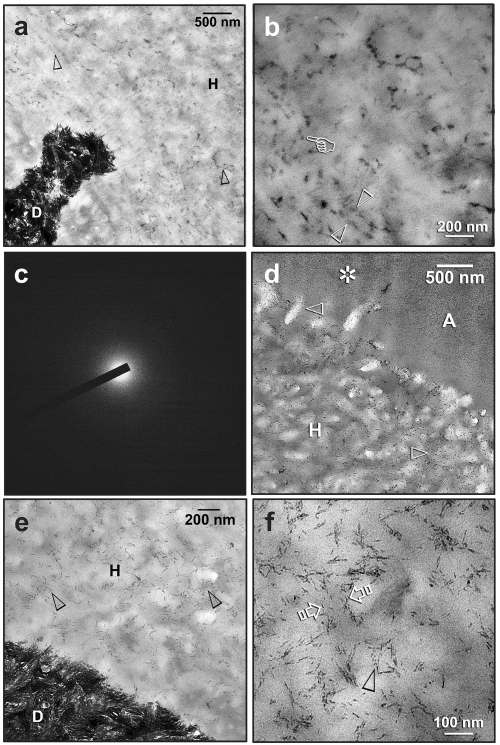
Progressive remineralization of the resin-dentin interfaces was identified for both adhesives. The first ultrastructural changes that occurred at 1 mo were subtle and not discernible below 5000X magnification. A specimen retrieved after 1 mo of biomimetic remineralization showed unusual electron densities along the interfibrillar spaces of the hybrid layer (Fig. 1a). A higher-magnification view (Fig. 1b) revealed electron-dense, amorphous structures that probably represent coalesced fluidic amorphous calcium phosphate nanoprecursors that had penetrated the incompletely infiltrated regions of the hybrid layer. Selected Area Electron Diffraction (SAED) of these amorphous structures resulted in a broad, diffuse pattern without concentric rings (Fig.1c), indicating their non-crystalline status. After 1 mo of remineralization, nanocrystals were observed within the interfibrillar spaces of the hybrid layer (Fig. 1d). Initially, these nanocrystals were much finer than the apatite platelets from the underlying mineralized dentin base (Fig. 1e). They underwent mesoscopic assembly (Niederberger and Cölfen, 2006; Xu et al., 2007) and were eventually transformed into larger needle-shaped crystallites (ca. 20 nm long) within the interfibrillar spaces (Fig. 1f).
Figure 1.
Experimental specimens that have undergone biomimetic remineralization for 1-2 mos revealed two subtle stages of remineralization within hybrid layers, the initial stage of amorphous nanoprecursor coalescence (a-c) and the subsequent initiation of interfibrillar remineralization (d-f). C, composite; A, unfilled adhesive; FA, filled adhesive; H, hybrid layer; D, mineralized dentin. (a) A One-Step specimen, taken at 1 mo after remineralization, showing interfibrillar spaces containing amorphous electron-dense structures (open arrowheads) in the hybrid layer. (b) High-magnification view of (a), showing the amorphous nature of the electron-dense structures (open arrowheads). They probably represent coalesced fluidic amorphous calcium phosphate nanoprecursors (pointer) that had penetrated the incompletely infiltrated regions of the hybrid layer. (c) Selected Area Electron Diffraction (SAED) of these amorphous structures resulted in a broad, diffuse pattern without concentric rings. (d) A One-Step specimen, taken 2 mos after remineralization, showing the presence of very fine needle-shaped nanocrystals (open arrowheads) within interfibrillar spaces of the hybrid layer. These initially formed nanocrystals were smaller than the apatites that are found in natural mineralized dentin. Fine mineral deposits could also be identified within the adhesive layer (asterisk). (e) A Single Bond Plus specimen, taken 2 mos after remineralization, showing evidence of initial interfibrillar remineralization (open arrowheads) at the base of the hybrid layer. (f) A high-magnification view of (e), showing crystallites with the interfibrillar spaces of a collagen fibril (between open arrows). Needle-shaped crystallites (ca. 20 nm long) appeared to have been produced by the fusion of nanocrystals (open arrowhead).
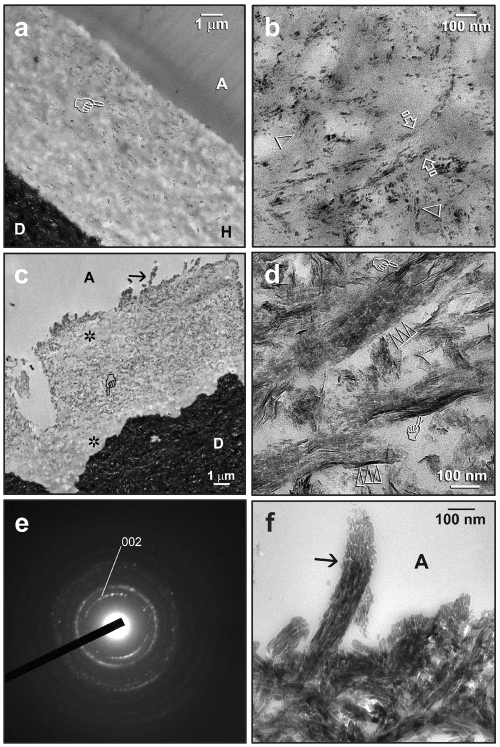
Intrafibrillar remineralization could be identified at lower magnifications (Fig. 2a) after 2-3 mos of remineralization. The earliest evidence of intrafibrillar remineralization was seen depicted as an ordered alignment of nanocrystals within the collagen fibril. These intrafibrillar nanocrystals co-exist with adjacent interfibrillar nanocrystals (Fig. 2b), both of which appeared to be much smaller than the intrafibrillar and inter-fibrillar apatite platelets seen in mineralized intertubular dentin. A more advanced stage of intrafibrillar remineralization (after 3 mos) is depicted in Fig. 2c. Remineralized regions within the hybrid layers could be readily discerned from those non-remineralized, better-resin-infiltrated regions. At a high magnification (Fig. 2d), mesoscopic transformation of the original nanocrystals had probably occurred, with the larger crystallite platelets (ca. 20 nm long) stacked in a repeating and orderly sequence within the collagen fibrils (ca. 100 nm in diameter). The crystallographic c-axes of these electron-dense platelets were well-aligned with the longitudinal axis of the collagen fibrils, thereby producing arc-shaped SAED patterns (Mishima and Sakae, 1986; Müller et al., 2007) along the (002) plane of apatite (Fig. 2e). Along the surface of the hybrid layer, heavily remineralized, shag-carpet-appearing collagen fibrils extended into the adhesive layer (Fig. 2f).
Figure 2.
Transmission electron micrographs showing examples of intrafibrillar remineralization in resin-dentin interfaces after 2-3 mos of biomimetic remineralization. C, composite; A, unfilled adhesive; FA, filled adhesive; H, hybrid layer; D, mineralized dentin. (a) A One-Step specimen taken after 2 mos, with remineralization (pointer) observable at low magnification. (b) High magnification of (a), showing nanocrystals along the interfibrillar spaces (between open arrows). The earliest stage of intrafibrillar remineralization could be recognized as an ordered alignment of nanocrystals (open arrowheads). (c) A One-Step specimen taken after 3 mos, with remineralization occurring in the middle of the hybrid layer and along the surface collagen fibrils (arrow). Regions that were not remineralized (asterisks) were probably better infiltrated with adhesive resin. (d) A high-magnification view of the region depicted by the “pointer” in (c), illustrating a more advanced stage of intrafibrillar remineralization. Crystallite platelets (ca. 20 nm long) were stacked in a repeating and orderly sequence (triple open arrowheads) within the collagen fibrils (ca. 100 nm in diameter). The crystallographic c-axes of these electron-dense platelets were well-aligned with the axis of the collagen fibrils. Larger needle-shaped crystallites (ca. 50 nm) could be seen (pointers) along the periphery of the fibrils (i.e., interfibrillar remineralization). (e) SAED of the region in (d). The arc-shaped diffraction patterns ascribed to the (002) plane of apatite suggest that the c-axes of the platelets have a preferential orientation. (f) A high-magnification view of (c), showing the density of apatite platelets within a remineralized collagen fibril (arrow) along the hybrid layer surface.
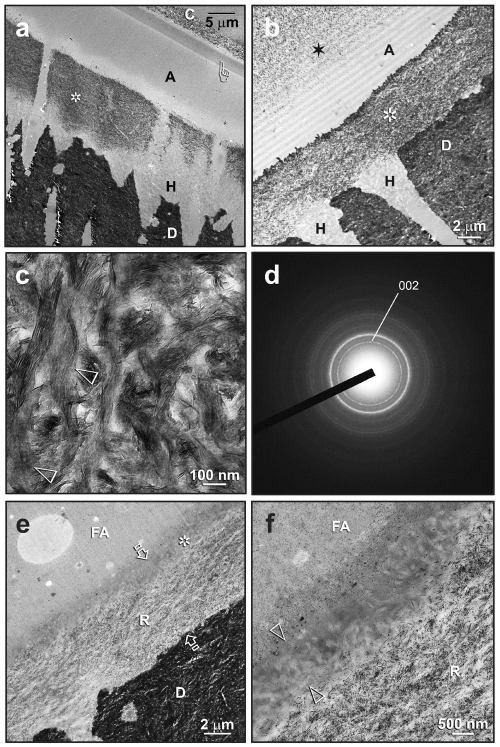
The extent of remineralization within the hybrid layers appeared to be complete after 4 mos. Resin-dentin interfaces of One-Step adhesive were retrieved after 3 and 4 mos of remineralization, respectively (Figs. 3a, 3b). Extensive remineralization had occurred in the top and middle parts of the hybrid layers. The bases of the hybrid layers did not remineralize, suggesting that these regions are better resin-infiltrated. Although intrafibrillar remineralization could still be recognized by the banded appearance in some collagen fibrils, it was largely masked by more extensive interfibrillar mineral deposits (Fig. 3c). These heavily remineralized regions revealed SAED ring patterns that were devoid of arc-shaped patterns (Fig. 3d), suggesting a more random crystallite arrangement contributed by the more extensive interfibrillar remineralization. For Single Bond Plus, remineralization occurred within the middle and base of the hybrid layer (Fig. 3e). The surface 1-2 µm of the hybrid layer appeared to be better infiltrated by the filled adhesive and was not remineralized (Fig. 3f).
Figure 3.
Transmission electron micrographs showing the extent of remineralization in resin-dentin interfaces after 3-4 mos of biomimetic remineralization. C, composite; A, unfilled adhesive; FA, filled adhesive; H, hybrid layer; D, mineralized dentin. (a) A One-Step specimen with extensive remineralization of the top and middle parts of the hybrid layer (asterisk). The base of the hybrid layer appeared to be better-infiltrated and did not remineralize. Initial evidence of mineralization of the adhesive layer could be seen as an electron-dense band (pointer) beneath the composite (see Fig. 4 for details). (b) Another One-Step specimen with even more profuse remineralization of the hybrid layer (asterisk). Extensive mineralization of the adhesive layer could also be seen (star). (c) High magnification of the heavily remineralized regions. Intrafibrillar remineralization could be recognized by the banded appearance of the collagen fibrils (open arrowheads), although this feature has largely been masked by heavy interfibrillar minerals. (d) SAED of these heavily remineralized regions revealed diffraction rings that are ascribed to the major 002 and 211 planes of apatites. Absence of arc-shaped patterns in the 002 ring suggests a more random, overall crystallite arrangement. (e) A Single Bond Plus specimen showing extensive remineralization within the middle and base of the hybrid layer (R). The total thickness of the hybrid layer is depicted by the two open arrows. The surface 2 µm of the hybrid layer (asterisk) appeared to be better-infiltrated by the filled adhesive and did not remineralize. (f) High magnification of (e), showing the absence of remineralization (between open arrowheads) from the surface of the hybrid layer.
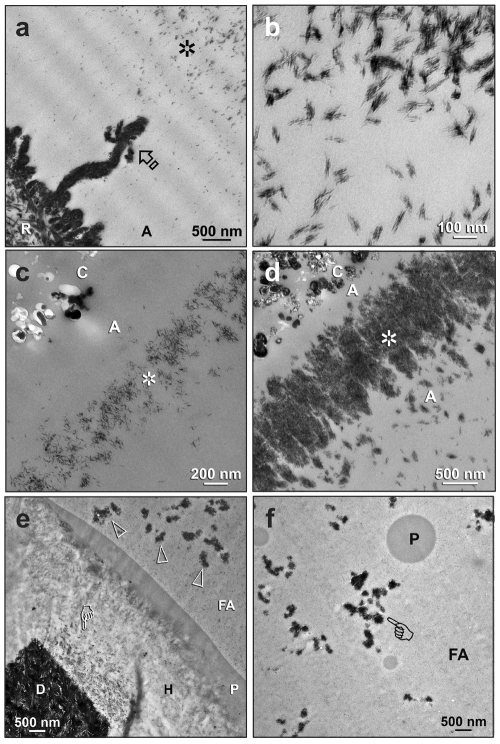
During the process of remineralization, apatite crystallites were deposited within the adhesive layers. For One-Step, the two predominant sites of deposition were the water-rich channels (water trees) close to the dentin surface (Figs. 4a,4b) and the water-rich zone directly beneath the resin composite (Figs. 4c,4d). Intra-resin apatite deposition in Single-Bond Plus was difficult to discern, since nanofillers were present in this filled adhesive. Nevertheless, water trees that originated from the surface of the hybrid layer were filled with minerals after remineralization (Figs. 4e,4f).
Figure 4.
During the process of biomimetic remineralization, intra-resin apatite depositions were unexpectedly created within the porous adhesive resin layers. C, composite; A, unfilled adhesive; FA, filled adhesive; H, hybrid layer; D, mineralized dentin. (a) A One-Step specimen after 3 mos of remineralization, showing a highly remineralized hybrid layer surface (R), a large remineralized water tree (arrow) that was filled with apatite platelets, and a region farther away that contained innumerable apatite clusters (asterisk). (b) At a high magnification, each of the apatite clusters depicted in (a) consisted of apatite platelets that were arranged into bundles. (c) A One-Step specimen after 2 mos of remineralization. A zone of crystallites (asterisk) could be seen in the adhesive layer beneath the resin composite. (d) Another One-Step specimen after 4 mos of remineralization, with a denser layer of crystallite deposits (asterisk) at almost the same site as the specimen in the previous Fig. (e) A Single-Bond Plus specimen retrieved after 2 mos, with partial remineralization along the base of the hybrid layer (pointer). Water trees on top of the hybrid layer were filled with electron-dense minerals (open arrowheads). P, polyalkenoic acid copolymer. (f) These mineral-containing water trees (pointer) extended through the entire adhesive layer. P, polyalkenoic acid copolymer.
Discussion
Although some fluoride-releasing adhesives may induce crystal growth within fluid-filled gaps of resin-dentin interfaces (Hashimoto et al., 2008), apatite deposition occurred with the use of non-fluoride-releasing adhesives, in the absence of interfacial gaps in the present study. For remineralization of defects to be obtained within resin-dentin bonds, the nanoprecursors had to penetrate the resin-sparse regions of the hybrid layer where the interfibrillar spaces are exposed. Being partially resin-infiltrated, the tortuosity of these spaces is probably longer than that present in uninfiltrated demineralized collagen matrices, making the penetration of the nanoprecursors more challenging. It is also notable how mineral deposition could have occurred within the adhesive layer, resulting in the mineralization of nanometer-sized, water-filled voids. The fluidic nature of these amorphous nanoprecursors provided a plausible explanation (Appendix 2) for how they could have followed the pathways of water movement within the polymerized adhesive and penetrated the water-filled nanovoids within the adhesive.
We hypothesized that the PVPA-containing analog molecules that diffuse into the denuded collagen matrix are bound initially to specific sites along the surfaces of the collagen fibrils (Appendix 2). This is analogous to the attachment of different ECM phosphoprotein molecules to specific collagen-binding sites (Dahl et al., 1998; Gajjeraman et al., 2007). Auto-transformation of the coalesced amorphous calcium phosphate nanoprecursors (Cölfen, 2008) into apatite results in their deposition within the interfibrillar spaces. In addition, we speculated that the PVPA-containing molecules also diffuse into the collagen fibrils and attach to the specific locations along the tropocollagen molecules (Appendix 2), thereby guiding mineral deposition within the gap regions of the collagen fibrils (Weiner, 2008). Since the initially formed nanocrystals were smaller than the dimensions of the intrafibrillar apatite platelets, we further hypothesized that the single-crystal apatite platelets represent the end-products of mesoscopic transformation of multiple, orderly arranged, and closely approximated polyacrylic-acid-stabilized mesocrystalline intermediates. This bricklaying, “Lego-like” modular assembly strategy through a particle-mediated approach probably proceeds via the formation of nanoscopic mineral bridges across the surfaces of the mesocrystals (Imai, 2007). Thus, with the use of two biomimetic analogs that are designed for different functions, we verified that the “bottom-up” particle-mediated crystallization pathway (Cölfen, 2007) permits hierarchical crystalline order (i.e., intrafibrillar and interfibrillar minerals; Traub et al., 1989) to be re-established in incompletely resin-infiltrated type-I collagen fibrils under ambient temperature. These remarkable features have never been demonstrated with the traditional technology of crystal growth that focused on the “top-down” fabrication of homogeneous, large-size single crystals.
Although the One-Step adhesive demonstrated a general trend of better infiltration at the base of the hybrid layers, the extent of remineralization was dependent upon the initial status of resin infiltration and varied considerably from specimen to specimen and even from location to location within a single specimen slab. Thus, nanoindentation experiments that examine the changes in mechanical properties within the hybrid layer as discrete value sets would not be sufficient to represent the complexity and variation of remineralized hybrid layers. Rather, nanoscopic Dynamic Mechanical Analysis that creates 3-D maps of the correlation between variations in mechanical properties and ultrastructural changes would be a more appropriate approach to be attempted in future studies.
The current biomimetic remineralization model provides a proof-of-concept for extending the longevity of resin-dentin bonds. In the current model, each resin-dentin slab is placed sideways on top of a set Portland cement block in the remineralization solution. This strategy is not possible clinically, and delivery systems that incorporate set Portland cement particles in either the adhesive or restorative material have to be considered for remineralization to proceed from the top of the adhesive-bonded dentin to the mineralized dentin base via diffusion of the amorphous calcium phosphate nanoprecursors. Moreover, additional strategies have to be considered for incorporating the biomimetic analogs as part of the delivery systems. Investigations into these procedures are in order.
Supplementary Material
Acknowledgments
We thank Bob Smith for TEM technical assistance and Michelle Barnes for secretarial support.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by Grant R21 DE019213-01 from the National Institute of Dental and Craniofacial Research (PI, Franklin R. Tay).
References
- Cai Y, Tang R. (2008). Calcium phosphate nanoparticles in biomineralization and biomaterials. J Mater Chem 18:3775-3787. [Google Scholar]
- Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. (2007). In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res 86:529-533. [DOI] [PubMed] [Google Scholar]
- Cölfen H. (2007). Bio-inspired mineralization using hydrophilic polymers. Top Curr Chem 271:1-77. [Google Scholar]
- Cölfen H. (2008). Single crystals with complex form via amorphous precursors. Angew Chem Int Ed Engl 13:2351-2353. [DOI] [PubMed] [Google Scholar]
- Cölfen H, Antonietti M. (2005). Mesocrystals: inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew Chem Int Ed Engl 44:5576-5591. [DOI] [PubMed] [Google Scholar]
- Dahl T, Sabsay B, Veis A. (1998). Type I collagen-phosphophoryn interactions: specificity of the monomer-monomer binding. J Struct Biol 123:162-168. [DOI] [PubMed] [Google Scholar]
- De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. (2005). A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res 84:118-132. [DOI] [PubMed] [Google Scholar]
- Eanes ED. (2001). Amorphous calcium phosphate. Monogr Oral Sci 18:130-147. [DOI] [PubMed] [Google Scholar]
- Gajjeraman S, Narayanan K, Hao J, Qin C, George A. (2007). Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem 282:1193-1204. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Nakamura K, Kaga M, Yawaka Y. (2008). Crystal growth by fluoridated adhesive resins. Dent Mater 24:457-463. [DOI] [PubMed] [Google Scholar]
- He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, et al. (2005). Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry 44:16140-16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H. (2007). Self-organized formation of hierarchical structures. Top Curr Chem 270:43-72. [Google Scholar]
- Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, et al. (2005). Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 26:6449-6459. [DOI] [PubMed] [Google Scholar]
- Kinney JH, Habelitz S, Marshall SJ, Marshall GW. (2003). The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res 82:957-961. [DOI] [PubMed] [Google Scholar]
- Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. (1990). Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Mater Res 24:721-734. [DOI] [PubMed] [Google Scholar]
- Mishima H, Sakae T. (1986). Demonstration of structural variation in rat incisor dentin as determined by the x-ray Laue method. J Dent Res 65:932-934. [DOI] [PubMed] [Google Scholar]
- Müller FA, Müller L, Caillard D, Conforto E. (2007). Preferred growth orientation of biomimetic apatite crystals. J Crystal Growth 304:464-471. [DOI] [PubMed] [Google Scholar]
- Munisamy S, Vaidyanathan TK, Vaidyanathan J. (2008). A bone-like precoating strategy for implants: collagen immobilization and mineralization on pure titanium implant surface. J Oral Implantol 34:67-75. [DOI] [PubMed] [Google Scholar]
- Niederberger M, Cölfen H. (2006). Oriented attachment and mesocrystals: non-classical crystallization mechanisms based on nanoparticle assembly. Phys Chem Chem Phys 8:3271-3287. [DOI] [PubMed] [Google Scholar]
- Olszta MJ, Odom DJ, Douglas EP, Gower LB. (2003). A new paradigm for biomineral formation: mineralization via an amorphous liquid-phase precursor. Connect Tissue Res 44(Suppl 1):326-334. [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. (2004).Collagen degradation by host-derived enzymes during aging. J Dent Res 83:216-221. [DOI] [PubMed] [Google Scholar]
- Rosenthal S, Spångberg L, Safavi K. (2004). Chlorhexidine substantivity in root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 98:488-492. [DOI] [PubMed] [Google Scholar]
- Tao J, Pan H, Zeng Y, Xu X, Tang R. (2007). Roles of amorphous calcium phosphate and biological additives in the assembly of hydroxyapatite nanoparticles. J Phys Chem B 111:13410-13418. [DOI] [PubMed] [Google Scholar]
- Tay FR, Moulding KM, Pashley DH. (1999). Distribution of nanofillers from a simplified-step adhesive in acid-conditioned dentin. J Adhes Dent 1:103-117. [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2002). Dental adhesives of the future. J Adhes Dent 4:91-103. [PubMed] [Google Scholar]
- Tay FR, Pashley DH. (2008). Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 29:1127-1137. [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Yoshiyama M. (2002). Two modes of nanoleakage expression in single-step adhesives. J Dent Res 81:472-476. [DOI] [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Suh BI, Hiraishi N, Yiu CK. (2005). Water treeing in simplified dentin adhesives—déjà vu? Oper Dent 30:561-579. [PubMed] [Google Scholar]
- Tay FR, Pashley DH, Rueggeberg FA, Loushine RJ, Weller RN. (2007). Calcium phosphate phase transformation produced by interaction of the Portland cement component of white MTA with a phosphate-containing fluid. J Endod 33:1347-1351. [DOI] [PubMed] [Google Scholar]
- Traub W, Arad T, Weiner S. (1989). Three-dimensional ordered distribution of crystals in turkey tendon collagen fibers. Proc Natl Acad Sci USA 86:9822-9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XT, Ker RF. (1995). Creep rupture of wallaby tail tendons. J Exp Biol 198(Pt 3):831-845. [DOI] [PubMed] [Google Scholar]
- Wang XT, Ker RF, Alexander RM. (1995). Fatigue rupture of wallaby tail tendons. J Exp Biol 198(Pt 3):847-852. [DOI] [PubMed] [Google Scholar]
- Weiner S. (2008). Biomineralization: a structural perspective. J Struct Biol 163:229-234. [DOI] [PubMed] [Google Scholar]
- Weiner S, Sagi I, Addadi L. (2005). Structural biology. Choosing the crystallization path less traveled. Science 309:1027-1028. [DOI] [PubMed] [Google Scholar]
- Xu A-W, MA Y, Cölfen H. (2007). Biomimetic mineralization. J Mater Chem 17:415-449. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.