SUMMARY
This study evaluated the micropermeability of six etch-and-rinse adhesives bonded to dentin. There were two principal groups: wet bonding with water or wet bonding with absolute ethyl alcohol. After bonding and the creation of composite build-ups, the pulp chambers were filled with 0.1% Lucifer Yellow. The contents of the pulp chamber were kept under 20 cm H2O pressure to simulate pulpal pressure for 3 h. The specimens were vertically sectioned into multiple 0.5 mm thick slabs that were polished and then examined in a two-photon confocal laser scanning microscope (TPCLSM). The results showed that specimens bonded with adhesives using the water wet-bonding condition all showed tracer taken up uniformly by the hybrid layer. This uptake of fluorescent tracer into the hybrid layer was quantified by computer software. The most hydrophobic experimental resins showed the highest fluorescent tracer uptake (ca. 1800 ± 160 arbitrary fluorescent units/std. surface area). The most hydrophilic experimental resins showed the lowest tracer uptake into water-saturated hybrid layers (ca. 1000 ± 100 units). When ethanol wet-bonding was used, significantly less tracer was seen in hybrid layers. The most hydrophilic experimental resins and Single Bond Plus only took up about 100 ± 20 units. Clearly, ethanol wet-bonding seals dentin significantly better than water-wet dentin regardless of the adhesive in etch-and-rinse systems.
Keywords: Micropermeability, Two-photon confocal microscopy, Ethanol-saturated dentin, Hydrophobic hybrid layer, Dentin sealing
INTRODUCTION
One of the assumptions in the “wet-bonding” technique is that solvated comonomer mixtures can displace all of the water from the interfibrillar spaces and from the top 10 µm of the acid-etched dentinal tubules during the resin-infiltration phase of dentin bonding. After evaporation of the solvent, the resin infiltration should ideally displace all of the water that had replaced all of the mineral extracted by acid-etching. That assumption has been tested using high-magnification nanoleakage studies with TEM and SEM.1,2 Nanoleakage studies using silver nitrate have identified water-filled channels within hybrid layers indicating that not all of the residual water was removed.3,4 However, whether done using SEM or TEM techniques, the results are not quantitative and are regarded as technique-sensitive.5 Recently a new form of optical microscopy named multi-photon laser fluorescence microscopy has been used for imaging biomaterials and resin-dentin interfaces.6 The two-photon laser fluorescence microscope uses a laser light source generated by a mode-locked Ti: sapphire laser operating in the near-infrared range with femto-second (10−17) pulses that allow deeper imaging with fewer artifacts induced by photo-damaging.7,8 This confocal laser microscope is equipped with sophisticated software that allow quantification of the magnitude of fluorescence in standardized area of interest.9,10
These microscopy systems can be used to measure the micropermeability of resin tags as they pass through the hybrid layer. This is done after resin-dentin bonding is completed by filling the pulp chamber of a bonded tooth with a water-soluble fluoroprobe such as lucifer yellow (LY).
This solution is placed under a physiologic pulpal pressure of 15–20 cm H2O11 for 3–4 h to facilitate the seepage of lucifer yellow into any water-filled spaces in the resin-bonded complex that remain in continuity with pulpal/dentinal fluid.12–14 If a resin-dentin bond is perfect, there should be no water-filled microporosities in continuity with Lucifer Yellow in the pulp chamber. Even after 3 h of exposure to a physiologic pulpal pressure, there should be no CLSM evidence of micropermeation of lucifer yellow into the resin bonded complex.
Recently, the wet-bonding technique15,16 has been modified by replacing water with absolute ethyl alcohol. That is, after rinsing the acid-etched cavity preparation with water, the water is replaced with an excess of 100% ethyl alcohol. The goal is to replace all water in interfibrillar spaces and in the tops of the dentinal tubules, with ethanol. Because ethanol is completely miscible with water, it is thought that any residual layer of ethanol on collagen fibrils will permit monomers to dissolve in the residual ethanol. This is unlikely if residual water covers collagen fibrils, because adhesive monomers are not very soluble in water even though some classify them as hydrophilic. Our intent is to not only displace water from collagen but to dissolve monomers in residual ethanol that is in intimate contact with collagen fibrils. The second advantage of ethanol-wet bonding is that one can infiltrate hydrophobic monomers that are not soluble in water, into ethanol-saturated demineralized dentin. Such hydrophobic monomers absorb much less water over time compared to more hydrophilic monomers. 17–18
The purpose of this study was to test the hypothesis that there is much less micropermeability of resin-bonded dentin hybrid layers created using ethanol wet-bonding compared to water wet-bonding. This hypothesis was tested using confocal two-photon laser scanning microscopy to evaluate the uptake of lucifer yellow in dentin bonds made with five experimental methacrylate adhesives and a one-bottle commercial adhesive applied to acid-etched dentin.
MATERIAL & METHODS
Dentin sample preparation
Extracted unerupted human third molars were selected for this study. All teeth were extracted after informed consent was obtained according to a treatment protocol approved by the Human Assurance Committee of the Medical College of Georgia. The teeth were stored at 4 °C in physiological saline for no longer than 1 month.
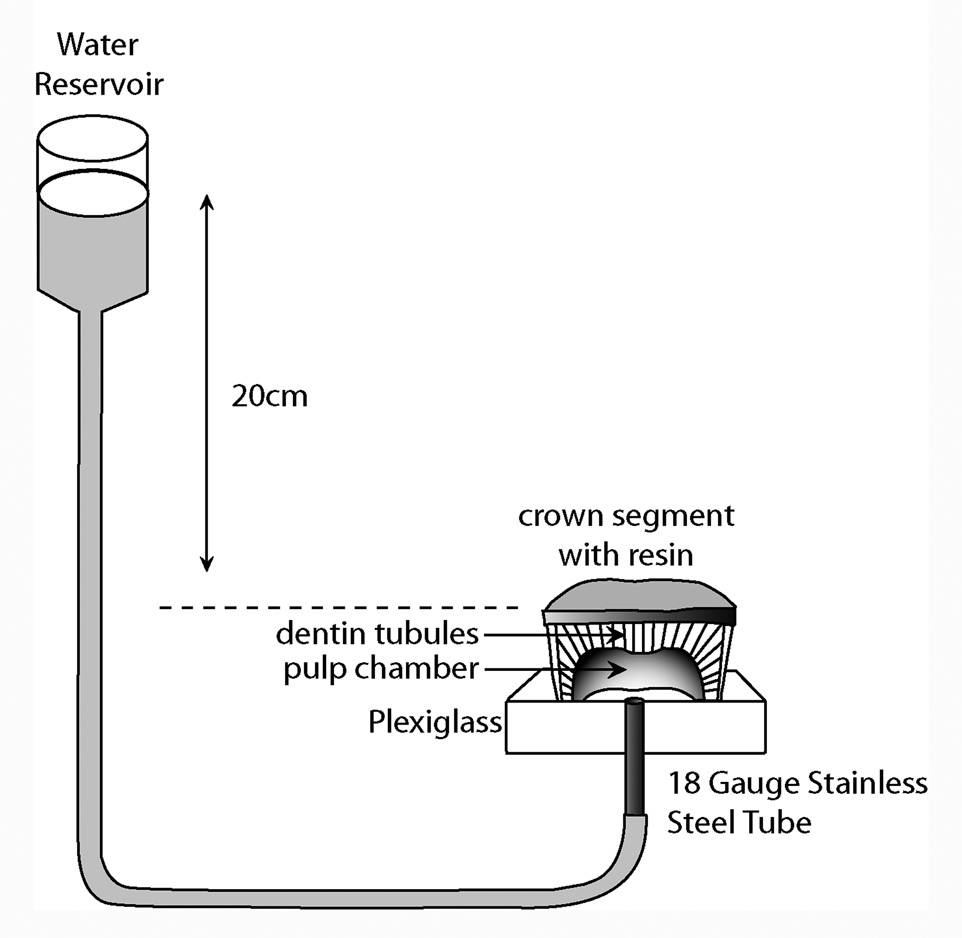
Seventy-two crown segments were obtained by sectioning the roots 1 mm beneath the cementum enamel junction (CEJ) and 1 mm below the deepest occlusal fissures using a low-speed water-cooled diamond saw (Isomet, Buehler Ltd., Lake Bluff, IL, USA) The thickness of the remaining deep dentin between the flat surface and highest pulp horn of the crown segments was 1.5 ± 0.5 mm. The pulpal tissue was removed with a small forceps, with care, to avoid crushing the pre-dentin. The crown segments were cemented to Perspex (Perspex Distributions, London, UK) supports (2 cm × 2 cm × 0.5 cm) penetrated by an 18 gauge stainless steel tube (Figure 1), using a viscous cyanocrylate (Zapit, Dental Ventures of America, Anaheim Hills, CA, USA). Deep dentin was chosen because it has the highest water content (Pashley, 1998) and is the most challenging bonding substrate.
Figure 1.
Schematic of how a crown segment was created from extracted third molars and mounted to a plastic platform penetrated by a short length of stainless steel tubing. The steel tubing permitted water containing lucifer yellow to fill the pulp chamber and the attached polyethylene tubing that was raised 20 cm above the pulp chamber.
The crown segments were then divided in two principal groups: water-wet dentin (n=36) or ethanol-saturated dentin (n=36). Each principal group was further divided in 6 sub-groups based on the tested adhesives (n=6/each). The compositions of the adhesive blends are listed in Table 1.
Table 1.
Composition of neat and solvated resins used in this study
| Neat Resin | Solvated Resin | |
|---|---|---|
| Single Bond Plus (3M ESPE, St. Paul, MN, USA) |
-- | 20 wt% BisGMA |
| -- | 15 wt% HEMA | |
| -- | 10 wt% polyalkenoic acid | |
| -- | 35wt% ethanol/water | |
| -- | 5–10% glycerol-1,3-dimethacrylate | |
| -- | 5–10% diurethane dimethacrylate | |
| Resin 1 (most hydrophobic) |
70 wt% E-BisADM | 34.4 wt% E-BisADM |
| 28.75% TEGDMA | 14.35% TEGDMA | |
| -- | 50 wt% ethanol | |
| Resin 2 (second most hydrophobic) |
70 wt% BisGMA | 34.4 wt% BisGMA |
| 28.75% TEGDMA | 14.35% TEGDMA | |
| -- | 50 wt% ethanol | |
| Resin 3 (mildly hydrophilic) |
70 wt% BisGMA | 34.4 wt% BisGMA |
| 28.75% HEMA | 14.35 wt% HEMA | |
| -- | 50 wt% ethanol | |
| Resin 4 (more hydrophilic) |
40 wt% BisGMA | 20 wt% BisGMA |
| 30 wt% TCDM | 14.4 wt% TCDM | |
| 28.75% HEMA | 14.35% HEMA | |
| -- | 50 wt% ethanol | |
| Resin 5 (most hydrophilic) |
40 wt% BisGMA | 20 wt% BisGMA |
| 30 wt% BisMP | 14.4 wt% BisMP | |
| 28.75% HEMA | 14.35% HEMA | |
| -- | 50 wt% ethanol |
All resin blends also contained 0.25 wt% camphorquinone and 1.0 wt% 2-ethyl-dimethyl-4-aminobenzoate.
Abbreviations: E-BisADM = ethoxylated Bisphenol A dimethacrylate; BisGMA = 2,2-bis[4-(2-hydroxy-3-methacryloylpropoxy)]-phenyl propane; TEGDMA = triethyleneglycol dimethacrylate; HEMA = 2-hydroxyethylmethacrylate; TCDM = di(hydroxyethyl-methacrylate) ester of 5-(2,5-dioxotetrahydrofurfuryl)-methyl-3-cyclohexane-1,2’-dicarboxylic acid ; BisMP = Bis [2-(methacryloyloxy)ethyl]phosphate.
Bonding procedures
A homogeneous smear layer was created on each dentin surface using 180-grit SiC abrasive paper for 30 s under continuous water irrigation. The specimens were then acid-etched for 15 s with 37% phosphoric acid (PA) and bonded using the ethanol wet-bonding technique or water wet-bonding technique. The ethanol wet-bonding substrate was achieved by covering the acid-etched water-rinsed dentin surfaces with excess absolute ethyl alcohol for 5 min. The dentin surface was always covered by a liquid to avoid surface tension forces, keeping it visibly moist prior to the application of the resin blends. The water wet-bonding substrate was achieved by water-rinsing the dentin surfaces and gently blowing off the excess water in order to leave a wet reflective surface (Figure 2).
Figure 2.
Micrographs showing representative degrees of wetness of water wet-bonding and ethanol wet-bonding procedures. A: shows the ethanol wet-surface meticulously performed to ensure that the dentin surface was always covered with a liquid phase to avoid surface tension forces by keeping it visibly moist prior to the application of the resin blends. B: shows water wet-substrate achieved by water-rinsing the dentin surfaces and gently blowing off the excess water in order to leave a wet reflective surface.
Two consecutive coats of one of five 50% ethanol/50% experimental primers were then applied to ethanol-saturated dentin or water-wet dentin. Excess solvent was evaporated with a gentle air stream for 5 sec. Subsequently, a layer of each respective neat comonomer adhesive was applied, spread thin with moisture-free air, and light-cured for 20 sec with the use of an Optilux 500 halogen light-curing unit (Demetron/Kerr, Danbury, CT, USA) with a power output of 600 mW/cm2. A commercial adhesive, Single Bond Plus (SBP) (3M ESPE, St. Paul, MN), was also applied per the manufacturer’s instructions on the ethanol-saturated dentin or water-wet dentin. Composite build-ups were constructed with a light-cured flowable resin composite (Wave-wave MV, SDI Limited, Australia) in two 2-mm-thick increments. The bonding procedures were performed in the absence of simulated pulpal pressure but with the pulp chambers filled with water
Confocal two-photon laser microscopy
The fluorochrome selected for the evaluation of micropermeability of the resin-dentin interfaces was a 0.1 wt% water solution of lucifer yellow CH (LY), ammonium salt (Sigma Chemicals Co., St. Louis, MO) due to its high water solubility. This fluorescent dye was used to trace the water-filled spaces in the hybrid layer from the pulp chamber through the tubules to the bonded interface for 3 h under 20 cm H2O of hydrostatic pressure (Figure 1). When using the aqueous solution of lucifer yellow in the pulp chamber, the comonomers in the primer and adhesive contained no dye. That is, when fluorescent tracer was used in the pulp chamber, no other dye was added to the comonomer blends. One hour after resin bonding was completed, the bonded built-up specimens were vertically sliced into multiple 0.5 mm slabs, slightly polished using wet 1200 grit silicon carbide paper and ultra-sonicated for 5 min. Two slabs from the center of each specimen were selected for confocal microscopy resulting in twelve slabs per sub-group.
Three images were obtained from each slice resulting in six images per specimen and thirty-six images per sub-group. These images were intended to be representative of the most common features observed in each specimen and for quantification of the micropermeability of lucifer yellow.
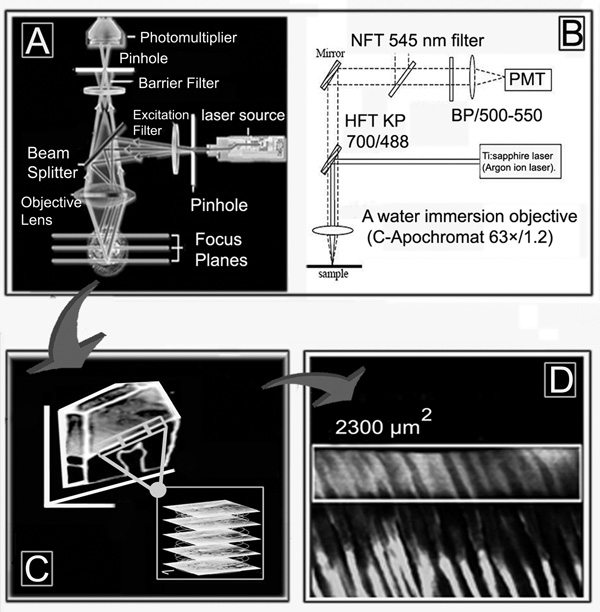
Microscopic examination and quantification of the intensity of lucifer yellow in the resin-bonded 0.5 mm thick slabs were performed using a two-photon laser confocal scanning microscope (TPLSM) (LSM 510 META, Carl Zeiss; Thornwood, NY, USA) coupled to a 10-W mode-locked Ti:sapphire laser (MIRA 900 System, Coherent Laser Group; Santa Clara CA, USA) pumped by an Argon ion laser. The excitation wavelength was 800 nm with pulse repetition rate of 90 MHz 8. A HFT KP 700/488 beam splitter-mirror (Carl Zeiss; Göttingen, Germany) was used to filter laser light at 488 nm. A band-pass (BP/500–550) filter was used to collect the emission of lucifer yellow8. A water immersion objective (C-Apochromat 63×/1.2 Zeiss, Canada ) was used for capturing images up to 20 µm deep, beginning 5 µm below the external surface to avoid superficial specimen preparation artifacts (i.e. polishing scratches), in a sequential series of images taken every 0.5 µm (Figure 3). Thus, 40 images were every 0.5 µm taken without any virtual separation between them, and then converted into a single projection.
Figure 3.
Schematic showing how the confocal two-photon microscopy was performed. A: shows the basic principles of confocal microscopy. B: shows the parameters used with the two-photon laser confocal scanning microscope (TPLSM) (LSM 510, Carl Zeiss; Thornwood, NY, USA). PMT is a photomultiplier‥ C: schematic showing how serial images were taken every 0.5 µm using standardized parameters beginning from 5 µm below the external at depth of 20 µm. D shows how the serial sections were converted into a single projection using the imaging software associated with the confocal microscope and where the region of interest (ROI) retangle was drawn around each hybrid layer to quantitate mean emission intensity.
Prior to the experiment, a pilot study was performed to standardize all the parameters for excitation and emission of the lucifer yellow infiltrated within the hybrid layer for accurate calculation of the micropermeability observed when using the two w e t-bonding techniques. The following standardized parameters were used for the entire experiment (excitation wavelength 800 nm at 25%; pinhole: 600 µm; stack size: 73.1 µm × 73.1 µm × 20 µm). The pilot study was also used to calibrate the power of the laser for the excitation in order to reduce the photo-bleaching and to obtain a good depth of z-penetration. This calibration indicated that the appropriate depth of penetration was 20–25 microns, to avoid too much difference in the excitation and emission intensity between the top and the bottom of the projections (i.e. first vs. last z-image).
The 20 µm deep z-sections were converted into a single projection and, with the use of the imaging software associated with the confocal microscope (LSM 510 META Image Browser), a region of interest (ROI) was drawn around each hybrid layer within a field of view and the mean fluorescent emission intensity was calculated.8
The images were taken to a depth of 20 µm in order to integrate more micropermeability data than could be obtained in a single plane 2D-image. Although there was a tendency of slightly less fluorescence with increasing subsurface depth, this factor was standardized during the pilot study to be kept constant in all resin-dentin specimens. The serial images were converted into a single projection in order to obtain an average emission intensity, that was not significantly different from the arithmetic mean of the individual images. Moreover, it was easier to calculate fluorescence intensity of single projections of several serial images than to calculate the average of every single serial image and then calculate a global average. The values of fluorescence emission intensity of the lucifer yellow of all of the experimental groups were statistically compared, by two-way ANOVA (resin as one factor and type of matrix saturation as the other factor). Because there were highly significant interactions between the factors (p<0.001), multiple comparisons were performed with the Least Square Means test. Least Square Means are the expected values of group or subgroup means that one expects for a balanced design involving the group variable, with all covariates at their mean values. In this multiple comparison test, the variance around the means is given as the standard error of the mean (S.E.M.), instead of the S.D. Statistical significance were set in advance at α = 0.05. A reflection light reference image was also captured from each specimen.
RESULTS
Two-photon confocal microscopy evaluation of micropermeability
Fluorescence emission intensity
The micropermeability of the five experimental resins and of the Single Bond Plus adhesive system applied with the ethanol wet-bonding or water wet-bonding technique was quantified by measuring the intensity of the fluorescence emission (λ) of the lucifer yellow that had been taken up by the hybrid layers.
The results (Table 2) showed different degrees of micropermeability between the resin-dentin interfaces created with the two wet-bonding techniques. The top half of Table 2 shows the results obtained with water-wet bonding. The highest uptake of lucifer yellow occurred using the two hydrophilic experimental resins #1 and #2. These values were significantly higher (p<0.05) compared to experimental resins #3–5 and Single Bond Plus. None of the permeability values of the latter four resins were significantly different from each other. Table 2 shows the results obtained with all six specimens in all five groups.
Table 2.
Intensity data of the fluorescence emission of the lucifer yellow that had been taken up by the hybrid layers of the adhesives applied with the water-wet or ethanol-saturated dentin technique.
| Water-wet echnique | Intensity data obtained from the fluorescence emission of the images of each sub-group | ||||||
|---|---|---|---|---|---|---|---|
| #[1°-2/6] | #[2°-2/6] | #[3°-2/6] | #[4°-2/6] | #[5°-2/6] | #[6°-2/6] | #Mean-SEM [12–36] | |
| Resin 1 | 2010 | 1876 | 2001 | 1886 | 1567 | 1912 | 1875 ± 43a |
| Resin 2 | 1559 | 1776 | 1991 | 1856 | 1577 | 1812 | 1762 ± 43a |
| Resin 3 | 1259 | 1172 | 1131 | 1251 | 1277 | 992 | 1168 ± 43b |
| Resin 4 | 1139 | 1112 | 981 | 1101 | 997 | 895 | 1038 ± 43b |
| Resin 5 | 1021 | 812 | 998 | 1111 | 977 | 1012 | 989 ± 43b |
| Single Bond Plus | 1032 | 947 | 1010 | 1211 | 1212 | 1092 | 1084 ± 43b |
|
Ethanol-saturated technique# |
#[1°-2/6] | #[2°-2/6] | #[3°-2/6] | #[4°-2/6] | #[5°-2/6] | #[6°-2/6] | #Mean-SD [12–36] |
| Resin 1 | 510 | 203 | 98 | 119 | 67 | 312 | 218 ± 43c |
| Resin 2 | 91 | 120 | 81 | 119 | 111 | 67 | 98 ± 43c |
| Resin 3 | 104 | 112 | 79 | 109 | 102 | 94 | 100 ± 43c |
| Resin 4 | 114 | 122 | 57 | 69 | 130 | 79 | 95 ± 43c |
| Resin 5 | 110 | 59 | 98 | 121 | 79 | 82 | 91 ± 43c |
| Single Bond Plus | 95 | 119 | 126 | 71 | 95 | 92 | 100 ± 43c |
Number of the specimen and number of slabs/number of images per each sub-group elaborated for the quantification of the fluorescence emission intensity of the lucifer yellow that had been taken up by the hybrid layers (arbitrary fluorescence units per 2300 µm2).
When the five experimental resin blends and Single Bond Plus were applied with the ethanol wet-bonding technique, the micropermeability was significantly lower (p<0.05) compared to the results obtained with the same resins used with water wet-bonding. There were no significant differences among the five resins in the ethanol wet-bonding group.
In Table 3, the means and standard errors of the means of the percent changes in micropermeability of each resin in the water vs. ethanol wet-bonding groups were compared and found to be significantly different (p <0.05).
Table 3.
Statistical analysis and percentage of intensity change between of the micropermeability of resinbonded dentin interfaces created with the ethanol-saturated and water-wet technique.
| * Micropermeability of resin-bonded dentin interfaces (Lucifer Yellow Intensity Emission)* | |||
|---|---|---|---|
| Adhesive Systems | Ethanol-saturated technique | Water-wet technique | % change |
| Resin 1 | 218 ± 43cB | 1875 ± 43aA | 88.4 |
| Resin 2 | 98 ± 43cB | 1762 ± 43aA | 94.4 |
| Resin 3 | 100 ± 43cB | 1168 ±43bA | 91.4 |
| Resin 4 | 95 ± 43cB | 1038 ± 43bA | 90.8 |
| Resin 5 | 91 ± 43cB | 989 ± 43bA | 90.8 |
| Single Bond Plus | 100 ± 43cB | 1084 ± 43bA | 90.8 |
The micropermeability of hydrophobic hybrid layers induced by pulpal pressure was quantified by the intensity of the fluorescence emission (λ) of the lucifer yellow that was taken up by the hybrid layer (arbitrary fluorescence units per 2300 µm2 surface area). % change was calculated as water-wet fluorescence emission intensity minus the ethanol-saturated fluorescence intensity divided by the water-wet fluorescence intensity times 100.
Different lower case letter indicate statistically significant differences between the adhesive systems applied on dentin with the same technique (p< 0.05)
Different upper case letter indicate statistically significant differences between the ethanol-saturated dentin vs. water-wet dentin technique used for each adhesive system (p< 0.05).
Multiple comparisons tests (Holm-Sidek) were performed to compare similarities and differences among the subgroup micropermeabilities. The power of these statistical analyses with α = 0.05 was 1.0 for resins, 1.0 for substrate solvents and 1.0 for their interactions.
The two-photon confocal microscopy
The confocal microscopy investigation showed different degrees of micropermeability within the entire thickness of the hybrid layer and around the resin tags of the resin-dentin interfaces created with ethanol wet-bonding or with the classic water wet-bonding technique.
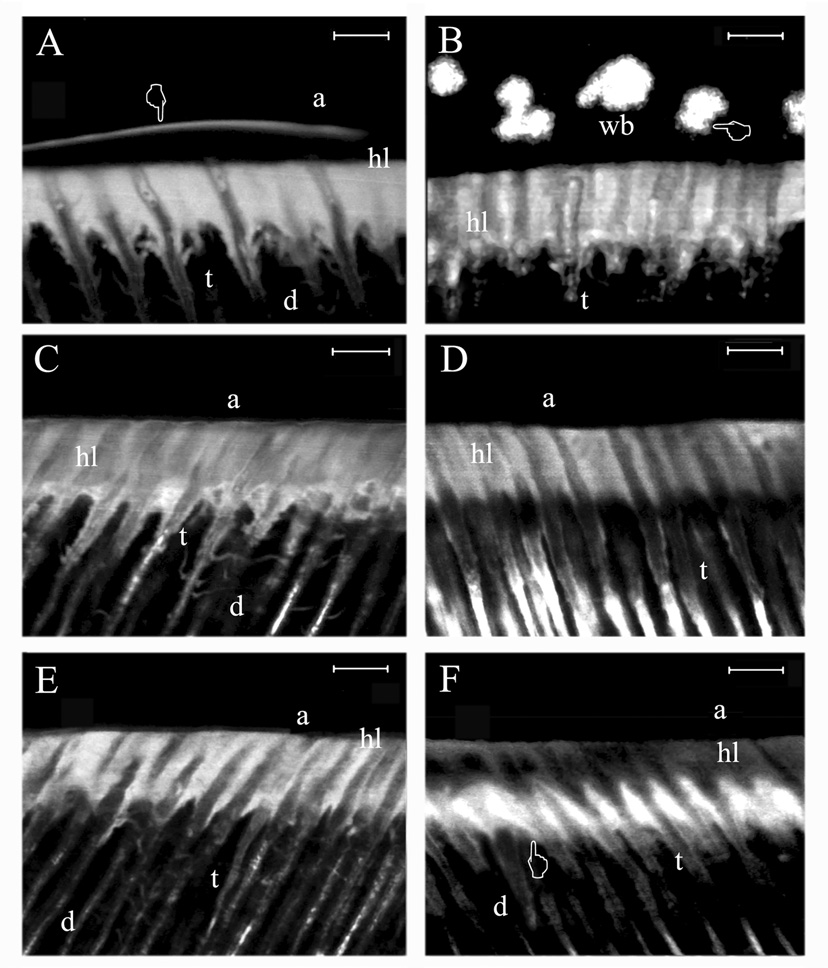
The appearance of lucifer yellow in the interfaces can be seen in the fluorescence mode in Figures 4A–F and Figures 5A,C,E. All five experimental resins and the one-bottle etch-and-rinse adhesive (SBP) applied with the water wet-bonding technique on acid-etched dentin created highly fluorescent hybrid layers due to the incomplete resin infiltration of the acid-etched dentin (Figure 4).
Figure 4.
Two-photon laser confocal single projections obtained in fluorescence mode using standardized parameters for evaluating the degree of micropermeability within the resin-dentin interfaces created with the water wet-bonding technique. Magnification bar = 4 µm throughout the figure.
A: Micropermeability observed using Single Bond Plus applied with the water wet-bonding technique. Note the severe micropermeability (i.e. high fluorescence staining) around the resin tags and within the hybrid layer (hl). The micropermeation of the fluorescent dye was detected throughout the entire thickness of the resin-dentin interface and within the adhesive (a) layer (pointer). B: Micropermeability of resin#1 applied to water-wet dentin. Since this resin is very hydrophobic, it created severe micropermeability around the resin tags and in microporosities located within the hybrid layer (hl). Fluorescent dye was also detected within the adhesive layer (pointer) showing lucifer yellow in water blisters (wb) entrapped within the polymeric-resin structure. C: Micropermeability observed using resin #2. Severe lucifer yellow permeation was seen around the resin tags (t) and within the hybrid layer (hl). Although this resin was almost as hydrophobic as resin 1, no phase separation was observed. D: Micropermeability observed using resin #3 applied with the water wet-bonding technique. Micropermeability was evident around the resin tags (t) and within the hybrid layer (hl). E: Micropermeability observed using resin #4 applied with the water wet-bonding technique. The micropermeability was similar to resin #3. F: Micropermeability created using resin #5, the most hydrophilic resin, applied with the water wet-bonding technique. Note that high micropermeability was only evident around the resin tags (t) and within the bottom of the hybrid layer (pointer) but was much less apparent in the top third of the hybrid layer (hl). This may indicate that the first coating of resin #5 displaced most of the water from the collagen and that the second coat may have infiltrated the collagen in the top half of the hybrid layer better than in the bottom half, preventing most of the lucifer yellow uptake in the top half.
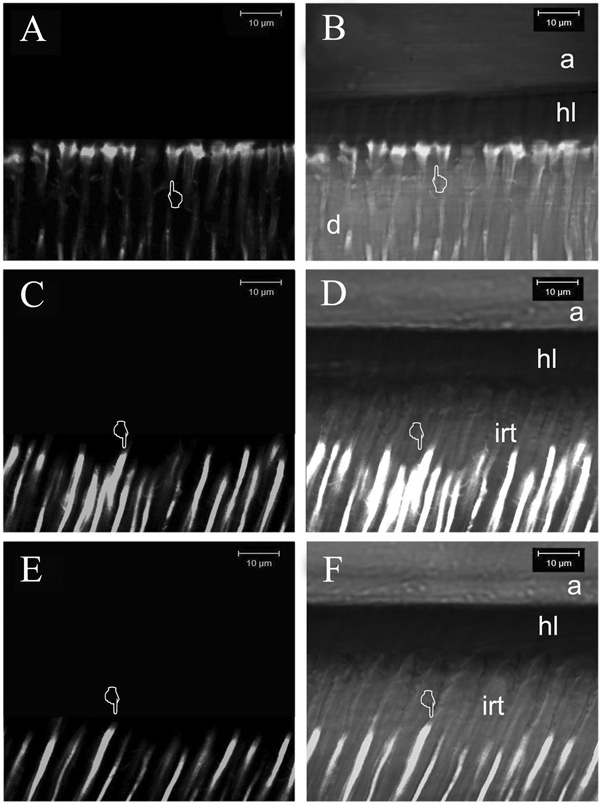
Figure 5.
Two-photon laser confocal single projections obtained in fluorescence mode (A,C,E) and in reflection/fluorescent mode (B,D,F) using standardized parameters for evaluating the degree of micropermeability within the resin-dentin interfaces created with the ethanol wet-bonding technique. A: Micropermeability observed in the resin-dentin interfaces created using the resin #2. Only slight evidence of micropermeability was detected round resin tags beneath the hybrid layer (pointer). B: Micropermeability created using the resin #2. Note the absence of lucifer yellow permeation within the hybrid layer (hl). This projection shows how the fluorescent dye penetrated only slightly around the resin tags (pointer). C: Micropermeability observed using resin #3. Note the presence of yellow lucifer filling only the dentinal tubules (pointer). D: Fluorescent/reflection single projection of resin #3 after ethanol wet-bonding. No micropermeability was seen within hybrid layer (hl). Because of the excellent sealing ability achieved between the resin tags (irt: impermeable resin tags) and the walls of the tubule, the fluorescent dye stopped several microns away from the hybrid layer (pointer). E: Micropermeability observed using resin #4. Yellow lucifer only filled the dentinal tubules (pointer). F: Fluorescent/reflection single projection of the previous image in showing a complete absence of micropermeability within the hybrid layer. Because of the excellent sealing ability achieved between the resin tags (irt: impermeable resin tags), the fluorescent dye stopped several microns away from the hybrid layer (pointer).
Furthermore, none of these adhesive resins was able to achieve anything close to perfect dentin sealing, especially, Single Bond Plus, which even showed water permeation between the hybrid and adhesive layers (Figure 4A). However, the most hydrophobic resin #1 showed the highest level of micropermeability, with evidence of adhesive phase separation and presence of water droplets within the entire thickness of the adhesive layer (Figure 4B).
Although the experimental resin #2 was the second most hydrophobic adhesive, there were no water droplets or evidence of phase separation within the resin-dentin interface (Figure 4C). When applied to water-saturated acid-etched dentin, it showed relatively high, uniform lucifer yellow uptake.
The experimental hydrophilic resins #3 and #4 also showed signs of micropermeability, exhibiting high uptake of lucifer yellow between the walls of the dentinal tubules and resin tags and throughout the entire thickness of adhesive and hybrid layers (Figure 4D, E). Resin #5, the most hydrophilic resin showed extensive micropermeability around the resin tags and the lower half of the hybrid layer but not in the upper half of the hybrid layer.
Figure 5B,D,F are combined reflectance and fluorescence modes that provide detail of the location of the interface between the adhesive and the top of the hybrid layer, between the bottom of the hybrid layer and the mineralized dentin and the extent of penetration of non-fluorescent resin tags into the underlying mineralized dentin.
When the five experimental resins and the one-bottle/etch-and-rinse adhesive (SBP) were applied with the ethanol wet-bonding technique on etched-dentin, much less porous hybrid layers, lower micropermeability and improved sealing ability were seen (Figure 5). The reflection/fluorescent images in Figure 5 delineated important morphologic details such as the thickness of the adhesive and hybrid layers that could not be seen in the fluorescence-only mode. When ethanol-saturated specimens were bonded with resin, the fluorescent dye was not able to infiltrate the hybrid layer of these interfaces due to the better resin infiltration into the acid-etched collagen fibril network, and consequently to the excellent sealing ability achieved with the ethanol-saturated dentin technique (Figure 5A).
In the case of the hydrophilic experimental resins #3 and #4, it was interesting to observe how little micropermeability was evident when compared with the hydrophobic resin #2. Indeed, the specimens imaged in Figure 5C (fluorescent mode) and Figure 5D (reflection/fluorescent mode) showed very low micropermeability in the resin-dentin interfaces created by the resin #3 applied with the ethanol wet-bonding technique.
The specimens imaged in Figures 5E (fluorescent mode) and Figure 5F (reflection/fluorescent mode) show the micropermeability in the resin-dentin interfaces created by the even more hydrophilic resin #4 applied with the ethanol wet-bonding technique.
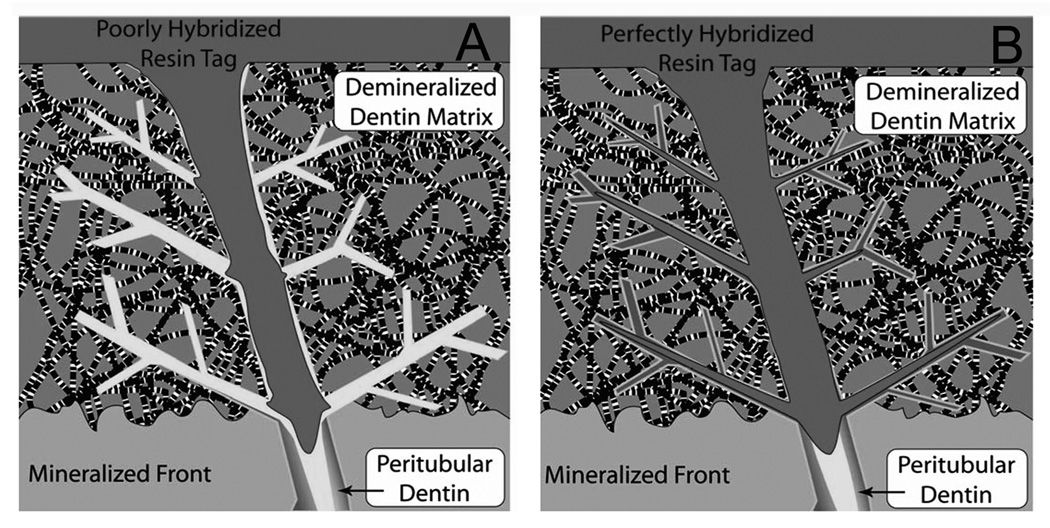
Figure 6 is a schematic that illustrates the differences in micropermeability of lucifer yellow in water-saturated dentin (Figure 6A) versus ethanol-saturated dentin (Figure 6B). In Figure 6A, the matrix was saturated with water. Water filled the lateral branches of the tubules and the interfibrillar spaces. The solvated resin was unable to displace the thin layer of water coating the collagen fibrils. Thus, the resin filled the interfibrillar spaces but did not actually bond to the collagen, leaving a nanometer sized gap between the collagen fibril and the resin. In Figure 6B, the water has been replaced by ethanol, an excellent solvent for all monomers. We speculate that this allows the monomers to truly coat the collagen fibrils, thereby eliminating any nanometer-sized gaps between the collagen fibrils and the resin, preventing lucifer yellow permeation.
Figure 6.
Schematic of micropermeability of resins bonded to water-saturated acid-etched dentin (A) versus ethanol-saturated acid-etched dentin (B). During the infiltration phase of dentin bonding, solvated comonomers diffuse down open tubules and into the interfibrillar spaces of superficial intertubular dentin. Deeper interfibrillar spaces seem to fill indirectly via radial diffusion through lateral branches of the tubules. Residual water in those diffusion channels may permit more water-soluble hydrophilic monomers to diffuse into these sites and exclude the more water-insoluble dimethacrylates. Residual ethanol in (B) would dissolve all comonomers and may coat the collagen fibrils with more resin.
DISCUSSION
It has recently been demonstrated that if the water in the demineralized dentin is replaced with 100% ethanol, the solubility parameters and the hydrophobicity of the ethanol-saturated dentin become closer to that of the adhesive resin blends.19–22 The closer the solubility parameters of adhesive blends are to acid-etched dentin, the better the resin-dentin hybridization19,22 and the less the nanoleakage expression within the hybrid layer.20
In the current confocal two-photon laser scanning microscopy study, the micropermeability of two hydrophobic experimental resins (#1 and 2), three hydrophilic experimental resins (#3, 4 and 5) and a commercial one-bottle (SBP) adhesive were evaluated on etch-and-rinsed, water or 100% ethanol-saturated dentin.
The term “micropermeability” was first used by Sidhu and Watson13 in the evaluation of the interfacial characteristics of dentin bonded with resin-modified glass ionomers using a solution of rhodamine B and confocal microscopy. This technique consists of placing a fluorescence dye (i.e. Rhodamine B or Lucifer Yellow) in the pulp chamber and evaluating any appearance of fluorescent tracer in the bonded interface that had to have seeped around resin tags to reach microporosities within the hybrid layer. The most important advantage of this technique is that it is possible to visualize this leakage with minimal specimen preparation.
The multi-photon confocal laser microscope used in this study allowed both the imaging of deep areas below the external surface of the specimens with fewer artifacts induced by photo-damage7,8 and the quantification of the micropermeability of bonded interfaces.
The results of two-photon confocal laser microscopy performed using the hydrophobic experimental resin blends (#1 and 2) applied using water saturated dentin showed severe micropermeability within the entire thickness of the interface with evidence of phase separation and water blisters within the adhesive. We assumed that the saturation of the acid-etched dentin with absolute ethyl alcohol would allow the resins to infiltrate into the demineralized collagen matrix more completely, creating a hybrid layer free of any nano- or microvoids.20,22,23 However, ethanol saturation of acid-etched dentin did not create hybrid layers that were completely free of microvoids, but it did decrease micropermeability of all tested resins more than 88% of that seen in water-saturated specimens and improved the infiltration of the acid-etched dentin. Apparently, ethanol wet bonding permitted the adhesives to achieve better sealing between the resin tags and the walls of the tubules which prevented the fluorescent dye from penetrating into the resin-dentin interface.
Acid-etched dentin is intrinsically micro-porous with the removal of the mineralized dentin matrix. There is a fluid-filled continuum from the lumen of the dentinal tubules into the adjacent interfibrillar spaces that connect the surface of the demineralised matrix to its mineralized base. When these spaces are filled with water, solvated adhesive comonomers may not be able to remove and replace all of the water. In contrast, when these spaces are filled with ethanol, it is likely that less residual water remains in those spaces (Fig. 6).
The high micropermeability observed in the water-saturated hybrid layers compared to the low micropermeability in ethanol-saturated hybrid layers created supports the test hypothesis that there is much less micropermeability in bonded interfaces created using ethanol wet bonding. This was true in six different adhesives (Table 2 and Table 3).
In addition to the excellent solvent properties of ethanol for adhesive comonomers, when acid-etched dentin is saturated with ethanol it permits sufficient interpeptide hydrogen bonding in the collagen matrix to slightly stiffen the matrix,21–27 thereby preventing matrix collapse during bonding and enhancing monomer infiltration. During the infiltration phase of dentin bonding, when the matrix is covered with water during wet-bonding, solvated comonomer mixtures in most etch-and-rinse adhesive systems contain no water. Ethanol is miscible with water and allow water to diffuse from the water-saturated matrix into the (initially) water-free solvated comonomers. The top of the demineralized matrix is likely to be chemically dehydrated first because it is closest to the applied adhesive. As water diffuses from the matrix, alcohol and comonomers will diffuse in the opposite direction. It is appropriate to call the hybrid layer an “inter-diffusion zone”28,29 because that is how it is created. The completeness of removal of water by solvated comonomers depends upon the time allowed for “infiltration” that also includes chemical dehydration of the matrix together with monomer infiltration, the volume of solvated comonomers applied, and their fractional concentration. In some adhesive blends, the solvents occupy about half the volume of the mixture, while in others, they only occupy 8 vol% 30. The larger the volume fraction (0.08–0.5) of water-free solvent, the more chemical dehydration that is possible. However, higher fractional volumes of solvents require more time for subsequent solvent evaporation and increase the probability of allowing more residual solvent to remain after incomplete solvent evaporation.31
When excess ethanol is applied to water-saturated dentin, the ethanol chemically dehydrates the matrix, osmotically shrinks the diameter of the collagen fibrils20 and fills the resulting larger interfibrillar spaces with 100% ethanol. Several authors have speculated that the interfibrillar spaces of acid-etched dentin matrix are not empty after acid-etching, but contain proteoglycan gels.32–37 Organic solvents such as ethanol are known to cause these hydrogels to collapse.38 In ethanol-saturated demineralized dentin matrix, there should be little or no unbound water coating each collagen fibril. Instead, ethanol should be in intimate contact with collagen. When ethanol-solvated comonomers diffuse into ethanol-saturated dentin, we speculate that the ethanol in the interfibrillar spaces will become saturated with the comonomers that are very soluble in ethanol. This should bring the monomers in direct contact with the matrix. In contrast, we speculate that there is a layer of water coating collagen fibrils in water wet-bonding conditions that is never removed or displaced by solvated resins during infiltration of solvated comonomers. We believe that this residual water layer on collagen fibrils provides the nanometer-sized spaces through which lucifer yellow is transported during evaluations of micropermeability. The much lower micropermeability seen in ethanol-saturated matrices is taken as evidence that such nanometer-sized channels are either smaller or less frequent or both.
The ethanol wet-bonding technique is not yet ready for clinical practice but it is evolving toward a practical technique. Currently, instead of inverting a specimen in 100% ethanol for 5 min, we are filling a cavity preparation with 100% ethanol from a dropper bottle for 1 min. This produces similar results. It is likely that the ethanol/water exchange could be done in 30 sec Clearly, ethanol wet-bonding brings about a series of events that decreases micropermeability, allows the creation of a more hydrophobic hybrid layer and increases resin-dentin bond strength.19 What remains to be determined is if this bonding technique can be shown to be superior to water wet-bonding in vivo and whether it will increase the durability of resin-dentin bonds.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Michelle Barnes for secretarial support. This work was supported by grant R01 DE014911 from the National Institute of Dental and Craniofacial Research (P.I. DHP).
REFERENCES
- 1.Sano H, Yoshiyama M, Ebisu S, Burrow MF, Takatsu T, Ciucchi B, Carvalho R, Pashley DH. Comparative SEM and SEM observations of nanoleakage within the hybrid layer. Oper Dent. 1995;20:160–167. [PubMed] [Google Scholar]
- 2.Tay FR, Pashley DH, Suh BI, Hiraishi N, Yiu CKY. Water treeing in simplified dentin adhesives – déjà vu? Buonocore Memorial Lecture. Oper Dent. 2005;30:561–579. [PubMed] [Google Scholar]
- 3.Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. J Dent Res. 2002;81:472–476. doi: 10.1177/154405910208100708. [DOI] [PubMed] [Google Scholar]
- 4.Tay FR, Pashley DH. Water-treeing – a potential mechanism for degradation of dentin adhesives. Am J Dent. 2003;16:6–12. [PubMed] [Google Scholar]
- 5.Van Meerbeek B. The “myth” of nanoleakage. J Adhes Dent. 2007;9:491–492. [PubMed] [Google Scholar]
- 6.Watson TF, Cook RJ, Festy F, Pilecki P, Sauro S. Optical imaging techniques for dental biomaterials interfaces, Chap. 2. In: Curtis RV, Watson TF, editors. Dental Biomaterials: Imaging, Testing and Modelling. Cambridge: Woodhead Publishing Ltd; 2008. pp. 37–57. [Google Scholar]
- 7.Watson TF, Azzopardi A, Etman M, Cheng PC, Sidhu SK. Confocal and multi-photon microscopy of dental hard tissues and biomaterials. Am J Dent. 2000;13:19D–24D. [PubMed] [Google Scholar]
- 8.D'Alpino PH, Pereira JC, Svizero NR, Rueggeberg FA, Carvalho RM, Pashley DH. A new technique for assessing hybrid layer interfacial micromorphology and integrity: two-photon laser microscopy. J Adhe Dent. 2006;8:279–284. [PubMed] [Google Scholar]
- 9.Miller JS, Béthencourt MI, Hahn M, Lee TR, West JL. Laser-scanning lithography (LSL) for the soft lithographic patterning of cell-adhesive self-assembled monolayers. Biotechnol Bioeng. 2006;93:1060–1068. doi: 10.1002/bit.20809. [DOI] [PubMed] [Google Scholar]
- 10.Karotki A, Khurana M, Lepock JR, Wilson BC. Simultaneous two-photon excitation of photofrin in relation to photodynamic therapy. Photochem Photobiol. 2006;82:443–452. doi: 10.1562/2005-08-24-RA-657. [DOI] [PubMed] [Google Scholar]
- 11.Ciucchi B, Bouillaguet S, Holz J, Pashley DH. Dentinal fluid dynamics in human teeth, in vivo. J Endod. 1995;21:191–194. doi: 10.1016/S0099-2399(06)80564-9. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths BM, Nassan M, Sherriff M, Watson TF. Variable polymerization shrinkage and the interfacial micropermeability of a dentine bonding system. J Adhes Dent. 1999;1:119–131. [PubMed] [Google Scholar]
- 13.Sidhu SK, Watson TF. Interfacial characteristics of resin-modified glass-ionomer materials: a study on fluid permeability using confocal fluorescence microscopy. J Dent Res. 1998;77:1749–1759. doi: 10.1177/00220345980770091101. [DOI] [PubMed] [Google Scholar]
- 14.Sauro S, Pashley DH, Monnocci F, Tay FR, Pilecki P, Watson TF. Water uptake and micropermeability of different classes of adhesives bonded to dentine: A comparison study using confocal reflection/fluorescence microscopy. Eur J Oral Sci. 2008;116:184–193. doi: 10.1111/j.1600-0722.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 15.Kanka J. Improved bond strength through acid-etching of dentin and bonding to wet dentin surfaces. J Am Dent Assoc. 1992;123:35–43. doi: 10.14219/jada.archive.1992.0248. [DOI] [PubMed] [Google Scholar]
- 16.Kanka J. Wet bonding: effect of drying time and distance. Am J Dent. 1996;9:273–276. [PubMed] [Google Scholar]
- 17.Ito S, Hashimoto M, Wadgaonkar B, Svizero N, Carvalho RM, Yiu C, Rueggeberg F, Foulger S, Saito T, Nishitani Y, Yoshiyama M, Tay FR, Pashley DH. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials. 2005;26:6449–6459. doi: 10.1016/j.biomaterials.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 18.Malacarne J, Carvalho RM, de Goes MF, Svizero N, Pashley DH, Tay FR, Yiu CK, Carrilho MR. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22:973–980. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Nishitani Y, Yoshiyama M, Donnelly AM, Agee KA, Sword J, Tay FR, Pashley DH. Effects of resin hydrophilicity on dentin bond strength. J Dent Res. 2006;85:1016–1021. doi: 10.1177/154405910608501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tay FR, Pashley DH, Kapur RR, Carrilho MRO, Hur YB, Garrett LV, Tay KCY. Bonding BisGMA to dentin – a proof of concept. J Dent Res. 2007;86:1034–1039. doi: 10.1177/154405910708601103. [DOI] [PubMed] [Google Scholar]
- 21.Sadek FT, Pashley DH, Nishitani Y, Carrilho MR, Donnelly A, Ferrari M, Tay FR. Application of hydrophobic resin adhesives to acid-etched dentin with an alternative wet bonding technique. J Biomed Mater Res A. 2008;84A:19–29. doi: 10.1002/jbm.a.31290. [DOI] [PubMed] [Google Scholar]
- 22.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carvalho RM, Donnelly A, Garcia-Godoy F. From dry bonding to wet bonding to ethanol-wet bonding: a review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–20. [PubMed] [Google Scholar]
- 23.Agee KA, Becker TD, Joyce AP, Rueggeberg FA, Borke JL, Waller JL, Tay FR, Pashley DH. Net expansion of dried demineralized dentin matrix produced by monomer/alcohol saturation and solvent evaporation. J Biomed Mater Res A. 2006;79A:349–358. doi: 10.1002/jbm.a.30752. [DOI] [PubMed] [Google Scholar]
- 24.Carrilho MR, Tay FR, Sword J, Donnelly AM, Agee KA, Nishitani Y, Sadek FT, Carvalho RM, Pashley DH. Dentine sealing provided by smear layer/smear plugs vs. adhesive resins/resin tags. Eur J Oral Sci. 2007;115:321–329. doi: 10.1111/j.1600-0722.2007.00465.x. [DOI] [PubMed] [Google Scholar]
- 25.Eddleston C, Hindle A, Rueggeberg FA, Agee K, Carvalho R, Tay FR, Pashley DH. Dimensional changes in acid-demineralized matrices following the use of HEMA-water vs. HEMA-alcohol primers. J Biomed Mater Res A. 2003;67A:267–282. doi: 10.1002/jbm.a.10151. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho RM, Mendonça JS, Santiago SL, Silveira RR, Garcia FC, Tay FR, Pashley DH. Effects of HEMA/solvent combinations on bond strength to dentin. J Dent Res. 2003;82:597–601. doi: 10.1177/154405910308200805. [DOI] [PubMed] [Google Scholar]
- 27.Garcia FC, Otsuki M, Pashley DH, Tay FR, Carvalho RM. Effects of solvents on the early stage stiffening rate of demineralized dentin matrix. J Dent. 2005;33:371–377. doi: 10.1016/j.jdent.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Van Meerbeek B, Inokoshi S, Braem M, Lambrecht’s P, Vanherle G. Morphological aspects of the resin-dentin inter-diffusion zone with different dentin adhesive systems. J Dent Res. 1992;71:1530–1540. doi: 10.1177/00220345920710081301. [DOI] [PubMed] [Google Scholar]
- 29.Van Meerbeek B, Dhem A, Goret-Nicaise M, Braem M, Lambrechts P, Vanherle G. Comparative SEM and TEM examination of the ultrastructure of the resin-dentin interdiffusion zone. J Dent Res. 1993;72:495–501. doi: 10.1177/00220345930720020501. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto M, Tay FR, Ito S, Sano H, Kaga M, Pashley DH. Permeability of adhesive resin films. J Biomed Mater Res B Appl Biomater. 2005;74B:699–705. doi: 10.1002/jbm.b.30301. [DOI] [PubMed] [Google Scholar]
- 31.Yiu CKY, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, Carvalho RM, Pashley DH, Tay FR. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 2005;26:6863–6872. doi: 10.1016/j.biomaterials.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg M, Takagi M. Dentine proteoglycans: composition, ultrastructure and function. Histochem J. 1993;25:781–806. [PubMed] [Google Scholar]
- 33.Embery G, Hall R, Waddlington R, Septier D, Goldberg M. Proteoglycans in dentinogenesis. Crit Rev Oral Biol Med. 2001;12:331–349. doi: 10.1177/10454411010120040401. [DOI] [PubMed] [Google Scholar]
- 34.Oyarzün A, Rathkamp H, Dreyer E. Immunohistochemical and ultrastructural evaluation of the effects of phosphoric acid etching on dentin proteoglycans. Eur J Oral Sci. 2000;108:546–554. doi: 10.1034/j.1600-0722.2000.00912.x. [DOI] [PubMed] [Google Scholar]
- 35.Breschi L, Lopes M, Gobbi P, Mazzotti G, Falconi M, Perdigão J. Dentin proteoglycans: An immunocytochemical FEISEM study. J Biomed Mater Res. 2002;61:40–46. doi: 10.1002/jbm.10102. [DOI] [PubMed] [Google Scholar]
- 36.Pereira PNR, Bedran-de-Castro AKB, Duarte W, Yamauchi M. Removal of noncollagenous components affects dentin bonding. J Biomed Mater Res B Appl Biomater. 2007;80B:86–91. doi: 10.1002/jbm.b.30572. [DOI] [PubMed] [Google Scholar]
- 37.Mazzoni A, Pashley DH, Ruggeri A, Jr, Vita F, Falconi M, Di Lenarda R, Breschi L. Adhesion to chondrotinase ABC-treated dentin. J Biomed Mater Res B Appl Biomater. 2008 doi: 10.1002/jbm.b.31010. [IN PRESS] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott JE, Thomlinson AM. The structure of interfibrillar proteoglycan bridges (shape modules) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J Anat. 1998;192:391–405. doi: 10.1046/j.1469-7580.1998.19230391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.