Summary
Background
Surgical intervention for advanced Parkinson's disease is an option if medical therapy fails to control symptoms adequately. We aimed to assess whether surgery and best medical therapy improved self-reported quality of life more than best medical therapy alone in patients with advanced Parkinson's disease.
Methods
The PD SURG trial is an ongoing randomised, open-label trial. At 13 neurosurgical centres in the UK, between November, 2000, and December, 2006, patients with Parkinson's disease that was not adequately controlled by medical therapy were randomly assigned by use of a computerised minimisation procedure to immediate surgery (lesioning or deep brain stimulation at the discretion of the local clinician) and best medical therapy or to best medical therapy alone. Patients were analysed in the treatment group to which they were randomised, irrespective of whether they received their allocated treatment. The primary endpoint was patient self-reported quality of life on the 39-item Parkinson's disease questionnaire (PDQ-39). Changes between baseline and 1 year were compared by use of t tests. This trial is registered with Current Controlled Trials, number ISRCTN34111222.
Findings
366 patients were randomly assigned to receive immediate surgery and best medical therapy (183) or best medical therapy alone (183). All patients who had surgery had deep brain stimulation. At 1 year, the mean improvement in PDQ-39 summary index score compared with baseline was 5·0 points in the surgery group and 0·3 points in the medical therapy group (difference −4·7, 95% CI −7·6 to −1·8; p=0·001); the difference in mean change in PDQ-39 score in the mobility domain between the surgery group and the best medical therapy group was −8·9 (95% CI −13·8 to −4·0; p=0·0004), in the activities of daily living domain was −12·4 (−17·3 to −7·5; p<0·0001), and in the bodily discomfort domain was −7·5 (−12·6 to −2·4; p=0·004). Differences between groups in all other domains of the PDQ-39 were not significant. 36 (19%) patients had serious surgery-related adverse events; there were no suicides but there was one procedure-related death. 20 patients in the surgery group and 13 in the best medical therapy group had serious adverse events related to Parkinson's disease and drug treatment.
Interpretation
At 1 year, surgery and best medical therapy improved patient self-reported quality of life more than best medical therapy alone in patients with advanced Parkinson's disease. These differences are clinically meaningful, but surgery is not without risk and targeting of patients most likely to benefit might be warranted.
Funding
UK Medical Research Council, Parkinson's UK, and UK Department of Health.
Introduction
Parkinson's disease is caused in part by loss of dopaminergic neurons in the substantia nigra pars compacta; the resultant abnormal neuronal oscillatory and synchronous activity between the subthalamic nucleus, globus pallidus pars interna, and cerebral cortex leads to increasing problems with tremor, rigidity, bradykinesia, and postural disturbances.1 Levodopa and other dopaminergic drugs relieve these movement disorders,2 but dyskinesia and motor fluctuations develop after a few years.
Most neurosurgery for Parkinson's disease has been done on the thalamus, globus pallidus pars interna, or subthalamic nucleus, using either lesioning or high frequency deep brain stimulation. In recent years, advances in imaging have increased the precision of surgical interventions; this and advances in the understanding of basal ganglia physiology3, 4, 5 have meant that deep brain stimulation of the subthalamic nucleus has been preferred.6
In the late 1990s, there was little reliable evidence from randomised trials on the efficacy and safety of surgery.7 Thus, we started the PD SURG trial with the aim of comparing the effect of surgery with best medical therapy in patients with advanced Parkinson's disease. This report presents the results at 1 year's follow-up.
Methods
Patients
PD SURG is a randomised, open-label trial. Patients with Parkinson's disease for whom current medical therapy was not providing adequate symptomatic control were eligible. Inclusion criteria were diagnosis of Parkinson's disease according to the UK Brain Bank criteria,8 age-adjusted score of greater than 5 on the dementia rating scale-II (DRS-II),9 and fitness for surgery.
For the trial protocol see http://www.pdsurg.bham.ac.uk/investigators/documentation
All patients gave written informed consent before randomisation. The trial was approved by the West Midlands multicentre research ethics committee and local ethics committees at each centre.
Randomisation and masking
Patients were randomly assigned by a telephone call made to the central trial office. Allocation (1:1) to surgery and best medical therapy (surgery group) or best medical therapy alone (medical therapy group) was done by use of a computerised minimisation procedure with the following categories: age at entry (<60, 60–69, and ≥70 years); years since diagnosis of Parkinson's disease (<5, 5–9, 10–14, and ≥15 years); Hoehn and Yahr stage10 in the on state (≤2·0, 2·5, 3·0, and ≥4·0); reason for considering surgery (tremor, dyskinesia, severe off periods, or other reasons); type of surgery (stimulation or lesion) and region to be targeted if allocated to surgery (subthalamic nucleus or globus pallidus pars interna); and drug therapy to be given if allocated to medical therapy (apomorphine or other standard drug treatments for Parkinson's disease). A pair-wise randomisation option was available so that centres could enter two patients together,11 with one allocated to surgery and one to medical therapy. Patients and clinicians were unmasked to treatment allocation.
Procedures
Patients allocated to surgery could receive any standard procedure in use at the time: either stimulation or lesioning of either the subthalamic nucleus or globus pallidus pars interna. Surgery was to be done within 4 weeks of random allocation. The local clinician selected the surgical techniques and postoperative management of stimulator settings for each patient.
Patients in both groups received medical therapy, which could include apomorphine according to local practice, other dopamine agonists, monoamine oxidase type B inhibitors, catechol-O-methyltransferase inhibitors, amantadine, or other drugs for treatment of Parkinson's disease symptoms. Levodopa equivalents were calculated on the basis of 100 mg/day of standard levodopa being equivalent to the following doses of other drugs: 133 mg controlled-release levodopa; 1 mg pergolide, pramipexole, cabergoline, or rasagiline; 1·25 mg sublingual selegiline; 2 mg benzhexol; 3·3 mg rotigotine; 5 mg ropinirole; 10 mg bromocriptine, oral selegiline, or apomorphine; and 100 mg amantadine. The total levodopa dose was multiplied by 1·33 for entacapone and by 1·5 for tolcapone.
Apart from the random treatment allocation, all other aspects of the management of patients were at the discretion of the local clinicians. Patients in the medical therapy group could cross over to receive surgery after about 1 year.
The primary endpoint was the patient's self-evaluation of their functional status by use of the 39-item Parkinson's disease questionnaire (PDQ-39).12 Secondary endpoints included clinical assessment of functioning (unified Parkinson's disease rating scale [UPDRS]13 in both on and off states) and cognitive status (DRS-II).9 The UPDRS was assessed in the on state (on medication) and off state (after overnight withdrawal of medication) at study entry, and in the on state (on medication and on stimulation if surgery was done) and off state (after overnight withdrawal of medication but on stimulation if surgery was done) at follow-up. Neuropsychological assessments were also done in a subset of patients and involved a clinical interview and a battery of 16 psychometric tests and questionnaires. Neuropsychological assessment could not be done on all patients because trained examiners were not available in some centres. For centres that did not have trained examiners, a similar method to that used in a previous multicentre randomised controlled trial was adopted:14 where possible, psychologists (who were based, trained, and supervised centrally in Oxford) visited the centres to complete assessments as required. We collected data on the type and dose of drug treatments for Parkinson's disease and on the incidence of serious adverse events (defined as any event that resulted in a prolonged stay in hospital or admission to hospital, was thought to be life-threatening, or resulted in death). Data on serious adverse events was collected on serious adverse events forms, annual follow-up forms (completed by the local clinician), resource usage forms (completed by the patients), and partial review of medical notes and information from family doctors. Data for non-serious adverse events was also collected for patients in the surgery group by use of post-operation forms (one immediately after surgery and one 6 months later) and subsequent annual follow-up forms. Patients in both groups were to be assessed at 1, 2, 3, 5, 7, and 9 years after randomisation. Here, we present data from the 1-year follow-up.
Statistical analysis
PD SURG was designed to detect a ten-point difference (regarded as clinically important) between groups in the PDQ-39 summary index. Assuming a standard deviation of 30 (two-sided p of 0·05 and 90% power), this required random allocation of about 400 patients in total.
An independent data monitoring committee reviewed efficacy and safety data annually. If large differences between the groups were observed, the data monitoring committee could recommend to the independent trial steering committee that enrolment to the trial be stopped early or modified as appropriate.
Patients were analysed in the treatment group to which they were randomised, irrespective of whether they received their allocated treatment, although patients without follow-up at 1 year could not be included in the analysis. For continuous variables, changes from baseline to 1 year were compared between the groups using t tests. Missing values in PDQ-39 domain scores were imputed by use of the expectation maximisation algorithm.15 Categorical data were analysed using χ2 tests or Fisher's exact tests. Subgroup analyses by protocol-specified stratification parameters were done to explore differences in treatment effect across subgroups, using tests of heterogeneity or tests for trend. Analyses were done using SAS version 9.1 (Cary, NC, USA).
This trial is registered with Current Controlled Trials, number ISRCTN34111222.
Role of the funding source
The study funding sources were not involved in the study design, data collection, data analysis, data interpretation, or the writing of the report, nor were they involved in the decision to submit the paper for publication. The manufacturers of the stimulators used in the trial had no role in the design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the paper for publication. The views expressed in this Article do not necessarily reflect those of the funding bodies. All authors had full access to the study data, read and approved the final version of the paper, and were responsible for the decision to submit the paper for publication.
Results
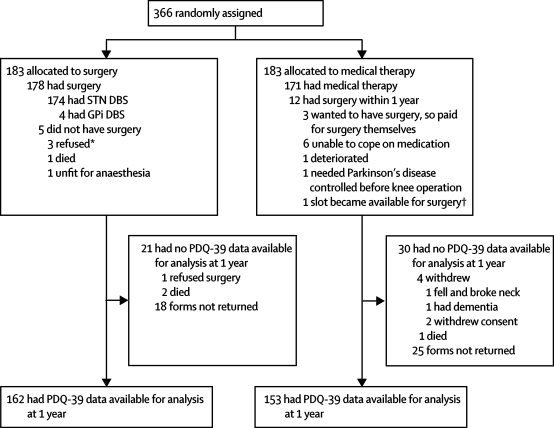
Between November, 2000, and December, 2006, 366 patients from 13 neurosurgical centres in the UK were randomly assigned to the surgery group or to the best medical therapy group (183 per group, figure 1). Baseline characteristics were similar between groups (Table 1, Table 2, Table 3). 348 of 366 patients were aged less than 70 years (mean age 59 years) and 341 patients had had Parkinson's disease for at least 5 years (mean duration 11·4 years). Dyskinesia (n=267) and severe off periods (n=280) were the most common reasons for considering surgery (table 1). In addition to levodopa, 357 of 366 patients had received previous therapy with a dopamine agonist, 197 with a monoamine oxidase type B inhibitor, 214 with a catechol-O-methyltransferase inhibitor, and 145 with apomorphine (90 were still on apomorphine at random allocation).
Figure 1.
Trial profile
PDQ-39=39-item Parkinson's disease questionnaire. STN=subthalamic nucleus. DBS=deep brain stimulation. GPi=globus pallidus pars interna. *1 patient who refused surgery then withdrew from the trial. †As slots for surgery became available around the 1 year timepoint they were used for patients in the medical therapy group. In one case, a slot became available earlier and the treating clinician decided to cross this patient over early (at 10 months).
Table 1.
Demographics and baseline characteristics
| Surgery (n=183) | Medical therapy (n=183) | ||
|---|---|---|---|
| Demographics | |||
| Age (years) | 59 (37–79) | 59 (36–75) | |
| Men | 125 (68%) | 135 (74%) | |
| Baseline characteristics | |||
| Duration of Parkinson's disease (years) | 11·5 (2·0–32·2) | 11·2 (1·0–30·0) | |
| Hoehn and Yahr stage (on state) | |||
| ≤2 | 66 (36%) | 68 (37%) | |
| 2·5 | 58 (32%) | 55 (30%) | |
| 3 | 54 (30%) | 54 (30%) | |
| 4 | 5 (3%) | 6 (3%) | |
| 5 | 0 (0%) | 0 (0%) | |
| Hoehn and Yahr stage (off state)* | |||
| ≤2 | 12 (7%) | 11 (6%) | |
| 2·5 | 19 (11%) | 29 (16%) | |
| 3 | 65 (38%) | 59 (34%) | |
| 4 | 54 (32%) | 55 (31%) | |
| 5 | 19 (11%) | 22 (13%) | |
| Previous treatment† | |||
| Dopamine agonist | 179 (98%) | 178 (97%) | |
| Monoamine oxidase type B inhibitor | 99 (54%) | 98 (54%) | |
| Catechol-O-methyltransferase inhibitor | 103 (56%) | 111 (61%) | |
| Apomorphine | 72 (39%) | 73 (40%) | |
| On apomorphine at study entry | 45 (25%) | 45 (25%) | |
| Reason for considering surgery† | |||
| Tremor | 73 (40%) | 73 (40%) | |
| Dyskinesia | 134 (73%) | 133 (73%) | |
| Severe off periods | 141 (77%) | 139 (76%) | |
| Other‡ | 16 (9%) | 17 (9%) | |
| PDQ-39 summary index | 37·5 (14·6) | 38·7 (13·7) | |
| UPDRS part III: motor (on) | 18·9 (11·4) | 20·1 (11·4) | |
| UPDRS part III: motor (off) | 47·6 (14·0) | 48·6 (14·3) | |
Data are mean (range), number (%), or mean (SD). UPDRS=unified Parkinson's disease rating scale. PDQ=Parkinson's disease questionnaire.
Based on baseline UPDRS part IV: complications of therapy, where patients are asked what their worst Hoehn and Yahr stage had been in the past week. Data were available for 169 of 183 patients in the surgery group and 176 of 183 patients in the medical therapy group.
Not mutually exclusive: more than one previous treatment and reason for considering surgery could apply.
Including motor complications (fluctuations or dystonia, n=12), rigidity (n=7), bradykinesia (n=5), gait problems (n=4), intolerance to drugs (n=3), goose stepping (n=1), and pain (n=1). Percentage values do not add up to 100 in some cases because of rounding.
Table 2.
Primary outcome (PDQ-39)
|
Baseline |
1 year |
Change between baseline and 1 year |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surgery (n=178) | Medical therapy (n=178) | Surgery (n=162) | Medical therapy (n=153) | Difference in means at 1 year (95% CI) | p | Surgery (n=160) | Medical therapy (n=150) | Difference in the mean change (95% CI) | p | |
| Summary index | 37·5 (14·6) | 38·7 (13·7) | 32·5 (15·8) | 38·1 (13·5) | −5·6 (−8·9 to −2·4) | 0·0008 | −5·0 (14·1) | −0·3 (11·1) | −4·7 (−7·6 to −1·8) | 0·001 |
| Mobility | 56·3 (22·5) | 60·1 (22·4) | 48·1 (25·2) | 60·2 (23·6) | −12·0 (−17·5 to −6·6) | <0·0001 | −8·2 (24·8) | 0·7 (18·9) | −8·9 (−13·8 to −4·0) | 0·0004 |
| Activities of daily living | 49·6 (21·4) | 51·4 (20·4) | 37·0 (21·6) | 51·0 (21·1) | −14·0 (−18·7 to −9·3) | <0·0001 | −12·3 (23·6) | 0·1 (20·3) | −12·4 (−17·3 to −7·5) | <0·0001 |
| Emotional wellbeing | 31·3 (19·7) | 31·0 (19·5) | 27·9 (21·1) | 28·6 (18·8) | −0·7 (−5·1 to 3·8) | 0·77 | −3·3 (20·9) | −1·2 (16·2) | −2·1 (−6·3 to 2·1) | 0·33 |
| Stigma | 33·2 (25·2) | 37·2 (26·0) | 25·5 (24·2) | 35·0 (24·6) | −9·5 (−14·9 to −4·1) | 0·0006 | −8·1 (24·4) | −3·0 (21·9) | −5·2 (−10·4 to 0·03) | 0·05 |
| Social support | 18·8 (19·0) | 16·6 (20·9) | 19·2 (20·5) | 16·8 (19·3) | 2·4 (−2·1 to 6·8) | 0·30 | 0·6 (18·4) | 0·5 (19·3) | 0·1 (−4·1 to 4·4) | 0·95 |
| Cognition | 30·4 (19·7) | 29·6 (20·5) | 28·6 (21·3) | 30·0 (19·4) | −1·4 (−5·9 to 3·1) | 0·54 | −1·7 (19·7) | 1·4 (19·2) | −3·0 (−7·4 to 1·3) | 0·17 |
| Communication | 31·9 (23·1) | 31·7 (23·1) | 34·3 (23·0) | 33·0 (21·0) | 1·3 (−3·6 to 6·2) | 0·61 | 2·9 (22·3) | 1·6 (18·0) | 1·4 (−3·2 to 5·9) | 0·55 |
| Bodily discomfort | 48·9 (23·1) | 52·1 (23·8) | 39·2 (23·7) | 50·0 (23·0) | −10·9 (−16·1 to −5·7) | <0·0001 | −9·8 (23·1) | −2·4 (22·6) | −7·5 (−12·6 to −2·4) | 0·004 |
Data are mean (SD). The PDQ-39 range is 0–100; the higher the score, the worse the self-reported quality of life; negative change=improvement. Ten baseline forms and 51 1-year forms were not returned. Five patients returned 1-year PDQ-39 forms, but did not return baseline PDQ-39 forms, so we were unable to calculate a change from baseline for these patients. Missing values in PDQ-39 domain scores were imputed using the expectation maximisation algorithm. PDQ-39=39-item Parkinson's disease questionnaire.
Table 3.
UPDRS and DRS-II scores
|
Baseline |
1 year |
Change between baseline and 1 year |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Surgery |
Medical therapy |
Surgery |
Medical therapy |
Difference in means at 1 year (95% CI) | p |
Surgery |
Medical therapy |
Difference in the mean change (95% CI) | p | |||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |||||
| UPDRS* | ||||||||||||||||
| Part I: mental | 176 | 2·3 (1·7) | 181 | 2·2 (1·6) | 154 | 2·3 (1·8) | 151 | 2·5 (1·7) | −0·2 (−0·6 to 0·2) | 0·29 | 148 | −0·01 (1·8) | 149 | 0·3 (1·6) | −0·3 (−0·7 to 0·1) | 0·15 |
| Part II: activities of daily living (on) | 170 | 9·9 (6·5) | 172 | 10·3 (6·0) | 150 | 9·9 (5·9) | 146 | 10·9 (6·5) | −1·0 (−2·4 to 0·4) | 0·16 | 142 | 0·3 (6·4) | 138 | 1·0 (5·1) | −0·6 (−2·0 to 0·7) | 0·36 |
| Part II: activities of daily living (off) | 162 | 23·8 (7·2) | 167 | 24·7 (7·3) | 130 | 17·6 (8·1) | 139 | 23·9 (7·6) | −6·3 (−8·2 to −4·4) | <0·0001 | 123 | −6·9 (7·2) | 129 | −0·5 (5·9) | −6·4 (−8·0 to −4·7) | <0·0001 |
| Part III: motor (on) | 165 | 18·9 (11·4) | 164 | 20·1 (11·4) | 146 | 16·0 (8·8) | 136 | 20·4 (10·8) | −4·5 (−6·8 to −2·2) | 0·0001 | 135 | −3·3 (9·4) | 124 | 0·8 (9·1) | −4·0 (−6·3 to −1·8) | 0·0006 |
| Part III: motor (off) | 161 | 47·6 (14·0) | 162 | 48·6 (14·3) | 126 | 30·6 (15·2) | 135 | 47·3 (15·4) | −16·6 (−20·4 to −12·9) | <0·0001 | 119 | −17·2 (13·1) | 123 | −0·4 (13·3) | −16·8 (−20·1 to −13·4) | <0·0001 |
| Part IV: complications of therapy | 145 | 9·0 (3·4) | 145 | 9·1 (3·4) | 119 | 4·5 (3·1) | 118 | 9·0 (3·7) | −4·6 (−5·4 to −3·7) | <0·0001 | 106 | −4·4 (3·8) | 112 | −0·2 (2·8) | −4·2 (−5·1 to −3·3) | <0·0001 |
| Total (I–III) score (on) | 161 | 30·9 (16·6) | 159 | 32·6 (16·3) | 141 | 28·1 (13·8) | 132 | 34·0 (15·6) | −5·9 (−9·5 to −2·4) | 0·001 | 128 | −2·8 (14·2) | 116 | 2·5 (11·5) | −5·4 (−8·6 to −2·1) | 0·002 |
| Total (I–III) score (off) | 155 | 73·1 (19·7) | 158 | 75·7 (20·0) | 121 | 50·9 (21·3) | 127 | 73·3 (21·7) | −22·4 (−27·8 to −17·1) | <0·0001 | 114 | −23·7 (17·5) | 113 | −0·5 (18·0) | −23·2 (−27·9 to −18·6) | <0·0001 |
| Total (I–IV) score (on) | 136 | 39·6 (17·8) | 129 | 39·5 (15·4) | 111 | 32·7 (14·6) | 107 | 41·6 (16·3) | −8·9 (−13·0 to −4·7) | <0·0001 | 95 | −6·6 (15·3) | 91 | 1·6 (12·2) | −8·3 (−12·3 to −4·3) | <0·0001 |
| Total (I–IV) score (off) | 137 | 81·5 (21·4) | 132 | 83·6 (21·0) | 100 | 55·6 (22·4) | 105 | 81·9 (24·3) | −26·3 (−32·8 to −19·9) | <0·0001 | 89 | −27·4 (18·9) | 91 | −0·9 (20·1) | −26·6 (−32·3 to −20·9) | <0·0001 |
| Cognition† | ||||||||||||||||
| DRS-II | 159 | 10·7 (2·8) | 156 | 10·4 (2·9) | 126 | 10·6 (3·2) | 144 | 10·1 (3·0) | 0·5 (−0·3 to 1·2) | 0·20 | 121 | −0·4 (3·5) | 133 | −0·4 (2·9) | 0·05 (−0·7 to 0·8) | 0·90 |
UPDRS=unified Parkinson's disease rating scale. DRS-II=dementia rating scale II.
177 patients in the surgery group and 181 in the medical therapy group were assessed at baseline, and 155 in the surgery group and 152 in the medical therapy group were assessed at 1 year. Data on 150 patients in each group were included in assessments of mean changes between baseline and 1 year. The numbers analysed for each part of the UPDRS are different because of missing data. There are no imputation methods for the UPDRS. UPDRS score ranges: mental 0–16; activities of daily living 0–52; motor 0–108; complications 0–23; total (parts I–III) 0–176; total (parts I–IV) 0–199 (high scores=worse clinical assessment of the patient's Parkinson's disease). UPDRS negative change=improvement.
When the trial started patients were allowed to complete either the mini-mental state examination or DRS-II; therefore data on the DRS-II were not available for all patients. DRS-II range: 0–18 (high score=better cognitive function). DRS-II negative change=deterioration.
Five patients in the surgery group did not have surgery: three refused surgery, one was unfit for anaesthesia, and one died before surgery (figure 1). 81 of 178 patients had surgery within 4 weeks of random allocation, 66 within 4–8 weeks, 22 within 8–16 weeks, and nine more than 16 weeks after random allocation. All 178 patients in the surgery group who received surgery had deep brain stimulation and in 174 the subthalamic nucleus was the surgical target. 176 of 178 procedures were bilateral; there was one staged procedure with electrodes implanted 10 months apart.
In the medical therapy group, 12 patients had surgery between baseline and 1 year (figure 1), of whom three received surgery between 10 months and 12 months after random allocation but completed 1-year assessments before surgery. 118 patients randomly allocated to medical therapy had surgery at or after 1 year.
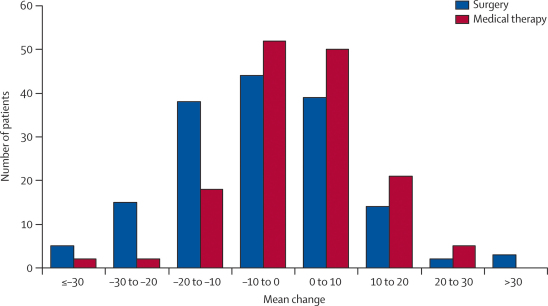
The mean change between baseline and 1 year on the PDQ-39 summary index was −5·0 points in the surgery group and −0·3 points in the medical therapy group (difference −4·7 points, 95% CI −7·6 to −1·8, p=0·001; table 2). The mean change in PDQ-39 summary index between baseline and 1 year ranged from less than −30 to more than 30 (figure 2). The difference in mean change in score between baseline and 1 year was −8·9 for the PDQ-39 domain of mobility (95% CI −13·8 to −4·0; p=0·0004), −12·4 for activities of daily living (−17·3 to −7·5; p<0·0001), −7·5 for bodily discomfort (−12·6 to −2·4; p=0·004), and −5·2 for stigma (−10·4 to 0·03; p=0·05).
Figure 2.
Mean change in 39-item Parkinson's disease questionnaire summary index score between baseline and 1 year
Negative scores=improvement.
For the PDQ-39 summary index score, there was no evidence that the size of the treatment effect in favour of surgery varied with age, duration of Parkinson's disease, Hoehn and Yahr stage, reasons for considering surgery, or whether apomorphine treatment was planned (figure 3). Full details of the subgroup analyses will be reported elsewhere.
Figure 3.
Subgroup analyses of 39-item Parkinson's disease questionnaire summary index score
The mean UPDRS (on) total (parts I–IV) score decreased between baseline and 1 year by 6·6 points in the surgery group (ie, improvement) and increased by 1·6 points in the medical therapy group (difference −8·3 points, 95% CI −12·3 to −4·3; p<0·0001; table 3). The difference in the mean change between baseline and 1 year between the groups was −4·0 (95% CI −6·3 to −1·8; p=0·0006) for the motor (on) subsection of the UPDRS and −4·2 (−5·1 to −3·3; p<0·0001) for the complications of therapy subsection. The mean UPDRS (off) total (parts I–IV) score decreased between baseline and 1 year by 27·4 points in the surgery group and by 0·9 points in the medical therapy group (difference −26·6 points, 95% CI −32·3 to −20·9; p<0·0001). We investigated the answers to UPDRS questions relating specifically to dyskinesia and off periods because these were the two main reasons that patients were considered for surgery. At 1 year, 75 patients in the surgery group and 21 in the medical therapy group reported no waking day dyskinesia (p<0·0001) and 45 in the surgery group and five in the medical therapy group reported no off time (p<0·0001; table 4).
Table 4.
Dyskinesia and off periods
|
Baseline |
1 year |
|||||
|---|---|---|---|---|---|---|
| Surgery | Medical therapy | p* | Surgery | Medical therapy | p* | |
| Dyskinesia | ||||||
| n | 177 | 180 | 0·93 | 155 | 151 | <0·0001 |
| None | 19 (11%) | 27 (15%) | .. | 75 (48%) | 21 (14%) | .. |
| 1–25% | 71 (40%) | 63 (35%) | .. | 65 (42%) | 56 (37%) | .. |
| 26–50% | 55 (31%) | 55 (31%) | .. | 10 (6%) | 44 (29%) | .. |
| 51–75% | 26 (15%) | 27 (15%) | .. | 4 (3%) | 27 (18%) | .. |
| 76–100% | 6 (3%) | 8 (4%) | .. | 1 (1%) | 3 (2%) | .. |
| Off time | ||||||
| n | 175 | 180 | 0·40 | 154 | 152 | <0·0001 |
| None | 6 (3%) | 2 (1%) | .. | 45 (29%) | 5 (3%) | .. |
| 1–25% | 66 (38%) | 68 (38%) | .. | 83 (54%) | 64 (42%) | .. |
| 26–50% | 85 (49%) | 88 (49%) | .. | 23 (15%) | 63 (41%) | .. |
| 51–75% | 16 (9%) | 20 (11%) | .. | 3 (2%) | 17 (11%) | .. |
| 76–100% | 2 (1%) | 2 (1%) | .. | 0 (0%) | 3 (2%) | .. |
Based on UPDRS part IV: complications of therapy (Q32: what proportion of the waking day is dyskinesia present? Q39: what proportion of the waking day is the patient off on average?).
χ2 test for the difference between the surgery group and the medical therapy group across all categories.
The DRS-II score decreased by 0·4 points (ie, deterioration) between baseline and 1 year in both groups (difference 0·05, 95% CI −0·7 to 0·8, p=0·90; table 3). 39 measures were compared in the neuropsychological assessments on up to 163 patients. The Delis-Kaplan executive function system phonemic mean score decreased by 6·5 points (SD 9·4) between baseline and 1 year in the surgery group and decreased by 0·6 points (8·7) in the medical therapy group (difference −5·9, 95% CI −8·9 to −2·9; p=0·0002). The mean change in score on the D-KEFS categorical verbal fluency between baseline and 1 year was −4·5 points (SD 7·8) in the surgery group and −0·2 (7·7) in the medical therapy group (difference −4·4, −6·9 to −1·8; p=0·001). On the Wechsler abbreviated scale of intelligence vocabulary, the mean change from baseline to 1 year was −1·5 (4·8) in the surgery group and 0·6 (4·9) in the medical therapy group (difference −2·1, −3·7 to −0·5; p=0·01).
At 1 year, patients in the surgery group were on a mean levodopa equivalent dose of 894 mg/day (SD 568) and those in the medical therapy group were on 1347 mg/day (585, p<0·0001). This difference of 453 mg/day (95% CI 328 to 580) at 1 year represents a 34% reduction in mean drug dose in the surgery group compared with the medical therapy group.
At baseline, 45 patients in each group were on apomorphine. By 1 year, this had decreased to 13 in the surgery group (ten were on apomorphine at baseline and three started on apomorphine after random allocation) and had increased to 63 in the medical therapy group (34 were on apomorphine at baseline and 29 started on apomorphine after randomisation). Of the patients on apomorphine at 1 year, 54 patients were on continuous drug infusions (six in the surgery group and 48 in the medical therapy group) and 22 were on intermittent dosing (seven in the surgery group and 15 in the medical therapy group).
36 of 178 patients in the surgery group had 43 surgery-related serious adverse events. There were no serious adverse events in the 12 patients in the medical therapy group who received surgery in the first year. The most common surgery-related serious adverse events were infections (n=16; table 5).
Table 5.
Serious adverse events in the first year
| Surgery (n=183) | Medical therapy (n=183) | |||
|---|---|---|---|---|
| Surgery-related | 43 events in 36 patients | 0 events in 12 patients* | ||
| Haemorrhage | 4 (including 1 death)† | 0 | ||
| Infection | 16 | 0 | ||
| DBS-specific adverse events | 13 events in 12 patients | 0 | ||
| Postoperative confusion | 5 | 0 | ||
| Neck pain | 2 | 0 | ||
| Seizures | 2 | 0 | ||
| Deteriorating control of Parkinson's disease because battery was switched off | 1 | 0 | ||
| Psychosis | 1 | 0 | ||
| Unresponsive on operating table (possibly because of levodopa withdrawal) | 1 | 0 | ||
| Visual neglect from oedema | 1 | 0 | ||
| General surgery problems | 10 events in 9 patients | 0 events | ||
| Urinary retention | 4 | 0 | ||
| Pulmonary embolism | 2 | 0 | ||
| Anxiety attack | 1 | 0 | ||
| Difficulty removing catheter | 1 | 0 | ||
| Postoperative hypotension | 1 | 0 | ||
| Pyrexia | 1 | 0 | ||
| Parkinson's-disease related and drug-related | 25 events in 20 patients | 14 events in 13 patients | ||
| Falls | 3 | 7 | ||
| Constipation | 4 in 3 patients | 2 | ||
| Worsening of Parkinson's disease symptoms or uncontrolled Parkinson's disease symptoms | 12 in 11 patients | 2 | ||
| Psychiatric problems | 4 | 1 | ||
| Neuropsychiatric disturbances (including hallucinations or paranoia) | 3 | 0 | ||
| Breakdown | 0 | 1 | ||
| Suicide attempt | 1 | 0 | ||
| Parkinson's disease drug-related | 2 | 2 | ||
| Other | 26 events‡ in 19 patients | 14 events§ in 13 patients | ||
| Deaths | 2 (haemorrhage and pneumonia) | 1 (stroke) | ||
| Total | 96 events in 65 patients | 29 events in 26 patients | ||
Serious adverse events were any event that prolonged a patient's stay in hospital, resulted in the patient being admitted to hospital, was considered to be life-threatening, or resulted in death. DBS=deep brain stimulation.
12 patients randomly assigned to medical therapy received surgery between baseline and 1 year.
One patient had a haemorrhage 5 months after surgery and this was probably not treatment related.
Five urinary problems; five leg swelling or knee swelling, or both; three pain; two chest pain or angina; two chest infection; one collapse; one deep vein thrombosis (more than 8 months after surgery); one pulmonary embolism (more than 8 months after surgery); one polymyalgia rheumatica; one vertigo; one renal colic; one fainting episode; one lacerated wound to forearm; and one head injury.
Four chest pain or angina; two urinary problems; two pain; one abscess on chest wall leading to infection; one deep vein thrombosis; one cauda equina syndrome; one fainting episode; one confusion; and one chest problems.
There were 39 Parkinson's disease-related and drug-related serious adverse events reported in 33 patients (25 events in 20 patients in the surgery group and 14 in 13 patients in the medical therapy group), the most common of which were worsening of Parkinson's disease symptoms or uncontrolled Parkinson's disease symptoms (12 events in 11 patients in the surgery group and two in the medical therapy group; table 5). There was one unsuccessful postoperative suicide attempt in a patient in the surgery group; however, this patient had previously attempted suicide before trial entry. Three patients died during the first year: one from haemorrhage during surgery, one in the surgery group from pneumonia 3 weeks after study entry before surgery was done, and one from stroke in the medical therapy group 10 months after entry.
Discussion
PD SURG was designed with quality of life as the primary endpoint to examine the efficacy of deep brain stimulation versus medical therapy on the daily lives of people with Parkinson's disease and thus provide practical information to inform future use. PD SURG included a representative sample of patients likely to be offered surgery at neuroscience centres in the UK, where apomorphine is readily available, thus enabling comparison of surgery with best medical therapy and providing evidence on the benefits of surgery in a real-world setting. The follow-up reported here was longer than in two other large trials of deep brain stimulation versus medical therapy,16, 17 and thus gives statistically more reliable results and provides evidence on the longer term benefits of surgery (with less likelihood of a so-called honeymoon effect18 in the period just after surgery). Although one trial has investigated the effects of surgery for Parkinson's disease to 18 months, only 20 patients were included and thus the trial was underpowered; also, patients with earlier stage disease were recruited.19
From a purely scientific perspective, a long-term trial of surgery versus medical therapy would have been ideal; however, a realistic design acceptable to both patients and clinicians was necessary, and so surgery was permitted after 1 year in the medical therapy group. There were clear advantages for surgery compared with medical therapy alone at 1 year, both in patient-assessed quality of life and on clinical assessment. These benefits are likely to be meaningful to patients, as measured by use of the PDQ-39,20 and were found in domains of the PDQ-39 that surgery would be expected to affect (eg, mobility and activities of daily living), but not in others (eg, social support, cognition, and communication). These findings were mirrored by clinically meaningful differences on the UPDRS,21 including the patient-rated UPDRS part IV, which showed substantial benefits of surgery in the time and severity of dyskinesia and off periods—the most common reasons for patients to be considered for surgery. Greater benefits for the surgery group than the medical therapy group were seen for off-medication UPDRS assessments. However, this represents an artificial situation, created by a temporary withdrawal of medication and does not indicate an absence of drug because the washout period was not long enough. When considering the real-life on-medication assessment, the magnitude of the benefit seen in our trial is smaller than perhaps anticipated from the numerous small uncontrolled series that have suggested large effects of surgery.22, 23
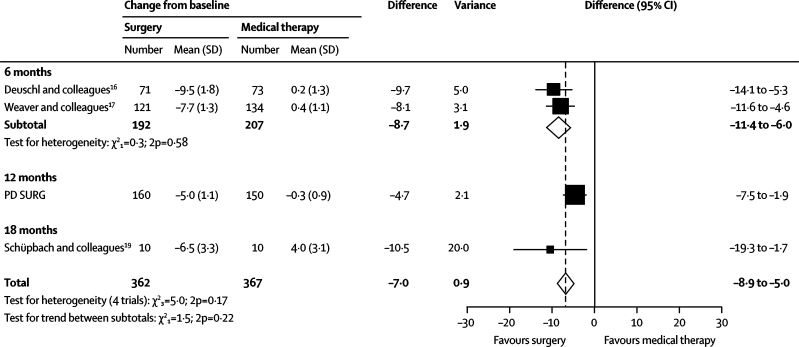
PD SURG, along with other reported randomised trials,16, 17, 19 shows benefits for surgery over best medical treatment in patients with advanced Parkinson's disease, even when apomorphine is available, while also confirming that there are risks associated with surgery. A meta-analysis of PDQ-39 summary index scores showed that the results of the trials are generally consistent with each other (test for heterogeneity, p=0·2; figure 4), although there is evidence of heterogeneity of treatment effect between the trials with 6 months of follow-up and PD SURG with 12 months of follow-up (test for interaction, p=0·04).
Figure 4.
Meta-analysis of 39-item Parkinson's disease questionnaire summary index score in trials of deep brain stimulation versus medical therapy
The PD SURG results at 1 year show smaller differences between the groups in the PDQ-39 summary index (4·7 points) than was seen in the two trials that reported results after 6 months (8·7 points).16, 17 Although from a statistical perspective this difference is not substantial and might be a chance effect, it is worth considering potential alternative explanations based on differences in the trial designs. First, it is possible that there is a large immediate effect of surgery, whether real or in part related to an early so-called honeymoon effect,18 which gradually decreases over time. If the benefits of surgery are relatively transient, this would call into question the long-term value of surgery. Second, there might have been better drug treatment of Parkinson's disease in the medical group of PD SURG than in the other two trials, because of the use of apomorphine in over one-third of patients in this group. Apomorphine is an effective drug in advanced Parkinson's disease2 and can be given by continuous infusion to enable a more constant dose to be delivered to the patient, thereby smoothing out on–off periods and fluctuations. However, apomorphine is expensive, and thus in the UK tends to be used only when other drugs have failed to control the symptoms of Parkinson's disease adequately; that is, it might be used in the same situations as surgery for patients with advanced Parkinson's disease. Hence, a comparison of the effects of surgery plus medical therapy versus medical therapy, in a population of patients whose treatment could have included apomorphine (as in PD SURG), provides better evidence on the relative benefits of surgery than a comparison with medical therapy not including apomorphine. However, apomorphine is less widely used outside the UK, and was not reported as being widely used in the other trials.16, 17 Administration of apomorphine is more complicated than for other Parkinson's disease drugs, requiring infusion and monitoring. Nevertheless, because of its efficacy, apomorphine use might become more common, and thus the results of PD SURG could have wider relevance in future. Optimisation of medical therapy might lead to a smaller comparative advantage for surgery. Nevertheless, surgery is still a valid treatment because patients would need to have only a one-off procedure (albeit with need for stimulator adjustment and replacement) rather than regular administration of an expensive drug. Whether technical aspects of the procedure, such as electrode location within the target site, are factors that could be improved are also important to consider.24, 25
Substantially more patients in the surgery group had serious adverse events than did patients in the medical therapy group, confirming that deep brain stimulation surgery for Parkinson's disease is not without risks.26, 27 Reporting of all serious adverse events, whether surgery related, disease related, or drug related, was mandatory in both the surgical and medical groups. Because a 6-month postoperation form that included serious adverse events was completed only in the surgical group, there could have been differential reporting of serious adverse events unrelated to surgery, despite efforts—through a case-note review—to collect these data in both groups. However, similar surgery-related serious adverse events were seen in the two other trials.16, 17 Furthermore, recently raised concerns about the suicide rate27, 28, 29 after surgery were not confirmed in our study (only one patient attempted suicide after surgery), and only one patient died as a result of the procedure. A limitation of the study is that adverse events that were not serious enough to cause or prolong a patient's stay in hospital were not routinely recorded. However, adverse events are difficult to record accurately (eg, there were three times more adverse events in one of two comparable trials than in the other)16, 17 and their combined effects should be reflected in the participants' perception of their quality of life.
Preliminary analysis of the neuropsychological outcomes, to be reported in detail elsewhere, did not suggest any major adverse effect of surgery other than on verbal fluency and vocabulary. The changes in group means for neuropsychological outcomes represent small decreases in individual scores that are not usually associated with clinically meaningful effects on any activities of daily living.30 Subclinical decreases in verbal fluency after deep brain stimulation or lesional surgery for Parkinson's disease have been reported,31, 32, 33 and are understood to be caused by disruption to projections from the basal ganglia to the prefrontal cortex, which are involved in language and executive skills. For example, after deep brain stimulation of the subthalamic nucleus, substantial associations have been reported between activation of areas including the dorsolateral prefrontal cortex and Broca's area, as measured with fluorine-18-labelled-fluorodeoxyglucose-PET, and performance on verbal fluency tasks.33 These changes are not associated with patient age, disease duration, or dose of dopaminomimetic drugs after surgery;34 however, a frequency-dependent reciprocal modulation of verbal fluency and motor functions in deep brain stimulation of the subthalamic nucleus has been reported.34
Discussions with potential candidates for surgery should include the potential risks and benefits of surgery. Deep brain stimulation is a costly procedure and therefore health economic issues need to be taken into account. However, the amount of drug therapy required in the surgery group was about one third lower than the amount required by those in the medical therapy group. Thus, the cost of surgery will be partly offset by the reduction in the amount of drug therapy required by patients who have had surgery. In particular, if apomorphine or continuous intestinal infusions of levodopa, with high recurrent costs, are the alternative drug treatment options, the cost-effectiveness equation might favour surgery (a full economic analysis of PD SURG will be reported elsewhere). Thus, it is important to identify patients who are or are not likely to benefit from surgery when the risks and costs are taken into account. Subgroup analyses are unreliable, with a high likelihood of chance effects being observed.35 The protocol-specified subgroup analyses did not provide clear evidence that the benefit of surgery differed in different types of patient, although, given the insensitivity of tests for interaction,36 the possible greater benefit in patients with more advanced disease, as measured by Hoehn and Yahr stage (p=0·2), is worthy of further investigation.
Some limitations in the design of PD SURG should be acknowledged, especially in relation to a potential placebo effect. Ideally, patients and assessors would have been masked to treatment allocation. However, sham surgery on patients in the medical therapy group (ie, to insert electrodes and stimulators but not switch them on) would not have been practical (eg, increased theatre time and cost). Furthermore, attempts at masking are likely to be ineffective because, in many cases, patients will be able to tell if their stimulator is switched on. Thus, patients' perception of their quality of life could have been influenced by their knowledge of the treatment they received. Use of independent masked assessors was beyond the resources available for this trial and, because the UPDRS was a secondary endpoint, was not considered essential. The use of the insensitive DRS-II as a measure of cognition was also a potential drawback, but this was deemed to be adequate to provide an overall assessment of the whole trial population, with a more detailed neuropsychological evaluation being done in a subset of patients. The absence of a standard definition of the on state in the protocol might also have been a limitation of the study, although all centres had experienced neurological teams familiar with doing the UPDRS and the comparative nature of the trial meant that any cross-centre differences would apply to both groups and would not introduce bias.
Follow-up of PD SURG will continue for several years and future papers will report on the longer term outcome of immediate surgery versus deferred surgery; subgroup and prognostic factor analyses; neuropsychological effects of surgery; the effect of surgery on carers; further details on the procedure and longer term outcomes; and health economic evaluation. Surgery is likely to remain an important treatment option for patients with Parkinson's disease, especially if the way in which deep brain stimulation exerts its therapeutic effects is better understood, if its use can be optimised by better electrode placement and settings, and if patients who would have the greatest benefit can be better identified.
Acknowledgments
Acknowledgments
PD SURG was supported by funding from the UK Medical Research Council and Parkinson's UK. The University of Birmingham Clinical Trials Unit receives support from the UK Department of Health. The trial also received funding from the UK Department of Health to cover some of the costs of surgery. We thank all the investigators who contributed to the trial and the patients who agreed to enter the study. The PDQ-39 questionnaire was developed by Crispin Jenkinson, Ray Fitzpatrick, and Viv Peto in 1993, who have asserted their moral rights in it, and the copyright, which is owned by Isis Innovation Limited. The questionnaire was first published in 1995 in the journal Quality of Life Research. No part of this questionnaire may be reproduced without the prior written consent of Isis Innovation Limited.
Contributors
AW (chief investigator), SG, TV, CJ, NQ, RM, JD, and KW (co-chief investigator) designed the trial. AW, NI, CR, JD, SP, and KW ran the trial and AW, SG, TV, NQ, and RM recruited patients. AW, SG, TV, CJ, NQ, RM, NI, CR, JD, SP, and KW interpreted the data and wrote the paper. NI and SP analysed the data. RBS designed and ran the neuropsychological substudy. The authors assume responsibility for the accuracy and completeness of the data and for the overall content and integrity of the paper.
Data monitoring committee: P Sandercock (chair), C Baigent, C Counsell, A Mendelow, O Rascol.
Steering committee: D Chadwick (chair), M Baker, K McPherson, J Pickard, C Polkey, M Sculpher, C Warlow.
Trial management centres
University of Birmingham (including the trial management centre at the Birmingham Clinical Trials Unit), Birmingham T Boodell, C E Clarke, J Daniels, R Gray, N Hilken, M Hyde, N Ives, G Madden, M Nixon, S Parsons, S Patel, C Rick, C Tomlinson, K Wheatley, A Williams; University of Oxford, Oxford R Fitzpatrick, A Gray, C Jenkinson (quality of life), E McIntosh (health economics), R Scott (neuropsychology).
Participating centres and PD SURG collaborative group members
Addenbrookes Hospital, Cambridge P Buttery, C Watts (principal investigator), H Watson (number of patients 12); Frenchay Hospital, Bristol S Gill (principal investigator), S Khan, N Heywood, L Mooney, N Patel, P Plaha (64); Hope Hospital, Salford P Cotton, J Dick (principal investigator), M Kellett, P Richardson, M Silverdale (7); John Radcliffe Infirmary, Oxford T Aziz (principal investigator), C Fletcher, R Gregory (38); King's College Hospital, London K Ashkan, C Clough, N Hulse, M Samuel, R Selway (principal investigator) (26); National Hospital for Neurology and Neurosurgery, London E Borrell, J Candalario, T Foltynie, M Hariz, A Lees, P Limousin (principal investigator), N Quinn, L Zrinzo (38); Newcastle General Hospital, Newcastle U Brechany, D Burn (principal investigator), A Jenkins (42); Ninewells Hospital, Dundee S Eljamel (principal investigator), R Swingler, C Young (4); Queen Elizabeth Hospital, Birmingham S Holloway, J Kausar, J Martey, R Mitchell (principal investigator), H Pall, A Williams (65); Queen's Medical Centre, Nottingham K Anderton, S Basu (principal investigator), G Sawle, J Wright (2); Royal Hallamshire Hospital, Sheffield A Franks, R Grunewald, C Keeley, L Nelson, M Radatz (principal investigator), J Rowe (19); Southern General Hospital, Glasgow L Dunn (principal investigator), D Grossett, T Murphy (9); Walton Centre for Neurology and Neurosurgery, Liverpool A Duffy, P Eldridge, N Fletcher, T R K Varma (principal investigator) (40).
Conflicts of interest
TV received travel grants from Medtronic. NQ received reimbursement fees from Medtronic for time spent collecting and analysing data as a member of the ad hoc adverse-event committee for another multicentre study on deep brain stimulation in Parkinson's disease. The other authors have no conflicts of interest.
References
- 1.Williams D, Tijssen M, van Bruggen G. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain. 2002;125:1558–1569. doi: 10.1093/brain/awf156. [DOI] [PubMed] [Google Scholar]
- 2.Poewe W. Treatments for Parkinson disease—past achievements and current clinical needs. Neurology. 2009;72(7 Suppl):S65–S73. doi: 10.1212/WNL.0b013e31819908ce. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell IJ, Clarke CE, Boyce S. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 1989;32:213–226. doi: 10.1016/0306-4522(89)90120-6. [DOI] [PubMed] [Google Scholar]
- 4.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 5.Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6:288–292. doi: 10.1002/mds.870060404. [DOI] [PubMed] [Google Scholar]
- 6.Limousin P, Krack P, Pollak P. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 7.Stowe RL, Wheatley K, Clarke CE. Surgery for Parkinson's disease: lack of reliable clinical trial evidence. J Neurol Neurosurg Psychiatry. 2003;74:519–521. doi: 10.1136/jnnp.74.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattis S. Dementia rating scale: professional manual. Psychological Assessment Resources; Odessa, FL, USA: 1988. [Google Scholar]
- 10.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 11.Daniels J, Wheatley K, Gray R. Pairwise randomisation to balance within centres without possible foreknowledge of allocation. Control Clin Trials. 2003;24(suppl 3S):104S–105S. (abstract P23). [Google Scholar]
- 12.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's disease questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing. 1997;26:353–357. doi: 10.1093/ageing/26.5.353. [DOI] [PubMed] [Google Scholar]
- 13.Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease The unified Parkinson's disease rating scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 14.Scott RB, Farmer E, Smiton A, Tovey C, Clarke M, Carpenter K. Methodology of neuropsychological research in multicentre randomized clinical trials: a model derived from the International Subarachnoid Aneurysm Trial. Clin Trials. 2004;1:31–39. doi: 10.1191/1740774504cn008xx. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson C, Hefferman C, Doll H, Fitzpatrick R. The Parkinson's disease questionnaire (PDQ-39): evidence for a method of imputing missing data. Age Ageing. 2006;35:497–502. doi: 10.1093/ageing/afl055. [DOI] [PubMed] [Google Scholar]
- 16.Deuschl G, Schade-Brittinger C, Krack P. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 17.Weaver FM, Follett K, Stern M. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson's disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetz CG, Wuu J, McDermott MP. Placebo response in Parkinson's disease: comparisons among 11 trials covering medical and surgical interventions. Mov Disord. 2008;23:690–699. doi: 10.1002/mds.21894. [DOI] [PubMed] [Google Scholar]
- 19.Schüpbach WM, Maltête D, Houeto JL. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology. 2007;68:267–271. doi: 10.1212/01.wnl.0000250253.03919.fb. [DOI] [PubMed] [Google Scholar]
- 20.Peto V, Jenkinson C, Fitzpatrick R. Determining minimally important differences for the PDQ-39 Parkinson's disease questionnaire. Age Ageing. 2001;30:299–302. doi: 10.1093/ageing/30.4.299. [DOI] [PubMed] [Google Scholar]
- 21.Schrag A, Sampaio C, Counsell N, Poewe W. Minimal clinically important change on the unified Parkinson's disease rating scale. Mov Disord. 2006;21:1200–1207. doi: 10.1002/mds.20914. [DOI] [PubMed] [Google Scholar]
- 22.Kleiner-Fisman G, Herzog J, Fisman DN. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21:S290–S304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- 23.Weaver F, Follett K, Hur K, Ippolito D, Stern M. Deep brain stimulation in Parkinson disease: a metaanalysis of patient outcomes. J Neurosurg. 2005;103:956–957. doi: 10.3171/jns.2005.103.6.0956. [DOI] [PubMed] [Google Scholar]
- 24.Lanotte MM, Rizzone M, Bergamasco B, Faccani G, Melcarne A, Lopiano L. Deep brain stimulation of the subthalamic nucleus: anatomical, neurophysiological, and outcome correlations with the effects of stimulation. J Neurol Neurosurg Psychiatry. 2002;72:53–58. doi: 10.1136/jnnp.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starr PA, Christine CW, Theodosopoulos PV. Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified lead locations. J Neurosurg. 2002;97:370–387. doi: 10.3171/jns.2002.97.2.0370. [DOI] [PubMed] [Google Scholar]
- 26.Hariz MI, Rehncrona S, Quinn NP, Speelman JD, Wensing C, Multicentre Advanced Parkinson's Disease Deep Brain Stimulation Group Multicenter study on deep brain stimulation in Parkinson's disease: an independent assessment of reported adverse events at 4 years. Mov Disord. 2008;23:416–421. doi: 10.1002/mds.21888. [DOI] [PubMed] [Google Scholar]
- 27.Appleby BS, Duggan PS, Regenberg A, Rabins PV. Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: a meta-analysis of ten years' experience. Mov Disord. 2007;22:1722–1728. doi: 10.1002/mds.21551. [DOI] [PubMed] [Google Scholar]
- 28.Burkhard PR, Vingerhoets FJ, Berney A, Bogousslavsky J, Villemure JG, Ghika J. Suicide after successful deep brain stimulation for movement disorders. Neurology. 2004;63:2170–2172. doi: 10.1212/01.wnl.0000145603.48221.b5. [DOI] [PubMed] [Google Scholar]
- 29.Voon V, Krack P, Lang AE. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson's disease. Brain. 2008;131:2720–2728. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contarino MF, Daniele A, Sibilia AH. Cognitive outcome 5 years after bilateral chronic stimulation of subthalamic nucleus in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:248–252. doi: 10.1136/jnnp.2005.086660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsons TD, Rogers SA, Braaten AJ, Woods SP, Tröster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a meta-analysis. Lancet Neurol. 2006;5:578–588. doi: 10.1016/S1474-4422(06)70475-6. [DOI] [PubMed] [Google Scholar]
- 32.Scott R, Gregory R, Hines N. Neuropsychological, neurological and functional outcome following pallidotomy for Parkinson's disease—a consecutive series of eight simultaneous bilateral and twelve unilateral procedures. Brain. 1998;121:659–675. doi: 10.1093/brain/121.4.659. [DOI] [PubMed] [Google Scholar]
- 33.Cilia R, Siri C, Marotta G. Brain networks underlining verbal fluency decline during STN-DBS in Parkinson's disease: an ECD-SPECT study. Parkinsonism Relat Disord. 2007;13:290–294. doi: 10.1016/j.parkreldis.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Wojtecki L, Timmermann L, Jorgens S. Frequency-dependent reciprocal modulation of verbal fluency and motor functions in subthalamic deep brain stimulation. Arch Neurol. 2006;63:1273–1276. doi: 10.1001/archneur.63.9.1273. [DOI] [PubMed] [Google Scholar]
- 35.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355:1064–1069. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]
- 36.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. John Wiley and Sons Ltd; Chichester, UK: 2000. [Google Scholar]