Abstract
Acyclovir-resistant herpes simplex virus (HSV) has become increasingly common, particularly among patients with human immunodeficiency virus (HIV). We present a case of acyclovir-resistant HSV treated with intralesional cidofovir.
REPORT OF A CASE
A 34-year-old HIV-positive man with a known history of recurrent genital HSV infection presented with an exophytic, left-sided nasal lesion with ulceration. Multiple biopsy specimens only showed extensive granulation tissue, and an indurated, ulcerated plaque recurred and progressed despite surgical removal and corticosteroid injections. His viral load at that time was undetectable, and his CD4 lymphocyte count was 400 cells/μL (to convert to X 109/L, multiply by 0.001). He had been treated for 3 years with antiretroviral therapy and had responded well.
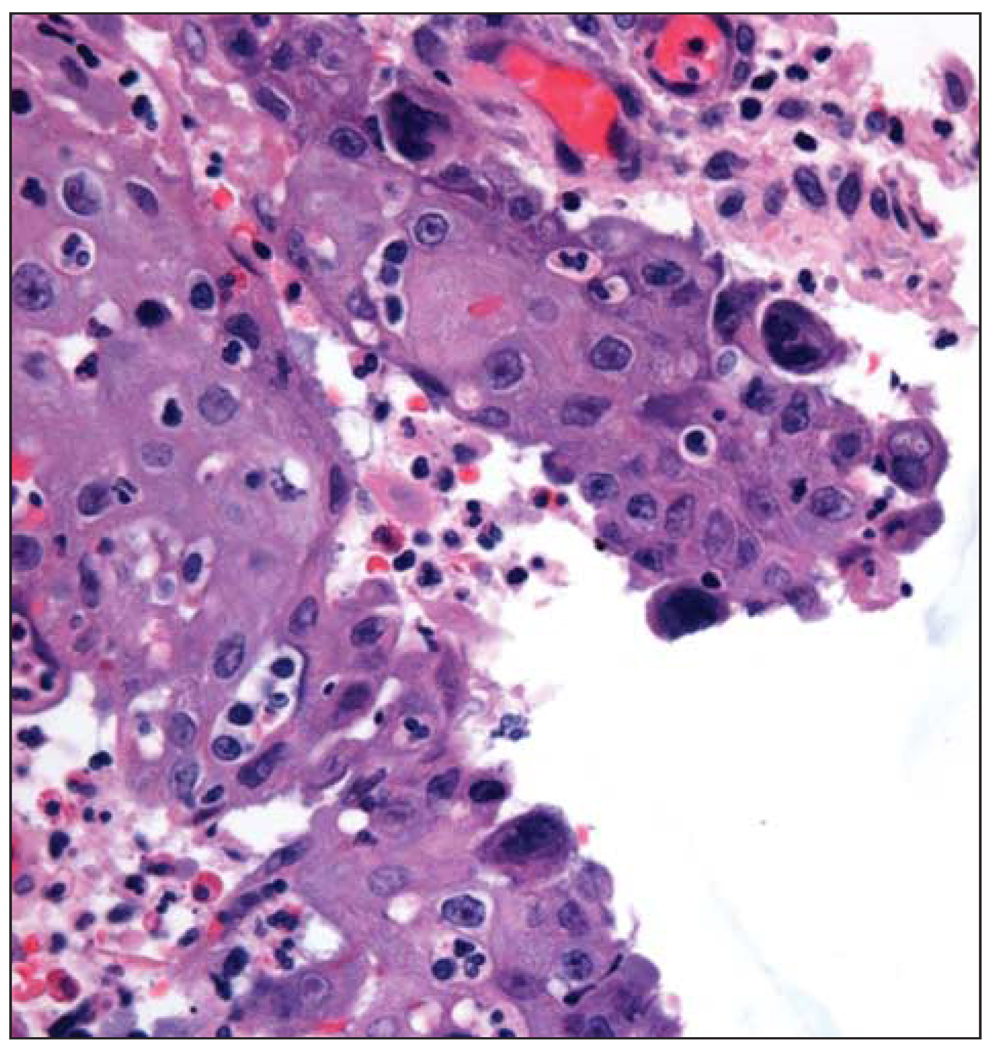
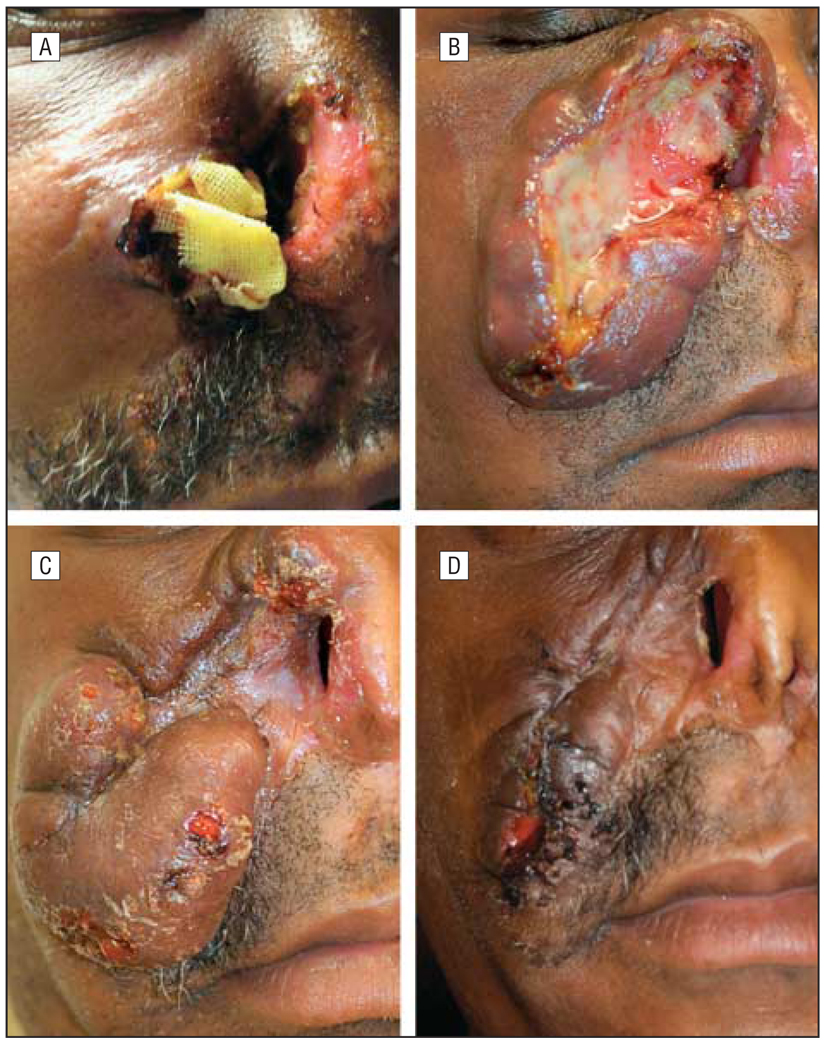
Fifteen months later the nasal lesion extended to the other nares and was again biopsied. Findings from pathologic examination showed HSV changes, as well as abundant granulation tissue without evidence of T-cell lymphoma or infection (Figure 1). As an outpatient this patient had been treated on multiple occasions with oral acyclovir for genital herpes simplex infections, without improvement of the nasal lesion. Despite subsequent treatment with intravenous (IV) acyclovir and a partial rhinectomy, the mass again recurred (Figure 2A). The patient was treated on multiple occasions with IV acyclovir, vancomycin hydrochloride, and further debridement. Results from repeated biopsies confirmed continued HSV infection and suggested clinical resistance to acyclovir. The patient began treatment with IV cidofovir and had slow, yet significant improvement of the lesion, with almost total clearance after 6 weeks of treatment. However, with the patient off therapy, the lesion quickly recurred, with rapid progression toward the lower eyelid, and treatment with IV cidofovir was again instituted (Figure 2B). Given the rapid progression of the lesion while off treatment, combined with risk of renal toxic effects with long term use of IV cidofovir, the clinicians considered other treatment options.
Figure 1.
Photomicrograph depicting biopsy specimen showing viral cytopathic change and multinucleated giant cells (hematoxylin-eosin, original magnification X400).
Figure 2.
Progression of patient’s herpes simplex virus lesion. A, Initial lesion after debridement. B, Recurrence of lesion after treatment with intravenous cidofovir. C, Progression of lesion after initiation of intralesional cidofovir. D, Skin lesion after final treatment with intralesional cidofovir.
THERAPEUTIC CHALLENGE
Although antiviral resistance in HSV has not been a major problem in immunocompetent patients, the problem of acyclovir resistance in immunocompromised patients is well documented. In these patients, alternative nucleoside analogues including cidofovir and pyrophosphate analogues such as foscarnet sodium have been used intravenously. However, the risk of adverse events, most prominently compromise of kidney function, makes these medications challenging to use. In our case the destruction of the patient’s nose and the persistence of HSV prompted us to consider all alternative local and systemic therapies (Figure 2). The idea for his subsequent treatment came from otolaryngologic consultation.
SOLUTION
The treating otolaryngologist suggested, after collaboration with infectious disease specialists, an alternate strategy from the ear, nose, and throat literature and applied it to this patient’s cutaneous HSV lesions.1 In the field of otolaryngology, cidofovir has been used intralesionally for laryngeal papillomatosis. Laryngeal papillomas are recurrent benign growths caused by human papillomavirus. 2 In patients with papillomas, intralesional (IL) cidofovir has been demonstrated to be effective in treating disease and alleviating the need in many for surgical excision. Importantly, patients receiving IL cidofovir do not show increased risk of adverse events3 including kidney dysfunction, nor are they at increased risk of malignancy. 4 Many patients have been observed for 10 years after therapy. After restarting IV cidofovir therapy, there was only minimal improvement in the lesion. At this point, our patient started to receive concurrent IL injections (75 mg/mL, diluted 1:4 with saline [2 mL of cidofovir in 8 mL of isotonic sodium chloride solution]) every 2 weeks for 3 injections. The lesion and surrounding tissue were infiltrated intradermally with a total of 10 mL of diluted cidofovir. The IL injection was performed in the outpatient setting without anesthesia and was well tolerated. The patient had monitoring of his creatinine and glomerular filtration rate on a weekly basis throughout both IV and IL cidofovir therapy and for 2 months after completion of therapy. After initiating IL cidofovir therapy, a great improvement of the lesion was noted.
After a 6-week treatment interruption and enlargement of the lesion, the concentration of the cidofovir injections was increased (75 mg/mL, diluted 1:1 with saline). Over the next 4 to 5 months, the lesion began to regress again. Intravenous cidofovir therapy was stopped owing to renal toxic effects 6 months after initial IL cidofovir therapy and 3 months after restarting IL cidofovir therapy. Our patient received only IL injections for 3 months after discontinuing IV cidofovir therapy on a monthly basis, with continued improvement in the lesion and recovery of his renal function (Figure 2C). As of this writing, the patients had completed IL cidofovir therapy 3 months prior and has been stable for 5 months, with a hypertrophic scar, and no HSV lesions have recurred (Figure 2D). In total, he received 6 months of combined IV and IL cidofovir therapy followed by 3 months of only IL therapy. A significant additional benefit was noted when IL injections were started, and considerable improvement continued with only IL injections.
COMMENT
We report the first case, to our knowledge, of acyclovir-resistant HSV treated with IL cidofovir. There is a precedent for using topical cidofovir, 1% and 3%, in dermatology for cutaneous HSV,5 human papillomavirus,6 and molluscum contagiosum.7 Intravenous cidofovir is also used in patients with acyclovir-resistant HSV skin disease.8,9 We decided that IL therapy would provide a more efficient and direct source of medication for this patient’s lesions because his response to the IV cidofovir seemed to be waning. Additional concern for nephrotoxicity was also considered. Cidofovir is a nucleoside analogue antiviral drug with broad spectrum activity against DNA viruses. It works by inactivating viral DNA polymerase. Pharmacokinetics has been reported for IV administration of cidofovir, but only bioavailability studies have been performed for topical and subcutaneous administration of cidofovir. On intact rabbit skin, the bioavailability of radiolabeled cidofovir has been shown to be 0.2% to 2.1% and is enhanced with vehicles containing propylene glycol.10 On nonintact skin, bioavailability increases to 41%. When given subcutaneously to African green monkeys (Cercopithecus aethiops), the bioavailability has been shown to be 98% without increase in systemic adverse effects.11 Similar studies show 91.5% bioavailability of subcutaneous bioavailability in rats.12 There have been no direct comparisons of skin tissue availability for IV or subcutaneous administration. A review of the otorhinology literature for children and adults receiving IL cidofovir for laryngeal papillomas showed that administration of IL cidofovir was safe, without increased risk of dysplasia or carcinoma. Increased bioavailability combined with greater safety enabled successful treatment of disfiguring HSV in an HIV-positive patient.
Our patient had completed therapy 3 months prior to this writing and has been free from HSV infection for 5 months. Intralesional therapy enabled us to treat the HSV lesion resistant to acyclovir therapy without further compromising this patient’s renal function. This patient will undergo reconstructive surgery after 6 months of being clear of HSV infection.
Footnotes
Author Contributions: Drs Castelo-Soccio, Bernardin, and Stern had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Castelo- Soccio, Stern, Goldstein, and Kovarik. Acquisition of data: Castelo-Soccio, Bernardin, and Goldstein. Analysis and interpretation of data: Goldstein. Drafting of the manuscript: Castelo-Soccio, Bernardin, and Kovarik. Critical revision of the manuscript for important intellectual content: Stern, Goldstein, and Kovarik. Administrative, technical, and material support: Castelo-Soccio, Bernardin, Stern, and Kovarik. Study supervision: Kovarik.
Financial Disclosure: None reported.
REFERENCES
- 1.Soma MA, Albert DM. Cidofovir: to use or not to use? Curr Opin Otolaryngol Head Neck Surg. 2008;16(1):86–90. doi: 10.1097/MOO.0b013e3282f43408. [DOI] [PubMed] [Google Scholar]
- 2.Tanna N, Sidell D, Joshi A, Bielamowicz SA. Adult intralesional cidofovir therapy for laryngeal papilloma: a 10-year perspective. Arch Otolaryngol Head Neck Surg. 2008;134(5):497–500. doi: 10.1001/archotol.134.5.497. [DOI] [PubMed] [Google Scholar]
- 3.Broekema FI, Dikkers FG. Side effects of cidofovir in the treatment of recurrent respiratory papillomatosis. Eur Arch Otorhinolaryngol. 2008;265(8):871–879. doi: 10.1007/s00405-008-0658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dikkers FG. Intralesional cidofovir does not increase the risk of laryngeal dysplasia or laryngeal carcinoma. Int J Pediatr Otorhinolaryngol. 2008;72(10):1581–1582. doi: 10.1016/j.ijporl.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Briand S, Milpied B, Navas D, Thomare P, Stalder JF. 1% topical cidofovir used as a last alternative to treat viral infections. J Eur Acad Dermatol Venereol. 2008;22(2):249–250. doi: 10.1111/j.1468-3083.2007.02304.x. [DOI] [PubMed] [Google Scholar]
- 6.Cha S, Johnston L, Natkunam Y, Brown J. Treatment of verruca vulgaris with topical cidofovir in an immunocompromised patient: a case report and review of the literature. Transpl Infect Dis. 2005;7(3–4):158–161. doi: 10.1111/j.1399-3062.2005.00099.x. [DOI] [PubMed] [Google Scholar]
- 7.Toro JR, Sanchez S, Turiansky G, Blauvelt A. Topical cidofovir for the treatment of dermatologic conditions: verruca, condyloma, intraepithelial neoplasia, herpes simplex and its potential use in smallpox. Dermatol Clin. 2003;21(2):301–309. doi: 10.1016/s0733-8635(02)00116-x. [DOI] [PubMed] [Google Scholar]
- 8.Kottke MD, Parker SR. Intravenous cidofovir induced resolution of disfiguring cutaneous human papilloma virus infection. J Am Acad Dermatol. 2006;55(3):533–536. doi: 10.1016/j.jaad.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Chilukuri S, Rosen T. Management of acyclovir resistant herpes simplex virus. Dermatol Clin. 2003;21(2):311–320. doi: 10.1016/s0733-8635(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 10.Cundy KC, Lynch G, Lee WA. Bioavailability and metabolism of cidofovir following topical administration to rabbits. Antiviral Res. 1997;35(2):113–122. doi: 10.1016/s0166-3542(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 11.Cundy KC, Li ZH, Hitchcock MJ, Lee WA. Pharmacokinetics of cidofovir in monkeys: evidence for prolonged elimination phase represents phosophorylated drug. Drug Metab Dispos. 1996;24(7):738–744. [PubMed] [Google Scholar]
- 12.Cundy KC, Bidgood AM, Lynch G, Shaw JP, Griffin L, Lee WA. Pharmacokinetics, bioavailability, metabolism and tissue distribution of cidofovir (HPMPC) and cyclic HPMPC in rats. Drug Metab Dispos. 1996;24(7):745–752. [PubMed] [Google Scholar]