Abstract
OBJECTIVE
To test a developmental model of neurobehavioral dysregulation relating prenatal substance exposure to behavior problems at age 7.
PATIENTS AND METHODS
The sample included 360 cocaine-exposed and 480 unexposed children from lower to lower middle class families of which 78% were African American. Structural equation modeling (SEM) was used to test models whereby prenatal exposure to cocaine and other substances would result in neurobehavioral dysregulation in infancy, which would predict externalizing and internalizing behavior problems in early childhood. SEM models were developed for individual and combined parent and teacher report for externalizing, internalizing, and total problem scores on the Child Behavior Checklist.
RESULTS
The Goodness of Fit Statistics indicated that all of the models met criteria for adequate fit with 7 of the 9 models explaining 18 to 60% of the variance in behavior problems at age 7. The paths in the models indicate that there are direct effects of prenatal substance exposure on 7-year behavior problems as well as indirect effects, including neurobehavioral dysregulation.
CONCLUSIONS
Prenatal substance exposure affects behavior problems at age 7 through two mechanisms. The direct pathway is consistent with a teratogenic effect. Indirect pathways suggest cascading effects where prenatal substance exposure results in neurobehavioral dysregulation manifesting as deviations in later behavioral expression. Developmental models provide an understanding of pathways that describe how prenatal substance exposure affects child outcome and have significant implications for early identification and prevention.
Keywords: Prenatal substance exposure, cocaine, neurobehavioral dysregulation, behavior problems
Follow-up studies relating prenatal cocaine and other substance exposures to behavioral problems during childhood typically use a behavioral teratology model.1–4 The goal here is to isolate the effects of a teratogen by controlling for effects of potential confounding variables through study design such as matching and/or statistics in which confounding variables are covaried. The variance in outcome explained by the confounding variables is essentially removed from the analysis, and the left over unexplained variance is attributed to the teratogen. For example, we found effects of prenatal cocaine exposure on trajectories of behavior problems from 3 to 7 years independent of the effects of prenatal exposure to alcohol and tobacco, as well as other potentially confounding variables.5 The behavioral teratology model is critically important because it enables us to determine, not only if there is a unique drug effect (i.e., drugs affect outcome when confounding factors are controlled), but also the magnitude of the drug effect (i.e., variability in the outcome measure explained by the drug alone).
A limitation of the behavioral teratology approach, however, is that it does not lend itself to study the developmental processes that lead from exposure to developmental outcome. In addition to direct drug effects there may be indirect effects that suggest how factors mediate the relationship between teratogenic effects and developmental outcome. In a developmental model, effects that are removed as confounding variables can be studied as factors that explain more of the variability in developmental outcome in the presence of teratogenic effects. In other words, these factors are included rather than controlled or removed. In addition, other factors that are hypothesized to be involved in these developmental models can also be included. Statistical techniques such as path analysis or structural equation modeling (SEM) are often used to study these pathways. In previous work, path models have shown that growth deficits associated with prenatal cocaine exposure are mediated, in part, by gestational age.6 In addition to direct effects of prenatal cocaine exposure on IQ, there are indirect effects mediated by head circumference, child behavior, and the home environment.7 In our work, the relationship between prenatal cocaine exposure and hypertension was mediated by body mass index.8
In the present study, we used SEM to test a developmental model relating prenatal cocaine and other substance exposure to behavior problems at age 7. The primary hypothesis was that prenatal exposure to cocaine and other substances would result in neurobehavioral dysregulation in infancy (i.e., problems with arousal and reactivity), which would predict externalizing and internalizing behavior problems in childhood. Externalizing and internalizing behavior problems are part of the neurobehavioral disinhibition profile that includes cognitive, emotional, and behavioral disturbances.9 This profile reflects diminished inhibitory control and is related to early onset of substance use.9–11 In the present study, we were interested in the behavioral antecedents of neurobehavioral disinhibition. Therefore, we predicted children who showed signs of neurobehavioral dysregulation at one month would have a difficult temperament at 4 months, leading to behavior problems at 3 and 7 years of age. Our long-term goal is to delineate developmental pathways in which children with prenatal cocaine exposure are at increased risk for adolescent substance use.
METHODS
Mothers and their infants were enrolled in the Maternal Lifestyle Study (MLS), a multisite longitudinal study of prenatal cocaine exposure conducted at four centers (i.e., Wayne State University, University of Tennessee at Memphis, University of Miami, and Brown University). Each participating center had approval for the study from the institutional review board and a certificate of confidentiality from the National Institute on Drug Abuse. Between May 1993 and 1995, mothers at these centers were enrolled into the study within 24 hours after delivery. Initial screening included the mother’s labor and delivery chart, newborn admission chart, and a meconium sample. A substance-use questionnaire that addressed the mother’s use of nicotine, alcohol, marijuana, cocaine, opiates, and other illicit substances was administered by research staff that were trained and certified in the reliable administration of the interview. Exposure was determined by mother’s verbal admittance of using cocaine during pregnancy and/or a positive meconium assay for cocaine metabolites including gas chromatography/mass spectrometry confirmation. Nonexposed children were born to mothers who denied cocaine use, confirmed by negative meconium test results. All demographic data were collected at the time of the infant’s birth.
As previously reported,12 participants for the longitudinal follow-up were recruited at a 1-month visit. The sample included a cohort of exposed infants (n = 658) who were matched within each site with a group of nonexposed comparison infants (n = 730) by gestational age categories (<32, 33–36, and >36 weeks) and child gender, race, and ethnicity. Of the 1,388 infants recruited, 955 had complete 7-year behavioral outcome data. Infants with prenatal opiate exposure (n = 115) were excluded because they constituted less than 10% of the original sample and differ from the rest of the sample on demographic characteristics (data not shown). The final sample for the current study included 360 cocaine exposed and 480 unexposed children followed from 1 month to age 7. There were no differences in neonatal characteristics between infants included and excluded from the current sample except for differences in APGAR 1 scores among the cocaine-exposed group and more first-born subjects in the comparison group excluded than included (Table 1). For maternal characteristics (Table 2), there were fewer African Americans in the cocaine exposed excluded than included group, more mothers in the 26–36 age range in the cocaine exposed included group than excluded group, and a higher percentage of families in the low SES range in the included than excluded comparison group.
Table 1.
Neonatal Characteristics of Infants Included and Excluded from the Study by Cocaine Exposure
| Cocaine Exposed | Comparison | |||||
|---|---|---|---|---|---|---|
| Included (n = 360) | Excluded (n = 183) | p | Included (n = 480) | Excluded (n = 250) | p | |
| Gestational age (wk) | 36.0 (4.1) | 36.2 (3.7) | .640 | 36.3 (4.1) | 36.4 (4.0) | .663 |
| Birth weight (g) | 2546 (780) | 2587 (656) | .544 | 2675 (881) | 2699 (835) | .719 |
| Length (cm) | 46.3 (5.0) | 46.6 (3.8) | .385 | 44.0 (5.3) | 47.20 (5.1) | .547 |
| Head circumference (cm) | 31.9 (2.9) | 32.1 (2.7) | .513 | 32.2 (3.2) | 32.3 (3.0) | .547 |
| Apgar 1 (median & range) | 8 (1–10) | 8 (1–10) | .033 | 8 (0–9) | 8 (1–9) | .269 |
| Apgar 5 (median & range) | 9 (3–10) | 9 (2–10) | .441 | 9 (4–10) | 9 (1–10) | .118 |
| Male, % | 190 (52.8%) | 98 (53.6%) | .864 | 231 (48.1%) | 114 (45.6%) | .517 |
| First Born, % | 31 (8.6%) | 19 (10.4%) | .500 | 134 (27.9%) | 89 (35.6%) | .032 |
Table 2.
Characteristics of Mothers Included and Excluded from the Study by Cocaine Exposure
| Cocaine Exposed | Comparison | |||||
|---|---|---|---|---|---|---|
| Included (n = 360) | Excluded (n = 183) | p | Included (n = 480) | Excluded (n = 250) | p | |
| Race: African American | 299 (83.1%) | 136 (74.3%) | .016 | 381 (79.4%) | 189 (75.6%) | .242 |
| Age: 26–36 yr | 255 (70.8%) | 111 (60.7%) | .017 | 204 (42.5%) | 90 (36.0%) | .089 |
| Marital Status: Single | 326 (90.8%) | 158 (86.3%) | .111 | 356 (74.2%) | 185 (74.3%) | .970 |
| Insurance: Medicaid | 313 (86.9%) | 158 (86.3%) | .844 | 374 (77.9%) | 193 (77.2%) | .825 |
| Education: < High School | 181 (50.3%) | 92 (50.3%) | .999 | 155 (32.4%) | 72 (28.8%) | .325 |
| Hollingshead SES: Low-V | 93 (27.6%) | 50 (29.2%) | .697 | 109 (22.9%) | 37 (15.2%) | .015 |
| Prenatal Drug use | ||||||
| Alcohol | 274 (76.1%) | 135 (73.8%) | .550 | 234 (48.8%) | 128 (51.2%) | .530 |
| Tobacco | 293 (81.4%) | 157 (85.8%) | .198 | 143 (29.8%) | 68 (27.2%) | .464 |
| Marijuana | 143 (39.7%) | 82 (44.8%) | .255 | 45 (9.4%) | 26 (10.4%) | .657 |
Measures
Medical Characteristics were collected at birth (Table 1).
Demographics
Caregiver age, race, marital status, education level, and Medicaid insurance status were collected at 1 month.
Socioeconomic status (SES) was measured using the Hollingshead Index of Social Position13, 14 at 7 years.
Prenatal substance exposure
Each of the four exposure substances (i.e., cocaine, tobacco, alcohol, and marijuana) was converted to a categorical scale (Y/N) to indicate use of the substance during pregnancy.
Neurobehavioral Dysregulation
The NICU Network Scale (NNNS)15 provides an assessment of infant neurologic, behavioral, and stress/abstinence function. The NNNS includes 13 summary scales with adequate psychometric properties.16 The NNNS was administered at 1 month in the hospital by a certified examiner masked to exposure status of the newborn.
Infant Temperament
A modified version of the Infant Behavior Questionnaire (IBQ)17 was administered as a caregiver-report measure of infant temperament at 4 months.* The IBQ summary scales included Distress to Novelty and Distress to Limitations to represent difficult temperament.
Child Behavior
The Child Behavior Checklists for Ages 2–3 and 4–18 (CBCL) are 99- and 118-item questionnaires, respectively, that assess child behavior using caregiver report.18, 19 Broadband scales for Internalizing, Externalizing, and Total Problems are derived. Test-retest reliability ranged from .74 to .96, and construct validity ranged from .84 to .90. The CBCL was administered to the caregiver at 3 and 7 years as well as the child’s teacher, who was unaware of the child’s drug exposure status, at 7 years.
Statistical Methods
The statistical analysis was a two step process using Mplus SEM software.20 First, latent variables, which are unobserved constructs representing statistically related observed variables, were developed to measure prenatal substance exposure, neurobehavioral dysregulation on the NNNS, difficult temperament on the IBQ, and behavior problems on the CBCL. Second, SEM was used to develop models that help examine the relationship between the latent variables, and Goodness of Fit statistics were used to test the adequacy of each model. The primary model tested was that prenatal substance exposure results in disorganization at 1 month (NNNS), which, in turn predicts difficult temperament at 4 months (IBQ), behavior problems at ages 3 and 7 (CBCL). Socioeconomic status was included in the model, and study site, birth weight, and out of home placement were tested for inclusion. Models were tested individually for caregiver and teacher reports as well as combined caregiver and teacher reports on the CBCL. Additional models were tested for each CBCL broadband scale. A total of nine models were examined for the present study. In these models, a direct effect exists when one latent variable predicts another latent variable, whereas an indirect effect occurs when a third variable meditates the relationship between two latent variables. A path coefficient is a measure of the magnitude of the effect between latent variables, and the total variance of a model indicates how much of an effect is due to all variables in the model.
RESULTS
Development of latent variables
Latent variables were successfully developed for the nine models. A single latent prenatal exposure variable was developed that included all four substances (i.e., cocaine, tobacco, alcohol and marijuana). On the NNNS, the latent variable was composed of two summary scales, including arousal and the number of stress abstinence signs. A positive score indicates infants who were highly aroused and stressed during the exam. The IBQ latent variable was composed of distress to novelty and distress to limits. A positive score describes infants who become upset in novel situations or when limits are set. A latent variable was not necessary for the 3 or 7 year caregiver or teacher CBCL because we developed separate models for the broadband scales. However, a latent variable was developed for the three models in which caregiver and teacher CBCL scores were used together. Positive scores indicated that both parents and teachers rated the child as having more externalizing, internalizing, or total behavior problems. The variables included in these factors were determined by the results of the exploratory and confirmatory factor analysis. Both factor loadings, the correlation between a variable and a latent variable, and Root Mean Square Residuals (RMSR), a statistic used to measure the appropriateness of a model, were adequate for each latent variable. For example, RMSR for the polydrug factor was 0.027, and the factor loadings were .874 for cocaine, .810 for tobacco, .539 for alcohol, and .669 for marijuana.
SEM findings
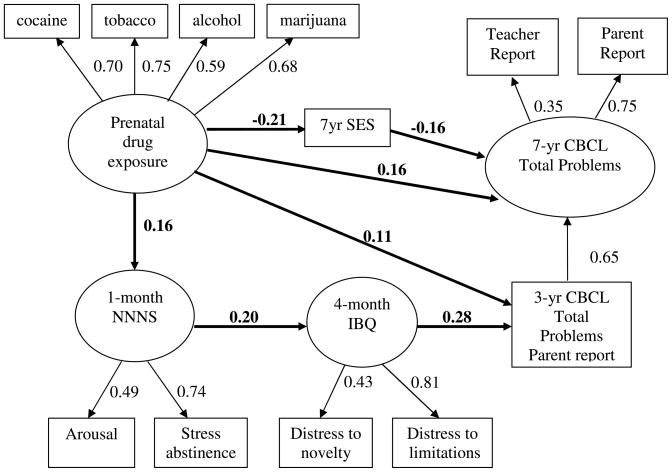
Goodness of fit results indicated all nine models met statistical criteria for an adequate fit (Table 3). For ease of interpretation, Figure 1 is a schematic of the model using combined caregiver and teacher report. Latent variables are in circles and observed variables are in squares. The figure shows four pathways (bolded) from prenatal drug exposure to behavior problems on the CBCL at 7 years: (1) prenatal substance exposure is related to lower SES (β = −.21) and, in turn, predicts behavior problems at 7 (β = −.16); (2) direct effect of prenatal substance exposure on behavior problems at 7 (β = .16); (3) direct effect of prenatal substance exposure on behavior problems at 3 (β = .11), which is related to behavior problems at 7 (β = .65); and (4) infants with prenatal substance exposure show higher arousal and more stress abstinence signs at 1 month on the NNNS (β = .16). Temperamentally, these infants showed distress to novelty and limitations at 4 months (β = .20). These temperamentally difficult infants showed behavior problems on the CBCL at 3 years (β = .28), which, in turn predicted behavior problems on the CBCL at 7 years (β = .65). The entire model explained approximately half (52%) of the variance in total behavior problems at 7 years.
Table 3.
Summary of Structural Equation Models Predicting Child Behavior at Age 7 With Prenatal Drug Exposure (N = 840, 360 cocaine exposed and 480 comparison)
| Path Coefficients in Each Model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Final Outcome Variables |
|||||||||
| Caregiver Report | Teacher Report | Caregiver and Parent Report | |||||||
| Paths in the Model | Externalizing | Internalizing | Total | Externalizing | Internalizing | Total | Externalizing | Internalizing | Total |
| Drug →7yr CBCL | 0.21 | n.s. | 0.16 | n.s. | n.s. | 0.11 | 0.34 | n.s. | 0.16 |
| Drug →3yr CBCL | n.s. | 0.11 | n.s. | 0.14 | n.s. | n.s. | 0.12 | n.s. | 0.11 |
| Drug → 7yr SES | −0.20 | −0.21 | −0.20 | −0.22 | −0.20 | −0.20 | −0.21 | −0.21 | −0.21 |
| 7yr SES →7y CBCL | −0.09 | −0.07 | −0.10 | −0.14 | −0.16 | −0.14 | −0.16 | −0.23 | −0.16 |
| Drug → NNNS | 0.16 | 0.15 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| NNNS → IBQ | 0.21 | 0.17 | 0.22 | 0.20 | 0.18 | 0.23 | 0.19 | 0.19 | 0.20 |
| IBQ→3yr CBCL | 0.27 | 0.25 | 0.29 | 0.28 | 0.27 | 0.31 | 0.29 | 0.27 | 0.28 |
| Total Variance Explained in the Final Outcome Variables | |||||||||
| R Square | .31 | .18 | .32 | .07 | .03 | .22 | .35 | .60 | .52 |
| Goodness of Fit Statistics | |||||||||
| CFI | 0.971 | 0.968 | 0.972 | 0.971 | 0.968 | 0.975 | 0.976 | 0.956 | 0.975 |
| TLI | 0.963 | 0.959 | 0.963 | 0.962 | 0.957 | 0.966 | 0.971 | 0.944 | 0.968 |
| P value | .001 | .001 | .001 | .002 | .001 | .005 | .006 | .000 | .004 |
| χ2/df | 2.04 | 2.07 | 2.04 | 1.94 | 2.02 | 1.83 | 1.73 | 2.22 | 1.80 |
| RMSEA | 0.035 | 0.036 | 0.035 | 0.033 | 0.035 | 0.031 | 0.030 | 0.038 | 0.031 |
Note: All path coefficients are significant (p < .05) unless otherwise indicated as nonsignficant (n.s.). Nonsignificant paths were removed from the final models.
Figure 1. Total Problems at 7 Years By Parent and Teacher Report.
Note. CFI = 0.975, TLI = 0.968, RMSEA = 0.031, Chi-square/df ratio = 1.80. All indicator loadings and path coefficients are significant, p < .05.
The standardized path coefficients for the models predicting externalizing, internalizing and total behavior problems were all statistically significant (p < .05) unless otherwise indicated (Table 3). Overall, 63 of the 72 paths (88%) were statistically significant and 7 of the 9 models explained between 18% and 60% of the variance in behavior problems at age 7. This suggests that the relationships among these variables were relatively stable across types of behavior problems (externalizing, internalizing, and total) and reporters (caregiver, teacher, and combined caregiver and teacher). All of the path coefficients along the indirect (developmental) path from prenatal substance exposure to NNNS, IBQ, 3-year CBCL, and 7-year CBCL were statistically significant. All path coefficients that were not statistically significant were direct paths from prenatal drug exposure to the 3- or 7-year CBCL (9 of the 18 possible paths), although the most robust finding was the direct path from substance exposure to caregiver and parent report of externalizing behavior problems at age 7. This may suggest that in these models, the indirect drug effects of prenatal substance exposure are more robust than the direct effects of prenatal substance exposure.
Testing alternative models
For all models (Table 3), we examined model fit statistics and parameter coefficients with and without the direct effects of poly substances on 3-year and/or 7-year CBCL outcomes and only included models with the best fit. We tested a number of alternative models including models with cocaine as the only substance, models with cocaine excluded from the latent drug variable, models for level of prenatal drug exposure (e.g. heavy exposure), separate models for boys and girls, models with birthweight as a mediator of drug effects on the CBCL, and models with other measures of the postnatal caregiving environment (e.g. quality of the home environment, parenting stress, and maternal psychopathology). These alternative models were rejected because there was no convergence, the model fit statistics did not meet criteria (CFI ≥ .95 and TLI ≥ .95), or they showed poorer fit statistics.
DISCUSSION
We found evidence for a developmental model suggesting both direct and indirect effects of prenatal exposure to cocaine and other substances on behavior problems in childhood. The indirect effects show a sequence of connected behavioral alterations starting in the neonatal period that lead to later behavior problems. Prenatal substance exposure predicted higher infant reactivity and stress at 1 month, which led to a more difficult temperament at 4 months. In turn, difficult temperament was associated with more behavior problems at 3 and 7 years. These indirect effects of prenatal substance exposure were observed in the presence of the direct effects of prenatal substance exposure and effects of SES on these behavioral outcomes. In other words, the indirect effects remained after controlling for the direct effects of prenatal substance exposure and SES. This suggests multiple pathways, both direct and indirect, from prenatal substance exposure to behavioral outcomes in childhood, and that the effects are cumulative.
The direct paths may be thought of as teratogenic effects and supports previous findings relating prenatal substance exposure to caregiver report of behavior problems in school-age children.5, 21, 22 Our findings suggest these may be “true” teratogenic effects because they remained when indirect effects were also included. On the other hand, half of the direct path coefficients failed to reach statistical significance in the SEMs (Table 3), suggesting these effects are less robust than when tested with more traditional regression analysis. These findings also provide an alternative complimentary model for studying unique (direct) effects of prenatal substance exposure. In addition, both direct and indirect effects were found in some models indicating they explain additive portions of the variance.
Our finding that SES mediates the effects of prenatal substance exposure on childhood behavioral problems is consistent with previous findings.23–25 Clearly, this is not a “causal” model; prenatal substance exposure does not “cause” low SES. Rather, SES is a proxy for postnatal environmental factors associated with substance use during pregnancy related to childhood behavior problems. Other factors, including quality of the home environment,26, 27 maternal psychopathology,28 and parenting stress29 associated with the caregiving environment of mothers who used substances during pregnancy were also examined, but these models did not meet statistical criteria or were weaker than models with SES.
We included teacher and caregiver report of behavior problems. In previous work, prenatal cocaine exposure has been related to behavior problems using teacher report.30, 31 In contrast, we averaged teacher and caregiver report because the combination of two independent reports of behavior in different settings might provide a more complete assessment than either report alone. This may explain the higher total variance explained in the models for the combined caregiver and teacher report than for the separate report models.
Our results demonstrate a logical sequence of cascading effects on developmental processes leading to behavior problems in children with exposure to cocaine and other substances. This pathway could explain some of the behavioral origins of neurobehavioral disinhibition in later childhood related to adolescent substance use.9–11 Neurobehavioral disinhibition includes many of the behavioral dimensions reminiscent of the behaviors measured in our study, including emotional liability, irritability, difficult temperament in infancy, and externalizing and internalizing behavior problems. In older children, these behaviors are subsumed under the broader categories of dysregulated emotion and behavior undercontrol. Neurobehavioral disinhibition is thought to be due to dysfunction of the prefrontal cortex and also includes deficits in executive function that were not measured in the current study. We need to continue to follow the children in our study to determine if this developmental pathway extends to the profile of neurobehavioral disinhibition and later substance use in adolescence. Nonetheless, we demonstrated that some precursors of neurobehavioral disinhibition have behavioral echoes in early infancy.
Study Limitations
The limitation of SEM is that alternative models could be developed that fit the data as well as or better than the model developed in this study. Although we examined alternative models (e.g., using measures of the caregiving environment), it is possible that other caregiving environment measures would also result in adequate models. The model we tested was theoretically based, and we acknowledge it could be modified. In addition, we did not measure genetic influences that are potentially involved in the development of behavior problems.
Three of the four measures of child behavior were based on caregiver or teacher report and could have been strengthened with the addition of more objective measures. The path coefficients between the 3- and 7-year CBCL scores were stronger when caregiver report was used at both ages than when caregiver report was related to teacher report (Table 3), suggesting possible reporter bias. An alternative explanation is that the observations of caregivers and teachers reflect the different contexts in which they interact with the children, supporting the decision to average caregiver and teacher scores.
Prenatal substance exposure was a single factor based on the presence or absence of each substance examined, including nicotine, alcohol, marijuana, and cocaine. We tested models that included estimates for each individual substance and the amount of exposure to each substance; however, these models did not have adequate goodness of fit statistics. The alternative would have been to use cocaine only and acknowledge the presence of other substances, but we thought it was more accurate to include all substances in a single model. It is interesting that the path coefficients for the four substances in the latent substance factor (Figure 1) are similar in size, suggesting the contribution of each substance to the factor is similar.
Implications
Early identification and prevention of behavior problems is in line with recent recommendations by the American Academy of Pediatrics32 and would address an important public health need. Our findings suggest it is possible to identify infants at 1 month who are on a path leading to behavior problems in childhood. Intervention studies could be developed to determine if altering behaviors measured by the NNNS (i.e., reducing arousal and stress) could prevent the development or reduce the magnitude of later child behavior problems. Our findings are optimistic because although we may not be able to treat the children who show direct effects of prenatal substance exposure on childhood behavior problems, we were able to identify children who left behavioral tracks in infancy that may be amenable to treatment. In the long term, altering the developmental trajectory of these children could address more severe conduct problems and substance use disorders in adolescence.
Acknowledgments
This study was conducted with support from the National Institutes of Health National Institute of Child Health and Human Development through cooperative agreements and interagency agreement with the National Institute on Drug Abuse; Administration on Children, Youth and Families; and Center for Substance Abuse Treatment. Participating institutions, grant awards, investigators, and key research personnel include the following: Brown University, U10 HD 27904, N01-HD-2-3159 (Barry M. Lester, PhD, Cindy Loncar, PhD, Linda LaGasse, PhD, and Jean Twomey, PhD); University of Miami, U10 HD 21397 (Charles R. Bauer, MD, Wendy Griffin, RN, and Elizabeth Jacque, RN); University of Tennessee, U10 HD 21415 (Henrietta S. Bada, MD, Charlotte Bursi, MSSW, Marilyn Williams, MSW, Deloris Lee, MSW, Lillie Hughey, MSW, and Kimberly Yolton, PhD), Wayne State University, U10 HD 21385 (Seetha Shankaran, MD, Eunice Woldt, MSN, and Jay Ann Nelson, BSN); RTI, International, U01 HD 36790 (W. Kenneth Poole, PhD, Abhik Das, PhD, and Jane Hammond, PhD); and National Institute of Child Health and Human Development (Linda L. Wright, MD, Rosemary Higgins, MD); and National Institute on Drug Abuse (Vincent L. Smeriglio, PhD).
Footnotes
The authors have indicated they have no financial disclosures or conflicts of interest relevant to this article to disclose.
Modifications, approved by M. K. Rothbart, included simplification of language for the MLS population and reduction of response scale to 5 points.
References
- 1.Lester BM, LaGasse LL, Seifer R. Cocaine exposure and children: the meaning of subtle effects. Science. 1998 Oct 23;282(5389):633–634. doi: 10.1126/science.282.5389.633. [DOI] [PubMed] [Google Scholar]
- 2.Tronick EZ, Messinger DS, Weinberg MK, et al. Cocaine exposure is associated with subtle compromises of infants’ and mothers’ social-emotional behavior and dyadic features of their interaction in the face-to-face still-face paradigm. Dev Psychol. 2005 Sep;41(5):711–722. doi: 10.1037/0012-1649.41.5.711. [DOI] [PubMed] [Google Scholar]
- 3.Schiller C, Allen PJ. Follow-up of infants prenatally exposed to cocaine. Pediatr Nurs. 2005 Sep-Oct;31(5):427–436. [PubMed] [Google Scholar]
- 4.Seifer R, LaGasse LL, Lester B, et al. Attachment status in children prenatally exposed to cocaine and other substances. Child Dev. 2004 May-Jun;75(3):850–868. doi: 10.1111/j.1467-8624.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 5.Bada HS, Das A, Bauer CR, et al. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007 Feb;119(2):348–359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- 6.Bandstra ES, Morrow CE, Anthony JC, et al. Intrauterine growth of full-term infants: impact of prenatal cocaine exposure. Pediatrics. 2001 Dec;108(6):1309–1319. doi: 10.1542/peds.108.6.1309. [DOI] [PubMed] [Google Scholar]
- 7.Azuma SD, Chasnoff IJ. Outcome of children prenatally exposed to cocaine and other drugs: a path analysis of three-year data. Pediatrics. 1993 Sep;92(3):396–402. [PubMed] [Google Scholar]
- 8.Shankaran S, Das A, Bauer C, et al. PAS. Washington, DC: 2005. Prenatal cocaine exposure predicts increased blood pressure at 9 yers of age. [Google Scholar]
- 9.Tarter RE, Kirisci L, Mezzich A, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003 Jun;160(6):1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 10.Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug Alcohol Depend. 2004 Feb 7;73(2):121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Tarter RE, Kirisci L, Reynolds M, Mezzich A. Neurobehavior disinhibition in childhood predicts suicide potential and substance use disorder by young adulthood. Drug Alcohol Depend. 2004 Dec 7;76 (Suppl):S45–52. doi: 10.1016/j.drugalcdep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Lester BM, Tronick EZ, LaGasse L, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002 Dec;110(6):1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 13.Holingshead A. A Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 14.Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment. 2002 Jun;9(2):145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- 15.Lester BM, Tronick EZ. The NICU network neurobehavioral scale (NNNS) Pediatrics. 2004;113 (Supplement):631–699. [PubMed] [Google Scholar]
- 16.Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003 Jun;111(6 Pt 1):1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- 17.Rothbart MK. Measurement of temperament in infancy. Child Dev. 1981;52:569–578. [Google Scholar]
- 18.Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 19.Achenbach TM. Manual for the Child Behavior Checklist/2–3. Burlington, VT: University of Vermont, Department of Psychiatry; 1992. [Google Scholar]
- 20.Muthen LK, Muthen BO. Mplus User’s Guide. 4. Los, Angeles, CA: Muthen & Muthen; pp. 1998–2006. [Google Scholar]
- 21.Sood B, Delaney-Black V, Covington C, et al. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. dose-response effect. Pediatrics. 2001 Aug;108(2):E34. doi: 10.1542/peds.108.2.e34. [DOI] [PubMed] [Google Scholar]
- 22.Linares TJ, Singer LT, Kirchner HL, et al. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006 Jan-Feb;31(1):85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arria AM, Derauf C, Lagasse LL, et al. Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) study. Matern Child Health J. 2006 May;10(3):293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- 24.Bor W, Najman JM, Andersen MJ, O’Callaghan M, Williams GM, Behrens BC. The relationship between low family income and psychological disturbance in young children: an Australian longitudinal study. Aust N Z J Psychiatry. 1997 Oct;31(5):664–675. doi: 10.3109/00048679709062679. [DOI] [PubMed] [Google Scholar]
- 25.Duncan GJ, Brooks-Gunn J, Klebanov PK. Economic deprivation and early childhood development. Child Dev. 1994 Apr;65(2 Spec):296–318. [PubMed] [Google Scholar]
- 26.Bendersky M, Bennett D, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006 Jan-Feb;31(1):71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett DS, Bendersky M, Lewis M. Children’s intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Dev Psychol. 2002 Sep;38(5):648–658. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warner TD, Behnke M, Hou W, Garvan CW, Wobie K, Eyler FD. Predicting caregiver-reported behavior problems in cocaine-exposed children at 3 years. J Dev Behav Pediatr. 2006 Apr;27(2):83–92. doi: 10.1097/00004703-200604000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagner DM, Sheinkopf SJ, Miller-Loncar C, et al. The Effect of Parenting Stress on Child Behavior Problems in High-Risk Children with Prenatal Drug Exposure. Child Psychiatry Hum Dev. 2008 Jul 15; doi: 10.1007/s10578-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delaney-Black V, Covington C, Templin T, et al. Teacher-assessed behavior of children prenatally exposed to cocaine. Pediatrics. 2000 Oct;106(4):782–791. doi: 10.1542/peds.106.4.782. [DOI] [PubMed] [Google Scholar]
- 31.Nordstrom Bailey B, Sood BG, Sokol RJ, et al. Gender and alcohol moderate prenatal cocaine effects on teacher-report of child behavior. Neurotoxicol Teratol. 2005 Mar-Apr;27(2):181–189. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Developmental surveillance and screening of infants and young children. Pediatrics. 2001 Jul;108(1):192–196. doi: 10.1542/peds.108.1.192. [DOI] [PubMed] [Google Scholar]