Abstract
Hyperactivation of ErbB signaling is implicated in metastatic breast cancer. However, the mechanisms that cause dysregulated ErbB signaling and promote breast carcinoma cell invasion remain poorly understood. One pathway leading to ErbB activation that remains unexplored in breast carcinoma cell invasion involves transactivation by G-protein-coupled receptors (GPCRs). Protease-activated receptor-1 (PAR1), a GPCR activated by extracellular proteases, is overexpressed in invasive breast cancer. PAR1 is also proposed to function in breast cancer invasion and metastasis, but how PAR1 contributes to these processes is not known. In this study, we report that proteolytic activation of PAR1 by thrombin induces persistent transactivation of EGFR and ErbB2/HER2 in invasive breast carcinoma, but not in normal mammary epithelial cells. PAR1-stimulated EGFR and ErbB2 transactivation leads to prolonged extracellular signal-regulated kinase-1 and −2 signaling and promotes breast carcinoma cell invasion. We also show that PAR1 signaling through Gαi/o and metalloprotease activity is critical for ErbB transactivation and cellular invasion. Finally, we demonstrate that PAR1 expression in invasive breast carcinoma is essential for tumor growth in vivo assessed by mammary fat pad xenografts. These studies reveal a critical role for PAR1, a receptor activated by tumor-generated proteases, in hyperactivation of ErbB signaling that promotes breast carcinoma cell invasion.
Keywords: thrombin, GPCR, metalloprotease, MDA-MB-231, G protein
Introduction
The molecular mechanisms underlying breast tumorigenesis, invasion and metastasis remain poorly understood. One pathway implicated in metastatic breast cancer and associated with poor clinical prognosis involves hyperactivation of ErbB signaling (Yarden, 2001). The ErbB family of receptor tyrosine kinases includes epidermal growth factor (EGF) receptor (EGFR)/ErbB1, ErbB2/HER2, ErbB3 and ErbB4. ErbB family members undergo ligand-induced dimerization, which provokes intrinsic kinase activation, transphosphorylation of cytoplasmic tyrosine residues and recruitment of signaling molecules. ErbB2/HER2 is overexpressed in ~20–30% of human invasive breast cancers and is correlated with increased metastatic potential and decreased patient survival (Cobleigh et al., 1999). Increased expression of EGFR and ErbB3 is also correlated with reduced breast cancer patient survival (Nicholson et al., 2001; Witton et al., 2003). ErbB2 does not bind ligand directly but exists in an extended conformation poised to signal by forming heterodimeric complexes with EGFR or ErbB3 (Burgess et al., 2003; Garrett et al., 2003). Humanized antibodies generated against ErbB2/HER2 termed trastuzumab or Herceptin reduce ErbB2 activity and expression at the cell surface and increase survival of patients with metastatic breast cancer (Cobleigh et al., 1999; Slamon et al., 2001). However, many patients who achieve initial response to trastuzumab-based therapies acquire resistance and many ErbB2/HER2-positive breast cancers do not respond to trastuzumab treatments (Nahta and Esteva, 2007). Thus, identifying other pathways that lead to hyperactivation of ErbB signaling is critical for identifying new drug targets and for improving treatment options for patients with metastatic breast cancer disease.
One pathway leading to ErbB signaling that has not been thoroughly investigated in breast cancer progression involves transactivation by G-protein-coupled receptors (GPCRs) (Wetzker and Bohmer, 2003). Transactivation of EGFR by GPCRs results from the activation of a disintegrin and metalloprotease (ADAM) and/or matrix metalloprotease (MMP) family members, which release membrane-anchored ligands such as heparin-binding-EGF or transforming growth factor-α (TGF-α) and through intracellular signaling pathways (Prenzel et al., 1999; Gschwind et al., 2003). Previous studies have shown that several GPCR agonists including thrombin are capable of inducing transactivation of EGFR in both transformed and nontransformed cells (Daub et al., 1996; Prenzel et al., 1999; Darmoul et al., 2004; Bergman et al., 2006). GPCR-mediated transactivation of EGFR through release of membrane-anchored ligands also functions in various pathophysiological diseases, including kidney deterioration, cardiac hypertrophy and cardioprotection (Asakura et al., 2002; Lautrette et al., 2005; Noma et al., 2007). However, the role of GPCR-mediated ErbB transactivation in breast carcinoma cell invasion has not been previously investigated.
Protease-activated receptor-1 (PAR1), a GPCR uniquely activated by proteolysis, has been implicated in invasive and metastatic breast cancer (Vu et al., 1991; Arora et al., 2007). PAR1 elicits cellular responses to multiple tumor-generated proteases, including plasmin, MMP-1 and coagulant proteases (Kuliopulos et al., 1999; Boire et al., 2005). An increase in PAR1 expression is positively correlated with invasiveness of primary breast tissue specimens and breast carcinoma cell lines (Even-Ram et al., 1998; Booden et al., 2004). Moreover, the ectopic expression of PAR1 in noninvasive MCF7 breast carcinoma is sufficient to promote tumor growth and invasion in a xenograft nude mouse model (Boire et al., 2005). Activated PAR1 also fails to downregulate in invasive breast carcinoma and consequently signals persistently to extracellular signal-regulated kinase-1 and -2 (ERK1/2) and promotes breast carcinoma invasion (Booden et al., 2004). The mechanism by which activated PAR1 induces prolonged ERK1/2 signaling and cellular invasion is not known.
In this study, we report that proteolytic activation of PAR1 by thrombin causes persistent transactivation of EGFR and ErbB2 that sustains ERK1/2 signaling and promotes breast carcinoma cell invasion. PAR1 expression in breast carcinoma is also essential for tumor growth in vivo. These findings reveal a critical role for PAR1 in mediating hyperactivation of ErbB signaling that promotes breast carcinoma cell invasion.
Results
EGFR and ErbB2/HER2 transactivation by PAR1
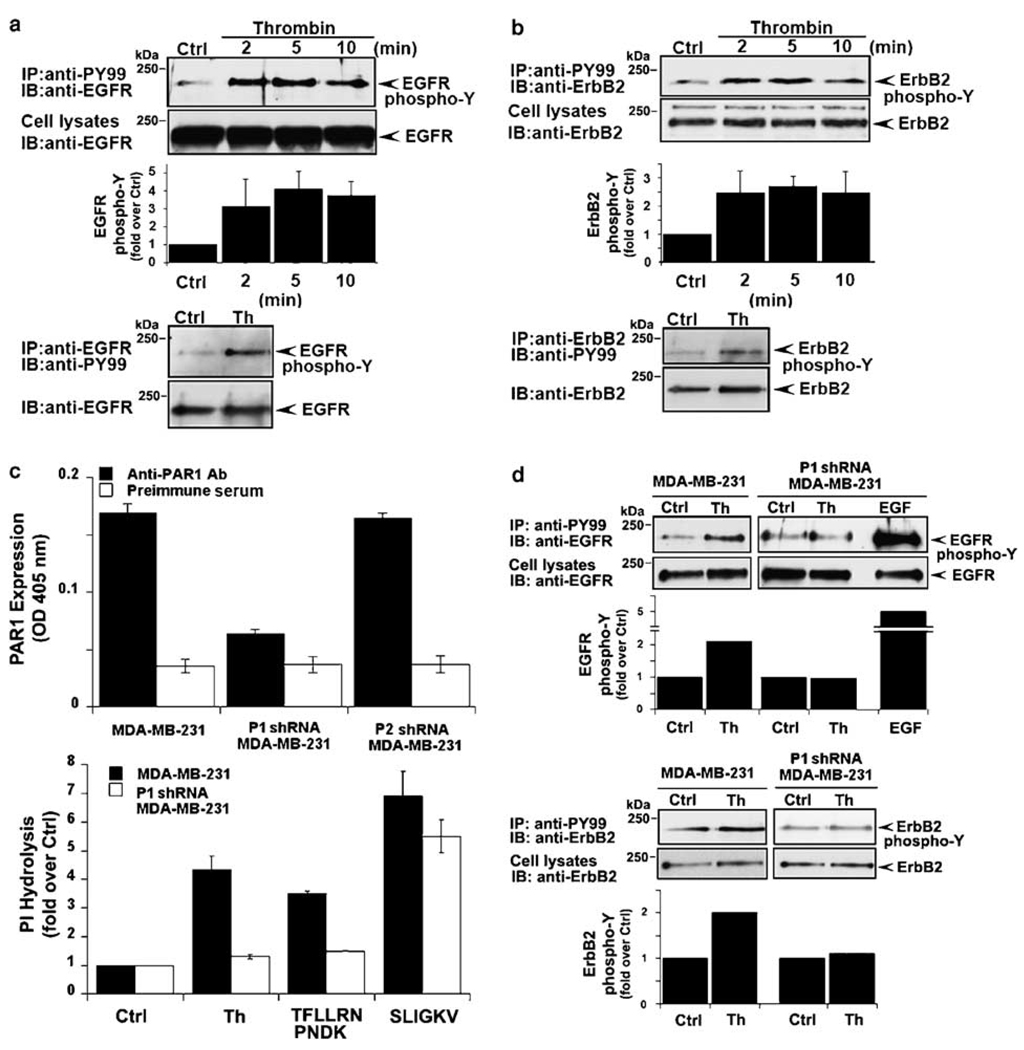
To understand the contribution of PAR1 to processes that lead to breast carcinoma invasion and metastasis, we first examined whether thrombin transactivates EGFR in invasive breast carcinoma. MDA-MB-231 cells were incubated with thrombin for various times at 37 °C and EGFR tyrosine phosphorylation was assessed. Thrombin caused a ~3-fold increase in EGFR tyrosine phosphorylation at 2 min that remained elevated over a 10 min time course (Figure 1a), consistent with the early increase in thrombin-stimulated EGFR transactivation previously observed in multiple cell types (Daub et al., 1996; Prenzel et al., 1999). Reciprocal immunoprecipitation experiments revealed a similar early increase in EGFR tyrosine phosphorylation following thrombin incubation (Figure 1a). The PAR1-specific agonist peptide TFLLRNPNDK also induced EGFR transactivation in a manner comparable to that observed with thrombin (Supplementary Figure 1a).
Figure 1.
Protease-activated receptor-1 (PAR1) is essential for thrombin-induced ErbB transactivation in breast carcinoma cells. Serum-deprived MDA-MB-231 cells were incubated with 10 nM thrombin (a and b) for various times at 37 °C. Cells were lysed, immunoprecipitated with anti-PY99 antibody and immunoblotted with anti-epidermal growth factor (EGF) receptor (EGFR) or anti-ErbB2 antibody. Total cell lysates were immunoblotted for EGFR or ErbB2 as a control. The time-course of thrombin-induced EGFR and ErbB2 transactivation shown is from a representative experiment. The data (mean ± s.e.) are expressed as fold increase over basal from three independent experiments. In reciprocal experiments, serum-deprived MDA-MB-231 cells were incubated with 10 nM thrombin for 4 min at 37 °C, lysed and immunoprecipitated with anti-EGFR or anti-ErbB2 antibodies and then immunoblotted with anti-PY99 antibody. (c) Control, P1 and P2 shRNA-expressing MDA-MB-231 cells were incubated with anti-PAR1 antibody or preimmune serum, fixed and the amount of antibody bound to the cell surface was measured by enzyme-linked immunosorbent assay (ELISA). The data (mean ± s.d., n = 3) shown are from a representative experiment repeated at least three times. MDA-MB-231 control and P1 shRNA cells labeled with myo-[3H]inositol were incubated in the absence or presence of 10 nM thrombin, 100 µM TFLLRNPNDK or 100 µM SLIGKV for 60 min at 37 °C and the amounts of [3H]inositol phosphates generated were then measured. The data (mean ± s.d., n = 3) are expressed as fold increase over untreated control and are representative of three different experiments. (d) Control and PAR1-deficient P1 shRNA-expressing MDA-MB-231 cells were incubated in the absence or presence of 10 nM thrombin or 16 nM EGF for 4 min at 37 °C, lysed and immunoprecipitated with anti-PY99 antibody and immunoblotted with anti-EGFR or anti-ErbB2 antibody. Cell lysates were immunoblotted for EGFR or ErbB2 as a control. The data are expressed as fold increase over untreated control and are representative of three independent experiments.
ErbB2 is overexpressed in metastatic breast carcinoma and signals by forming heterodimeric complexes with EGFR or ErbB3; raising the possibility that thrombin might induce ErbB2 transactivation in invasive breast carcinoma. Remarkably, thrombin stimulated an ~2-fold increase in ErbB2 tyrosine phosphorylation at 2 min that was sustained for 10 min in MDA-MB-231 cells (Figure 1b). ErbB2 transactivation by thrombin was also confirmed in reciprocal immunoprecipitation experiments (Figure 1b). The PAR1-specific agonist peptide TFLLRNPNDK also stimulated ErbB2 transactivation with a magnitude and duration similar to that observed with thrombin (Supplementary Figure 1b). These studies are the first to demonstrate thrombin-induced ErbB2 transactivation in invasive breast carcinoma.
To directly test whether PAR1 mediates thrombin-induced ErbB transactivation, we used MDA-MB-231 breast carcinoma cells deficient in endogenous PAR1 expression. MDA-MB-231 cells expressing a short-hairpin loop PAR1 (P1) small interfering RNA (siRNA) showed a substantial reduction in PAR1 cell surface expression compared to control cells or cells stably transduced with PAR2-specific P2 short hairpin RNA (shRNA) (Figure 1c). Thrombin- and TFLLRNPNDK-stimulated phosphoinositide hydrolysis were also virtually abolished in P1 shRNA-expressing cells compared to control cells (Figure 1c), whereas signaling induced by the PAR2-selective peptide agonist SLIGKV remained intact. Together these findings are consistent with a loss of functional PAR1 in P1 shRNA-expressing MDA-MB-231 cells.
We next assessed thrombin-induced ErbB transactivation in PAR1-deficient MDA-MB-231 cells. Thrombin stimulated an ~2-fold increase in EGFR tyrosine phosphorylation in control cells but failed to induce EGFR transactivation in PAR1-deficient cells (Figure 1d). In contrast, addition of EGF ligand caused a robust increase in EGFR tyrosine phosphorylation in P1 shRNA-expressing cells (Figure 1d), indicating that EGFR activity remained intact in PAR1-deficient cells. Thrombin-stimulated ErbB2 transactivation was also completely inhibited in PAR1-deficient cells compared to control cells (Figure 1d). We also found that MDA-MB-231 cells express PAR1 and PAR2, but not other PARs, consistent with a role for PAR1 in thrombin-stimulated responses (Supplementary Figure 2). Together, these data strongly suggest a critical role for PAR1, and not another receptor or factor, in thrombin-induced EGFR and ErbB2 transactivation in invasive breast carcinoma cells.
EGFR kinase activity is required for thrombin-induced ErbB transactivation
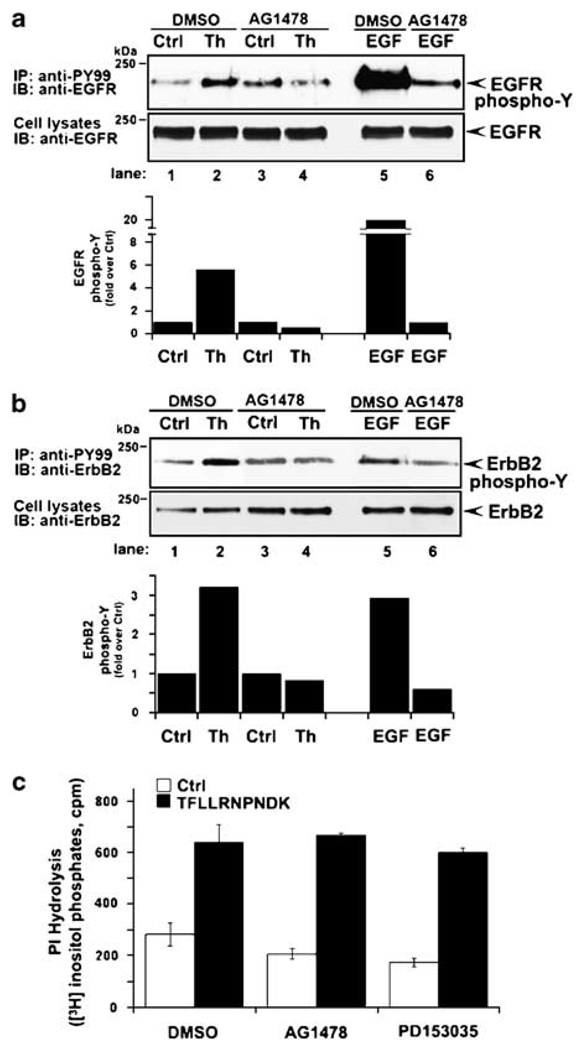
To determine whether EGFR kinase activity is required for thrombin-stimulated ErbB transactivation we used the tyrphostin AG1478 compound, a selective EGFR kinase inhibitor. AG1478 substantially inhibited EGF-induced EGFR tyrosine phosphorylation (Figure 2a, lanes 5–6), consistent with inhibition of EGFR kinase activity. EGF-stimulated ErbB2 tyrosine phosphorylation was also blocked by AG1478 (Figure 2b, lanes 5 and 6), suggesting that EGFR kinase activity mediates ErbB2 transphosphorylation. AG1478 also caused marked inhibition of EGFR and ErbB2 tyrosine phosphorylation induced by thrombin (Figure 2a and b, lanes 1–4). These findings reveal a critical role for EGFR kinase activity in thrombin-induced EGFR and ErbB2 transactivation in invasive breast carcinoma. Similar effects were observed with the PD153035 compound, another specific inhibitor of EGFR kinase activity (data not shown). However, neither AG1478 nor PD153035 affected PAR1-stimulated phosphoinositide hydrolysis, excluding the possibility of global inhibitor effects on PAR1 function (Figure 2c). Thus, EGFR tyrosine kinase activity is critical for thrombin-stimulated EGFR and ErbB2 transactivation in highly invasive breast carcinoma cells.
Figure 2.
Thrombin-induced epidermal growth factor (EGF) receptor (EGFR) and ErbB2 transactivation requires EGFR kinase activity. (a and b) MDA-MB-231 cells were pretreated with 0.1% dimethyl sulfoxide (DMSO) or 2 µM AG1478 for 2 h at 37 °C. Cells were then incubated with or without 10 nM thrombin or 16 nM EGF for 4 min at 37 °C and EGFR and ErbB2 tyrosine phosphorylation was detected as described in Figure 1. The data are expressed as fold increase over untreated control and are representative of three independent experiments. (c) MDA-MB-231 cells labeled with myo-[3H]inositol were incubated with 0.1% DMSO, 2 µM AG1478 or 1 µM PD153035 for 2 h at 37 °C. Cells were then stimulated with 100 µM TFLLRNPNDK for 60 min at 37 °C and the amounts of [3H]inositol phosphates generated were then measured.
Thrombin induces persistent ErbB transactivation in invasive breast carcinoma
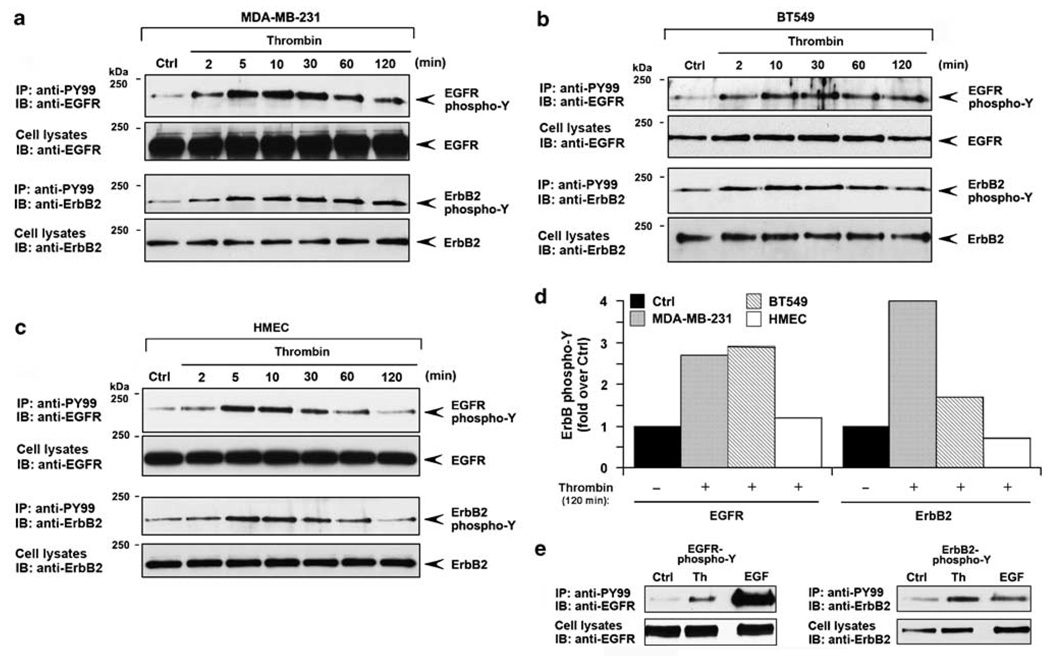
Hyperactivation of ErbB signaling is associated with invasive breast cancer (Yarden, 2001). To determine whether activation of ErbB2 signaling by thrombin is dysregulated in invasive breast carcinoma we examined the kinetics of ErbB transactivation. In MDA-MB-231 cells, thrombin caused prolonged EGFR and ErbB2 transactivation that was detected as early as 2 min and remained markedly sustained for at least 2 h (Figure 3a). A similar rapid and persistent increase in EGFR and ErbB2 tyrosine phosphorylation induced by thrombin was observed in invasive BT549 breast carcinoma (Figure 3b). In contrast, in normal human mammary epithelial cells (HMECs) thrombin elicited a transient increase in EGFR and ErbB2 transactivation, which peaked at 5 min and was substantially diminished by 1 h (Figures 3c and d). These results suggest that proteolytic activation of PAR1 induces persistent ErbB signaling in invasive breast carcinoma but not in normal breast epithelial cells. Moreover, the extent of ErbB2 transactivation stimulated by thrombin was comparable to that induced by saturating concentrations of EGF ligand (Figure 3e). These data suggest that the persistent ErbB2 transactivation induced by thrombin occurs through a highly efficient mechanism in invasive breast carcinoma.
Figure 3.
Persistent transactivation of epidermal growth factor (EGF) receptor (EGFR) and ErbB2 induced by thrombin in invasive breast carcinoma. Serum-starved MDA-MB-231 cells (a), BT549 cells (b) and normal human mammary epithelial cells (HMECs) (c) were incubated with 10 nM thrombin various times at 37 °C. Cells were lysed and EGFR and ErbB2 tyrosine phosphorylation were detected as described in Figure 1. (d) The data shown are from the thrombin treated 120 min time point and is expressed as the fold increases over untreated control. (e) MDA-MB-231 cells were incubated in the absence or presence of 10 nM thrombin or 16 nM EGF for 4 min at 37 °C and EGFR and ErbB2 tyrosine phosphorylation was examined. The data shown are representative of at least three independent experiments.
PAR1 promotes ERK1/2 signaling and breast carcinoma invasion through ErbB transactivation
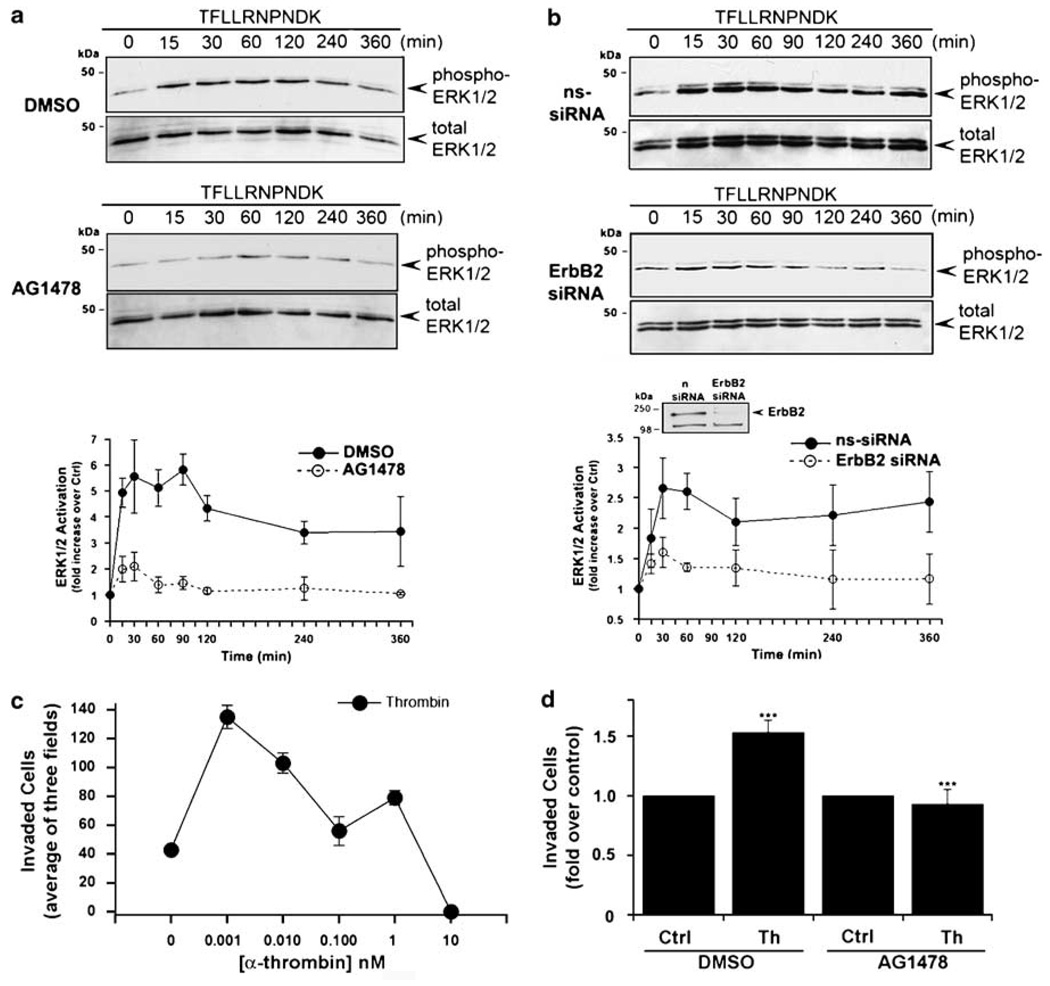
We previously showed that PAR1 is not downregulated and signals persistently to ERK1/2 in invasive breast carcinoma (Booden et al., 2004). To examine the role of EGFR and ErbB2 transactivation to PAR1-mediated ERK1/2 activation, we used AG1478 to inhibit EGFR kinase activity and siRNA to deplete cells of ErbB2 expression. In control cells, the PAR1-specific agonist peptide increased ERK1/2 activity within 15 min that remained elevated for 6 h (Figure 4a), consistent with that previously reported (Booden et al., 2004). The sustained activation of ERK1/2 induced by activated PAR1 was considerably reduced by AG1478 (Figure 4a). We next used siRNA-targeting ErbB2 to assess the role of ErbB2 in PAR1-induced ERK1/2 activation. MDA-MB-231 cells electroporated with ErbB2 siRNA showed a substantial reduction in ErbB2 expression compared to nonspecific siRNA control cells (Figure 4b, inset). The early increase in ERK1/2 activity induced by agonist peptide was diminished but still detectable in ErbB2 siRNA-treated cells, whereas the sustained ERK1/2 activity was considerably inhibited (Figure 4b). Thus, EGFR and ErbB2 transactivation induced by thrombin contributes to prolonged ERK1/2 signaling in invasive breast carcinoma.
Figure 4.
Protease-activated receptor-1 (PAR1)-stimulated epidermal growth factor (EGF) receptor (EGFR) and ErbB2 transactivation contributes to sustained extracellular signal-regulated kinase-1 and -2 (ERK1/2) signaling and promotes breast carcinoma cell invasion. (a) MDA-MB-231 cells were pretreated with 0.1% dimethyl sulfoxide (DMSO) or 2 µM AG1478 for 2 h at 37 °C and then incubated with 100 µM TFLLRNPNDK for various times at 37 °C. Cell lysates were immunoblotted with anti-phospho-p44/42 mitogen-activated protein kinase (MAPK) (ERK1/2) antibody. Membranes were stripped and reprobed with anti-p44/42 MAPK (ERK1/2) to control for loading. The data (mean ± s.e.) shown are expressed as fold increase over basal from at least three independent experiments. (b) MDA-MB-231 cells were electroporated with 600 nM ErbB2-specific or nonspecific siRNA. After 48 h, serum-deprived cells were incubated with or without 100 µM TFLLRNPNDK for various times at 37 °C and ERK1/2 activity was measured and quantified as described above. The inset confirms the loss of ErbB expression in ErbB2 siRNA-treated cells as detected by immunoblotting. (c) Serum-starved MDA-MB-231 cells were left untreated or treated with varying concentrations of thrombin at 37 °C and then added to the upper well and cellular invasion toward NIH 3T3-conditioned medium (CM) was assessed. Representative data from one experiment is shown. (d) MDA-MB-231 cells were pretreated with 0.1% DMSO or 2 µM AG1478 for 30 min at 37 °C. After the treatment, cells were incubated in the absence or presence of 1 pM thrombin and then added the upper well and cellular invasion toward NIH 3T3 fibroblast-CM present in the lower well with or without AG1478 was assessed. The data (mean ± s.e.) shown are expressed as fold increase over untreated control from three independent experiments. The difference between thrombin-stimulated cellular invasion in control and AG1478-treated cells was significant (***P<0.005).
We next examined the contribution of EGFR transactivation to thrombin-induced breast carcinoma invasion. MDA-MB-231 cells were treated with varying concentrations of thrombin to determine the optimal dose for inducing cellular invasion. We found that low thrombin concentrations induced significant increases in breast carcinoma cell invasion compared to untreated control cells, whereas higher thrombin concentrations actually inhibited cellular invasion (Figure 4c). These findings suggest that the extent of PAR1 activation is important for conferring positive or negative regulation of cellular invasion. We then examined the capacity of thrombin to induce cellular invasion following inhibition of EGFR kinase activity with AG1478. Thrombin-induced a significant increase in invasion of control cells, which was markedly inhibited by AG1478 (Figure 4d). These studies are the first to demonstrate a critical role for EGFR transactivation in thrombin-induced breast carcinoma cell invasion.
PAR1 signaling through Gαi/o and metalloprotease activity mediate ErbB transactivation and breast carcinoma cell invasion
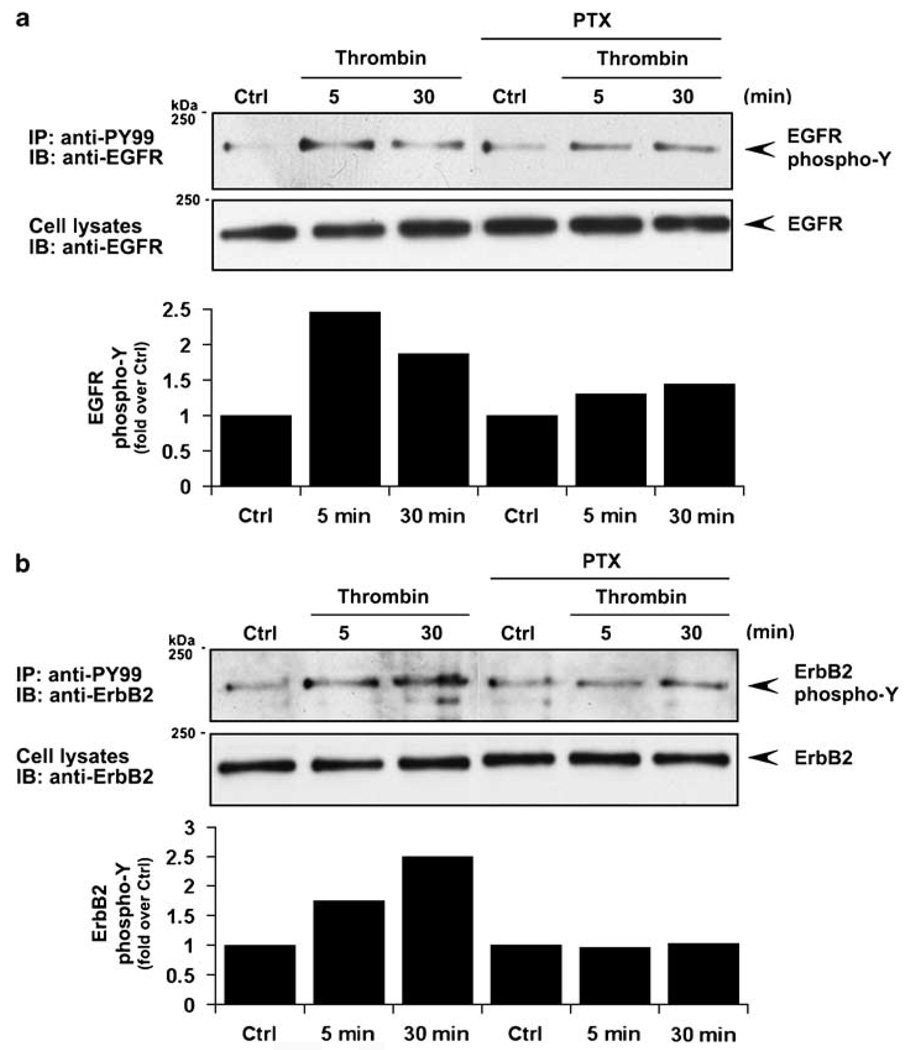
To define the mechanism by which PAR1 stimulates ErbB transactivation we examined the function of Gαi/o using pertussis toxin, which catalyses ADP ribosylation and inactivation of Gαi/o proteins. Thrombin-induced EGFR transactivation was substantially diminished in MDA-MB-231 cells pretreated with pertussis toxin compared to control cells (Figure 5a). ErbB2 transactivation induced by thrombin was also virtually ablated in pertussis toxin-treated cells (Figure 5b). These findings are consistent with a critical role for Gαi/o signaling in thrombin-induced ErbB transactivation in invasive breast carcinoma.
Figure 5.
Protease-activated receptor-1 (PAR1) signaling through Gαi/o is critical for epidermal growth factor (EGF) receptor (EGFR) and ErbB2 transactivation. (a and b) Serum-starved MDA-MB-231 cells were pretreated with 100 ng/ml pertussis toxin for 18 h at 37 °C or left untreated and then incubated in the absence or presence of 10 nM thrombin for 5 or 30 min at 37 °C. Cells were processed and EGFR and ErbB2 tyrosine phosphorylation was measured as described in Figure 1.
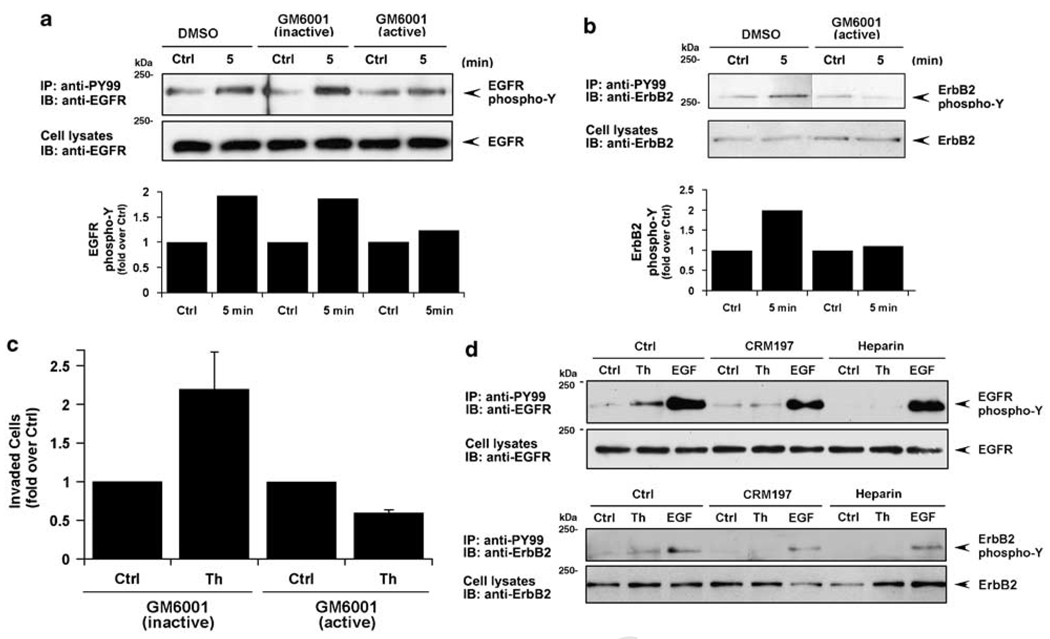
To determine whether PAR1-stimulated EGFR and ErbB2 transactivation occurred in a metalloprotease-dependent manner we used the broad-spectrum metalloprotease inhibitor GM6001. EGFR transactivation induced by thrombin was substantially inhibited in cells treated with active GM6001 compared to inactive agent or vehicle control-treated cells (Figure 6a). Thrombin-stimulated ErbB2 transactivation was similarly inhibited by GM6001 pretreatment compared to control cells (Figure 6b). We next examined whether thrombin-induced cellular invasion was dependent on metalloprotease activity. Thrombin stimulated an ~2-fold increase in MDA-MB-231 cellular invasion under control conditions that was virtually abolished in GM6001-treated cells (Figure 6c). To identify the ErbB ligands released by MDA-MB-231 cells in response to thrombin, we used heparin and the diphtheria toxin mutant CRM197. Both EGFR and ErbB2 transactivation induced by thrombin were virtually ablated following preincubation with heparin and CRM197 (Figure 6d), suggesting that HB-EGF is the critical mediator of this process. These studies strongly suggest a critical role for metalloprotease activity in thrombin-induced ErbB transactivation and breast carcinoma cell invasion.
Figure 6.
Metalloprotease activity is required for thrombin-induced epidermal growth factor (EGF) receptor (EGFR) and ErbB2 transactivation and cellular invasion. (a and b) Serum-deprived MDA-MB-231 cells were preincubated with 0.1% dimethyl sulfoxide (DMSO) (vehicle control), 10 µM GM6001 active or inactive agent for 1 h at 37 °C and then treated with or without 10 nM thrombin for 5 min at 37 °C. EGFR and ErbB2 tyrosine phosphorylation was detected as described in Figure 1. The data are expressed as fold increase over control and are representative of three different experiments. (c) MDA-MB-231 cells were pretreated for 1 h with 10 mM GM6001 active or inactive control agent then incubated with 1 pM thrombin at 37 °C. Cells were then added to the upper well and cellular invasion toward conditioned medium (CM) containing inactive or active GM6001 was assessed. (d) Serum-starved MDA-MB-231 cells were preincubated with 20 µg/ml of CRM 197, 100 ng/ml heparin or vehicle control for 30 min at 37 °C and then treated in the absence (Ctrl) or presence of 10 nM Th or 16 nM EGF ligand for 5 min at 37 °C. ErbB tyrosine phosphorylation was then examined as described above.
PAR1 expression is essential for ErbB transactivation and cellular invasion induced by fibroblasts secreted factors in vitro and for tumor growth in vivo
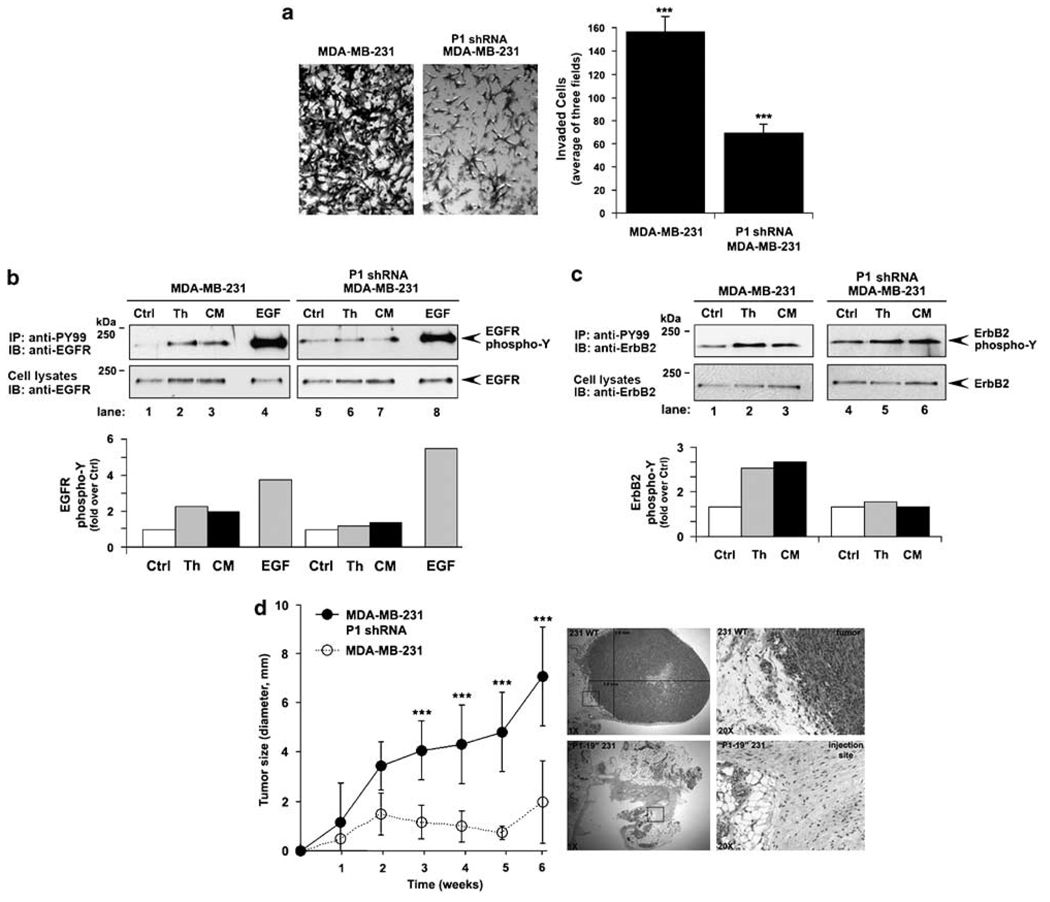
Stromal factors secreted into the tumor microenvironment promote tumor progression. Previous studies have also demonstrated that breast carcinoma invasion toward factors secreted by stromal fibroblasts is positively correlated with increased PAR1 expression (Even-Ram et al., 1998). We therefore examined whether PAR1 is necessary for transactivation of ErbB induced by factors secreted by NIH 3T3 fibroblasts. PAR1-deficient breast carcinoma exhibited a significant reduction in invasion toward conditioned medium compared to control cells (Figure 7a), suggesting a critical role for PAR1 in cellular invasion induced by factors secreted by fibroblasts. We next examined whether fibroblast-secreted factors induced ErbB trans-activation through a PAR1-dependent mechanism. Interestingly, our results indicate that NIH 3T3 fibro-blast-conditioned medium stimulated EGFR and ErbB2 transactivation comparable to that induced by thrombin (Figures 7b and c). In contrast, conditioned medium failed to stimulate EGFR and ErbB2 transactivation in cells lacking PAR1 (Figures 7b and c). However, EGF-induced EGFR tyrosine phosphorylation remained intact. Thus, in addition to thrombin, factors secreted by fibroblasts present in conditioned media induce ErbB transactivation through a PAR1-dependent mechanism.
Figure 7.
Protease-activated receptor-1 (PAR10 mediates conditioned media-induced ErbB transactivation and cellular invasion in vitro, and tumor growth in vivo. (a) The basal invasion of control and PAR1-deficient P1 shRNA-expressing MDA-MB-231 cells toward NIH 3T3 fibroblast-conditioned medium (CM) was assessed. The data (mean ± s.d., n = 5) shown are the number of invaded cells representative of at least three independent experiments. The difference between invasion observed with control and PAR1-deficient MDA-MB-231 cells was significant (***P<0.005). (b and c) Serum-deprived control and P1 shRNA-expressing cells were incubated in the absence or presence of 10 nM thrombin, CM or 16 nM EGF for 8 min at 37 °C, and epidermal growth factor (EGF) receptor (EGFR) and ErbB2 tyrosine phosphorylation was measured and quantified as described in Figure 1. The data shown are expressed as fold increase over control and are representative of at least three independent experiments. (d) PAR1-deficient and control MDA-MB-231 cells were implanted into the left and right mammary fat pad of immunodeficient severe combined immunodeficiency (SCID) mice, respectively. Tumor growth was measured weekly and the data (mean ± s.e., n = 3) are representative of two independent experiments. The difference between tumor size measured in PAR1-deficient versus control MDA-MB-231 cell implants was significant (***P<0.005). Tumors were excised from the mammary fat pads at 6-weeks postimplantation, fixed and stained with hematoxylin and eosin (H&E) and imaged at ×1 and boxed areas at ×20 magnification and examined for morphology and the invasive edge.
Our in vitro studies strongly suggest an important role for PAR1 in thrombin-induced ErbB transactivation and breast carcinoma cell invasion. To determine whether PAR1 contributes to breast carcinoma invasion in vivo, we examined the ability of PAR1-deficient cells to confer tumor growth and invasion using an orthotopic mammary fat pad xenograft model in immunodeficient mice. MDA-MB-231 cells implanted into the mouse mammary fat pad generate tumors and develop metastases with a high frequency (Price et al., 1990). PAR1-deficient and control cells were implanted into the left and right mammary fat pads, respectively of 6-to 8-week-old female immunodeficient mice and tumor growth was measured weekly. Tumor nodules were present in all mice implanted with cells within 1 week. Surprisingly, however, PAR1-deficient cells showed a significant reduction in tumor growth compared to control cells measured over a 6-week period. In addition, hematoxylin and eosin (H&E) staining of tumor sections prepared 6-weeks postimplantation revealed invasive growth of control cells (Figure 7d), whereas tumor growth and invasion was not detected in sections derived from sites implanted with PAR1-deficient cells. Similarly, PAR1-deficient cells displayed attenuated tumor growth compared to vector control cells examined by subcutaneous flank injection using immunodeficient mice (data not shown). These studies are the first to demonstrate a critical role for PAR1 in tumor growth in a mammary fat pad xenograft model using immunodeficient mice.
Discussion
In the present study, we demonstrate that proteolytic activation of PAR1 by thrombin induces persistent EGFR and ErbB2 transactivation in invasive breast cancer cells, which is distinct from transient EGFR and ErbB2 transactivation observed in normal mammary epithelial cells. We also show that PAR1-stimulated EGFR and ErbB2 transactivation sustains ERK1/2 signaling and promotes breast carcinoma invasion. In addition, PAR1 signaling through Gαi/o and metalloprotease activity is critical for transactivation of EGFR and ErbB2 and induction of cellular invasion. Interestingly, transactivation of ErbB2 induced by thrombin occurred to a similar extent, as that observed with EGF ligand, indicating that GPCR transactivation of ErbB2 is an efficient mode of activation in invasive breast carcinoma. Finally, we report that PAR1 expression in breast carcinoma is essential for tumor growth in vivo, suggesting a critical function for PAR1 in processes that promote breast cancer progression. Thus, our study establishes an important link between PAR1, a receptor activated by tumor-generated proteases, and hyperactivation of ErbB signaling that contribute to processes associated with progression of metastatic breast cancer.
Overexpression of ErbB2/HER2 leads to hyperactivation of ErbB signaling and is implicated in metastatic breast cancer disease (Yarden, 2001; Burgess et al., 2003). However, the mechanism(s) that lead to activation of ErbB signaling in breast cancers are varied and poorly understood. Our study establishes an important role for PAR1 in mediating persistent activation of ErbB signaling in invasive breast carcinoma. ErbB2 does not signal alone, but preferentially forms heterodimeric signaling complexes with EGFR or ErbB3 (Yarden, 2001; Burgess et al., 2003). Strikingly, proteolytic activation of PAR1 by thrombin is sufficient to activate and sustain ErbB2 signaling through a mechanism that requires EGFR activation. This is consistent with ErbB2 heterodimerization with EGFR to form a potent signaling complex. We show that thrombin-induced ErbB2 transactivation is dependent on EGFR kinase activity, metalloprotease activity and release of HB-EGF ligand. Moreover, expression of ErbB3, another preferred binding partner of ErbB2, was not detectable in MDA-MB-231 cells (data not shown). Thus, thrombin-induced EGFR transactivation is critical for activation of ErbB2, a receptor that cannot bind ligand directly. We also report that thrombin-induced ErbB2 transactivation is comparable to that stimulated by EGF ligand. These findings suggest that proteolytic activation of PAR1 by thrombin is capable of efficient ErbB2 activation through a process that requires EGFR activation. Our studies further reveal that EGFR activity is critical for thrombin-induced breast carcinoma invasion.
The mechanism by which PAR1 persistently transactivates EGFR and ErbB2 in invasive breast cancer cells involves Gαi/o signaling, metalloprotease activity and release of HB-EGF ligand. We show that inactivation of Gαi/o with pertussis toxin inhibits thrombin-induced EGFR and ErbB2 transactivation, consistent with a role for Gαi and Gβγ signaling to metalloproteases. GPCRs regulate metalloprotease-dependent EGFR transactivation through multiple pathways, including increases in intracellular Ca2+, activation of protein kinase C, generation of reactive oxygen species and the activation of the nonreceptor tyrosine kinases Src and Pyk2 (Ohtsu et al., 2006). A previous study demonstrated that two metalloproteases ADAM15 and ADAM 17 (also known as TACE) mediate release of HB-EGF and EGFR transactivation following thrombin stimulation (Hart et al., 2005). However, the role of these effectors in ErbB2 transactivation was not examined. Finally, we previously demonstrated that activated PAR1 trafficking is severely altered in metastatic breast carcinoma but not in nonmetastatic or normal breast epithelial cells (Booden et al., 2004). Consequently, the proteolytically activated PAR1 is not downregulated and signals continuously to multiple effectors that likely mediate persistent ErbB transactivation.
In addition to Gαi/o, PAR1 may signal through other G-protein subtypes to promote breast carcinoma invasion and metastasis. Kelley et al. (2006) recently showed that the Gα12 proteins are upregulated in invasive breast tissue specimens and that constitutively active forms of Gα12 and Gα13 promote invasion of MDA-MB-231 and BT549 cells. These studies further showed that thrombin-induced breast carcinoma invasion was dependent on Gα12 proteins and Rho activation. Thus, Gα12 proteins and Rho signaling have critical functions in invasive processes mediated by PAR1. These findings also raise the possibility that Gα12 proteins and Rho signaling may also be important mediators of ErbB transactivation induced by PAR1. Thrombin-induced ErbB transactivation could also mediate Rho activation to promote breast carcinoma invasion. Thus, the function of Gα12 proteins and Rho signaling in thrombin-induced ErbB transactivation in invasive breast carcinoma will be important to determine.
Our in vitro studies provide an important link between PAR1 and hyperactivation of ErbB signaling, which promote breast carcinoma invasion. Intriguingly, our in vivo work also indicates a critical role for PAR1 in breast tumor growth. Previous studies have reported that ectopic expression of PAR1 in noninvasive MCF-7 breast carcinoma is sufficient to induce tumor growth (Boire et al., 2005) and that anti-sense reduction of PAR1 expression in melanoma cells causes an attenuation of tumor growth in vivo (Salah et al., 2007). However, the mechanism by which PAR1 regulates breast tumor growth and the contribution of ErbB transactivation in vivo is not known. Interestingly, Borrell-Pages et al., 2003 previously showed that TACE/ADAM17 is overexpressed in human breast tumors and mediates TGF-α shedding, which contributes to breast tumor formation in vivo. PAR1 also has been shown to activate TACE/ADAM17 in breast cancer cell lines (Hart et al., 2005), but whether this pathway is involved in PAR1-mediated tumor growth remains to be determined. Previous in vivo studies have also demonstrated important roles for transactivation of ErbB family members by GPCRs in several pathophysiological conditions, including kidney diseases, cardiac hypertrophy and cardioprotection (Asakura et al., 2002; Lautrette et al., 2005; Noma et al., 2007). Together these studies indicate that ErbB transactivation by PAR1 could have significant function in several different pathophysiologies including breast cancer progression.
Our study establishes a critical function for PAR1 in persistent activation of ErbB signaling in invasive breast carcinoma. We found that proteolytic activation of PAR1 by thrombin in invasive breast carcinoma is critical for ErbB transactivation and cellular invasion in vitro. PAR1 expression in invasive breast carcinoma is also essential for tumor growth in vivo. These results strongly suggest a critical role for PAR1 in breast tumorigenesis and invasion. Further studies to define the function of PAR1 to tumor progression and metastasis in vivo will provide new insights into the molecular basis of breast cancer disease and provide new targets for anticancer drug discovery.
Materials and methods
Reagents and antibodies
α-Thrombin was from Enzyme Research Laboratories. The agonist peptides, TFLLRNPNDK (PAR1 specific) and SLIGKV (PAR2 specific), were synthesized as the carboxyl amide and purified by reverse-phase high-pressure liquid chromatography (UNC Peptide Facility, Chapel Hill, NC, USA). EGF, heparin and diphtheria toxin cross-reacting material CRM 197 were from Sigma. AG1478, PD153035 and GM6001 compounds were from Calbiochem. Pertussis toxin was from List Biological Laboratories Inc.
Anti-ErbB2 antibody was from LabVision Corporation. Anti-EGFR polyclonal antibody was a gift from H Shelton Earp III (UNC, Chapel Hill, NC, USA). Anti-phosphotyrosine antibody (PY99) was from Santa Cruz Biotechnology. Monoclonal anti-phospho-p44/42 mitogen-activated protein kinase (MAPK) antibody and polyclonal anti-p44/42 MAPK antibody were from Cell Signaling Technology Inc. The anti-PAR1 polyclonal antibody was generated as described (Paing et al., 2006). Horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit antibodies were from Bio-Rad.
Cell lines
MDA-MB-231, BT549 and NIH 3T3 cells were obtained from American Type Culture Collection (Manassas, VA, USA) and maintained according to their instructions. HMECs immortalized with human telomerase reverse transcriptase were maintained in HuMEC medium containing HuMEC Supplement Kit (growth factors, hydrocortisone, isoproterenol, transferrin, insulin and bovine pituitary extract) purchased from Invitrogen.
MDA-MB-231 cells expressing P1 and P2 shRNA were generated as follows. The shRNA specific for PAR1 (termed P1) or PAR2 (termed P2) were generated by insertion of an shRNA encoding either the PAR1-specific mRNA sequence 5′-AGAUUAGUCUCCAUCAAUA-3′ and PAR2-specific mRNA sequence 5′-GGAAGAAGCCUUAUUGGUA-3′ into pSilencer 5.1-U6 Retro (Ambion Inc.) using the Bam HI and Hind III sites. The insertion sequence was confirmed by dideoxy sequencing. Retroviruses were generated using the PA317 packaging cells and were used to infect MDA-MB-231 cells. Mass populations of cells stably transduced with pSilencer 5.1 encoding shRNA were selected with 0.6 µg/ml of puromycin.
Immunoprecipitation and immunoblotting
Cells grown in 10 cm2 dishes were serum starved. After various treatments, cells were lysed in buffer containing 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10% glycerol, 10 mM NaPP, 10 mM NaF with freshly added 2 mM sodium orthovanadate, 1 mM phenylmethylsulphonylfluoride and protease inhibitor cocktail. Equivalent amounts of cell lysates were immunoprecipitated with various antibodies. Immunoprecipitates were resolved on 9% SDS-polyacrylamide gel electrophoresis, transferred to membranes, immunoblotted and developed with enhanced chemiluminescence (Amersham) and quantified by densitometry. Total cellular lysates were analysed as described above.
ERK1/2 activity was determined from serum-starved cells plated at 6 × 104 cells per well in 24-well dishes. Cells were lysed in ×2 sample buffer and processed as described above. Activated ERK1/2 was detected by immunoblotting with anti-phospho-p44/42 MAPK (ERK1/2) antibody. Membranes were stripped and reprobed with anti-p44/42 MAPK (ERK1/2) antibody. Immunoblots were quantitated by densitometry.
Small interfering RNA electroporation
The following siRNA sequences targeting nonspecific 5′-CTACGTCCAGGAGCGCACC-3′ or ErbB2 mRNA sequence 5′-AAAUUCCAGUGGCCAUCAA-3′ were obtained from Dharmacon Inc. and introduced into MDA-MB-231 cells by electroporation as described (Morris et al., 2006).
Phosphoinositide hydrolysis
MDA-MB-231 cells were plated at 6×104 cells per well in 24-well plates. Cells labeled overnight with 1 µCi/ml of myo-[3H]inositol (American Radiolabeled Chemicals) and accumulated [3H]inositol phosphates were measured as described (Paing et al., 2002).
Cell surface ELISA
Cells plated at 1.6 × 105 cells per well of 24-well dishes were fixed and processed as previously described (Morris et al., 2006).
Invasion assays
Serum-starved MDA-MB-231 cells (1.5 × 105) were added together with agonists to the upper chamber of matrigel-coated transwells in a 24-well format (8-µm pore size polycarbonate filter; BD Biosciences) and incubated for ~40 h at 37 °C. The bottom chamber contained 5-µg/ml fibronectin diluted in 700 µl of NIH 3T3 fibroblast-conditioned media. Cells were then fixed, stained with 0.4% crystal violet and noninvading cells were removed as described (Morris et al., 2006). At least five fields of invaded cells were photographed at × 10 magnification using an Olympus IX71 inverted microscope fitted with a UPlanFl × 10 objective and a Hamamatsu ORCA-ER digital camera and counted.
Conditioned media
NIH 3T3 fibroblast-conditioned media was prepared from exponentially growing cells maintained in phenol red-free Dulbecco’s modified Eagle’s medium containing 10 mM HEPES, pH 7.4 and 1 mg/ml bovine serum albumin for 24 h. Conditioned media was collected, aliquoted and stored at −20 °C.
Orthotopic mammary fat pad xenografts
Four- to six-week-old female ICR-severe combined immunodeficiency (SCID) mice were obtained from Taconic (German-town, NY, USA) and maintained in a pathogen-free environment. All animal studies were carried out in accordance with Institutional Animal Care and Use Committee guidelines. Groups of three to four mice received two mammary fat pad inoculations consisting of 2 × 106 control and P1 shRNA-expressing cells resuspended in 50 µl media and mixed with equal volume of matrigel. Cells were implanted orthotopically into the left and right intact fourth inguinal mammary fat pad of 6- to 8-week-old female SCID mice. The growth of mammary fat pad tumors was measured weekly with calipers. Tumors were excised from the killed mice 6-weeks postimplantation, formalin fixed and embedded in paraffin for sectioning. The UNC Lineberger Comprehensive Cancer Center Animal Histopathology Core Facility performed the embedding, sectioning and H&E staining.
Data analysis
Data were analysed using Prism 3.0 software and statistical significance was determined using InStat 3.0 (GraphPad, San Diego, CA, USA). Group comparisons were made using an unpaired t-test. Final composite images were created with Photoshop 7.0 (Adobe).
Supplementary Material
Acknowledgements
We thank Dr Pilar Blancafort, Dr Lee Graves and Dr Shelton Earp III for advice and generously providing reagents. This study was supported by NIH grant HL073328, Susan G Komen Breast Cancer Foundation Award and an American Heart Association-established Investigator Award (to JT) and NIH grants GM30324 and DK37871 (to GLJ).
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
References
- Arora P, Ricks TK, Trejo J. Protease-activated receptor signalling, endocytic sorting and dysregulation in cancer. J Cell Sci. 2007;120:921–928. doi: 10.1242/jcs.03409. [DOI] [PubMed] [Google Scholar]
- Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, et al. Cardiac hypertrophy is inhibited by antagonisms of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- Bergman S, Junker K, Henklein P, Hollenberg MD, Settmacher U, Kaufman R. PAR-type thrombin receptors in renal carcinoma cells: PAR1-mediated EGFR activation promotes cell migration. Oncol Rep. 2006;15:889–893. [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Booden MA, Ekert L, Der CJ, Trejo J. Persistent signaling by dysregulated thrombin receptor trafficking promotes breast carcinoma cell invasion. Mol Cell Biol. 2004;24:1990–1999. doi: 10.1128/MCB.24.5.1990-1999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell-Pages M, Rojo F, Albanell J, Baselga J, Arribas J. TACE is required for the activation of EGFR by TGF-α in tumors. EMBO J. 2003;22:1114–1124. doi: 10.1093/emboj/cdg111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess AW, Cho H-S, Eigenbrot C, Ferguson KM, Garret TPJ, Leahy DJ, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- Darmoul D, Gratio V, Devaud H, Peiretti F, Laburthe M. Activation of proteinase-activated receptor 1 promotes human colon cancer cell proliferation through epidermal growth factor receptor transactivation. Mol Cancer Res. 2004;2:514–522. [PubMed] [Google Scholar]
- Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909–914. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11:495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- Gschwind A, Hart S, Fischer OM, Ullrich A. TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO J. 2003;22:2411–2421. doi: 10.1093/emboj/cdg231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart S, Fischer OM, Prenzel N, Zwick-Wallasch E, Schneider M, Henninghausen L, et al. GPCR-induced migration of breast carcinoma cells depends on both EGFR signal transactivation and EGFR-independent pathways. Biol Chem. 2005;386:845–855. doi: 10.1515/BC.2005.099. [DOI] [PubMed] [Google Scholar]
- Kelley P, Moeller BJ, Juneja J, Booden MA, Der CJ, Daaka Y, et al. The G12 family of heterotrimeric G proteins promotes breast cancer invasion and metastasis. Proc Natl Acad Sci USA. 2006;103:8173–8178. doi: 10.1073/pnas.0510254103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliopulos A, Covic L, Seely SK, Sheridan PJ, Helin J, Costello CE. Plasmin desensitization of the PAR1 thrombin receptor: kinetics, sites of truncation, and implications for thrombolytic therapy. Biochemistry. 1999;38:4572–4585. doi: 10.1021/bi9824792. [DOI] [PubMed] [Google Scholar]
- Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration and invasion of breast cancer cells. Cancer Res. 2006;66:307–314. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26:3637–3643. doi: 10.1038/sj.onc.1210379. [DOI] [PubMed] [Google Scholar]
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37:S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, et al. Arrestin-mediated β1-adrenergic receptor tranactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291:C1–C10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- Paing MM, Johnston CA, Siderovski DP, Trejo J. Clathrin adaptor AP2 regulates thrombin receptor constitutive internalization and endothelial cell resensitization. Mol Cell Biol. 2006;28:3221–3242. doi: 10.1128/MCB.26.8.3231-3242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J. β-Arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem. 2002;277:1292–1300. doi: 10.1074/jbc.M109160200. [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, et al. EGF receptor transactivation by G protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- Salah Z, Maoz M, Pokroy E, Lotem M, Bar-Shavit R, Uziely B. Protease-activated receptor-1 (hPar1), a survival factor eliciting tumor progression. Mol Cancer Res. 2007;5:229–240. doi: 10.1158/1541-7786.MCR-06-0261. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones D, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpress HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Wetzker R, Bohmer F-D. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol. 2003;4:651–657. doi: 10.1038/nrm1173. [DOI] [PubMed] [Google Scholar]
- Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61:1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.