Abstract
Traumatic brain injury (TBI) is a major cause of morbidity and mortality in the United States. Current clinical therapy is focused on optimization of the acute/subacute intracerebral milieu, minimizing continued cell death, and subsequent intense rehabilitation to ameliorate the prolonged physical, cognitive, and psychosocial deficits that result from TBI. Adult progenitor (stem) cell therapies have shown promise in pre-clinical studies and remain a focus of intense scientific investigation. One of the fundamental challenges to successful translation of the large body of pre-clinical work is the delivery of progenitor cells to the target location/organ. Classically used vehicles such as intravenous and intra arterial infusion have shown low engraftment rates and risk of distal emboli. Novel delivery methods such as nanofiber scaffold implantation could provide the structural and nutritive support required for progenitor cell proliferation, engraftment, and differentiation. The focus of this review is to explore the current state of the art as it relates to current and novel progenitor cell delivery methods.
Keywords: Traumatic brain injury, Stem cells, Progenitor cells, Delivery, Scaffold, Review, Therapy, Treatment
Introduction
Traumatic brain injury (TBI) is a major source of morbidity and mortality in the United States, affecting 1.5 million new patients yearly. The annual mortality derived from TBI approaches 50,000 with an additional 230,000 patients requiring hospitalization [1]. The overall prevalence of TBI is estimated to be 6.5 million people [2]. Acute treatment of TBI is supportive and requires control of intracranial pressure and maintenance of cerebral perfusion. Long term care involving rehabilitation decreases the physical, cognitive, and psychosocial deficits caused by TBI [3], however, neurons show little ability for repair and no therapy is currently available to reverse neuronal injury.
Neuronal injury after TBI is commonly classified into immediate (primary) injury and delayed (secondary) injury. Mechanical force causing shear and compression of neural and vascular tissue leads to an immediate site of nervous tissue necrosis [4]. The mechanical insult causes acute, subacute, and delayed release of inflammatory/biochemical mediators that alter the regional milieu leading to delayed neuronal injury. Preliminary pre clinical research has shown potential benefit from progenitor (stem) cell therapeutics for treatment of TBI [5–7].
By definition, progenitor cells are multipotent (able to differentiate down multiple cell lineages) and capable of self-renewal [8]. Progenitor cells are maintained in anatomical niches throughout the body to protect against cell line depletion and/or over proliferation. Tissue regeneration, maintenance, and repair is regulated by the adult progenitor cell niches [9]. Adult stem cell niches and respective progenitor cell populations currently under investigation include bone marrow derived cells (mesenchymal stem cells (MSCs), multipotent adult progenitor cells (MAPCs) and hematopoeitic stem cells (HSCs)), subventricular zone (neural stem cells (NSCs)), umbilical cord blood mononuclear fraction (MSCs and HSCs), umbilical cord blood and adipose tissue. The capabilities to self renew and differentiate down multiple cell lineages have made progenitor cells attractive targets for TBI therapy.
Preclinical research into progenitor cell transplantation after TBI has shown cell engraftment with functional neurologic improvement [10]; however, the therapeutic mechanism remains controversial. While initial research showed tissue specific progenitor cells such as MSCs to phenotypically adopt neural cell characteristics (10), the frequency of engraftment and clinical significance of transdifferentiation remains controversial [11]. More recent research has shown increased growth factor secretion [12], modulation of the inflammatory response [12], and progenitor cell to resident cell contact/fusion [13] may be other more likely potential mechanisms of benefit. The observed progenitor cell therapeutic benefit is possibly derived from changes in the local/regional cellular milieu promoting resident niche stem cell populations to stimulate tissue regeneration and repair.
The interaction between progenitor cells and resident cell niches requires an effective delivery vehicle. An ideal delivery method would be minimally invasive and lead to high levels of cell engraftment. This review will investigate the efficacy and barriers to implementation of current cell delivery methods including direct implantation, intravenous, intra-arterial, and intra-thecal infusions as well as novel progenitor cell delivery methods using engineered scaffolding constructs.
Current Stem Cell Delivery Methods
Intravenous Infusion
Intravenous infusion is an attractive delivery vehicle due to ease of access and potential distribution to multiple organ systems including bone marrow, spleen, brain, cardiomyocytes, and mesenchymal tissues [7, 14, 15]. After controlled cortical impact (CCI) injury in rats, MSC infusion reduced both motor and neurological deficits with preferential engraftment into injured brain parenchyma [6]. Intravenous infusion of human umbilical cord blood (HUCB) after induced middle cerebral artery occlusion in rats has shown modulation of the systemic inflammatory response leading to improved neurological functioning [16].
MSC infusion after total body irradiation in baboons has shown up to 2.7% engraftment in multiple tissues [17]. Preliminary research has shown engraftment of MSCs in the injured brain parenchyma after intravenous infusion [18]. However, the frequency of engraftment remains controversial as further studies have failed to show the presence of transplanted progenitor cells in the brain [19]. The observed failure of MSC engraftment after intravenous infusion could be secondary to a significant first pass pulmonary effect since the average MSC diameter is larger than the average pulmonary capillary diameter [20]. In a murine model, infusion of labeled 4, 10, and 15 μm microspheres with and without vasodilator pretreatment (sodium nitroprusside) was followed by lung tissue harvest to analyze the frequency of microsphere sequestration. No 4 μm microspheres were found, however 10 and 15 μm microspheres were sequestered in the lungs. The frequency of 10 and 15 μm microsphere sequestration decreased after sodium nitroprusside pretreatment. Data analysis estimated a pulmonary capillary diameter of 5–9 μm while MSC diameter averages 15–19 μm [21]. The difference between pulmonary capillary and MSC diameter would explain the high rate of pulmonary microvasculature entrapment and lack of significant cell engraftment in the brain parenchyma. Figure 1 outlines the advantages and possible untoward effects of intravenous progenitor cell delivery. Intravenous infusion offers a minimally invasive vehicle for progenitor cell delivery; however, further investigation into pharmacologic intervention to decrease first pass pulmonary effect is needed to improve the efficiency of cellular engraftment.
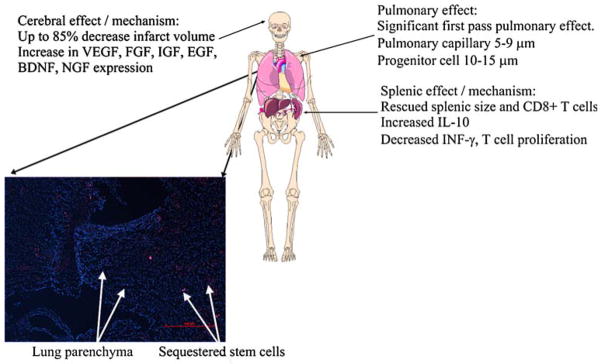
Fig. 1.
Mechanisms of action, advantages, and untoward effects of systemic stem cell therapy on brain, spleen (inflammatory and immune responses), and lung. In addition, lung cross section showing sequestration of stem cells in pulmonary capillaries. (Lung parenchyma nuclei labeled with DAPI and stained blue. Stem cells labeled with quantum dots and stained red.) [167]
Intra-arterial Infusion
Intra arterial infusion of progenitor cells allows for more focused delivery and could bypass the significant first pass pulmonary effect seen with intravenous infusion. Intra carotid infusion of MSCs in rat TBI and stroke models has shown up to 21% engraftment in the injury boundary zone, corpus callosum, and ipsilateral hemisphere [22, 23]. The Steinberg laboratory recently used fluorescent activated cell sorting to enrich an NSC population for CD49d (a cellular surface integrin known to increase inflammatory cell endothelial attachment via interaction with vascular cell adhesion molecule 1 (VCAM-1)). The enriched NSC population was infused after hypoxic ischemic injury. There was increased engraftment in the area of cerebral ischemia with improved behavioral functioning as compared to CD49d negative controls [24].
Although intra carotid infusion improves progenitor cell engraftment, the generation of distal emboli remains a potential threat. Recent research using a transcarotid laser Doppler to measure cerebral blood flow after intra carotid infusion of MSCs in a stroke model showed up to an 80% decrease in Doppler signal associated with high levels of progenitor cell engraftment. Cerebral blood flow decreased in an MSC dose dependent fashion leading to significant neurological morbidity [19]. Figure 2 outlines the advantages and possible untoward effects of intra carotid infusion of progenitor cells. Intra arterial infusion of progenitor cells could bypass significant pulmonary effect and enhance cellular engraftment, however, the potential risk of distal emboli and decreased cerebral blood flow need to be assessed.
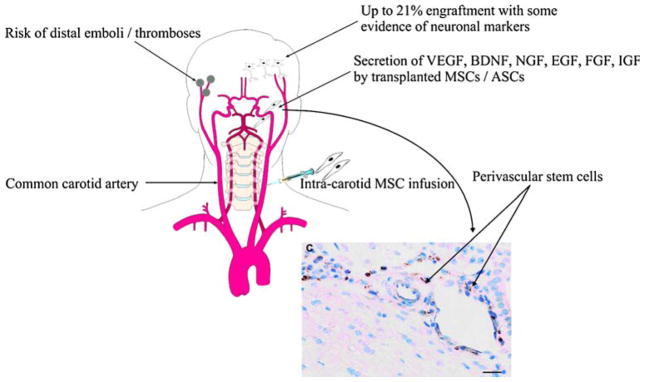
Fig. 2.
Advantages and possible untoward effects of intra-carotid transfusion of stem cells to treat ischemic or traumatic brain injury with cross section of vessel showing stem cells located in a perivascular niche which are a likely source of growth factor secretion. (BrdU labeled stem cells stained brown.) [23, 167]
Direct Implantation
Direct intracerebral implantation of progenitor cells maximizes delivery at the injury site allowing for enhanced cellular engraftment. Direct implantation of MSCs 24 h after CCI in a rat brain has shown MSC engraftment, proliferation, and significant functional motor improvement [25, 26]. After CCI in a rat model, neural stem cells (NSCs) directly implanted into the injury site showed up to 1.9% engraftment 2 weeks after injury with improvements in motor and cognitive functioning [27]. Snyder et al. implanted NSCs 3 days after CCI in either the ipsilateral or contralateral cerebral hemispheres. Histological analysis of both implantation groups showed progenitor cell survival for up to 13 weeks in the hippocampus and/or area adjacent to injury site with differentiation into neural and/or glial cells [28]. Figure 3 displays NSC engraftment in a rat brain CCI model after direct implantation.
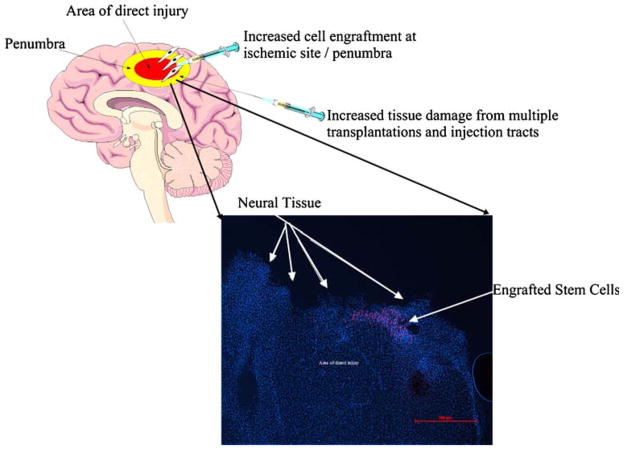
Fig. 3.
Advantages and possible untoward effects of direct transplantation of stem cells for treatment of traumatic brain injury with neuronal cross section showing engraftment of transplanted NSCs. (DAPI stained neuronal nuclei appear blue. Quantum dot labeled stem cells stained red.) [27, 167]
Preclinical research has shown potential improvement in progenitor cell engraftment and motor/cognitive functioning after direct implantation. Prior to wide scale clinical trials, further investigation into progenitor cell tumorigenesis needs to be completed. Preliminary research with human MSCs has shown spontaneous transformation into cancer cells upon expansion in vitro for 4–5 months [29]. Further investigation has shown the development of fibrosarcomas after subcutaneous injection of MSCs [30]. Creating multiple traumatic needle tracts in order to stereotactically implant progenitor cells throughout a large injury cavity will likely extend the initial injury area. Direct implantation allows focused progenitor cell delivery without the significant first pass pulmonary effect seen with cell infusion, however; further pre clinical research to ensure the safety of this method needs to be completed.
Intrathecal Infusion
Intrathecal infusion offers a minimally invasive delivery vehicle that could bypass first pass pulmonary effect and lead to increased progenitor cell engraftment. Intrathecal infusion of NSCs after hemisection of the cervical spinal cord has shown migration to the site of injury with cellular engraftment at up to 5 weeks later [31]. In a rabbit TBI model, intrathecal infusion of MSCs showed increased cellular engraftment with improved motor functioning [32]. While intrathecal delivery offers easy access and allows for the delivery of a large number of cells to an area near the injury, the safety of this approach, along with the ultimate effectiveness of delivery to the optimal location, remain unknown.
Novel Stem Cell Delivery Methods
Scaffold Implantation
Cellular therapy will likely require the introduction of progenitor cells en masse. The field of tissue engineering has been developed to investigate and optimize this form of therapy [33–35]. Engineered scaffolding constructs must supply structural and microenvironmental support to direct and maintain the viability of implanted progenitor cells [36]. In many ways, the scaffold must act as a temporary and artificial extra-cellular matrix (ECM) [37]. The intimate relationship between structure and function makes tissue scaffolding an exercise in creating shapes that match the functional goals of therapy and cellular engraftment [38, 39].
There is a great deal of variability in scaffold design to meet the specific requirements for different tissues. Overall shape may be unimportant for constructs designed for relatively amorphous organs such as brain or liver where the main emphasis is simple delivery. Cells must be delivered via a scaffold that enhances both engraftment and development while providing adequate strength. Scaffold materials and constructs intended for implantation must meet certain criteria including:
Capable of delivery without loss of function
Adequate cellular adhesion to the construct
Influence cell differentiation down desired cell lineages via mechanical or chemical interaction with support matrix [40–42]
Capacity for spontaneous in-vivo degradation
1. Capacity for delivery
For therapeutic applications, scaffolding constructs must be capable of injection, topical placement, or direct implantation into a necrotic defect without losing functional integrity. Furthermore, the geometry of scaffolding must be compatible with stereotactic or surgical delivery to the site of implantation. If the area of therapy is sufficiently large or complex, a piecewise means of treatment using multiple scaffolds may be necessary. Overall, the delivery vehicle of the scaffolding depends upon the nature of the injured tissue and the accessibility of the defect.
2. Cell localization through adhesion
The interface between progenitor cell and scaffolding is one of the most important features of a tissue engineering system with interface strength being a primary concern [43]. A proper scaffold should be effective in keeping cells localized to a particular area through strong adherence. Adhesion mechanisms include cell adhesion molecules (CAMs) such as integrins and cadherins binding to proteins (fibronectin, vitronectin) naturally present in the ECM [44]. Identical or analogous compounds should be present in the scaffold, either through absorption or based upon its native structure that allow for enhanced progenitor cell adherence.
3. Mechanical strength
Scaffolds must withstand the mechanical loads applied by native tissue and during fixation/implantation. For example, a construct implanted to repair a complex defect must be able to be incorporated into surrounding tissue. Therefore, it must have enough strength and flexibility to accept fixation. A porous scaffold of pure hydroxyapatite, while attractive from a biological point of view, is brittle and will shatter under torsion when screws are applied. More flexible materials such as thin layers of poly(vinyl alcohol) (PVA), or poly(glycerol sebacate) (PGS) give a scaffold more elasticity.
4. Mechanotransduction
Cell/scaffold interaction also plays a major role in gene expression due to mechanotransductive processes [42, 43]. Evidence of such a relationship is seen in vitro where confluence induces contact inhibition, presumably due to paracrine effects. Cell shape and cytoskeletal tension also influence the differentiation of multipotent cells [44]. These two properties are highly dependent on the microenvironment of the cell and are influenced by the scaffolds. The dynamic response of a scaffold to applied forces also affects the differentiation pathway [45]. For example, engineered blood vessels designed for arterial implantation must be developed under pulsatile flow conditions for proper engraftment [46]. The material must match the flexibility of surrounding arteries; long experience with hemodialysis vascular grafts shows that aneurysms inevitably form at the junction of normal artery and stiff implant material [47].
5. Capacity for degradation
A final requirement is that scaffolding must be degraded and replaced with native tissue or ultimately incorporated into the tissue itself. Therefore, the scaffold material must be bioresorbable or comprised of stromal material derived from the target tissue (hydroxyapatite/nacre for bone or collagen for most other tissues). Many artificial materials used for cell scaffolds are broken down through hydrolytic mechanisms that clip the ester bonds forming the backbone of the polymer chain [48]. The polymer chain is degraded into primary subunits that are excreted through renal and/or respiratory mechanisms. Composites of different subunits within the polymer allow adaptation of the degradation rate to meet design specifications [49]. The degradation rate can be controlled in some copolymer mixtures (such as poly(D, L-lactic acid co-glycolic acid) (PLGA) by modifying the ratio of the two constituents [49]. An important point is that degradation brings about an inevitable change in the scaffold structure and tissue microenvironment. As hydrolysis of the biomaterials progresses, swelling of the scaffold may occur [50]. In addition, byproducts of scaffolding degradation could potentially alter the loco regional milieu changing progenitor cell behavior [51].
Scaffold Materials
Optimal production of a scaffolding construct requires careful material selection based upon therpeutic indication and location and mode of deployment. Both natural and biosynthetic reabsorbable materials have been developed and used in clinical practice for more than 30 years. Traditionally, scaffolds were fabricated from synthetic hydrocarbon based fibers; however, the recent combination of tissue engineering and cell therapeutics has increased interest in naturally occurring materials that more readily degrade in vivo without compromising structural integrity or cellular support. Table 1 lists currently used synthetic and natural materials and will be discussed below.
Table 1.
Common natural and synthetic construct materials
| Natural materials | Synthetic materials |
|---|---|
| Collagen | Polycaprolactone (PCL) |
| Alginate | Polydioxanone (PDO) |
| Elastin | Polyglycolic acid (PGA) |
| Fibrinogen | Poly l-lactic acid (PLLA) |
| Chitosan | Poly (D,L-lactic acid co-glycolic acid) (PLGA) |
| Poly (glycerol sebacate) (PGS) | |
| Gelatin-siloxane (GS) |
Natural Materials
1. Collagen
Collagen is the most abundant mammalian protein comprising the majority of connective tissue [52]. Although the main types of interstitial collagen (Type 1 (skin), Type 2 (connective tissue), and Type 3/fetal collagen (blood vessels, skin and organ parenchyma)) are derived from different genes, their basic structure is similar, consisting of a triple helical domain made from three chains [53]. A major component of the extracellular matrix, collagen contains many chemoattractant binding domains that are key to cellular adhesion, proliferation, and differentiation [52, 54]. The indigenous nature of collagen and potential ability to supply both structural and nutritive support make it an attractive material for scaffold fabrication [55].
Initial in vitro research has shown long term survival of MSCs implanted onto three dimensional (3D) collagen scaffolds with terminal differentiation into osteoblasts and adipocytes [56]. In addition, the Potts laboratory showed maturation of MSCs down vascular cell lineages when cultured on 3D type I collagen scaffolds. Scanning electron microscopy showed smooth walled cylindrical tube like structures with smooth muscle cells consistent with microvessel morphogenesis [57]. Bolliet et al. transfected MSCs impregnated on collagen scaffolds with plasmids for glial cell line derived neurotrophic factor (pGDNF). Such a combination of tissue engineering with enhanced growth factor production offers theoretical promise for neural tissue defects after tumor excision or penetrating trauma [58].
Preliminary in vivo studies using rodent stroke, spinal cord injury, and TBI models have shown the potential neuroprotective effects of progenitor cell laden collagen scaffolds. The Evans laboratory implanted scaffolds seeded with neural stem cells (NSCs) into the infarction cavity of murine brains after hypoxic ischemic injury. Multiple reciprocal interactions between the implanted NSCs and damaged host parenchymal tissue were noted with a decrease in infarction cavity volume and regeneration of cortical tissue [59]. In addition, injection of NSCs in fibronectin treated collagen matrices improved progenitor cell survival and migration up to 3 weeks after murine cortical injury [60]. Yoshii et al. implanted collagen filament nerve scaffolds into the spinal cords of rabbits after transection showing axonal regeneration and functional neurologic recovery [61].
Another potential mechanism for progenitor cell/scaffold neuroprotection is the stimulation of vasculogenesis. Injection of enriched CD133+ progenitor cells into the ischemic hindlimb of athymic rats showed improved restoration of the vascular network [62]. The structural and nutritive support afforded by collagen scaffolds could potentially enhance progenitor cell engraftment and function leading to improved vasculogenesis and resident neural cell regeneration; thus, warranting further investigation for the treatment of CNS injury.
2. Alginate
Alginate is a naturally occurring polymer rendered from the brown algae of seaweed. Classically, it has been used to fabricate high detail dental casts [63]. Due to cast stability at room/body temperature and construct porosity, alginate is an attractive material for scaffold fabrication. Culture of MSCs in 3D alginate beads modified with the tripeptide arginine-glycine-aspartic acid showed high progenitor cell viability with little cellular proliferation. Upon dissolution of the alginate construct, MSCs returned to monolayer culture offering a functional model to evaluate 3D cultures of progenitor cells [64]. In addition, culture of newborn rat hepatocytes and progenitor cells in a macroporous alginate scaffold promoted maturation into functional hepatic tissue; an effect not seen in collagen constructs [65]. The Cohen laboratory incorporated microspheres into porous alginate scaffold capable of the controlled release of pro-angiogenic growth factors such as basic fibroblast growth factor (bFGF). After construct implantation into rat peritoneum, a four fold increase in penetrating capillaries was observed [66].
Implantation of alginate scaffolds seeded with NSCs into the spinal cord defect after transection in a rat model has shown axonal regeneration and progenitor cell differentiation into astrocytes [67]. Preliminary research has shown the ability of alginate scaffolds to promote angiogenesis as well as progenitor cell viability, maturation, and differentiation; thus warranting further investigation into the potential role of alginate constructs for CNS injury.
3. Elastin
Elastin fibers are components of the extracellular matrix with the ability to stretch up to 1.5 times their original length [68]. The fibers are located throughout all connective tissues accounting for arterial pulsation, venous capacitance, and pulmonary compliance. A large body of research has been completed to evaluate combination collagen/elastin scaffolds for usage as vascular grafts. While pure collagen constructs have proved too weak to be used as vascular grafts for in vivo studies, the addition of elastin increased construct strength and improved viscoelastic properties [69]. The Wang laboratory coated elastin scaffold tubules with the biodegradable elastomer, poly (glycerol sebacate), and subsequently seeded the lumen of the tubes with smooth muscle cells. After 1 week in culture with gentle perfusion, the scaffold lumen was impregnated with endothelial progenitor cells under pulsatile flow conditions. At 8 weeks, a confluent cellular lumen was noted with compliance comparable to human arteries [70]. Furthermore, implantation of pure elastin tubes filled with aragose gel containing bFGF implanted in subdermal patches in adult rats showed increased cellular infiltration and neovascularization when compared to controls [71]. The potential benefit of improved angiogenesis leading to neural cell repair after CNS insult warrants investigation into the role of pure elastin or elastin hybrid scaffolds for the treatment of TBI.
4. Fibrin
Fibrin or factor 1a is a glycoprotein derived from fibrinogen and synthesized in the liver. It plays a central role in hemostasis by catalyzing the formation of thrombin. Classically, fibrin scaffolds have been used in multiple tissue engineering fields including bone, cardiac tissue, cartilage, and ligaments [72]. The Krishnan laboratory observed differentiation of peripheral blood mononuclear cells into endothelial cells and smooth muscle cells when cultured in a fibrin gel scaffold [73]. In addition, culture of human MSCs in fibrin constructs have been shown to maintain viability and differentiate into chondrocytes [74] and osteoblasts [75]. Willerth et al. cultured embryonic stem cells in a fibrin matrix and observed progenitor cell differentiation into neurons and astrocytes [76]. Although initial in vitro research has shown promise for progenitor cell viability and differentiation in a fibrin construct, little in vivo work has been completed using CNS injury models.
5. Chitosan
Chitosan is a linear polysaccharide with procoagulant properties derived from the exoskeleton of crustaceans. Classically, chitosan based bandages have been used for hemostasis in cases of acute hemorrhage [77]; however, recent work has investigated the potential role of chitosan based contructs for tissue engineering. Culture of both adipose tissue derived stem cells (ADSCs) and MSCs on chitosan scaffolds showed progenitor cell adhesion, proliferation, and differentiation [78, 79]. Recent in vivo work colonized chitosan channels with NSCs and implanted the contructs into a spinal cord injury defect in rats. Progenitor cells engrafted, survived and were found to differentiate into oligodendrocytes and astrocytes [80]; however, no locomotor improvement was observed [81]. While preliminary research has shown potential efficacy using spinal cord injury model, further investigation using TBI and stroke models is warranted.
Synthetic Materials
Synthetic bioresorbable materials have been developed and used in clinical practice for more than 30 years in the form of sutures, screws and meshes. Synthetic materials can be fabricated to swell or shrink when exposed to different temperatures, pH, ionic strength, electric field, and light. The compositions of the synthetic polymers can be designed to minimize immune response and degrade at different rates (Table 2). Synthetic polymers can be manufactured to combine the properties that are unique to each construct [82]. The majority of the applications of these materials have been with regards to bone, vascular, and cartilage repair/reconstruction; correlations and applicability of each of these materials will be made to the central nervous system (CNS), when applicable.
Table 2.
Synthetic polymer degradation times
| Material | Product names | Degradation time |
|---|---|---|
| Polycaprolactone (PCL) | MONOCRYL® | >20 months |
| Polydioxanone (PDO) | PDS® BIOSYN® | 6 months |
| Polyglycolic acid (PGA) | DEXON® | 1–4 months |
| Poly l-lactic acid (PLLA) | BioScrew®, PL-FIX | 20–60 months |
| Poly (D,L-lactic acid co-glycolic acid) (PLGA) | VICRYL® (90% Glycolide 10% Lactide) | 2 months |
| Poly (glycerol sebacate) (PGS) | 2 months | |
| Gelatin-siloxane (GS) | >2 months |
1. Polycaprolactone [PCL]
PCL is a polymer with minimal bio-reactivity that has been utilized in a wide variety of biomedical applications including the construction of absorbable sutures (Monocryl) and scaffolds [83, 84]. One of the advantages of PCL is the relative ease to form polymer blends with other materials. Degradation does not result in acidic byproducts, and metabolism of the byproducts is via the tri carboxylic acid cycle with renal excretion. Its high rate of permeability has made it an attractive option for drug delivery systems. [83, 85] PCL based microspheres have been used as a vehicle for the delivery of nerve growth factor, which may promote axonal regeneration after CNS injury [86]. Additionally, PCL based matrices have been used as nerve guides and as a scaffold for tissue regeneration after control cortical impact induced TBI and spinal cord transection [87–89].
Properties that may serve well in the CNS include its low rate of hydrolytic degradation (up to 1 year) (secondary to its hydrophobic and high crystalline properties) that can be enhanced by the copolymers (i.e., poly (lactic acid) or poly (glycolic acid)). PCL scaffold have shown the capacity to support a wide variety of cell types, including fibroblasts and MSCs [85, 90, 91]. Studies have confirmed the ability of MSCs to differentiate down multiple developmental lineages on PCL based scaffolds [92]. In addition, extensive in vivo and in vitro testing has confirmed the relative inertness of the compound [84, 93].
2. Polydioxanone [PDO]
PDO is a flexible synthetic, absorbable polymer that has been used in the construction of scaffolds and suture material (PDS). It degrades via hydrolysis [94] at a moderate rate (approximately 6 months) and is believed to have little immunological and inflammatory reactivity. Long term (i.e. 6 months) studies in animals have demonstrated no significant toxicity [95]. It has significant shape memory, which may prove valuable in tissue engineering applications, particularly scaffold construction [96].
PDO based scaffolds have been engineered with electro-spinning and utilized for the construction of vascular grafts, repair of tendon and bony defects, and as nerve guides [96–100]. PDO based monofilaments have been utilized as nerve grafts, [101] able to maintain viability of implanted Schwann cells [102]. In addition, PDO based scaffolds have been utilized as a matrix for MSCs for the repair of osteochondral defects [103]. Complexing PDO based polymers with natural polymers (i.e., elastin) allows for increased bioreactivity and tissue in-growth [104]. Composite PDO/elastin constructs could offer potential enhancement of progenitor cell viability and function.
3. Polyglycolic acid [PGA]
PGA is another synthetic, biodegradable polymer (Dexon). PGA is easy to shape and is relatively porous allowing for the diffusion of nutrients to support tissue growth [105]. Degradation generates acidic breakdown products that could potentially result in a local inflammatory reaction; however, testing has confirmed the relative inert nature of this compound. There is rapid loss of strength over a period of 2 to 4 weeks, which may prove valuable for short term applications and in minimizing native tissue bio-reactivity/immune response. Degradation of PGA based polymers can be controlled by manipulating the lactic/glycolic acid ratio [59, 105].
PGA based scaffolds have been used successfully to culture progenitor cells allowing for differentiation [106]; however a potential increase in the local inflammatory response may occur [107]. In addition, cells seeded on PGA based constructs have shown a decreased rate of proliferation [106, 108]. PGA complexed with a hydrogel allows for structural integrity; however it is important to note that the formation of three dimensional structures may be difficult secondary to its relatively weak intrinsic strength as compared to other synthetic polymers [105].
The majority of PGA based scaffold applications have been in the field of orthopedics. They have also been used successfully in composite for progenitor cell based articular cartilage regeneration [109]. PGA scaffolds implanted with NSCs demonstrated beneficial effects in a murine model of hypoxic ischemic injury, including differentiation into neurons and modulation of the inflammatory response [59].
4. Poly l-lactic acid [PLLA]
PLLA is a hydrophobic, biocompatible synthetic polymer [110]. It is degradable, porous, flexible, and can maintain structural integrity [111]. The hydrophobic nature of PLLA may prove detrimental as compared to other polymers, with regards to apopotosis, proliferation and cell viability [112]. Breakdown may result in the release of acidic byproducts that could potentially enhance the local inflammatory response [113]; however, long term studies utilizing PLLA medullary rods demonstrate minimal inflammatory reaction [114]. In addition, seeding PLLA scaffolds with chondrocytes has shown a decrease in the local inflammatory response after subcutaneous implantation [115].
PLLA constructs have a longer degradation time when compared to other polymers, having shown to be present at 3 years after implantation. Its structural characteristics have proven useful for the construction of orthopedic hardware [115]. PLLA based scaffolds have been shown to support NSC culture [116] and have also been used as conduits for nerve regeneration [111].
5. Poly (D,L-lactic acid co-glycolic acid) [PLGA]
PLGA, an amorphous copolymer, is formed by polymerizing poly (glycolic acid) and poly (lactic acid). The lack of a definite repeating form, shape, or structure causes this polymer to be less rigid, weaker, and more easily deformed. An advantage of copolymerization is the ability to control the rate of degradation of PLGA by adjusting the ratio of PLLA/PGA.
PLGA has been used as a scaffold for tissue engineering and has the ability to support growth of multiple cell types [117–122]. In addition, cell proliferation, differentiation, and function can be mediated by incorporation of bioactive molecules onto the scaffold surface [120–122].
PLGA scaffolds have been widely investigated for neural tissue engineering applications. Electrospun tubes made from a blend of PLGA/PCL implanted in vivo into a 10-mm nerve gap in a rat sciatic nerve induced neural regeneration and functional reconnection of the two severed sciatic nerve tracts. Myelination and collagen IV deposition were detected in the regenerated fibers and neural tracers revealed restoration of functional neuronal connections and reinnervation of the target muscles. There was no significant inflammatory response due to the scaffold. This study showed that electrospun tubes, with no additional biological coating or drug loading treatment, are promising scaffolds for functional nervous regeneration [123].
Neurite growth has been studied using micropatterns on PLGA films coated with collagen type I or laminin peptide using a laser ablation method. The micropatterned PLGA films provided a substrate that guided both the neurite outgrowth and elongation [124]. Neuronal repair, regeneration, and function after peripheral nerve or spinal cord injury was studied by delivering cellular and trophic factors to the site of injury using PLGA scaffolds seeded with genetically engineered green fluorescent protein (GFP) rat MSCs transfected with an adenoviral vector for nerve growth factor. Morphology, viability, and growth kinetics were maintained when cells were grown on the PLGA scaffold. Levels of secreted NGF from MSCs were physiologically relevant and functionally active [125].
6. Poly (glycerol sebacate) [PGS]
PGS is a biodegradable elastomer that is able to recover from deformation and can effectively distribute stress evenly throughout regenerating tissues. PGS absorbs minimal water and does not swell during degradation. PGS scaffold induces a minimal immune response, has high metabolic activity, allows cell attachment and proliferation, and does not induce apoptosis [126–128].
PGS has been used as a nerve guidance conduit showing no negative effects on resident Schwann cells. In vitro, PGS construct behavior was similar to that of PLGA; however, in vivo testing showed significantly less inflammation and fibrosis without detectable swelling during degradation compared to that of PLGA [129].
7. Gelatin-siloxane (GS)
GS is a three-dimensional scaffold that is synthesized from the integration of gelatin and 3-(glycidoxypropyl) trimethoxysilane (GPSM). GS scaffolding has both strength and slow biodegradation that is easily controlled by the amount of bridging bonds or cross-link density between gelatin chains and GPSM molecules. Altering the degradation rate has shown no inhibitory effect on cell proliferation in vitro [130].
GS hybrid implantation has been used to investigate resident neuron regeneration in a cortical defect. The implant maintained cortical shape while attaching to the surrounding neurons. Scaffolds stained with bromodeoxyuridine showed newly formed vascular endothelial cells as well as both astroglial and microglial cells. In addition, resident dendrites were found to communicate with the newly formed neuronal cells. Furthermore, GS scaffolds embedded with bFGF and epidermal growth factor (EGF) showed a dose-dependent enhancement of cell growth [131].
GS constructs coated with vascular endothelial growth factor (VEGF) have been implanted into neural defects in both TBI and stroke models. Thirty days after implantation, adult neuronal cells were noted in the scaffolds. Immunohistochemical techniques found endothelial, astroglial, and microglial cells. VEGF was found to have a dose dependent effect upon the proliferation of the observed neuronal and endothelial cells [132].
Scaffold Fabrication
Some scaffold fabrication methods yield morphologically suitable structures with randomly assorted channels and pathways such as foaming of polymers induced by phase change [133, 134] or other physical events designed to create porosity in a substrate [135]. Some groups have even attempted to use samples of coral to act as a scaffold for neural cells [136]. Creation of chaotically distributed channels may be suitable for in vivo use given extensive empirical testing and stringent adherence to good manufacturing practices (GMP). A few of these methods are reviewed below.
1. Phase Separation
Phase separation is a common technique used to create structures with nano- and micro-scale features. This method takes advantage of differing physical properties between two or more materials. Typically one of the materials is intended for implantation, and the other(s) acts as a porogen. There are several different classes and subclasses of phase separation that have a significant effect on the outcome of the polymer scaffold.
Phase separation as it pertains to tissue scaffold fabrication can be considered solid-liquid or liquid-liquid. Solid-liquid phase separation involves the freezing and subsequent sublimation of the solvent. This method is commonly known as freeze drying or lypholization. The morphology of the pores created is a direct result of the crystalline structure formed as the solvent freezes [137].
Schugens et al. introduced a method of freeze drying solutions of poly (l-lactide) (PLLA) in 1,4 Dioxane to create scaffolds that can exhibit directional porosity. Specifically, they determined that the direction of cooling guided the direction of macropore formation. The average macropore diameter was 100 μm. The concentration of PLLA was found to be optimal at 1%, although it had a limited effect on scaffold properties. This study represents the first significant work related to phase separation methods for the fabrication of tissue scaffolds.
Teng et al. [138] used Schugens’ solid-liquid phase separation method with PLGA in their two layer scaffold system for spinal cord repair to create an oriented pore structure with the intent to direct axonal growth along the long axis of the spinal cord. They created these channels by slowly lowering tubes into a cold solution of ethanol and dry ice (−78C), and then sublimated in a freeze dryer. The result was axially oriented pores that replicated the white matter of the spinal cord. The inner grey matter of the spinal cord was fabricated using a salt-leaching process, as described later.
Liquid-liquid phase separation is more complicated in that there are two different subclasses, each resulting in different polymer morphology. One subclass undergoes phase separation through a crystal growth process at specific points of nucleation. The alternative is known as spinodal decomposition, where the separation occurs throughout the solution spontaneously and not at discrete nucleation sites. Both classes of liquid-liquid phase separation usually occur through cooling the solution to within a narrow temperature range. After phase separation, the solvent is removed through successive dilution and with a liquid that does not dissolve the scaffold material. Spinodal decomposition yields nanofibers that are desirable in scaffolds intended for CNS applications, namely fibers that are on the order of hundreds of nanometers in diameter, with porosities greater than 80%.
Liu et al. [139] used liquid-liquid phase separation and leaching with PLLA and microspheres of gelatin to create a nano-fibrous matrix with surface modifications for a bone scaffold. The gelatin microspheres were designed to create voids in the scaffold to increase porosity. The first stage of phase separation involved mixing a solution of PLLA dissolved in Tetrahydrofuran (THF) at 60°C with the gelatin microspheres. The combined solution was then placed in a −76°C freezer to initiate a phase change of the solvent, leaving PLLA in a nanofibrous structure. Cyclohexane was used to replace the THF. The gelatin was leached out of the scaffold to create interconnected pores throughout the scaffold.
Yang et al. [140] also used PLLA in solution with THF to create a nanofibrous scaffold for nerve tissue engineering. The solution was cast onto a glass petri dish and rapidly cooled, then diluted and freeze dried. Neonatal mouse cerebellum stem cells were seeded at a concentration of 3.77 *10^4 cells/cm^2. Neurite outgrowth initiated shortly after seeding.
2. Leaching
Leaching is another method that has been used extensively in the fabrication of tissue scaffolds. The process involves casting the polymer mixed with a salt into a desired shape, and then introducing a solvent that would dissolve the salt and leave the polymer intact, leaving a foamlike structure. The pore size distribution is dependant upon the morphology of the salt crystals. Weight percentages of 80% or higher of salt can be used to create highly porous structures, many times with interconnected pores.
Teng’s [138] spinal cord scaffold used the salt leaching process to build the gray matter section, using PLGA dissolved in chloroform over NaCl with crystal diameters ranging from 250–500 μm. Upon evaporation of the chloroform, water was introduced to dissolve the salt. This hybrid design is noteworthy because it uses two separate fabrication methods to create a composite scaffold, thereby taking advantage of the structural differences each material and method provides.
3. Electrospinning
Another method to create tissue scaffolds in a semi-repeatable fashion is to utilize the process of electrospinning. Although the concept was developed more than a century ago [141] and has found utility in textile research [142], electrospinning has only recently been applied to create engineered tissue scaffolds [143]. Electrospinning has the capability to create fibers from the nanometer to micrometer scale [144] that are structured in various configurations, matching the dimensions of the ECM natively found in tissue.
Conceptually, electrospinning is simply the extrusion of a polymer due to the repulsive surface charges generated by an electric field. The process is initiated by injecting a solution of a polymer dissolved in a volatile solvent slowly through a charged needle (on the order of tens of kV). A pendant drop forms at the tip of the needle, at which point electrostatic forces pull a stream of the solution towards a grounded structure. As the stream moves through the air, the solvent evaporates, leaving a thin fiber of the polymer. Dalton [145] has created a system where the polymer is melted prior to ejection and propelled electrostatically as a fluid jet where it quickly cools and solidifies, eliminating the need for the solvent altogether.
The simple nature of fabrication allows the electrospun fibers to be arranged in a number of different configurations. If the electrospun fibers are deposited on a flat grounded plate, they will arrange in a nonwoven mat with random fiber orientation. Cylinders of nanofibers are formed by depositing the fibers on a grounded rotating mandrel. The alignment of fibers on the cylinder can be adjusted by varying the speed of rotation. Panseri fabricated cylinders from a blend of PLGA and PCL fibers to create a sciatic nerve graft. In their case, the mandrel was simply a 16 gauge bare copper wire. Other groups have built larger cylinders for use as scaffolds for regenerating blood vessels.
Aligned fibers can be formed by rotating a mandrel at high speeds, or alternatively by using two parallel grounded plates separated by a small distance. Fibers would span the gap between the two plates. Aligned fibers are important for structural tissue such as cartilage or muscle, but it can also be important for CNS applications since cells have the tendency to align along the direction of the nanofibers. Other configurations include woven strands [146], stacked layers [147] and coaxial tubes [148].
Fiber diameter and other characteristics of the nanofibers can be controlled by modifying variables of the setup, including applied voltage, spacing between needle and plate, needle size and solute concentration. Zhao et al. [149] has shown how the variables effect electrospinning of PLGA, and Theron et al. provide the results of a study that experimented with a number of different polymers including poly (ethylene oxide) (PEO), PVA, and PCL, focusing on the electrical properties of the solution [150]. Mathematical modeling has been performed to predict the behavior of the nanofiber as it is propelled by the electric field [151]. Teo and Ramakrishna provide a comprehensive review of the unique setups [152].
While the use of electrospun fibers as a cell scaffold has been investigated in many settings, limited research has been performed using CNS models. Yang et al. [116] electrospun PLLA and investigated the effect of fiber diameter and alignment with respect to murine NSC growth. A comparison was made between four groups: Aligned and randomly oriented fiber mats of 300 nm and 1.5 μm average diameter fibers. The fiber diameter was changed by altering the concentration of the polymer in solution (2% for smaller fibers versus 5% for the larger fibers). Alignment was controlled using the rotating mandrel method previously described. Collecting the fibers on a rotating platform caused a slight reduction in fiber diameter compared to their randomly oriented counterpart. It was surmised that a stretching force was being applied to the fibers due to the rotation of the mandrel. The NSCs were implanted on each of the four study groups. It was found that cell differentiation is based upon fiber diameter, and not the degree of fiber alignment. Neurite outgrowth length was 20% greater in the aligned 300 nm diameter fiber group compared to the other three groups. The authors conclusion was to focus investigation of nanoscale diameter aligned fibers for neural tissue engineering.
Nisbet et al. [153] investigated the response of murine embryonic cortical neurons on both PLLA and PLGA electrospun scaffolds. They performed surface modification of the nanofibers using potassium hydroxide to change hydrophilicity. They concluded that a reduction surface tension (i.e. an increase in scaffold hydrophilicity) leads to a quicker outgrowth of neurites from the seeded cells. Another observation was the path of neurite extension followed fibers directly if the fiber concentration was low, but crossed perpendicular to fibers if the density was greater. Highly dense regions of fibers were avoided by neurite outgrowths. This led the authors to conclude that control of the fiber density could allow the designer a limited degree of controlling neurite outgrowth direction. A solid foundation has been established by these and other investigators in the field of electrospinning tissue scaffolds. However, much more work needs to be done on improving the process and control of scaffold fabrication using this method.
4. Rapid Prototyping
The tissue fabrication methods described above have been shown to work well within various areas of the CNS. However, they lack the ability to control the specific shape of the construct other than molding it within a customized container. Techniques developed in fields outside of biology are finding utility within the microscale world of tissue engineering [154]. One field which has transformed the capabilities of tissue engineering is rapid prototyping (RP). RP describes a number of different manufacturing processes that allow automated fabrication using unique methods of material bonding or deposition. RP systems are capable of producing objects with geometry that make it difficult, even impossible, to be created using the “traditional” machining methods of milling, turning, or drilling. Furthermore, RP methods enable users to fabricate such geometries directly from files generated using standard computer aided design (CAD) software [155]. Many RP techniques are additive rather than subtractive, and typically build an object one thin layer at a time. RP methods give a designer flexibility to create geometries that would previously be impossible to achieve using any amount of labor and/or non-repeatable.
RP methods were not originally designed with medical applications in mind. As a result, these methods each have drawbacks that limit their potential as therapeutic devices for use in the CNS. A reduction in build volume and increased resolution is often necessary for suitable scaffolds to be created [156]. SLA fabrication requires the use of cytotoxic photopolymers that prevents concurrent deposition of cells during fabrication. Furthermore, the original photopolymers developed for SLA were never intended to be biodegradable. The SLS fabrication process occurs at temperatures far above the upper limit for human cells. Researchers are striving to eliminate these barriers [157]. Many problems can be bypassed by completing fabrication prior to cell seeding. Additionally, much of the work using RP methods in cellular scaffolding has been directed towards orthopedic therapies such as osteogenesis or articular cartilage regeneration, since most RP techniques produce parts that are rather stiff and strong. Such mechanically robust structures are not optimal for the brain. The topic of RP methods and their utility in cell scaffolding has been well covered in numerous review articles [158–160].
Selective laser sintering (SLS) is a subset of RP that uses a laser to fuse powdered material into geometries specified by the user in a layer by layer fashion. Prior to fabrication, the user would design the shape to be created in a CAD program and send it to a special program that slices the part into 2-dimensional shapes that will subsequently be used by the fabrication system. A SLS machine consists of three chambers, two feed chambers and one fabrication chamber. The feed chambers are filled with powdered material, and have a piston in each chamber that moves the contents upwards. All three chambers are heated to a temperature just below the melting temperature of the fabrication material. A roller transfers a thin layer of powder from one feed chamber to the fabrication chamber. A laser then raster scans the thin sheet of powder, melting and fusing the powder in a pattern determined by a computer program. Once the scanning is complete, the fabrication chamber is lowered slightly, and a new layer of powder is deposited for the next scan. This process is repeated until all parts have been completed. The end result is parts surrounded by un-sintered powder that acts as a structural support during the fabrication steps. The unused powder can be recycled for later use.
Tight control of temperature and laser power is required to ensure accurate fabrication of parts. Additionally, the powdered material must be homogeneous in its size distribution to generate repeatable geometries. Many machines are optimized to work with proprietary materials that are not designed for direct implantation. However, most SLS control programs allow the user to modify settings and experiment with different materials. Some materials may be problematic if they are unable to be ground to a powder like consistency (i.e. are gummy or have the tendency to clump together). The smallest feature size is dependent upon the material being used, the step size of the powder layers, and the properties of the laser (beam width, power output etc.)
Biomaterials can be quite expensive, and most SLS systems are large in size. This mandates that the build volume be reduced to minimize the requisite amount of powder needed for a scaffold to be made. Zhou et al. [156] modified their SLS system to perform research with PLLA/hydroxyapatite microspheres with the application naturally being a bone scaffold. They also had to reduce the set temperature of the machine to match the material’s properties. Their results showed that porous bone scaffolds can be made successfully using a modified SLS system.
5. Stereolithography
Stereolithography (SLA) uses a laser and a photosensitive liquid polymer solution to fabricate parts. The main difference between SLA and SLS is that the precursor material is a liquid rather than a solid. Fabrication occurs by a piston lowering step by step in a bath of the photocurable solution as a UV laser scans over the top surface of the solution, solidifying the material. If the part being built is inherently unstable, support structures are required to keep the part upright during the build process, and are removed after fabrication is complete. After the part has been fabricated it must undergo a post-cure treatment where it is heated in a UV oven. The feature size of a production grade SLA system (3D Systems) can reach as small as .005″, which is the minimum layer thickness that can be generated. Some research groups have built customized systems to create scaffolds with improved resolution.
Lu [161] created a system utilizing a digital micro-mirror array instead of a conventional raster scanning laser to build scaffolds using poly(ethylene diacrylate) as the crosslinking polymer. With this system the micromirror selectively masks UV light to crosslink the polymer one layer at a time. The resulting scaffold has a feature size of approximately 20 μ. Although their goal was to create a scaffold for an osteogenic differentiation of MSCs, this method could be easily adapted for other areas of the body.
6. Three dimensional (3D) printing (3DP)
3DP is mechanically very similar to the SLS system in that it feeds a thin layer of powder across an area where an object is to be fabricated. However, instead of using a laser, a liquid binder is deposited onto the powder using methods commonly found in standard inkjet printers. The additive process is repeated until the part is complete. Factors such as temperature are not as important in this method. However, the final part is not as structurally strong as an SLS part, and may need to be reinforced with an infiltrating material. From a tissue engineering standpoint, this factor may not be critical.
Wong et al. [88] used a 3D printing system to mold scaffolds of PCL in various geometries to investigate importance of scaffold geometry in a cortical injury application. Various types of grooves and channels were created using the 3D printer. Wong observed that actrocyte elongation into the scaffold occurred more often in porous scaffolds with integrated channels containing microgrooves oriented in the desired direction of elongation.
Progenitor Cell/Scaffold Immunogenicity
The harvest of bone marrow to generate MSCs is an invasive process that can cause significant pain. In addition, while still controversial, the number of bone marrow derived MSCs has been shown to decline with increased donor age [162]. The ready availability and increased yield of progenitor cells derived from alternate sources such as human umbilical cord blood and rodent bone marrow make both allografts and xenografts attractive therapeutic models. Potential immunogenecity, however, is a concern.
Initial in vitro studies have shown umbilical cord derived MSCs (UC-MSCs) to lack expression of major histocompatibility complex II (MHC II), CD 40, and CD 80. In addition, allogenic UC-MSCs did not stimulate mononuclear cell proliferation when cultured with donor blood and suppressed the function of mature dendritic cells [163]. Direct implantation of both human and rat MSCs into rat striatum showed very few dendritic and T cells in the neural tissue consistent with the hypoimmunogenicity observed with in vitro studies [164].
To further evaluate the potential immunomodulatory effect of MSC grafts, irradiated rodents received allogeneic bone marrow transplants with and without host or donor MSCs. Transplantation of autologous MSCs was associated with increased graft survival with tolerance of donor antigens; however, allogenic MSCs were associated with a significant increase in graft rejection. Furthermore, allogenic MSC transplantation into naïve animals was showed an increase in memory T cells [165].
Both allogenic and autologous ADSCs seeded on collagen scaffolds and implanted into spinal cord defects in rats were associated with accelerated spinal fusion. Although allogenic ADSCs potentiated an increase in IgG antibodies, they were found to be non cytotoxic in the presence of complement [166].
The immunogenicity and potential immunomodulatory effect of progenitor cell therapy remains a controversial subject. The majority of studies have investigated bone marrow derived MSCs and UC-MSCs with little work completed to evaluate progenitor cells derived from separate niches. In addition, little investigation into the immunomodulatory effects of scaffolds seeded with progenitor cells has been completed to date. Additional in vitro and in vivo studies evaluating progenitor cell immunogenicity need to be completed prior to the implementation of multicenter trials using allografts or xenografts for CNS injury.
Future Considerations
Initial research showing benefit of progenitor cell therapy for the treatment of TBI indicated transdifferentiation of the stromal cells into neurons as the likely mechanism of action. However, the current consensus is that the frequency of transdifferentiation is far too low to account for the observed therapeutic benefit. The effect is more likely due to alterations in the intracellular milieu of the CNS via modulation of the inflammatory response, production of neurotrophic growth factors, stimulation of angiogenesis, or transfer of genes/proteins via cellular fusion. Regardless of the mechanism, optimal therapeutic effect requires progenitor cell delivery to the injury site while maintaining viability and cellular function.
The optimal method of progenitor cell delivery for CNS injury remains controversial. Intravenous infusion offers minimally invasive access with the promise of widespread cellular distribution; however, previous work has shown low levels of engraftment at the injury site likely due to a large first pass pulmonary effect. While intra arterial infusion allows more focused delivery with enhanced engraftment, there is a significant risk of distal cellular emboli and reduced cerebral blood flow for the treatment of brain injury. Direct progenitor cell implantation offers increased engraftment but is invasive and could require multiple injections (needle tracts) for a large injury cavity. Initial investigation using intrathecal delivery has shown promise, but more work needs to be completed to evaluate engraftment efficiency.
The implantation of scaffolds seeded with progenitor cells could potentially act as an artificial ECM providing both structural and nutritive support. While initial construct production used mainly synthetic materials, preliminary investigation with natural materials and synthetic/natural hybrid constructs has shown promise for enhanced cellular engraftment and function. In addition, new manufacturing techniques such as electrospinning and RP afford increased control of construct shape and fiber orientation. Such control of construct characteristics could allow for scaffold fabrication to fit the specifications of a specific CNS defect and allow for axonal growth parallel to construct fibers. While initial studies have shown benefit with both NSCs and MSCs seeded onto scaffold constructs for the treatment of CNS lesions, additional investigation into optimal progenitor cell type and concentration needs to be conducted.
TBI and spinal cord injury are major sources of morbidity worldwide with no treatment currently available to combat the observed secondary neuronal injury. The implantation of nanofiber scaffolds seeded with progenitor cells could have neuroprotective effects mitigating the chronic motor, cognitive, and psychosocial deficits seen with CNS diseases. Future in vivo and in vitro investigation into optimal progenitor cell type, production material, and fabrication technique to ensure optimal cell delivery, viability, and function needs to be completed prior any multicenter clinical trial.
Contributor Information
Peter A. Walker, Department of Surgery, University of Texas Medical School at Houston, Houston, TX, USA. Department of Pediatric Surgery, University of Texas Medical School at Houston, 6431 Fannin Street, MSB 5.236, Houston, TX 77030, USA
Kevin R. Aroom, Department of Surgery, University of Texas Medical School at Houston, Houston, TX, USA. Department of Pediatric Surgery, University of Texas Medical School at Houston, 6431 Fannin Street, MSB 5.236, Houston, TX 77030, USA
Fernando Jimenez, Department of Surgery, University of Texas Medical School at Houston, Houston, TX, USA. Department of Pediatric Surgery, University of Texas Medical School at Houston, 6431 Fannin Street, MSB 5.236, Houston, TX 77030, USA.
Shinil K. Shah, Department of Surgery, University of Texas Medical School at Houston, Houston, TX, USA. Department of Pediatric Surgery, University of Texas Medical School at Houston, 6431 Fannin Street, MSB 5.236, Houston, TX 77030, USA
Matthew T. Harting, Department of Surgery, University of Texas Medical School at Houston, Houston, TX, USA. Department of Pediatric Surgery, University of Texas Medical School at Houston, 6431 Fannin Street, MSB 5.236, Houston, TX 77030, USA
Brijesh S. Gill, Department of Surgery, University of Texas Medical School at Houston, Houston, TX, USA
Charles S. Cox, Jr, Email: charles.s.cox@uth.tmc.edu, Department of Surgery, University of Texas Medical School at Houston, Houston, TX, USA. Department of Pediatric Surgery, University of Texas Medical School at Houston, 6431 Fannin Street, MSB 5.236, Houston, TX 77030, USA.
References
- 1.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. Journal of Head Trauma Rehabilitation. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Consensus conference. Rehabilitation of persons with traumatic brain injury. NIH consensus development panel on rehabilitation of persons with traumatic brain injury. Journal of the American Medical Association. 1999;282:974–983. [PubMed] [Google Scholar]
- 3.Gray DS, Burnham RS. Preliminary outcome analysis of a long-term rehabilitation program for severe acquired brain injury. Archives of Physical Medicine and Rehabilitation. 2000;81:1447–1456. doi: 10.1053/apmr.2000.16343. [DOI] [PubMed] [Google Scholar]
- 4.Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. British Journal of Anaesthesia. 2007;99:18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- 5.Gao J, Prough DS, McAdoo DJ, Grady JJ, Parsley MO, Ma L, et al. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Experimental Neurology. 2006;201:281–292. doi: 10.1016/j.expneurol.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–1203. discussion 203–4. [PubMed] [Google Scholar]
- 7.Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, Sanchez-Ramos J, et al. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplantation. 2002;11:275–281. [PubMed] [Google Scholar]
- 8.Weiner LP. Definitions and criteria for stem cells. Methods in Molecular Biology. 2008;438:3–8. doi: 10.1007/978-1-59745-133-8_1. [DOI] [PubMed] [Google Scholar]
- 9.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 10.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. Journal of Neurosurgery. 2006;104:272–277. doi: 10.3171/jns.2006.104.2.272. [DOI] [PubMed] [Google Scholar]
- 11.Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297:1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- 12.Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, et al. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27:355–363. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, et al. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allers C, Sierralta WD, Neubauer S, Rivera F, Minguell JJ, Conget PA. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation. 2004;78:503–508. doi: 10.1097/01.tp.0000128334.93343.b3. [DOI] [PubMed] [Google Scholar]
- 15.Boomsma RA, Swaminathan PD, Geenen DL. Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. International Journal of Cardiology. 2007;122:17–28. doi: 10.1016/j.ijcard.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Vendrame M, Gemma C, Pennypacker KR, Bickford PC, Davis Sanberg C, Sanberg PR, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Experimental Neurology. 2006;199:191–200. doi: 10.1016/j.expneurol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 18.Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. NeuroReport. 2001;12:559–563. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- 19.Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harting MT, Jimenez F, Cox CS., Jr The pulmonary first-pass effect, xenotransplantation and translation to clinical trials—a commentary. Brain. 2008;131:e100. doi: 10.1093/brain/awn142. author reply e1. [DOI] [PubMed] [Google Scholar]
- 21.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplantation Proceedings. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- 23.Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. Journal of Neurotrauma. 2001;18:813–819. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- 24.Guzman R, De Los Angeles A, Cheshier S, Choi R, Hoang S, Liauw J, et al. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008;39:1300–1306. doi: 10.1161/STROKEAHA.107.500470. [DOI] [PubMed] [Google Scholar]
- 25.Mahmood A, Lu D, Yi L, Chen JL, Chopp M. Intracranial bone marrow transplantation after traumatic brain injury improving functional outcome in adult rats. Journal of Neurosurgery. 2001;94:589–595. doi: 10.3171/jns.2001.94.4.0589. [DOI] [PubMed] [Google Scholar]
- 26.Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 27.Harting MT, Sloan LE, Jimenez F, Baumgartner J, Cox CS., Jr Subacute neural stem cell therapy for traumatic brain injury. Journal of Surgical Research. 2008;144:425. doi: 10.1016/j.jss.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riess P, Zhang C, Saatman KE, Laurer HL, Longhi LG, Raghupathi R, et al. Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery. 2002;51:1043–1052. doi: 10.1097/00006123-200210000-00035. discussion 52–4. [DOI] [PubMed] [Google Scholar]
- 29.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, et al. Spontaneous human adult stem cell transformation. Cancer Research. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R, et al. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Research. 2007;67:10889–10898. doi: 10.1158/0008-5472.CAN-07-2665. [DOI] [PubMed] [Google Scholar]
- 31.Lepore AC, Bakshi A, Swanger SA, Rao MS, Fischer I. Neural precursor cells can be delivered into the injured cervical spinal cord by intrathecal injection at the lumbar cord. Brain Research. 2005;1045:206–216. doi: 10.1016/j.brainres.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Jiang X, Fu X, Cui S, Du M, Cai Y, et al. Bone marrow stromal cells can be delivered to the site of traumatic brain injury via intrathecal transplantation in rabbits. Neuroscience Letters. 2008;434:160–164. doi: 10.1016/j.neulet.2007.12.067. [DOI] [PubMed] [Google Scholar]
- 33.Guillot PV, Cui W, Fisk NM, Polak DJ. Stem cell differentiation and expansion for clinical applications of tissue engineering. Journal of Cellular and Molecular Medicine. 2007;11:935–944. doi: 10.1111/j.1582-4934.2007.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 35.Griffith LG, Naughton G. Tissue engineering—current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 36.Sands RW, Mooney DJ. Polymers to direct cell fate by controlling the microenvironment. Current Opinion in Biotechnology. 2007;18:448–453. doi: 10.1016/j.copbio.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao S, Li B, Ma Z, Wei H, Chan C, Ramakrishna S. Biomimetic electrospun nanofibers for tissue regeneration. Biomed Mater. 2006;1:R45–R53. doi: 10.1088/1748-6041/1/3/R01. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Engineering Part B Reviews. 2008;14:61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 39.Nishimura I, Garrell RL, Hedrick M, Iida K, Osher S, Wu B. Precursor tissue analogs as a tissue-engineering strategy. Tissue Engineering. 2003;9(Suppl 1):S77–S89. doi: 10.1089/10763270360696996. [DOI] [PubMed] [Google Scholar]
- 40.Chai C, Leong KW. Biomaterials approach to expand and direct differentiation of stem cells. Molecular Therapy. 2007;15:467–480. doi: 10.1038/sj.mt.6300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 42.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophysical Journal. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burdick JA, Vunjak-Novakovic G. Review: engineered Microenvironments for controlled stem cell differentiation. Tissue Engineering Part A. 2008 doi: 10.1089/ten.tea.2008.0131. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aplin AE, Howe AK, Juliano RL. Cell adhesion molecules, signal transduction and cell growth. Current Opinion in Cell Biology. 1999;11:737–744. doi: 10.1016/s0955-0674(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 45.Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnology and Bioengineering. 2004;88:359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 46.Xu ZC, Zhang WJ, Li H, Cui L, Cen L, Zhou GD, et al. Engineering of an elastic large muscular vessel wall with pulsatile stimulation in bioreactor. Biomaterials. 2008;29:1464–1472. doi: 10.1016/j.biomaterials.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 47.Flora HS, Talei-Faz B, Ansdell L, Chaloner EJ, Sweeny A, Grass A, et al. Aneurysm wall stress and tendency to rupture are features of physical wall properties: an experimental study. Journal of Endovascular Therapy. 2002;9:665–675. doi: 10.1177/152660280200900518. [DOI] [PubMed] [Google Scholar]
- 48.Gopferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- 49.Lavik E, Teng YD, Snyder E, Langer R. Seeding neural stem cells on scaffolds of PGA, PLA, and their copolymers. Methods in Molecular Biology. 2002;198:89–97. doi: 10.1385/1-59259-186-8:89. [DOI] [PubMed] [Google Scholar]
- 50.Yoon JJ, Park TG. Degradation behaviors of biodegradable macroporous scaffolds prepared by gas foaming of effervescent salts. Journal of Biomedical Materials Research. 2001;55:401–408. doi: 10.1002/1097-4636(20010605)55:3<401::aid-jbm1029>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 51.Mohammadi YJE. Monte carlo simulation of degradation of porous Poly(lactide) scaffolds, 1:effect of porosity on pH. Macromolecular Theory and Simulations. 2006;15:643–653. [Google Scholar]
- 52.Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. Journal of Biological Chemistry. 2002;277:4223–4231. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 53.Brodsky B, Ramshaw JA. The collagen triple-helix structure. Matrix Biology. 1997;15:545–554. doi: 10.1016/s0945-053x(97)90030-5. [DOI] [PubMed] [Google Scholar]
- 54.Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 55.Zhong S, Teo WE, Zhu X, Beuerman RW, Ramakrishna S, Yung LY. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. Journal of Biomedical Materials Research A. 2006;79:456–463. doi: 10.1002/jbm.a.30870. [DOI] [PubMed] [Google Scholar]
- 56.Neuss S, Stainforth R, Salber J, Schenck P, Bovi M, Knuchel R, et al. Long-term survival and bipotent terminal differentiation of human mesenchymal stem cells (hMSC) in combination with a commercially available three-dimensional collagen scaffold. Cell Transplantation. 2008;17:977–986. doi: 10.3727/096368908786576462. [DOI] [PubMed] [Google Scholar]
- 57.Valarmathi MT, Davis JM, Yost MJ, Goodwin RL, Potts JD. A three-dimensional model of vasculogenesis. Biomaterials. 2009;30:1098–1112. doi: 10.1016/j.biomaterials.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 58.Bolliet C, Bohn MC, Spector M. Non-viral delivery of the gene for glial cell line-derived neurotrophic factor to mesenchymal stem cells in vitro via a collagen scaffold. Tissue Engineering Part C Methods. 2008;14:207–219. doi: 10.1089/ten.tec.2008.0168. [DOI] [PubMed] [Google Scholar]
- 59.Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nature Biotechnology. 2002;20:1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 60.Tate MC, Shear DA, Hoffman SW, Stein DG, Archer DR, LaPlaca MC. Fibronectin promotes survival and migration of primary neural stem cells transplanted into the traumatically injured mouse brain. Cell Transplantation. 2002;11:283–295. [PubMed] [Google Scholar]
- 61.Yoshii S, Ito S, Shima M, Taniguchi A, Akagi M. Functional restoration of rabbit spinal cord using collagen-filament scaffold. Journal of Tissue Engineering and Regenerative Medicine. 2009;3:19–25. doi: 10.1002/term.130. [DOI] [PubMed] [Google Scholar]
- 62.Suuronen EJ, Veinot JP, Wong S, Kapila V, Price J, Griffith M, et al. Tissue-engineered injectable collagen-based matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation. 2006;114:I138–I144. doi: 10.1161/CIRCULATIONAHA.105.001081. [DOI] [PubMed] [Google Scholar]
- 63.Shaba OP, Adegbulugbe IC, Oderinu OH. Dimensional stability of alginate impression material over a four hours time frame. Nigerian Quarterly Journal of Hospital Medicine. 2007:1–4. doi: 10.4314/nqjhm.v17i1.12531. [DOI] [PubMed] [Google Scholar]
- 64.Duggal S, Fronsdal KB, Szoke K, Shahdadfar A, Melvik JE, Brinchmann JE. Phenotype and gene expression of human mesenchymal stem cells in alginate scaffolds. Tissue Engineering Part A. 2008 doi: 10.1089/ten.tea.2008.0306. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 65.Dvir-Ginzberg M, Elkayam T, Cohen S. Induced differentiation and maturation of newborn liver cells into functional hepatic tissue in macroporous alginate scaffolds. FASEB Journal. 2008;22:1440–1449. doi: 10.1096/fj.07-9277com. [DOI] [PubMed] [Google Scholar]
- 66.Novikova LN, Novikov LN, Kellerth JO. Biopolymers and biodegradable smart implants for tissue regeneration after spinal cord injury. Current Opinion in Neurology. 2003;16:711–715. doi: 10.1097/01.wco.0000102620.38669.3e. [DOI] [PubMed] [Google Scholar]
- 67.Wu S, Suzuki Y, Kitada M, Kitaura M, Kataoka K, Takahashi J, et al. Migration, integration, and differentiation of hippocampus-derived neurosphere cells after transplantation into injured rat spinal cord. Neuroscience Letters. 2001;312:173–176. doi: 10.1016/s0304-3940(01)02219-4. [DOI] [PubMed] [Google Scholar]
- 68.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nature Genetics. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 69.Berglund JD, Nerem RM, Sambanis A. Incorporation of intact elastin scaffolds in tissue-engineered collagen-based vascular grafts. Tissue Engineering. 2004;10:1526–1535. doi: 10.1089/ten.2004.10.1526. [DOI] [PubMed] [Google Scholar]
- 70.Gao J, Crapo P, Nerem R, Wang Y. Co-expression of elastin and collagen leads to highly compliant engineered blood vessels. Journal of Biomedical Materials Research A. 2008;85:1120–1128. doi: 10.1002/jbm.a.32028. [DOI] [PubMed] [Google Scholar]
- 71.Kurane A, Simionescu DT, Vyavahare NR. In vivo cellular repopulation of tubular elastin scaffolds mediated by basic fibroblast growth factor. Biomaterials. 2007;28:2830–2838. doi: 10.1016/j.biomaterials.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Engineering Part B Reviews. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 73.Sreerekha PR, Divya P, Krishnan LK. Adult stem cell homing and differentiation in vitro on composite fibrin matrix. Cell Proliferation. 2006;39:301–312. doi: 10.1111/j.1365-2184.2006.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pelaez D, Huang CY, Cheung HS. Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells Development. 2008 doi: 10.1089/scd.2008.0030. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 75.Trombi L, D’Alessandro D, Pacini S, Fiorentino B, Scarpellini M, Fazzi R, et al. Good manufacturing practice-grade fibrin gel is useful as a scaffold for human mesenchymal stromal cells and supports in vitro osteogenic differentiation. Transfusion. 2008;48:2246–2251. doi: 10.1111/j.1537-2995.2008.01829.x. [DOI] [PubMed] [Google Scholar]
- 76.Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006;27:5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Englehart MS, Cho SD, Tieu BH, Morris MS, Underwood SJ, Karahan A, et al. A novel highly porous silica and chitosan-based hemostatic dressing is superior to HemCon and gauze sponges. Journal of Trauma. 2008;65:884–890. doi: 10.1097/TA.0b013e318187800b. discussion 90–2. [DOI] [PubMed] [Google Scholar]
- 78.Zhu Y, Liu T, Song K, Jiang B, Ma X, Cui Z. Collagen-chitosan polymer as a scaffold for the proliferation of human adipose tissue-derived stem cells. Journal of Materials Science Materials in Medicine. 2009;20:799–808. doi: 10.1007/s10856-008-3636-6. [DOI] [PubMed] [Google Scholar]
- 79.Moreau JL, Xu HH. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate—Chitosan composite scaffold. Biomaterials. 2009;30:2675–2682. doi: 10.1016/j.biomaterials.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zahir T, Nomura H, Guo XD, Kim H, Tator C, Morshead C, et al. Bioengineering neural stem/progenitor cell-coated tubes for spinal cord injury repair. Cell Transplantation. 2008;17:245–254. doi: 10.3727/096368908784153887. [DOI] [PubMed] [Google Scholar]
- 81.Nomura H, Zahir T, Kim H, Katayama Y, Kulbatski I, Morshead CM, et al. Extramedullary chitosan channels promote survival of transplanted neural stem and progenitor cells and create a tissue bridge after complete spinal cord transection. Tissue Engineering Part A. 2008;14:649–665. doi: 10.1089/tea.2007.0180. [DOI] [PubMed] [Google Scholar]
- 82.Nisbet DR, Crompton KE, Horne MK, Finkelstein DI, Forsythe JS. Neural tissue engineering of the CNS using hydrogels. Journal of Biomedical Materials Research Part B Applied Biomaterials. 2008;87:251–263. doi: 10.1002/jbm.b.31000. [DOI] [PubMed] [Google Scholar]
- 83.Kweon H, Yoo MK, Park IK, Kim TH, Lee HC, Lee HS, et al. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials. 2003;24:801–808. doi: 10.1016/s0142-9612(02)00370-8. [DOI] [PubMed] [Google Scholar]
- 84.Bezwada RS, Jamiolkowski DD, Lee IY, Agarwal V, Persivale J, Trenka-Benthin S, et al. Monocryl suture, a new ultra-pliable absorbable monofilament suture. Biomaterials. 1995;16:1141–1148. doi: 10.1016/0142-9612(95)93577-z. [DOI] [PubMed] [Google Scholar]
- 85.Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A. Poly-epsilon-caprolactone microspheres and nanospheres: an overview. International Journal of Pharmaceutics. 2004;278:1–23. doi: 10.1016/j.ijpharm.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 86.Cao X, Schoichet MS. Delivering neuroactive molecules from biodegradable microspheres for application in central nervous system disorders. Biomaterials. 1999;20:329–339. doi: 10.1016/s0142-9612(98)00172-0. [DOI] [PubMed] [Google Scholar]
- 87.Bender MD, Bennett JM, Waddell RL, Doctor JS, Marra KG. Multi-channeled biodegradable polymer/CultiSpher composite nerve guides. Biomaterials. 2004;25:1269–1278. doi: 10.1016/j.biomaterials.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 88.Wong DY, Krebsbach PH, Hollister SJ. Brain cortex regeneration affected by scaffold architectures. Journal of Neurosurgery. 2008;109:715–722. doi: 10.3171/JNS/2008/109/10/0715. [DOI] [PubMed] [Google Scholar]
- 89.Wong DY, Leveque JC, Brumblay H, Krebsbach PH, Hollister SJ, Lamarca F. Macro-architectures in spinal cord scaffold implants influence regeneration. Journal of Neurotrauma. 2008;25:1027–1037. doi: 10.1089/neu.2007.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mas Estelles J, Vidaurre A, Meseguer Duenas JM, Castilla Cortazar I. Physical characterization of poly-caprolactone scaffolds. Journal of Materials Science Materials in Medicine. 2008;19:189–195. doi: 10.1007/s10856-006-0101-2. [DOI] [PubMed] [Google Scholar]
- 91.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 92.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158–5166. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 93.Sun H, Mei L, Song C, Cui X, Wang P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27:1735–1740. doi: 10.1016/j.biomaterials.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 94.Smith MJ, White KL, Jr, Smith DC, Bowlin GL. In vitro evaluations of innate and acquired immune responses to electrospun polydioxanone-elastin blends. Biomaterials. 2009;30:149–159. doi: 10.1016/j.biomaterials.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 95.Molea G, Schonauer F, Bifulco G, D’Angelo D. Comparative study on biocompatibility and absorption times of three absorbable monofilament suture materials (Polydioxanone, Poliglecaprone 25, Glycomer 631) British Journal of Plastic Surgery. 2000;53:137–141. doi: 10.1054/bjps.1999.3247. [DOI] [PubMed] [Google Scholar]
- 96.Boland ED, Coleman BD, Barnes CP, Simpson DG, Wnek GE, Bowlin GL. Electrospinning polydioxanone for biomedical applications. Acta Biomaterialia. 2005;1:115–123. doi: 10.1016/j.actbio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 97.Greisler HP, Tattersall CW, Klosak JJ, Cabusao EA, Garfield JD, Kim DU. Partially bioresorbable vascular grafts in dogs. Surgery. 1991;110:645–654. discussion 54–5. [PubMed] [Google Scholar]
- 98.Sell SA, McClure MJ, Barnes CP, Knapp DC, Walpoth BH, Simpson DG, et al. Electrospun polydioxanone-elastin blends: potential for bioresorbable vascular grafts. Biomedical Materials. 2006;1:72–80. doi: 10.1088/1748-6041/1/2/004. [DOI] [PubMed] [Google Scholar]
- 99.Curtis AS, Wilkinson CD, Crossan J, Broadley C, Darmani H, Johal KK, et al. An in vivo micro-fabricated scaffold for tendon repair. Eur Cell Mater. 2005;9:50–57. doi: 10.22203/ecm.v009a07. discussion 7. [DOI] [PubMed] [Google Scholar]
- 100.Christgau M, Bader N, Felden A, Gradl J, Wenzel A, Schmalz G. Guided tissue regeneration in intrabony defects using an experimental bioresorbable polydioxanon (PDS) membrane. A 24-month split-mouth study. Journal of Clinical Periodontology. 2002;29:710–723. doi: 10.1034/j.1600-051x.2002.290808.x. [DOI] [PubMed] [Google Scholar]
- 101.Arai T, Lundborg G, Dahlin LB. Bioartificial nerve graft for bridging extended nerve defects in rat sciatic nerve based on resorbable guiding filaments. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery. 2000;34:101–108. doi: 10.1080/02844310050159936. [DOI] [PubMed] [Google Scholar]
- 102.Shen ZL, Berger A, Hierner R, Allmeling C, Ungewickell E, Walter GF. A Schwann cell-seeded intrinsic framework and its satisfactory biocompatibility for a bioartificial nerve graft. Microsurgery. 2001;21:6–11. doi: 10.1002/1098-2752(2001)21:1<6::aid-micr1001>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 103.Jeong WK, Oh SH, Lee JH, Im GI. Repair of osteochondral defects with a construct of mesenchymal stem cells and a polydioxanone/poly(vinyl alcohol) scaffold. Biotechnology and Applied Biochemistry. 2008;49:155–164. doi: 10.1042/BA20070149. [DOI] [PubMed] [Google Scholar]
- 104.Smith MJ, McClure MJ, Sell SA, Barnes CP, Walpoth BH, Simpson DG, et al. Suture-reinforced electrospun polydioxanone-elastin small-diameter tubes for use in vascular tissue engineering: a feasibility study. Acta Biomater-ialia. 2008;4:58–66. doi: 10.1016/j.actbio.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 105.Mooney DJ, Mazzoni CL, Breuer C, McNamara K, Hern D, Vacanti JP, et al. Stabilized polyglycolic acid fibre-based tubes for tissue engineering. Biomaterials. 1996;17:115–124. doi: 10.1016/0142-9612(96)85756-5. [DOI] [PubMed] [Google Scholar]
- 106.Hannouche D, Terai H, Fuchs JR, Terada S, Zand S, Nasseri BA, et al. Engineering of implantable cartilaginous structures from bone marrow-derived mesenchymal stem cells. Tissue Engineering. 2007;13:87–99. doi: 10.1089/ten.2006.0067. [DOI] [PubMed] [Google Scholar]
- 107.Seo YK, Yoon HH, Park YS, Song KY, Lee WS, Park JK. Correlation between scaffold in vivo biocompatibility and in vitro cell compatibility using mesenchymal and mononuclear cell cultures. Cell Biology and Toxicology. 2008;24:471–474. doi: 10.1007/s10565-008-9105-7. [DOI] [PubMed] [Google Scholar]
- 108.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 109.Zhou XZ, Leung VY, Dong QR, Cheung KM, Chan D, Lu WW. Mesenchymal stem cell-based repair of articular cartilage with polyglycolic acid-hydroxyapatite biphasic scaffold. International Journal of Artificial Organs. 2008;31:480–489. doi: 10.1177/039139880803100603. [DOI] [PubMed] [Google Scholar]
- 110.Jung HJ, Park K, Kim JJ, Lee JH, Han KO, Han DK. Effect of RGD-immobilized dual-pore poly(L-lactic acid) scaffolds on chondrocyte proliferation and extracellular matrix production. Artificial Organs. 2008;32:981–989. doi: 10.1111/j.1525-1594.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- 111.Evans GR, Brandt K, Niederbichler AD, Chauvin P, Herrman S, Bogle M, et al. Clinical long-term in vivo evaluation of poly(L-lactic acid) porous conduits for peripheral nerve regeneration. Journal of Biomaterials Science Polymer Ed. 2000;11:869–878. doi: 10.1163/156856200744066. [DOI] [PubMed] [Google Scholar]
- 112.Bhang SH, Lim JS, Choi CY, Kwon YK, Kim BS. The behavior of neural stem cells on biodegradable synthetic polymers. Journal of Biomaterials Science Polymer Ed. 2007;18:223–239. doi: 10.1163/156856207779116711. [DOI] [PubMed] [Google Scholar]
- 113.Willerth SM, Sakiyama-Elbert SE. Approaches to neural tissue engineering using scaffolds for drug delivery. Advanced Drug Delivery Reviews. 2007;59:325–338. doi: 10.1016/j.addr.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Matsusue Y, Yamamuro T, Oka M, Shikinami Y, Hyon SH, Ikada Y. In vitro and in vivo studies on bioabsorbable ultra-high-strength poly(L-lactide) rods. Journal of Biomedical Materials Research. 1992;26:1553–1567. doi: 10.1002/jbm.820261203. [DOI] [PubMed] [Google Scholar]
- 115.Fujihara Y, Asawa Y, Takato T, Hoshi K. Tissue reactions to engineered cartilage based on poly-L-lactic acid scaffolds. Tissue Engineering Part A. 2008 doi: 10.1089/ten.tea.2008.0154. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 116.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 117.Chen G, Tanaka J, Tateishi T. Osteochondral tissue engineering using a PLGA-collagen hybrid mesh. Materials Science and Engineering. 2006;26:124–129. [Google Scholar]
- 118.Park GE, Pattison Megan, Park K, Webster TJ. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials. 2005;26:3075–3082. doi: 10.1016/j.biomaterials.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 119.Chen G, Sato T, Ushida T, Ochiai N, Tateishi T. Tissue engineering of cartilage using a hybrid scaffold of synthetic polymer and collagen. Tissue Engineering. 2004;10:323–330. doi: 10.1089/107632704323061681. [DOI] [PubMed] [Google Scholar]
- 120.Kim SH, Yoon SJ, Choi B, Ha HJ, Rhee JM, Kim MS, et al. Evaluation of various types of scaffold for tissue engineered intervertebral disc. Advances in Experimental Medicine and Biology. 2006;585:169–181. doi: 10.1007/978-0-387-34133-0_12. [DOI] [PubMed] [Google Scholar]
- 121.Lee SJ, Lee I, Lee YM, Lee HB, Khang G. Macroporous biodegradable natural/synthetic hybrid scaffolds as small intestine mucosa impregnated poly(lactide0 co-glycolide) for tissue engineered bone. Journal of Biomaterials science Polymer Ed. 2004;15:1003–1017. doi: 10.1163/1568562041526487. [DOI] [PubMed] [Google Scholar]
- 122.Jang JW, Park KS, Kim SH. Tissue engineered bone regeneration using DBP-loaded PLGA scaffold in rabbit model. Tissue Engineering and Regenerative. 2005;2:34–40. [Google Scholar]
- 123.Panseri S, Cunha C, Lowery J, Del Carro U, Taraballi F, Amadio S, et al. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnology. 2008;8:39. doi: 10.1186/1472-6750-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yao L, Wang S, Cui W, Sherlock R, O’Connell C, Damodaran G, et al. Effect of functionalized micropatterned PLGA on guided neurite growth. Acta Biomaterialia. 2009;5:580–588. doi: 10.1016/j.actbio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 125.Rooney GE, Moran C, McMahon SS, Ritter T, Maenz M, Flugel A, et al. Gene-modified mesenchymal stem cells express functionally active nerve growth factor on an engineered poly lactic glycolic acid (PLGA) substrate. Tissue Engineering Part A. 2008;14:681–690. doi: 10.1089/tea.2007.0260. [DOI] [PubMed] [Google Scholar]
- 126.Pomerantseva I, Krebs N, Hart A, Neville CM, Huang AY, Sundback CA. Degradation behavior of poly(glycerol sebacate) Journal of Biomedical Materials Research A. 2008 doi: 10.1002/jbm.a.32327. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 127.Engelmayr GC, Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nature Matters. 2008;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ifkovits JL, Padera RF, Burdick JA. Biodegradable and radically polymerized elastomers with enhanced processing capabilities. Biomedical Materials. 2008;3:034104. doi: 10.1088/1748-6041/3/3/034104. [DOI] [PubMed] [Google Scholar]
- 129.Sundback CA, Shyu JY, Wang Y, Faquin WC, Langer RS, Vacanti JP, et al. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454–5464. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 130.Ren L, Tsuru K, Hayakawa S, Osaka A. Novel approach to fabricate porous gelatin-siloxane hybrids for bone tissue engineering. Biomaterials. 2002;23:4765–4773. doi: 10.1016/s0142-9612(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 131.Deguchi K, Tsuru K, Hayashi T, Takaishi M, Nagahara M, Nagotani S, et al. Implantation of a new porous gelatin-siloxane hybrid into a brain lesion as a potential scaffold for tissue regeneration. Journal of Cerebral Blood Flow and Metabolism. 2006;26:1263–1273. doi: 10.1038/sj.jcbfm.9600275. [DOI] [PubMed] [Google Scholar]
- 132.Zhang H, Kamiya T, Hayashi T, Tsuru K, Deguchi K, Lukic V, et al. Gelatin-siloxane hybrid scaffolds with vascular endothelial growth factor induces brain tissue regeneration. Current Neurovascular Research. 2008;5:112–117. doi: 10.2174/156720208784310204. [DOI] [PubMed] [Google Scholar]
- 133.Sachlos E, Wahl DA, Triffitt JT, Czernuszka JT. The impact of critical point drying with liquid carbon dioxide on collagen-hydroxyapatite composite scaffolds. Acta Biomaterialia. 2008;4:1322–1331. doi: 10.1016/j.actbio.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 134.Tomita M, Lavik E, Klassen H, Zahir T, Langer R, Young MJ. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells. 2005;23:1579–1588. doi: 10.1634/stemcells.2005-0111. [DOI] [PubMed] [Google Scholar]
- 135.Wang M. Composite scaffolds for bone tissue engineering. American Journal of Biochemistry and Biotechnology. 2006;2:80–84. [Google Scholar]
- 136.Peretz H, Blinder P, Baranes D, Vago R. Aragonite crystalline matrix as an instructive microenvironment for neural development. Journal of Tissue Engineering and Regenerative Medicine. 2008;2:463–471. doi: 10.1002/term.118. [DOI] [PubMed] [Google Scholar]
- 137.Schugens C, Maquet V, Grandfils C, Jerome R, Teyssie P. Biodegradable and macroporous polylactide implants for cell transplantation.1. Preparation of macroporous polylactide supports by solid-liquid phase-separation. Polymer. 1996;37:1027–1038. doi: 10.1002/(SICI)1097-4636(199604)30:4<449::AID-JBM3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 138.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu X, Won Y, Ma PX. Porogen-induced surface modification of nano-fibrous poly(L-lactic acid) scaffolds for tissue engineering. Biomaterials. 2006;27:3980–3987. doi: 10.1016/j.biomaterials.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 140.Yang F, Murugan R, Ramakrishna S, Wang X, Ma YX, Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 141.Cooley JF. Apparatus for electrically dispersing fluids. 1902:692631. [Google Scholar]
- 142.Graham K, Gogins M, Schreuder-Gibson H. Incorporation of electrospun nanofibers into functional structures. International Nonwoven Technical Conference.2003. [Google Scholar]
- 143.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. Journal of Biomedical Materials Research. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 144.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Engineering. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 145.Dalton PD, Klinkhammer K, Salber J, Klee D, Moller M. Direct in vitro electrospinning with polymer melts. Biomacromolecules. 2006;7:686–690. doi: 10.1021/bm050777q. [DOI] [PubMed] [Google Scholar]
- 146.Dalton PD, Klee D, Moller M. Electrospinning with dual collection rings. Polymer. 2005;46:611–614. [Google Scholar]
- 147.Srouji S, Kizhner T, Suss-Tobi E, Livne E, Zussman E. 3-D Nanofibrous electrospun multilayered construct is an alternative ECM mimicking scaffold. Journal of Materials Science Materials in Medicine. 2008;19:1249–1255. doi: 10.1007/s10856-007-3218-z. [DOI] [PubMed] [Google Scholar]
- 148.Li S, Sun B, Li X, Yuan X. Characterization of electrospun core/shell poly(vinyl pyrrolidone)/poly(L-lactide-co-epsilon-caprolactone) fibrous membranes and their cytocompatibility in vitro. Journal of Biomaterials Science Polymer Ed. 2008;19:245–258. doi: 10.1163/156856208783432499. [DOI] [PubMed] [Google Scholar]
- 149.Zhao L, He C, Gao Y, Cen L, Cui L, Cao Y. Preparation and cytocompatibility of PLGA scaffolds with controllable fiber morphology and diameter using electrospinning method. Journal of Biomedical Materials Research Part B Applied Biomaterials. 2008;87:26–34. doi: 10.1002/jbm.b.31060. [DOI] [PubMed] [Google Scholar]
- 150.Theron SA, Zussman E, Yarin AL. Experimental investigation of the governing parameters in the electrospinning of polymer solutions. Polymer. 2004;45:2017–2030. [Google Scholar]
- 151.Rutledge GC, Fridrikh SV. Formation of fibers by electrospinning. Advanced Drug Delivery Reviews. 2007;59:1384–1391. doi: 10.1016/j.addr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 152.Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. IOP Nanotechnology. 2006;17:R89–R106. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 153.Nisbet DR, Pattanawong S, Ritchie NE, Shen W, Finkelstein DI, Horne MK, et al. Interaction of embryonic cortical neurons on nanofibrous scaffolds for neural tissue engineering. Journal of Neural Engineering. 2007;4:35–41. doi: 10.1088/1741-2560/4/2/004. [DOI] [PubMed] [Google Scholar]
- 154.Manaresi N. A CMOS chip for individual cell manipulation and detection. IEEE Journal of Solid State Circuits. 2003;38:2297–2305. [Google Scholar]
- 155.Wang X, Yan Y, Zhang R. Rapid prototyping as a tool for manufacturing bioartificial livers. Trends in Biotechnology. 2007;25:505–513. doi: 10.1016/j.tibtech.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 156.Zhou WY, Lee SH, Wang M, Cheung WL, Ip WY. Selective laser sintering of porous tissue engineering scaffolds from poly(L: -lactide)/carbonated hydroxyapatite nano-composite microspheres. Journal of Materials Science Materials in Medicine. 2008;19:2535–2540. doi: 10.1007/s10856-007-3089-3. [DOI] [PubMed] [Google Scholar]
- 157.Schuster M, Turecek C, Mateos A, Stampfl J, Liska R, Varga F. Evaluation of biocompatible photo-polymers II: further reactive diluents. Chemical Monthly. 2007;138:261–268. [Google Scholar]
- 158.Warren SM, Hedrick MH, Sylvester K, Longaker MT, Chen CM. New directions in bioabsorbable technology. Journal of Neurosurgery. 2002;97:481–489. doi: 10.3171/spi.2002.97.4.0481. [DOI] [PubMed] [Google Scholar]
- 159.Hollister SJ. Porous scaffold design for tissue engineering. Nature Matters. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 160.Peltola SM, Melchels FP, Grijpma DW, Kellomaki M. A review of rapid prototyping techniques for tissue engineering purposes. Annals of Medicine. 2008;40:268–280. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 161.Lu Y, Mapili G, Suhali G, Chen S, Roy K. A digital micro-mirror device-based system for the microfabrication of complex, spatially patterned tissue engineering scaffolds. Journal of Biomedical Materials Research A. 2006;77:396–405. doi: 10.1002/jbm.a.30601. [DOI] [PubMed] [Google Scholar]
- 162.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mechanisms of Ageing and Development. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 163.Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220–232. doi: 10.1111/j.1365-2567.2008.02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rossignol J, Boyer C, Thinard R, Remy S, Dugast AS, Dubayle D, et al. Mesenchymal stem cells induce a weak immune response in the rat striatum after allo or xenotransplantation. Journal of Cellular and Molecular Medicine. 2008 doi: 10.1111/j.1582-4934.2008.00657.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McIntosh KR, Lopez MJ, Borneman JN, Spencer ND, Anderson PA, Gimble JM. Immunogenicity of allogeneic adipose-derived stem cells in a rat spinal fusion model. Tissue Engineering Part A. 2008 doi: 10.1089/ten.TEA.2008.0566. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Walker PA, Shah SK, Harting MT, Cox CS. Progenitor cell therapies for traumatic brain injury: barriers and opportunities in translation. Disease Models & Mechanisms. 2009;2:23–38. doi: 10.1242/dmm.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]