Abstract
Impaired cognitive functions are well-described in the aging process. GABA(B) antagonists can facilitate learning and memory in young subjects, but these agents have not been well-characterized in aging. Here we show a complete reversal of olfactory discrimination learning deficits in cognitively-impaired aged Fischer 344 rats using the GABA(B) antagonist CGP55845, such that drug treatment restored performance to that on par with young and cognitively-unimpaired aged subjects. There was no evidence that this improved learning was due to enhanced olfactory detection abilities produced by the drug. These results highlight the potential of targeting GABA(B) receptors to ameliorate age-related cognitive deficits and demonstrate the utility of olfactory discrimination learning as a preclinical model for testing novel therapies to improve cognitive functions in aging.
Keywords: CGP55845, aging, GABA(B) receptor, cognitive enhancement, odor, learning
The number of people over 65 living in the US is expected to increase from 35 to 72 million by 2030, comprising almost 20% of the US population (US Census Bureau, 2004). Further estimates suggest that as many as 30% of these individuals will develop cognitive decline ranging from severe dementia associated with pathological conditions such as Alzheimer’s disease (8-9%) (Freedman et al., 2002, Centers for Disease Control and Prevention and The Merck Company Foundation, 2007), to mild cognitive impairment (MCI, 20%) (Lopez et al., 2003, Morris, 2005). Cognitive disabilities associated with aging create a significant burden for individuals and family members as well as a financial strain on the healthcare system. As such, there is significant interest in identifying therapies to slow and/or counteract loss of learning and memory capacities associated with the aging process.
Basal forebrain cholinergic and GABAergic projection neurons are well- positioned to directly impact mnemonic function in hippocampus and other medial temporal lobe structures, the functions of which are sensitive to precipitous decline in aging (e.g., explicit/declarative and spatial learning and memory; Frotscher, 1986, Freund, 1988, Baxter et al., 1996, Pang and Nocera, 1999). As such, these transmitter systems are logical targets for therapies to improve cognitive capacities in aging. Indeed, drugs that enhance cholinergic activity (either through direct agonistic actions at cholinergic receptors or by increasing ACh availability by inhibiting acetylcholinesterase (AChE) activity) enhance a variety of cognitive functions across species (For review, please see Parent and Baxter, 2004) and most currently available treatments for age-related cognitive decline are AChE inhibitors (Fischer et al., 1989, Smith and Booze, 1995, Gibbs, 1998, Gilmor et al., 1999, Doggrell and Evans, 2003, Jones, 2003, Parent and Baxter, 2004). Indeed, MCI patients taking AChE inhibitors show increased hippocampal activity and improved performance on explicit memory tasks, demonstrating that these drugs do offer clinical benefit (e.g.,Gron et al., 2006). However, this therapeutic avenue in isolation has limitations, as the cognitive enhancing effects of AChE inhibitors in aged individuals are transient; after 3 years, MCI patients with and without AChE inhibitor treatment are cognitively equivalent (Petersen, 2005). Moreover, this class of drugs only appears effective in improving mild loss of cognitive functions and offers little benefit to aged individuals with moderate to severe learning and memory deficits (Kaduszkiewicz et al., 2005, Jacqueline and Leon, 2006, Pelosi et al., 2006, Raschetti et al., 2007).
Given that data from lesion studies in young subjects indicate that coordinated actions of cholinergic and GABAergic signaling are critical to many aspects of cognition affected by age (Baxter et al., 1995, Pang and Nocera, 1999, Pang et al., 2001, Parent and Baxter, 2004, Yoder and Pang, 2005), drug therapies targeting the GABAergic system may offer novel but complementary treatment avenues for dementia. Specifically, antagonists at the GABA(B) receptor appear to be promising candidates, as compounds from this drug class reportedly enhance cognitive function across a wide range of tasks and species in young subjects (Mondadori et al., 1996a, Mondadori et al., 1996b, Flood, 1998, Getova, 1998, Escher, 2004, Froestl et al., 2004, Helm et al., 2005, Berta et al., 2009). For example, in young rodents and non-human primates, the most well-studied GABA(B) receptor antagonist, SGS742 (CGP36742), improves performance in a two-way active avoidance task and spatial reference memory in the eight-arm radial and Morris water mazes (Getova and Bowery, 2001, Froestl et al., 2002, Helm et al., 2005, Chan et al., 2006). In the Helm et al. (2005) study, improved memory was associated with decreased hippocampal CREB2 (ATF4) activity, indicating one site of action and possible mechanism following systemic administration of this compound (Vernon et al., 2001, Chen et al., 2003, Helm et al., 2005). The clinical utility of this class of pharmaceuticals is further supported by the wide range of effective doses at which enhanced learning and memory is observed in young subjects and few side effects associated with the efficacious doses (Blake et al., 1993, Mondadori et al., 1996a, Mondadori et al., 1996b, Getova and Bowery, 2001, Helm et al., 2005, Chan et al., 2006, Emson et al., 2007). Nevertheless, surprisingly few studies have examined GABA(B) antagonists as a possible treatment for age-related cognitive deficits. This was the goal of the current report.
In this study, the GABA(B) antagonist CGP55845, a compound that to date has not been investigated within the context of aging, was assessed for its ability to improve odor discrimination learning deficits in a subset of aged F344 rats. In humans, olfactory functions are increasingly being recognized as vulnerable to age, and olfactory identification and discrimination deficits have been linked to other types of more troublesome learning and memory dysfunction such as declarative memory processes mediated by the medial temporal lobe (Gabrieli, 1996, Freedman et al., 2002, Eibenstein et al., 2005, Wilson et al., 2006). In agreement with these results, our group recently reported such a relationship in aged F344 rats (LaSarge et al., 2007). As observed among humans, considerable variability naturally occurs among the aged F344 rat population such that some aged rats maintain cognitive abilities on par with young cohorts while others develop marked and significant cognitive impairment with advancing age (Bizon et al., 2009). We observed that the same sub-population of aged F344 rats that demonstrates impaired spatial reference memory is also impaired in the ability to discriminate odors despite the fact that these rats have comparable odor detection abilities and can discriminate other sensory stimuli as well as young and aged-unimpaired cohorts (LaSarge et al., 2007).
Notably, in our previous study, odor discrimination learning abilities in individual aged F344 rats were highly consistent across novel odor discrimination pairs. Aged rats classified as “learning-impaired” eventually reached criterion levels of performance on a given discrimination problem, but there appeared to be neither savings of prior learning rules nor a practice effect across subsequently presented odor pairs. Indeed, these rats were just as impaired on their third odor discrimination problem as on their first. As such, the olfactory discrimination task presented itself as particularly well-suited for assessing the ability of pharmacological agents to improve age-related cognitive impairment. First, the task is as effective as water maze for identifying learning-impaired rats within the aged F344 study population. Second, the reliability of the olfactory discrimination learning deficit in aged rats allows for the use of a within-subject experimental design in which performance of each subject can be evaluated with and without drug treatment. Using this design, we report here that acute administration of the GABA(B) antagonist CGP55845 completely reverses olfactory discrimination learning deficits in aged learning-impaired rats, returning performance to that on par with young subjects.
Methods
Young adult (6 mo, n=10) and aged (22 mo, n=17) male F344 rats obtained from the National Institute on Aging colony (Harlan, IN, USA) were individually housed in the AALAC-accredited Psychology Department vivarium at Texas A&M University with a regular 12:12h light/dark cycle (lights on 08:00) and climate control at 25 °C. Rats were given free access to food and water except during discrimination testing, when they were food-restricted to 80% of their free-feeding weights. All rats were screened daily for health problems including but not limited to cataracts, jaundice, food and water intake, and the appearance of tumors. Sentinel rats housed in the same room were further screened for a range of pathogens, and all blood work was negative throughout testing. All animal procedures were conducted in accordance with approved institutional animal care procedures and NIH guidelines.
Olfactory discrimination learning was tested according to LaSarge et al., (2007). Briefly, the test apparatus consisted of an opaque plastic box (49 × 33 × 28 cm) divided by an opaque Plexiglas barrier into holding (16 cm) and test (33 cm) compartments, the latter of which contained two terra cotta flower pots arranged side-by-side against the rear wall. Behavior was scored by an experimenter blind to drug treatment using a video feed to a TV monitor that allowed the rats to be viewed through the rear wall of the test compartment.
Initially, rats were shaped to dig for a food reward (1/4 of a Froot Loop, Kellog’s, Battle Creek, MI) buried at varying depths in the pots, which were filled with home cage bedding (wood shavings). Raising the Plexiglas barrier marked the start of each trial and rats were considered shaped to dig when they successfully obtained the food reward buried 2 cm below the surface of both pots in under 2 minutes.
For discrimination problems, pots were filled with clean home cage bedding and the rims of the pots were scented with two different odorants (e.g., rose+ and citrus-). Odorants used were perfume oils obtained from The Bath Junkie and The Body Shop and 10 l of the full strength oil was applied to pots. Novel odors were used for each discrimination problem (i.e. – each odor was used only once). Only one pot contained the food reward (+), and the odor of the food was disguised by crushed Froot Loops sprinkled over the bedding filling both pots. The position (left or right) of the rewarded pot was varied pseudo-randomly across trials. Criterion performance on each problem consisted of six consecutive trials in which the correct (baited) pot was chosen. Rats were considered shaped to discriminate after reaching criterion performance on two odor problems prior to the onset of pharmacological testing.
Pharmacological testing began on a separate day after completion of shaping. Young and aged rats received i.p. injections of one of three doses of the GABA(B) receptor antagonist CGP55845 (0.001, 0.01 or 0.1 mg/kg; Tocris, Ballwin, MO) or 0.9% saline vehicle alone (1 ml/kg) 40 min prior to testing. The number of trials to reach criterion was used as the measure of performance. The order of injections was as follows: CGP55845, saline, CGP55845, saline, CGP55845, saline, with the order of presentation of the doses of CGP55845 randomized across rats and age groups. Only one dose of CGP55845 or saline was given each day and a 48 hour washout period was interposed between injections (during which no testing was conducted). A second cohort of animals was tested with an additional .001 mg/kg dosage. This dosage was added to provide a more comprehensive dose response curve after it was clear in the initial cohort that both 0.01 mg/kg and 0.1 mg/kg doses significantly improved performance.
After completion of discrimination testing, a subset of rats were tested for their ability to detect and respond to decreasing concentrations of odorants following vehicle (saline) and the highest effective dose of CGP55845 (0.1 mg/kg). Significant attrition due to the length of time necessary to complete odor discrimination testing and test odor detection abilities with and without drug resulted in only a subset of subjects (N=5 young adult; N= 4 aged learning-unimpaired and N=3 aged learning-impaired) completing this testing. Only animals that completed all testing, including the doses of CGP 55845, washout, saline, and odor detection assessment with and without drug were included in this latter analysis. For these tests, rats were trained on two new odor discrimination problems as described above (one for saline and one for CGP5585) using novel full strength odorants paired with mineral oil (unscented pot). For both problems, the novel odor was rewarded. After reaching criterion performance on the new problem, rats were assessed for their ability to respond to decreasing concentrations of the same odorant (diluted 1:10, 1:100, or 1:1000 in mineral oil) versus mineral oil alone. Rats received 16 trials at each dilution and the percentage of correct responses was used to assess performance.
Note that saline and CGP 55845 odor detection testing was performed on different days with at least a 48 hour interval between assessments and that assessment of performance at descending dilutions was performed immediately after reaching criterion on the initial problem.
Results
In agreement with our previous report (LaSarge et al., 2007), significantly greater variance in performance was observed among aged rats compared to young adult rats following vehicle injections (Levine’s Test of Equality of Variance performed on mean trials to criterion on saline problems: F(1,28)=6.55, p<0.05)) with some aged rats performing on par with young cohorts (hereon referred to as aged learning-unimpaired rats) and others demonstrating marked and consistent impairment across multiple discrimination problems (hereon referred to as aged learning-impaired rats). As shown in Fig. 1, young adult rats (N= 10) averaged 10.15 +/- 3.22 (S.D.) trials-to-criterion on saline problems. Performance of each aged learning-unimpaired rats (N=11) fell no more than one standard deviation above young adult performance (mean =8.26 +/- 1.89 S.D.; animals under line in Fig. 1). All other aged rats were classified as aged learning-impaired (mean trials to criterion = 19.35 +/- 4.40 S.D.; N=9; animals above line on Fig.1).
Fig. 1.
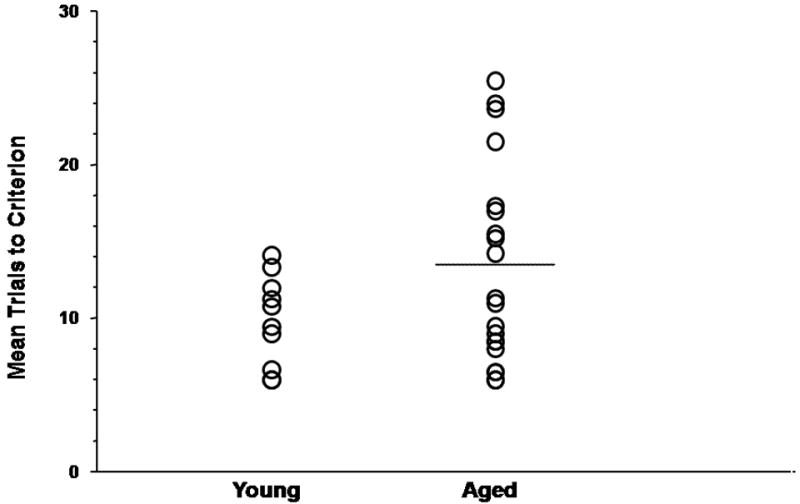
Mean trials to criterion of individual young (n=9) and aged (n=20) rats across saline odor discrimination sessions. Black lines indicate the classification of aged subjects into aged learning-unimpaired and aged learning-impaired groups. All aged-impaired rats fell outside the young mean + S.D., while all aged-unimpaired rats performed within that criterion (see text).
All rats received odor discrimination sessions following saline and two doses of CGP55845 (0.1 mg/kg and 0.01 mg/kg). As shown in Fig. 2, although aged learning-impaired rats needed more trials to reach criterion after saline compared to young adult and aged learning-unimpaired rats, after both doses of the GABA(B) antagonist, aged learning-impaired rats performed on par with the other two groups. These observations were confirmed using a two-factor repeated measures ANOVA (Cognitive Age Group × Drug Condition). The ANOVA revealed main effects of Cognitive Age Group (F(2,57)=7.34, p < .01) and Drug Condition (F(2,54)= 3.25, p < .05), as well as an interaction between Cognitive Age Group and Drug Condition, such that the drug effect on performance differed across Cognitive Age Groups (F(4,54)= 5.22, p < .01). To confirm that the interaction was a result of the drug improving the performance (trials to criterion) of aged learning-impaired rats, a series of one-factor repeated measures ANOVAs within each Cognitive Age Group with Drug Condition as the sole within-subjects factor were performed. There was a main effect of drug condition in the aged learning-impaired group (F(2,16)= 11.73, p<0.01) but not the young or aged learning-unimpaired groups (Fs<0.58, n.s.). Post hoc pair-wise comparisons confirmed that the aged learning-impaired group performed significantly better with both the 0.01 and 0.1 mg/kg dose of CGP 55845 when compared to their saline control trials (all ps<0.05).
Fig. 2.
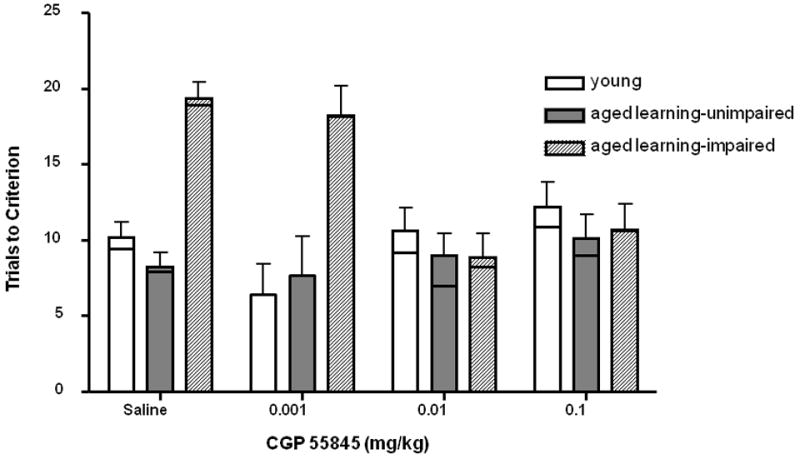
Mean (+/- S.D.) odor discrimination performance after administration of the GABA(B) antagonist CGP55845 or saline vehicle in young adult (open bar), aged learning-unimpaired (solid grey bar), and aged learning-impaired (hatched grey bar) rats. There was a significant Cognitive Age Group by Drug Condition interaction such that treatment with 0.01 and 0.1 mg/kg of CGP558445 improved trials to criterion specifically in the aged learning-impaired group. Black lines indicate means in the subset of rats tested with the 0.001 mg/kg dose, which as shown was ineffective in reversing the learning deficit in the aged learning-impaired group.
Given that both doses of CGP55845 initially tested were effective in reversing the learning deficit in aged learning-impaired rats, additional testing was performed with a lower dose of CGP55845 (0.001 mg/kg) to better define the minimum effective dose of this drug. Only a subset of animals were tested at this dose (N=5 young adult; N=3 aged learning-unimpaired; N=5 aged learning-impaired). Note that these animals received all three doses of CGP55845 and saline in the randomized order as described above. A repeated measures ANOVA (Cognitive Age Group × Drug Condition) was performed on only those subjects that received all three doses of CGP55845 to test efficacy of the lower dose. Although no main effect of drug was observed (F(3, 30) = 1.22, n.s.), the ANOVA did reveal a main effect of Cognitive Age Group (F(2,10)= 11.96, p <.01) and a significant interaction between Cognitive Age Group and Drug Condition (F (6, 30) = 2.41, p =.05). To confirm that the interaction was consistent with the larger dataset, and that it resulted from the drug improving the performance (trials to criterion) of aged learning-impaired rats, a series of one-factor repeated measures ANOVAs were conducted within each Cognitive Age Group with Drug Condition as the within-subjects factor. There was a main effect of drug condition in the aged learning-impaired group (F(3,12)= 3.80, p<0.05) but not the young or aged learning-unimpaired groups (Fs<0.998, n.s.). Post hoc pair-wise comparisons showed that even with the small group size there was still a significant improvement in performance of aged learning-impaired rats with the 0.01 mg/kg dose of CGP 55845 when compared to saline (p<0.05); the additional lower dose of 0.001 mg/kg was not effective.
We have previously found no evidence for olfactory detection deficits in aged learning-impaired F344 rats (LaSarge et al., 2007). Nevertheless, it is possible that CGP55845 enhanced olfactory discrimination learning in this subgroup by enhancing olfactory detection abilities. Hence, after completion of discrimination testing, subsets of rats from each Cognitive Age Group (N=5 young adult; N=4 aged learning-unimpaired and N=3 aged learning-impaired) were tested for their ability to detect and respond to decreasing concentrations of odorants following vehicle and the highest effective dose of CGP55845 (0.1 mg/kg). As shown in Fig. 3A and in agreement with the above data, on the discriminations performed prior to odor detection testing, a two-way ANOVA of Cognitive Age Group and Drug Condition revealed main effects of Cognitive Age Group (F (2, 8) = 5.77, p <.01) and Drug Condition (F (1, 8) = 7.18, p <.01), and an interaction between Cognitive Age Group and Drug Condition (F (2, 8) = 12.29, p <.01). Fisher’s PLSD post hoc analyses confirmed that aged learning-impaired rats performed significantly worse than young adult and aged learning-unimpaired rats in acquiring the problem after saline (ps< 0.05 in both cases) but not on the problem acquired after CGP55845. Fig. 3B shows the percent accuracy on the two discrimination problems (saline and 0.1 CGP55845) on which the odor predictive of the reward was progressively diluted (1:10, 1:100 and 1:1000 of the full strength odor). A repeated measures ANOVA revealed no main effects nor interactions involving Drug Condition, suggesting that the drug did not influence odor detection abilities. In addition, a planned comparison of performance on descending dilutions of only the aged learning-impaired rats (the group in which performance was selectively enhanced by CGP55845) after saline and CGP55845 also revealed no main effects or interaction, including at the 1:1000 dilution (F (1, 5) = 0.45, n.s.), suggesting that the improved olfactory discrimination learning observed following CGP55845 in this study was not likely a result of the drug altering gross olfactory abilities.
Fig. 3.
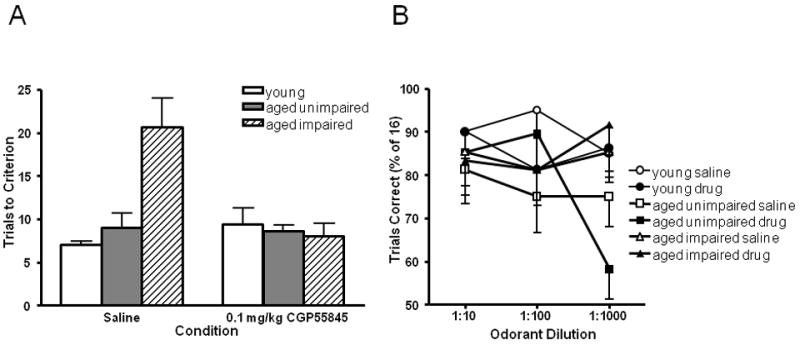
Olfactory Detection Testing With and Without GABA(B) Antagonist. (A.) Mean (+/- S.D.) odor discrimination performance in young adult (open bar), aged learning-unimpaired (solid grey bar), and aged-learning impaired (hatched grey bar) in rats after injection with either saline (control condition) or 0.1 mg/kg CGP55845 (highest effective dose shown in Fig. 1) on discrimination problems prior to odor detection testing. The aged learning-impaired group performed significantly worse than both young and aged-unimpaired under the control condition (saline), an impairment that was once again reversed under 0.1mg/kg CGP 55845. (B.) After all rats reached criterion performance on the odor discrimination problems with saline or CGP55845, the rats were assessed for their ability to detect odors at decreasing dilutions. There were no differences between Cognitive Age Groups in their ability to detect an odorant at 1:10, 1:100, or even a 1:1000 dilution of the full strength odor.
Discussion
Results from the current study are the first to demonstrate that acute treatment with the GABA(B) antagonist CGP55845 can completely ameliorate robust discrimination learning deficits that reliably occur in a subset of aged F344 rats. Notably, this enhancement of learning performance was selective to aged learning-impaired rats as performance of young and aged learning-unimpaired cohorts was not significantly affected by CGP55845 at any dose. Moreover, the reversal of learning deficits by CGP55845 in aged learning-impaired rats did not appear to be a result of the drug influencing gross olfactory detection ability, as all cognitive age groups performed similarly at a 1000-fold dilution of the odorants used for drug testing with or without the presence of the compound.
In addition to providing support for the use of this GABA(B) antagonist as a treatment for age-related learning and memory impairment, these data also demonstrate the utility of the simultaneous two-choice odor discrimination task in the F344 aging model for preclinical assessment of pharmacological interventions to improve learning deficits in aging. Reliably, approximately half of the aged subjects are cognitively impaired in this naturalistic aging model, with the same subset of aged subjects demonstrating marked spatial reference memory deficits assessed in the Morris water maze and olfactory discriminations using the simultaneous two-choice design implemented in the current report (Fig. 1; LaSarge et al., 2007, Bizon et al., 2009). In agreement with our findings, Robitsek et al. (2008) recently reported that a subset of aged rats impaired in the water maze task also performed poorly in an odor recall task, further implicating a role of hippocampus in odor learning. Unlike in the water maze, however, the ability to alternate vehicle (no drug) sessions with drug sessions and to observe a consistent return to baseline performance in the absence of drug within individual subjects even after numerous (upwards of 10) novel discrimination problems allows for stringent interpretation that the drug is responsible for changes in learning performance. Indeed, when using novel odors, there appears to be little to no savings from learning of prior odor discrimination problems.
The relationship between performance on the two tasks (water maze and the simultaneous two-choice odor discrimination task) in aged rats does suggest that the septohippocampal pathway critical for spatial learning may also be involved in the odor discrimination task used here. Indeed, Eichenbaum et al. (1988) found that lesions of the fimbria fornix, which result in deafferentation of both cholinergic and GABAergic projections from basal forebrain to hippocampus, impair learning in a very similar simultaneous two-choice odor discrimination task (Eichenbaum et al., 1988, Eichenbaum et al., 1989). A number of studies have identified the hippocampus as a common site of action following systemic administration of GABA(B) antagonists, through mechanisms such as disinhibition of excitatory presynaptic terminals and direct actions on post-synaptic hippocampal neurons (Kulik et al., 2003). GABA(B) receptor activation in the pre-synaptic terminals causes a decrease in Ca2+ conductance which inhibits neurotransmitter release, such that blockade of these receptors has a net effect of enhancing hippocampal signaling. Post-synaptic activation of hippocampal GABA(B) receptors increases K+ conductance and CREB2 activation, a transcription factor implicated as a regulator of memory suppressor genes (White et al., 2000, Bettler et al., 2004, Helm et al., 2005, Emson et al., 2007). In support of the involvement of such a mechanism in the effects observed in the present study, Helm et al. (2005) showed that systemic administration of the GABA(B) antagonist SGS742 both facilitated memory and suppressed hippocampal CREB2 activation through its interaction with CRE, reducing basal CRE binding in young rats. While involvement of the hippocampus is suggested, there is also evidence that lesions of the dorsal striatum, and not hippocampus, disrupt simultaneous two-choice odor discrimination learning (Jonasson et al., 2004, Broadbent et al., 2007). Further studies using selective lesions in combination with this specific odor discrimination task are currently being conducted to determine if the task is dependent specifically on the fornix or hippocampus and/ or if the hippocampus modulates the beneficial effects of the GABA(B) antagonists.
The absence of memory facilitation in young subjects in the current study in comparison to Helm et al. (2005) could be reflective of the different drugs used but is more likely due to differences in task difficulty between the two studies. In the current study, young and aged learning-unimpaired rats performed proficiently in the odor discrimination task, producing very few errors even in the absence of the drug. Thus, there was very little room in which to observe an enhancement of performance in these groups. Notably, there was a modest trend toward enhancement of learning in young rats at the lowest dose (which was ineffective for aged learning-impaired rats) and performance in young and aged learning-unimpaired rats was numerically worse at the highest dose of the drug tested here (0.1 mg/kg) relative to saline performance. Together with the Helm study, these data suggest that there may be an optimal level of signaling via the GABA(B) receptor that affords maximally proficient cognitive performance in both young and aged subjects.
The SGS742 compound was previously in clinical trials to treat some aspects of cognitive decline in aging, with limited success (Sabbagh, 2009). However, this is the first study to show that the CGP55845 compound specifically and robustly improves learning in aged subjects with already pronounced learning impairment. In addition, one potential role for GABA(B) antagonists in treating age-related cognitive impairment which to our knowledge has not been thus far explored is the possibility that GABA(B) antagonists may augment and/or extend the efficacy of other known cognitive enhancers in aging, most specifically those targeting the cholinergic system. Lesion studies in young subjects indicate that coordinated action of cholinergic and GABAergic transmission is critical for many of the cognitive functions vulnerable to age-related decline (Baxter et al., 1995, Pang and Nocera, 1999, Pang et al., 2001, Yoder and Pang, 2005). Moreover, one study in young rats assessed working memory following administration of SGS742, an AChE inhibitor with a combination of an AChE inhibitor and the GABA(B) antagonist, and reported greater enhancement in performance following the two drugs in combination compared to either drug alone (Helm et al., 2005). Ongoing studies in our laboratory are exploring this approach in the F344 rat cognitive aging model.
Abbreviations
- MCI
mild cognitive impairments
- AChE
acetylcholinesterase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baxter M, Bucci D, Gorman L, Wiley R, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav Neurosci. 1995;109:714–722. doi: 10.1037//0735-7044.109.4.714. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Sobel TJ, Williams MJ, Gorman LK, Gallagher M. Intact spatial learning following lesions of basal forebrain cholinergic neurons. NeuroReport. 1996;7:1417. doi: 10.1097/00001756-199605310-00019. [DOI] [PubMed] [Google Scholar]
- Berta S, Ki-Shuk S, Gunyong A, Harald H, Gert L. Hippocampal levels of phosphorylated protein kinase a (phosphor-S96) are linked to spatial memory enhancement by SGS742. Hippocampus. 2009;19:90–98. doi: 10.1002/hipo.20484. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular Structure and Physiological Functions of GABAB Receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiology of Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake JF, Cao CQ, Headley PM, Collingridge GL, Brugger F, Evans RH. Antagonism of baclofen-induced depression of whole-cell synaptic currents in spinal dorsal horn neurones by the potent GABAB antagonist CGP55845. Neuropharmacology. 1993;32:1437–1440. doi: 10.1016/0028-3908(93)90042-2. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Rats depend on habit memory for discrimination learning and retention. 2007;14:145–151. doi: 10.1101/lm.455607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, The Merck Company Foundation. The State of Aging and Health in America 2007. Whitehouse Station, NJ: The Merck Company Foundation; 2007. [Google Scholar]
- Chan KFY, Burnham WM, Jia Z, Cortez MA, Snead Iii OC. GABAB receptor antagonism abolishes the learning impairments in rats with chronic atypical absence seizures. European Journal of Pharmacology. 2006;541:64–72. doi: 10.1016/j.ejphar.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Chen A, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, Yonan AL, Vronskaya S, Grody MB, Cepeda I, Gilliam TC, Kandel ER. Inducible Enhancement of Memory Storage and Synaptic Plasticity in Transgenic Mice Expressing an Inhibitor of ATF4 (CREB-2) and C/EBP Proteins. 2003;39:655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- Doggrell SA, Evans S. Treatment of dementia with neurotransmission modulation. Expert Opinion on Investigational Drugs. 2003;12:1633–1654. doi: 10.1517/13543784.12.10.1633. [DOI] [PubMed] [Google Scholar]
- Eibenstein A, Fioretti AB, Simaskou MN, Sucapane P, Mearelli S, Mina C, Amabile G, Fusetti M. Olfactory screening test in mild cognitive impairment. Neurological Sciences. 2005;26:156–160. doi: 10.1007/s10072-005-0453-2. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Fagan A, Mathews P, Cohen NJ. Hippocampal system dysfunction and odor discrimination learning in rats: Impairment of facilitation depending on representational demands. Behavioral Neuroscience. 1988;102:331–339. doi: 10.1037//0735-7044.102.3.331. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Mathews P, Cohen NJ. Further Studies of Hippocampal Representation During Odor Discrimination Learning. Behavioral neuroscience. 1989;103:1207–1216. doi: 10.1037//0735-7044.103.6.1207. [DOI] [PubMed] [Google Scholar]
- Emson PC, James M, Tepper EDA, Bolam JP. Progress in Brain Research. Vol. 160. Elsevier; 2007. GABAB receptors: structure and function; pp. 43–57. [DOI] [PubMed] [Google Scholar]
- Escher T, Mittleman G. Effects of ethanol and GABA(B) drugs on working memory in C57BL/6J and DBA/2J mice. Psychopharmacology. 2004;176:166–174. doi: 10.1007/s00213-004-1875-x. [DOI] [PubMed] [Google Scholar]
- Fischer W, Gage FH, Bjorklund A. Degenerative changes in forebrain cholinergic nuclei correlate with cognitive impairments in aged rats. Eur J Neurosci. 1989;1:34–45. doi: 10.1111/j.1460-9568.1989.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Flood JF, Farr SA, Uezu K, Morley JE. Age-related changes in septal serotonergic, GABAergic and glutamatergic facilitation of retention in SAMP8 mice. Mechanisms of Ageing and Development. 1998;105:173–188. doi: 10.1016/s0047-6374(98)00098-0. [DOI] [PubMed] [Google Scholar]
- Freedman VA, Martin LG, Schoeni RF. Recent trends in disability and functioning among older adults in the United States: a systematic review. JAMA. 2002;288:3137–3146. doi: 10.1001/jama.288.24.3137. [DOI] [PubMed] [Google Scholar]
- Freund TF, A M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Froestl W, Gallagher M, Jenkins H, Madrid A, Melcher T, Teichman S, Mondadori CG, Pearlman R. SGS742: the first GABAB receptor antagonist in clinical trials. Biochemical Pharmacology. 2004;68:1479–1487. doi: 10.1016/j.bcp.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Froestl W, Mickel SJ, von Sprecher G, Diel PJ, Hall RG, Maier L, Strub D, Melillo V, Baumann PA. Phosphinic Acid Analogs of GABA. 2. Selective, Orally Active GABAB Antagonists. Journal of Medicinal Chemistry. 2002;38:3313–3331. doi: 10.1021/jm00017a016. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. The cholinergic innervation of the rat fascia dentata: identification of target structures on granule cells by combining choline acetyltransferase immunocytochemistry and Golgi impregnation. The Journal of Comparitive Neurology. 1986;243:58–70. doi: 10.1002/cne.902430106. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD. Memory systems analyses of mnemonic disorders in aging and age-related diseases. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13534–13540. doi: 10.1073/pnas.93.24.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getova D, Bowery N. Effects of high-affinity GABAB receptor antagonists on active and passive avoidance responding in rodents with gamma-hydroxybutyrolactone-induced absence syndrome. Psychopharmacology. 2001;157:89–95. doi: 10.1007/s002130100766. [DOI] [PubMed] [Google Scholar]
- Getova D, Bowery NG. The modulatory effects of high-affinity GABA(B) receptor antagonists in an active acoidance learning paradigm in rats. Psychopharmacology. 1998;137:369–373. doi: 10.1007/s002130050632. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Impairment of basal forebrain cholinergic neurons associated with aging and long-term loss of ovarian function. Exp Neurol. 1998;151:289–302. doi: 10.1006/exnr.1998.6789. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Erickson JD, Varuqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey AI. Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheirmer’s disease. J Comp Neurol. 1999;411 [PubMed] [Google Scholar]
- Gron G, Brandenburg I, Wunderlich AP, Riepe MW. Inhibition of hippocampal function in mild cognitive impairment: targeting the cholinergic hypothesis. Neurobiology of Aging. 2006;27:78–87. doi: 10.1016/j.neurobiolaging.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Helm KA, Haberman RP, Dean SL, Hoyt EC, Melcher T, Lund PK, Gallagher M. GABAB receptor antagonist SGS742 improves spatial memory and reduces protein binding to the cAMP response element (CRE) in the hippocampus. Neuropharmacology. 2005;48:956–964. doi: 10.1016/j.neuropharm.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Jacqueline B, Leon F. Cochrane Database Syst Rev. Vol. 3. John Wiley & Sons, Ltd; 2006. Donepezil for mild cognitive impairment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson Z, Ballantyne JK, Baxter MG. Preserved anterograde and retrograde memory of rapidly acquired olfactory discrminations after neurotoxic hippocampal lesions. 2004;14:28–39. doi: 10.1002/hipo.10146. [DOI] [PubMed] [Google Scholar]
- Jones RW. Have cholinergic therapies reached their clinical boundary in Alzheimer’s disease? International Journal of Geriatric Psychiatry. 2003;18:S7–S13. doi: 10.1002/gps.936. [DOI] [PubMed] [Google Scholar]
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt H, van der Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ. 2005;331:321–327. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Vida I, Lujan R, Haas CA, Lopez-Benito G, Shigemoto R, Frotscher M. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSarge CL, Montgomery KS, Tucker C, Slaton GS, Griffith WH, Setlow B, Bizon JL. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiology of Aging. 2007;28:928–936. doi: 10.1016/j.neurobiolaging.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Risk Factors for Mild Cognitive Impairment in the Cardiovascular Health Study Cognition Study: Part 2. Arch Neurol. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- Mondadori C, Mobius H-J, Borkowski J. The GABAB receptor antagonist CGP 36 742 and the nootropic oxiracetam facilitate the formation of long-term memory. Behavioural Brain Research. 1996a;77:223–225. doi: 10.1016/0166-4328(95)00222-7. [DOI] [PubMed] [Google Scholar]
- Mondadori C, Moebius H-J, Zingg M. CGP 36 742, an orally active GABAB receptor antagonist, facilitates memory in a social recognition test in rats. Behavioural Brain Research. 1996b;77:227–229. doi: 10.1016/0166-4328(95)00226-x. [DOI] [PubMed] [Google Scholar]
- Morris JC. Mild cognitive impairment and preclinical Alzheimer’s disease. Geriatrics. 2005 June;:9–14. [PubMed] [Google Scholar]
- Pang K, Nocera R, Secor AJ, Yoder RM. GABAergic septohippocampal neurons are not necessary for spatial memory. Hippocampus. 2001;11:814–827. doi: 10.1002/hipo.1097. [DOI] [PubMed] [Google Scholar]
- Pang KC, Nocera R. Interactions between 192-IgG saporin and intraseptal cholinergic and GABAergic drugs: role of cholinergic medial septal neurons in spatial working memory. Behav Neurosci. 1999;113:265–275. doi: 10.1037//0735-7044.113.2.265. [DOI] [PubMed] [Google Scholar]
- Parent MB, Baxter MG. Septohippocampal Acetylcholine: Involved in but not Necessary for Learning and Memory? Learn Mem. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi AJ, McNulty SV, Jackson GA. Role of cholinesterase inhibitors in dementia care needs rethinking. BMJ. 2006;333:491–493. doi: 10.1136/bmj.38945.478160.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey AI, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamine E and donepezil for the treatment of mild cognitive impairment. New England Journal of Medicine. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Raschetti R, Albanese E, Vanacore N, Maggini M. Cholinesterase Inhibitors in Mild Cognitive Impairment: A Systematic Review of Randomised Trials. PLoS Med. 2007;4:e338. doi: 10.1371/journal.pmed.0040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive Aging: A Common Decline of Episodic Recollection and Spatial Memory in Rats. 2008;28:8945–8954. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MN. Drug development for Alzheimer’s disease: Where are we now and where are we headed? The American Journal of Geriatric Pharmacotherapy. 2009;7:167–185. doi: 10.1016/j.amjopharm.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Booze RM. Cholinergic and GABAergic neurons in the nucleus basalis region of young and aged rats. Neuroscience. 1995;67:679–688. doi: 10.1016/0306-4522(95)00076-u. [DOI] [PubMed] [Google Scholar]
- Vernon E, Meyer G, Pickard L, Dev K, Molnar E, Collingridge GL, Henley JM. GABAB Receptors Couple Directly to the Transcription Factor ATF4. Molecular and Cellular Neuroscience. 2001;17:637–645. doi: 10.1006/mcne.2000.0960. [DOI] [PubMed] [Google Scholar]
- White JH, McIllhinney RAJ, Wise A, Ciruela F, Chan W-Y, Emson PC, Billinton A, Marshall FH. The GABAB receptor interacts directly with the related transcription factors CREB2 and ATFx. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13967–13972. doi: 10.1073/pnas.240452197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Tang Y, Bennerr DA. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26:61–67. doi: 10.1159/000090250. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Pang KC. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15:381–392. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]


